Abstract
Sucralose is partially absorbed after oral ingestion, with the majority excreted in the feces. We aimed to measure plasma sucralose concentrations following ingestion of doses reflecting a range of consumption (from one can of diet soda up to multiple sodas over the course of a day) and to compare concentrations in children and adults. Eleven adults (7 females, 4 males) consumed 355 mL water containing 0 mg sucralose (control) or 68, 170, or 250 mg sucralose (equivalent to 1–4 diet sodas). A second group of adults (n=11, 6 females and 5 males) consumed 355 mL Diet Rite Cola™ (68 mg sucralose and 41 mg acesulfame-potassium (ace-K)) or 68 mg sucralose and 41 mg ace-K in seltzer. Beverages were provided at separate visits in randomized order, prior to an oral glucose tolerance test. Eleven children (7 females and 4 males) consumed 0 or 68 mg sucralose in 240 mL water, in an identical study design. Blood was collected before beverage ingestion and serially for 120 min. Sucralose doses (corrected for weight) resulted in similar plasma concentrations in children and adults. Children reached peak concentrations of 145–400 ng/mL after 68 mg (mean 262.3 ± 24.6 ng/mL). Most adults reached similar peak concentrations (200–400 ng/mL after 250 mg (365.6 ± 69.9 ng/mL)) with the exception of two adults (1520 ng/mL and 1557 ng/mL, respectively). Concentrations were comparable whether sucralose was administered in water, combined with ace-K, or in diet soda. Due to their lower body weight and blood volume, children have markedly higher plasma sucralose concentrations after consumption of a typical diet soda, emphasizing the need to determine the clinical implications of sucralose use in children.
Keywords: Non-nutritive sweeteners, diet soda, sweetener absorption, pediatric
Introduction
Sucralose, the active ingredient in Splenda™, is a popular artificial sweetener used in many foods and beverages, including in baked goods and soft drinks (Schiffman and Rother 2013). The majority of orally ingested sucralose is excreted in the feces in humans, as well as in animal models including rats, dogs, and rabbits (Grice and Goldsmith 2000). In rats, it has been shown that sucralose undergoes uptake into enterocytes with efflux back into the intestinal lumen by the transporter P-glycoprotein (P-gp) and metabolism by intestinal cytochrome P-450 (CYP) enzymes (Abou-Donia et al. 2008). In humans, these mechanisms have not yet been explored. However, in a small pharmacokinetic study of eight men, 14% of ingested sucralose was absorbed, and excreted via the kidneys over 120 hours (Roberts et al. 2000).
Despite its approval as a food additive following submission of detailed toxicological data to the United States Food and Drug Administration (FDA), concerns about its safety and health effects have remained (Schiffman and Abou-Donia 2012, Swithers 2013). For example, data from in vitro models demonstrate that sucralose exposure stimulates insulin release (Nakagawa et al. 2009) and promotes adipogenesis (Simon et al. 2013). Findings from animal models demonstrate that sucralose induces P-glycoprotein and CYP3A, alters the gut microbiome, and promotes weight gain and glucose intolerance (Abou-Donia et al. 2008, Suez et al. 2014)
In light of the increasing use of sucralose by children and its potential health effects (Sylvetsky, Rother, and Brown 2011), this study was conducted to extend previous work (Roberts et al. 2000) investigating plasma sucralose concentrations in adults, and to compare these results with measurements obtained in children at doses commonly consumed. We also evaluated whether plasma sucralose concentrations differed depending on the composition of the beverage (water, carbonated water (seltzer), combined with ace-K, or commercially-available diet soda).
Methods
Twenty-two adults and eleven children were enrolled in a randomized same-subject crossover study. All adult subjects (and parents/guardians) provided written informed consent. Assent was obtained from all children. The protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Adults between 18 and 45 years old and with no known active medical conditions were included. Pregnant or lactating women and individuals using medication other than oral contraceptives were excluded. For children, inclusion criteria were age 6–12 years, weight ≥ 17 kg, and being pre-pubertal (defined by Tanner staging). Any child using medication, with elevated liver enzymes (ALT or AST > 1.5 times the upper limit of normal) or abnormal glucose tolerance was excluded. Details of the study design have been published elsewhere (Brown, Walter, and Rother 2012, 2009).
