Abstract
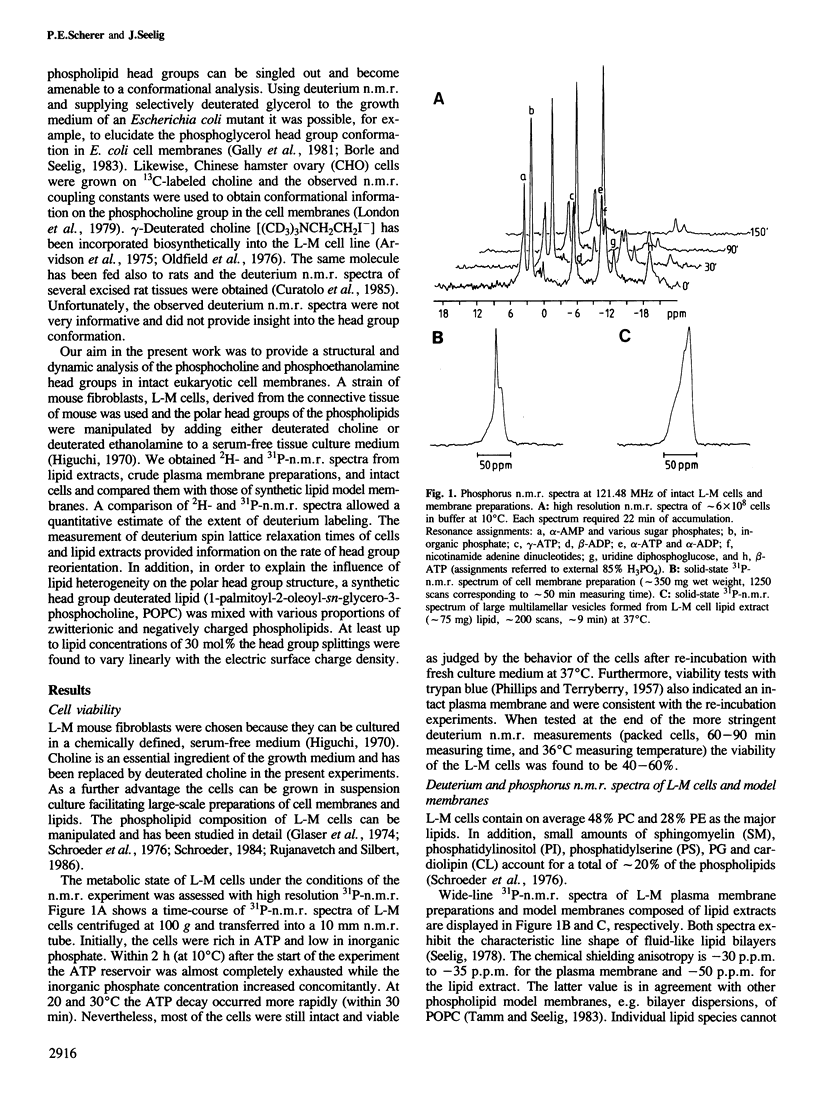
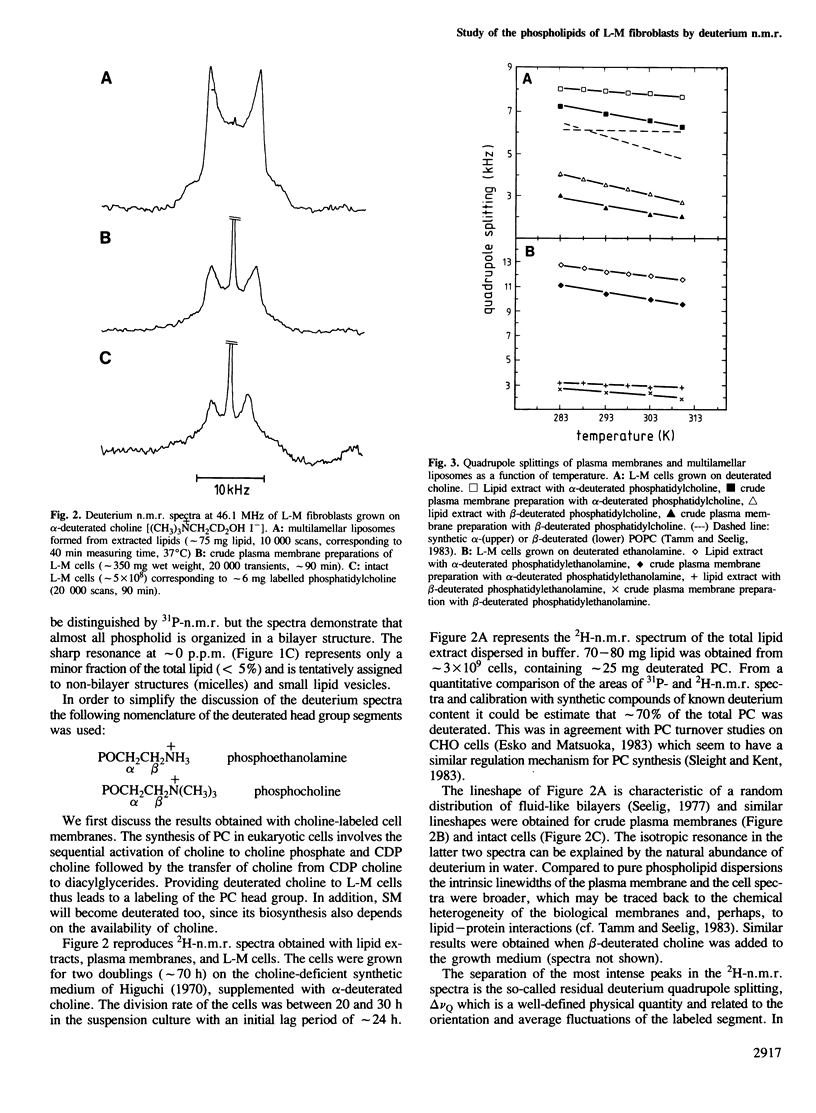
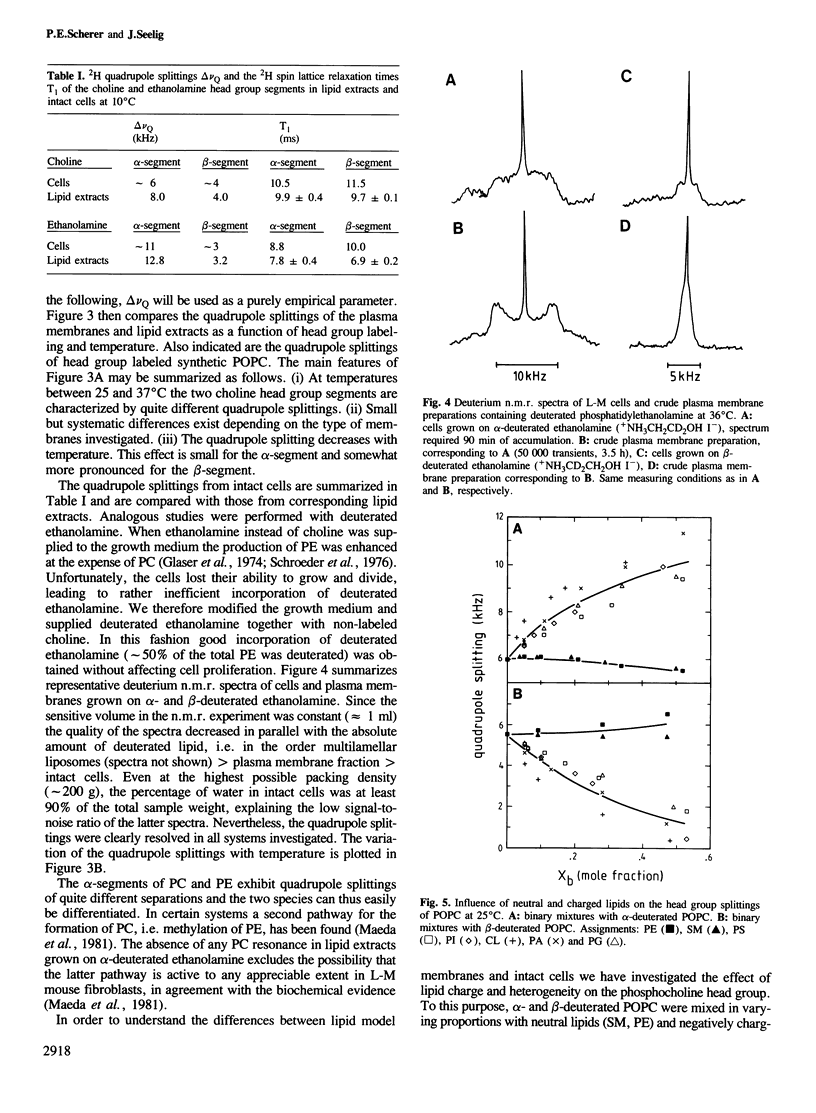
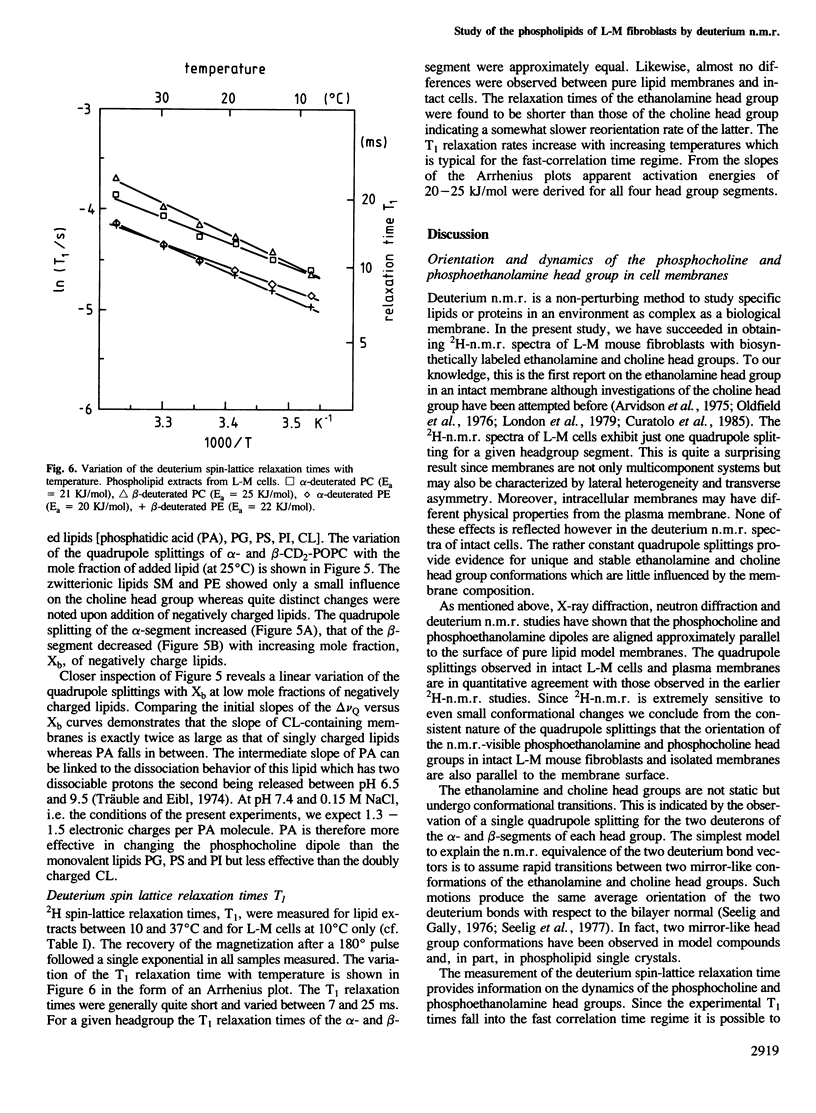
Mouse fibroblast L-M cells were grown in tissue culture medium containing selectively deuterated choline or ethanolamine. Both compounds were incorporated into the corresponding phospholipids at levels greater than 50% thus leading to a selective deuteration of these phospholipid head groups. Choline and ethanolamine were labeled at either the alpha- or the beta-carbon atom and well-resolved deuterium and phosphorus n.m.r. spectra were obtained from intact cells, crude plasma membranes and lipid extracts, leading to the following conclusions. (i) A large fraction, if not all, of the phospholipids in the intact L-M cell membranes were organized in a liquid crystalline bilayer. (ii) The phosphoethanolamine and the phosphocholine head group conformation were found to be remarkably similar in pure lipid bilayers and in intact L-M cell membranes with the head group dipoles being oriented parallel to the membrane surface. (iii) The deuterium T1 spin lattice relaxation times fell in the range of 7-25 ms and were similar in intact L-M cells and in pure lipid model membranes, suggesting that the two head groups are not involved in strong interactions with membrane proteins. The rotational diffusion rate of the two head groups was reduced by at least a factor of 10 compared to molecules of the same size in aqueous solution. (iv) The phosphocholine head group was sensitive to the size and sign of membrane surface charges as verified in mixing experiments with charged lipids. In L-M cell membranes the phosphocholine appeared to sense an electrically neutral environment in spite of the fact that L-M cell membranes contain 10-20% negatively charged lipids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akutsu H., Seelig J. Interaction of metal ions with phosphatidylcholine bilayer membranes. Biochemistry. 