Abstract
Joint replacement is a commonly performed, highly successful orthopaedic procedure, for which surgeons have a large choice of different materials and implant designs. The materials used for joint replacement must be both biologically acceptable to minimize adverse local tissue reactions, and robust enough to support weight bearing during common activities of daily living. Modern joint replacements are made from metals and their alloys, polymers, ceramics, and composites. This review focuses on the biological response to the different biomaterials used for joint replacement. In general, modern materials for joint replacement are well tolerated by the body as long as they are in bulk (rather than in particulate or ionic) form, are mechanically stable and noninfected. If the latter conditions are not met, the prosthesis will be associated with an acute/chronic inflammatory reaction, peri-prosthetic osteolysis, loosening and failure. This article (Part 1 of 2) is dedicated to the use of metallic devices in orthopaedic surgery including the associated biological response to metallic byproducts is a review of the basic science literature regarding this topic.
Keywords: orthopaedic implants, biological response, foreign body response, inflammation, biomaterials
INTRODUCTION
The use of implanted devices in surgery has increased dramatically; when considering the field of orthopaedic surgery alone, the underlying industry is expected to grow to $41.1 billion by 2016.1 Briefly, the goals of total joint replacement (TJR) implants are to relieve pain and restore function, with limited impact on the surrounding tissues. Studies focusing on long-term outcomes of total knee replacement have shown survivorship after 15 years ranging from 81.7 to 98.14%.2–4 Similarly, studies for total hip replacement showed survivorship after 15 years ranging above 90%.5,6 Several materials are currently available for the surgeons and bioengineers when designing a TJR. As a support for artificial joints, metal implants are widely used in orthopaedic surgery. Metal implants can be monobloc (one piece), modular (different pieces put together) or articulate to each other. As implanted in a living body, metal implants may have adverse effect. Metal alloys are mainly used to replace the bone (e.g., femoral stem and acetabular shell), metal alloys or ceramics replace the articular surface on the femoral side, and polyethylene (PE) or ceramics replace the articular surface on the acetabular side. Polymethylmethacrylate (PMMA) can be used to cement the implants within the bone.
Historically, the use of metal alloys started in 1923 when Smith-Peterson introduced the cobalt–chromium alloy (Vitallium, Howmedica). Stainless steel was introduced in 1942 and titanium alloy in 1951. Animal experiments conducted in the early 1960s by Laing et al.7 showed that the tissue reaction to various metallic implants can be classified according to the degree of severity of the reaction. In general, the degree of tissue reaction was proportionate to the amounts of constituent elements released by corrosion of a pure metal or an alloy. Discussing the electrochemical fundamentals of implant metal performance, Pourbaix8 later pointed out that both electrochemical thermodynamics (determining corrosion tendencies) and electrochemical kinetics (determining corrosion rates) are important to consider in order to better understand and predict the corrosion behavior of metals and alloys in the presence of body fluids. This article identified 13 metals that may theoretically be considered for use as surgical implants and dental alloys. 8 of them (Au, Ir. Pt Rh. Ru, Pd, Ag, and Os) are noble metals, which keep a pure metallic surface. The remaining 5 (Ti, Ta, Nb, Zr, and Cr) are passive metals, which are covered by a layer of protective oxide. Subsequent electrochemical tests by Zitter and Plenk9 demonstrated that the use of passive metals is more preferable for surgical implants because of their protective surface layers of semi- or nonconductive oxides that prevent any exchange of electrons and thus any redox reaction at the surface. The electrochemical “inertness” ranking of the metal surfaces tested was increasing in the order of gold, stainless steel, the cobalt-based alloy, and the TiAlV alloy, with the pure metals Ti, Nb, and Ta being the most favorable. The authors concluded that this rating of metallic implant materials based on in vitro measurements of current densities was in good accordance with their biocompatibility rating reported from in vivo experiences. These electrochemical studies in the mid-1980s also pointed out that “more basic work remains to be done for studying thoroughly the conditions of crevice corrosion and fretting corrosion of metallic surgical implant materials”8 and that “the effect of a redox reaction taking place without corrosion is same as that of a corrosion reaction: in both cases the most significant chemical changes are an increase in the pH value at the cathode and a decrease in this value at the anode.”9 All these considerations became even more important in the following years when the use of modular implant devices increased significantly. 10–12 Therefore, the selection of metal to be used in a TJR is highly important for bioengineers who design the implant. The goal being to provide the patient a harmless long lasting implant.
This review of the basic science literature will focus on the mechanisms of metal ion and particle generation during TJR (e.g., modular junction corrosion and metal wear), most frequent use metals in TJR, as well as the effect of these metals on the surrounding cells and tissues. Polymers, ceramics, and composites will be the subject of a second review (see Part II).
MODULAR JUNCTION CORROSION IN TJRs
Corrosion of orthopaedic implants was first reported in 1956 after discovering that metal ions had harmful effects on the soft tissues.13 Five different modes of corrosion are described: galvanic, fretting, crevice, pitting, and intergranular.14–18 In an oxygen rich environment, the surface of metal implants is covered in a thin layer of a metal oxide that protects the underlying metal from further oxidative damage. This oxidized protective layer is the by-product of passivation (Figure 1). If this oxide layer is damaged, the surface of the metal implant re-oxidizes (re-passivates) to recreate the protective oxide layer. When the re-passivation process is disrupted, corrosion can occur.19 Corrosion and subsequent production of metal ions or even implant fracture is seen at all modular junctions utilized in TJR: head–neck junction (i.e., trunnion), neck–stem junction (i.e., dual modular implants), and metaphyseal–diaphyseal junction. Studies show that modularity can lead to increased corrosion of all metal implants in a highly design-dependent fashion (Figure 2).20–24 The emergence of mechanically assisted corrosion as a failure mechanism for the trunnion and dual modular junctions has forced manufacturers to re-evaluate their existing designs.
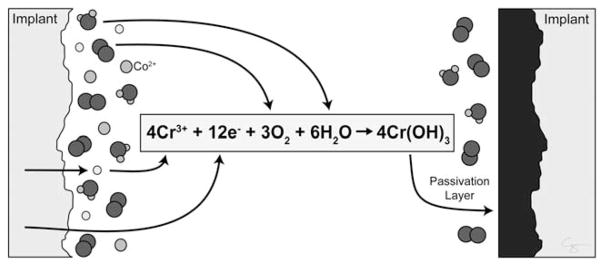
FIGURE 1.
The passivation layer is an oxidized metal (black). The passivation layer prevents further degradation of the underlying coalloy (Implant, gray). Metal implanted into the body has reacted with oxygen and is passivated. However, damage to the passivation layer results in exposure of the reactive coalloy surface, which reacts oxygen (O2) and water (H2O) resulting in repassivation. Illustration created by Chrisoula Toupadakis Skouritakis, Ph.D.
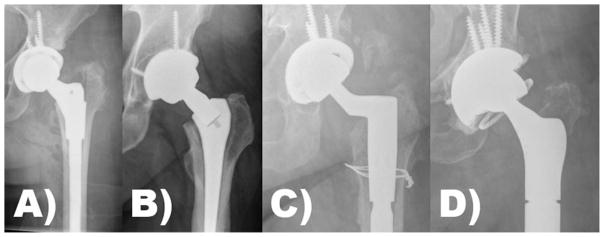
FIGURE 2.
Examples of the types of modular junctions in total hip arthroplasty: (A) S-ROM illustrating proximal metaphyseal modularity, (B) dual modular design illustrating neck–stem modularity, (C) fluted tapered modular stem illustrating distal diaphyseal modularity, and (D) total femoral replacement illustrating modularity at its extreme. Note, examples A–D all illustrate head–neck modularity using the trunnion of the stem to accept a modular femoral head.
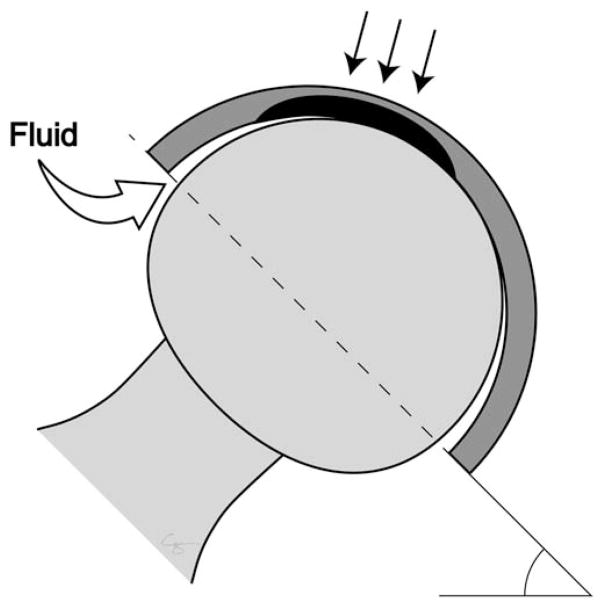
All modular junctions are susceptible to crevice corrosion. At modular junctions machined with tight tolerances, such as the head–neck junction, the space between the two surfaces is partially shielded from the aqueous chemical environment of the body fluid. This microisolation of the tiny gaps (or crevices) between the two surfaces of the implant is the source of crevice corrosion.25 The imbalance of oxygen between the cathode (the surface of the implant outside the crevice) and the anode (inside the crevice) creates a current even if each part of the modular junction is made from the same metal (Figure 3). At the anode, oxygen is low, while the concentration of metal, hydrogen, and chloride ions are high. In this oxygen-poor environment, the surface of the implant cannot repassivate.26–28 If the metal ion released is chromium, it reacts with phosphate to form a black or green, tarry, or glassy precipitate (i.e., chromium III phosphate and/or other metal oxides) near the crevice and on the surface of the implant.29–31 Hence, the serum chromium level remains normal or only slightly elevated in patients with corrosion at modular junctions, whereas the serum cobalt level rises due to its increased solubility. 20,29,30 This fact can be used to distinguish a failed metal-on-metal articulation, which in general will have an equivalent rise in serum cobalt and chromium levels, from a failed modular junction, which in general will have a differential elevation of the serum cobalt level several fold above that of the serum chromium level.32,33
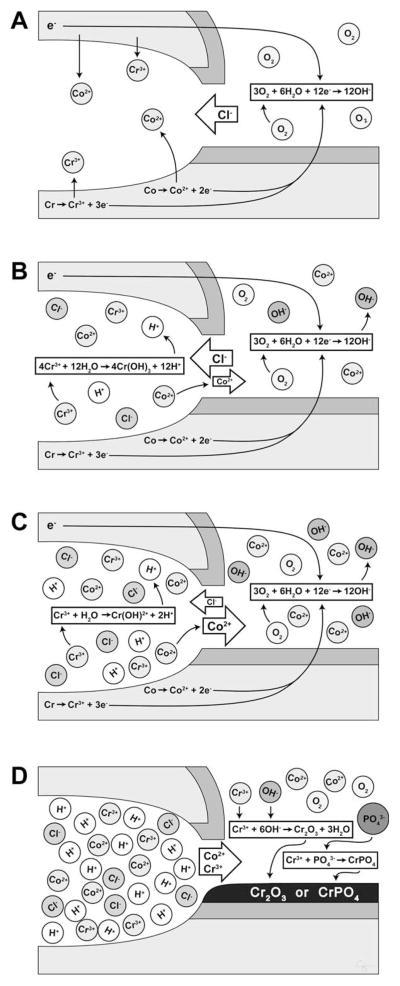
FIGURE 3.
