Abstract
Oesophageal adenocarcinoma is rapidly increasing in Western countries. This tumour frequently presents late in its course with metastatic disease and has a very poor prognosis. Barrett’s oesophagus is an acquired condition whereby the native squamous mucosa of the lower oesophagus is replaced by columnar epithelium following prolonged gastro-oesophageal reflux and is the recognised precursor lesion for oesophageal adenocarcinoma. There are multiple national and society guidelines regarding screening, surveillance and management of Barrett’s oesophagus, however all are limited regarding a clear evidence base for a well-demonstrated benefit and cost-effectiveness of surveillance, and robust risk stratification for patients to best use resources. Currently the accepted risk factors upon which surveillance intervals and interventions are based are Barrett’s segment length and histological interpretation of the systematic biopsies. Further patient risk factors including other demographic features, smoking, gender, obesity, ethnicity, patient age, biomarkers and endoscopic adjuncts remain under consideration and are discussed in full. Recent evidence has been published to support earlier endoscopic intervention by means of ablation of the metaplastic Barrett’s segment when the earliest signs of dysplasia are detected. Further work should concentrate on establishing better risk stratification and primary and secondary preventative strategies to reduce the risk of adenocarcinoma of the oesophagus.
Keywords: Barrett’s oesophagus, Gastroenterology, Endoscopy, Oesophageal adenocarcinoma, Dysplasia
Core tip: Oesophageal adenocarcinoma is increasing in incidence especially in Western populations. Barrett’s oesophagus is the identifiable pre-malignant condition which allows periodic surveillance and secondary prevention to be undertaken to reduce cancer risk. There has been recent evidence supporting earlier endoscopic intervention for dysplastic changes in Barrett’s oesophagus, but the high burden of surveillance prompts increased efforts to identify individuals at highest cancer risk to concentrate resources on those patients who will derive the greatest benefit.
INTRODUCTION
Barrett’s oesophagus is an acquired oesophageal condition characterized by the presence of metaplastic columnar epithelium in the distal oesophagus which replaces normal stratified squamous mucosa[1].
It is associated with prolonged gastro-oesophageal reflux and a risk of development of adenocarcinoma of the oesophagus[1]. Diagnosis is made by oesophagogastroduodenoscopy and biopsy sampling to allow histological examination of the oesophageal mucosa (Figure 1). There is a histological spectrum of appearances of the Barrett’s epithelium spanning benign changes to adenocarcinoma which is classified using the Modified Vienna Criteria into one of five categories[2] (Table 1).
Figure 1.
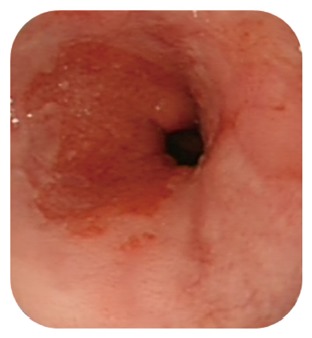
Barrett’s oesophagus on endoscopy.
Table 1.
Modified Vienna Criteria
| Category | Description |
| 1 | No dysplasia |
| 2 | Indefinite for dysplasia |
| 3 | Low-grade intraepithelial neoplasia (low-grade adenoma/dysplasia) |
| 4 | High-grade intraepithelial neoplasia (high-grade adenoma/dysplasia, non-invasive carcinoma, or suspicion of invasive carcinoma) |
| 5 | Invasive epithelial neoplasia (intramucosal carcinoma, submucosal carcinoma, or beyond) |
Following initial diagnosis and confirmation of the histological findings[3-6], the management of Barrett’s oesophagus will include consideration of periodic surveillance of the Barrett’s mucosa, measures to control gastro-oesophageal reflux, chemo-protective strategies, ablation of the metaplastic segment, endoscopic resection and surgical resection of the oesophagus.
AREAS OF DEBATE
Six decades have passed since Normal Barrett described this eponymous condition[7], yet there are still several areas of controversy surrounding definitions, formal diagnostic criteria, the role of screening, the scope of primary and/or secondary prevention and how to undertake surveillance. The pathogenesis of Barrett’s oesophagus is also debateable but will not be addressed fully in this article as the focus is clinical. The most controversial areas surround the fact that overall risk has been established but calculating individualised risk is limited as it is based on crude markers of perceived risk. Also, new evidence for earlier ablation of dysplasia has changed the goals of surveillance; the best risk modification to reduce the risk of dysplasia is not widely practiced; sampling error and pathological interpretation are subject to significant errors; and adjuncts to these methods not being widely taken up.
DEFINITIONS IN BARRETT’S OESOPHAGUS
The definition of Barrett’s oesophagus is controversial. There are several definitions of Barrett’s oesophagus and without comprehensive population-based studies it is difficult to define the true incidence of the disease. Overall, it is fundamentally the presence of metaplastic columnar epithelium in the distal oesophagus.
However, other factors which are not part of the definition are oftentimes included in the requirements for consideration in surveillance including the precise location of the oesophageal landmarks, the required extent of metaplastic mucosa and the presence of intestinal metaplasia.
Different studies use different clinical end points when examining outcomes in Barrett’s oesophagus. Some use cancer incidence or mortality, whereas others use the development of dysplasia (to improve study power or due to therapeutic interventions). Interventional trials may use endpoints such as macroscopic eradication of columnar mucosa, a reduction in Barrett’s extent or absence of intestinal metaplasia or dysplasia on biopsies. There is also considerable variability in biopsy protocol and histological grading of biopsy findings.
SCREENING FOR BARRETT’S OESOPHAGUS
Screening identifies the possible presence of disease in asymptomatic individuals to facilitate earlier intervention and management with the aim of reducing morbidity and mortality. The criteria required for a valid screening programme are listed in Table 2.
Table 2.
The Wilson-Jungner criteria for appraising the validity of a screening programme
| The Wilson-Jungner Screening Criteria | Achieved for Barrett’s oesophagus? |
| The condition being screened for should be an important health problem | + |
| The natural history of the condition should be well understood | +/- |
| There should be a detectable early stage | + |
| Treatment at an early stage should be of more benefit than at a later stage | + |
| A suitable test should be devised for the early stage | + |
| The test should be acceptable | + |
| Intervals for repeating the test should be determined | +/- |
| Adequate health service provision should be made for the extra clinical workload resulting from screening | + |
| The risks, both physical and psychological, should be less than the benefits | + |
| The costs should be balanced against the benefits | - |
WHAT IS THE PREVALENCE OF BARRETT’S OESOPHAGUS?
Several studies have established that the prevalence of Barrett’s oesophagus in the unselected general population is between 1%-2% in European studies (Italian 1.3%, n = 1033 and Swedish 1.6%, n = 1000)[8,9]. It is 5.6% in the United States[10]. The factors associated with Barrett’s oesophagus are gastro-oesophageal reflux disease (GORD) symptoms[11-16], older age[11-13], and the male gender[11,12,17]. Studies have revealed an association with central obesity (waist to hip ratio or abdominal circumference, but less clearly to body-mass index or overall body fat content), tobacco smoking, the Caucasian race and a positive family history. Conversely, alcohol consumption does not appear to be a strong risk factor. Studies have also found potential risk factors including metabolic syndrome, type 2 diabetes mellitus, and sleep apnoea[18-21]. It has been suggested that the difference in prevalence between the United States and Europe is due to a higher prevalence of associated risk factors (GORD, obesity, diet, smoking); and can explain the reason behind the difference in prevalence between the West and Asia or Africa[8,22]. Nevertheless, data from meta-analyses on the difference in cancer incidence between countries across the world do not show a difference in cancer risk[23]. However, there are likely to be differences between individual studies so further individualised risk stratification is needed with a possible inclusion on geographical location.
In examining the risk of development of oesophageal adenocarcinoma in the general population: a large case-control study found that the OR of developing oesophageal adenocarcinoma for patients with GORD symptoms at least once a week was 7.7 (95%CI: 5.3-11.4) compared to individuals without GORD symptoms[24].
In summary, Barrett’s oesophagus is an important health problem as it is an identifiable premalignant leading to oesophageal adenocarcinoma[1]. There is a detectable early stage where an effective intervention would be more beneficial than at a later stage as it would reduce the risk of malignant progression.
NATURAL HISTORY OF BARRETT’S OESOPHAGUS
There is an asymptomatic but detectable early stage which offers a window for treatment. Treatment of cancer/dysplasia is more beneficial the earlier it is given[25]. Subsequently, the natural history is now often interrupted by interventions made when dysplasia is identified[26]. Evidence for the efficacy of various interventions (endoscopic, pharmacological and surgical) on the natural history is currently being studied.
Two specific major United Kingdom trials currently underway are: the Barrett's Oesophagus Surveillance Study (BOSS) which randomises patients to standard surveillance vs endoscopy at time of need and the Aspirin and Esomeprazole Cancer Chemoprevention Trial[27,28] which is discussed in the section on Secondary prevention.
Nevertheless, for now, it is agreed that oesophageal adenocarcinoma develops by a multistep process where a normal stratified squamous cell in the distal oesophagus becomes metaplastic columnar epithelium under the environmental assault of gastric acid, made more likely on a background of genetic and non-modifiable risk factor predisposition[29] and onward to neoplasia (Table 1). The process is dependent on defective genes amongst those which control the cell cycle where genomic instability results in multiple aneuploid populations of cells; which will genetically acquire the ability to invade and metastasize[30-32].
A number of studies have reported resolution of dysplastic changes and whilst regression to a less severe dysplastic stage may be plausible, the absence of dysplasia (which by definition is neoplastic with genetic changes) is more likely to be due to sampling error or variability in histopathological interpretation[30,32-34]. Several papers conclude that the natural history of Barrett’s oesophagus is not known with an unpredictable progression[29]. Moreover, attempting to understand the natural history becomes more difficult on an individual patient basis as it would require consideration of genetic, environmental and behavioural factors[35]. Despite the uncertainty, a study in Northern Ireland found that the annual risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus was 0.38% per year (when intestinal metaplasia is present) compared to 0.07% in patients without intestinal metaplasia[36]. The lifetime risk was 5.8% in males and 3.0% in females. Overall, there are many questions surrounding the pathogenesis which require further research into.
TARGETTED SCREENING
A decision analytical model established that a one-time screening endoscopy for Barrett’s oesophagus was cost-effective[37]. Nonetheless, there is a debate on the target population for screening and most guidelines advocate targetting individuals with certain risk factors rather than the general population to maximise its yield. One of the important risk factors considered includes GORD. There is evidence lacking for the most suitable tests and potential methods for screening include endoscopy but this is generally considered to be too expensive, invasive and cumbersome where a study found that the cost of endoscopic screening in GORD patients was $24718 per life-year saved[38]. A similar figure ($22200) was also arrived at in another study where the population screened incorporated a number of the key risk factors (50-year-old white men with a history of GORD)[39,40]. Another study investigating the possibility of streamlining the number of patients requiring endoscopic surveillance has positive results. They limited the surveillance cohort after an initial endoscopy to patients with 2 cm of columnar metaplasia or more, and limited again after the second endoscopy by excluding patients without intestinal metaplasia. Results showed that when the risk was stratified in this way, the percentage requiring endoscopy was reduced by 33% and the procedure becomes cost-effective[41].