1981 Dec 22;20(26):7366–7373. doi: 10.1021/bi00529a007. [DOI] [PubMed] [Google Scholar]
- Altenbach C., Seelig J. Ca2+ binding to phosphatidylcholine bilayers as studied by deuterium magnetic resonance. Evidence for the formation of a Ca2+ complex with two phospholipid molecules. Biochemistry. 1984 Aug 14;23(17):3913–3920. doi: 10.1021/bi00312a019. [DOI] [PubMed] [Google Scholar]
- Arvidson G., Lindblom G., Drakenberg T. A novel approach to the study of mammalian cell-membranes using deuterium NMR. FEBS Lett. 1975 Jun 15;54(2):249–252. doi: 10.1016/0014-5793(75)80085-8. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boulanger Y., Schreier S., Smith I. C. Molecular details of anesthetic--lipid interaction as seen by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry. 1981 Nov 24;20(24):6824–6830. doi: 10.1021/bi00527a013. [DOI] [PubMed] [Google Scholar]
- Büldt G., Gally H. U., Seelig A., Seelig J., Zaccai G. Neutron diffraction studies on selectively deuterated phospholipid bilayers. Nature. 1978 Jan 12;271(5641):182–184. doi: 10.1038/271182a0. [DOI] [PubMed] [Google Scholar]
- Büldt G., Gally H. U., Seelig J., Zaccai G. Neutron diffraction studies on phosphatidylcholine model membranes. I. Head group conformation. J Mol Biol. 1979 Nov 15;134(4):673–691. doi: 10.1016/0022-2836(79)90479-0. [DOI] [PubMed] [Google Scholar]
