Abstract
Purpose
The human orbit is an environment that is vulnerable to inflammation and edema in the setting of autoimmune thyroid disease. Our study investigated the tenet that orbital adipose tissue lacks lymphatic vessels and analyzed the clinicopathologic differences between patients with acute and chronic thyroid eye disease (TED). The underlying molecular mediators of blood and lymphatic vessel formation within the orbital fat were also evaluated.
Design
Retrospective cohort study
Participants
The study included fat specimens from 26 orbits of 15 patients with TED undergoing orbital decompression. Orbital fat specimens from patients without TED as well as cadaveric orbital fat served as controls.
Methods
Tissue specimens were processed as formalin-fixed paraffin-embedded sections (FFPE) or frozen cryosections for immunohistochemistry. Total RNA was extracted and analyzed via quantitative (real-time) reverse transcription polymerase chain reaction (qRT-PCR). Clinicopathological correlation was made by determining the Clinical Activity Score (CAS) of each patient with TED.
Main Outcome Measures
Samples were examined for vascular and lymphatic markers including podoplanin, LYVE-1, and CD31 by immunohistochemistry, as well as for mRNA levels of VEGF, VEGF receptors, SEMA-3F, NRP-1, NRP-2, podoplanin and LYVE-1 by qRT-PCR.
Results
Clinicopathological correlation revealed increased staining of CD31-positive blood vessels in patients with acute TED with CAS > 4, as well as rare staining of podoplanin-positive lymphatic vessels within acutely inflamed orbital fat tissue. Additionally, qRT-PCR analysis demonstrated increased expression of vascular endothelial growth factor receptor 2 (VEGFR-2) as well as VEGF signaling molecules: VEGF-A, VEGF-C, and VEGF-D.
Conclusions
In acute TED, compared to chronic TED and control orbital fat, there is increased blood vessel density suggesting neovascularization and rare lymphatic vessels suggestive of limited lymphangiogenesis. This pro-angiogenic and pro-lymphangiogenic microenvironment is likely due to the increased expression of VEGFR-2 and VEGF-A, VEGF-C, and VEGF-D. These findings imply that orbital edema in acute TED may be mediated, in part, by both the formation of new, immature blood vessels and the formation of lymphatic capillaries that are functionally incapable of draining interstitial fluid.
Introduction
TED is a potentially sight-threatening condition that has perplexed physicians for centuries. The estimated annual incidence rate of TED is 16 cases per 100,000 women and 3 cases per 100,000 men. Approximately 10–20 percent of patients who suffer with this systemic autoimmune condition will develop severe inflammation in the orbit that can lead to disabling double vision or irreversible vision loss.1, 2 Orbital involvement in TED can consist of extraocular muscle enlargement as well as adipogenesis, the proliferation of fat cells, due to soluble factors upregulated during inflammation and found in the edematous milieu. In severe cases, both of these changes can lead to significant exophthalmos as well as sight threatening optic neuropathy.
TED is most often characterized by an acute inflammatory phase followed by a prolonged state of chronic inflammation and fibrosis. It is generally during the acute phase when patients may experience the most catastrophic effects of TED. Standard treatment during this acute period consists of systemic corticosteroids, external beam radiation therapy and/or urgent surgical decompression of the orbit. Although these non-targeted therapies can help diminish the inflammatory changes and the ensuing compressive sequelae in the orbit, research has not yet elucidated the factors that make the orbit a susceptible microenvironment for this condition. Previous studies of orbital soft tissues have reported the following: (1) orbital fat and extraocular muscle lack lymphatic vessels,3 (2) inflammation can induce both angiogenesis, the growth of new blood vessels, and lymphangiogenesis, the formation of lymphatic vessels, in certain ocular tissues such as the cornea,4–6 and (3) lymphangiogenesis can occur in orbits that are acutely inflamed from orbital infection.7
It is thought that orbital soft tissues do not contain lymphatic vessels except around the dura mater surrounding the optic nerve8 and the lacrimal gland.9 In contrast, other fat depots throughout the body contain both blood and lymphatic vessels.10 The lymphatic system consists of thin-walled, low-pressure vessels that collect and drain protein-rich fluid from the interstitial space and return it to the venous system via the thoracic duct. It plays a dual role as it not only drains interstitial fluid from tissues by way of blind-ended sacs, or terminal lymphatics,11 but also participates in the immune response. In order to evaluate whether the proliferation of blood vessels and/or the absence of native lymphatic capillaries could contribute to the severity of TED, we examined human orbital tissue specimens obtained from normal controls and subjects with acute and chronic TED. Specifically, we investigated the molecular mechanisms of angiogenesis and lymphangiogenesis in TED to potentially identify targets or agents that may alter the clinical course of the disease.
Methods
Human subjects and specimen collection
Orbital fat samples were obtained from patients undergoing either urgent or elective orbital decompression for TED by three surgeons at Massachusetts Eye and Ear (MEE) between 2012 and 2016. Patients were excluded if they had previous radiation to the orbit, previous unrelated orbital surgery or trauma (not including strabismus surgery), or previous orbital infection. We included patients regardless of whether they were or were not they had been treated with systemic corticosteroids at the time of decompression surgery. Smoking status was noted, but was not considered in the inclusion or exclusion criteria.
