Highlights
-
•
Lung resection for lung cancer is associated with marked reductions in exercise capacity.
-
•
Exercise training increased exercise capacity in people with non-small cell lung cancer.
-
•
Exercise training did not improve other outcomes.
Keywords: Lung neoplasms, Carcinoma, Non-small cell, Exercise training, Rehabilitation
Abstract
Objective
In people following curative intent treatment for non-small cell lung cancer, to investigate the effects of supervised exercise training on exercise capacity, physical activity and sedentary behavior, peripheral muscle force, health-related quality of life, fatigue, feelings of anxiety and depression, and lung function.
Method
This pilot randomized controlled trial included participants 6–10 weeks after lobectomy for non-small cell lung cancer or, for those who required adjuvant chemotherapy, 4–8 weeks after their last cycle. Participants were randomized to either 8 weeks of supervised exercise training (exercise group) or 8 weeks of usual care (control group). Prior to and following the intervention period, both groups completed measurements of exercise capacity, physical activity and sedentary behavior, quadriceps and handgrip force, HRQoL, fatigue, feelings of anxiety and depression, and lung function. Intention-to-treat analysis was undertaken.
Results
Seventeen participants (mean age 67, SD = 9 years; 12 females) were included. Nine and eight participants were randomized to the exercise and control groups, respectively. Four participants (44%) adhered to exercise training. Compared with any change seen in the control group, those in the exercise group demonstrated greater gains in the peak rate of oxygen consumption (mean difference, 95% confidence interval for between-group difference: 0.19 [0.04–0.33] L min−1) and 6-minute walk distance (52 [12–93] m). No other between-group differences were demonstrated.
Conclusions
In people following curative intent treatment for non-small cell lung cancer, 8 weeks of supervised exercise training improved exercise capacity, measured by both laboratory- and field-based exercise tests. These results suggest that this clinical population may benefit from attending exercise training programs.
Introduction
Lung cancer is the leading cause of death for malignancy worldwide.1 Data from the Australian Institute of Health and Welfare revealed that the 5-year survival of people with lung cancer is 15%.2 In people diagnosed with lung cancer, 85% of cases are non-small cell lung cancer (NSCLC).3 Importantly, for people diagnosed with early stage NSCLC, surgical resection of the tumor, with or without adjuvant chemotherapy, is considered to be a curative intent treatment,4 and the 5-year survival of people following lung resection is up to 80%.4
Lung resection is associated with marked reductions in exercise capacity (i.e., peak rate of oxygen consumption [VO2peak])5, 6, 7 and health-related quality of life (HRQoL).8, 9 Although there is strong evidence that exercise training improves exercise capacity and HRQoL in people with chronic respiratory conditions such as chronic obstructive pulmonary disease (COPD)10 and interstitial lung disease,11 there are few studies investigating the role of exercise training for people who have recently completed curative intent treatment for NSCLC. Preliminary data suggests that exercise training may play an important role for individuals with a variety of cancer diagnoses.12, 13 A recent Cochrane systematic review, which included three randomized controlled trials (RCT) of exercise training in people following lung resection for NSCLC, demonstrated an increase in six-minute walk distance (6MWD).14 However, this finding needs to be interpreted with caution due to methodological shortcomings of the included studies, such as lack of computer-generated randomization sequence and blinding of outcome assessors, per-protocol analysis, and selective reporting of results. Further, in the three studies included in the review, outcome measures were limited to exercise capacity, muscle strength, and HRQoL.
Therefore, the aim of this pilot study was to investigate the effects of supervised exercise training on a wide range of outcomes such as exercise capacity, physical activity and sedentary behavior, peripheral muscle force, HRQoL, fatigue, feelings of anxiety and depression, and lung function in people following curative intent treatment for NSCLC. We sought to use a design that would overcome some of the methodological shortcomings evident in earlier work by concealing the computer-generated randomization sequence, blinding the outcome assessor, and analyzing the data according to the intention-to-treat (ITT) principle.