- Büldt G., Seelig J. Conformation of phosphatidylethanolamine in the gel phase as seen by neutron diffraction. Biochemistry. 1980 Dec 23;19(26):6170–6175. doi: 10.1021/bi00567a034. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Jungalwala F. B., Sears B., Tuck L., Neuringer L. J. Deuterium NMR spectroscopy of biosynthetically deuterated mammalian tissues. Biochemistry. 1985 Jul 30;24(16):4360–4364. doi: 10.1021/bi00337a017. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Matsuoka K. Y. Biosynthesis of phosphatidylcholine from serum phospholipids in Chinese hamster ovary cells deprived of choline. J Biol Chem. 1983 Mar 10;258(5):3051–3057. [PubMed] [Google Scholar]
- Gally H. U., Niederberger W., Seelig J. Conformation and motion of the choline head group in bilayers of dipalmitoyl-3-sn-phosphatidylcholine. Biochemistry. 1975 Aug 12;14(16):3647–3652. doi: 10.1021/bi00687a021. [DOI] [PubMed] [Google Scholar]
- Gally H. U., Pluschke G., Overath P., Seelig J. Structure of Escherichia coli membranes. Glycerol auxotrophs as a tool for the analysis of the phospholipid head-group region by deuterium magentic resonance. Biochemistry. 1981 Mar 31;20(7):1826–1831. doi: 10.1021/bi00510a017. [DOI] [PubMed] [Google Scholar]
- Glaser M., Ferguson K. A., Vagelos P. R. Manipulation of the phospholipid composition of tissue culture cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4072–4076. doi: 10.1073/pnas.71.10.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Griffin R. G. Improved method for the synthesis of phosphatidylcholines. J Lipid Res. 1984 Oct;25(10):1140–1142. [PubMed] [Google Scholar]
- Higuchi K. An improved chemically defined culture medium for strain L mouse cells based on growth responses to graded levels of nutrients including iron and zinc ions. J Cell Physiol. 1970 Feb;75(1):65–72. doi: 10.1002/jcp.1040750108. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. B., Mason R., Thomas K. M., Shipley G. G. Structural chemistry of 1,2 dilauroyl-DL-phosphatidylethanolamine: molecular conformation and intermolecular packing of phospholipids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3036–3040. doi: 10.1073/pnas.71.8.