Control samples included eyelid skin, eyelid pre-aponeurotic fat, and subcutaneous neck fat from patients without thyroid disease undergoing unrelated procedures (blepharoplasty and excision of prolapsed orbital fat) as well as cadaveric orbital fat from patients without thyroid disease. Electronic medical records were reviewed for demographic information, prior history of medical and surgical treatments for thyroid disease, clinical exam findings and photos. Clinical Activity Score (CAS) was calculated for each patient based on documented exam findings and photos by assigning one point each for retrobulbar pain, pain with eye movement, redness of eyelids, diffuse injection of conjunctiva involving at least one quadrant, eyelid edema, chemosis, caruncular swelling, increased proptosis of 2mm or more during a period of 1–3 months, decrease in extraocular motility greater than 5 degrees during a period of 1–3 months, and decrease in visual acuity by one or more line on Snellen chart during a period of 1–3 months for a maximum total of 10 points according to Mourits et al.12 Collection and evaluation of protected patient health information were in compliance with the rules and regulations of the Health Insurance Portability and Accountability Act. The MEE and the Massachusetts General Hospital Human Studies Committee completed an administrative review of the study and Institutional Review Board (IRB) approval was obtained. Informed consent was obtained from each subject for use of tissue for research purposes. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Immunohistochemistry (IHC)
Tissue specimens were collected and processed as either formalin-fixed paraffin-embedded sections (FFPE) or cryosections. For FFPE sections, serial sections (4 µm) were cut and deparaffinized in 100% xylene, then rehydrated in a series of ethanol baths and washed with phosphate buffered saline (PBS). Slides were incubated in 3% H2O2 in methanol to quench endogenous peroxidases and blocked in TNB protein blocking solution (Thermo-Fisher Scientific, Waltham, MA). Primary antibody (podoplanin 1:25, Covance Laboratories, Dedham, MA) was incubated overnight at 4°C. The following day, sections were incubated in biotinylated secondary antibody (1:200, Vector Laboratories, Burlingame, CA) followed by alkaline phosphatase-conjugated avidin (Vectastain ABC-AP Universal Kit; Vector Laboratories, Burlingame, CA). Expression was visualized with the Vector Red chromogenic substrate kit (Vector Laboratories, Burlingame, CA) and counterstaining was performed using Gill no. 3 hematoxylin (Sigma-Aldrich, St. Louis, MO).
For frozen tissue, serial cryosections (8 – 10 µm) were cut and stored at −80°C prior to use. Slides were air-dried at room temperature (RT), fixed in 100% acetone, washed with PBS, incubated in 3% H2O2 in methanol, and blocked in TNB. Primary antibodies cluster of differentiation 31 (CD31 (1:200 Dako, Carpinteria, CA) and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) (1:200 ReliaTech GmbH, Wolfenbuttel, Germany) were added for two hours at RT. Sections were then incubated in biotinylated secondary antibody (1:200, Vector Laboratories, Burlingame, CA) followed by alkaline phosphatase-conjugated avidin. Vector Red chromogenic substrate kit was used for visualization followed by a counterstain with Gill no. 3 hematoxylin. All imaging was performed using an Axioskop 2 MOT Plus microscope (Carl Zeiss Inc., Thornwood, NY).
RNA extraction and quantitative real-time PCR (qPCR)
Total RNA was extracted from samples using TRIzol® (Invitrogen, Carlsbad, CA) and PureLink® RNA Mini Kit (Ambion, Foster City, CA). Primers for vascular endothelial growth factor receptor 1 (VEGFR-1), VEGFR-2, VEGFR-3, neuropilin 1 (NRP1), NRP2, semaphorin-3F (SEMA3F), vascular endothelial growth factor A (VEGF-A), VEGF-C, VEGF-D, podoplanin, and LYVE-1 were designed using the MGH Primer Bank. cDNA was prepared using 800 ng of RNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA) and probed for quantitative (real-time) reverse transcription polymerase chain reaction (qRT-PCR) using Faststart Universal SYBR Green Master (Hoffmann-La Roche, Basel, Switzerland). Fold changes were calculated as the ratio of 2-ΔΔCt and normalized to the housekeeping genes GAPDH, HPRT1 and B2M and compared between acute (CAS > 4) and chronic (CAS < 4) cases of TED, and normal control specimens.
Results
Clinical demographics
Tissues from 15 TED patients (G1–15) and control orbital tissue samples (C1–4) were included as specimens in this study and their clinical and demographic information is summarized in Table 1. A majority of the patients were female (11/15). Ages ranged from 33 to 77 years with a mean of 55.9±13.5 years. Among the 15 patients, 7 had acute compressive optic neuropathy (CON) requiring an urgent orbital decompression. All of the patients with CON were found to have a CAS of 5 or greater with a mean score of 5.8±1.0. One additional patient was in the acute phase of TED but did not have CON. The remaining seven patients who underwent balanced decompression for stable exophthalmos during the chronic phase had CAS ranging from 1 to 3 with an average of 1.9±0.7. All but one patient received systemic treatment for their Graves’ disease. Patients with active disease had an average elapsed time from the initial onset of symptoms of TED to surgical management of 9 months, while patients in the chronic group had an average elapsed time of 38 months.