The junction between the passivated femoral head and stem trunnion (dark gray) results in the microisolation of the corrosive environment that establishes an oxygen gradient between the interstitial fluid (“normoxic”) and the crevice fluid (“anoxic”). (A) The lack of oxygen inside the crevice leads to the separation of the anodic process (metal dissolution inside the crevice) and the cathodic process (oxygen reduction outside the crevice). The excess of positive ions in the crevice solution is balanced by an influx of negatively charged ions, primarily chloride ions (Cl−). The electrons generated by the anodic process is picked up by oxygen at the cathodic sites on the metal surface outside the crevice. (B) The hydrolysis of the chromium produces chromium ion (Cr3+) during the anodic process results in hydrogen ion (H+) generation inside the crevice, dropping the pH of the microenvironment and accelerating metal ion release. (C) As the crevice solution becomes more acidic, the hydrolysis of chromium becomes less and less complete, and the rate of acidification decreases. (D) As the critical pH is reached, the chromium ions are no longer hydrolyzed and crevice corrosion progresses at the rate of metal ion (CO2+, Cr3+, and so forth) diffusion. The metal ions then can react with organic and inorganic anions in the vicinity of the crevice opening, and—in the case of chromium ions—form insoluble precipitates containing chromium oxide (Cr2O3) and chromium phosphate (CrPO4) on the metal surface. Illustration created by Chrisoula Toupadakis Skouritakis, Ph.D.
Modular junctions are susceptible to fretting corrosion. During fretting corrosion, surface pressure between contacting surfaces at modular articulations cause friction. Surface pressure is often initiated by micromotion at the interfaces during mechanical loading. This micromotion can physically disrupt the passivation layer, leading to metal particle release and facilitation of crevice corrosion.34 Micromotion is not a result of a loose implant. Rather, when the joint is loaded, the various components of the modular implant flex (slip region) while remaining together (stick region, Figure 4).
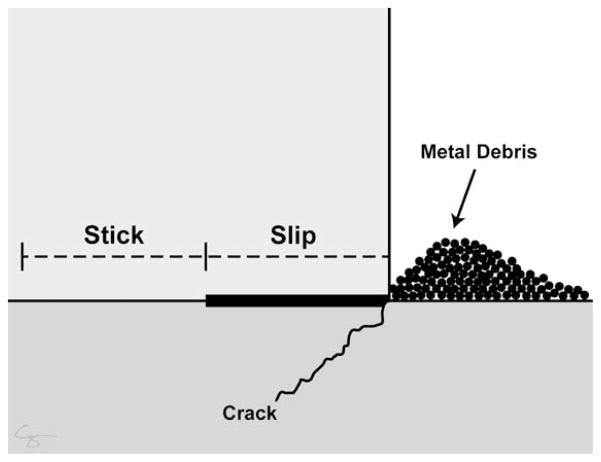
FIGURE 4.
When a modular junction is loaded, the implant flexes (slip region) while remaining together (stick region). This micromotion can physically disrupt the passivation layer, leading to metal wear or implant fracture. Micromotion does not equate to loosening. Illustration created by Chrisoula Toupadakis Skouritakis, Ph.D.
METAL WEAR DURING TJR
There are different modes for wear, including adhesive, abrasive, third body, fatigue, and corrosion. Metal wear occurs between metal-on-metal articulation and between metal-on-polymer (MOP) articulation as well. In the case of a metal-on-metal articulation, adhesive wear predominates, suboptimal implant positioning leads to additional wear.35 After the initial placement of a metal-on-metal articulation (Figure 5), “bedding-in” occurs with high wear conditions and metal particle production. During this time one metal component wears into another until an “optimal contact area” develops and the wear rate is reduced, contact stress is decreased, and lubrication conditions improve (Figure 6).36 The bedding-in period is followed by a much lower steady-state wear rate that continues for the life of the metal-on-metal articulation.37 The transition from the bedding-in period to the steady-state phase represents a change in the mode of lubrication from high friction boundary lubrication to low friction fluid-film lubrication.38
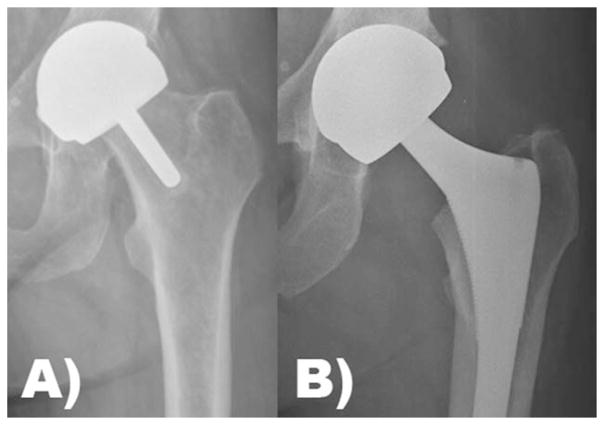
FIGURE 5.
(A) Hip resurfacing arthroplasty and (B) total hip arthroplasty with a metal head and metal acetabular liner are types of metal-on-metal articulations in total joint arthroplasty.
FIGURE 6.
A polar bearing prosthesis leads to proper bedding-in and low steady-state wear as well as fluidfilm lubrication assuming proper implant positioning. Illustration created by Chrisoula Toupadakis Skouritakis, Ph.D.
Corrosion and wear act synergistically leading to even greater metal breakdown in the presence of proteinaceous solutions.27 Tribocorrosion involves similar corrosive modes present in modular junction corrosion.39 Tribocorrosion occurs during fluid film lubrication even when the metal components are not in contact. Tribocorrosion is the irreversible transformation of a material due to the simultaneous action of corrosion and wear taking place in a sliding tribological contact area.40 In the case of metal-on-metal articulations, the two metal surfaces along with the synovial fluid react to form what is known as a tribomaterial. This material has an acidic nanocrystalline structure and consists of metallic and organic constituents, which are liberated within the surrounding soft tissues.41 In the case of metal-on-polymer articulations, metal wear and tribocorrosion occur as well. Using hip stimulators, De Villiers et al.42 showed an increased cobalt release in MOP arthroplasty with large femoral head, which was even worse with third body abrasives. Moreover, Savarino et al.43 showed a significant increase of Co and Cr in serum of patients with MOP arthroplasty compared to subjects with no implant.
METALS UTILIZED DURING TJR
The most common metal alloys used in TJR are cobalt–chromium–molybdenum (CoCr or CoCrMo) and titanium-6 aluminum- 4 vanadium (Ti6Al4V). Other metals or alloys include iron–chromium–nickel (stainless steel, AISI 316 L), commercially pure titanium (cpTi), titanium–aluminum–niobium (Ti6Al7Nb), and tantalum (Ta). The above alloys are generally named by the most representative elements forming their composition and as the percentage of the remaining elements (aluminum, vanadium, niobium, and so forth). These alloys exist in different compositions and structures. Currently, the chemical, mechanical, and metallurgical requirements for implant quality stainless steel are documented in F138-13a. Similarly, there are two representatives of CoCr based alloys: cast CoCrMo and the wrought MP35N, the main difference being the percentage of Cr and how the alloys are processed. Ti6Al4V alloy (ASTM F136) is the most common alloy of titanium in medical implants.44 Furthermore, as shown by Rae et al.45,46 there are differences in the biological responses to bulk and particulate metal, so each will be discussed separately.
Titanium and titanium alloys
Bulk
The biological response to bulk cpTi, Ti6Al4V, and Ti6Al7Nb involves transient inflammation but little cytotoxicity. A cylinder of bulk cpTi placed in bone demonstrated hypocellular fibrous tissue surrounding the implant with little inflammation.47 MC3T3-E1 preosteoblast cells do not exhibit cytotoxicity when cultured on cpTi unless a cathodic voltage is applied.48–50 As bulk, the advantage of Ti-alloy as a stem is lower modulus of elasticity, and its disadvantage as a head is poor wear resistance.51,52 Moreover, as shown by Tengvall et al.53 the Fenton reaction (decomposition of H2O2 by metal surfaces or metal ions leading to the production of harmful hydroxyl radicals) is diminished at a titanium surface making this metal highly biocompatible. Goldberg et al.54 investigated, the issue of corrosion and self-passivation of Ti6Al4V alloy and CoCrMo alloy. They concluded that Ti6Al4V re-passivation takes slightly longer to occur and more electrons are released during this process. However, Ti6Al4V appears to be more resistant to corrosion if the oxide on the surface is not mechanically disturbed.
