Abstract
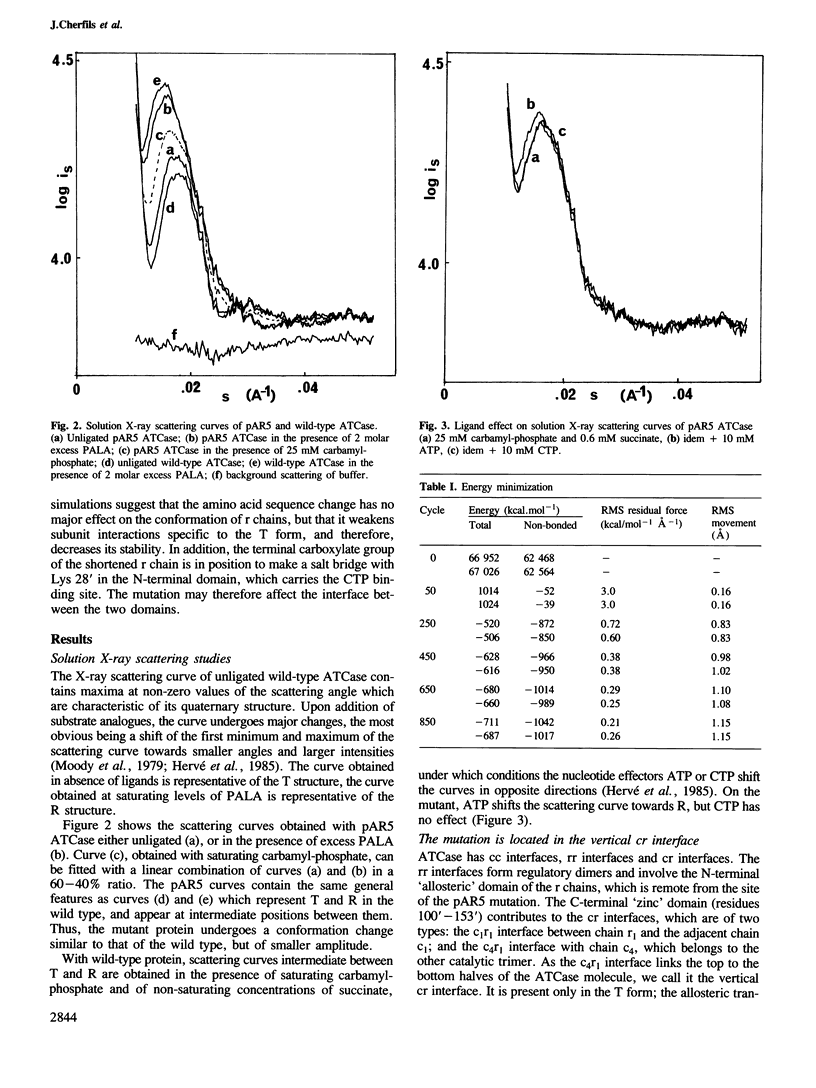
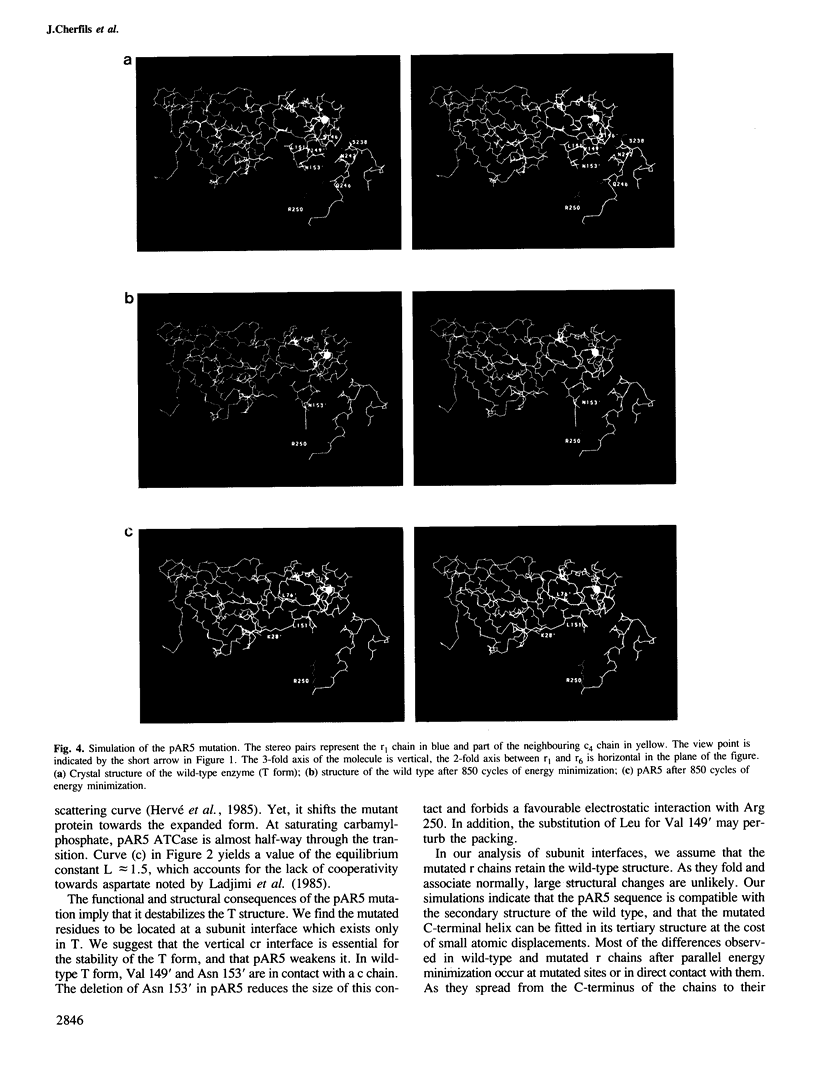
Mutation pAR5 replaces residues 145'-153' at the C terminus of the regulatory (r) chains of Escherichia coli ATCase by a new sequence of six residues. The mutated enzyme has been shown to lack substrate cooperativity and inhibition by CTP. Solution X-ray scattering curves demonstrate that, in the absence of ligands, its structure is intermediate between the T form and the R form. In the presence of N-phosphonacetyl-L-aspartate, the mutant is similar to the wild type. An examination of the crystal structure of unligated ATCase reveals that the mutated site is at an interface between r and catalytic (c) chains, which exists only in the T allosteric form. A computer simulation by energy minimization suggests that the pAR5 mutation destabilizes this interface and induces minor changes in the tertiary structure of r chains. The resulting lower stability of the T form explains the loss of substrate cooperativity. The lack of allosteric inhibition may be related to a new electrostatic interaction made in mutant r chains between the C-terminal carboxylate and a lysine residue of the allosteric domain.
Full text
PDF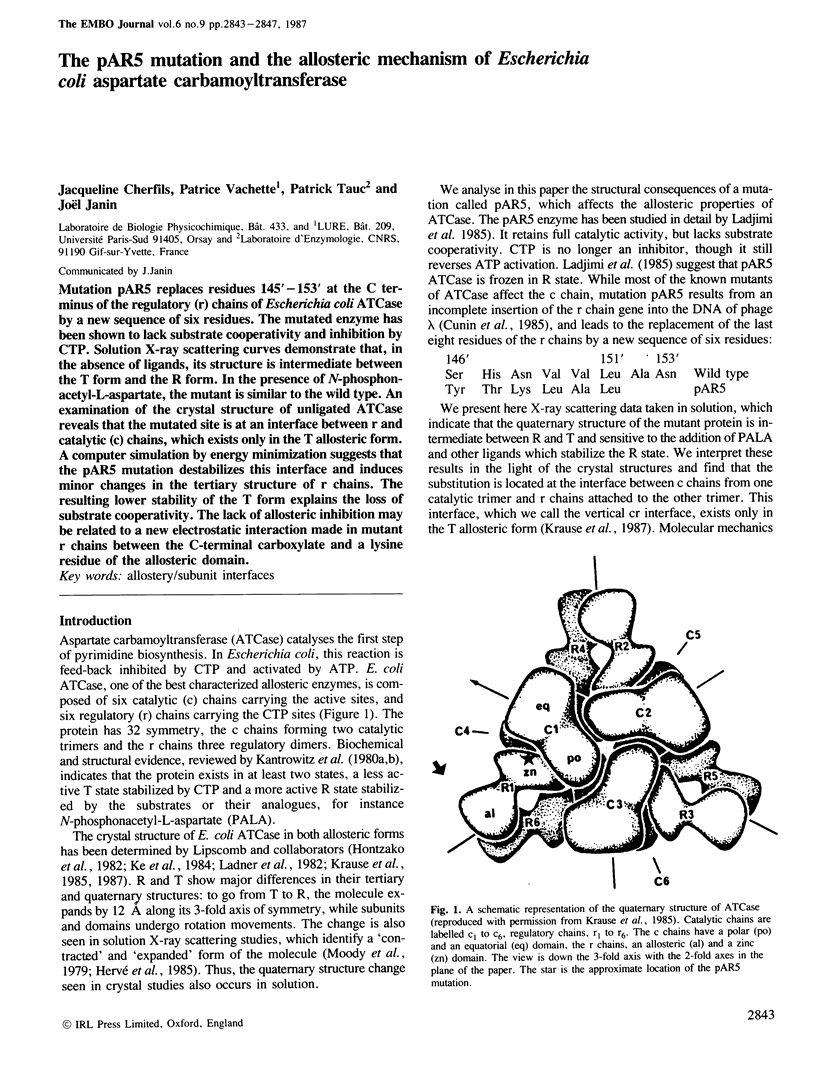


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R. B., Ladner J. E., Lipscomb W. N. Quaternary structural changes in aspartate carbamoyltransferase of Escherichia coli at pH 8.3 and pH 5.8. Biochem Biophys Res Commun. 1982 Sep 30;108(2):592–595. doi: 10.1016/0006-291x(82)90869-5. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bode W., Chen Z., Bartels K., Kutzbach C., Schmidt-Kastner G., Bartunik H. Refined 2 A X-ray crystal structure of porcine pancreatic kallikrein A, a specific trypsin-like serine proteinase. Crystallization, structure determination, crystallographic refinement, structure and its comparison with bovine trypsin. J Mol Biol. 1983 Feb 25;164(2):237–282. doi: 10.1016/0022-2836(83)90077-3. [DOI] [PubMed] [Google Scholar]