Table 1.
Clinical Characteristics
| Patient | Gender | Age | Laterality | Thyroid treatment |
Time dx to surgery |
Steroids Y/N |
Smoking Y/N |
CON | CAS |
|---|---|---|---|---|---|---|---|---|---|
| C1 | F | 69 | N/A | N/A | N/A | N | Unk | N/A | N/A |
| C2 | M | 54 | N/A | N/A | N/A | N | Unk | N/A | N/A |
| C3 | M | 41 | N/A | N/A | N/A | N | Unk | N/A | N/A |
| C4 | M | 61 | N/A | N/A | N/A | N | Unk | N/A | N/A |
| G1 | F | 59 | OU | RAI, methimazole, thyroidectomy | 13 mo | Y | N | Y | 7 |
| G2 | M | 49 | OU | Tapazole | 10 mo | Y | N | Y | 5 |
| G3 | F | 58 | OS | Methimazole | 9 mo | Y | Y | Y | 7 |
| G4 | M | 48 | OD | Methimazole | 10 mo | N | Y | N | 2 |
| G5 | F | 67 | OU | RAI | 36 mo | N | Y | N | 1 |
| G6 | F | 35 | OU | Methimazole, propranolol | 13 mo | N | N | N | 1 |
| G7 | F | 65 | OU | none | 3 mo | Y | N | Y | 5 |
| G8 | F | 70 | OU | Methimazole | 4 mo | Y | N | N | 2 |
| G9 | F | 64 | OS | Methimazole | 3 mo | N | N | Y | 7 |
| G10 | M | 49 | OU | Tapazole | 20 mo | Y | N | Y | 5 |
| G11 | F | 61 | OU | RAI | 2 mo | Y | Y | Y | 5 |
| G12 | F | 66 | OU | Thyroidectomy | 15 mo | N | N | N | 5 |
| G13 | F | 33 | OU | RAI | 84 mo | N | N | N | 2 |
| G14 | M | 37 | OS | Thyroidectomy | 96 mo | N | N | N | 3 |
| G15 | F | 77 | OU | Methimazole | 24 mo | Y | N | N | 2 |
CAS=clinical activity score; CON=compressive optic neuropathy; F=Female; M=male; mo=month; N=no; N/A=not applicable; OD=right eye; OS=left eye; OU=both eyes; RAI=radioactive iodine; Unk=unknown; Y=yes
Four control specimens were obtained from cadaveric intraconal fat (C2 and C3) or from live patients undergoing removal of prolapsed orbital fat (C1 and C4). There were three male and one female control subjects with ages ranging from 41 to 69 years (mean 56.3 years). None of these subjects had a history of thyroid disease or TED.
Immunohistological characterization of lymphatic vessels
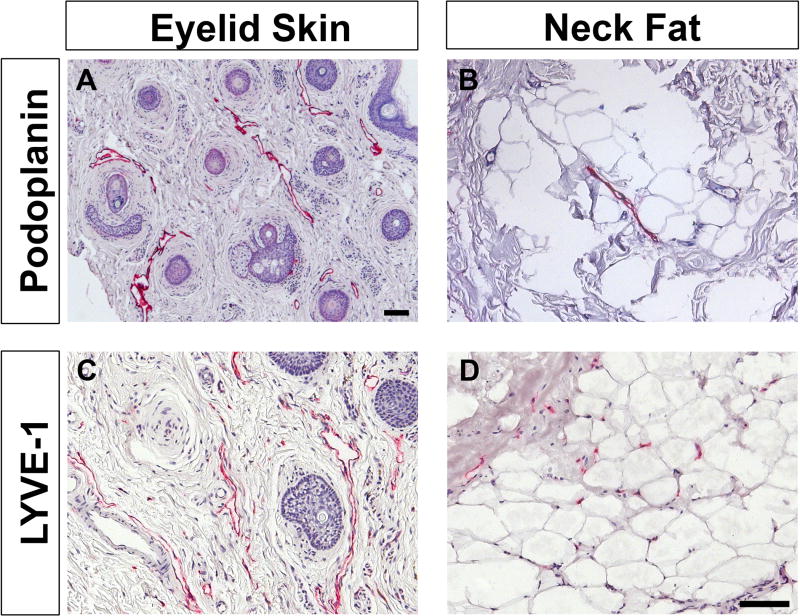
Specimens from anatomic locations known to contain lymphatic vessels were stained with podoplanin and LYVE-1 as positive controls (Figure 1). Eyelid skin from blepharoplasty specimens clearly showed positive staining along open-lumened, vessel-like structures with both podoplanin and LYVE-1 (Figure 1A, 1C). Subcutaneous neck fat also showed positive podoplanin staining in vessels though there were fewer lymphatic structures within this tissue (Figure 1B). LYVE-1 staining of neck fat revealed small vascular structures as well as staining of single cells suggestive of macrophages and/or lymphatic capillaries (Figure 1D).