Cytosponge
The Cytosponge is a device encased within a pill attached to a piece of string which, when swallowed, dissolves to reveal an expandable sponge which scrapes off up to 500000 cells when withdrawn up the oesophagus by the string[42]. Whilst being withdrawn, it collects cells along the entire oesophagus rather than just point samples from endoscopy. Studies have already shown that the Cytosponge technique is able to overcome the sampling bias of endoscopy and is able to reflect the entire clonal architecture[43].
The initial study (BEST1) of 500 patients between 50 to 70 year olds found that 99% were able to swallow the device without issues. A larger study (BEST2) was conducted involving 1110 patients with Barrett’s oesophagus (n = 647) or GORD but not investigations for Barrett’s oesophagus (n = 463) where both groups swallowed the Cytosponge (93.9% swallowed successfully) and underwent an endoscopy. Results showed that the Cytosponge was as accurate as endoscopy and was preferred to endoscopy in over 90% of patients[44].
The sensitivity of the device was 79.9% which rose to 87.2% for patients with more than 3 cm of circumferential Barrett’s oesophagus. It rose further again to 89.7% when the Cytosponge was swallowed twice during the study (n = 107). Specificity was unchanged (92.4%). The study demonstrated that the Cytosponge is safe and acceptable and comparable to other screening options[40]; however, it was a case-control study rather than a population-based study which limits the amount generalization that can be made to a primary care population[44]. Therefore, the BEST3 Trial is in place to investigate its use in a primary care setting and evaluate its cost effectiveness. A small study found that 16% of 161 endoscopy referrals were suitable for triage to use of the Cytosponge[45].
Targetted population
Cost effective identification of patients at highest risk will involve symptoms, demographics and other associated factors (as previously discussed). A meta-analysis of five case-control studies (1189 oesophageal adenocarcinoma patients and 4666 controls) revealed that patients with weekly GORD symptoms were five times more likely (OR = 4.9) to develop oesophageal adenocarcinoma than their counterparts with less frequent or no symptoms[46]. The question remains as to whether a single (one off) screening test is appropriate or whether repetition should be undertaken. Studies show that the mean age at the time of diagnosis is approximately 55 years[47] and whilst children may have patches of columnar epithelium in the oesophagus or distal oesophageal columnarised segments, it is rare before five years of age[48]. This epidemiology suggests that Barrett’s oesophagus is an acquired condition and provides insight into informing the age at which screening should start.
The absolute risk for development of adenocarcinoma in individuals with GORD symptoms less than once per week is very low at 0.1 to 15.4 per 100000 for men (aged 30-80 years), and 0 to 2.3 per 100000 for women (aged 30-80 years)[49]. Over 40% of patients with oesophageal adenocarcinoma do not have a history of heartburn, and a study in 2000 found that fewer than 5% of patients with oesophageal adenocarcinoma were known to have had Barrett’s oesophagus before they presented with symptoms[24,50]. Subsequently, a targetted screening (or surveillance) programme will only detect some of the individuals at risk[49].
The lack of utility in screening all GORD patients was echoed in the guidelines from the American Gastroenterological Association (AGA)[6] and the American College of Physicians[45] which state that endoscopy should be offered to patients with risk factors for adenocarcinoma. According to both guidelines, these include chronic GORD, hiatal hernia (Figure 2), age 50 years and over, male gender, Caucasian race, and intra-abdominal body fat distribution. Interestingly, the American College of Gastroenterology (ACG)[3] also supports the use of endoscopy as a screening tool but only if there are GORD symptoms in the presence of alarm symptoms (dysphagia, weight loss, and signs of gastrointestinal bleeding). The British Society of Gastroenterology (BSG) also arrived at similar conclusions where it was decided that endoscopic screening is unfeasible and unjustified in an unselected population with GORD symptoms but should be considered in patients with chronic GORD and multiple risk factors (at least three of age 50 years or older, Caucasian race, male sex and obesity)[4]. The report also mentioned that the threshold should be lowered if there is a family history of Barrett’s oesophagus or oesophageal adenocarcinoma; and that life expectancy of the individual should be considered in view of screening. The National Institute of Health and Care Excellence also has guidelines on a “two-week wait referral for suspected upper gastrointestinal cancer” where endoscopy is urgently arranged for patients with the following symptoms: dysphagia or aged 55 and over with weight loss and upper abdominal pain, reflux or dyspepsia[51].
Figure 2.
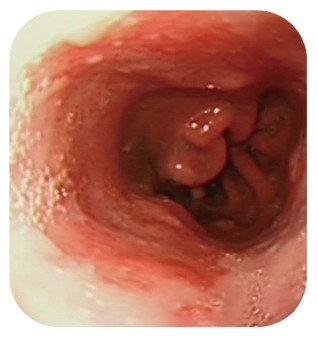
Barrett’s oesophagus extending above a hiatus hernia.
DIAGNOSIS AND CONSIDERATION OF ENTRANCE INTO SURVEILLANCE PROGRAMMES
Controversies around the entrance into a surveillance programmes focus on the relevance of intestinal metaplasia and the minimum length of Barrett’s oesophagus needed. Following endoscopic and histological diagnosis, as per the BSG, AGA, ACG and American Society for Gastrointestinal Endoscopy (ASGE)[3-6], intestinal metaplasia is not compulsory but helpful, and patients with at least 1 cm of columnar metaplasia of the oesophagus may be considered for surveillance.
Relevance of intestinal metaplasia
There have been differences in opinion on the importance of the detection of in short (arbitrarily defined as < 3 cm) segments when considering the management of Barrett’s oesophagus.
Intestinal metaplasia is the transformation of oesophageal or stomach epithelium into that which resembles intestinal epithelium (with goblet cells identified in the sampled columnar epithelium). Its requirement for consideration of surveillance arises from a suspected lower risk of development of oesophageal adenocarcinoma in short segment Barrett’s epithelium without features of intestinal metaplasia. Recommendations on surveillance based on the presence of intestinal metaplasia have not been included in previous BSG guidelines[4] due concern that in the process to confirm intestinal metaplasia, there would be limitations from sampling errors in mucosal biopsy samples alongside some studies suggesting it does not influence cancer risk[52]. Moreover, a studies showed that initially the rate of developing dysplasia or cancer was the same in patients with or without intestinal metaplasia[53,54].
Furthermore, in one study which undertook a survival analysis, over 50% of those without intestinal metaplasia initially had evidence of it within 5 years and there was a cancer risk in patients where intestinal metaplasia had not been detected. The study demonstrated that a low number of biopsy samples (fewer than 8) is not enough to exclude intestinal metaplasia, especially if the segment of Barrett’s oesophagus is short[55]. It has also been demonstrated that DNA content abnormalities are comparable in both metaplastic epithelia without goblet cells and metaplastic epithelia with goblet cells; however, another study has shown that cancer is commonly found with the surrounding presence of goblet cells[56-58]. Subsequently, a large study from Northern Ireland found that the incidence of high grade dysplasia and cancer in patients with intestinal metaplasia is five times higher than those without intestinal metaplasia (0.38% vs 0.07%)[36,56].
The other issue surrounding the use of intestinal metaplasia in the definition is distinguishing between true Barrett’s oesophagus and intestinal metaplasia of the cardia of the stomach. It is notoriously difficult to distinguish between the two on a gastro-oesophageal junction biopsy sample as the different forms of intestinal metaplasia occur at both sites and unless native oesophageal structures are seen by the histopathologist, there is a lack of reliable markers which distinguish between intestinal metaplasia of the oesophagus and cardia requiring accurate endoscopic technique when sampling this dynamic organ[59-62].
It thus follows that although intestinal metaplasia is not a prerequisite for the definition of Barrett’s oesophagus, it could and should be taken in consideration when determining the frequency and necessity of follow-up of patients as there is evidence that it affects cancer risk long term following the results of the large study from Northern Ireland[36].
1-cm threshold and “long segment” Barrett’s oesophagus
Another area of contention is the use of a 1-cm threshold. The use of 1-cm stems from studies which have shown that segments below 1 cm have very high levels of inter-observer variability, and are at very low risk of development of oesophageal adenocarcinoma, and do not show that they are at an increased risk of developing dysplasia[63]. Therefore, they are not considered as Barrett’s oesophagus but as “specialized intestinal metaplasia of the oesophagogastric junction”, an irregular z-line or “ultra-short segment Barrett’s oesophagus”. “Long segment Barrett’s oesophagus” describes metaplastic segments of 3 or more centimetres in length. Increased segment lengths have been associated with higher dysplasia and cancer risk and subsequently the presence of intestinal metaplasia is not considered a pre-requisite for either diagnosis or enrolment into surveillance programmes[64,65].
PRIMARY PREVENTION
Some of the most important risk factors (male gender, older age) for developing Barrett’s oesophagus cannot be modified which limits the scope for primary intervention to preventing GORD by maintaining a healthy weight and not smoking. However, the influence that these risk factors have on the probability of developing Barrett’s needs to be investigated to ascertain whether differential efforts need to go into the respective risk factors as smoking confers a greater risk for Barrett’s oesophagus in non-GORD controls.
SURVEILLANCE
Oesophageal adenocarcinoma is a tumour which tends to spread early before dysphagic symptoms become apparent with lymph node metastasis being a very poor prognostic factor. The goals of surveillance are to detect dysplasia and early cancer before distant disease has developed[66]. Lymphatic invasion may occur very early in oesophageal tumours (when the tumour has reached the submucosa) which is one of the main reasons for the frequent presentation of advanced disease and poor prognosis[67]. For this reason, there is immense benefit in the early detection of cancer or pre-cancer where intervention may be curative. Tumours detected within Barrett’s oesophagus surveillance programmes are in general at an earlier stage than those detected de novo[68].
Endoscopy is the main method of surveillance in Barrett’s oesophagus with biopsy sampling using the Seattle protocol which consists of four-quadrant biopsies taken every 2 cm or every 1 cm in cases of dysplasia[69]. The purpose of surveillance is to detect dysplasia and at present, the frequency of surveillance is generally based on the grade of dysplasia detected. Prior to surveillance, it is imperative that GORD (if present) is medically controlled as active inflammation makes it very difficult to differentiate between dysplasia and reparation. The biopsies taken should then classed using the five-tier system of the Vienna classification[2] (Table 1).