Particulate titanium alloy
Particulate cpTi, Ti6Al4V, and Ti6Al4Nb stimulate inflammation and bone resorption. Clinically relevant particles of cpTi placed in bone were phagocytized by macrophages without an acute inflammatory response (i.e., no polymorphonuclear leukocytes (PMNs) or lymphocytes), fibrosis, or granuloma.55 However, with increased concentration of Ti6Al4V particles, the cortical endosteal surfaces showed evidence of scalloping and resorption.56 The macrophages responded to cpTi particles with increased release of prostaglandin E2 (PGE2), interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α).57,58 The release of the cytokines was associated with an increase in tyrosine phosphorylation and mitogen-activated protein kinase (MAPK) activation.59 Interestingly, inhibition of phagocytosis with antibodies to CD11b/CD18 receptors did not decrease the release of IL-6 and TNF-α indicating a phagocytosis-independent mechanism of macrophage activation by cpTi particles. This was also supported by data from co-culture of macrophages and lymphocytes, which demonstrated a dose-dependent release of IL-6 and TNF-α.59,60 Osteoblasts exposed to cpTi particles respond with acute increase in nuclear factor (nuclear factor IL-6 (NF-IL-6) and nuclear factor-kappa B (NFκB)) translocation, IL-6 release, and type-1 collagen expression.61–63 The addition of cpTi particles was not associated with increased cell death, nitric oxide production, and alkaline phosphatase (ALP) or osteocalcin production expression.62 Fibroblasts cultured with cpTi particles release monocyte chemoattractant protein 1 (MCP-1) and increased expression of bone-resorbing metal-loproteinase in a dose- and time-dependent manner, which coincided with the release of IL-6.64–66 However, exposure of fibroblasts to cpTi particles did not induce MCP-2, monocyte inflammatory protein 1 alpha (MIP-1α), or RANTES (regulated on activation, normal T cell expressed and secreted, also known as CCL5). A murine model using a cpTi intramedullary femoral rod demonstrated that addition of cpTi particles resulted in release of MCP-1, macrophage colony stimulating factor (M-CSF), and IL-6 without evidence of cell necrosis.67 However, cpTi particles downregulate the expression of toll-like receptor (TLR) proteins and decrease the number of TLR immunoreactive cells.68 This unexpected result is possibly due to a positive feedback loop to inhibit excessive inflammation. Titanium particles appear to induce pro-inflammatory cytokine production, including IL-6, TNF-α, and MCP-1, and induce a minor inflammatory reaction. This reaction is distinctly different from that seen with cobalt chrome based alloys in particulate form, which are associated with significant cell necrosis. Blocking titanium-induced osteoclastogenesis and titaniuminduced release of TNF-α could potentially mitigate the adverse effects of titanium particles.69–71
Cobalt–chromium alloy
Bulk
The biological response to bulk CoCr involves cytotoxicity and inflammation. Bulk CoCr placed in bone evoked a discontinuous fibrous tissue layer mainly composed of collagen fibers with decreased cellularity.47 Bulk CoCr in different fluids (e.g., human serum, fetal bovine serum, synovial fluid, and water) showed that the thickest calcium deposit was obtained with human serum, but metal ions were trapped as hydroxides and phosphates.72 Corrosion of bulk CoCr affects osteoprogenitor viability and adhesion at the cathodic edge of voltage through a time-lapse study.73,74 The presence of CoCr corrosion contributes to increased local tissue inflammation.75 Interestingly, Willert et al.76 have shown that CoCr particles as well as bulk material undergo corrosion and repassivation, releasing metal ions. The same group also demonstrated that hypersensitivity reactions to metal ions “start immediately after implantation, accelerate or at least facilitate sensitization and the consequent immunological response.”77
Particulates and ions
Co and Cr are specifically toxic at high concentrations, and can affect tissue that is both local to the modular implant and remotely. Metal ions can travel via lymph and blood to various sites throughout the body including bone marrow, lymph nodes, spleen, liver, and heart.78–80 Chromium ions have varying effects on the body depending on ionic valence. Hexavalent chromium is classified as a group 1 carcinogen and can cause pulmonary epithelial cancer, while trivalent chromium is less harmful.81 Merritt and Brown82 discovered that hexavalent chromium is the principal chromium ion that is released during the corrosion of stainless steel and CoCr implants. However, hexavalent chromium is quickly reduced to trivalent chromium in red blood cells, and thus the trivalent chromium ion is often detected in the cells surrounding TJR.83 Co ions from MOM implants inhibit osteoblast function,84 alter lymphocyte function and chemokine secretion,85,86 as well as cause neurological, thyroid, and cardiac dysfunction.87,88 CoCr implants pose three main threats to human cells: genotoxicity, cytotoxicity, and hypersensitivity.
Genotoxicity
Co and Cr are genotoxic, which means they can damage the genetic makeup of a cell and thus cause cancer, and there is some concern that this is possible even at concentrations that are considered “subtoxic.”89,90 Co and Cr damage tissues and DNA by oxidative stress.91 Cr specifically can release free radicals, which can cause breakage in DNA strands.92 Both Co and Cr ions inhibit DNA repair pathways, which results in defective gene expression.93 Co also inhibits topoisomerase II, which aids in DNA replication. 94 Some basic science studies suggest metal-on-metal articulations may put individuals at risk for developing cancer or other genetic defects. One study, found a decrease in DNA synthesis when cells were exposed to Co and Cr.95 A similar study by Dunstan et al. found that individuals with modular THA implants had a greater number of chromosomal aberrations compared to individuals who did not have implants.96 In addition, individuals with implants exhibited a 2.5-fold greater risk of aneuploidy when compared to individuals without implants, as well as a 3.5-fold greater risk of chromosomal translocations.97
Lewis et al.98 challenged primary human fibroblasts with CoCr particles that had been previously incubated with either human serum or minimal essential media. When particles were preincubated with human serum, they were phagocytozed less readily. They also found a significant increase in DNA damage when cells were exposed to CoCr particles compared to untreated cells. The size of the particles also plays a role on the damage to the surrounding cells. Papageorgiou et al. investigated the effect of CoCr particles on human fibroblasts of different ages.99 They found that the level of DNA damage was similar in both groups but older cells experienced a greater loss of viability and demonstrated more complex aneuploidy. This was confirmed by Raghunathan et al.100 and Behl et al.101 who also found an increase in reactive oxygen species (ROS) production which was dose-dependent after 24 h of exposure. Finally, Posada et al.102 found significantly higher cellular uptake of Co than Cr regardless of the concentration. They also showed an increase apoptosis after 48 h of exposure.
Despite the above reports, it is still unknown if genotoxicity from modular hip implants is a serious threat clinically. One study analyzed over 80 published papers to determine if there is a relationship between CoCr implants and an increase in cancer. They found that the Co and Cr ion concentration in the bodies of those with meal-on-metal articulations were far too small to substantially increase the risk of developing systemic cancer.103 In vitro and in vivo assays showed that DNA defects would be very unlikely to occur due to the increase in Co or Cr ion concentration as a result of a MOM articulation.103 Moreover, in 40 tumor bioassays where Co and Cr ion concentrations were significantly higher than those in hip implants, none reported a significant increase in systemic cancer.103 A Finnish study also looked at 2,000 patients roughly 15 years after a first generation MOM articulation and found that there was no increase in the incidence of cancer. The authors concluded that factors other than the TJR surgery are responsible for the development of cancer in hip implant patients.104 Similarly, studies of patients with more modern metal-on-metal articulations failed to suggest an increased risk of cancer.105 Lastly, DNA damage as a result of modular implants is not necessarily predictive of cancer risk. Christian et al.103 noted that DNA damage has been seen in patients with implants that were made of materials other than Co and Cr. They concluded that effects on DNA in the blood surrounding metal-on-metal articulations is not due to the metal ions that are released, but is instead due to local effects, such as inflammation, that are unrelated to the implant material.103 Although there is concern that Co and Cr ions could cause cancer, thus far no definite conclusions can be drawn.
Cytotoxicity
Co and Cr ions are very cytotoxic, with the former being more toxic than the latter. Co ions, even at smaller concentrations, can result in greater macrophage and lymphocyte death than Cr ions.36,106,107 Both ions stimulate TNF-α secretion and macrophage apoptosis.108 Several in vitro studies suggest that Co and Cr ions lead to macrophage apoptosis even after being incubated for both 24 and 48 h. DNA laddering indicates that DNA fragmentation declines after 48 h, which could be a sign of necrosis. 36,107,108 Co and Cr particles reduced the viability of histiocytes by 97% and fibroblasts by 95%.109 CoCr alloy was shown to be much less toxic than Co or Ni alone in primary fibroblasts.110 CoCr alloy particle injection within the knee joint resulted in large zones of necrosis with infiltrating macrophages and lymphocytes.111 Haynes et al.112 studied the effect of age of CoCr particles under physiological conditions with human monocytes. Their results showed that (1) Co particles were more significantly released than Cr particles, (2) this release decreased with time, (3) aged particles were less toxic than freshly produced particles, and (4) aged particles stimulated the release of IL-6 and PGE2. Kaufman et al.113 demonstrated that CoCr particles were “mildly stimulatory” for cytokine release: when challenged with CoCr particles, macrophages released two- to five fold greater levels of IL-1a, IL-6, IL-10, and granulocyte–macrophage CSF (GM-CSF) compared to unchallenged cells. Papageorgiou et al.114 used nano- and microparticles in an in vitro study with human fibroblasts. Nanoparticles were found to release significantly more free radicals and to induce more DNA damage than the microparticles. Unexpectedly, exposure to CoCr particles did not increase the release of IL-6, IL-10, TNF-α, or FGF-23, possibly due to cell death.
Hypersensitivity
Hypersensitivity refers to the immune system overreacting to an allergen and producing an undesired reaction. Metal allergies (or hypersensitivities) are characterized by contact allergies to Cr, Co, or Ni, and are found in roughly 10–15% of the general population.115,116 Metal allergy can develop from extended exposure to items, such as jewelry, cell phones, and clothing fasteners.116–123 This is of concern to orthopaedic surgeons because if a patient has a metal allergy, implanting a metal hip could potentially have negative consequences, such as hives, eczema, redness, and itching (in <1% joint replacement patients).124 Ni is often found in the CoCr alloys that are used in orthopaedic implants, and has been shown to be the strongest immunological sensitizing metal when compared with Co and Cr. Metal ions cannot initiate an immune response by themselves. Instead, they bind to proteins and form haptens. It is the metal-bound denatured protein, such as albumin, that is large enough to elicit an immune response.125 CoCr particles also have an effect on T-cells. As shown by Hallab et al.126 metalloprotein complexes made from CoCr degradation byproducts are capable of inducing a lymphocyte proliferative response. This response was greatest with high molecular weight protein (180–250 kDa); a possible trigger of the response is a crosslinking reaction with lymphocyte receptors (BV17 or CDR1 T-cell receptor).