Figure 1. Immunohistological characterization of control specimens, eyelid skin and subcutaneous fat obtained from the neck.
Localization of podoplanin and LYVE-1 (red) confirm the presence of lymphatic vessels in these samples, as expected, and proves the utility of these markers. Samples are counterstained with hematoxylin (blue). Scale bar = 100 µm
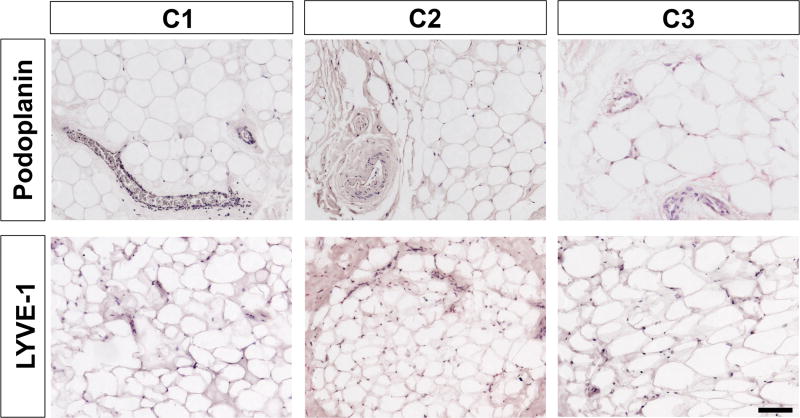
Podoplanin and LYVE-1 were used to stain control periocular fat from patients with prolapsed orbital fat and from cadaver intraconal orbital fat (Figure 2) as negative controls. In these control specimens, there was no positive staining in areas that contained vascular structures, which is consistent with previous reports.
Figure 2. Immunohistological characterization of orbital fat obtained from control patients without TED.
Staining for podoplanin and LYVE-1 (red) show no positive staining, indicating the absence of lymphatic vessels, which is consistent with previous reports. Samples are counterstained with hematoxylin (blue). Scale bar = 100 µm
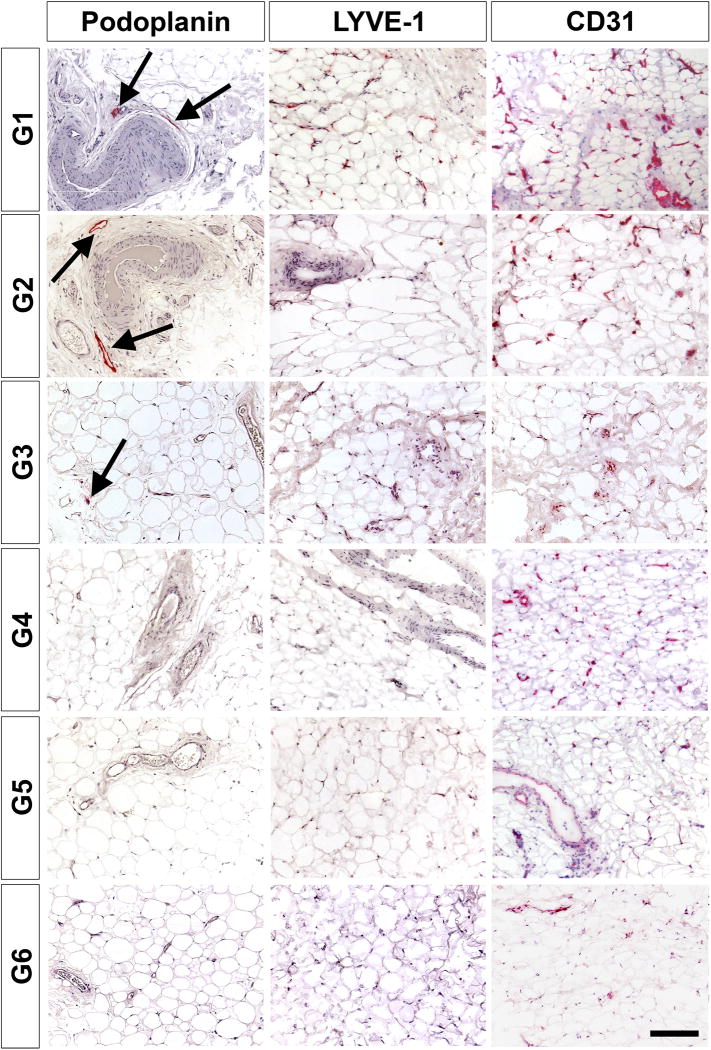
When evaluating the first six patients with Graves’ disease and TED (G1-G6), podoplanin staining only identified rare lymphatic vessels in G1, G2, and G3 which were acute TED patients who underwent urgent decompression for compressive optic neuropathy with a CAS of 7, 5, and 7, respectively (Figure 3). The lymphatic vessel staining failed to identify any positive vessels in the last three patients with chronic TED who underwent elective decompression for chronic disease (G4-G6), with a CAS of 2, 1, and 1 respectively.
Figure 3. Immunohistological characterization of orbital fat from patients with TED.