Group A (11 adults) was randomized to consume 355 mL water mixed with 0 mg, 68 mg, 170 mg, or 250 mg sucralose. Group B (11 adults) was randomized to consume 355 mL seltzer water, 355 mL caffeine-free Diet Rite Cola™ sweetened with 68 mg sucralose and 41 mg ace-K, or 68 mg sucralose and 41 mg ace-K in 355 mL of seltzer water. Group C (11 children) was randomized to consume 0 mg or 68 mg sucralose in 240 mL water. In all three groups, study visits were scheduled at least one-week apart, to avoid potential carry-over effects. All study beverages were administered 10 minutes prior to a 2-hour oral glucose tolerance test (OGTT). The current investigation was performed as sub-analysis of a larger study designed to evaluate changes in glucose-stimulated glucagon-like peptide 1 (GLP-1) response following ingestion of sucralose (manuscript under review). Adults ingested 75 g glucose in 300 mL, while children were dosed 1.75 g/kg body weight, up to 75 g. Further details of OGTTs (Brown, Walter, and Rother 2012, 2009) have been described elsewhere. Sucralose was analyzed using liquid chromatography–mass spectrometry (relative standard deviation (RSD) across all samples and replicates was < 4%). Sucralose was extracted from plasma by vortexing a 50μL aliquot of plasma and 500 μL methanol containing D6-sucralose internal standard for 5 minutes. The tube was then centrifuged at 14,000 RPM for 10 min. Supernatant (300uL) was transferred to a HPLC vial and sealed. Assays were performed with an Acquity I-Class UPLC (Waters Corp., Milford, MA) and an Acquity UPLC BEH C-18 column (2.1 mm × 50 mm, 1.7 μm) coupled with a Q-Exactive MS (Thermo Scientific, Waltham, MA) with an HESI-II electrospray source.
Results
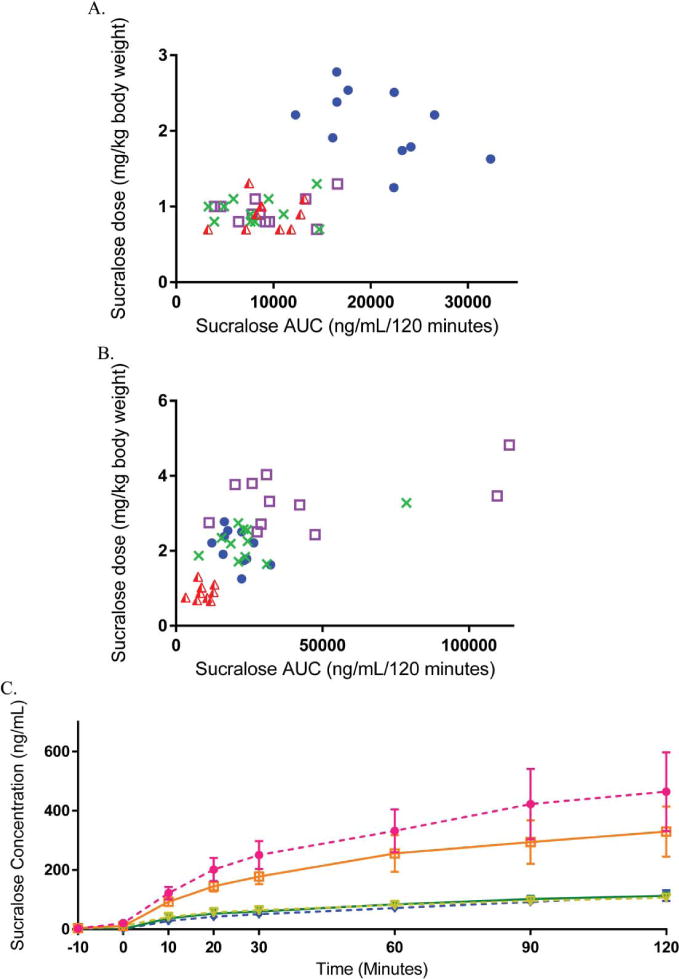
Characteristics of the study participants are shown in Table 1. Sucralose doses in adults ranged from 0.66 – 1.31 mg/kg for the 68 mg dose, 1.65 – 3.28 mg/kg for the 170 mg dose, and 2.51 – 4.82 mg/kg for the 250 mg dose. In children, sucralose doses ranged from 1.25 – 2.78 mg/kg (68 mg dose only). As shown in Figure 1A and Table 2, absolute sucralose plasma concentrations following 68 mg were markedly higher in children compared to adults (mean peak 262.3 ± 24.6 in kids vs. 108.2 ± 13.7 ng/mL in adults, p<0.0001; mean AUC0–120 20,911 ± 5,741 vs. 9,059 ± 3740 ng/mL/120 min, p<0.0001), but were similar in children and adults when AUC was divided by the individual dosage (mg/kg body weight) (10,838.3 ± 1466.4 ng/mL/120 minutes in children vs. 10,547.5 ± 1313.6 ng/mL/120 min, p=0.89 when plasma sucralose AUC following 68 mg was divided by mg/kg dose provided). The extent to which the higher sucralose concentrations observed among children were related to lower body weight (rather than their younger age) is further demonstrated in Figure 1B. Plasma sucralose concentrations after ingestion of 68 mg were inversely correlated with blood volume (calculated as 86.5 mL/kg body weight in children and 78 mL/kg body weight in adults, r= −0.616, p = 0.003), but were not associated with waist circumference or neck circumference, used as proxy measures of adiposity (adults only).
