Abstract
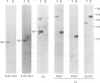
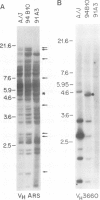
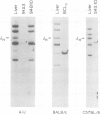
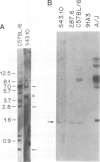
Deletion mapping analyses have been employed to order the heavy chain variable region (VH) gene families in three inbred murine strains. These nine VH gene families have been positioned with respect to the J558 and 3660 VH families in A/J (Ighe) as follows: 3609-J558-(J606,VGAM3-8,S107)-3660-(X24,Q52,7183 )-DH. Maps generated with respect to J558 in the BALB/c (Igha) and C57BL/6 (Ighb) strains are consistent with these results. The organization of the VH complex produced by deletion mapping is quite different from the accepted map generated by other methods, particularly in that J558 is more DH distal and 3660 is more DH proximal than previously thought. The order presented here is compatible with VH rearrangement frequencies suggesting preferential utilization of DH-proximal VH gene segments. Our data also indicate that interspersion of some VH family members may be a common feature of the murine VH complex since the 3609 VH family is interdigitated in the three strains and a Q52 VH gene segment is interspersed in C57BL/6.
Full text
PDF

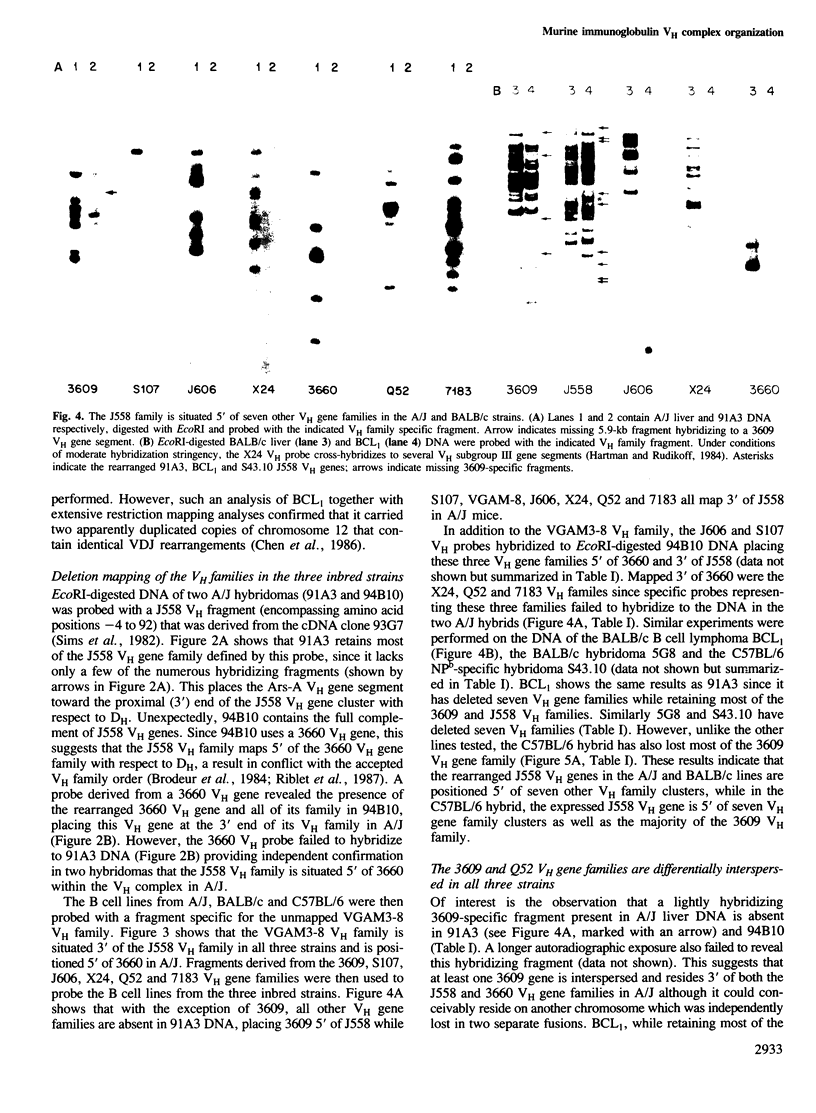
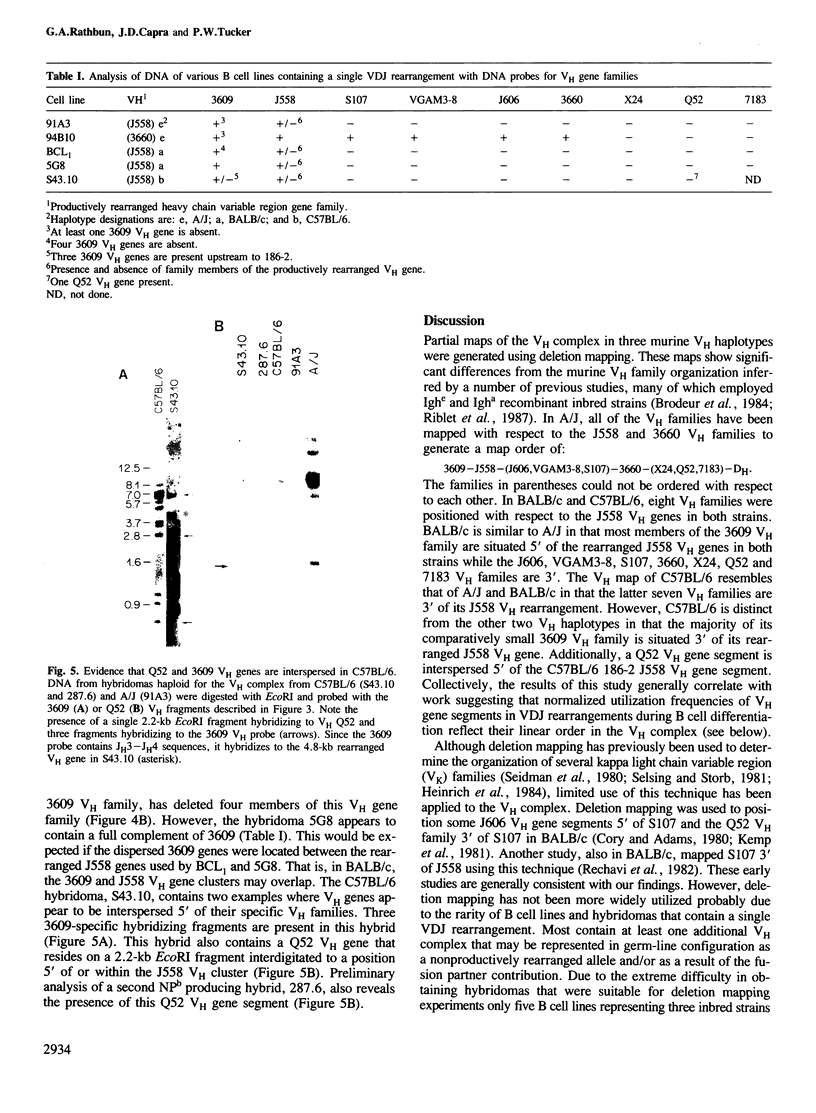



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Berek C., Eichmann K. Structural studies on induced antibodies with defined idiotypic specificities. III. N-terminal amino acid sequence of the heavy and light chains of mouse anti-streptococcal antibodies--A5A, S8, and S117. J Immunol. 1976 Jul;117(1):7–10. [PubMed] [Google Scholar]
- Chen Y. W., Word C., Dev V., Uhr J. W., Vitetta E. S., Tucker P. W. Double isotype production by a neoplastic B cell line. II. Allelically excluded production of mu and gamma 1 heavy chains without CH gene rearrangement. J Exp Med. 1986 Aug 1;164(2):562–579. doi: 10.1084/jem.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Deletions are associated with somatic rearrangement of immunoglobulin heavy chain genes. Cell. 1980 Jan;19(1):37–51. doi: 10.1016/0092-8674(80)90386-4. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Dzierzak E. A., Brodeur P., Marion T., Janeway C. A., Jr, Bothwell A. Molecular characterization of antibodies bearing Id-460. II. Molecular basis for Id-460 expression. J Exp Med. 1985 Nov 1;162(5):1494–1511. doi: 10.1084/jem.162.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estess P., Nisonoff A., Capra J. D. Structural studies on induced antibodies with defined idiotypic specificities--VIII. NH2-terminal amino acid sequence analysis of the heavy and light chain variable regions of monoclonal anti-para-azophenylarsonate antibodies from A/J mice differing with respect to a cross-reactive idiotype. Mol Immunol. 1979 Dec;16(12):1111–1118. doi: 10.1016/0161-5890(79)90045-2. [DOI] [PubMed] [Google Scholar]