Method
Study design and participants
This study was a pilot single-blinded RCT approved by the Ethics Committees of Sir Charles Gairdner Hospital (SCGH) and Royal Perth Hospital (RPH), Perth, WA, Australia (approval numbers 2011/105 and RA-11/033) and Curtin University, Perth, WA, Australia (approval number HR178/2011). The trial was prospectively registered (15/08/2011) with the Australian New Zealand Clinical Trials Registry (ACTRN12611000864921).
Data collection was performed between February 2012 and April 2014. Measurements were collected in people 6–10 weeks after lobectomy for NSCLC (stages I–IIIA) or, for those who required post-operative chemotherapy, 4–8 weeks after their last chemotherapy cycle. Exclusion criteria comprised: presence of any co-morbid condition that could compromise safety during assessments; severe neuromusculoskeletal limitations; participation in a program of supervised exercise training in the last 3 months; and inability to understand spoken or written English. Participants were recruited from outpatient clinics and referrals to the pulmonary rehabilitation programs at two hospitals and a private thoracic surgery clinic.
Protocol and measurements
After obtaining written informed consent, baseline assessments were undertaken over 2–3 days, with a minimum of 24 h between each assessment day. Participants were then randomized to an exercise group (EG) or a control group (CG). The randomization sequence was generated and managed by an independent researcher using a computer and concealed using sequentially numbered opaque envelopes. The sequence was stratified according to the hospital from which the participant was recruited and for the use (or not) of adjuvant chemotherapy.
Participants were reassessed on completion of the 8-week intervention period. The primary outcome was exercise capacity. Secondary outcomes comprised physical activity and sedentary behavior, peripheral muscle force, HRQoL, fatigue, feelings of anxiety and depression, and lung function. The primary investigator, who was responsible for the baseline and post-intervention period assessments, was not aware of whether a participant had been allocated to the EG or the CG. For both baseline and post-intervention period assessments, the first and second assessment days took place at the hospital at which the participants had received their treatment.
Measurements performed on the first assessment day were:
-
(i)
6MWD (45-m straight course within an enclosed corridor. Two tests, separated by a 30-min rest period, were conducted and the best 6MWD was recorded as the test result).15, 16
-
(ii)
HRQoL (Medical Outcomes Study Short-Form 36 general health survey (SF-36),17 Functional Assessment of Cancer Therapy – Lung scale (FACT-L),18 and European Organisation for Research and Treatment of Cancer, Quality of Life Questionnaire Core-30 [EORTC QLQ-C30]19).
-
(iii)
Feelings of anxiety and depression (Hospital Anxiety and Depression Scale [HADS]20).
-
(iv)
Fatigue (Functional Assessment of Chronic Illness Therapy – Fatigue subscale [FACIT-Fatigue]21).