When individuals with a metal allergy are exposed to immunoreactive haptens that are made from Ni, Co, or Cr particles, a hypersensitivity reaction occurs. This is characterized by the activation of T-cell mediated delayed-type hypersensitivity lymphocytes by antigens, which leads to cytokine release and the activation of macrophages. A hypersensitivity reaction can either be an immediate humoral response or a delayed cell-mediated response, but hypersensitivity reactions as a result of orthopaedic implants are usually a cell-mediated type-IV delayed hypersensitivity response.127 A hypersensitivity reaction is initiated when haptenic antigens activate T-cell mediated delayed-type hypersensitivity lymphocytes, which are a subtype of CD4+ helper T-cell lymphocytes. These lymphocytes are presented with a hapten, and interact with a major histocompatibility complex (MHC) class II molecule to release interferon-gamma. Interferon-gamma activates macrophages to secrete various cytokines, such as granulocyte–macrophage colony stimulating factors, tumor necrosis factors, monocyte chemotactic factors, and migration inhibitory factors. These cytokines recruit cytotoxic T-cells that are involved in the cell-mediated type IV-hypersensitivity reaction. The macrophages are also able to activate T-cell mediated delayed-type hypersensitivity lymphocytes, which will then activate more macrophages, and a vicious cycle develops. Thus, an immunologic overreaction occurs.128,129 Patients who are sensitive to Ni often exhibit crossreactivity with Co and Cr.130 This is because when patients react to a hapten, they actually are reacting to the denatured carrier. Ni, Co, and Cr all have similar mechanisms by which they denature carrier proteins, and thus cross-reactivity occurs.131,132
Stainless Steel
Bulk
Stainless steel is mainly used in polished tapered cemented femoral stems.133 The main components of stainless steel are iron (Fe), chromium (Cr), nickel (Ni), and Copper (Cu). The biological response to bulk stainless steel involves transient inflammation and lacks cytotoxicity. Macrophages demonstrated increased IL-1β release and late apoptosis when exposed to ingots of stainless steel.134 Interestingly, Hierholzer et al.135 have shown that the inflammatory reaction associated with infection causes an increased dissolution of the metal ions from stainless steel implants. Karov et al.28 investigated corrosion and self-passivation under physiological conditions. Their results showed that self-passivation can take up to 10 s and continue to evolve thereafter. The authors stated that a fast repassivation rate is an important biocompatibility requirement for alloys used to manufacture dental and orthopaedic devices.
Particulates and ions
The rate of stainless steel ion release depends on several factors including alloy composition and surface finish. The ions release by stainless steel alloy and its pure constituents after immersion in a synthetic biological medium to mimic the reaction within the human body show Ni release in the first hour followed by Fe and Cr which increase for four hours prior to assuming a steady state concentration (with Fe being the highest).136 When the surface was abraded the rate of ion release was 3- to 5-fold higher.
Stainless steel corrosion products affect osteoblast survival and stimulate macrophages-depended inflammation. Osteoblasts exposed to stainless steel corrosion products have lower DNA content, decreased ALP activity, and failure to mineralize. The effect of 316 L stainless steel and nitro-genated stainless steel (NSS) alloys (both in particles and bulk shapes) found that 316 L stainless steel particles were more toxic than NSS particles to macrophages. However, both of the alloys induced significant increase in IL-1β and TNF-α expression.134 Stainless steel particles cultured with osteoclasts increased IL-1β, IL-6, and TNF-α expression.137 Osteoclasts induced the corrosion of stainless steel particles and led to a significant release of Cr, Ni, and Mn ions into the supernatant.138 With regards to corrosion, Woodman et al.139 have shown that the predominant form of the corrosion product of these metals is an organometallic complex with serum proteins.
ADVERSE LOCAL TISSUE
Adverse local tissue reaction (ALTR) refers to untoward histological reactions (often associated with clinical manifestations) that occur when metal implants corrode to produce metallic byproducts including particles, complexes, and ions. ALTR is a contributing factor to TJA failure.140 It is unknown what the relationship is between ALTR and genotoxicity, cytotoxicity, and metal hypersensitivity, but ALTR is often associated with pain in the groin, hip, thigh, and buttock. 141 There are three major types of ALTR: aseptic lymphocyte-dominated vasculitis-associated lesions (ALVaL), pseudotumors, and osteolysis.
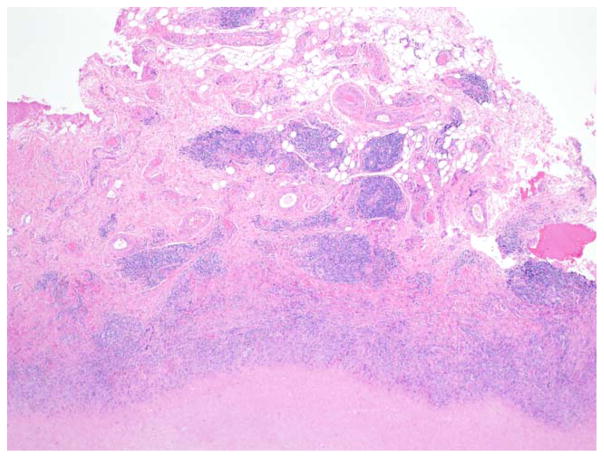
ALVaL describes a periprosthetic inflammatory reaction that is similar to a type IV hypersensitivity response in the soft tissue surrounding metal-on-metal implants.142,143 It is characterized by soft tissue necrosis, lymphocytic infiltration, and abnormal joint fluid. In TJR patients who needed revision surgery due to symptoms of implant failure, tissue samples showed perivascular infiltrates of T-cell and B-cell lymphocytes, as well as an accumulation of plasma cells and macrophages (Figure 7).144
FIGURE 7.
4× magnification of the diffuse perivascular infiltrates of T-and B-lymphocytes along with plasma cells as well as macrophages with or without metal debris that is characteristic of aseptic lymphocyte dominated vasculitis-associated lesions (ALVAL).
Pseudotumors are large, cystic masses that are often seen in patients with ALTR (Figure 8). Pseudotumors result in symptoms similar to ALVaL, but the lymphocyte infiltration and necrosis is much broader and more extensive. Pseudotumors may be characterized by pain, nerve palsy, palpable mass, and spontaneous dislocation. Various studies have attempted to determine the prevalence of pseudotumors amongst hip implant patients, and thus far the results have varied widely. Pandit et al.145 estimated that 1% of patients with metal-on-metal implants develop a pseudotumor within 5 years, while a study by Hart et al.146 found that roughly 60% of patients with metal-on-metal implants develop a pseudotumor. Van der Weegen et al. reported a rate of 37% of asymptomatic pseudotumors in their cohort of metal-on-metal implants.147
FIGURE 8.
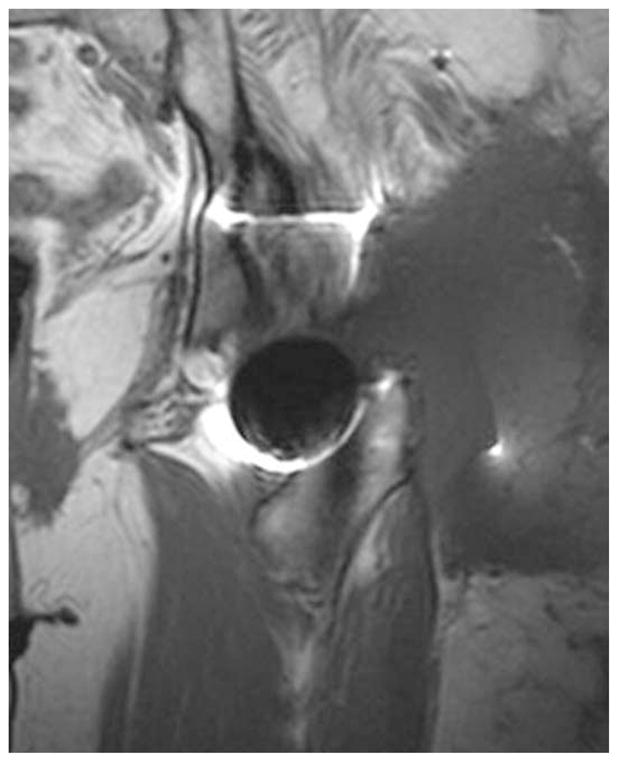
T1-weighted coronal section from a metal artifact reduction sequence (MARS) MRI demonstrating large pseudotumors involving the gluteus maximus muscle and lateral left hip.
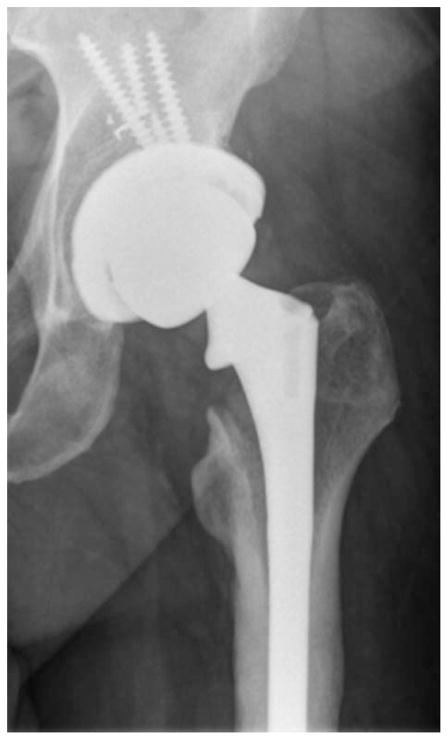
Osteolysis is the destruction of bone, resulting from osteoclast resorption.148 Cystic bone lesions and radiolucent regions near the femoral calcar and acetabular components are strong signs of osteolysis (Figure 9). Osteolysis can be asymptomatic, but can also be associated with pain if the bone loss affects the implant stability.148 It is difficult to determine the prevalence of osteolysis, as it exists in many asymptomatic patients. However, in Sweden, osteolysis was the cause of revision surgery for 75% of patients with metal-on-metal articulation.149,150
FIGURE 9.
Severe osteolysis of the calcar and failure of acetabular fixation.
CONCLUSIONS
Due to their mechanical strength and durability, metal alloys are widely used in TJR. Titanium alloy, cobalt–chromium alloy, and stainless steel in bulk form all induce a mild inflammatory response. However, byproducts of these metal alloys can have increased harmful effects including various degrees of cytotoxicity, genotoxicity and induce adverse local tissue reactions. Future implants, bearing surfaces and modular junctions should be designed to minimize the generation of metallic byproducts and the potential resultant consequences.