Patients G1, G2 and G3 are in the acute, inflammatory phase of the disease and all exhibit podoplanin-positive vessel-like structures (red, arrows). Patients G4, G5, and G6 are in the chronic stage of disease and do not exhibit any podoplanin-positive cells. Middle and rightmost panels shows LYVE-1 and CD31 staining for patients G1 – G6 respectively. Samples are counterstained with hematoxylin (blue). Scale bar = 100 µm
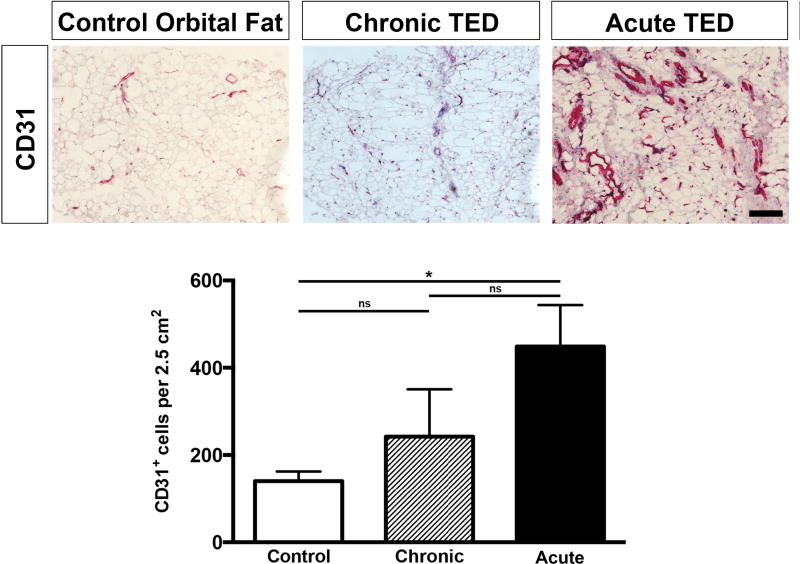
Immunohistological characterization of quantification of CD31+ vessels
In the inflamed orbit of patients with acute TED, there appeared to be evidence of rare but new lymphatic vessel formation. This prompted investigation of potential blood vessel formation within acute TED. There was significantly increased expression of the pan-endothelial cell marker CD31 in acute TED (G1-G3) compared to chronic TED (G4-G6) (Figure 3, rightmost column) and normal controls (Figure 4). Quantification of CD31+ staining of orbital fat samples revealed an average of 140 CD31+ cells per 2.5 cm2 in the control, 242 CD31+ cells per 2.5 cm2 in the chronic TED patients, and 448 CD31+ cells per 2.5 cm2 in the acute TED patients (Figure 4). There was a statistically significant increase in CD31+ staining in the acute TED patients when compared against the controls. These data suggest that the surge in CD31 expression, especially in the acute, inflammatory phase of TED, signifies angiogenesis and these new vessels are somewhat sustained in the chronic phase compared to controls.
Figure 4. Increased vasculature in tissue from patients with acute TED.
Patients with TED have increased staining of CD31+ cells with dilated blood vessels, when compared against control fat specimens and orbital fat from patients with chronic TED. Quantification of staining revealed a statistically significant increase in CD31+ staining in the acute TED patients when compared against the controls. Samples are counterstained with hematoxylin (blue). Scale bar = 100 µm
qRT-PCR analysis of orbital specimens
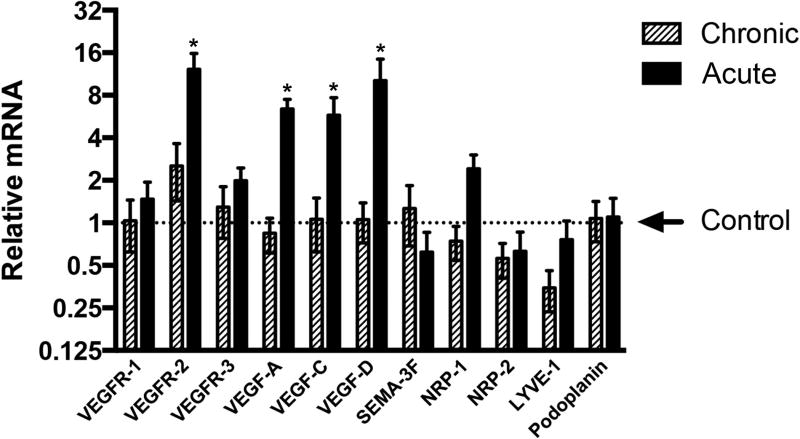
Patients with acute TED (CAS > 4): subjects G3, G7, G9, G11, G12, and patients with chronic TED (CAS < 4): subjects G6, G8, G13, G14, G15, were grouped and relative mRNA expression of VEGF, VEGF receptors, SEMA-3F, NRP-1, NRP-2, LYVE-1 and podoplanin were evaluated (Figure 5). There was insufficient tissue remaining after immunohistology to conduct qRT-PCR analysis on subjects G1, G2, G4, and G5. There was no statistically significant difference between acute and chronic TED in the expression of VEGFR-1 and VEGFR-3. In contrast, there was a significant 12.24 ± 3.57-fold increase in VEGFR-2 expression within the acute TED patients when compared to control, while there was only a 2.53 ± 1.10-fold increase of VEGFR-2 expression within patients with chronic TED compared to control. In addition, there was a significant relative increase in VEGF-A (6.41 ± 1.10 fold), VEGF-C (5.78 ± 1.92 fold), and VEGF-D (10.17 ±0.17 fold) mRNA expression in the acute TED patients when compared to control. Within patients with chronic TED, there was no significant increase in VEGF-A (0.84 ± 0.23), VEGF-C (1.10 ± 0.44), and VEGF-D (1.05 ± 0.33) mRNA expression. In addition, there was a trend toward increased expression of NRP-1 and LYVE-1 in the acute TED patients when compared against chronic TED. A significant difference in NRP-2 or podoplanin expression between the two cohorts was not seen. Intriguingly, there appeared to be a trend towards increased mRNA expression of SEMA-3F, an inhibitor of lymphangiogenesis, in chronic TED subjects when compared against acute TED subjects.