- Cunin R., Jacobs A., Charlier D., Crabeel M., Hervé G., Glansdorff N., Piérard A. Structure-function relationship in allosteric aspartate carbamoyltransferase from Escherichia coli. I. Primary structure of a pyrI gene encoding a modified regulatory subunit. J Mol Biol. 1985 Dec 20;186(4):707–713. doi: 10.1016/0022-2836(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Hervé G., Moody M. F., Tauc P., Vachette P., Jones P. T. Quaternary structure changes in aspartate transcarbamylase studied by X-ray solution scattering. Signal transmission following effector binding. J Mol Biol. 1985 Sep 5;185(1):189–199. doi: 10.1016/0022-2836(85)90190-1. [DOI] [PubMed] [Google Scholar]
- Honzatko R. B., Crawford J. L., Monaco H. L., Ladner J. E., Ewards B. F., Evans D. R., Warren S. G., Wiley D. C., Ladner R. C., Lipscomb W. N. Crystal and molecular structures of native and CTP-liganded aspartate carbamoyltransferase from Escherichia coli. J Mol Biol. 1982 Sep 15;160(2):219–263. doi: 10.1016/0022-2836(82)90175-9. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Honzatko R. B., Lipscomb W. N. Structure of unligated aspartate carbamoyltransferase of Escherichia coli at 2.6-A resolution. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4037–4040. doi: 10.1073/pnas.81.13.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. L., Volz K. W., Lipscomb W. N. 2.5 A structure of aspartate carbamoyltransferase complexed with the bisubstrate analog N-(phosphonacetyl)-L-aspartate. J Mol Biol. 1987 Feb 5;193(3):527–553. doi: 10.1016/0022-2836(87)90265-8. [DOI] [PubMed] [Google Scholar]