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelusky E. C., Smith I. C. The influence of local anesthetics on molecular organization in phosphatidylethanolamine membranes. Mol Pharmacol. 1984 Sep;26(2):314–321. [PubMed] [Google Scholar]
- Macdonald P. M., Seelig J. Calcium binding to mixed phosphatidylglycerol-phosphatidylcholine bilayers as studied by deuterium nuclear magnetic resonance. Biochemistry. 1987 Mar 10;26(5):1231–1240. doi: 10.1021/bi00379a005. [DOI] [PubMed] [Google Scholar]
- Maeda T., Balakrishnan K., Mehdi S. Q. A simple and rapid method for the preparation of plasma membranes. Biochim Biophys Acta. 1983 May 26;731(1):115–120. doi: 10.1016/0005-2736(83)90404-2. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Wang E. Biosynthesis of long-chain (sphingoid) bases from serine by LM cells. Evidence for introduction of the 4-trans-double bond after de novo biosynthesis of N-acylsphinganine(s). J Biol Chem. 1986 Mar 15;261(8):3764–3769. [PubMed] [Google Scholar]
- Mischel M., Seelig J., Braganza L. F., Büldt G. A neutron diffraction study of the headgroup conformation of phosphatidylglycerol from Escherichia coli membranes. Chem Phys Lipids. 1987 May;43(4):237–246. doi: 10.1016/0009-3084(87)90020-x. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Meadows M., Glaser M. Deuterium magnetic resonance spectroscopy of isotopically labeled mammalian cells. J Biol Chem. 1976 Oct 10;251(19):6147–6149. [PubMed] [Google Scholar]
- PHILLIPS H. J., TERRYBERRY J. E. Counting actively metabolizing tissue cultured cells. Exp Cell Res. 1957 Oct;13(2):341–347. doi: 10.1016/0014-4827(57)90013-7. [DOI] [PubMed] [Google Scholar]
