Abstract
Anthranilate phosphoribosyltransferase (TrpD) is involved in tryptophan biosynthesis, catalyzing the transfer of a phosphoribosyl group to anthranilate, leading to the generation of phosphoribosyl anthranilate. TrpD belongs to the phosphoribosyltransferase (PRT) superfamily and is the only member of the structural class IV. X‐ray structures of TrpD from seven species have been solved to date. Here, functional and structural characterization of a recombinant TrpD from hyperthermophilic archaeon Thermococcus kodakarensis KOD1 (TkTrpD) was carried out. Contrary to previously characterized Mg2+‐dependent TrpD enzymes, TkTrpD was found to have a unique divalent cation dependency characterized by maximum activity in the presence of Zn2+ (1580 μmol·min−1·mg−1, the highest reported for any TrpD) followed by Ca2+ (948 μmol·min−1·mg−1) and Mg2+ (711 μmol·min−1·mg−1). TkTrpD displayed an unusually low thermostability compared to other previously characterized proteins from T. kodakarensis KOD1. The crystal structure of TkTrpD was determined in free form and in the presence of Zn2+ to 1.9 and 2.4 Å resolutions, respectively. TkTrpD structure displayed the typical PRT fold similar to other class IV PRTs, with a small N‐terminal α‐helical domain and a larger C‐terminal α/β domain. Electron densities for Zn2+ were identified at the expected zinc‐binding motif, DE(217–218), of the enzyme in each subunit of the dimer. Two additional Zn2+ were found at a new dimer interface formed in the presence of Zn2+. A fifth Zn2+ was found bound to Glu118 at crystal lattice contacts and a sixth one was ligated with Glu235. Based on the TkTrpD–Zn2+ structure, it is suggested that the formation of a new dimer may be responsible for the higher enzyme activity of TkTrpD in the presence of Zn2+ ions.
Keywords: crystal structure, thermophilicity, tryptophan biosynthesis, zinc binding
Abbreviations
- LB
Luria–Bertani
- PRA
phosphoribosyl anthranilate
- PRPP
phosphoribosyl pyrophosphate
- PRTs
phosphoribosyltransferases
- rmsd
root mean square deviation
- TrpD
anthranilate phosphoribosyltransferase
Anthranilate phosphoribosyltransferase (TrpD, EC2.4.2.18) catalyzes the second step in tryptophan biosynthesis, which involves the transfer of a phosphoribosyl group to anthranilate to generate phosphoribosyl anthranilate (PRA), the basic skeleton of tryptophan (Fig. S1). TrpD belongs to the functional superfamily of phosphoribosyltransferases (PRTs) 1, which play important role in the metabolism of nucleotides and amino acids 2.
Phosphoribosyltransferases have been divided into four different classes on the basis of their tertiary structures 3, 4. Class I has a common α/β fold and comprises uracil, orotate, and purine PRTs. Class II has an N‐terminal α/β sandwich domain and a C‐terminal α/β TIM barrel domain. This class includes the quinolinate and nicotinic acid PRTs. Class III has a unique domain structure and includes ATP‐PRTase. Class IV PRTs are limited to TrpD 5 and exhibit a homodimeric structure and a novel PRT fold, consisting of a small N‐terminal α‐helical domain connected to a large C‐terminal α/β domain by a hinge region 6. The X‐ray structures of TrpD enzymes from Sulfolobus solfataricus (SsTrpD; PDB entry 2GVQ) 7, Pectobacterium carotovorum (PcTrpD; PDB entry 1KHD) 8, Mycobacterium tuberculosis (MtbTrpD; PDB entry 4X5B) 9, Thermus thermophilus (TtTrpD; PDB entry 1V8G; Shimizu and Kunishima, 2004, RIKEN Structural Genomics/Proteomics Initiative, unpublished), Acinetobacter sp. ADP1 (AsTrpD; PDB entry 4YI7; Evans et al., 2015, unpublished), Xanthomonas campestris (XcTrpD; PDB entry 4HKM; Ghosh et al., 2012; New York Structural Genomics Research Consortium, unpublished), and Nostoc sp. (NsTrpD; PDB entry 1VQU; Joint Center for Structural Genomics, 2005, unpublished) have been solved.
Most of PRTs have been shown to utilize Mg2+ as divalent cation for enzyme activity. However, Salmonella typhimurium and P. carotovorum TrpD enzymes have been found to utilize other metal ions, including Mn2+ and Co2+ in addition to Mg2+, for enzyme activity 8, 10. These divalent cations have been implicated in phosphoribosyl pyrophosphate (PRPP) complexation, which induces prominent ordering of a conserved Gly‐rich loop GTGGD in TrpD 7.
Here, we report the biochemical and structural characterization of TrpD (TkTrpD) from the hyperthermophilic archaeon Thermococcus kodakarensis KOD1, an obligate heterotroph that grows optimally at 85 °C and pH 6.5 11. The gene encoding TkTrpD was expressed in Escherichia coli and the recombinant gene product was purified, characterized, crystallized and its crystal structure was determined in free form as well as in the presence of Zn2+ to 1.9 and 2.4 Å resolutions, respectively. The results would provide a better understanding of the TrpD family of enzymes and help in biotechnological applications to synthesize compounds for use in biochemical assays 12, 13. Moreover, TrpD has also emerged as a potential candidate for biomedical applications. The importance of TrpD has been emphasized by a genome‐wide transposon mutagenesis study in M. tuberculosis 14, which showed that the enzymes responsible for the biosynthesis of PRPP as well as biosynthetic enzymes that use PRPP, such as TrpD, are essential for mycobacterial growth 14, 15.
Results and Discussion
Production and purification of TkTrpD
The TkTrpD gene (KEGG entry: TK0253) consists of an open reading frame (ORF) of 978 nucleotides, encoding for a polypeptide of 325 amino acid residues with a theoretical molecular mass of 34346.16 Da and pI of 4.9. TkTrpD was produced in E. coli and purified to homogeneity using heat treatment and ion‐exchange chromatography. Purified recombinant TkTrpD exhibited a molecular weight of about 36 kDa (Fig. 1), matching the molecular weight calculated from the amino acid sequence. By gel filtration chromatography, the molecular mass of TkTrpD was estimated to be 70 kDa, indicating that TkTrpD is a homodimer in solution (Fig. S2).
Figure 1.
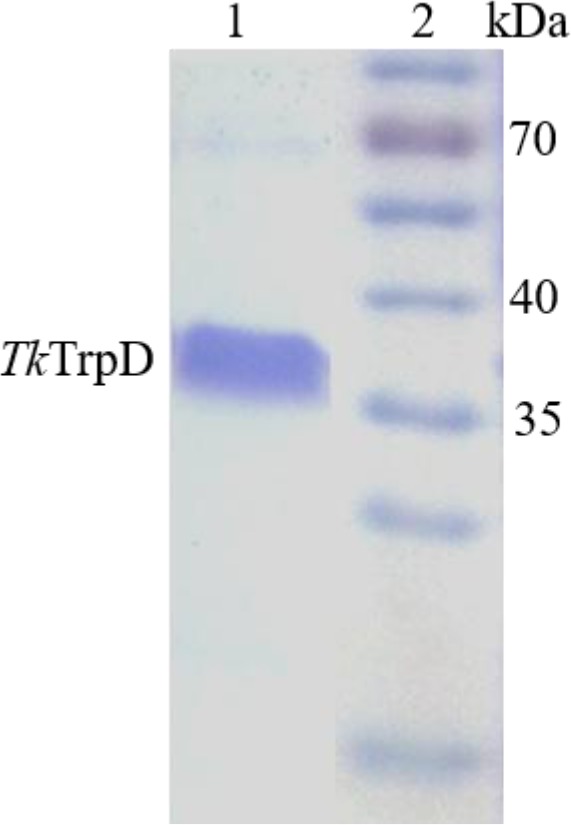
Purity of TkTrpD in SDS/PAGE stained with Coomassie Brilliant Blue. Lane 1, Purified TkTrpD eluted after ResQ column chromatography; Lane 2, molecular mass marker (Page Ruler prestained protein ladder # SM 0671, Fermentas).