In most guidelines, surveillance is every 2-5 years if there is no dysplasia, every 6-12 mo for low grade dysplasia (unless an endoscopic intervention has been undertaken) and every 3 mo for high grade dysplasia with most patients undergoing endoscopic therapy rather than continued surveillance. Indefinite changes for dysplasia prompt an early repeat endoscopy (typically at 6 mo) with maximal control of reflux in the interim period to help clarify the histological features and allow more accurate interpretation. The evidence base behind the surveillance intervals arises from decision analytical models which found that surveillance every 5 years was on the only viable strategy with the greatest quality adjusted life[69]; informing the maximal interval of 5 years. Another model found that there was little benefit of surveillance of Barrett’s oesophagus patients without dysplasia as there is a low incidence of adenocarcinoma in the group. It demonstrated that if there is no dysplasia, surveillance intervals longer than 5 years are associated with costs outweighing the marginal increases in quality adjusted life years[37].
Observer variability
Surveillance relies on histologic evaluation of dysplasia which unfortunately attracts downsides as there are pathologic limitations and diagnostic variability in assessing the presence and grading of dysplasia[70]. This is worsened by the fact that the changes which occur as Barrett’s oesophagus progresses are subtle and accompanied by a wide range of morphological patterns of atypia thus introducing intra- and inter-observer variability[71,72]. The impact of the variability is most obvious at the lower end of the scale where it becomes challenging to differentiate regeneration from dysplasia but not the extremes where agreement is generally very high[73]. A study into the variability found that amongst eight expert oesophageal histopathologists, there was only 60% agreement in drawing a distinction between no dysplasia detected from indefinite changes for dysplasia and low grade dysplasia[74]. The effect is a predilection to a provisional diagnosis of indefinite changes for dysplasia. Various studies have investigated whether the variability in the diagnosis of dysplasia can be decreased but there are yet to be substantial solutions[75]. There is clearly the need for less subjective markers to determine the risk of malignant progression Barrett’s oesophagus.
BIOPSY PROTOCOL AND ADJUNCTS TO STANDARD SYSTEMATIC BIOPSY
There have been some studies examining the utility of a systematic biopsy protocol in comparison to a random or targetted approach[76]. One study found that four-quadrant biopsy detected dysplasia in Barrett’s oesophagus in 13 times more patients than non-systematic biopsy surveillance. There is also discussion around the benefit of targetted biopsy samples where adjuncts are used alongside endoscopy to better visualise the oesophageal mucosa.
Chromo-endoscopy
Chromo-endoscopy is founded on the current use of acetic acid to stain abnormal tissues during an examination of the cervix to whiten immature (young) and dysplastic cells. When acetic acid is used in the gastrointestinal tract via a spray catheter in the endoscope, both the oesophageal and gastric mucosae turn white (as in the cervix) but once a few minutes have passed, normal mucosa remains white whereas Barrett’s mucosa transiently turns red, as does gastric columnar mucosa[77]. Its use can be improved by the addition of indigo carmine to better visualise early gastric cancer and as a mucolytic to remove mucus obscuring the mucosa[78]. Dysplasia may be found where there are areas of surface irregularity, changes in the vascular pattern or variability of staining.
A retrospective study (n = 982) involving patients with Barrett’s oesophagus under surveillance found that dysplasia was detected in 41/327 (13%) patients where acetic acid was used as an adjunct vs only 13/655 (2%) in the random biopsy group[79]. Moreover, in the initial detection of Barrett’s oesophagus, targetted biopsies using acetic acid more than doubles the yield of detection (57% vs 26%)[80]. Other studies have shown similar results for acetic acid chromo-endoscopy which was found to detect dysplasia and neoplasia better than white light endoscopy[81], with another study showing that it requires 15 times fewer biopsies per neoplasia detected[79]. In another study where 263 procedures were examined with neoplasia in 143, acetic acid chromo-endoscopy correctly identified 96% of these cases vs 55% with white light endoscopy.
Other dyes which have been used include methylene blue, toluidine blue, cresyl violet, crystal violet, Congo red, phenol red and Lugol’s solution. Lugol’s solution contains potassium iodide and iodine, both of which attach avidly to glycogen in non-keratinised squamous epithelium and so studies have found it is extremely effective for detecting squamous lesions (sensitivity 9% vs specificity 40%-95%)[82] and it can also be used in post-ablation Barrett’s oesophagus patients to distinguish between regenerative squamous epithelium and areas of residual Barrett’s mucosa (which do not take up the dye). Despite its benefits, safety studies have shown that its use may cause retrosternal pain which is attenuated by sodium thiosulfate[83]. Methylene blue is only taken up by tissue which is actively absorbing (small intestinal and colonic epithelium) and so can be used to find Barrett’s mucosa (metaplastic absorptive mucosa). Although indigo carmine is predominately used in investigating the colon by visualizing pit patterns to distinguish between different types of polyps, it can also be used to identify Barrett’s oesophagus when used in conjunction with high-magnification endoscopy and Lugol’s solution[84]. Both cresyl violet and crystal violet stain cell nuclei thus aiding in identifying Barrett’s metaplastic mucosa[84,85]. Less commonly used adjuncts to endoscopy are Congo red and phenol red which are both pH indicators used to detect areas of ectopic acid secretion.
Although chromo-endoscopy offers benefits to aid in the screening or surveillance of Barrett’s oesophagus, it does have a number of shortcomings that limit its use. Unfortunately, the procedure is very subjective and subject to inter-observer variability and a study found that even when blinding techniques are employed, there was no increase in the numbers of cases of Barrett’s oesophagus detected and no widely accepted standardisation of their application[86-90].
Narrow band imaging
Narrow band imaging (NBI) is an alternative technique where lights of specific blue (wavelength = 440-460 nm) and green (wavelength = 540-560 nm) wavelengths are used to enhance the detail of the mucosa and blood vessels. This works because the wavelengths correlate with the peak light absorption of haemoglobin hence will appear very dark thus improving their visibility and easing the identification of neighbouring structures.
The other methods which can be employed for surveillance of Barrett’s oesophagus include endosonography [endoscopic ultrasound (EUS)], optical coherence tomography (OCT), confocal microendoscopy, auto-fluorescence endoscopy and computed virtual chromo-endoscopy (CVC).
Endoscopic ultrasound
Studies have shown that EUS to screen patients with Barrett’s oesophagus is neither justified nor cost-effective but does play a role when there is high grade dysplasia or intramucosal carcinoma[91]. Conversely, in terms of superiority, OCT is above EUS as its resolution is better as once can see the layers of the oesophageal wall can be visualised with good correlation to histologic structures thus allowing endoscopists to detect high grade dysplasia earlier. The sensitivity of detecting dysplasia was 68% and specificity was 28%[92].
Computed virtual chromo-endoscopy
CVC enhances mucosal surface contrasts and vascular pattern variability without the use of dye as is standard in chromo-endoscopy. Its utility was demonstrated in a randomised control trial where 57 patients with Barrett’s oesophagus and a history of high grade intraepithelial neoplasia/early cancer were allocated to undergo acetic acid chromo-endoscopy or CVC with re-examination after 4-6 wk with the other procedure. The positive predictive value for the former was 39% and 37% for the latter with comparable sensitivities at 83% and 92% respectively[92]. The study thus shows that CVC is not only useful as an adjunct but provides comparable results to conventional chromo-endoscopy in the detection of high grade dysplasia/early cancer.
Auto-fluorescence
Auto-fluorescence endoscopy is a technique incorporating a real time wide angle view allowing the endoscopist to rapidly go from standard white lighting to auto-fluorescence and to very quickly examine large areas of gastrointestinal mucosa. However, there was no clear superiority over conventional white-light imaging (whether or not it is used in conjunction with NBI)[93]. On the other hand, confocal microendoscopy is where the resolution and contrast of imaging is augmented by eliminating out of focus light by the addition of a spatial pinhole at the confocal plane of the lens. A study determined that this method is very accurate and reliable (sensitivity 88% and specificity 96%) for the diagnosis of neoplasia[94].
ENDOSCOPIC SURVEILLANCE INTERVALS
Non-dysplastic (no dysplasia detected)
The BSG, ASGE and AGA are all in agreement on the management which follows for each biopsy category[3-6]. If biopsies show non-dysplastic Barrett’s oesophagus (no dysplasia detected), surveillance (every 2-5 years) is offered following a discussion about its benefits and risks. The Barrett’s segment length is incorporated into guidelines too. Australian and British guidelines state that endoscopy should be repeated 3-5 years if the maximal length is less than 3 cm, and every 2-3 years if above or equal to 3 cm[4,95]. The AGA and ACG does not delineate surveillance for no dysplasia detected by segment length (3-5 years for all lengths); however, the latter differentiates the number if biopsies by segment length (4 biopsies for every 2 cm of segment length, or at least 8 biopsies if the segment is less than 2 cm at the initial exam) which should reduce sampling error for detection of intestinal metaplasia[53].
Indefinite changes for dysplasia, Low grade dysplasia, High grade dysplasia and Adenocarcinoma
If biopsies are indefinite for dysplasia, American and British guidelines emphasise maximal acid suppression with a PPI to reduce the misleading effects of reflux oesophagitis on the oesophageal mucosa. After adequate acid suppression (BSG and Australian guidelines: 6 mo; ACG: 3-6 mo), further biopsies should be taken using the Seattle biopsy protocol and if they are still indefinite, the diagnosis should be confirmed by an expert oesophageal histopathologist. If the diagnosis is clarified with classification to another group on the second biopsy, the appropriate pathway (no dysplasia detected, low grade dysplasia, high grade dysplasia, adenocarcinoma) should be taken.
If low grade dysplasia or high grade dysplasia/intramucosal carcinoma is seen, the findings must be confirmed with an expert oesophageal histopathologist and the Seattle protocol used to obtain further systematic biopsies (due to the risk of sampling error and confirm the degree of dysplasia) with endoscopic resection of any mucosal irregularities. Guidelines from the United States and the United Kingdom recommend that low grade dysplasia patients are given the option of either surveillance every six months or endoscopic eradication. For high grade dysplasia/intramucosal carcinoma patients, guidelines recommend an intervention to the dysplastic mucosa at this time due to the risk of an occult carcinoma or disease progression[64,96]. Although endoscopic eradication is recommended for high grade dysplasia, endoscopic surveillance is advocated in some units. The evidence comes from a study over a period of 20 years where of 75 patients with high grade dysplasia underwent surveillance over an average of 7.3 years, and 12 developed adenocarcinoma which was curable by ablation in all but 1 who was lost to follow-up[97]. In another study of 45 patients with diagnosed cancer from high grade dysplasia, 13 were detected at the initial endoscopy whereas 32 were found during surveillance and of the 32, only one patient had metastatic disease when first seen on surveillance[98].
Patients who are found to have frank oesophageal adenocarcinoma need to undergo staging investigations with a frank discussion on possible treatment options. The radical options include chemo-radiotherapy, radiotherapy, an oesophagectomy or endoscopic resection/ablation of disease confined to the mucosa. Quality of life is slow to return after an oesophagectomy and not regained in patients surviving less than 2 years[99].
