Abstract
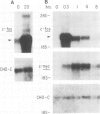
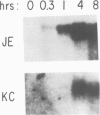
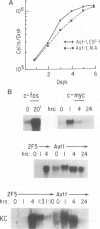
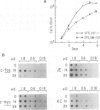
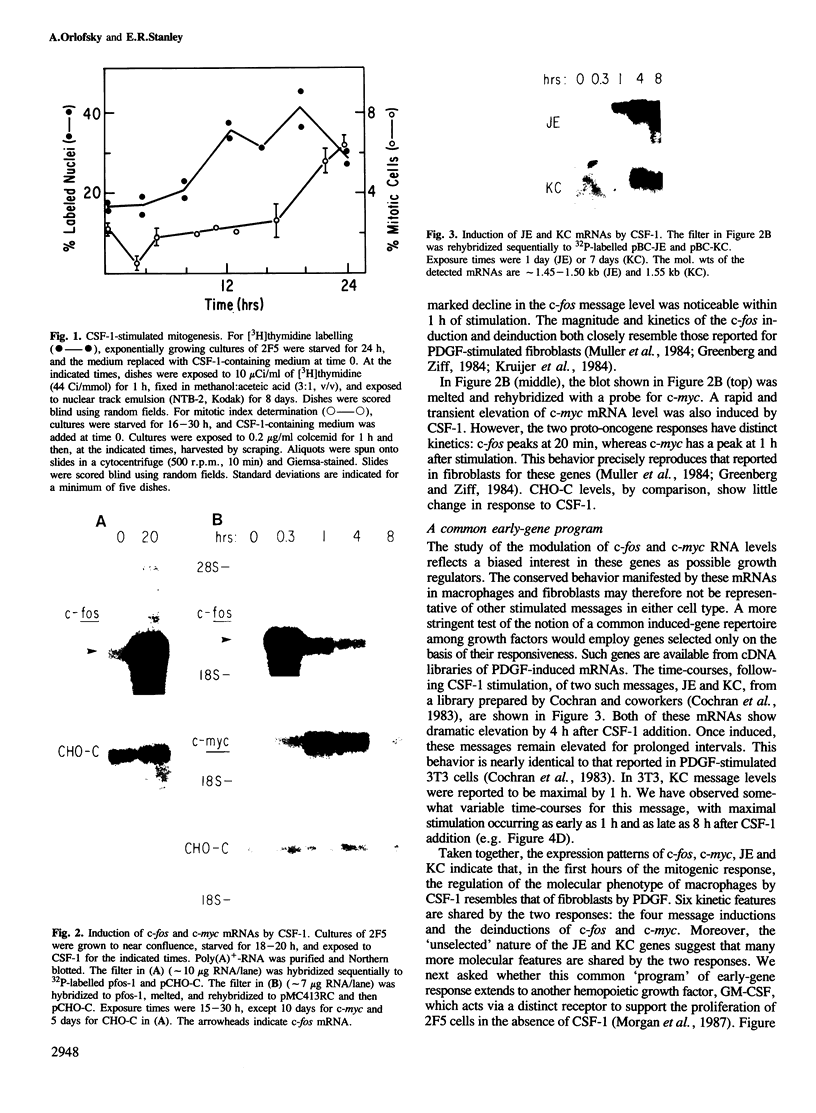
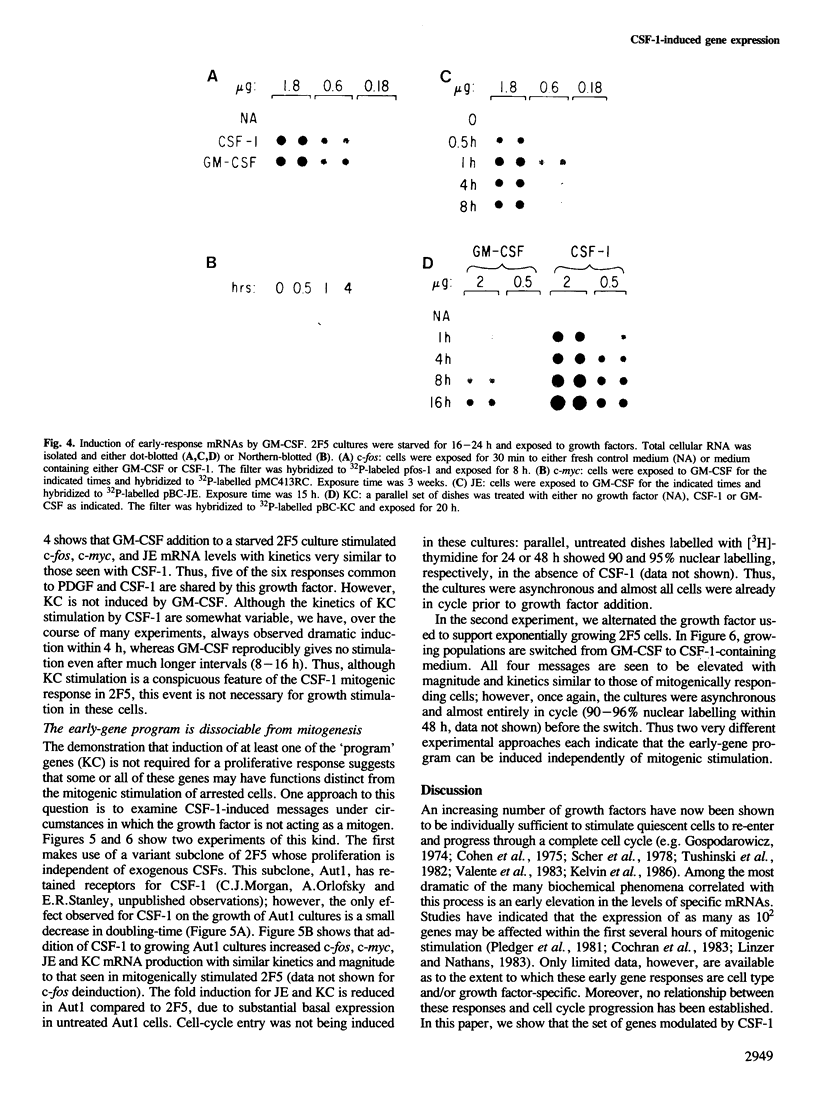
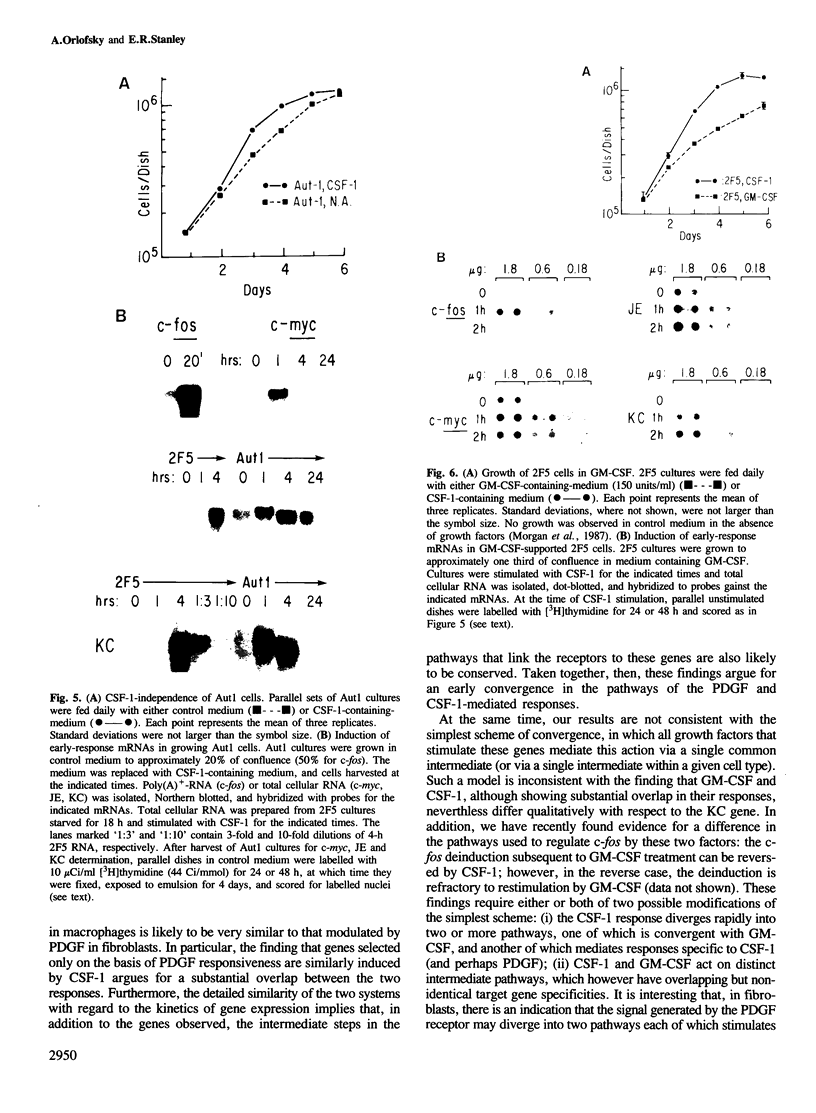
Early gene expression associated with the mitogenic response to colony stimulating factor-1 (CSF-1) has been examined in BAC1.2F5, a CSF-1-dependent murine macrophage cell line. Stimulation of arrested cells by CSF-1 resulted in acute, transient elevation in c-fos and subsequently in c-myc mRNA levels. Dramatic, sustained elevations were observed for JE and KC mRNAs, which are induced by platelet-derived growth factor (PDGF) in 3T3 cells. The kinetics of expression of all four messages were similar to those reported in PDGF-stimulated fibroblasts, implying a program of gene expression common to these two mitogens. Granulocyte-macrophage CSF (GM-CSF) can replace CSF-1 in stimulating the growth of 2F5 cells. It induced mRNAs for c-fos, c-myc and JE but not KC. Therefore KC expression, although correlated with mitogenesis, is not required for proliferation. The effects of CSF-1 were also examined in cells cycling continuously in its absence: 2F5 cells incubated in GM-CSF and an autonomous variant subclone of 2F5. In either case, the only detected growth effect of CSF-1 was a reduction in doubling-time. Nevertheless, all four of the mRNAs induced by CSF-1 in arrested cultures of 2F5 were strongly induced with the same kinetics in these cycling cells. Thus it would appear that the functions mediated by this early-gene program are not restricted to the mitogenic stimulation of arrested cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Expression of c-fos in NIH3T3 cells is very low but inducible throughout the cell cycle. EMBO J. 1986 Apr;5(4):695–700. doi: 10.1002/j.1460-2075.1986.tb04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Brooks R. F., Bennett D. C., Smith J. A. Mammalian cell cycles need two random transitions. Cell. 1980 Feb;19(2):493–504. doi: 10.1016/0092-8674(80)90524-3. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., Lembach K. J. Interaction of epidermal growth factor (EGF) with cultured fibroblasts. Adv Metab Disord. 1975;8:265–284. doi: 10.1016/b978-0-12-027308-9.50024-x. [DOI] [PubMed] [Google Scholar]
- Colletta G., Cirafici A. M., Vecchio G. Induction of the c-fos oncogene by thyrotropic hormone in rat thyroid cells in culture. Science. 1986 Jul 25;233(4762):458–460. doi: 10.1126/science.3726540. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Martinotti S., Gallo R. C., Erikson J., Croce C. M. Translocation and rearrangements of the c-myc oncogene locus in human undifferentiated B-cell lymphomas. Science. 1983 Feb 25;219(4587):963–967. doi: 10.1126/science.6401867. [DOI] [PubMed] [Google Scholar]
- Das S. K., Stanley E. R. Structure-function studies of a colony stimulating factor (CSF-1). J Biol Chem. 1982 Nov 25;257(22):13679–13684. [PubMed] [Google Scholar]
- Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974 May 10;249(453):123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Stanley E. R., Burgess A. W., Shadduck R. K. Stimulation of macrophage plasminogen activator activity by colony-stimulating factors. J Cell Physiol. 1980 Jun;103(3):435–445. doi: 10.1002/jcp.1041030309. [DOI] [PubMed] [Google Scholar]
- Han J. H., Rall L., Rutter W. J. Selective expression of rat pancreatic genes during embryonic development. Proc Natl Acad Sci U S A. 1986 Jan;83(1):110–114. doi: 10.1073/pnas.83.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann S. R., Thompson C. B., Eisenman R. N. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. 1985 Mar 28-Apr 3Nature. 314(6009):366–369. doi: 10.1038/314366a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Johnsson A., Wennergren S., Wernstedt C., Betsholtz C., Westermark B. A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature. 1986 Feb 6;319(6053):511–514. doi: 10.1038/319511a0. [DOI] [PubMed] [Google Scholar]