-
(v)
Isometric handgrip force (measured using a hydraulic hand dynamometer [Jamar; JA Preston Corporation; USA]). Peak handgrip force was assessed bilaterally, with the elbow at 90° flexion and the forearm and wrist in a neutral position.22
Participants were also given two physical activity monitors (the SenseWear armband23, 24 and the Stepwatch activity monitor25) to be worn over 7 consecutive days. A minimum of 4 full days of data (defined as ≥10 h/day of monitoring), including one weekend day, were required for participants’ data to be included in analyses. Data on energy expenditure (i.e., metabolic equivalent units [MET]), provided by the SenseWear armband, and daily step count, provided by the Stepwatch, were averaged for analysis. Using measures of MET derived from the SenseWear armband, proportion of time spent in three domains were calculated: (i) sedentary behavior (≤1.5 MET); (ii) light intensity physical activity (>1.5 and ≤3 MET); and (iii) moderate-to-vigorous intensity physical activity (>3 MET).26
Following the completion of 7 days of activity monitoring, participants returned for the second assessment day, during which measures were made of spirometry, lung volumes, and gas transfer.27, 28, 29, 30 The Medgraphics Elite Series DX plethysmograph (Medical Graphics Corporation, USA) was used. Thereafter, a symptom-limited ramp cycle-ergometry cardiopulmonary exercise test (CPET) was undertaken on an electronically braked bicycle ergometer (Corival; Lode, The Netherlands) in accordance with published guidelines.31 Breath-by-breath measurements were collected (Ultima™ CardiO2®; MGC-Diagnostics, USA). Blood pressure was measured every 2 min by automated sphygmomanometry. Twelve-lead electrocardiography was used and arterial oxygen saturation measured via pulse oximetry (SpO2) was continuously monitored (Radical; Masimo Corporation, USA). The modified BORG scale (0–10) was used to quantify dyspnea and leg fatigue prior to starting the test, each minute during the test, and on test completion. Measures were collected of peak rate of oxygen consumption (VO2peak), VO2 at the anaerobic threshold (AT) maximum work rate (Wmax), and oxygen pulse (O2 pulse), which was calculated by dividing the VO2peak by the maximal heart rate.31 The Wmax and measures of VO2peak were expressed in absolute values and as a percentage of the predicted value in a healthy population.32
The third assessment day comprised the measurement of isometric quadriceps muscle torque.33 It was performed in the upright seated position using the HUMAC NORM isokinetic dynamometer (CSMi; Stoughton, USA). The dominant leg was chosen and participants were asked to perform five maximum contractions of the quadriceps at 60° knee flexion. Each contraction was separated by 60 s. The contraction that generated the highest torque, and was within 5% of another effort, was recorded as the test result. Measures were expressed in absolute values and as a percentage of the predicted value in a healthy population.33 The measurement of isometric quadriceps muscle torque took place at the University. As the University is approximately 15 km from either of the hospitals, participants were given the option to decline this assessment.
Exercise group
Participants in the EG underwent an 8-week exercise training program aimed at improving aerobic capacity and muscle strength. This program was embedded within the exercise training programs at SCGH and RPH. It comprised individual, supervised training three times per week delivered by senior physical therapists. Each session was 60 min in duration. In the event that a participant could only attend two supervised sessions per week, they were provided with a cycle ergometer (OBK600A; Orbit fitness equipment, Perth, WA, Australia) to use at home for one training session per week. Each class comprised aerobic (walking/cycling) and resistance training (upper/lower limbs). Adherence to exercise training was defined as a completion rate of ≥60% of training sessions (i.e., ≥15 training sessions) and reported by the senior physical therapists to the investigators.
Participants walked in a 100-m long corridor or on a treadmill for 20 min. For corridor walking, the initial average speed was set at 80% of the average 6MWT speed.34 For instance, for someone with a baseline 6MWD = 450 m, the walking goal was calculated as follows:
Therefore, during the 20 min of walking, this person would be instructed to walk 1200 m (i.e., 6 laps).
For treadmill walking, the initial average speed was set at 70% of the average 6MWT speed.34 Average walking speed was increased if the participant was able to walk for 20 min continuously providing symptoms and SpO2 were within acceptable limits (≥88%). Cycling consisted of 10 min of endurance training (initial work rate was set at 60% of the Wmax achieved during the CPET) and two periods of 2 min of power training (initial work rate was set at 80% of the Wmax achieved during the CPET performed at the baseline assessment).
The resistance training comprised step-ups (undertaken within parallel bars in two sets of 10 repetitions) and exercises with hand weights for the biceps brachii muscle (elbow flexion) and deltoid muscle (short-lever shoulder abduction). Upper limb training was undertaken in three sets of 10 repetitions (initial weights: 1.5 kg for women and 2 kg for men).
Control group
Participants in the CG were instructed to continue to perform their usual activities during the period of the study. They received weekly phone calls from a research assistant, which consisted of general conversation as well as standardized questions about their health and well-being. These phone calls allowed the investigators to maintain contact with those in the CG and optimize their retention in the study and also served to minimize bias resulting from differences in attention provided by the investigators to the participants during the intervention period.