Unfortunately, abiding by comprehensive systematic biopsy protocols is very challenging because there is a substantial time and resource implication to taking multiple biopsies including time to process and interpret results. Moreover, there is no widely utilised system for targetted biopsies.
SURVEILLANCE-DETECTED CANCERS
Overall, studies have already demonstrated that survival rates are markedly better in patients with Barrett’s oesophagus where endoscopic surveillance has detected oesophageal adenocarcinoma compared to patients not undergoing surveillance[100-105]. Despite the potential for the findings from these studies to be explained away with lead time and length time biases, the findings were maintained even after correcting for these biases. Nevertheless, there are costs associated with surveillance including the small morbidity associated with surveillance and biopsy, the resource use and associated anxiety. There are also the limitations associated with surveillance programmes in their goals of detecting dysplasia and early cancer. There is an ongoing randomised control trial (BOSS Trial) comparing survival rates in 3400 patients with Barrett’s oesophagus in a standardised 2-year endoscopic surveillance group vs an “at need endoscopy” group[27]. Results from the study will contribute towards the settling the debate on the need and benefit of surveillance to cancer incidence or survival.
SECONDARY PREVENTION
Unfortunately, the incidence of Barrett’s oesophagus and oesophageal adenocarcinoma are on the rise[46].
Medical control of reflux
Acid suppression with PPIs is a fundamental part of the management of patients with Barrett’s oesophagus, and PPIs have been shown to be superior to histamine receptor antagonists[106]. It is known that PPI use relieves symptoms associated with GORD but its effect on the risk of progression to cancer is not known. It has been postulated that if PPI treatment could reduce the stage of dysplasia or the length of Barrett’s mucosa, it would contribute to a reduction in the cancer risk[107,108]. At present, studies show that PPI use promotes squamous re-epithelialization next to and on top of Barrett’s mucosa but does not cause regression hence surveillance would still be necessary[109-111].
Chemoprevention
There are data that suggest that non-steroidal anti-inflammatory drugs (NSAIDs, particularly aspirin and COX inhibitors) and statins reduce the risk of malignant progression which was seen in a study of 570 Barrett’s oesophagus patients who were investigated across 4.5 years[112]. The study demonstrated that the use of both pharmacological agents together had an additive protective effect. These findings and suggestions have been replicated in several other studies supporting the potential implementation of chemoprevention into guidelines[113-118] including one which found that aspirin chemoprevention was more effective and less expensive than endoscopic surveillance alone[119]. However, there are data which suggest the opposite or discuss it in general[120-122]. Table 3 summarises all the studies[113,115-117,121,123]. Nevertheless, at present, the BSG, AGA and ACG guidelines do not recommend chemoprevention.
Table 3.
Studies investigating chemoprevention in Barrett’s oesophagus
| Ref. | Type | Sample size | Chemo-prevention | Effect on risk | Overall |
| Nguyen et al[113], 2010 | Cohort | 812 | NSAID and aspirin | Filled NSAID/aspirin prescriptions were associated with a reduced risk of oesophageal adenocarcinoma (adjusted incidence density ratio, 0.64; 95%CI: 0.42-0.97) | Reduces risk |
| Filled statin prescriptions were associated with a reduction in EAC risk (0.55; 95%CI: 0.36-0.86) | |||||
| Corley et al[115], 2003 | Meta-analysis of 9 studies | 1813 | NSAID and aspirin | Protective association between any use of aspirin/NSAID and oesophageal adenocarcinoma (OR = 0.57; 95%CI: 0.47-0.71) | Reduces risk |
| Intermittent (OR = 0.82; CI: 0.67-0.99) and frequent medication use were protective (OR = 0.54; 95%CI: 0.43-0.67) | |||||
| Any use was protective against both oesophageal adenocarcinoma (OR = 0.67; 95%CI: 0.51-0.87) and squamous cell carcinoma (OR = 0.58; 95%CI: 0.43-0.78) | |||||
| Alexandre et al[116], 2012 | Meta-analysis of 2 studies | 1382 | Statin | Pooled effect size of 0.53 (95%CI: 0.36-0.78, P = 0.001, I2 = 0%) for risk of oesophageal adenocarcinoma with prior statin use | Reduces risk |
| Alexandre et al[116], 2012 | Meta-analysis of 3 studies | 35214 | Statin | Pooled effect size of 0.86 (95%CI: 0.78-0.94, P = 0.001, I2 = 0%) for risk of oesophageal adenocarcinoma wth prior statin use | Reduces risk |
| Beales et al[117], 2012 | Case-control | 85 | Statin | Regular statin use was associated with a significantly lower incidence of oesophageal adenocarcinoma (OR = 0.45, 95%CI: 0.24-0.84) | Reduces risk |
| After NSAID/aspirin confounding correction: OR = 0.57, 95%CI: 0.28-0.94 | |||||
| Heath et al[121], 2007 | Randomised control trial | 100 | NSAID (celecoxib) | No difference in the proportion of biopsy samples with dysplasia or cancer between treatment groups in either the low-grade (median change with celecoxib = -0.09); or high-grade (median change with celecoxib = 0.12) stratum | No effect |
| Singh et al[123], 2013 | Meta-analysis of 13 studies | 9285 | Statin | A 28% reduction in the risk of oesophageal adenocarcinoma among patients who took statins (adjusted OR = 0.72; 95%CI: 0.60-0.86) | Reduces risk |
NSAID: Non-steroidal anti-inflammatory drug.
Anti-reflux surgery
Anti-reflux surgery (fundoplication) has been shown to offer some benefits to patients with Barrett’s oesophagus which is mostly symptomatic relief[124-127]. However, at present, there is conflicting evidence with some studies (including meta-analyses) showing that anti-reflux surgery does not reduce the risk or incidence of adenocarcinoma but others do show a lower cancer risk[128-130].
TREATMENT OF DYSPLASIA
In the past, an oesophagectomy was the preferred option for the management of dysplasia in Barrett’s oesophagus but nowadays, it can be managed using endoscopic techniques such as ablation or resection. Ablative therapy uses energy to destroy the Barrett’s mucosa (without damaging the deeper oesophageal wall) but does not provide a tissue sample.
The most commonly used endoscopic ablative therapy is radiofrequency ablation (RFA) and studies demonstrate that patients with low- and high-grade dysplasia treated with RFA were less likely to undergo malignant progression of their disease than controls[129]. A meta-analysis looking into the efficacy of radiofrequency ablation found that 91% of patients across 20 studies had complete eradication of dysplastic Barrett’s mucosa[130]. However, recurrence is an issue as a study of 246 patients with high grade dysplasia or intramucosal carcinoma found that despite initial eradication in 80% of cases, neoplastic recurrence was at 25% by 5 years and metaplastic recurrence was 50% by 4 years[131]. Until recently the role of endoscopic ablation of low grade dysplasia was controversial, but this has changed with the recently published outcomes from the SURF study (Surveillance vs Radiofrequency Ablation)[132].
This randomized control trial which compared surveillance with radio-frequency ablation for low grade dysplasia. The trial was undertaken at 9 Barrett treatment centres in Europe where eligible patients had confirmed low grade dysplasia Barrett’s oesophagus (seen on endoscopy within the previous 18 mo). Patients were excluded if they had previous endoscopic treatment for Barrett’s oesophagus, a history of high grade dysplasia or adenocarcinoma, active secondary malignancy, an estimated life expectancy of less than 2 years, and who were under 18 years or over 85 years. Randomization was in 1:1 ratio into either the ablation group or the endoscopic surveillance (control) group.
The trial found that the ablation resulted in a reduced risk of neoplastic progression (high grade dysplasia or adenocarcinoma) over 3 years of follow-up [high grade dysplasia: 1.5% ablation group (n = 1) vs 26.5% control group (n = 18), P < 0.001; and adenocarcinoma: 1.5% ablation group (n = 1) vs 8.8% control group (n = 6), P = 0.03][132]. The number needed to treat to prevent one case of high grade dysplasia was 4.0 and adenocarcinoma was 13.6. Moreover, the dysplasia and intestinal metaplasia were completely eradicated and remained so in the majority of patients in the ablation group. The data effectively suggests that ablative treatment is superior to endoscopic surveillance in patients with Barrett’s oesophagus and low grade dysplasia. Nevertheless, no patient in the control group had unresectable cancer or cancer-related death.
Endoscopic resection of specific lesions has been successfully reported (and the resected tissue can be examined by the pathologist). Resection of the entire or circumferential Barrett’s mucosa is not recommended due to the risk of stricture formation. It has been reported that complete eradication of high grade dysplasia/early cancer or Barrett’s mucosa was achieved in 95% and 89% of patients respectively and the remaining Barrett’s mucosa may be treated with ablative therapy[133].
Nevertheless, there are issues around current ablative therapies which include not having an examinable sample; having to wait for the epithelium to regenerate before repeat sampling can take place and the risk of buried dysplastic or neoplastic cells and glands which have the potential to progress undetected. Moreover, there are risks associated with the procedure itself (pain, bleeding, perforation and stricturing), difficulty in interpreting the sampled findings, and undemonstrated long-term outcomes[134].
One of the more novel approaches to ablation involves the use of cryotherapy where tissue is rapidly cooled by liquid nitrogen spray or carbon dioxide gas. Studies demonstrate success rates which are comparable to aforementioned ablative techniques in the treatment of Barrett’s oesophagus with high-grade dysplasia (complete eradication of dysplasia in 87%-96% of treated patients and complete eradication of intestinal metaplasia in 57%-96% of treated patients). This success has also been replicated in early-stage oesophageal adenocarcinoma where mucosal cancer was completely eradicated in 75% of patients which included patients that were unsuccessful with other therapies. Cryotherapy is generally tolerated well by patients according to studies but these studies tend to have small sample sizes and short periods of follow-up so the need for more robust studies remains[135].
FUTURE DEVELOPMENTS
As mentioned earlier, many institutions are not able to undertake full Seattle biopsy protocol systematic biopsies. There are adjuncts but their use is limited because they are only used in specialist institutions in the context of research projects and there is a lack of recommendation in the guidelines.
Biomarkers
The endoscopic detection of Barrett’s oesophagus and grading of dysplasia are not as reliable as they could be. The need for reliable biomarkers is critical in being able to distinguish Barrett’s oesophagus patients who are at risk of developing oesophageal adenocarcinoma[136,137]. The number of publications discussing a potential biomarker for Barrett’s oesophagus have increase exponentially over the last 30 years from 1 in 1981 to 1069 in total in 2011 which reflects the fact that Barrett’s oesophagus needs a clinically validated prognostic tool such as an effective biomarker to aid in defining risk.