- Herman B., Harrington M. A., Olashaw N. E., Pledger W. J. Identification of the cellular mechanisms responsible for platelet-derived growth factor induced alterations in cytoplasmic vinculin distribution. J Cell Physiol. 1986 Jan;126(1):115–125. doi: 10.1002/jcp.1041260116. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek L., Hyland J. K., Watt R., Rosenberg M., Baserga R. Microinjected c-myc as a competence factor. Science. 1985 Jun 14;228(4705):1313–1315. doi: 10.1126/science.4001943. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kelvin D. J., Chance S., Shreeve M., Axelrad A. A., Connolly J. A., McLeod D. Interleukin 3 and cell cycle progression. J Cell Physiol. 1986 Jun;127(3):403–409. doi: 10.1002/jcp.1041270308. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Todd J. A., Hesketh T. R., Metcalfe J. C. c-fos and c-myc gene activation, ionic signals, and DNA synthesis in thymocytes. J Biol Chem. 1986 Jun 25;261(18):8158–8162. [PubMed] [Google Scholar]
- Morgan C., Pollard J. W., Stanley E. R. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC1.2F5. J Cell Physiol. 1987 Mar;130(3):420–427. doi: 10.1002/jcp.1041300316. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Hart C. A., Locatell K. L., Scher C. D. Platelet-derived growth factor-modulated proteins: constitutive synthesis by a transformed cell line. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4358–4362. doi: 10.1073/pnas.78.7.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Pledger W. J., Martin P., Antoniades H., Stiles C. D. Transforming viruses directly reduce the cellular growth requirement for a platelet derived growth factor. J Cell Physiol. 1978 Dec;97(3 Pt 1):371–380. doi: 10.1002/jcp.1040970312. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Stone M. E., Stiles C. D. Platelet-derived growth factor prevents G0 growth arrest. Nature. 1979 Oct 4;281(5730):390–392. doi: 10.1038/281390a0. [DOI] [PubMed] [Google Scholar]