References
- 1.Richards RG, Moriarty TF, Miclau T, McClellan RT, Grainger DW. Advances in biomaterials and surface technologies. J Orthop Trauma. 2012;26:703–707. doi: 10.1097/BOT.0b013e31826e37a2. [DOI] [PubMed] [Google Scholar]
- 2.Epinette JA, Manley MT. Hydroxyapatite-coated total knee replacement: Clinical experience at 10 to 15 years. J Bone Joint Surg Br. 2007;89:34–38. doi: 10.1302/0301-620X.89B1.17864. [DOI] [PubMed] [Google Scholar]
- 3.Attar FG, Khaw F-M, Kirk LMG, Gregg PJ. Survivorship analysis at 15 years of cemented press-fit condylar total knee arthroplasty. J Arthroplasty. 2008;23:344–349. doi: 10.1016/j.arth.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Melton JTK, Mayahi R, Baxter SE, Facek M, Glezos C. Long-term outcome in an uncemented, hydroxyapatite-coated total knee replacement: A 15- to 18-year survivorship analysis. J Bone Joint Surg Br. 2012;94:1067–1070. doi: 10.1302/0301-620X.94B8.28350. [DOI] [PubMed] [Google Scholar]
- 5.El Masri F, Kerboull L, Kerboull M, Courpied JP, Hamadouche M. Is the so-called ‘French paradox’ a reality? Long-term survival and migration of the Charnley–Kerboull stem cemented line-to-line. J Bone Joint Surg Br. 2010;92:342–348. doi: 10.1302/0301-620X.92B3.23151. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin JR, Lee KR. The outcome of total hip replacement in obese and non-obese patients at 10- to 18-years. J Bone Joint Surg Br. 2006;88:1286–1292. doi: 10.1302/0301-620X.88B10.17660. [DOI] [PubMed] [Google Scholar]
- 7.Laing PG, Ferguson AB, Hodge ES. Tissue reaction in rabbit muscle exposed to metallic implants. J Biomed Mater Res. 1967;1:135–149. doi: 10.1002/jbm.820010113. [DOI] [PubMed] [Google Scholar]
- 8.Pourbaix M. Electrochemical corrosion of metallic biomaterials. Biomaterials. 1984;5:122–134. doi: 10.1016/0142-9612(84)90046-2. [DOI] [PubMed] [Google Scholar]
- 9.Zitter H, Plenk H. The electrochemical behavior of metallic implant materials as an indicator of their biocompatibility. J Biomed Mater Res. 1987;21:881–896. doi: 10.1002/jbm.820210705. [DOI] [PubMed] [Google Scholar]
- 10.Hench LL, Thompson I. Twenty-first century challenges for biomaterials. J R Soc Interface. 2010;7(Suppl 4):S379–S391. doi: 10.1098/rsif.2010.0151.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzapfel BM, Reichert JC, Schantz J-T, Gbureck U, Rackwitz L, Nöth U, Jakob F, Rudert M, Groll J, Hutmacher DW. How smart do biomaterials need to be? A translational science and clinical point of view. Adv Drug Deliv Rev. 2013;65:581–603. doi: 10.1016/j.addr.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Mantripragada VP, Lecka-Czernik B, Ebraheim NA, Jayasuriya AC. An overview of recent advances in designing orthopedic and craniofacial implants. J Biomed Mater Res A. 2013;101:3349– 3364. doi: 10.1002/jbm.a.34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cater WH, Hicks JH. The recent history of corrosion in metal used for internal fixation. Lancet (London, England) 1956;271:871–873. doi: 10.1016/s0140-6736(56)91043-1. [DOI] [PubMed] [Google Scholar]
- 14.Reclaru L, Lerf R, Eschler PY, Blatter A, Meyer JM. Pitting, crevice and galvanic corrosion of REX stainless-steel/CoCr orthopedic implant material. Biomaterials. 2002;23:3479–3485. doi: 10.1016/s0142-9612(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 15.Brown SA, Flemming CA, Kawalec JS, Placko HE, Vassaux C, Merritt K, Payer JH, Kraay MJ. Fretting corrosion accelerates crevice corrosion of modular hip tapers. J Appl Biomater. 1995;6:19–26. doi: 10.1002/jab.770060104. [DOI] [PubMed] [Google Scholar]
- 16.Willert HG, Semlitsch M. Tissue reactions to plastic and metallic wear products of joint endoprostheses. Clin Orthop Relat Res. 1996:4–14. [PubMed] [Google Scholar]
- 17.Lucas LC, Buchanan RA, Lemons JE, Griffin CD. Susceptibility of surgical cobalt–base alloy to pitting corrosion. J Biomed Mater Res. 1982;16:799–810. doi: 10.1002/jbm.820160606. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert JL, Buckley CA, Jacobs JJ, Bertin KC, Zernich MR. Intergranular corrosion-fatigue failure of cobalt–alloy femoral stems. A failure analysis of two implants. J Bone Joint Surg Am. 1994;76:110–115. doi: 10.2106/00004623-199401000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Liao Y, Hoffman E, Wimmer M, Fischer A, Jacobs J, Marks L. CoCrMo metal-on-metal hip replacements. Phys Chem Chem Phys. 2013;15:746–756. doi: 10.1039/c2cp42968c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper HJ, Della Valle CJ, Berger RA, Tetreault M, Paprosky WG, Sporer SM, Jacobs JJ. Corrosion at the head–neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94:1655–1661. doi: 10.2106/JBJS.K.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck–body junction in a dual-taper stem with a cobalt–chromium modular neck. J Bone Joint Surg Am. 2013;95:865–872. doi: 10.2106/JBJS.L.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Houwelingen AP, Duncan CP, Masri BA, Greidanus NV, Garbuz DS. High survival of modular tapered stems for proximal femoral bone defects at 5 to 10 years follow up. Clin Orthop Relat Res. 2013;471:454–462. doi: 10.1007/s11999-012-2552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakstein D, Eliaz N, Levi O, Backstein D, Kosashvili Y, Safir O, Gross AE. Fracture of cementless femoral stems at the mid-stem junction in modular revision hip arthroplasty systems. J Bone Joint Surg Am. 2011;93:57–65. doi: 10.2106/JBJS.I.01589. [DOI] [PubMed] [Google Scholar]
- 24.Kop AM, Keogh C, Swarts E. Proximal component modularity in THA—At what cost? An implant retrieval study. Clin Orthop Relat Res. 2012;470:1885–1894. doi: 10.1007/s11999-011-2155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown SA, Simpson JP. Crevice and fretting corrosion of stainless-steel plates and screws. J Biomed Mater Res. 1981;15:867–878. doi: 10.1002/jbm.820150611. [DOI] [PubMed] [Google Scholar]
- 26.Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33:1531–1536. doi: 10.1007/s00264-009-0729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathew MT, Abbey S, Hallab NJ, Hall DJ, Sukotjo C, Wimmer MA. Influence of pH on the tribocorrosion behavior of CpTi in the oral environment: Synergistic interactions of wear and corrosion. J Biomed Mater Res B. 2012;100:1662–1671. doi: 10.1002/jbm.b.32735. [DOI] [PubMed] [Google Scholar]
- 28.Karov J, Sinclair A, Hinberg I. Repassivation of a high chromium stainless steel orthopaedic alloy. Biomed Mater Eng. 2002;12:375–386. [PubMed] [Google Scholar]
- 29.Kop AM, Swarts E. Corrosion of a hip stem with a modular neck taper junction: A retrieval study of 16 cases. J Arthroplasty. 2009;24:1019–1023. doi: 10.1016/j.arth.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Molloy DO, Munir S, Jack CM, Cross MB, Walter WL, Walter WK. Fretting and corrosion in modular-neck total hip arthroplasty femoral stems. J Bone Joint Surg Am. 2014;96:488–493. doi: 10.2106/JBJS.L.01625. [DOI] [PubMed] [Google Scholar]
- 31.Urban RM, Jacobs JJ, Gilbert JL, Galante JO. Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J Bone Joint Surg Am. 1994;76:1345–1359. doi: 10.2106/00004623-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Campbell PA, Kung MS, Hsu AR, Jacobs JJ. Do retrieval analysis and blood metal measurements contribute to our understanding of adverse local tissue reactions? Clin Orthop Relat Res. 2014;472:3718–3727. doi: 10.1007/s11999-014-3893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perino G, Ricciardi BF, Jerabek SA, Martignoni G, Wilner G, Maass D, Goldring SR, Purdue PE. Implant based differences in adverse local tissue reaction in failed total hip arthroplasties: A morphological and immunohistochemical study. BMC Clin Pathol. 2014;14:39. doi: 10.1186/1472-6890-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg JR, Gilbert JL. In vitro corrosion testing of modular hip tapers. J Biomed Mater Res B. 2003;64:78–93. doi: 10.1002/jbm.b.10526. [DOI] [PubMed] [Google Scholar]
- 35.Chiu A, Shi XL, Lee WKP, Hill R, Wakeman TP, Katz A, Xu B, Dalal NS, Robertson JD, Chen C, Chiu N, Donehower L. Review of chromium (VI) apoptosis, cell-cycle-arrest, and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2010;28:188–230. doi: 10.1080/10590501.2010.504980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catelas I, Petit A, Vali H, Fragiskatos C, Meilleur R, Zukor DJ, Antoniou J, Huk OL. Quantitative analysis of macrophage apoptosis vs. necrosis induced by cobalt and chromium ions in vitro. Biomaterials. 2005;26:2441–2453. doi: 10.1016/j.biomaterials.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Tipper JL, Firkins PJ, Ingham E, Fisher J, Stone MH, Farrar R. Quantitative analysis of the wear and wear debris from low and high carbon content cobalt chrome alloys used in metal on metal total hip replacements. J Mater Sci Mater Med. 1999;10:353–362. doi: 10.1023/a:1026473723777. [DOI] [PubMed] [Google Scholar]
- 38.Jin ZM, Dowson D, Fisher J. Analysis of fluid film lubrication in artificial hip joint replacements with surfaces of high elastic modulus. Proc Inst Mech Eng H. 1997;211:247–256. doi: 10.1243/0954411971534359. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res. 1993;27:1533–1544. doi: 10.1002/jbm.820271210. [DOI] [PubMed] [Google Scholar]