The Early Detection Research Network has recommended five phases of study before a biomarker can be used clinically[138]. Phase 1 is exploratory to identify markers, phase 2 is for the development of a clinical assay, phase 3 is for retrospective validation, phase 4 is for prospective validation and phase 5 is to test the biomarker on the population with the disease. At present, most biomarkers are in phase 3 and 4. Preclinical studies have been successful in detecting certain biomarkers which contribute to the malignant progression of Barrett’s oesophagus but their widespread clinical use is very limited by differences in reproducibility, low sample sizes and the need for multi-centre prospective studies[139-141]. Table 4 is a summary of the biomarkers studied to date[137,142].
Table 4.
Summary of molecular biomarkers predicting malignant progression
| Biomarker | Phase | Sample size | End-point |
| Biomarker panels | |||
| 8-gene methylation panel | 3 | 195 | High grade dysplasia/adenocarcinoma |
| DNA content abnormalities and loss of heterozygosity | 4 | 243 | Adenocarcinoma |
| Expert low grade dysplasia, aneuploidy, Aspergillus oryzae lectin | 3 | 380 | Adenocarcinoma |
| DNA content abnormalities | |||
| Aneupolidy/tetraploidy | 4 | 322 | Adenocarcinoma |
| Tumour suppressor loci | |||
| p53 loss of heterozygosity | 4 | 256 | Adenocarcinoma |
| p53 staining | 4 | 48 | High grade dysplasia/adenocarcinoma |
| Epigenetics | |||
| P16 methylation | 3 | 53 | HD/adenocarcinoma |
| Proliferation | |||
| Mcm2 | 3 | 27 | Adenocarcinoma |
| Clonal diversity | |||
| Clonal diversity measures | 4 | 239 | Adenocarcinoma |
| Cell cycle markers | |||
| Cyclin A | 3 | 48 | High grade dysplasia/adenocarcinoma |
| Cyclin D1 | 3 | 307 | Adenocarcinoma |
| Serum biomarkers | |||
| Leukocyte telomere length | 4 | 300 | Adenocarcinoma |
| Selenoprotein P | 4 | 361 | Adenocarcinoma |
Overall, the desire to predict which Barrett’s oesophagus patients will progress to oesophageal adenocarcinoma is palpable but remains a target and not a reality. The ideal biomarker as with all potential screening options should be cost-effective, minimally invasive, easily administered and have comparable or superior outcomes to what biopsies currently offer. More work is necessary to ensure that successful biomarkers are smoothly translated into widespread clinical practice.
Metabolomics
Metabolomics is the scientific study of the set of metabolites present within an organism, cell, or tissue and they could play a role in the discovery for a biomarker in Barrett’s oesophagus as they are key players in biological systems which are disrupted in disease[143]. A study using urinary metabolomics found that it was possible to separately distinguish Barrett’s oesophagus and oesophageal adenocarcinoma from controls as they had different urinary signatures[143]. This suggests that urinary metabolomics and other may have a future role in the pursuit of a non-invasive screening option for Barrett’s oesophagus.
Virtual biopsies
Studies have worked on trying to differentiate squamous and columnar epithelia based on their electrical characteristics using electrical impedance via a probe[144]. The aim is to reduce discrepancy from inter- and intra-observer variability by having an objective measurement to categorise the epithelium. Magnification endoscopy provides an even more detailed image by optically enlarging the mucosal surface area and studies found that low and high grade dysplasia were consistently identified in Barrett’s using this technique but missed using standard endoscopy alone[145]. Confocal laser endomicroscopy (CLE) is a novel technique combining standard white light endoscopy with confocal laser microendoscopy[146]. CLE has demonstrated a high diagnostic value for digestive diseases including Barrett’s oesophagus[147-151].
CONCLUSION
Progress has been made in further understanding Barrett’s oesophagus since it was first described in 1950. It is a large and increasing health problem with multiple modifiable risk factors, yet there remain several unanswered questions regarding a formal definition, diagnostic criteria, and screening and surveillance needs and methods. Although endoscopy with systematic biopsy and standard pathological examination is currently the mainstay of screening and surveillance for Barrett’s oesophagus, there is still the need for a more cost-effective, less invasive, less cumbersome and more reliable way to conduct diagnosis, screening and surveillance. Primary prevention of Barrett’s oesophagus and adenocarcinoma is also of huge interest and potential with studies focussing on the medical treatment of reflux, chemoprevention and anti-reflux surgery.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Conflict-of-interest statement: Amadi C and Gatenby P declare no conflicts of interest related to this publication.
Peer-review started: February 8, 2017
First decision: March 3, 2017
Article in press: July 4, 2017
P- Reviewer: Boger PC, Chen X, Herszenyi L S- Editor: Gong ZM L- Editor: A E- Editor: Li D
References
- 1.Nelsen EM, Hawes RH, Iyer PG. Diagnosis and management of Barrett’s esophagus. Surg Clin North Am. 2012;92:1135–1154. doi: 10.1016/j.suc.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111:30–50; quiz 51. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 5.ASGE Standards of Practice Committee, Evans JA, Early DS, Fukami N, Ben-Menachem T, Chandrasekhara V, Chathadi KV, Decker GA, Fanelli RD, Fisher DA, Foley KQ, Hwang JH, Jain R, Jue TL, Khan KM, Lightdale J, Malpas PM, Maple JT, Pasha SF, Saltzman JR, Sharaf RN, Shergill A, Dominitz JA, Cash BD; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in Barrett’s esophagus and other premalignant conditions of the esophagus. Gastrointest Endosc. 2012;76:1087–1094. doi: 10.1016/j.gie.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 6.American Gastroenterological Association, Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Barrett NR. Chronic peptic ulcer of the oesophagus and ‘oesophagitis’. Br J Surg. 1950;38:175–182. doi: 10.1002/bjs.18003815005. [DOI] [PubMed] [Google Scholar]
- 8.Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Vieth M, Stolte M, Talley NJ, Agréus L. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 9.Zagari RM, Fuccio L, Wallander MA, Johansson S, Fiocca R, Casanova S, Farahmand BY, Winchester CC, Roda E, Bazzoli F. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354–1359. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 10.Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23:451–457. doi: 10.1111/j.1442-2050.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelstein ZR, Bronner MP, Rosen SN, Vaughan TL. Risk factors for Barrett’s esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. Am J Gastroenterol. 2009;104:834–842. doi: 10.1038/ajg.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerson LB, Edson R, Lavori PW, Triadafilopoulos G. Use of a simple symptom questionnaire to predict Barrett’s esophagus in patients with symptoms of gastroesophageal reflux. Am J Gastroenterol. 2001;96:2005–2012. doi: 10.1111/j.1572-0241.2001.03933.x. [DOI] [PubMed] [Google Scholar]
- 13.Eloubeidi MA, Provenzale D. Clinical and demographic predictors of Barrett’s esophagus among patients with gastroesophageal reflux disease: a multivariable analysis in veterans. J Clin Gastroenterol. 2001;33:306–309. doi: 10.1097/00004836-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Avidan B, Sonnenberg A, Schnell TG, Sontag SJ. Hiatal hernia and acid reflux frequency predict presence and length of Barrett’s esophagus. Dig Dis Sci. 2002;47:256–264. doi: 10.1023/a:1013797417170. [DOI] [PubMed] [Google Scholar]
- 15.Smith KJ, O’Brien SM, Smithers BM, Gotley DC, Webb PM, Green AC, Whiteman DC. Interactions among smoking, obesity, and symptoms of acid reflux in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:2481–2486. doi: 10.1158/1055-9965.EPI-05-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor JB, Rubenstein JH. Meta-analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett’s esophagus. Am J Gastroenterol. 2010;105:1729, 1730–1737; quiz 1738. doi: 10.1038/ajg.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett’s esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol. 2005;162:1050–1061. doi: 10.1093/aje/kwi325. [DOI] [PubMed] [Google Scholar]
- 18.Leggett CL, Nelsen EM, Tian J, Schleck CB, Zinsmeister AR, Dunagan KT, Locke GR, Wang KK, Talley NJ, Iyer PG. Metabolic syndrome as a risk factor for Barrett esophagus: a population-based case-control study. Mayo Clin Proc. 2013;88:157–165. doi: 10.1016/j.mayocp.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer PG, Borah BJ, Heien HC, Das A, Cooper GS, Chak A. Association of Barrett’s esophagus with type II Diabetes Mellitus: results from a large population-based case-control study. Clin Gastroenterol Hepatol. 2013;11:1108–1114.e5. doi: 10.1016/j.cgh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leggett CL, Gorospe EC, Calvin AD, Harmsen WS, Zinsmeister AR, Caples S, Somers VK, Dunagan K, Lutzke L, Wang KK, et al. Obstructive sleep apnea is a risk factor for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:583–588.e1. doi: 10.1016/j.cgh.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drahos J, Ricker W, Parsons R, Pfeiffer RM, Warren JL, Cook MB. Metabolic syndrome increases risk of Barrett esophagus in the absence of gastroesophageal reflux: an analysis of SEER-Medicare Data. J Clin Gastroenterol. 2015;49:282–288. doi: 10.1097/MCG.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fock KM, Ang TL. Global epidemiology of Barrett’s esophagus. Expert Rev Gastroenterol Hepatol. 2011;5:123–130. doi: 10.1586/egh.10.82. [DOI] [PubMed] [Google Scholar]
- 23.Thomas T, Abrams KR, De Caestecker JS, Robinson RJ. Meta analysis: Cancer risk in Barrett’s oesophagus. Aliment Pharmacol Ther. 2007;26:1465–1477. doi: 10.1111/j.1365-2036.2007.03528.x. [DOI] [PubMed] [Google Scholar]
- 24.Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 25.Fléjou JF. Barrett’s oesophagus: from metaplasia to dysplasia and cancer. Gut. 2005;54 Suppl 1:i6–12. doi: 10.1136/gut.2004.041525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milind R, Attwood SE. Natural history of Barrett’s esophagus. World J Gastroenterol. 2012;18:3483–3491. doi: 10.3748/wjg.v18.i27.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Old O, Moayyedi P, Love S, Roberts C, Hapeshi J, Foy C, Stokes C, Briggs A, Jankowski J, Barr H; BOSS Trial Team. Barrett’s Oesophagus Surveillance versus endoscopy at need Study (BOSS): protocol and analysis plan for a multicentre randomized controlled trial. J Med Screen. 2015;22:158–164. doi: 10.1177/0969141315575052. [DOI] [PubMed] [Google Scholar]
- 28.Das D, Chilton AP, Jankowski JA. Chemoprevention of oesophageal cancer and the AspECT trial. Recent Results Cancer Res. 2009;181:161–169. doi: 10.1007/978-3-540-69297-3_15. [DOI] [PubMed] [Google Scholar]
- 29.Geboes K, Paepe PD. Is the natural history of high-grade dysplasia known? OESO 2015 [Google Scholar]
- 30.Krishnadath KK, Tilanus HW, van Blankenstein M, Hop WC, Teijgeman R, Mulder AH, Bosman FT, van Dekken H. Accumulation of genetic abnormalities during neoplastic progression in Barrett’s esophagus. Cancer Res. 1995;55:1971–1976. [PubMed] [Google Scholar]
- 31.Reid BJ, Blount PL, Rubin CE, Levine DS, Haggitt RC, Rabinovitch PS. Flow-cytometric and histological progression to malignancy in Barrett’s esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology. 1992;102:1212–1219. [PubMed] [Google Scholar]
- 32.Neshat K, Sanchez CA, Galipeau PC, Cowan DS, Ramel S, Levine DS, Reid BJ. Barrett’s esophagus: a model of human neoplastic progression. Cold Spring Harb Symp Quant Biol. 1994;59:577–583. doi: 10.1101/sqb.1994.059.01.065. [DOI] [PubMed] [Google Scholar]
- 33.Peters FT, Kleibeuker JH. Barrett’s oesophagus and carcinoma. Recent insights into its development and possible prevention. Scand J Gastroenterol Suppl. 1993;200:59–64. [PubMed] [Google Scholar]
- 34.Phillips RW, Wong RK. Barrett’s esophagus. Natural history, incidence, etiology, and complications. Gastroenterol Clin North Am. 1991;20:791–816. [PubMed] [Google Scholar]
- 35.Appelman HD, Umar A, Orlando RC, Sontag SJ, Nandurkar S, El-Zimaity H, Lanas A, Parise P, Lambert R, Shields HM. Barrett’s esophagus: natural history. Ann N Y Acad Sci. 2011;1232:292–308. doi: 10.1111/j.1749-6632.2011.06057.x. [DOI] [PubMed] [Google Scholar]
- 36.Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, Murray LJ. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inadomi JM, Sampliner R, Lagergren J, Lieberman D, Fendrick AM, Vakil N. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 38.Soni A, Sampliner RE, Sonnenberg A. Screening for high-grade dysplasia in gastroesophageal reflux disease: is it cost-effective? Am J Gastroenterol. 2000;95:2086–2093. doi: 10.1111/j.1572-0241.2000.02173.x. [DOI] [PubMed] [Google Scholar]
- 39.Benaglia T, Sharples LD, Fitzgerald RC, Lyratzopoulos G. Health benefits and cost effectiveness of endoscopic and nonendoscopic cytosponge screening for Barrett’s esophagus. Gastroenterology. 2013;144:62–73.e6. doi: 10.1053/j.gastro.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 40.Gerson LB, Groeneveld PW, Triadafilopoulos G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2004;2:868–879. doi: 10.1016/s1542-3565(04)00394-5. [DOI] [PubMed] [Google Scholar]
- 41.Lindblad M, Bright T, Schloithe A, Mayne GC, Chen G, Bull J, Bampton PA, Fraser RJ, Gatenby PA, Gordon LG, et al. Toward More Efficient Surveillance of Barrett’s Esophagus: Identification and Exclusion of Patients at Low Risk of Cancer. World J Surg. 2017;41:1023–1034. doi: 10.1007/s00268-016-3819-0. [DOI] [PubMed] [Google Scholar]
- 42.University of Cambridge. ‘Pill on a string’ could help spot early signs of cancer of the gullet. Accessed January 7 2017. Available from: http://www.cam.ac.uk/research/news/pill-on-a-string-could-help-spot-early-signs-of-cancer-of-the-gullet.