Effect of pH and temperature
The optimal pH for TkTrpD activity was found to be 8.5–9.0 (Fig. S3). The effect of temperature on TkTrpD activity was examined at optimal pH. TkTrpD exhibited highest activity at 55 °C (Fig. 2) although the optimal growth temperature of T. kodakarensis is 85 °C. This result is in contrast to most of the enzymes from hyperthermophiles but similar to ribose‐5‐phosphate pyrophosphokinases from T. kodakarensis 16 and Pyrobaculum calidifontis 12, and phosphoribosyl diphosphate synthase from S. solfataricus 17. It should be noted that a protective mechanism of protein stabilization in hyperthermophiles has been suggested involving the secretion of small‐molecule osmolytes in stressful conditions 18. A similar mechanism may apply to TkTrpD to increase its stability and prevent unfolding at elevated temperatures.
Figure 2.
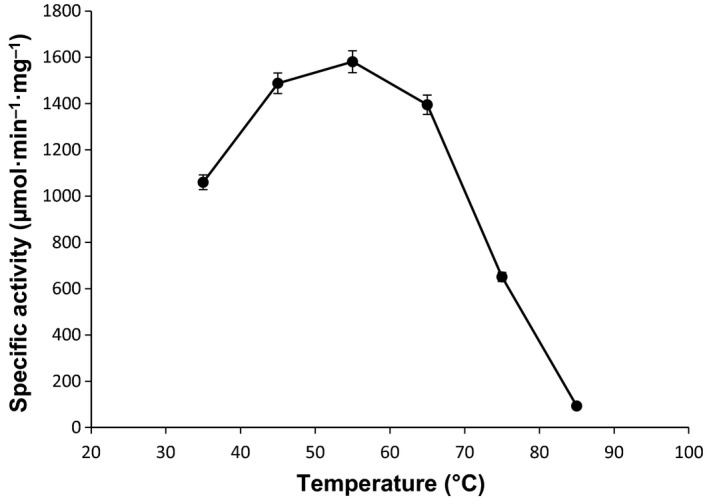
Optimal temperature for TkTrpD enzymatic activity. The activity assays were conducted in triplicate at pH 8.5 and at various temperatures (35–85 °C).
Cation dependency
Anthranilate phosphoribosyltransferase enzymes from E. coli, S. typhimurium, Saccharomyces cerevisiae, S. solfataricus, and M. tuberculosis have been reported to be dependent on Mg2+ for enzymatic activity 15, 19, 20. Pectobacterium carotovorum and S. typhimurium TrpDs have been reported to be activated by Mn2+ 8, 10. Addition of EDTA completely inhibited the enzymatic activity of TkTrpD, indicating the dependency of the enzyme on metal cations. The effect of various cations on TkTrpD was therefore examined (Fig. 3). Surprisingly, addition of Zn2+ and Ca2+ led to higher specific activities than Mg2+, whereas Cu2+, Ni2+, Co2+, and Mn2+ showed lower activities. The decrease in enzyme activity in the presence of Co2+ and Mn2+ may be attributed to the slight precipitation of TkTrpD in the presence of these metal ions.
Figure 3.
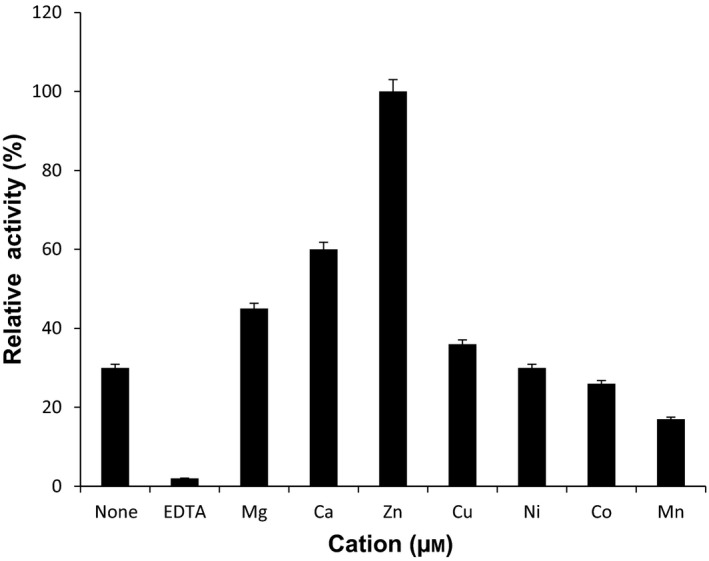
Effect of metal cations on TkTrpD activity. Reactions were performed at pH 8.5 and temperature of 55 °C. Chloride salt of each cation and EDTA were used at 100 μm concentration. Each measurement is the average value of three independent experiments.
Effect of cation concentration
TkTrpD activity increased with the addition of Zn2+ until the Zn2+ concentration reached 100 μm. Higher concentrations of Zn2+ significantly inhibited the reaction (Fig. 4A). In the case of Mg2+, the activity was maximal at 200 μm; however, concentrations above 200 μm also decreased enzyme activity (Fig. 4B).
Figure 4.
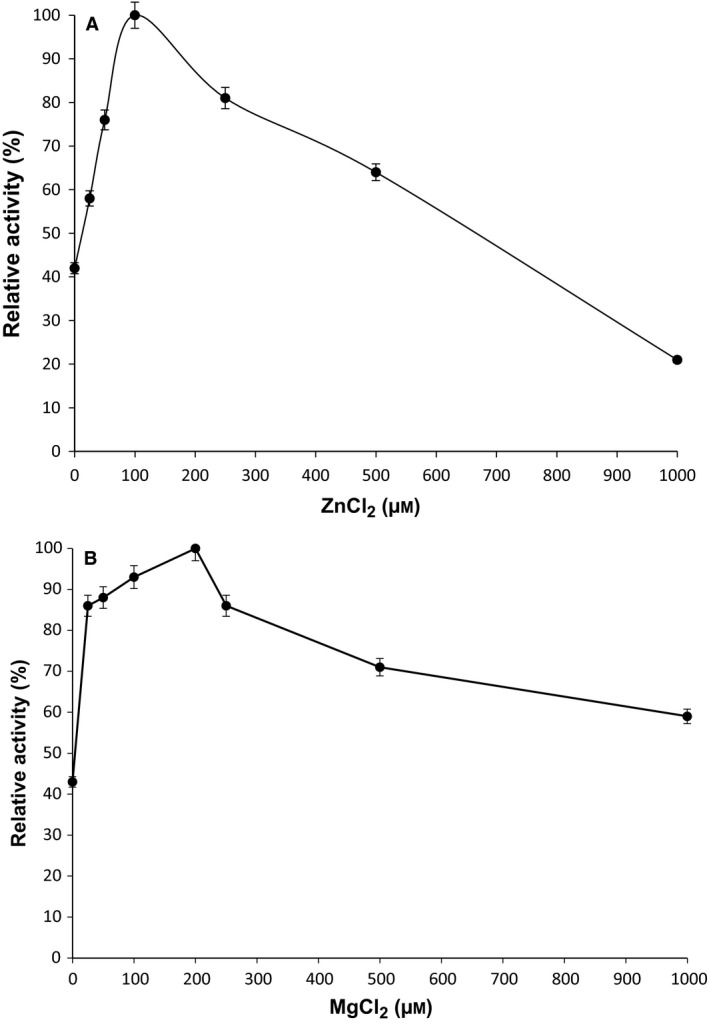
(A) Effect of ZnCl2 on TkTrpD activity. The activity assays were conducted with various concentrations of ZnCl2 at pH 8.5 and temperature of 55 °C in triplicate. (B) Effect of MgCl2 on TkTrpD enzyme activity. The activity assays were conducted with various concentrations of MgCl2 at pH 8.5 and temperature of 55 °C in triplicate.