Table 1.
Characteristics of Study Participants
| Variable | Group A (11 adults) | Group B (11 adults) | Group C (11 children) |
|---|---|---|---|
| Age (years, mean ± SD) | 31.8 ± 7.5 | 27.3 ± 7.3 | 9.1 ± 1.2 |
| Female Gender | 64% | 55% | 64% |
| Race (%) | |||
| White | 64% | 55% | 73% |
| Black | 27% | 27% | 18% |
| Other | 9% | 18% | 9% |
| Weight Status (%, (mean BMI ± SD)) | |||
| Normal Weight | 46% (22.5 ± 1.9) | 36% (22.3 ± 1.6) | 82% |
| Overweight | 27% (26.9 ± 0.8) | 36% (26.6 ± 1.0) | 9% |
| Obese | 27% (32.5 ± 1.6) | 27% (33.3 ± 2.0) | 9% |
| NNS Beverage Consumption (%)1 | |||
| Never | 46% | 27% | 50% |
| ≥ 1/month | 9% | 27% | 10% |
| ≥ 1/week | 18% | 46% | 30% |
| ≥ 1/day | 27% | 0% | 10% |
| NNS Packet Use (%)1 | |||
| Never | 55% | 64% | 100% |
| ≥ 1/month | 9% | 9% | 0% |
| ≥ 1/week | 27% | 9% | 0% |
| ≥ 1/day | 9% | 18% | 0% |
Different NNS intake questionnaires were used in Group A and Group B
Mean BMI in each weight status category are for adults (Group A and Group B) only
Figure 1. Plasma sucralose concentrations in adults and children.
1A (top). Plasma sucralose area-under-the-curve for each subject is shown following 68 mg of sucralose in water in adults (red), 68 mg sucralose in diet soda in adults (green), 68 mg of sucralose mixed with 41 mg ace-K in water in adults (purple), and 68 mg sucralose in water in children (blue). 1B (bottom). Average plasma sucralose concentrations in adults following 250 mg (pink) 170 mg (orange), or 68 mg (yellow) sucralose in water, 68 mg sucralose in diet soda (blue), or 68 mg sucralose mixed with 41 mg ace-K in water (green).
In adults (Figure 1C), sucralose concentrations increased in a dose-dependent manner (mg/kg) with concentrations proportional to dose in normal weight, overweight, and obese individuals alike. Sucralose concentrations continued to increase over the 120-minute time course indicating that peak levels may not have been reached at the 2-hour time point. Average plasma sucralose concentrations were 1.4-fold higher after the 250 mg dose compared to 170 mg and 3-fold higher after 170 mg compared to 68 mg. However, in two adults, ingestion of the 250 mg sucralose dose (4.82 mg/kg and 3.46 mg/kg, respectively) resulted in 6-fold higher plasma concentrations (peak 1520 ng/mL and 1557 ng/mL and AUC 113,779 ng/mL/120 min and 109,588 ng/mL/120 min in these two adults versus mean peak 365.6 ± 69.9 ng/mL and mean AUC 29,619 ± 3,382 ng/mL/120 min in the other 9 of 11 adults) (Supplemental Figure 1). Concentrations were comparable whether administered in water, in seltzer and combined with ace-K, or when consumed in diet soda.
Discussion
Plasma sucralose concentrations were similar whether provided alone, mixed with ace-K or in diet soda and increased proportionately to the amount ingested and were comparable in adults and children when dosage was adjusted for body weight. Thus the two-fold higher absolute plasma concentrations in pediatric volunteers after intake of 68 mg was likely a result of lower body weight and lower blood volume in children. While the clinical relevance of markedly higher sucralose concentrations in children is unknown, this finding is noteworthy because diet beverages and sucralose-containing foods are commonly consumed by youths (Sylvetsky et al. 2012, Sylvetsky et al. 2014).
While mean peak plasma sucralose concentrations following ingestion of approximately 1 mg/kg sucralose in our study were similar to those reported previously by Roberts et al. (Roberts et al. 2000), the results are irreconcilable with those obtained by Baird et al. (Baird et al. 2000). In the latter study, 10 mg/kg were administered, but plasma concentrations were two orders of magnitude greater, which may be due to differences type of sample measured, the analytic method, and the correction factor used.