- Pascher I., Sundell S., Harlos K., Eibl H. Conformation and packing properties of membrane lipids: the crystal structure of sodium dimyristoylphosphatidylglycerol. Biochim Biophys Acta. 1987 Jan 9;896(1):77–88. doi: 10.1016/0005-2736(87)90358-0. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Rujanavech C., Silbert D. F. Effect of sterol structure on the partition of sterol between phospholipid vesicles of different composition. J Biol Chem. 1986 Jun 5;261(16):7215–7219. [PubMed] [Google Scholar]
- Schroeder F. Fluorescent sterols: probe molecules of membrane structure and function. Prog Lipid Res. 1984;23(2):97–113. doi: 10.1016/0163-7827(84)90009-2. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Perlmutter J. F., Glaser M., Vagelos P. R. Isolation and characterization of subcellular membranes with altered phospholipid composition from cultured fibroblasts. J Biol Chem. 1976 Aug 25;251(16):5015–5026. [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Seelig J., Gally G. U., Wohlgemuth R. Orientation and flexibility of the choline head group in phosphatidylcholine bilayers. Biochim Biophys Acta. 1977 Jun 2;467(2):109–119. doi: 10.1016/0005-2736(77)90188-2. [DOI] [PubMed] [Google Scholar]
- Seelig J., Gally H. Investigation of phosphatidylethanolamine bilayers by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry. 1976 Nov 30;15(24):5199–5204. doi: 10.1021/bi00669a001. [DOI] [PubMed] [Google Scholar]
- Seelig J., Tamm L., Hymel L., Fleischer S. Deuterium and phosphorus nuclear magnetic resonance and fluorescence depolarization studies of functional reconstituted sarcoplasmic reticulum membrane vesicles. Biochemistry. 1981 Jun 23;20(13):3922–3932. doi: 10.1021/bi00516a040. [DOI] [PubMed] [Google Scholar]