Kinetic parameters
The effect of substrate concentration on TkTrpD activity was investigated in the presence of Zn2+. Anthranilate and PRPP were the two substrates used in the assays. The first substrate, PRPP, was kept constant at 1 mm during the measurement of the kinetic parameters toward anthranilate. Similarly, the second substrate, anthranilate, was kept constant at 4 μm when the kinetic parameters toward PRPP were measured. Anthranilate concentrations above 4 μm resulted in reduced enzymatic activity, suggesting substrate inhibition by anthranilate as observed also in M. tuberculosis TrpD 21. Apparent K m values for anthranilate and PRPP were 2.2 μm and 250 μm, respectively (Fig. 5). TkTrpD was highly active with specific activity of 1580 μmol·min−1·mg−1. To the best of our knowledge, this is the highest enzyme activity for any TrpD reported so far. A comparison of kinetic parameters and specific activities of characterized TrpDs from various sources is shown in Table 1.
Figure 5.
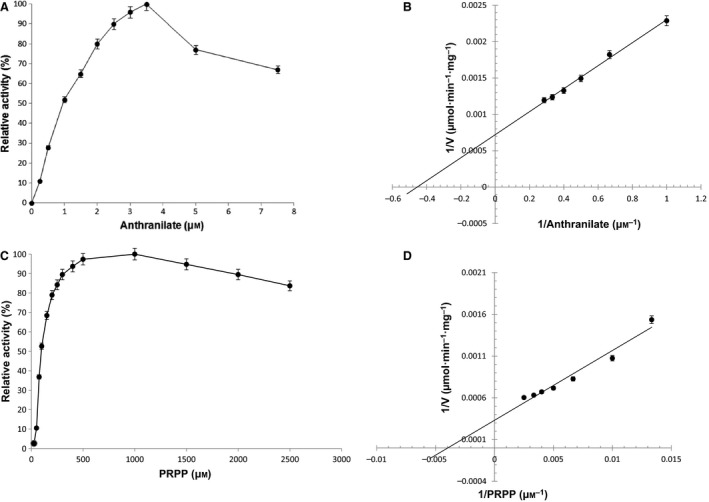
Effect of anthranilate and PRPP on TkTrpD activity. (A) and (C) show relative activity, whereas (B) and (D) show Lineweaver–Burk plot of steady‐state kinetic analysis. The kinetic parameters were examined at temperature of 55 °C and pH 8.5, in the presence of 100 μm ZnCl2 in triplicate.
Table 1.
Comparison of kinetic parameters of TrpD from various organisms. ND: no data available
| Organisms | K m anthranilate (μm) | K m PRPP (μm) | Specific activity (μmol·min−1·mg−1) | Reference |
|---|---|---|---|---|
| Thermococcus kodakarensis | 2.2 | 250 | 1580 | This study |
| Sulfolobus solfataricus | 0.085 | 180 | ND | 2 |
| Escherichia coli | 0.28 | 50 | ND | 39 |
| Salmonella typhimurium | 5.9 | 3.8 | 1350 | 40 |
| Pectobacterium carotovorum | ND | ND | 24.5 | 41 |
| Serratia marcescens | ND | ND | 409 | 42 |
| Aerobacter aerogenes | ND | ND | 734 | 43 |
| Neurospora crassa | ND | ND | 16 | 44 |
| Salmonella enterica subsp. enterica serovar typhimurium | ND | ND | 1.54 | 45 |
| Saccharomyces cerevisiae | 1.6 | 22.4 | 1.58 | 19 |
| Hansenula henricii | 4.6 | 880 | 0.4 | 46 |
| Corynebacterium glutamicum | ND | ND | 0.049 | 47 |
Quality of the TkTrpD structure
TkTrpD crystallizes with four molecules (A, B, C, and D) in the asymmetric unit that form two homodimers (A–C and B–D) (Fig. 6). The Matthews coefficient V M 22 for four molecules in the asymmetric unit is 2.4 Å3·Da−1, corresponding to a solvent content of ∼48.5%. The refined structure shows a root mean square deviation (rmsd) of 0.012 Å and 1.15° from the ideal values of bond lengths and angles, respectively. The observed crystal form of TkTrpD soaked with ZnCl2 has a dimer (A, B) in the asymmetric unit. The Matthews coefficient V M for two molecules in the asymmetric unit is 2.3 Å3·Da−1, corresponding to a solvent content of ~ 46.2%. As the crystals used for the Zn2+ soaking had been grown in different conditions, unsoaked crystals were tested and found to have similar space group and cell dimensions as those of the free TkTrpD, suggesting that soaking with Zn2+ induced a rearrangement of the crystal packing. The refined structure shows an rmsd of 0.010 Å and 1.41° from the ideal values of bond lengths and bond angles, respectively. Detailed statistics of data collection and refinement for both structures are presented in Table 2.
Figure 6.
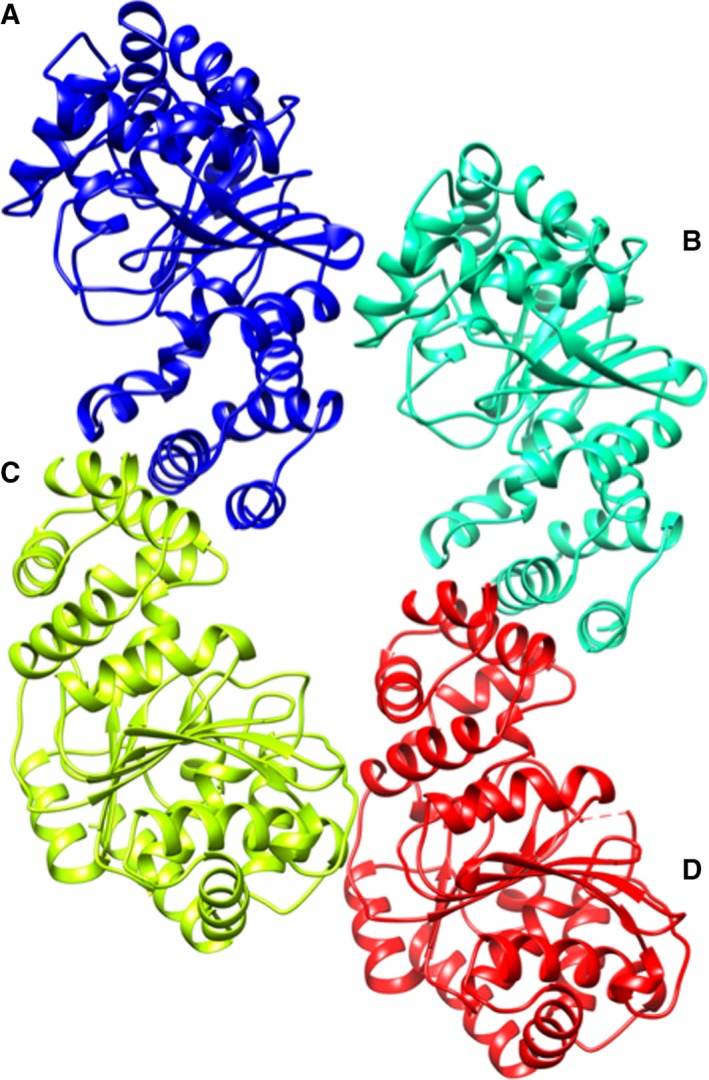
Ribbon diagram of TkTrpD tetramer in the asymmetric unit. Each subunit of the tetramer is shown in different color with the functional homodimers formed between A–C and B–D. Figure was created using UCSF Chimera 50.
Table 2.