Statistical analyses
Statistical analyses were performed using SPSS® (Statistical Package for Social Sciences, version 22.0). As this is a pilot RCT, sample size was determined by the number of participants recruited during the period allowed for commencement and completion of the study (i.e., from February 2012 to April 2014). Analyses were undertaken according to the intention-to-treat principle. The distribution of data was analyzed via frequency histograms and the Shapiro–Wilk test. For normally distributed data, both within- and between-group differences were assessed using two-way repeated measures ANOVA. Between-group differences are reported as the mean difference and 95% confidence interval (CI) (F values are provided in the tables). Regarding non-normally distributed data, within-group differences were assessed using a Wilcoxon test whereas between-group differences were assessed using a Mann–Whitney test. For all analyses, a p value <0.05 was considered significant. Data are expressed as either mean ± standard deviation or median [interquartile range].
Results
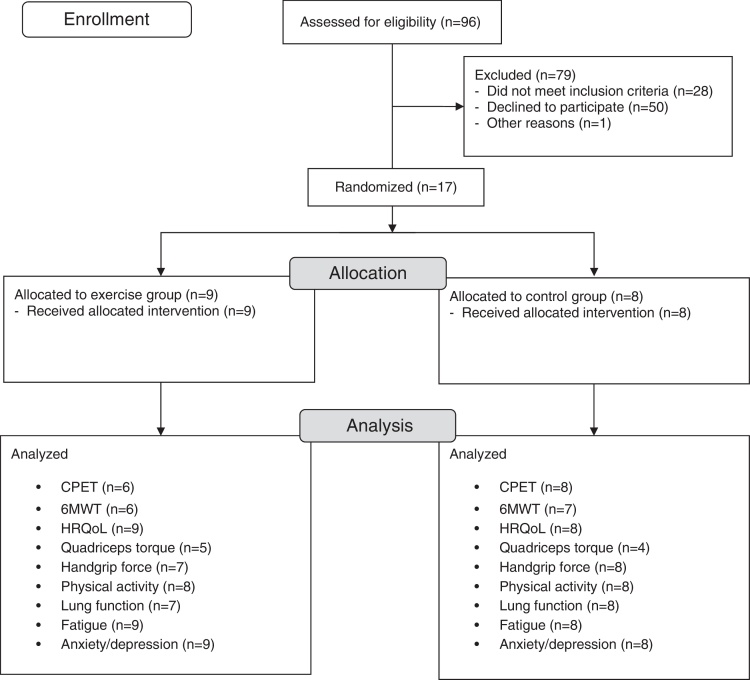
The study flow diagram is presented in Fig. 1. Seventeen participants (12 females) were randomized to the EG (n = 9) or the CG (n = 8). Baseline characteristics of the participants are summarized in Table 1.
Figure 1.
Study flow diagram.
Table 1.
Characteristics at baseline.