- 40.Diomidis N, Mischler S, More NS, Roy M. Tribo-electrochemical characterization of metallic biomaterials for total joint replacement. Acta Biomater. 2012;8:852–859. doi: 10.1016/j.actbio.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 41.Kaddick C, Wimmer MA. Hip simulator wear testing according to the newly introduced standard ISO 14242. Proc Inst Mech Eng H. 2001;215:429–442. doi: 10.1243/0954411011536019. [DOI] [PubMed] [Google Scholar]
- 42.de Villiers D, Traynor A, Collins SN, Shelton JC. The increase in cobalt release in metal-on-polyethylene hip bearings in tests with third body abrasives. Proc Inst Mech Eng H. 2015;229:611– 618. doi: 10.1177/0954411915595433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savarino L, Granchi D, Ciapetti G, Cenni E, Nardi Pantoli A, Rotini R, Veronesi CA, Baldini N, Giunti A. Ion release in patients with metal-on-metal hip bearings in total joint replacement: A comparison with metal-on-polyethylene bearings. J Biomed Mater Res. 2002;63:467–474. doi: 10.1002/jbm.10299. [DOI] [PubMed] [Google Scholar]
- 44.Navarro M, Michiardi A, Castano O, Planell JA. Biomaterials in orthopaedics. J R Soc Interface. 2008;5:1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rae T. A study on the effects of particulate metals of orthopaedic interest on murine macrophages in vitro. J Bone Joint Surg Br. 1975;57:444–450. [PubMed] [Google Scholar]
- 46.Rae T. Comparative laboratory studies on the production of soluble and particulate metal by total joint prostheses. Arch Orthop Trauma Surg. 1979;95:71–79. doi: 10.1007/BF00379173. [DOI] [PubMed] [Google Scholar]
- 47.Goodman SB, Fornasier VL, Lee J, Kei J. The effects of bulk versus particulate titanium and cobalt chrome alloy implanted into the rabbit tibia. J Biomed Mater Res. 1990;24:1539–1549. doi: 10.1002/jbm.820241109. [DOI] [PubMed] [Google Scholar]
- 48.Oya K, Tanaka Y, Moriyama Y, Yoshioka Y, Kimura T, Tsutsumi Y, Doi H, Nomura N, Noda K, Kishida A, et al. Differences in the bone differentiation properties of MC3T3-E1 cells on polished bulk and sputter-deposited titanium specimens. J Biomed Mater Res A. 2010;94:611–618. doi: 10.1002/jbm.a.32751. [DOI] [PubMed] [Google Scholar]
- 49.Haeri M, Wollert T, Langford GM, Gilbert JL. Voltage-controlled cellular viability of preosteoblasts on polarized cpTi with varying surface oxide thickness. Bioelectrochemistry. 2013;94:53–60. doi: 10.1016/j.bioelechem.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Sivan S, Kaul S, Gilbert JL. The effect of cathodic electrochemical potential of Ti-6Al-4V on cell viability: Voltage threshold and time dependence. J Biomed Mater Res B. 2013;101:1489–1497. doi: 10.1002/jbm.b.32970. [DOI] [PubMed] [Google Scholar]
- 51.Scales JT. Black staining around titanium alloy prostheses—An orthopaedic enigma. J Bone Joint Surg Br. 1991;73:534–536. doi: 10.1302/0301-620X.73B4.2071632. [DOI] [PubMed] [Google Scholar]
- 52.Witt JD, Swann M. Metal wear and tissue response in failed titanium alloy total hip replacements. J Bone Joint Surg Br. 1991;73:559–563. doi: 10.1302/0301-620X.73B4.2071635. [DOI] [PubMed] [Google Scholar]
- 53.Tengvall P, Lundstrom I, Sjoqvist L, Elwing H, Bjursten LM. Titanium- hydrogen peroxide interaction: Model studies of the influence of the inflammatory response on titanium implants. Biomaterials. 1989;10:166–175. doi: 10.1016/0142-9612(89)90019-7. [DOI] [PubMed] [Google Scholar]
- 54.Goldberg JR, Gilbert JL. The electrochemical and mechanical behavior of passivated and TiN/AlN-coated CoCrMo and Ti6Al4V alloys. Biomaterials. 2004;25:851–864. doi: 10.1016/s0142-9612(03)00606-9. [DOI] [PubMed] [Google Scholar]
- 55.Goodman SB, Davidson JA, Fornasier VL. Histological reaction to titanium alloy and hydroxyapatite particles in the rabbit tibia. Biomaterials. 1993;14:723–728. doi: 10.1016/0142-9612(93)90035-z. [DOI] [PubMed] [Google Scholar]
- 56.Goodman SB, Davidson JA, Song Y, Martial N, Fornasier VL. Histomorphological reaction of bone to different concentrations of phagocytosable particles of high-density polyethylene and Ti- 6Al-4V alloy in vivo. Biomaterials. 1996;17:1943–1947. doi: 10.1016/0142-9612(96)00013-0. [DOI] [PubMed] [Google Scholar]
- 57.Maloney WJ, James RE, Smith RL. Human macrophage response to retrieved titanium alloy particles in vitro. Clin Orthop Relat Res. 1996:268–278. [PubMed] [Google Scholar]
- 58.Lee SH, Brennan FR, Jacobs JJ, Urban RM, Ragasa DR, Glant TT. Human monocyte/macrophage response to cobalt–chromium corrosion products and titanium particles in patients with total joint replacements. J Orthop Res. 1997;15:40–49. doi: 10.1002/jor.1100150107. [DOI] [PubMed] [Google Scholar]
- 59.Nakashima Y, Sun DH, Trindade MC, Maloney WJ, Goodman SB, Schurman DJ, Smith RL. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J Bone Joint Surg Am. 1999;81:603–615. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Trindade MC, Lind M, Sun D, Schurman DJ, Goodman SB, Smith RL. In vitro reaction to orthopaedic biomaterials by macrophages and lymphocytes isolated from patients undergoing revision surgery. Biomaterials. 2001;22:253–259. doi: 10.1016/s0142-9612(00)00181-2. [DOI] [PubMed] [Google Scholar]
- 61.Shida J, Trindade MC, Goodman SB, Schurman DJ, Smith RL. Induction of interleukin-6 release in human osteoblast-like cells exposed to titanium particles in vitro. Calcif Tissue Int. 2000;67:151–155. doi: 10.1007/s00223001125. [DOI] [PubMed] [Google Scholar]
- 62.Ramachandran R, Goodman SB, Smith RL. The effects of titanium and polymethylmethacrylate particles on osteoblast phenotypic stability. J Biomed Mater Res A. 2006;77:512–517. doi: 10.1002/jbm.a.30649. [DOI] [PubMed] [Google Scholar]
- 63.Yao J, Cs-Szabo G, Jacobs JJ, Kuettner KE, Glant TT. Suppression of osteoblast function by titanium particles. J Bone Joint Surg Am. 1997;79:107–112. doi: 10.2106/00004623-199701000-00011. [DOI] [PubMed] [Google Scholar]
- 64.Yao J, Glant TT, Lark MW, Mikecz K, Jacobs JJ, Hutchinson NI, Hoerrner LA, Kuettner KE, Galante JO. The potential role of fibroblasts in periprosthetic osteolysis: Fibroblast response to titanium particles. J Bone Miner Res. 1995;10:1417–1427. doi: 10.1002/jbmr.5650100920. [DOI] [PubMed] [Google Scholar]
- 65.Yaszay B, Trindade MC, Lind M, Goodman SB, Smith RL. Fibroblast expression of C–C chemokines in response to orthopaedic biomaterial particle challenge in vitro. J Orthop Res. 2001;19:970–976. doi: 10.1016/S0736-0266(01)00003-1. [DOI] [PubMed] [Google Scholar]
- 66.Fritz EA, Glant TT, Vermes C, Jacobs JJ, Roebuck KA. Titanium particles induce the immediate early stress responsive chemokines IL-8 and MCP-1 in osteoblasts. J Orthop Res. 2002;20:490–498. doi: 10.1016/S0736-0266(01)00154-1. [DOI] [PubMed] [Google Scholar]
- 67.Warme BA, Epstein NJ, Trindade MCD, Miyanishi K, Ma T, Saket RR, Regula D, Goodman SB, Smith RL. Proinflammatory mediator expression in a novel murine model of titanium-particle-induced intramedullary inflammation. J Biomed Mater Res B. 2004;71:360–366. doi: 10.1002/jbm.b.30120. [DOI] [PubMed] [Google Scholar]
- 68.Pajarinen J, Mackiewicz Z, Pollanen R, Takagi M, Epstein NJ, Ma T, Goodman SB, Konttinen YT. Titanium particles modulate expression of Toll-like receptor proteins. J Biomed Mater Res A. 2010;92:1528–1537. doi: 10.1002/jbm.a.32495. [DOI] [PubMed] [Google Scholar]
- 69.Kim JA, Ihn HJ, Park J-Y, Lim J, Hong JM, Kim SH, Kim S-Y, Shin H-I, Park EK. Inhibitory effects of triptolide on titanium particle-induced osteolysis and receptor activator of nuclear factor-κB ligand-mediated osteoclast differentiation. Int Orthop. 2015;39:173–182. doi: 10.1007/s00264-014-2596-3. [DOI] [PubMed] [Google Scholar]
- 70.Shin D-K, Kim M-H, Lee S-H, Kim T-H, Kim S-Y. Inhibitory effects of luteolin on titanium particle-induced osteolysis in a mouse model. Acta Biomater. 2012;8:3524–3531. doi: 10.1016/j.actbio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 71.Jin S, Park JY, Hong JM, Kim TH, Shin HI, Park EK, Kim SY. Inhibitory effect of (−)-epigallocatechin gallate on titanium particle-induced TNF-α ± release and in vivo osteolysis. Exp Mol Med. 2011;43:411–418. doi: 10.3858/emm.2011.43.7.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis AC, Heard PJ. The effects of calcium phosphate deposition upon corrosion of CoCr alloys and the potential for implant failure. J Biomed Mater Res A. 2005;75:365–373. doi: 10.1002/jbm.a.30430. [DOI] [PubMed] [Google Scholar]
- 73.Haeri M, Wollert T, Langford GM, Gilbert JL. Electrochemical control of cell death by reduction-induced intrinsic apoptosis and oxidation-induced necrosis on CoCrMo alloy in vitro. Biomaterials. 2012;33:6295–6304. doi: 10.1016/j.biomaterials.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 74.Haeri M, Gilbert JL. Study of cellular dynamics on polarized CoCrMo alloy using time-lapse live-cell imaging. Acta Biomater. 2013;9:9220–9228. doi: 10.1016/j.actbio.2013.06.040. [DOI] [PubMed] [Google Scholar]
- 75.Gilbert JL, Sivan S, Liu Y, Kocagoz SB, Arnholt CM, Kurtz SM. Direct in vivo inflammatory cell-induced corrosion of CoCrMo alloy orthopedic implant surfaces. J Biomed Mater Res A. 2015;103:211–223. doi: 10.1002/jbm.a.35165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Willert HG, Buchhorn GH, Gobel D, Koster G, Schaffner S, Schenk R, Semlitsch M. Wear behavior and histopathology of classic cemented metal on metal hip endoprostheses. Clin Orthop Relat Res. 1996:S160–S186. doi: 10.1097/00003086-199608001-00016. [DOI] [PubMed] [Google Scholar]