- Gallarda J. L., Gleason K. S., Knight K. L. Organization of rabbit immunoglobulin genes. I. Structure and multiplicity of germ-line VH genes. J Immunol. 1985 Dec;135(6):4222–4228. [PubMed] [Google Scholar]
- Givol D., Zakut R., Effron K., Rechavi G., Ram D., Cohen J. B. Diversity of germ-line immunoglobulin VH genes. Nature. 1981 Jul 30;292(5822):426–430. doi: 10.1038/292426a0. [DOI] [PubMed] [Google Scholar]
- Gronowicz E. S., Doss C. A., Howard F. D., Morrison D. C., Strober S. An in vitro line of the B cell tumor BCL1 can be activated by LPS to secrete IgM1. J Immunol. 1980 Sep;125(3):976–980. [PubMed] [Google Scholar]
- Gulig P. A., Patrick C. C., Hermanstorfer L., McCracken G. H., Jr, Hansen E. J. Conservation of epitopes in the oligosaccharide portion of the lipooligosaccharide of Haemophilus influenzae type b. Infect Immun. 1987 Mar;55(3):513–520. doi: 10.1128/iai.55.3.513-520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman A. B., Rudikoff S. VH genes encoding the immune response to beta-(1,6)-galactan: somatic mutation in IgM molecules. EMBO J. 1984 Dec 1;3(12):3023–3030. doi: 10.1002/j.1460-2075.1984.tb02249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich G., Traunecker A., Tonegawa S. Somatic mutation creates diversity in the major group of mouse immunoglobulin kappa light chains. J Exp Med. 1984 Feb 1;159(2):417–435. doi: 10.1084/jem.159.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Campbell J. H., Elgin S. C. The organization, expression, and evolution of antibody genes and other multigene families. Annu Rev Genet. 1975;9:305–353. doi: 10.1146/annurev.ge.09.120175.001513. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Tyler B., Bernard O., Gough N., Gerondakis S., Adams J. M., Cory S. Organization of genes and spacers within the mouse immunoglobulin VH locus. J Mol Appl Genet. 1981;1(3):245–261. [PubMed] [Google Scholar]
- Kleinfield R., Hardy R. R., Tarlinton D., Dangl J., Herzenberg L. A., Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. 1986 Aug 28-Sep 3Nature. 322(6082):843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- Knapp M. R., Liu C. P., Newell N., Ward R. B., Tucker P. W., Strober S., Blattner F. Simultaneous expression of immunoglobulin mu and delta heavy chains by a cloned B-cell lymphoma: a single copy of the VH gene is shared by two adjacent CH genes. Proc Natl Acad Sci U S A. 1982 May;79(9):2996–3000. doi: 10.1073/pnas.79.9.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaira M., Kinashi T., Umemura I., Matsuda F., Noma T., Ono Y., Honjo T. Organization and evolution of variable region genes of the human immunoglobulin heavy chain. J Mol Biol. 1986 Aug 20;190(4):529–541. doi: 10.1016/0022-2836(86)90239-1. [DOI] [PubMed] [Google Scholar]