| Variables Mean (SD) |
Total sample (n = 17) | Exercise group (n = 9) | Control group (n = 8) |
|---|---|---|---|
| Age (year) | 67 (9) | 66 (10) | 68 (9) |
| Height (cm) | 165 (12) | 164 (14) | 166 (11) |
| Weight (kg) | 72 (21) | 67 (14) | 77 (27) |
| BMI (kg m−2) | 26 (6) | 25 (5) | 27 (6) |
| Smoking (pack years) | 35 (15) | 49 (27) | 35 (24) |
| FEV1 (L) | 1.65 (0.49) | 1.53 (0.47) | 1.78 (0.50) |
| FEV1 (%pred) | 66 (17) | 61 (17) | 71 (15) |
| FVC (L) | 2.68 (0.69) | 2.51 (0.76) | 2.87 (0.58) |
| FVC (%pred) | 80 (12) | 74 (11) | 86 (10) |
| FEV1/FVC (%) | 62 (12) | 62 (14) | 62 (11) |
| TLC (L) | 4.73 (1.19) | 4.58 (1.56) | 4.91 (0.64) |
| TLC (%pred) | 86 (14) | 82 (16) | 90 (12) |
| FRC (L) | 2.94 (0.78) | 2.87 (1.00) | 3.02 (0.47) |
| FRC (%pred) | 97 (20) | 94 (20) | 100 (21) |
| DLCO (mL min−1 mmHg−1) | 13.7 (3.6) | 12.8 (3.3) | 14.7 (3.8) |
| DLCO (%pred) | 53 (12) | 49 (11) | 57 (13) |
| MVV (L min−1) | 62 (21) | 60 (25) | 64 (28) |
| MVV (%pred) | 63 (16) | 59 (15) | 67 (18) |
| n | % | n | % | n | % | |
|---|---|---|---|---|---|---|
| Gender, male/female | 5/12 | 29/71 | 3/6 | 30/70 | 2/6 | 25/75 |
| COPD | 9 | 53 | 5 | 56 | 4 | 50 |
| Type of NSCLC | ||||||
| Adenocarcinoma | 10 | 59 | 5 | 56 | 5 | 63 |
| Squamous cell carcinoma | 6 | 35 | 4 | 44 | 2 | 25 |
| Large cell carcinoma | 1 | 6 | 0 | 0 | 1 | 12 |
| NSCLC stage | ||||||
| I | 14 | 82 | 7 | 78 | 7 | 88 |
| II | 2 | 12 | 2 | 22 | 0 | 0 |
| IIIA | 1 | 6 | 0 | 0 | 1 | 12 |
| Types of surgery | ||||||
| Open | 8 | 47 | 4 | 44 | 4 | 50 |
| VATS | 9 | 53 | 5 | 56 | 4 | 50 |
| Adjuvant chemotherapy | 2 | 12 | 1 | 11 | 1 | 12 |
BMI, body-mass index; COPD, chronic obstructive pulmonary disease; DLCO, single breath diffusing capacity for carbon monoxide; FEV1, forced expiratory volume in one second; FRC, functional residual capacity; FVC, forced vital capacity; MVV, maximum voluntary ventilation; NSCLC, non-small cell lung cancer; SD, standard deviation; TLC, total lung capacity; VATS, video-assisted thoracoscopic surgery.
Of the nine participants randomized to the EG, four (44%) adhered to exercise training by completing 15 or more training sessions (i.e., ≥60%). The mean number of sessions that these four participants completed was 17 ± 3. Of the remaining five participants, one completed 10 sessions and stopped training as they contracted pertussis. One participant completed four sessions and another completed six sessions. Both stopped training as they felt unwell. They completed some of the post-intervention assessments and were later diagnosed with a primary cancer other than lung cancer. One participant completed four sessions and decided to cease training stating they were too busy. The final participant declined participation in exercise training as they were unwilling to travel to the hospital. In order to facilitate ITT analysis, all participants were encouraged to attend the post-intervention assessments, regardless of their adherence to the exercise training.
Regarding the phone calls scheduled for those in the CG, three participants were available for all eight calls, one participant was available for six calls, two participants were available for five calls, and two participants were available for four calls. The reasons for missing phone calls were that they were either away on vacation or busy with family-related responsibilities. In order to facilitate ITT analysis, all participants were encouraged to attend the post-intervention assessments, regardless of their adherence with the phone calls.
Primary outcome – exercise capacity
Baseline and post-intervention period measures are presented in Table 2.
Table 2.