TkTrpD data collection and refinement statisticsa
| Data collection | Free TkTrpD | TkTrpD‐Zn2+ |
|---|---|---|
| Wavelength (Å) | 0.96598 | 1.03320 |
| Beamline | MASSIF‐1, ESRF | P13, PETRA III |
| Detector | PILATUS 2M | PILATUS 6M |
| Temperature (K) | 100 | 100 |
| Space group | P212121 | P22121 |
| Unit cell, a, b, c (Å) | 83.9, 85.6, 180.8 | 42.6, 81.3, 179.4 |
| α, β, γ (°) | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 |
| Mosaicity (°) | 0.05 | 0.19 |
| Resolution range (Å) | 49.27–1.91 (1.98–1.91) | 48.19–2.42 (2.50–2.42) |
| Total no. of measurements | 453 345 | 172 488 |
| No. of unique reflections | 100 253 | 24 674 |
| Completeness (%) | 98.8 (95.9) | 99.8 (99.0) |
| Multiplicity | 4.5 (4.6) | 7.0 (7.2) |
| <I/σ (I)> | 9.5 (1.5) | 9.6 (1.4) |
| R meas 48 (%) | 10.2 (113) | 17.2 (152) |
| CC1/2 49 | 0.997 (0.536) | 0.997 (0.467) |
| Overall B factor from Wilson plot (Å2) | 28.9 | 59.0 |
| Refinement | ||
| Resolution | 49.27–1.91 (1.98–1.91) | 48.19–2.42 (2.50–2.42) |
| No. of reflections (working/test) | 95 240/4930 | 23 378/1230 |
| R cryst/R free (%) | 18.6/23.5 | 20.4/25.3 |
| No. of protein atoms | 9635 | 4835 |
| No. of water molecules | 971 | 127 |
| No. of protein ligands | – | 9 (6Zn2+, 2Na+, 1Cl−) |
| rmsd in bond lengths (Å) | 0.012 | 0.010 |
| rmsd in bond angles (°) | 1.16 | 1.41 |
| Residues in most favorable regions (%) | 96.0 | 96.0 |
| Residues in additionally allowed regions (%) | 3.3 | 3.9 |
| Average B factor (Å2) | ||
| Protein | 35.9 | 52.6 |
| Water | 41.9 | 52.4 |
| Ligands | – | 66.3 |
| PDB id | 5NOE | 5NOF |
Values in parentheses are for the outer resolution shell.
Overall structure
TkTrpD structure displays the PRT fold similar to other PRTs. Each TkTrpD molecule consists of 325 residues arranged in two domains (Fig. 7): a small N‐terminal α‐helical domain containing four helices and a large C‐terminal α/β domain with a central sheet of seven β‐strands (six parallel and one antiparallel) surrounded by eight α‐helices. A hinge region (α4‐β1, β3‐α8, and α9–β4) connects the two domains.
Figure 7.
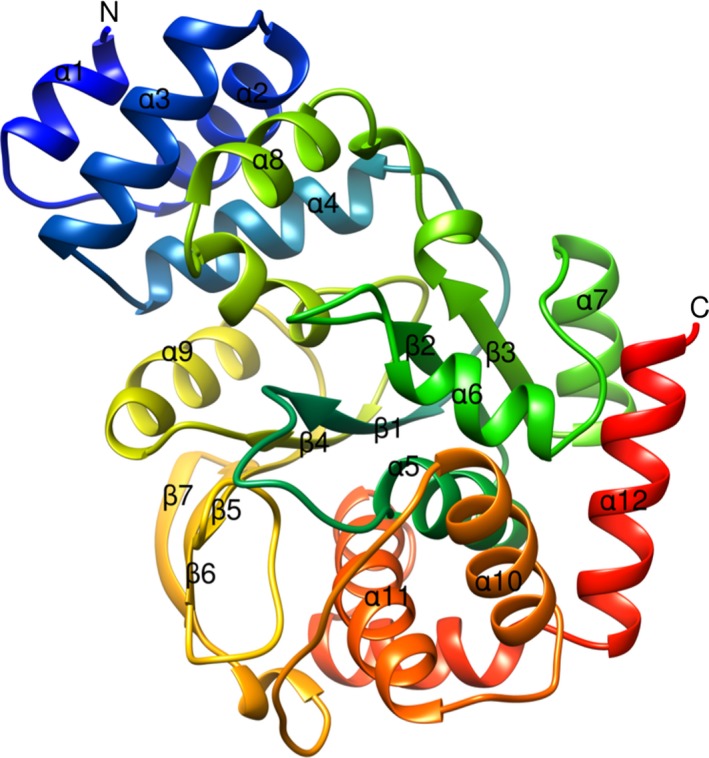
Ribbon diagram of X‐ray crystal structure of TkTrpD. Only one subunit of the homodimer is shown, with the amino acid chain colored from blue at the N terminus to red at the C terminus. Each subunit consists of a small α‐helical domain containing four helices (α1, α2, α3, and α4) and a larger C‐terminal α/β domain with a central β‐sheet containing seven β‐strands (six parallel and one antiparallel) surrounded by eight α‐helices. Figure was created using UCSF Chimera.
The N‐terminal domain is involved in dimer formation in TrpD enzymes (SsTrpD, MtbTrpD, TtTrpD, AsTrpD, XcTrpD, NsTrpD, and PcTrpD). Similarly, TkTrpD subunits also associate with each other at their N‐terminal ends through their small α‐helical domains (C‐terminal end of α1, α3, and α8). In SsTrpD, residues Ile36 and Met47 have been shown to be involved in dimerization. Mutations of these residues resulted in loss of dimeric form with decreased thermal stability 2. Both of these residues are not conserved in TrpD family. The corresponding residues in TkTrpD are Val31 and Thr42. Analysis of protein–protein interactions with PDBsum 23 shows that Ala35, Thr42 (located at the N and C termini of α3, respectively) and Leu162 (C‐terminal end of α8) are found to form highest number of inter‐subunit interactions, showing that mostly hydrophobic residues are involved in inter‐subunit interactions in TkTrpD dimer formation in agreement with dimer formation in other TrpD enzymes.
Structural comparison
Structure‐based sequence alignment of TkTrpD (Fig. 8) shows highest homology with SsTrpD (45%) followed by TtTrpD (43%), NsTrpD (39%), XcTrpD (39%), PcTrpD (37%), AsTrpD (36%), and MtbTrpD (34%). Several conserved sequences were found that play an important role in catalysis. When superimposed with SsTrpD, TkTrpD shows rmsd of 1.15 Å for 256 Cα‐atoms. At the C‐terminal end of SsTrpD, there is a small helix of 10 residues which is absent in TkTrpD and all other reported TrpD structures.
Figure 8.