- 43.Ross-Innes CS, Becq J, Warren A, Cheetham RK, Northen H, O’Donovan M, Malhotra S, di Pietro M, Ivakhno S, He M, et al. Whole-genome sequencing provides new insights into the clonal architecture of Barrett’s esophagus and esophageal adenocarcinoma. Nat Genet. 2015;47:1038–1046. doi: 10.1038/ng.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross-Innes CS, Debiram-Beecham I, O’Donovan M, Walker E, Varghese S, Lao-Sirieix P, Lovat L, Griffin M, Ragunath K, Haidry R, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med. 2015;12:e1001780. doi: 10.1371/journal.pmed.1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saravanapavan H, Hoare JM. Cytosponge instead of Endoscopy in Symptomatic Patients: a Feasibility Study. Gut. 2013;62:A269. [Google Scholar]
- 46.Modiano N, Gerson LB. Barrett’s esophagus: Incidence, etiology, pathophysiology, prevention and treatment. Ther Clin Risk Manag. 2007;3:1035–1145. [PMC free article] [PubMed] [Google Scholar]
- 47.Spechler SJ. Barrett’s esophagus. Semin Gastrointest Dis. 1996;7:51–60. [PubMed] [Google Scholar]
- 48.Hassall E. Columnar-lined esophagus in children. Gastroenterol Clin North Am. 1997;26:533–548. doi: 10.1016/s0889-8553(05)70312-5. [DOI] [PubMed] [Google Scholar]
- 49.Sharma P, Sidorenko EI. Are screening and surveillance for Barrett’s oesophagus really worthwhile? Gut. 2005;54 Suppl 1:i27–i32. doi: 10.1136/gut.2004.041566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther. 2010;32:1222–1227. doi: 10.1111/j.1365-2036.2010.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.National Institute for Health and Care Excellence 2015. Suspected cancer: recognition and referral. Accessed January 7 2017. Available from: https://www.nice.org.uk/guidance/ng12/resources/suspected-cancer-recognition-and-referral-1837268071621. [PubMed]
- 52.Kelty CJ, Gough MD, Van Wyk Q, Stephenson TJ, Ackroyd R. Barrett’s oesophagus: intestinal metaplasia is not essential for cancer risk. Scand J Gastroenterol. 2007;42:1271–1274. doi: 10.1080/00365520701420735. [DOI] [PubMed] [Google Scholar]
- 53.Gatenby PA, Ramus JR, Caygill CP, Shepherd NA, Watson A. Relevance of the detection of intestinal metaplasia in non-dysplastic columnar-lined oesophagus. Scand J Gastroenterol. 2008;43:524–530. doi: 10.1080/00365520701879831. [DOI] [PubMed] [Google Scholar]
- 54.DeMeester SR, DeMeester TR. The diagnosis and management of Barrett’s esophagus. Adv Surg. 1999;33:29–68. [PubMed] [Google Scholar]
- 55.Harrison R, Perry I, Haddadin W, McDonald S, Bryan R, Abrams K, Sampliner R, Talley NJ, Moayyedi P, Jankowski JA. Detection of intestinal metaplasia in Barrett’s esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol. 2007;102:1154–1161. doi: 10.1111/j.1572-0241.2007.01230.x. [DOI] [PubMed] [Google Scholar]
- 56.Rugge M, Fassan M, Cavallin F, Zaninotto G. Re: Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2012;104:1771–1772. doi: 10.1093/jnci/djs426. [DOI] [PubMed] [Google Scholar]
- 57.Liu W, Hahn H, Odze RD, Goyal RK. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell-containing epithelium. Am J Gastroenterol. 2009;104:816–824. doi: 10.1038/ajg.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bandla S, Peters JH, Ruff D, Chen SM, Li CY, Song K, Thoms K, Litle VR, Watson T, Chapurin N, et al. Comparison of cancer-associated genetic abnormalities in columnar-lined esophagus tissues with and without goblet cells. Ann Surg. 2014;260:72–80. doi: 10.1097/SLA.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oh DS, DeMeester SR, Tanaka K, Marjoram P, Kuramochi H, Vallbohmer D, Danenberg K, Chandrasoma PT, DeMeester TR, Hagen JA. The gene expression profile of cardia intestinal metaplasia is similar to that of Barrett’s esophagus, not gastric intestinal metaplasia. Dis Esophagus. 2011;24:516–522. doi: 10.1111/j.1442-2050.2010.01176.x. [DOI] [PubMed] [Google Scholar]
- 60.Ormsby AH, Vaezi MF, Richter JE, Goldblum JR, Rice TW, Falk GW, Gramlich TL. Cytokeratin immunoreactivity patterns in the diagnosis of short-segment Barrett’s esophagus. Gastroenterology. 2000;119:683–690. doi: 10.1053/gast.2000.16482. [DOI] [PubMed] [Google Scholar]
- 61.Ormsby AH, Goldblum JR, Rice TW, Richter JE, Falk GW, Vaezi MF, Gramlich TL. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol. 1999;30:288–294. doi: 10.1016/s0046-8177(99)90007-2. [DOI] [PubMed] [Google Scholar]
- 62.Glickman JN, Wang H, Das KM, Goyal RK, Spechler SJ, Antonioli D, Odze RD. Phenotype of Barrett’s esophagus and intestinal metaplasia of the distal esophagus and gastroesophageal junction: an immunohistochemical study of cytokeratins 7 and 20, Das-1 and 45 MI. Am J Surg Pathol. 2001;25:87–94. doi: 10.1097/00000478-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 63.Jung KW, Talley NJ, Romero Y, Katzka DA, Schleck CD, Zinsmeister AR, Dunagan KT, Lutzke LS, Wu TT, Wang KK, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. Am J Gastroenterol. 2011;106:1447–1455; quiz 1456. doi: 10.1038/ajg.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pohl H, Pech O, Arash H, Stolte M, Manner H, May A, Kraywinkel K, Sonnenberg A, Ell C. Length of Barrett’s oesophagus and cancer risk: implications from a large sample of patients with early oesophageal adenocarcinoma. Gut. 2016;65:196–201. doi: 10.1136/gutjnl-2015-309220. [DOI] [PubMed] [Google Scholar]
- 65.Kastelein F, van Olphen S, Steyerberg EW, Sikkema M, Spaander MC, Looman CW, Kuipers EJ, Siersema PD, Bruno MJ, de Bekker-Grob EW; ProBar-study group. Surveillance in patients with long-segment Barrett’s oesophagus: a cost-effectiveness analysis. Gut. 2015;64:864–871. doi: 10.1136/gutjnl-2014-307197. [DOI] [PubMed] [Google Scholar]
- 66.Playford RJ. New British Society of Gastroenterology (BSG) guidelines for the diagnosis and management of Barrett’s oesophagus. Gut. 2006;55:442. doi: 10.1136/gut.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol. 2012;107:850–862; quiz 863. doi: 10.1038/ajg.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology. 1993;105:40–50. doi: 10.1016/0016-5085(93)90008-z. [DOI] [PubMed] [Google Scholar]
- 69.Provenzale D, Schmitt C, Wong JB. Barrett’s esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 70.Odze RD. Diagnosis and grading of dysplasia in Barrett’s oesophagus. J Clin Pathol. 2006;59:1029–1038. doi: 10.1136/jcp.2005.035337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, Lamps LW, Lauwers GY, Lazenby AJ, Lewin DN, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 72.Naini BV, Chak A, Ali MA, Odze RD. Barrett’s oesophagus diagnostic criteria: endoscopy and histology. Best Pract Res Clin Gastroenterol. 2015;29:77–96. doi: 10.1016/j.bpg.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 73.Reid BJ, Haggitt RC, Rubin CE, Roth G, Surawicz CM, Van Belle G, Lewin K, Weinstein WM, Antonioli DA, Goldman H. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 74.Guda NM, Partington S, Vakil N. Inter- and intra-observer variability in the measurement of length at endoscopy: Implications for the measurement of Barrett’s esophagus. Gastrointest Endosc. 2004;59:655–658. doi: 10.1016/s0016-5107(04)00182-8. [DOI] [PubMed] [Google Scholar]
- 75.Montgomery E. Is there a way for pathologists to decrease interobserver variability in the diagnosis of dysplasia? Arch Pathol Lab Med. 2005;129:174–176. doi: 10.5858/2005-129-174-ITAWFP. [DOI] [PubMed] [Google Scholar]
- 76.Abela JE, Going JJ, Mackenzie JF, McKernan M, O’Mahoney S, Stuart RC. Systematic four-quadrant biopsy detects Barrett’s dysplasia in more patients than nonsystematic biopsy. Am J Gastroenterol. 2008;103:850–855. doi: 10.1111/j.1572-0241.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 77.Bhandari P, Kandaswamy P, Cowlishaw D, Longcroft-Wheaton G. Acetic acid-enhanced chromoendoscopy is more cost-effective than protocol-guided biopsies in a high-risk Barrett’s population. Dis Esophagus. 2012;25:386–392. doi: 10.1111/j.1442-2050.2011.01267.x. [DOI] [PubMed] [Google Scholar]
- 78.Sakai Y, Eto R, Kasanuki J, Kondo F, Kato K, Arai M, Suzuki T, Kobayashi M, Matsumura T, Bekku D, et al. Chromoendoscopy with indigo carmine dye added to acetic acid in the diagnosis of gastric neoplasia: a prospective comparative study. Gastrointest Endosc. 2008;68:635–641. doi: 10.1016/j.gie.2008.03.1065. [DOI] [PubMed] [Google Scholar]