Figure 5. Changes in gene expression associated with TED.
Orbit specimens of five patients with acute TED (CAS > 4) and five patients with chronic TED (CAS < 4) were collected and relative mRNA expression was evaluated. The values were averaged within the chronic and acute groups. qRT-PCR analysis of genes revealed a significant increase in expression of VEGFR-2, VEGF-A, VEGF-C, and VEGF-D in acute TED when compared to chronic disease and control specimens.
Discussion
The pathogenesis and management of TED have challenged physicians for many years. Although corticosteroids have been used to temporize acute, inflammatory TED, some patients eventually require orbital decompression surgery either urgently for compressive optic neuropathy or in the chronic stage of disease for persistent exophthalmos. Some severe cases of TED are refractory to currently available therapies and a larger proportion of patients with other comorbidities including diabetes are unable to tolerate systemic corticosteroids. Hence this study investigated the angiogenic and lymphangiogenic factors of orbital fat in TED, with the hope of finding markers with the potential for targeted therapy.
The study of lymphatic vessel formation has been facilitated by the identification of a variety of lymphatic EC markers including Prox-1,13 podoplanin,14 LYVE-1,15 and VEGFR-3.16 Most vascularized tissues, such as subcutaneous and abdominal fat contain lymphatic vessels, yet orbital fat appears to lack lymphatic vessels under normal conditions. We evaluated orbital adipose specimens from patients with chronic TED, acute TED, and normal controls using immunohistochemistry specifically in search of lymphatic vessels. Our data demonstrate that during the acute, inflammatory stage of TED, there is formation of both rare lymphatic vessels and robust blood vessels. We discovered increased numbers of CD31+ vessels in patients with acute TED and high CAS (Figures 3 and 4). Similarly, using the lymphatic markers podoplanin and LYVE-1, we confirmed the presence of lymphatic vessels from control specimens including eyelid skin and subcutaneous fat obtained from the neck (Figure 1). In contrast, orbital fat obtained from control patients without TED did not exhibit positive podoplanin nor LYVE-1 staining, consistent with previous reports (Figure 2).9 In patients with acute TED, we were able to detect lymphatic vessels that were podoplanin positive, while no lymphatic vessels were identified with podoplanin staining in patients with chronic TED (Figure 3). LYVE-1, on the other hand, which stains both lymphatic capillaries as well as macrophages,5 exhibited variable, non-specific staining throughout all TED specimens, though the staining appeared to be more robust in single cells in acute TED as opposed to frank vessels. Variable LYVE-1-positive staining in all TED specimens is likely indicative of inflammation-induced macrophage infiltration rather than presence of lymphatic endothelial cells (ECs). Although our studies have shown positive podoplanin staining of orbital adipose tissue in inflamed TED states, these new lymphatic vessels are very rare and they do not appear to impart any functional advantage within the course of the disease as there does not appear to be a significant decrease of edema in these orbits. These subjects exhibited relatively high CAS and required urgent surgical decompression. This may be due to the fact that there are simply not enough lymphatic vessels, or that these are non-functional lymphatic trunks that lack the capillary network to drain interstitial fluid. Regardless, by the time the disease stabilizes and becomes chronic, these lymphatic channels likely regress, as we did not discover any lymphatic vessels in patients with chronic TED.
Similarly, in other ocular tissues that are usually devoid of vascular structures such as the cornea, there has been strong evidence of angiogenesis and lymphangiogenesis within acute, inflammatory conditions using a suture model6, 17 or alkali burn model.18, 19 It has been shown that with a temporary insult to the cornea, the outgrowth of blood vessels and lymphatic vessels occurs as early as 2 days and peaks around day 14. Thereafter, regression of lymphatics starts earlier and is more pronounced than that of blood vessels.20 Both local anti-angiogenic and anti-lymphangiogenic approaches have been taken in order to regulate potential graft rejection. Similarly, modulation of these angiogenic and lymphangiogenic processes within the orbit may yield new therapeutic approaches for TED by decreasing orbital inflammation and edema in acute TED.