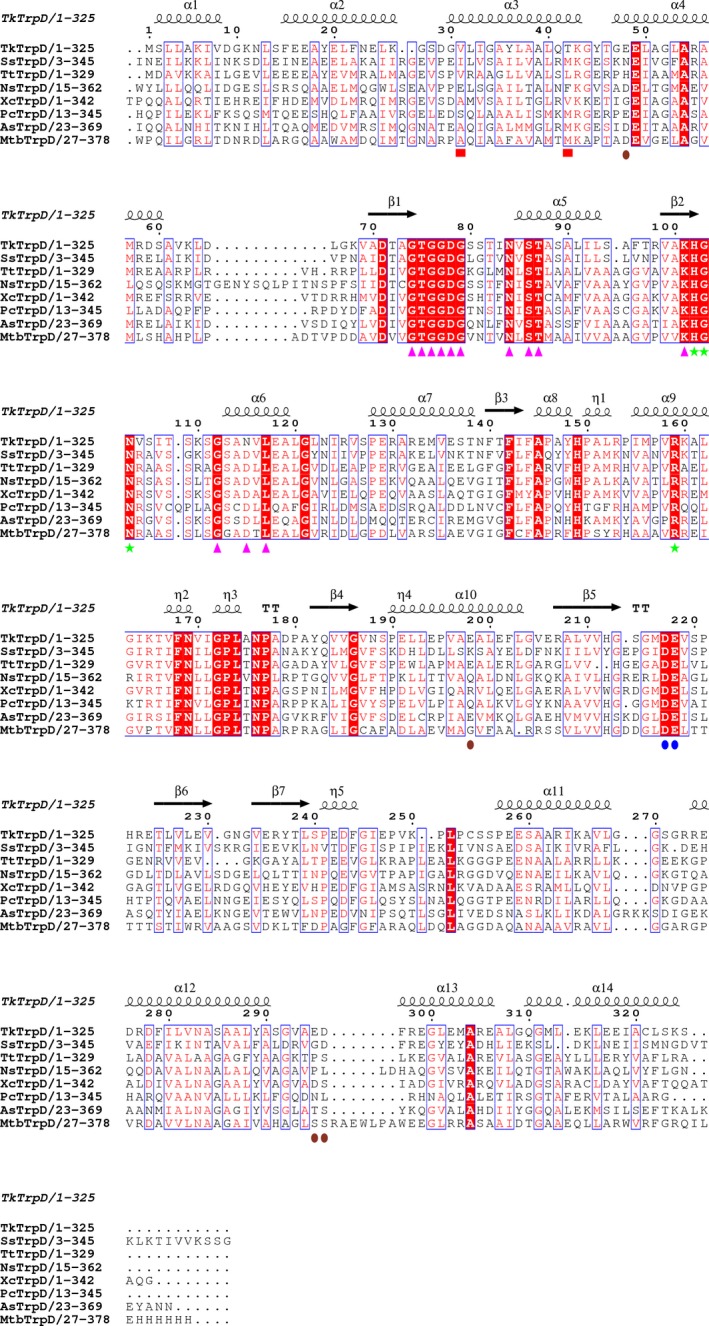
Structure‐based sequence alignment of TkTrpD. The enzymes used are SsTrpD (PDB entry 2GVQ) 7, TtTrpD (PDB entry 1V8G; Shimizu et al., 2004, unpublished), NsTrpD (PDB entry 1VQU; Joint Center for Structural Genomics, 2005, unpublished), XcTrpD (PDB entry 4HKM; Ghosh et al., 2012, unpublished), PcTrpD (PDB entry 1KHD) 8, AsTrpD (PDB entry 4YI7; Evans et al., 2015, unpublished), and MtbTrpD (PDB entry 4X5B) 9. Figure was created using espript 3.0 51. Conserved residues are indicated by white letters on a red background (strictly conserved) or red letters on a white background (global similarity score, 0.7) and framed in blue boxes. Markers indicate residues postulated to be involved in PRPP binding (magenta arrows), anthranilate binding (green stars), metal binding (blue ovals), and dimerization (red boxes). Residues involved in Zn2+ binding at the TrpD–Zn2+ dimer interface are shown with brown ovals.
Active site
Each monomer has an active site in a cleft found in the hinge region. In TkTrpD, substrate (anthranilate + PRPP) binding positions were found conserved as in other TrpDs. A conserved anthranilate binding motif (KHGN(101–104)) was found in β2–α6 loop. Lys101, in particular, is involved in anthranilate binding and HGN(102–104) in PRPP binding. This motif has been determined to be involved in catalysis in previously determined TrpD structures (e.g., SsTrpD, MtbTrpD). Arg159 in TkTrpD found on helix α8 is also conserved and in previous structures 7, 15 has been shown to be involved in anthranilate binding by forming hydrogen bond to it and is essential for catalytic function. The corresponding residues in MtbTrpD and SsTrpD are Arg193 and Arg164, respectively. A highly conserved Gly‐rich sequence GTGGD(74–78) found in TkTrpD in β1–α5 loop is considered as a signature motif of TrpD family and is involved in PRPP binding. Identical sequences have been found in MtbTrpD (GTGGD(107–111)) and in SsTrpD (GTGGD(79–83)). The first Gly of this region, in particular, is known to interact with the PPi group of PRPP via its peptide amino group and also with the amino group of anthranilate.
Divalent ion binding sites
Metal ions bind to two sites in the TrpD family. The first metal ion binds to pyrophosphate and ribose oxygen atoms of PRPP, and this site is common in PRT superfamily. The second site is specific for TrpD family and involves a conserved DE motif whose residues are key to metal binding and are invariant in all TrpD enzymes structurally characterized until now (Table 3).
Table 3.
Divalent ion binding sites in TrpD family
| Ions | Contacts | Reference |
|---|---|---|
| MtbTrpD | ||
| Mg2+‐I | S119, E252, and PRPP | 15 |
| Mg2+‐II | DE(251–252) | |
| SsTrpD | ||
| Mg2+‐I | OH− groups of ribose and pyrophosphate oxygens of PRPP | 7 |
| Mg2+‐II | DE(223–224) | |
| PcTrpD | ||
| Mn2+‐I | S103, E237, and PRPP | 8 |
| Mn2+‐II | DE(236–237) | |
| TkTrpD | ||
| Zn2+‐I | DE(217–218), D78 (subunit A) | This study |
| Zn2+‐II | E118 (subunit A at crystal lattice contacts with E118 from a symmetry molecule) | |
| Zn2+‐III | E235 (subunit B) | |
| Zn2+‐IV | DE(217–218), D78 (subunit B) | |
| Zn2+‐V | E198‐A and E295‐B | |
| Zn2+‐VI | ED(295–296)‐B, E48‐A, E198‐A | |
Soaking of TkTrpD with a ZnCl2 solution resulted in the identification of a total of six Zn2+ ions in the dimer, while in other TrpDs only four Zn2+ ions per dimer are present. In each subunit of TkTrpD, one Zn2+ ion was found in the primary metal binding site, involving the conserved DE(217–218) motif and Asp78. As PRPP is not present in the structure, no additional Zn2+ ion was found in the vicinity of the primary metal binding site. The structure of TkTrpD soaked with zinc has a dimer in the asymmetric unit. Gel filtration has also shown that in the presence of Zn2+, TkTrpD exists as a dimer in solution (Fig. S2). Structural comparison of the Zn2+‐free and Zn2+‐bound structures at subunit level shows low rmsd between Cα atoms (0.63 Å), suggesting no significant changes. Notable changes, however, were identified in the position of helices α8 and α9 that move toward the active site in the Zn2+‐bound structure. Most importantly, following superposition with the Zn2+‐free TkTrpD (Fig. 9A), the structure solution of the Zn2+‐bound TkTrpD revealed a different arrangement of the two subunits compared to the typical dimer found in other TrpD enzymes. Interestingly, two Zn2+ ions (V and VI) were found at the interface between Glu48 and Glu198 of subunit A and ED(295–296) of subunit B in the dimer. The two zinc ions are close to each other with a distance of 2.9 Å (Fig. 9B) and have similar B‐factors (43.7 and 46.0 Å2, respectively). These two additional Zn2+ binding sites in TkTrpD may therefore explain the effect of Zn2+ on TkTrpD by promoting a different dimer formation. At present, we cannot conclude whether this property is shared by this enzyme from other sources as well, or whether it is a unique property of TkTrpD. However, the structure‐based alignment (Fig. 8) shows that the ED(295–296) motif is not conserved, and therefore, other TrpDs may be unable to adopt the same dimer arrangement. Sequence variations are also evident for Glu48 and Glu198.
Figure 9.