- Landolfi N. F., Capra J. D., Tucker P. W. Germ-line sequence of the DH segment employed in Ars-A antibodies: implications for the generation of junctional diversity. J Immunol. 1986 Jul 1;137(1):362–365. [PubMed] [Google Scholar]
- Livant D., Blatt C., Hood L. One heavy chain variable region gene segment subfamily in the BALB/c mouse contains 500-1000 or more members. Cell. 1986 Nov 7;47(3):461–470. doi: 10.1016/0092-8674(86)90603-3. [DOI] [PubMed] [Google Scholar]
- Lötscher E., Grzeschik K. H., Bauer H. G., Pohlenz H. D., Straubinger B., Zachau H. G. Dispersed human immunoglobulin kappa light-chain genes. Nature. 1986 Apr 3;320(6061):456–458. doi: 10.1038/320456a0. [DOI] [PubMed] [Google Scholar]
- Milner E. C., Capra J. D. A serologic marker for the Ars-C family of anti-arsonate antibodies. Ann Immunol (Paris) 1984 Jan-Feb;135C(1):11–16. doi: 10.1016/s0769-2625(84)80006-9. [DOI] [PubMed] [Google Scholar]
- Milner E. C., Capra J. D. VH families in the antibody response to p-azophenylarsonate: correlation between serology and amino acid sequence. J Immunol. 1982 Jul;129(1):193–199. [PubMed] [Google Scholar]
- Mäkelä O., Seppälä I. J., Pelkonen J., Kaartinen M., Cazenave P. A., Gefter M. L. Crossing-over frequency in the Igh region of the mouse genome. Ann Immunol (Paris) 1984 Jan-Feb;135C(1):169–173. doi: 10.1016/s0769-2625(84)80028-8. [DOI] [PubMed] [Google Scholar]
- Near R. I., Juszczak E. C., Huang S. Y., Sicari S. A., Margolies M. N., Gefter M. L. Expression and rearrangement of homologous immunoglobulin VH genes in two mouse strains. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2167–2171. doi: 10.1073/pnas.81.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech M., Zachau H. G. Immunoglobulin genes of different subgroups are interdigitated within the VK locus. Nucleic Acids Res. 1984 Dec 21;12(24):9229–9236. doi: 10.1093/nar/12.24.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Pohlenz H. D., Straubinger B., Thiebe R., Pech M., Zimmer F. J., Zachau H. G. The human V kappa locus. Characterization of extended immunoglobulin gene regions by cosmid cloning. J Mol Biol. 1987 Jan 20;193(2):241–253. doi: 10.1016/0022-2836(87)90216-6. [DOI] [PubMed] [Google Scholar]