- 77.Willert H-G, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 78.Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, Solomon L. Widespread dissemination of metal debris from implants. J Bone Joint Surg Br. 1994;76:701–712. [PubMed] [Google Scholar]
- 79.Langkamer VG, Case CP, Heap P, Taylor A, Collins C, Pearse M, Solomon L. Systemic distribution of wear debris after hip replacement. A cause for concern? J Bone Joint Surg Br. 1992;74:831–839. doi: 10.1302/0301-620X.74B6.1447243. [DOI] [PubMed] [Google Scholar]
- 80.Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am. 2000;82:457–476. doi: 10.2106/00004623-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 81.Scharf B, Clement CC, Zolla V, Perino G, Yan B, Elci SG, Purdue E, Goldring S, Macaluso F, Cobelli N, et al. Molecular analysis of chromium and cobalt-related toxicity. Sci Rep. 2014;4:5729. doi: 10.1038/srep05729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Merritt K, Brown SA. Release of hexavalent chromium from corrosion of stainless steel and cobalt–chromium alloys. J Biomed Mater Res. 1995;29:627–633. doi: 10.1002/jbm.820290510. [DOI] [PubMed] [Google Scholar]
- 83.Keegan GM, Learmonth ID, Case CP. A systematic comparison of the actual, potential, and theoretical health effects of cobalt and chromium exposures from industry and surgical implants. Crit Rev Toxicol. 2008;38:645–674. doi: 10.1080/10408440701845534. [DOI] [PubMed] [Google Scholar]
- 84.Queally JM, Devitt BM, Butler JS, Malizia AP, Murray D, Doran PP, O’Byrne JM. Cobalt ions induce chemokine secretion in primary human osteoblasts. J Orthop Res. 2009;27:855–864. doi: 10.1002/jor.20837. [DOI] [PubMed] [Google Scholar]
- 85.Daou S, El Chemaly A, Christofilopoulos P, Bernard L, Hoffmeyer P, Demaurex N. The potential role of cobalt ions released from metal prosthesis on the inhibition of Hv1 proton channels and the decrease in Staphyloccocus epidermidis killing by human neutrophils. Biomaterials. 2011;32:1769–1777. doi: 10.1016/j.biomaterials.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 86.Devitt BM, Queally JM, Vioreanu M, Butler JS, Murray D, Doran PP, O’Byrne JM. Cobalt ions induce chemokine secretion in a variety of systemic cell lines. Acta Orthop. 2010;81:756–764. doi: 10.3109/17453674.2010.537806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mao X, Wong AA, Crawford RW. Cobalt toxicity—An emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194:649–651. doi: 10.5694/j.1326-5377.2011.tb03151.x. [DOI] [PubMed] [Google Scholar]
- 88.Tower SS. Arthroprosthetic cobaltism: Neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: A case report. J Bone Joint Surg Am. 2010;92:2847–2851. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 89.Colognato R, Bonelli A, Ponti J, Farina M, Bergamaschi E, Sabbioni E, Migliore L. Comparative genotoxicity of cobalt nanoparticles and ions on human peripheral leukocytes in vitro. Mutagenesis. 2008;23:377–382. doi: 10.1093/mutage/gen024. [DOI] [PubMed] [Google Scholar]
- 90.Magaye R, Zhao J, Bowman L, Ding M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp Ther Med. 2012;4:551–561. doi: 10.3892/etm.2012.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics. 2011;74:2313–2323. doi: 10.1016/j.jprot.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 92.Daley B, Doherty AT, Fairman B, Case CP. Wear debris from hip or knee replacements causes chromosomal damage in human cells in tissue culture. J Bone Joint Surg Br. 2004;86:598–606. [PubMed] [Google Scholar]
- 93.Polyzois I, Nikolopoulos D, Michos I, Patsouris E, Theocharis S. Local and systemic toxicity of nanoscale debris particles in total hip arthroplasty. J Appl Toxicol. 2012;32:255–269. doi: 10.1002/jat.2729. [DOI] [PubMed] [Google Scholar]
- 94.Ahmad M, Afzal M, Tabassum S, Kalińska B, Mrozinski J, Bharadwaj PK. Synthesis and structure elucidation of a cobalt(II) complex as topoisomerase I inhibitor: In vitro DNA binding, nuclease and RBC hemolysis. Eur J Med Chem. 2014;74:683–693. doi: 10.1016/j.ejmech.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 95.Hodges NJ, Chipman JK. Down-regulation of the DNA-repair endonuclease 8-oxo-guanine DNA glycosylase 1 (hOGG1) by sodium dichromate in cultured human A549 lung carcinoma cells. Carcinogenesis. 2002;23:55–60. doi: 10.1093/carcin/23.1.55. [DOI] [PubMed] [Google Scholar]
- 96.Dunstan E, Ladon D, Whittingham-Jones P, Carrington R, Briggs TWR. Chromosomal aberrations in the peripheral blood of patients with metal-on-metal hip bearings. J Bone Joint Surg Am. 2008;90:517–522. doi: 10.2106/JBJS.F.01435. [DOI] [PubMed] [Google Scholar]
- 97.Doherty AT, Howell RT, Ellis LA, Bisbinas I, Learmonth ID, Newson R, Case CP. Increased chromosome translocations and aneuploidy in peripheral blood lymphocytes of patients having revision arthroplasty of the hip. J Bone Joint Surg Br. 2001;83:1075–1081. doi: 10.1302/0301-620x.83b7.10102. [DOI] [PubMed] [Google Scholar]
- 98.Lewis AC, Ladon D, Heard PJ, Peto L, Learmonth I. The role of the surface chemistry of CoCr alloy particles in the phagocytosis and DNA damage of fibroblast cells. J Biomed Mater Res A. 2007;82:363–372. doi: 10.1002/jbm.a.31064. [DOI] [PubMed] [Google Scholar]
- 99.Papageorgiou I, Yin Z, Ladon D, Baird D, Lewis AC, Sood A, Newson R, Learmonth ID, Case CP. Genotoxic effects of particles of surgical cobalt chrome alloy on human cells of different age in vitro. Mutat Res. 2007;619:45–58. doi: 10.1016/j.mrfmmm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 100.Raghunathan VK, Devey M, Hawkins S, Hails L, Davis SA, Mann S, Chang IT, Ingham E, Malhas A, Vaux DJ, Lane JD, Case CP. Influence of particle size and reactive oxygen species on cobalt chrome nanoparticle-mediated genotoxicity. Biomaterials. 2013;34:3559– 3570. doi: 10.1016/j.biomaterials.2013.01.085. [DOI] [PubMed] [Google Scholar]
- 101.Behl B, Papageorgiou I, Brown C, Hall R, Tipper JL, Fisher J, Ingham E. Biological effects of cobalt–chromium nanoparticles and ions on dural fibroblasts and dural epithelial cells. Biomaterials. 2013;34:3547–3558. doi: 10.1016/j.biomaterials.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 102.Posada OM, Gilmour D, Tate RJ, Grant MH. CoCr wear particles generated from CoCr alloy metal-on-metal hip replacements, and cobalt ions stimulate apoptosis and expression of general toxicology-related genes in monocyte-like U937 cells. Toxicol Appl Pharmacol. 2014;281:125–135. doi: 10.1016/j.taap.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 103.Christian WV, Oliver LD, Paustenbach DJ, Kreider ML, Finley BL. Toxicology-based cancer causation analysis of CoCr-containing hip implants: A quantitative assessment of genotoxicity and tumorigenicity studies. J Appl Toxicol. 2014;34:939–967. doi: 10.1002/jat.3039. [DOI] [PubMed] [Google Scholar]
- 104.Visuri T, Pukkala E, Paavolainen P, Pulkkinen P, Riska EB. Cancer risk after metal on metal and polyethylene on metal total hip arthroplasty. Clin Orthop Relat Res. 1996:S280–S289. doi: 10.1097/00003086-199608001-00025. [DOI] [PubMed] [Google Scholar]
- 105.Smith AJ, Dieppe P, Porter M, Blom AW National Joint Registry of England and Wales. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: Linkage study between the National Joint Registry of England and Wales and hospital episode statistics. BMJ (Clinical research ed) 2012;344:e2383. doi: 10.1136/bmj.e2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petit A, Mwale F, Zukor DJ, Catelas I, Antoniou J, Huk OL. Effect of cobalt and chromium ions on bcl-2, bax, caspase-3, and caspase-8 expression in human U937 macrophages. Biomaterials. 2004;25:2013–2018. doi: 10.1016/j.biomaterials.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 107.Catelas I, Petit A, Zukor DJ, Huk OL. Cytotoxic and apoptotic effects of cobalt and chromium ions on J774 macrophages— Implication of caspase-3 in the apoptotic pathway. J Mater Sci Mater Med. 2001;12:949–953. doi: 10.1023/a:1012800813662. [DOI] [PubMed] [Google Scholar]
- 108.Catelas I, Petit A, Zukor DJ, Antoniou J, Huk OL. TNF-alpha secretion and macrophage mortality induced by cobalt and chromium ions in vitro-qualitative analysis of apoptosis. Biomaterials. 2003;24:383–391. doi: 10.1016/s0142-9612(02)00351-4. [DOI] [PubMed] [Google Scholar]
- 109.Germain MA, Hatton A, Williams S, Matthews JB, Stone MH, Fisher J, Ingham E. Comparison of the cytotoxicity of clinically relevant cobalt–chromium and alumina ceramic wear particles in vitro. Biomaterials. 2003;24:469–479. doi: 10.1016/s0142-9612(02)00360-5. [DOI] [PubMed] [Google Scholar]
- 110.Evans EJ, Thomas IT. The in vitro toxicity of cobalt–chromemolybdenum alloy and its constituent metals. Biomaterials. 1986;7:25–29. doi: 10.1016/0142-9612(86)90084-0. [DOI] [PubMed] [Google Scholar]
- 111.Howie DW, Vernon-Roberts B. Synovial macrophage response to aluminium oxide ceramic and cobalt–chrome alloy wear particles in rats. Biomaterials. 1988;9:442–448. doi: 10.1016/0142-9612(88)90010-5. [DOI] [PubMed] [Google Scholar]