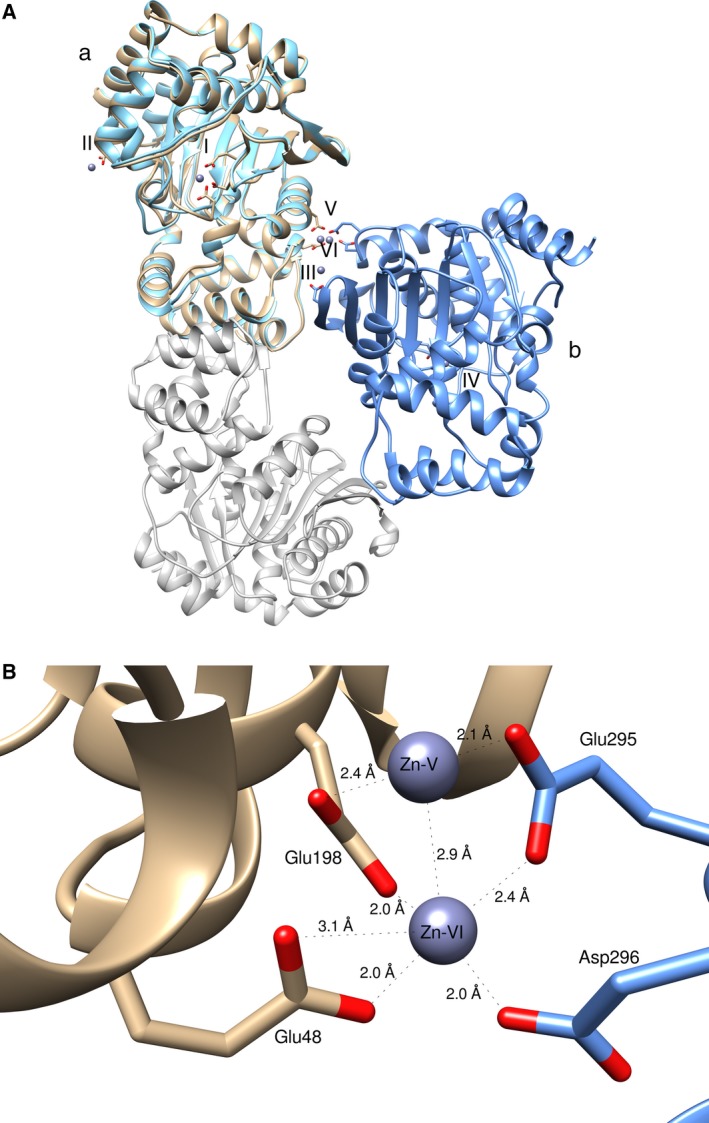
(A) Superposition of TkTrpD dimers with and without Zn2+. Subunit (a) is shown in brown (with Zn2+) and cyan (without Zn2+). The Zn2+‐binding sites are labeled with latin numbers as in the text. The second subunit of the dimer is colored in gray (without Zn2+, it corresponds to C as in Fig. 6) and in blue (with Zn2+) (B) The interface of TkTrpD with the bound Zn2+ ions. The subunits are colored differently. Zn2+ ions are shown as dark slate blue spheres. Distances are depicted. Figures were created using UCSF Chimera.
In conclusion, the biochemical and structural characterization of TkTrpD reported here may lead to new strategies to alter TrpD enzymatic activity. The new subunit–subunit interface may play a role in the increased activity of TkTrpD in the presence of Zn2+. For example, Glu198 belongs to helix α10 and slight alterations upon Zn2+ binding and dimer rearrangement could be traversed to the active site through the α8 and α9 helices. Alternatively, formation of the new dimer may affect the position of helix α8, which in the typical TrpD dimer is part of the conventional interface. In the new dimer, helix α8 becomes free from any interactions with a neighboring subunit, and therefore, it may be able to adopt more favorable positions for substrate binding. Further studies, however, are needed to elucidate the precise role of the Zn2+‐binding sites and their potential direct and indirect effects on the active site of the enzyme.
Materials and methods
Chemicals and materials used in this study were purchased from either Thermo‐Fisher Scientific (Leicestershire, UK), Fluka (Buchs, Switzerland), Merck (Darmstadt, Germany), or Sigma‐Aldrich (St. Louis, MO, USA). Gene‐specific primers were commercially synthesized by Macrogen Inc (Seoul, Korea). Escherichia coli strains used were DH5α and BL21 Codon Plus (DE3)‐RIL (Stratagene, La Jolla, CA, USA). Luria–Bertani (LB) medium was used for the cultivation of E. coli strains.
Gene cloning
Gene encoding TkTrpD was amplified from genomic DNA of T. kodakarensis, using sequence‐specific forward FTkTrpD (CATATGAGCCTTCTTGCGAAGATCGTCGATGG), which include a NdeI recognition site (shown in boldface) and reverse RTkTrpD (TCAGCTTTTTGAGAGGCATGCTATCTCCTC) primers. PCR‐amplified gene product was ligated to cloning vector pTZ57R/T. The resultant recombinant plasmid PTZ‐TkTrpD was digested with NdeI and HindIII to liberate TkTrpD, which was cloned into the expression vector pET21a(+) using the same restriction sites. pET‐TkTrpD name was assigned to the resultant recombinant expression plasmid. Presence of TkTrpD in the expression plasmid was subsequently confirmed by restriction analysis and DNA sequencing.
Gene expression and protein purification
Escherichia coli BL21 CodonPlus (DE3)‐RIL cells were transformed with recombinant pET‐TkTrpD. Expression of gene was induced by 0.5 mm isopropyl‐β‐d‐thiogalactoside (IPTG). After induction for 6 h, cells grown in LB medium were harvested and resuspended in 50 mm Tris/HCl pH 8.5 buffer containing 1 mm DTT, 1 mm PMSF, and 20% v/v glycerol. For purification, soluble portion obtained after sonication was heat‐treated at 65 °C for 25 min and centrifuged (15 000 g for 15 min). ÄKTA Purifier chromatography system (GE Healthcare, Uppsala, Sweden) was used for further purification. Heat‐treated supernatant was applied to anion‐exchange QFF (6 mL) column (GE Healthcare) and the recombinant TkTrpD was eluted with a linear gradient of 0–1 m NaCl. Fractions containing TkTrpD were desalted by dialysis against 50 mm Tris/HCl (pH 8.5) buffer containing 1 mm DTT, 1 mm PMSF, and 20% v/v glycerol. Dialyzed TkTrpD samples were applied to Resource Q (1 mL) column (GE Healthcare), and the protein was eluted with a linear gradient of 0–1 m NaCl. Analysis of the purified TkTrpD was performed by SDS/PAGE. Molecular weight and oligomeric nature of TkTrpD were determined by gel filtration chromatography column Superdex 75 10/300 GL attached to ÄKTA purifier (GE Healthcare). The standard curve was obtained with bovine pancreas chymotrypsinogen A (25 kDa), chicken egg white ovalbumin (48 kDa), and bovine serum albumin (63 kDa). Their gel‐phase distribution coefficient (K av) values were calculated and plotted against the log of their molecular weight (Fig. S3). Protein concentration was determined spectrophotometrically at every step of purification by Bradford reagent 24.
Enzyme assays
TkTrpD activity was determined fluorometrically by measuring the decrease in the concentration of anthranilic acid. Anthranilic acid reacts with PRPP, leading to the production of PRA (Fig. S1). The initial rate of decrease in anthranilate was measured, as anthranilate is utilized by TkTrpD to form PRA, resulting in a decrease in emission/fluorescence at 390 nm. The activation and emission wavelengths for anthranilate were 315 and 390 nm, respectively. A standard curve was then used to convert fluorescent intensity to anthranilate concentration. The reaction mixture contained 4 μm anthranilate, 1 mm PRPP, 100 μm ZnCl2, 100 mm Tris/HCl buffer (pH 8.5), and 5 μg of TkTrpD. The reaction mixture without PRPP was incubated at 55 °C for 5 min. The reaction started by adding PRPP at 55 °C and continued for 2.5 min. Two control experiments were carried out: one without enzyme and one without PRPP. The half‐life of PRPP is 56 min at 60 °C 20, suggesting that at 55 °C and for the time used for the reaction, no significant hydrolysis of PRPP is expected.
Effect of temperature, pH, and metal ions
For the measurement of optimal temperature, enzyme assays were performed at various temperatures ranging from 35 to 85 °C keeping the pH constant. For the estimation of optimal pH, assays were performed at various pH values keeping the temperature unchanged at 55 °C. The following buffers were used: Na‐phosphate (pH: 6.0–7.0), Tris/HCl (pH: 7.0–9.0), and Na‐bicarbonate (pH: 9.0–10.0). The effect of divalent metal ions on the enzyme activity was investigated in the presence of either 50 or 100 μm of ZnCl2, MgCl2, CaCl2, MnCl2, NiCl2, CoCl2, and CuCl2. In case of EDTA, the final EDTA concentration was 100 and 2.5 mm. The effect of Zn2+ and Mg2+ concentration on the enzyme activity was measured in the range of 0–1 mm.