- 79.Tholoor S, Bhattacharyya R, Tsagkournis O, Longcroft-Wheaton G, Bhandari P. Acetic acid chromoendoscopy in Barrett’s esophagus surveillance is superior to the standardized random biopsy protocol: results from a large cohort study (with video) Gastrointest Endosc. 2014;80:417–424. doi: 10.1016/j.gie.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 80.Hoffman A, Korczynski O, Tresch A, Hansen T, Rahman F, Goetz M, Murthy S, Galle PR, Kiesslich R. Acetic acid compared with i-scan imaging for detecting Barrett’s esophagus: a randomized, comparative trial. Gastrointest Endosc. 2014;79:46–54. doi: 10.1016/j.gie.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 81.Longcroft-Wheaton G, Duku M, Mead R, Poller D, Bhandari P. Acetic acid spray is an effective tool for the endoscopic detection of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2010;8:843–847. doi: 10.1016/j.cgh.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 82.Dawsey SM, Fleischer DE, Wang GQ, Zhou B, Kidwell JA, Lu N, Lewin KJ, Roth MJ, Tio TL, Taylor PR. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220–231. [PubMed] [Google Scholar]
- 83.Kondo H, Fukuda H, Ono H, Gotoda T, Saito D, Takahiro K, Shirao K, Yamaguchi H, Yoshida S. Sodium thiosulfate solution spray for relief of irritation caused by Lugol’s stain in chromoendoscopy. Gastrointest Endosc. 2001;53:199–202. doi: 10.1067/mge.2001.110730. [DOI] [PubMed] [Google Scholar]
- 84.Furuta Y, Kobori O, Shimazu H, Morioka Y, Okuyama Y. A new in vivo staining method, cresyl violet staining, for fiberoptic magnified observation of carcinoma of the gastric mucosa. Gastroenterol Jpn. 1985;20:120–124. doi: 10.1007/BF02776674. [DOI] [PubMed] [Google Scholar]
- 85.Amano Y, Kushiyama Y, Ishihara S, Yuki T, Miyaoka Y, Yoshino N, Ishimura N, Fujishiro H, Adachi K, Maruyama R, et al. Crystal violet chromoendoscopy with mucosal pit pattern diagnosis is useful for surveillance of short-segment Barrett’s esophagus. Am J Gastroenterol. 2005;100:21–26. doi: 10.1111/j.1572-0241.2005.40028.x. [DOI] [PubMed] [Google Scholar]
- 86.Meining A, Rösch T, Kiesslich R, Muders M, Sax F, Heldwein W. Inter- and intra-observer variability of magnification chromoendoscopy for detecting specialized intestinal metaplasia at the gastroesophageal junction. Endoscopy. 2004;36:160–164. doi: 10.1055/s-2004-814183. [DOI] [PubMed] [Google Scholar]
- 87.dos Santos CE, Lima JC, Lopes CV, Malaman D, Salomão AD, Garcia AC, Teixeira CR. Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol. 2010;22:1364–1371. doi: 10.1097/MEG.0b013e32833a5d63. [DOI] [PubMed] [Google Scholar]
- 88.Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, Tanioka D, Tsuzuki T, Uemura M, Ohara N, et al. Narrow-band imaging provides reliable screening for esophageal malignancy in patients with head and neck cancers. Am J Gastroenterol. 2009;104:2942–2948. doi: 10.1038/ajg.2009.426. [DOI] [PubMed] [Google Scholar]
- 89.Matsumoto T, Esaki M, Fujisawa R, Nakamura S, Yao T, Iida M. Chromoendoscopy, narrow-band imaging colonoscopy, and autofluorescence colonoscopy for detection of diminutive colorectal neoplasia in familial adenomatous polyposis. Dis Colon Rectum. 2009;52:1160–1165. doi: 10.1007/DCR.0b013e31819ef6fe. [DOI] [PubMed] [Google Scholar]
- 90.Togashi K, Osawa H, Koinuma K, Hayashi Y, Miyata T, Sunada K, Nokubi M, Horie H, Yamamoto H. A comparison of conventional endoscopy, chromoendoscopy, and the optimal-band imaging system for the differentiation of neoplastic and non-neoplastic colonic polyps. Gastrointest Endosc. 2009;69:734–741. doi: 10.1016/j.gie.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 91.Savoy AD, Wolfsen HC, Raimondo M, Woodward TA, Noh K, Pungpapong S, Hemminger LL, Wallace MB. The role of surveillance endoscopy and endosonography after endoscopic ablation of high-grade dysplasia and carcinoma of the esophagus. Dis Esophagus. 2008;21:108–113. doi: 10.1111/j.1442-2050.2007.00763.x. [DOI] [PubMed] [Google Scholar]
- 92.Pohl J, May A, Rabenstein T, Pech O, Nguyen-Tat M, Fissler-Eckhoff A, Ell C. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett’s esophagus. Endoscopy. 2007;39:594–598. doi: 10.1055/s-2007-966649. [DOI] [PubMed] [Google Scholar]
- 93.Falk GW. Autofluorescence endoscopy. Gastrointest Endosc Clin N Am. 2009;19:209–220. doi: 10.1016/j.giec.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 94.Wallace MB, Sharma P, Lightdale C, Wolfsen H, Coron E, Buchner A, Bajbouj M, Bansal A, Rastogi A, Abrams J, et al. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe-based confocal laser endomicroscopy. Gastrointest Endosc. 2010;72:19–24. doi: 10.1016/j.gie.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whiteman DC, Appleyard M, Bahin FF, Bobryshev YV, Bourke MJ, Brown I, Chung A, Clouston A, Dickins E, Emery J, et al. Australian clinical practice guidelines for the diagnosis and management of Barrett’s esophagus and early esophageal adenocarcinoma. J Gastroenterol Hepatol. 2015;30:804–820. doi: 10.1111/jgh.12913. [DOI] [PubMed] [Google Scholar]
- 96.Pellegrini CA, Pohl D. High-grade dysplasia in Barrett’s esophagus: surveillance or operation? J Gastrointest Surg. 2000;4:131–134. doi: 10.1016/s1091-255x(00)80048-7. [DOI] [PubMed] [Google Scholar]
- 97.Schnell TG, Sontag SJ, Chejfec G, Aranha G, Metz A, O’Connell S, Seidel UJ, Sonnenberg A. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 98.Reid BJ, Blount PL, Feng Z, Levine DS. Optimizing endoscopic biopsy detection of early cancers in Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:3089–3096. doi: 10.1111/j.1572-0241.2000.03182.x. [DOI] [PubMed] [Google Scholar]
- 99.Blazeby JM, Farndon JR, Donovan J, Alderson D. A prospective longitudinal study examining the quality of life of patients with esophageal carcinoma. Cancer. 2000;88:1781–1787. [PubMed] [Google Scholar]
- 100.Incarbone R, Bonavina L, Saino G, Bona D, Peracchia A. Outcome of esophageal adenocarcinoma detected during endoscopic biopsy surveillance for Barrett’s esophagus. Surg Endosc. 2002;16:263–266. doi: 10.1007/s00464-001-8161-3. [DOI] [PubMed] [Google Scholar]
- 101.Ferguson MK, Durkin A. Long-term survival after esophagectomy for Barrett’s adenocarcinoma in endoscopically surveyed and nonsurveyed patients. J Gastrointest Surg. 2002;6:29–35; discussion 36. doi: 10.1016/s1091-255x(01)00052-x. [DOI] [PubMed] [Google Scholar]
- 102.van Sandick JW, van Lanschot JJ, Kuiken BW, Tytgat GN, Offerhaus GJ, Obertop H. Impact of endoscopic biopsy surveillance of Barrett’s oesophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut. 1998;43:216–222. doi: 10.1136/gut.43.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peters JH, Clark GW, Ireland AP, Chandrasoma P, Smyrk TC, DeMeester TR. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg. 1994;108:813–821; discussion 821-822. [PubMed] [Google Scholar]
- 104.Bhat SK, McManus DT, Coleman HG, Johnston BT, Cardwell CR, McMenamin U, Bannon F, Hicks B, Kennedy G, Gavin AT, et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett’s oesophagus: a population-based study. Gut. 2015;64:20–25. doi: 10.1136/gutjnl-2013-305506. [DOI] [PubMed] [Google Scholar]
- 105.Castell DO, Katzka DA. Barrett’s esophagus: continuing questions and controversy. Gastrointest Endosc. 1999;49:S5–S8. doi: 10.1016/s0016-5107(99)70516-x. [DOI] [PubMed] [Google Scholar]
- 106.Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798–1810. doi: 10.1053/gast.1997.v112.pm9178669. [DOI] [PubMed] [Google Scholar]
- 107.Iftikhar SY, James PD, Steele RJ, Hardcastle JD, Atkinson M. Length of Barrett’s oesophagus: an important factor in the development of dysplasia and adenocarcinoma. Gut. 1992;33:1155–1158. doi: 10.1136/gut.33.9.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Menke-Pluymers MB, Hop WC, Dees J, van Blankenstein M, Tilanus HW. Risk factors for the development of an adenocarcinoma in columnar-lined (Barrett) esophagus. The Rotterdam Esophageal Tumor Study Group. Cancer. 1993;72:1155–1158. doi: 10.1002/1097-0142(19930815)72:4<1155::aid-cncr2820720404>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 109.Cooper BT, Neumann CS, Cox MA, Iqbal TH. Continuous treatment with omeprazole 20 mg daily for up to 6 years in Barrett’s oesophagus. Aliment Pharmacol Ther. 1998;12:893–897. doi: 10.1046/j.1365-2036.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- 110.Biddlestone LR, Barham CP, Wilkinson SP, Barr H, Shepherd NA. The histopathology of treated Barrett’s esophagus: squamous reepithelialization after acid suppression and laser and photodynamic therapy. Am J Surg Pathol. 1998;22:239–245. doi: 10.1097/00000478-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 111.Sampliner RE, Steinbronn K, Garewal HS, Riddell RH. Squamous mucosa overlying columnar epithelium in Barrett’s esophagus in the absence of anti-reflux surgery. Am J Gastroenterol. 1988;83:510–512. [PubMed] [Google Scholar]
- 112.Kastelein F, Spaander MC, Biermann K, Steyerberg EW, Kuipers EJ, Bruno MJ; Probar-study Group. Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology. 2011;141:2000–2008; quiz e13-e14. doi: 10.1053/j.gastro.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 113.Nguyen DM, Richardson P, El-Serag HB. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. Gastroenterology. 2010;138:2260–2266. doi: 10.1053/j.gastro.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Winberg H, Lindblad M, Lagergren J, Dahlstrand H. Risk factors and chemoprevention in Barrett’s esophagus--an update. Scand J Gastroenterol. 2012;47:397–406. doi: 10.3109/00365521.2012.667145. [DOI] [PubMed] [Google Scholar]