- 112.Haynes DR, Crotti TN, Haywood MR. Corrosion of and changes in biological effects of cobalt chrome alloy and 316L stainless steel prosthetic particles with age. J Biomed Mater Res. 2000;49:167–175. doi: 10.1002/(sici)1097-4636(200002)49:2<167::aid-jbm3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 113.Kaufman AM, Alabre CI, Rubash HE, Shanbhag AS. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: Analysis of multiple cytokines using protein arrays. J Biomed Mater Res A. 2008;84:464–474. doi: 10.1002/jbm.a.31467. [DOI] [PubMed] [Google Scholar]
- 114.Papageorgiou I, Brown C, Schins R, Singh S, Newson R, Davis S, Fisher J, Ingham E, Case CP. The effect of nano- and micron-sized particles of cobalt–chromium alloy on human fibroblasts in vitro. Biomaterials. 2007;28:2946–2958. doi: 10.1016/j.biomaterials.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 115.Thyssen JP, Linneberg A, Menné T, Johansen JD. The epidemiology of contact allergy in the general population—Prevalence and main findings. Contact Derm. 2007;57:287–299. doi: 10.1111/j.1600-0536.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 116.Thyssen JP, Menné T. Metal allergy—A review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol. 2010;23:309–318. doi: 10.1021/tx9002726. [DOI] [PubMed] [Google Scholar]
- 117.Hindsén M, Persson L, Gruvberger B. Allergic contact dermatitis from cobalt in jewellery. Contact Derm. 2005;53:350–351. doi: 10.1111/j.0105-1873.2005.0592a.x. [DOI] [PubMed] [Google Scholar]
- 118.Meijer C, Bredberg M, Fischer T, Widström L. Ear piercing, and nickel and cobalt sensitization, in 520 young Swedish men doing compulsory military service. Contact Derm. 1995;32:147–149. doi: 10.1111/j.1600-0536.1995.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 119.Seishima M, Oyama Z, Oda M. Cellular phone dermatitis with chromate allergy. Dermatology. 2003;207:48–50. doi: 10.1159/000070941. [DOI] [PubMed] [Google Scholar]
- 120.Suneja T, Flanagan KH, Glaser DA. Blue-jean button nickel: Prevalence and prevention of its release from buttons. Dermatitis. 2007;18:208–211. doi: 10.2310/6620.2007.07013. [DOI] [PubMed] [Google Scholar]
- 121.Thyssen JP, Jakobsen SS, Engkilde K, Johansen JD, Søballe K, Menné T. The association between metal allergy, total hip arthroplasty, and revision. Acta Orthop. 2009;80:646–652. doi: 10.3109/17453670903487008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thyssen JP, Uter W, Schnuch A, Linneberg A, Johansen JD. 10-year prevalence of contact allergy in the general population in Denmark estimated through the CE-DUR method. Contact Derm. 2007;57:265–272. doi: 10.1111/j.1600-0536.2007.01218.x. [DOI] [PubMed] [Google Scholar]
- 123.Thyssen JP, Maibach HI. Nickel release from earrings purchased in the United States: The San Francisco earring study. J Am Acad Dermatol. 2008;58:1000–1005. doi: 10.1016/j.jaad.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 124.Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83-A:428–436. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 125.Adala R, Chakravarthy M, Srinivas V, Pai S. Orthopaedic surgery in a patient with metal sensitivity. J Cutaneous Aesthet Surg. 2011;4:67–68. doi: 10.4103/0974-2077.79202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hallab NJ, Mikecz K, Vermes C, Skipor A, Jacobs JJ. Orthopaedic implant related metal toxicity in terms of human lymphocyte reactivity to metal–protein complexes produced from cobaltbase and titanium-base implant alloy degradation. Mol Cell Biochem. 2001;222:127–136. [PubMed] [Google Scholar]
- 127.Hensten-Pettersen A. Allergy and hypersensitivity. In: Morrey BF, editor. Biological, Material, and Mechanical Considerations of Joint Replacements. New York: Raven Press; 1993. pp. 353–360. [Google Scholar]
- 128.Hallab NJ, Mikecz K, Vermes C, Skipor A, Jacobs JJ. Differential lymphocyte reactivity to serum-derived metal–protein complexes produced from cobalt-based and titanium-based implant alloy degradation. J Biomed Mater Res. 2001;56:427–436. doi: 10.1002/1097-4636(20010905)56:3<427::aid-jbm1112>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 129.Thierse H-J, Moulon C, Allespach Y, Zimmermann B, Doetze A, Kuppig S, Wild D, Herberg F, Weltzien HU. Metal–protein complex-mediated transport and delivery of Ni2+ to TCR/MHC contact sites in nickel-specific human T cell activation. J Immunol. 2004;172:1926–1934. doi: 10.4049/jimmunol.172.3.1926. [DOI] [PubMed] [Google Scholar]
- 130.Merritt K, Rodrigo JJ. Immune response to synthetic materials. Sensitization of patients receiving orthopaedic implants. Clin Orthop Relat Res. 1996:71–79. [PubMed] [Google Scholar]
- 131.Heikkila RE, Cabbat FS, Cohen G. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem. 1976;251:2182–2185. [PubMed] [Google Scholar]
- 132.crMerritt K, Rodrigo JJ. Immune response to synthetic materials. Sensitization of patients receiving orthopaedic implants. Clin Orthop Relat Res. 1996:71–79. [PubMed] [Google Scholar]
- 133.Goodman SB, Gomez Barrena E, Takagi M, Konttinen YT. Biocompatibility of total joint replacements: A review. J Biomed Mater Res A. 2009;90:603–618. doi: 10.1002/jbm.a.32063. [DOI] [PubMed] [Google Scholar]
- 134.Bailey LO, Lippiatt S, Biancanello FS, Ridder SD, Washburn NR. The quantification of cellular viability and inflammatory response to stainless steel alloys. Biomaterials. 2005;26:5296–5302. doi: 10.1016/j.biomaterials.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 135.Hierholzer S, Hierholzer G, Sauer KH, Paterson RS. Increased corrosion of stainless steel implants in infected plated fractures. Arch Orthop Trauma Surg. 1984;102:198–200. doi: 10.1007/BF00575233. [DOI] [PubMed] [Google Scholar]
- 136.Herting G, Wallinder IO, Leygraf C. Metal release rate from AISI 316L stainless steel and pure Fe, Cr and Ni into a synthetic biological medium—A comparison. J Environ Monit. 2008;10:1092–1098. doi: 10.1039/b805075a. [DOI] [PubMed] [Google Scholar]
- 137.Cadosch D, Chan E, Gautschi OP, Simmen H-P, Filgueira L. Biocorrosion of stainless steel by osteoclasts—In vitro evidence. J Orthop Res. 2009;27:841–846. doi: 10.1002/jor.20831. [DOI] [PubMed] [Google Scholar]
- 138.Magone K, Luckenbill D, Goswami T. Metal ions as inflammatory initiators of osteolysis. Arch Orthop Trauma Surg. 2015;135:683– 695. doi: 10.1007/s00402-015-2196-8. [DOI] [PubMed] [Google Scholar]
- 139.Woodman JL, Black J, Jiminez SA. Isolation of serum protein organometallic corrosion products from 316LSS and HS-21 in vitro and in vivo. J Biomed Mater Res. 1984;18:99–114. doi: 10.1002/jbm.820180110. [DOI] [PubMed] [Google Scholar]
- 140.Prieto HA, Berbari EF, Sierra RJ. Acute delayed infection: Increased risk in failed metal on metal total hip arthroplasty. J Arthroplasty. 2014;29:1808–1812. doi: 10.1016/j.arth.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 141.Pivec R, Meneghini RM, Hozack WJ, Westrich GH, Mont MA. Modular taper junction corrosion and failure: How to approach a recalled total hip arthroplasty implant. J Arthroplasty. 2014;29:1–6. doi: 10.1016/j.arth.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 142.Kolatat K, Perino G, Wilner G, Kaplowitz E, Ricciardi BF, Boettner F, Westrich GH, Jerabek SA, Goldring SR, Purdue PE. Adverse local tissue reaction (ALTR) associated with corrosion products in metal-on-metal and dual modular neck total hip replacements is associated with upregulation of interferon gamma-mediated chemokine signaling. J Orthop Res. 2015;33:1487–1497. doi: 10.1002/jor.22916. [DOI] [PubMed] [Google Scholar]
- 143.Lawrence H, Deehan D, Holland J, Kirby J, Tyson-Capper A. The immunobiology of cobalt: Demonstration of a potential aetiology for inflammatory pseudotumours after metal-on-metal replacement of the hip. Bone Joint J. 2014;96-B:1172–1177. doi: 10.1302/0301-620X.96B9.33476. [DOI] [PubMed] [Google Scholar]
- 144.De Smet KA. Belgium experience with metal-on-metal surface arthroplasty. Orthop Clin North Am. 2005;36:203–213. doi: 10.1016/j.ocl.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 145.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CLM, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 146.Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, Cobb JP, Skinner JA, Mitchell AW. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: A case–control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94:317–325. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- 147.van der Weegen W, Brakel K, Horn RJ, Hoekstra HJ, Sijbesma T, Pilot P, Nelissen RGHH. Asymptomatic pseudotumours after metal-on-metal hip resurfacing show little change within one year. Bone Joint J. 2013;95-B:1626–1631. doi: 10.1302/0301-620X.95B12.32248. [DOI] [PubMed] [Google Scholar]
- 148.Ries MD, Link TM. Monitoring and risk of progression of osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2012;94:2097–2105. [PMC free article] [PubMed] [Google Scholar]
- 149.Malchau H, Herberts P, Eisler T, Garellick G, Söderman P. The Swedish total hip replacement register. J Bone Joint Surg Am. 2002;84-A(Suppl 2):2–20. doi: 10.2106/00004623-200200002-00002. [DOI] [PubMed] [Google Scholar]
- 150.Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83:312–316. doi: 10.1136/pgmj.2006.053215. [DOI] [PMC free article] [PubMed] [Google Scholar]