Baseline and post-intervention measures of exercise capacity.
| Variable | Exercise group |
Control group |
F; p value | MD [95% CI] | F; p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Baseline | Post-intervention | MD [95% CI] | Baseline | Post-intervention | MD [95% CI] | Within-group | Between-group | Between-group |
| Exercise capacity (CPET) | n = 6 | n = 6 | n = 8 | n = 8 | |||||
| VO2peak (L min−1) | 0.96 ± 0.22 | 1.09 ± 0.28 | 0.14 [−0.01 to 0.28] | 1.08 ± 0.40 | 1.03 ± 0.30 | −0.05 [−0.15 to 0.05] | 1.5; 0.24 | 0.2 [0.03 to 0.33] | 7.2; 0.02 |
| VO2peak (mL kg−1 min−1) | 15.7 ± 3.1 | 17.0 ± 2.5 | 1.3 [−0.1 to 1.8] | 13.9 ± 2.6 | 13.3 ± 2.1 | −0.5 [−2.1 to 1.0] | 0.7; 0.41 | 1.8 [−0.1 to 3.7] | 4.4; 0.06 |
| VO2peak (%pred) | 62 ± 18 | 70 ± 21 | 8 [2 to 15] | 64 ± 17 | 62 ± 13 | −2 [−9 to 5] | 2.8; 0.12 | 10 [2 to 19] | 6.7; 0.02 |
| Wmax (W) | 72 ± 28 | 77 ± 26 | 5 [−6 to 17] | 77 ± 32 | 68 ± 21 | −9 [−24 to 7] | 0.1; 0.73 | 14 [−5 to 33] | 2.6; 0.13 |
| Wmax (%pred) | 73 ± 25 | 78 ± 30 | 6 [−4 to 15] | 69 ± 15 | 64 ± 14 | −5 [−18 to 8] | 0.0; 0.90 | 10 [−6 to 26] | 2.0; 0.19 |
| BORGd CPET | 6.8 ± 2.0 | 6.8 ± 1.7 | 0.0 [−3.1 to 3.1] | 5.8 ± 3.0 | 6.1 ± 1.7 | 0.4 [−1.0 to 1.7] | 0.1; 0.77 | −0.4 [−3.0 to 2.3] | 0.1; 0.77 |
| BORGf CPET | 5.0 ± 2.5 | 6.8 ± 1.9 | 1.8 [−1.6 to 5.3] | 7.4 ± 2.1 | 6.9 ± 2.0 | −0.5 [−3.1 to 2.1] | 0.6; 0.46 | 2.3 [−1.5 to 6.1] | 1.8; 0.21 |
| Nadir SpO2 (%) | 94 ± 2 | 94 ± 4 | 1 [−4 to 5] | 94 ± 6 | 95 ± 3 | 1 [−6 to 7] | 0.2; 0.64 | −0 [−8 to 7] | 0.0; 0.99 |
| HRmax (bpm) | 130 ± 20 | 124 ± 19 | −6 [−13 to 2] | 127 ± 18 | 128 ± 18 | 1 [−15 to 17] | 0.3; 0.61 | −7 [−24 to 11] | 0.6; 0.44 |
| BR (%) | 27 ± 12 | 28 ± 13 | 1 [−14 to 16] | 32 ± 14 | 43 ± 10 | 11 [−0 to 23] | 2.7; 0.12 | −10 [−27 to 6] | 1.9; 0.19 |
| O2 pulse (mL beat−1) | 7 ± 2 | 9 ± 2 | 2 [0 to 2] | 8 ± 3 | 8 ± 3 | 0 [−1 to 1] | 3.4; 0.09 | 2 [1 to 3] | 8.7; 0.01 |
| AT (%VO2peak) | 60 ± 9 | 71 ± 8 | 11 [7 to 15] | 63 ± 10 | 63 ± 10 | 0 [−8 to 9] | 6.4; 0.03 | 11 [1 to 21] | 5.9; 0.03 |
| VEmax/MVV (%) | 73 ± 12 | 72 ± 13 | −1 [−16 to 14] | 68 ± 14 | 58 ± 12 | −9 [−20 to 1] | 2.3; 0.15 | 0.1 [−0.1 to 0.3] | 1.4; 0.26 |
| Exercise capacity (6MWT) | n = 6 | n = 6 | n = 7 | n = 7 | |||||
| 6MWD (m) | 540 ± 71 | 585 ± 77 | 45 [6 to 83] | 477 ± 78 | 469 ± 105 | −8 [−36 to 20] | 3.9; 0.07 | 52 [12 to 93] | 8.1; 0.02 |