It is also noteworthy that two of the eleven participants who had received varying doses of sucralose in water (Group A) had significantly higher plasma concentrations in comparison to the other adult subjects. This inter-individual variation in plasma sucralose concentration was observed only in the 250 mg condition and not at lower sucralose doses (e.g. 68 mg). No clinical, demographic, dietary, or biochemical differences were identified to explain the higher sucralose absorption in these two individuals. Previously, a positive association between obesity and greater sucralose absorption (‘leaky gut’) had been hypothesized (Gummesson et al. 2011), but no correlation between BMI and sucralose concentrations was observed in our cohort. This may be due to higher sucralose doses administered (1000 mg) in the prior study, and our relatively small sample size with a limited range of BMI.
It has been suggested that sucralose absorption is relatively lower with higher dosage (Roberts et al. 2000). In fact, when varying doses of sucralose (10 mg/kg versus 1 mg/kg) were compared previously, proportionately less sucralose was absorbed at the higher dose, which may be due to upregulation of intestinal P-gp (Abou-Donia et al. 2008), also known as multidrug resistance protein 1 (MDR1) or ATP-binding cassette sub-family B member 1 (ABCB1) (Thiebaut et al. 1987, Suzuki and Sugiyama 2000). We did not observe a dose-related reduction in plasma sucralose concentrations within the doses provided in the current study (maximum 4.8 mg/kg). This may imply that sucralose-induced up-regulation of P-glycoprotein (Abou-Donia et al. 2008) occurs at higher doses and/or may require repeated exposure. However, there are presently no clinical data available to document these effects.
Our findings demonstrate that compared to adults, the pediatric subjects had two-fold higher plasma sucralose concentrations after ingestion of the amount of sucralose found in a single twelve ounce can of commercially-available diet soda. This is particularly important because the acceptable daily intake (ADI) for sucralose (5 mg/kg in the US) is based on body weight and assumes that sucralose is absorbed, distributed, and excreted similarly regardless of age, body composition, and other characteristics. However, considerable differences exist between children and adults; for example, infants and young children (up to 2 years of age) have a significantly lower glomerular filtration rate compared to adults and are therefore expected to excrete sucralose more slowly.
Early life sucralose exposure may influence a child’s future taste preferences, diet, and metabolic health (Rother, Sylvetsky, and Schiffman 2015, Araujo, Martel, and Keating 2014, Zheng and Sarr 2013). Furthermore, we have recently reported that sucralose is present in human breast milk (Sylvetsky et al. 2015) at concentrations well-above the sweetness detection threshold (Rother, Sylvetsky, and Schiffman 2015). Given that children are typically exposed to the same volume of diet soda (there are no kid sized cans of diet soda) and given that children have a much higher preference for sweetness and lower sensitivity to high sucrose concentrations than adults (Mennella, 2005; Joseph, 2016), it is likely that children consume similar volumes of a sucralose-containing beverage as adults. Therefore, they have higher circulating plasma concentrations, considering their lower body weight and blood volume. While data on the volumes of diet beverages typically consumed by children on a daily basis do not exist, the assumption that they are similar to that consumed by adults is supported by the similar quantities of sugar-sweetened beverages consumed by children and adults (Ogden et al. 2011).
Limitations of this study include the small sample size, lack of data on sucralose excretion in urine and feces, and the short time course of sucralose measurements. While the clinical significance of higher sucralose plasma concentrations among children has not yet been investigated, our findings reiterate the need to evaluate the role of early life sucralose exposure, both during infancy and later in childhood, on dietary patterns and metabolic health. Our preliminary findings also highlight the importance of conducting more systematic examinations of sucralose pharmacokinetics to identify factors explaining markedly different sucralose concentrations, as exemplified by the two adult ‘outliers.’ This data will be integral in the design and interpretation of future human intervention studies in which sucralose is administered at physiologic concentrations, as the effects of sucralose may differ amongst individuals, in part due to marked inter-individual fluctuations in circulating sucralose concentrations. It is also critical to measure plasma sucralose concentration and distribution over a longer time period in future studies.
Supplementary Material
Acknowledgments
This work was supported in part by the intramural research program at the National Institute of Diabetes, Digestive and Kidney Diseases at the National Institutes of Health (Bethesda, MD).