Crystallization
Purified TkTrpD was concentrated to 12 mg·mL−1 in 10 mm Tris/HCl (pH 8.0) buffer containing 0.1 m NaCl and 0.002% (w/v) NaN3. PACT screen (Molecular Dimensions, Suffolk, UK) was performed in 96‐well plate using the sitting drop vapor diffusion method. Promising crystals found in solution 79 (0.2 m sodium acetate (pH 7.5), 20% (w/v) PEG 3350) were optimized by the hanging‐drop vapor diffusion method at 16 °C in Linbro 24‐well cell culture plates. The reservoir solution consisted of 0.6 mL of condition 79 mixed with 0.2 mL of MilliQ water and the drops comprised 2 μL of 12 mg·mL−1 TkTrpD mixed with 2 μL of reservoir solution. Crystals appeared after 1 day and were harvested after ~ 4 weeks for X‐ray data collection. Crystals were transferred to a reservoir solution supplemented with 20% v/v glycerol and flash‐cooled in liquid N2.
ZnCl2 crystal soaking
TkTrpD crystals obtained from solution 89 of the PACT screen (0.2 m sodium nitrate, 0.1 m Bistris‐propane buffer (pH 8.5), 20% (w/v) PEG 3350) were used after soaking in 100 mm ZnCl2 for ~ 5–10 min. The crystals were subsequently transferred to a reservoir solution supplemented with 23% v/v glycerol and flash‐cooled in liquid N2.
Data collection and structure determination
Diffraction data for the free TkTrpD were collected at ESRF (Grenoble) on the fully automatic high‐throughput MASSIF‐1 beamline 25 from a crystal that diffracted to 1.9 Å. xds 26 was used to index and integrate the data and aimless 27 for merging and scaling. The crystal was found to belong to the P212121 space group. SsTrpD (PDB entry 2GVQ) 7 was found to be the best matched search model by molrep 28 as implemented in mrbump 29 from CCP4 30 and was used to obtain initial phases. After the solution was found, buccaneer 31 was employed for initial model building and automatic refinement with refmac5 32. Further refinement was carried out using phenix and water molecules were added with tools in phenix 33. Manual rebuilding and structure visualization was performed by coot 34. The progress of refinement was monitored using the R free 35 with 5% of the reflections set aside.
ZnCl2 soaking
Data from a crystal soaked with ZnCl2 were collected at EMBL Hamburg (c/o DESY, Hamburg, Germany) on the P13 beamline at PETRA III and processed as before. Chain A of free TkTrpD crystal structure was used as search model in phaser for structure determination by molecular replacement. The best solution was found in the orthorhombic P22121 space group. Refinement was initially carried out using phenix and water molecules were added with tools in phenix. At the final stages of refinement, PDB_REDO 36 was employed and refmac 32 was used. Manual rebuilding and structure visualization was performed by coot 34. The progress of refinement was monitored with the R free.
Structure analysis
Interfaces were analyzed by PDBePISA 37. Structural superpositions were performed with PDBeFold 38 as implemented in coot 34. The superimposed structures were visually inspected using coot.
Data Accessibility
Structural data are available in the Protein Data Bank under the accession numbers 5NOE and 5NOF.
Author contributions
SP performed experiments, analyzed data, and wrote the manuscript. NR analyzed data, planned experiments, and wrote the manuscript. XFT performed experiments and analyzed data. TI planned experiments and analyzed data. ACP performed and planned experiments, analyzed data, and wrote the manuscript.
Supporting information
Fig. S1. Reaction catalyzed by TrpD.
Fig. S2. Gel filtration elution profile of TkTrpD with and without Zn2+.
Fig. S3. Determination of optimal pH for TkTrpD enzymatic activity.
Acknowledgements
We thank the EXPERTS4Asia Program for a scholarship to SP and the Biocenter Finland for structural biology infrastructure support. Access to EMBL Hamburg was provided by iNEXT (H2020 Project #653706).
References
- 1. Lambrecht JA and Downs DM (2013) Anthranilate phosphoribosyl transferase (TrpD) generates phosphoribosylamine for thiamine synthesis from enamines and phosphoribosyl pyrophosphate. ACS Chem Biol 8, 242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwab T, Skegro D, Mayans O and Sterner R (2008) A rationally designed monomeric variant of anthranilate phosphoribosyltransferase from Sulfolobus solfataricus is as active as the dimeric wild‐type enzyme but less thermostable. J Mol Biol 376, 506–516. [DOI] [PubMed] [Google Scholar]
- 3. Schramm VL and Grubmeyer C (2004) Phosphoribosyltransferase mechanisms and roles in nucleic acid metabolism. Prog Nucleic Acid Res Mol Biol 78, 261–304. [DOI] [PubMed] [Google Scholar]
- 4. Hove‐Jensen B, Andersen KR, Kilstrup M, Martinussen J, Switzer RL and Willemoës M (2017) Phosphoribosyl diphosphate (PRPP): biosynthesis, enzymology, utilization, and metabolic significance. Microbiol Mol Biol Rev 81, e00040‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castell A, Short FL, Evans GL, Cookson TVM, Bulloch EMM, Joseph DDA, Lee CE, Parker EJ, Baker EN and Lott JS (2013) The substrate capture mechanism of Mycobacterium tuberculosis anthranilate phosphoribosyltransferase provides a mode for inhibition. Biochemistry 52, 1776–1787. [DOI] [PubMed] [Google Scholar]
- 6. Schlee S, Deuss M, Bruning M, Ivens A, Schwab T, Hellmann N, Mayans O and Sterner R (2009) Activation of anthranilate phosphoribosyltransferase from Sulfolobus solfataricus by removal of magnesium inhibition and acceleration of product release. Biochemistry 48, 5199–5209. [DOI] [PubMed] [Google Scholar]
- 7. Marino M, Deuss M, Svergun DI, Konarev PV, Sterner R and Mayans O (2006) Structural and mutational analysis of substrate complexation by anthranilate phosphoribosyltransferase from Sulfolobus solfataricus . J Biol Chem 281, 21410–21421. [DOI] [PubMed] [Google Scholar]
- 8. Kim C, Xuong NH, Edwards S, Madhusudan Yee MC, Spraggon G and Mills SE (2002) The crystal structure of anthranilate phosphoribosyltransferase from the enterobacterium Pectobacterium carotovorum . FEBS Lett 523, 239–246. [DOI] [PubMed] [Google Scholar]
- 9. Cookson TVM, Evans GL, Castell A, Baker EN, Lott JS and Parker EJ (2015) Structures of Mycobacterium tuberculosis anthranilate phosphoribosyltransferase variants reveal the conformational changes that facilitate delivery of the substrate to the active site. Biochemistry 54, 6082–6092. [DOI] [PubMed] [Google Scholar]
- 10. Robison PD and Levy HR (1976) Metal ion requirement and tryptophan inhibition of normal and variant anthranilate synthase‐anthranilate 5‐phosphoribosylpyrophosphate phosphoribosyltransferase complexes from Salmonella typhimurium . Biochim Biophys Acta 445, 475–485. [DOI] [PubMed] [Google Scholar]
- 11. Atomi H, Fukui T, Kanai T, Morikawa M and Imanaka T (2004) Description of Thermococcus kodakaraensis sp. nov., a well‐studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bibi T, Perveen S, Aziz I, Bashir Q, Rashid N, Imanaka T and Akhtar M (2016) Pcal_1127, a highly stable and efficient ribose‐5‐phosphate pyrophosphokinase from Pyrobaculum calidifontis . Extremophiles 20, 821–830. [DOI] [PubMed] [Google Scholar]
- 13. Perveen S, Rashid N and Papageorgiou AC (2016) Crystal structure of a phosphoribosyl anthranilate isomerase from the hyperthermophilic archaeon Thermococcus kodakaraensis . Acta Crystallogr F Struct Biol Commun 72, 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sassetti CM, Boyd DH and Rubin EJ (2003) Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol 48, 77–84. [DOI] [PubMed] [Google Scholar]