- Rechavi G., Bienz B., Ram D., Ben-Neriah Y., Cohen J. B., Zakut R., Givol D. Organization and evolution of immunoglobulin VH gene subgroups. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4405–4409. doi: 10.1073/pnas.79.14.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. G., Jackson S., Alt F. W. VHDJH formation and DJH replacement during pre-B differentiation: non-random usage of gene segments. EMBO J. 1986 Sep;5(9):2131–2138. doi: 10.1002/j.1460-2075.1986.tb04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Hämmerling G. J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978 Jun;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- Riley S. C., Connors S. J., Klinman N. R., Ogata R. T. Preferential expression of variable region heavy chain gene segments by predominant 2,4-dinitrophenyl-specific BALB/c neonatal antibody clonotypes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2589–2593. doi: 10.1073/pnas.83.8.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Nau M. M., Norman B., Kwan S. P., Scharff M., Leder P. Immunoglobulin V/J recombination is accompanied by deletion of joining site and variable region segments. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6022–6026. doi: 10.1073/pnas.77.10.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Storb U. Mapping of immunoglobulin variable region genes: relationship to the 'deletion' model of immunoglobulin gene rearrangement. Nucleic Acids Res. 1981 Nov 11;9(21):5725–5735. doi: 10.1093/nar/9.21.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J., Rabbitts T. H., Estess P., Slaughter C., Tucker P. W., Capra J. D. Somatic mutation in genes for the variable portion of the immunoglobulin heavy chain. Science. 1982 Apr 16;216(4543):309–311. doi: 10.1126/science.6801765. [DOI] [PubMed] [Google Scholar]
- Slaughter C. A., Capra J. D. Amino acid sequence diversity within the family of antibodies bearing the major antiarsonate cross-reactive idiotype of the A strain mouse. J Exp Med. 1983 Nov 1;158(5):1615–1634. doi: 10.1084/jem.158.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Stephan D., Fischer Lindahl K. Gene organization and recombinational hotspots in the murine major histocompatibility complex. Cell. 1986 Mar 28;44(6):895–904. doi: 10.1016/0092-8674(86)90012-7. [DOI] [PubMed] [Google Scholar]
- Winter E., Radbruch A., Krawinkel U. Members of novel VH gene families are found in VDJ regions of polyclonally activated B-lymphocytes. EMBO J. 1985 Nov;4(11):2861–2867. doi: 10.1002/j.1460-2075.1985.tb04015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C., Tonegawa S. Diversity and joining segments of mouse immunoglobulin heavy chain genes are closely linked and in the same orientation: implications for the joining mechanism. Proc Natl Acad Sci U S A. 1983 May;80(10):3030–3034. doi: 10.1073/pnas.80.10.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. E., Paige C. J. VH gene family utilization in colonies derived from B and pre-B cells detected by the RNA colony blot assay. EMBO J. 1986 Dec 20;5(13):3475–3481. doi: 10.1002/j.1460-2075.1986.tb04672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]