- Schwarzbaum S., Halpern R., Diamond B. The generation of macrophage-like cell lines by transfection with SV40 origin defective DNA. J Immunol. 1984 Mar;132(3):1158–1162. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Soreq H., Harpold M., Wilson M., Darnell J. E., Jr Rate of synthesis and concentration of specific mRNA sequences in cultured Chinese hamster ovary cells compared to liver cells. Biochem Biophys Res Commun. 1980 Jan 29;92(2):485–491. doi: 10.1016/0006-291x(80)90359-9. [DOI] [PubMed] [Google Scholar]
- Sparrow L. G., Metcalf D., Hunkapiller M. W., Hood L. E., Burgess A. W. Purification and partial amino acid sequence of asialo murine granulocyte-macrophage colony stimulating factor. Proc Natl Acad Sci U S A. 1985 Jan;82(2):292–296. doi: 10.1073/pnas.82.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Stanley E. R. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Stroobant P., Waterfield M. D. Purification and properties of porcine platelet-derived growth factor. EMBO J. 1984 Dec 1;3(12):2963–2967. doi: 10.1002/j.1460-2075.1984.tb02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studzinski G. P., Brelvi Z. S., Feldman S. C., Watt R. A. Participation of c-myc protein in DNA synthesis of human cells. Science. 1986 Oct 24;234(4775):467–470. doi: 10.1126/science.3532322. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982 Jan;28(1):71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Stanley E. R. The regulation of mononuclear phagocyte entry into S phase by the colony stimulating factor CSF-1. J Cell Physiol. 1985 Feb;122(2):221–228. doi: 10.1002/jcp.1041220210. [DOI] [PubMed] [Google Scholar]
- Valente W. A., Vitti P., Kohn L. D., Brandi M. L., Rotella C. M., Toccafondi R., Tramontano D., Aloj S. M., Ambesi-Impiombato F. S. The relationship of growth and adenylate cyclase activity in cultured thyroid cells: separate bioeffects of thyrotropin. Endocrinology. 1983 Jan;112(1):71–79. doi: 10.1210/endo-112-1-71. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]