- Krause K. L., Volz K. W., Lipscomb W. N. Structure at 2.9-A resolution of aspartate carbamoyltransferase complexed with the bisubstrate analogue N-(phosphonacetyl)-L-aspartate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1643–1647. doi: 10.1073/pnas.82.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladjimi M. M., Ghellis C., Feller A., Cunin R., Glansdorff N., Piérard A., Hervé G. Structure-function relationship in allosteric aspartate carbamoyltransferase from Escherichia coli. II. Involvement of the C-terminal region of the regulatory chain in homotropic and heterotropic interactions. J Mol Biol. 1985 Dec 20;186(4):715–724. doi: 10.1016/0022-2836(85)90391-2. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Kitchell J. P., Honzatko R. B., Ke H. M., Volz K. W., Kalb A. J., Ladner R. C., Lipscomb W. N. Gross quaternary changes in aspartate carbamoyltransferase are induced by the binding of N-(phosphonacetyl)-L-aspartate: A 3.5-A resolution study. Proc Natl Acad Sci U S A. 1982 May;79(10):3125–3128. doi: 10.1073/pnas.79.10.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. F., Vachette P., Foote A. M. Changes in the x-ray solution scattering of aspartate transcarbamylase following the allosteric transition. J Mol Biol. 1979 Oct 9;133(4):517–532. doi: 10.1016/0022-2836(79)90405-4. [DOI] [PubMed] [Google Scholar]
- Vincent-Fiquet O., Leflon P., Plaquet R., Biserte G. Caractérisation de l'activité L-thréonine désaminasique du foie de cobaye induite par un régime hyperprotidique. Biochimie. 1984 Jan;66(1):43–48. doi: 10.1016/0300-9084(84)90190-1. [DOI] [PubMed] [Google Scholar]
- Warshel A., Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976 May 15;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]