- Sixl F., Watts A. Headgroup interactions in mixed phospholipid bilayers. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1613–1615. doi: 10.1073/pnas.80.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixl F., Watts A. Interactions between phospholipid head groups at membrane interfaces: a deuterium and phosphorus nuclear magnetic resonance and spin-label electron spin resonance study. Biochemistry. 1982 Dec 7;21(25):6446–6452. doi: 10.1021/bi00268a020. [DOI] [PubMed] [Google Scholar]
- Sleight R., Kent C. Regulation of phosphatidylcholine biosynthesis in mammalian cells. I. Effects of phospholipase C treatment on phosphatidylcholine metabolism in Chinese hamster ovary cells and LM mouse fibroblasts. J Biol Chem. 1983 Jan 25;258(2):824–830. [PubMed] [Google Scholar]
- Tamm L. K., Seelig J. Lipid solvation of cytochrome c oxidase. Deuterium, nitrogen-14, and phosphorus-31 nuclear magnetic resonance studies on the phosphocholine head group and on cis-unsaturated fatty acyl chains. Biochemistry. 1983 Mar 15;22(6):1474–1483. doi: 10.1021/bi00275a023. [DOI] [PubMed] [Google Scholar]
- Träuble H., Eibl H. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proc Natl Acad Sci U S A. 1974 Jan;71(1):214–219. doi: 10.1073/pnas.71.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker D. R. Phosphatidylserine functions as the major precursor of phosphatidylethanolamine in cultured BHK-21 cells. Proc Natl Acad Sci U S A. 1984 May;81(9):2669–2673. doi: 10.1073/pnas.81.9.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth R., Waespe-Sarcevic N., Seelig J. Bilayers of phosphatidylglycerol. A deuterium and phosphorus nuclear magnetic resonance study of the head-group region. Biochemistry. 1980 Jul 8;19(14):3315–3321. doi: 10.1021/bi00555a033. [DOI] [PubMed] [Google Scholar]