- 115.Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 116.Alexandre L, Clark AB, Cheong E, Lewis MP, Hart AR. Systematic review: potential preventive effects of statins against oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2012;36:301–311. doi: 10.1111/j.1365-2036.2012.05194.x. [DOI] [PubMed] [Google Scholar]
- 117.Beales IL, Vardi I, Dearman L. Regular statin and aspirin use in patients with Barrett’s oesophagus is associated with a reduced incidence of oesophageal adenocarcinoma. Eur J Gastroenterol Hepatol. 2012;24:917–923. doi: 10.1097/MEG.0b013e3283543f01. [DOI] [PubMed] [Google Scholar]
- 118.Gordon V, Jankowski J. Chemoprevention in Barrett’s oesophagus. Best Pract Res Clin Gastroenterol. 2011;25:569–579. doi: 10.1016/j.bpg.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 119.Choi SE, Perzan KE, Tramontano AC, Kong CY, Hur C. Statins and aspirin for chemoprevention in Barrett’s esophagus: results of a cost-effectiveness analysis. Cancer Prev Res (Phila) 2014;7:341–350. doi: 10.1158/1940-6207.CAPR-13-0191-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Falk GW, Buttar NS, Foster NR, Ziegler KL, Demars CJ, Romero Y, Marcon NE, Schnell T, Corley DA, Sharma P, et al. A combination of esomeprazole and aspirin reduces tissue concentrations of prostaglandin E(2) in patients with Barrett’s esophagus. Gastroenterology. 2012;143:917–926.e1. doi: 10.1053/j.gastro.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Heath EI, Canto MI, Piantadosi S, Montgomery E, Weinstein WM, Herman JG, Dannenberg AJ, Yang VW, Shar AO, Hawk E, et al. Secondary chemoprevention of Barrett’s esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst. 2007;99:545–557. doi: 10.1093/jnci/djk112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gatenby P, Soon Y. Barrett’s oesophagus: Evidence from the current meta-analyses. World J Gastrointest Pathophysiol. 2014;5:178–187. doi: 10.4291/wjgp.v5.i3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:620–629. doi: 10.1016/j.cgh.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jamieson GG, France M, Watson DI. Results of laparoscopic antireflux operations in patients who have Barrett’s esophagus. Chest Surg Clin N Am. 2002;12:149–155. doi: 10.1016/s1052-3359(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 125.Desai KM, Soper NJ, Frisella MM, Quasebarth MA, Dunnegan DL, Brunt LM. Efficacy of laparoscopic antireflux surgery in patients with Barrett’s esophagus. Am J Surg. 2003;186:652–659. doi: 10.1016/j.amjsurg.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 126.Hofstetter WL, Peters JH, DeMeester TR, Hagen JA, DeMeester SR, Crookes PF, Tsai P, Banki F, Bremner CG. Long-term outcome of antireflux surgery in patients with Barrett’s esophagus. Ann Surg. 2001;234:532–538; discussion 538-539. doi: 10.1097/00000658-200110000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chang EY, Morris CD, Seltman AK, O’Rourke RW, Chan BK, Hunter JG, Jobe BA. The effect of antireflux surgery on esophageal carcinogenesis in patients with barrett esophagus: a systematic review. Ann Surg. 2007;246:11–21. doi: 10.1097/01.sla.0000261459.10565.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maret-Ouda J, Konings P, Lagergren J, Brusselaers N. Antireflux Surgery and Risk of Esophageal Adenocarcinoma: A Systematic Review and Meta-analysis. Ann Surg. 2016;263:251–257. doi: 10.1097/SLA.0000000000001438. [DOI] [PubMed] [Google Scholar]
- 129.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 130.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1245–1255. doi: 10.1016/j.cgh.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Small AJ, Sutherland SE, Hightower JS, Guarner-Argente C, Furth EE, Kochman ML, Forde KA, Bewtra M, Falk GW, Ginsberg GG. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high-grade dysplasia versus intramucosal carcinoma in Barrett’s esophagus. Gastrointest Endosc. 2015;81:1158–1166.e1-4. doi: 10.1016/j.gie.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 132.Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, Fullarton G, Di Pietro M, Ravi N, Visser M, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 133.Chadwick G, Groene O, Markar SR, Hoare J, Cromwell D, Hanna GB. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett’s esophagus: a critical assessment of histologic outcomes and adverse events. Gastrointest Endosc. 2014;79:718–731.e3. doi: 10.1016/j.gie.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 134.Shaheen NJ, Greenwald BD, Peery AF, Dumot JA, Nishioka NS, Wolfsen HC, Burdick JS, Abrams JA, Wang KK, Mallat D, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71:680–685. doi: 10.1016/j.gie.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Greenwald BD, Dumot JA. Cryotherapy for Barrett’s esophagus and esophageal cancer. Curr Opin Gastroenterol. 2011;27:363–367. doi: 10.1097/MOG.0b013e328347bae8. [DOI] [PubMed] [Google Scholar]
- 136.Reid BJ, Blount PL, Rabinovitch PS. Biomarkers in Barrett’s esophagus. Gastrointest Endosc Clin N Am. 2003;13:369–397. doi: 10.1016/s1052-5157(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 137.Timmer MR, Sun G, Gorospe EC, Leggett CL, Lutzke L, Krishnadath KK, Wang KK. Predictive biomarkers for Barrett’s esophagus: so near and yet so far. Dis Esophagus. 2013;26:574–581. doi: 10.1111/dote.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 139.Ong CA, Lao-Sirieix P, Fitzgerald RC. Biomarkers in Barrett’s esophagus and esophageal adenocarcinoma: predictors of progression and prognosis. World J Gastroenterol. 2010;16:5669–5681. doi: 10.3748/wjg.v16.i45.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baron JA. Screening for cancer with molecular markers: progress comes with potential problems. Nat Rev Cancer. 2012;12:368–371. doi: 10.1038/nrc3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Williams PM, Lively TG, Jessup JM, Conley BA. Bridging the gap: moving predictive and prognostic assays from research to clinical use. Clin Cancer Res. 2012;18:1531–1539. doi: 10.1158/1078-0432.CCR-11-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fouad YM, Mostafa I, Yehia R, El-Khayat H. Biomarkers of Barrett’s esophagus. World J Gastrointest Pathophysiol. 2014;5:450–456. doi: 10.4291/wjgp.v5.i4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Monteiro MS, Carvalho M, Bastos ML, Guedes de Pinho P. Metabolomics analysis for biomarker discovery: advances and challenges. Curr Med Chem. 2013;20:257–271. doi: 10.2174/092986713804806621. [DOI] [PubMed] [Google Scholar]
- 144.González-Correa CA, Brown BH, Smallwood RH, Kalia N, Stoddard CJ, Stephenson TJ, Haggie SJ, Slater DN, Bardhan KD. Virtual biopsies in Barrett’s esophagus using an impedance probe. Ann N Y Acad Sci. 1999;873:313–321. doi: 10.1111/j.1749-6632.1999.tb09479.x. [DOI] [PubMed] [Google Scholar]
- 145.Bruno MJ. Magnification endoscopy, high resolution endoscopy, and chromoscopy; towards a better optical diagnosis. Gut. 2003;52 Suppl 4:iv7–i11. doi: 10.1136/gut.52.suppl_4.iv7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kiesslich R, Goetz M, Vieth M, Galle PR, Neurath MF. Confocal laser endomicroscopy. Gastrointest Endosc Clin N Am. 2005;15:715–731. doi: 10.1016/j.giec.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 147.Qumseya BJ, Wang H, Badie N, Uzomba RN, Parasa S, White DL, Wolfsen H, Sharma P, Wallace MB. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol. 2013;11:1562–1570.e1-e2. doi: 10.1016/j.cgh.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dong YY, Li YQ, Yu YB, Liu J, Li M, Luan XR. Meta-analysis of confocal laser endomicroscopy for the detection of colorectal neoplasia. Colorectal Dis. 2013;15:e488–e495. doi: 10.1111/codi.12329. [DOI] [PubMed] [Google Scholar]
- 149.Wu J, Pan YM, Wang TT, Hu B. Confocal laser endomicroscopy for detection of neoplasia in Barrett’s esophagus: a meta-analysis. Dis Esophagus. 2014;27:248–254. doi: 10.1111/dote.12085. [DOI] [PubMed] [Google Scholar]
- 150.Wanders LK, East JE, Uitentuis SE, Leeflang MM, Dekker E. Diagnostic performance of narrowed spectrum endoscopy, autofluorescence imaging, and confocal laser endomicroscopy for optical diagnosis of colonic polyps: a meta-analysis. Lancet Oncol. 2013;14:1337–1347. doi: 10.1016/S1470-2045(13)70509-6. [DOI] [PubMed] [Google Scholar]
- 151.Gatenby P, Bhattacharjee S, Wall C, Caygill C, Watson A. Risk stratification for malignant progression in Barrett’s esophagus: Gender, age, duration and year of surveillance. World J Gastroenterol. 2016;22:10592–10600. doi: 10.3748/wjg.v22.i48.10592. [DOI] [PMC free article] [PubMed] [Google Scholar]