| 6MWD (%pred) | 88 ± 9 | 96 ± 5 | 8 [3 to 14] | 77 ± 11 | 76 ± 16 | −1 [−6 to 4] | 5.4; 0.04 | 9 [3 to 16] | 9.1; 0.01 |
| BORGd 6MWT | 3.3 ± 2.0 | 2.8 ± 1.2 | −0.5 [−2.5 to 1.5] | 3.4 ± 1.5 | 3.7 ± 2.4 | 0.3 [−1.2 to 1.9] | 0.2; 0.89 | −0.9 [−3.0 to 1.3] | 0.8; 0.40 |
| BORGf 6MWT | 1.5 ± 1.9 | 2.3 ± 1.8 | 0.8 [−1.3 to 2.8] | 3.4 ± 1.9 | 4.1 ± 1.6 | 0.7 [−1.2 to 2.6] | 1.6; 0.22 | 0.0 [−2.4 to 2.5] | 0.0; 0.97 |
| Nadir SpO2 (%) | 92 ± 4 | 92 ± 3 | 0 [−2 to 1] | 92 ± 2 | 93 ± 1 | 1 [−0 to 2] | 0.6; 0.46 | −1 [−2 to 1] | 1.5; 0.25 |
| Peak HR (bpm) | 125 ± 16 | 126 ± 15 | 1 [−13 to 15] | 122 ± 11 | 121 ± 18 | 0 [−9 to 8] | 0.0; 0.95 | 2 [−12 to 15] | 0.1; 0.80 |
6MWD, six-minute walk distance; 6MWT, six-minute walk test; AT, anaerobic threshold as a percentage of the VO2peak; BORGd, dyspnea; BORGf, fatigue; BR, breathing reserve; CI, confidence interval; CPET, cardiopulmonary exercise test; HR, heart rate; HRmax, maximal heart rate; MD, mean difference; O2 pulse, oxygen pulse; SD, standard deviation; SpO2, arterial oxygen saturation measured via pulse oximetry; VEmax/MVV, maximum minute ventilation, maximum voluntary ventilation ratio; VO2peak, peak rate of oxygen consumption; Wmax, maximum work rate.
Cardiopulmonary exercise test
At baseline, the VO2peak of the EG and the CG was 62 ± 18 and 64 ± 17%pred, respectively (p = 0.74 for between-group difference). Compared with any change observed in the CG, greater gains were demonstrated in the EG in VO2peak (mean difference [95% CI] 0.19 [0.04–0.33] L min−1), O2 pulse (2 [0–3] mL beat−1), and AT (11 [1–21]% of VO2peak).
Six-minute walk test
At baseline, the 6MWD of the EG and the CG was 88 ± 9 and 77 ± 11%pred, respectively (p = 0.09 for between-group difference). Compared with any change observed in the CG, greater gains were demonstrated in the EG in 6MWD (mean difference [95% CI of difference] 52 [12–93] m).
Secondary outcomes
On completion of the intervention period, no between-group differences were observed in light intensity physical activity, moderate-to-vigorous intensity physical activity, sedentary behavior and daily steps (Table 3). At baseline, isometric quadriceps torque and isometric handgrip force in both groups were >90%pred (Table 3). On completion of the intervention period, no between-group differences were observed in these outcome measures (Table 3).
Table 3.