Like angiogenesis,21 lymphangiogenesis, is modulated by a balance of both stimulators and inhibitors. The VEGF family plays a crucial role in the proliferation of both blood and lymphatic vessels. There are 3 main receptors for VEGF: VEGFR-1, VEGFR-2, and VEGFR-3. VEGFR-1 is a negative regulator of VEGF-A activity in ECs. The ECs of blood vessels and lymphatic vessels express common receptors such as VEGFR-2 and NRP-2, and both respond to VEGF-A.22 VEGF-A increases vascular permeability,23 can act as a chemotactic factor for monocytes,24 and can directly induce expression of adhesion molecules such as the selectins, VE-Cadherin (VE-CAD), vascular cell adhesion molecule 1 (VCAM-1), and intercellular adhesion molecule 1 (ICAM-1) in ECs.25 The elevated mRNA expression of VEGF-A in patients in the acute inflammatory phase of TED may indicate that these new blood vessels are dilated, leaky, and may cause orbital edema with infiltration of leukocytes, and thereby contributing to the orbital congestion found in TED. We also found elevated mRNA expression of VEGFR-2, but did not find increased expression of NRP-2 or podoplanin in subjects with acute TED. This is consistent with our histopathological findings that there was increased blood vessel formation and very rare lymphatic vessel formation in patients with acute TED when compared with patients with chronic TED.
Besides VEGF-A, other major lymphangiogenic factors include VEGF-C26 and VEGF-D27 which bind VEGFR-3 and/or VEGFR-2 receptors expressed by lymphatic ECs and promote their proliferation, migration, and survival.28, 29 VEGF-C can also act as a chemoattractant of activated macrophages that express VEGFR-3.30 Experimental models of VEGF-C overexpression in tumor cells have demonstrated increased lymphatic metastasis.31 Therefore, increased expression of VEGF-C and VEGF-D as found in patients with acute TED suggests the presence of a pro-lymphangiogenic environment. However, histopathologically, we found limited lymphatic vessels in patients with acute TED, and no lymphatic vessels in patients with chronic TED. Limited lymphatic vessel formation may be due to limited expression of VEGFR-3 and NRP-2 confirming the paucity of lymphatic ECs within the orbit. Moreover, SEMA-3F, which is an inhibitor of lymphangiogenesis, trended towards an increased expression in chronic TED. We speculate that the decrease in SEMA-3F expression in acute TED may account for the formation of some lymphatic vessels in patients with acute TED, while the increase in SEMA-3F expression in chronic TED may be involved in lymphatic vessel regression.
Study strengths and limitations
The strengths of this study include the number and types of control tissues that were collected and the validation of the antisera that were used. Orbital adipose tissue from cadavers generated the purest form of control as they were collected from the area of the orbit that is most congruous to an orbital decompression and had no other confounding conditions. Another strength was the spectrum of TED patients that were included in this study and their detailed clinicopathological information, albeit, the study was retrospective in nature. Depending on the size of the sample that was collected during surgery, it was not possible to perform immunohistochemistry and mRNA extraction on every single specimen.
In conclusion, inflamed orbits in acute TED, as compared to chronic TED and control orbits, exhibit increased blood vessels likely mediated by VEGFR-2 and increased VEGF-A signaling. Further, we discovered new evidence of lymphatic vessels in acute TED orbits likely due to a combination of elevated expression of pro-lymphangiogenic signaling (VEGF-C and VEGF-D) and decreased expression of anti-lymphangiogenic signaling (SEMA-3F). Angiogenesis and lymphangiogenesis in TED have not previously been considered as a component in the pathophysiology of TED and our data could plausibly expand our therapeutic options through regulation of these processes.
Acknowledgments
Sources of public and private financial support to disclose:
Funded by the American Thyroid Association Grant (L.A.K.), the Massachusetts Lions Eye Research Foundation (L.A.K.), the Vascular Biology Program at Boston Children’s Hospital (D.R.B.), and the National Eye Institute R01 (EY005318) (P.A.D.), core grant (P30EY003790), K12 (EY16335) (L.A.K.) and R21 (EY027061) (L.A.K.)
List of abbreviations
- TED
thyroid eye disease
- CAS
clinical activity score
- CD31
cluster of differentiation 31
- CON
compressive optic neuropathy
- EC
endothelial cell
- FFPE
formalin-fixed paraffin-embedded sections
- IHC
immunohistochemistry
- LYVE
lymphatic vessel endothelial hyaluronan receptor
- NRP
neuropilin
- SEMA
semaphorin
- qRT-PCR
quantitative (real-time) reverse transcription polymerase chain reaction
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- VE-CAD
vascular endothelial cadherin
- VCAM-1
vascular cell adhesion molecule 1
- ICAM-1
intercellular adhesion molecule 1
Footnotes
Meeting Presentation: Presented in part at the American Academy of Ophthalmology Annual Meeting, November 15, 2015, Las Vegas, Nevada and the International Thyroid Congress, October 18, 2015.
Sources of conflicts of interest to disclose: None
The sponsor or funding organization had no role in the design or conduct of this research.