References
- Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, McLendon RE, Schiffman SS. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome p-450 in male rats. J Toxicol Environ Health A. 2008;71(21):1415–29. doi: 10.1080/15287390802328630. [DOI] [PubMed] [Google Scholar]
- Araujo JR, Martel F, Keating E. Exposure to non-nutritive sweeteners during pregnancy and lactation: Impact in programming of metabolic diseases in the progeny later in life. Reprod Toxicol. 2014;49C:196–201. doi: 10.1016/j.reprotox.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Baird IM, Shephard NW, Merritt RJ, Hildick-Smith G. Repeated dose study of sucralose tolerance in human subjects. Food Chem Toxicol. 2000;38(Suppl 2):S123–9. doi: 10.1016/s0278-6915(00)00035-1. [DOI] [PubMed] [Google Scholar]
- Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care. 2009;32(12):2184–6. doi: 10.2337/dc09-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RJ, Walter M, Rother KI. Effects of diet soda on gut hormones in youths with diabetes. Diabetes Care. 2012;35(5):959–64. doi: 10.2337/dc11-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice HC, Goldsmith LA. Sucralose--an overview of the toxicity data. Food Chem Toxicol. 2000;38(Suppl 2):S1–6. doi: 10.1016/s0278-6915(00)00023-5. [DOI] [PubMed] [Google Scholar]
- Gummesson A, Carlsson LM, Storlien LH, Backhed F, Lundin P, Lofgren L, Stenlof K, Lam YY, Fagerberg B, Carlsson B. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring) 2011;19(11):2280–2. doi: 10.1038/oby.2011.251. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4(4):e5106. doi: 10.1371/journal.pone.0005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Kit BK, Carroll MD, Park S. Consumption of sugar drinks in the United States, 2005–2008. NCHS Data Brief. 2011;(71):1–8. [PubMed] [Google Scholar]
- Roberts A, Renwick AG, Sims J, Snodin DJ. Sucralose metabolism and pharmacokinetics in man. Food Chem Toxicol. 2000;38(Suppl 2):S31–41. doi: 10.1016/s0278-6915(00)00026-0. [DOI] [PubMed] [Google Scholar]
- Rother KI, Sylvetsky AC, Schiffman SS. Non-nutritive sweeteners in breast milk: perspective on potential implications of recent findings. Arch Toxicol. 2015;89(11):2169–71. doi: 10.1007/s00204-015-1611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS, Abou-Donia MB. Sucralose revisited: rebuttal of two papers about Splenda safety. Regul Toxicol Pharmacol. 2012;63(3):505–8. doi: 10.1016/j.yrtph.2012.05.002. author reply 509–13. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Rother KI. Sucralose, a synthetic organochlorine sweetener: overview of biological issues. J Toxicol Environ Health B Crit Rev. 2013;16(7):399–451. doi: 10.1080/10937404.2013.842523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon BR, Parlee SD, Learman BS, Mori H, Scheller EL, Cawthorn WP, Ning X, Gallagher K, Tyrberg B, Assadi-Porter FM, Evans CR, MacDougald OA. Artificial sweeteners stimulate adipogenesis and suppress lipolysis independently of sweet taste receptors. J Biol Chem. 2013;288(45):32475–89. doi: 10.1074/jbc.M113.514034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y, Harmelin A, Kolodkin-Gal I, Shapiro H, Halpern Z, Segal E, Elinav E. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181–6. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sugiyama Y. Role of metabolic enzymes and efflux transporters in the absorption of drugs from the small intestine. Eur J Pharm Sci. 2000;12(1):3–12. doi: 10.1016/s0928-0987(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Swithers SE. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab. 2013;24(9):431–41. doi: 10.1016/j.tem.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvetsky AC, Gardner AL, Bauman V, Blau JE, Garraffo HM, Walter PJ, Rother KI. Nonnutritive Sweeteners in Breast Milk. J Toxicol Environ Health A. 2015;78(16):1029–32. doi: 10.1080/15287394.2015.1053646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvetsky AC, Greenberg M, Zhao X, Rother KI. What Parents Think about Giving Nonnutritive Sweeteners to Their Children: A Pilot Study. Int J Pediatr. 2014;2014:819872. doi: 10.1155/2014/819872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96(3):640–6. doi: 10.3945/ajcn.112.034751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvetsky A, Rother KI, Brown R. Artificial sweetener use among children: epidemiology, recommendations, metabolic outcomes, and future directions. Pediatr Clin North Am. 2011;58(6):1467–80. xi. doi: 10.1016/j.pcl.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84(21):7735–8. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Sarr MG. Effect of the artificial sweetener, acesulfame potassium, a sweet taste receptor agonist, on glucose uptake in small intestinal cell lines. J Gastrointest Surg. 2013;17(1):153–8. doi: 10.1007/s11605-012-1998-z. discussion p 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.