- 15. Lee CE, Goodfellow C, Javid‐Majd F, Baker EN and Shaun Lott J (2006) The crystal structure of TrpD, a metabolic enzyme essential for lung colonization by Mycobacterium tuberculosis, in complex with its substrate phosphoribosylpyrophosphate. J Mol Biol 355, 784–797. [DOI] [PubMed] [Google Scholar]
- 16. Rashid N, Morikawa M and Imanaka T (1997) Gene cloning and characterization of recombinant ribose phosphate pyrophosphokinase from a hyperthermophilic archaeon. J Ferment Bioeng 83, 412–418. [Google Scholar]
- 17. Andersen RW, Leggio Lo L, Hove‐Jensen B and Kadziola A (2015) Structure of dimeric, recombinant Sulfolobus solfataricus phosphoribosyl diphosphate synthase: a bent dimer defining the adenine specificity of the substrate ATP. Extremophiles 19, 407–415. [DOI] [PubMed] [Google Scholar]
- 18. Faria TQ, Lima JC, Bastos M, Maçanita AL and Santos H (2004) Protein stabilization by osmolytes from hyperthermophiles: effect of mannosylglycerate on the thermal unfolding of recombinant nuclease A from Staphylococcus aureus studied by picosecond time‐resolved fluorescence and calorimetry. J Biol Chem 279, 48680–48691. [DOI] [PubMed] [Google Scholar]
- 19. Hommel U, Lustig A and Kirschner K (1989) Purification and characterization of yeast anthranilate phosphoribosyltransferase. FEBS J 180, 33–40. [DOI] [PubMed] [Google Scholar]
- 20. Ivens A, Mayans O, Szadkowski H, Wilmanns M and Kirschner K (2001) Purification, characterization and crystallization of thermostable anthranilate phosphoribosyltransferase from Sulfolobus solfataricus . FEBS J 268, 2246–2252. [DOI] [PubMed] [Google Scholar]
- 21. Cookson TVM, Castell A, Bulloch EMM, Evans GL, Short FL, Baker EN, Lott JS and Parker EJ (2014) Alternative substrates reveal catalytic cycle and key binding events in the reaction catalysed by anthranilate phosphoribosyltransferase from Mycobacterium tuberculosis . Biochem J 461, 87–98. [DOI] [PubMed] [Google Scholar]
- 22. Matthews BW (1968) Solvent content of protein crystals. J Mol Biol 33, 491–497. [DOI] [PubMed] [Google Scholar]
- 23. Laskowski RA, Chistyakov VV and Thornton JM (2005) PDBsum more: new summaries and analyses of the known 3D structures of proteins and nucleic acids. Nucleic Acids Res 33, D266–D268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 25. Svensson O, Malbet‐Monaco S, Popov A, Nurizzo D and Bowler MW (2015) Fully automatic characterization and data collection from crystals of biological macromolecules. Acta Crystallogr D Biol Crystallogr 71, 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kabsch W (2010) XDS. Acta Crystallogr D Biol Crystallogr 66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evans PR and Murshudov GN (2013) How good are my data and what is the resolution? Acta Crystallogr D Biol Crystallogr 69, 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vagin A and Teplyakov A (1997) MOLREP: an automated program for molecular replacement. J Appl Crystallogr 30, 1022–1025. [Google Scholar]
- 29. Keegan RM and Winn MD (2008) MrBUMP: an automated pipeline for molecular replacement. Acta Crystallogr D Biol Crystallogr 64, 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AGW and McCoy A (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 67, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cowtan K (2006) The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr D Biol Crystallogr 62, 1002–1011. [DOI] [PubMed] [Google Scholar]
- 32. Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F and Vagin AA (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L‐W, Kapral GJ, Grosse‐Kunstleve RW et al (2010) PHENIX: a comprehensive Python‐based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Emsley P and Cowtan K (2004) Coot: model‐building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- 35. Brünger AT (1992) Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature 355, 472–475. [DOI] [PubMed] [Google Scholar]
- 36. Joosten RP, Long F, Murshudov GN and Perrakis A (2014) The PDB_REDO server for macromolecular structure model optimization. IUCrJ 1, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krissinel E and Henrick K (2007) Inference of macromolecular assemblies from crystalline state. J Mol Biol 372, 774–797. [DOI] [PubMed] [Google Scholar]
- 38. Krissinel E and Henrick K (2004) Secondary‐structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr 60, 2256–2268. [DOI] [PubMed] [Google Scholar]
- 39. Gonzalez JE and Somerville RL (1986) The anthranilate aggregate of Escherichia coli: kinetics of inhibition by tryptophan of phosphoribosyltransferase. Biochem Cell Biol 64, 681–691. [DOI] [PubMed] [Google Scholar]
- 40. Henderson EJ, Zalkin H and Hwang LH (1970) The anthranilate synthetase‐anthranilate 5‐phosphoribosylpyrophosphate phosphoribosyltransferase aggregate. Catalytic and regulatory properties of aggregated and unaggregated forms of anthranilate 5‐phosphoribosylpyrophosphate phosphoribosyltransferase. J Biol Chem 245, 1424–1431. [PubMed] [Google Scholar]
- 41. Largen M, Mills SE, Rowe J and Yanofsky C (1978) Purification and properties of a third form of anthranilate‐5‐phosphoribosylpyrophosphate phosphoribosyltransferase from the Enterobacteriaceae. J Biol Chem 253, 409–412. [PubMed] [Google Scholar]
- 42. Largen M, Mills SE, Rowe J and Yanofsky C (1976) Purification, subunit structure and partial amino‐acid sequence of anthranilate‐5‐phosphoribosylpyrophosphate phosphoribosyltransferase from the enteric bacterium Serratia marcescens . Eur J Biochem 67, 31–36. [DOI] [PubMed] [Google Scholar]
- 43. Egan AF and Gibson F (1972) Anthranilate synthase‐anthranilate 5‐phosphoribosyl 1‐pyrophosphate phosphoribosyltransferase from Aerobacter aerogenes . Biochem J 130, 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wegman J and DeMoss JA (1965) The enzymatic conversion of anthranilate to indolylglycerol phosphate in Neurospora crassa . J Biol Chem 240, 3781–3788. [PubMed] [Google Scholar]
- 45. Marcus SL and Balbinder E (1972) Purification of anthranilate 5‐phosphoribosylpyrophosphate phosphoribosyltransferase from Salmonella typhimurium using affinity chromatography: resolution of monomeric and dimeric forms. Biochem Biophys Res Commun 47, 438–444. [DOI] [PubMed] [Google Scholar]
- 46. Bode R and Birnbaum D (1979) Enzymes of the aromatic amino acid biosynthesis in Hansenula henricii: determination and characterization of the pretyrosine pathway enzymes. Z Allg Mikrobiol 19, 83–88. [DOI] [PubMed] [Google Scholar]
- 47. O'Gara JP and Dunican LK (1995) Mutations in the trpD gene of Corynebacterium glutamicum confer 5‐methyltryptophan resistance by encoding a feedback‐resistant anthranilate phosphoribosyltransferase. Appl Environ Microbiol 61, 4477–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weiss MS (2001) Global indicators of X‐ray data quality. J Appl Crystallogr 34, 130–135. [Google Scholar]
- 49. Diederichs K and Karplus PA (2013) Better models by discarding data? Acta Crystallogr D Biol Crystallogr 69, 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC and Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- 51. Robert X and Gouet P (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42, W320–W324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Reaction catalyzed by TrpD.
Fig. S2. Gel filtration elution profile of TkTrpD with and without Zn2+.
Fig. S3. Determination of optimal pH for TkTrpD enzymatic activity.
Data Availability Statement
Structural data are available in the Protein Data Bank under the accession numbers 5NOE and 5NOF.