References
- 1.Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994;92:477–588. [PMC free article] [PubMed] [Google Scholar]
- 2.Bartley GB, Fatourechi V, Kadrmas EF, et al. The incidence of Graves' ophthalmopathy in Olmsted County, Minnesota. Am J Ophthalmol. 1995;120(4):511–7. doi: 10.1016/s0002-9394(14)72666-2. [DOI] [PubMed] [Google Scholar]
- 3.Nakao S, Hafezi-Moghadam A, Ishibashi T. Lymphatics and lymphangiogenesis in the eye. J Ophthalmol. 2012;2012:783163. doi: 10.1155/2012/783163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SP, Dana R. Corneal lymphangiogenesis: implications in immunity. Semin Ophthalmol. 2009;24(3):135–8. doi: 10.1080/08820530902801320. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115(9):2363–72. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruyama K, Nakazawa T, Cursiefen C, et al. The maintenance of lymphatic vessels in the cornea is dependent on the presence of macrophages. Invest Ophthalmol Vis Sci. 2012;53(6):3145–53. doi: 10.1167/iovs.11-8010. [DOI] [PubMed] [Google Scholar]
- 7.Fogt F, Zimmerman RL, Daly T, Gausas RE. Observation of lymphatic vessels in orbital fat of patients with inflammatory conditions: a form fruste of lymphangiogenesis? Int J Mol Med. 2004;13(5):681–3. doi: 10.3892/ijmm.13.5.681. [DOI] [PubMed] [Google Scholar]
- 8.Killer HE, Laeng HR, Groscurth P. Lymphatic capillaries in the meninges of the human optic nerve. J Neuroophthalmol. 1999;19(4):222–8. [PubMed] [Google Scholar]
- 9.Gausas RE, Gonnering RS, Lemke BN, et al. Identification of human orbital lymphatics. Ophthal Plast Reconstr Surg. 1999;15(4):252–9. doi: 10.1097/00002341-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Harvey NL. The link between lymphatic function and adipose biology. Ann N Y Acad Sci. 2008;1131:82–8. doi: 10.1196/annals.1413.007. [DOI] [PubMed] [Google Scholar]
- 11.Suami H, Taylor GI, Pan WR. The lymphatic territories of the upper limb: anatomical study and clinical implications. Plast Reconstr Surg. 2007;119(6):1813–22. doi: 10.1097/01.prs.0000246516.64780.61. [DOI] [PubMed] [Google Scholar]
- 12.Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73(8):639–44. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong YK, Detmar M. Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res. 2003;314(1):85–92. doi: 10.1007/s00441-003-0747-8. [DOI] [PubMed] [Google Scholar]
- 14.Schacht V, Dadras SS, Johnson LA, et al. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166(3):913–21. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detmar M, Hirakawa S. The formation of lymphatic vessels and its importance in the setting of malignancy. J Exp Med. 2002;196(6):713–8. doi: 10.1084/jem.20021346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaipainen A, Vlaykova T, Hatva E, et al. Enhanced expression of the tie receptor tyrosine kinase mesenger RNA in the vascular endothelium of metastatic melanomas. Cancer Res. 1994;54(24):6571–7. [PubMed] [Google Scholar]
- 17.Bock F, Onderka J, Dietrich T, et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest Ophthalmol Vis Sci. 2007;48(6):2545–52. doi: 10.1167/iovs.06-0570. [DOI] [PubMed] [Google Scholar]
- 18.Rho CR, Choi JS, Seo M, et al. Inhibition of Lymphangiogenesis and Hemangiogenesis in Corneal Inflammation by Subconjunctival Prox1 siRNA Injection in Rats. Invest Ophthalmol Vis Sci. 2015;56(10):5871–9. doi: 10.1167/iovs.14-14433. [DOI] [PubMed] [Google Scholar]
- 19.Seo M, Choi JS, Rho CR, et al. MicroRNA miR-466 inhibits Lymphangiogenesis by targeting prospero-related homeobox 1 in the alkali burn corneal injury model. [Accessed May 11, 2016];J Biomed Sci. 2015 22:3. doi: 10.1186/s12929-014-0104-0. Available at http://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-014-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cursiefen C, Maruyama K, Jackson DG, et al. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25(4):443–7. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 21.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3(7):643–51. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 22.Nagy JA, Vasile E, Feng D, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196(11):1497–506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246(4935):1309–12. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 24.Sawano A, Iwai S, Sakurai Y, et al. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97(3):785–91. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- 25.Detmar M, Brown LF, Schon MP, et al. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111(1):1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 26.Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 27.Haiko P, Makinen T, Keskitalo S, et al. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol Cell Biol. 2008;28(15):4843–50. doi: 10.1128/MCB.02214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achen MG, Jeltsch M, Kukk E, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95(2):548–53. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20(17):4762–73. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skobe M, Hamberg LM, Hawighorst T, et al. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159(3):893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huggenberger R, Siddiqui SS, Brander D, et al. An important role of lymphatic vessel activation in limiting acute inflammation. Blood. 2011;117(17):4667–78. doi: 10.1182/blood-2010-10-316356. [DOI] [PMC free article] [PubMed] [Google Scholar]