Baseline and post-intervention measures of physical activity, sedentary behavior and peripheral muscle force.
| Variable | Exercise group (n = 8) |
Control group (n = 8) |
F; p value | MD [95% CI] | F; p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Physical activity and sedentary behavior Mean ± SD |
Baseline | Post-intervention | MD [95% CI] | Baseline | Post-intervention | MD [95% CI] | Within-group | Between-group | Between-group |
| Number of days wearing monitor | 6.6 ± 0.5 | 6.5 ± 0.5 | −0.0 [−1.0 to 1.0] | 6.3 ± 1.1 | 6.5 ± 1.0 | −0.2 [−1.1 to 1.2] | 0.9; 0.35 | 0.2 [−0.8 to 1.3] | 0.2; 0.63 |
| Monitor wear time (h/day) | 13.8 ± 1.2 | 13.0 ± 1.1 | −0.8 [−2.0 to 0.4] | 13.3 ± 1.4 | 13.2 ± 1.5 | −0.4 [−1.3 to 0.5] | 2.7; 0.12 | −0.8 [−1.8 to 0.2] | 1.8; 0.20 |
| Stepwatch activity monitor | |||||||||
| Daily steps | 9357 ± 4195 | 9816 ± 4382 | 460 [−153 to 1073] | 6282 ± 2331 | 8020 ± 3864 | 1738 [−455 to 3931] | 5.2; 0.04 | −1278 [−3344 to 786] | 1.8; 0.21 |
| SenseWear armband | |||||||||
| Sedentary behavior (%) | 62 ± 16 | 59 ± 16 | −3 [−7 to 1] | 74 ± 12 | 67 ± 14 | −7 [−13 to 1] | 7.3; 0.02 | 4 [−4 to 11] | 1.1; 0.31 |
| Light intensity PA (%) | 21 ± 11 | 25 ± 11 | 4 [−3 to 11] | 20 ± 7 | 26 ± 11 | 6 [−1 to 12] | 6.9; 0.02 | 4 [−10 to 6] | 0.3; 0.58 |
| Moderate-to-vigorous intensity PA (%) | 17 ± 13 | 16 ± 8 | −1 [−6 to 4] | 6 ± 6 | 7 ± 4 | 1 [−3 to 5] | 0.3; 0.86 | −2 [−7 to 5] | 0.3; 0.60 |
| Variable | Exercise group |
Control group |
p value | ||
|---|---|---|---|---|---|
| Peripheral muscle force Median [IQR] |
Baseline | Post-intervention | Baseline | Post-intervention | Between-group |
| Isometric quadriceps force | n = 5 | n = 5 | n = 4 | n = 4 | |
| Torque (Nm) | 101 [70–132] | 112 [82–142] | 151 [91–238] | 153 [101–210] | 0.536 |
| Torque (%pred) | 103 [87–160] | 114 [100–171] | 99 [93–104] | 97 [82–114] | 0.190 |
| Isometric handgrip force | n = 7 | n = 7 | n = 8 | n = 8 | |
| Torque (Nm) | 32 [18–34] | 33 [20–35] | 26 [20–30] | 26 [19–31] | 0.072 |
| Torque (%pred) | 91 [78–115] | 93 [78–127] | 97 [83–111] | 100 [83–107] | 0.281 |
%-, percentage of waking hours; CI, confidence interval; IQR, interquartile range; MD, mean difference; PA, physical activity; SD, standard deviation. No within- or between-group differences were observed in physical activity, sedentary behavior, isometric quadriceps torque or isometric handgrip force. Definitions: sedentary behavior–energy expenditure ≤1.5 metabolic equivalent units (MET); light intensity PA – energy expenditure >1.5 and ≤3 MET; moderate-to-vigorous intensity PA – energy expenditure >3 MET.
No between-group differences were observed in the component summary scores or individual domains of the SF-36, the scores of the FACT-L and EORTC QLQ-C30 (Table 4).
Table 4.
Baseline and post-intervention measures of health-related quality of life.
| Variable | Exercise group (n = 9) |
Control group (n = 8) |
F; p value | MD [95% CI] | F; p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Baseline | Post-intervention | MD [95% CI] | Baseline | Post-intervention | MD [95% CI] | Within-group | Between-group | Between-group |
| HRQoL (SF-36) | |||||||||
| PCSa | 46 ± 6 | 48 ± 8 | 2 [−3 to 6] | 42 ± 5 | 40 ± 9 | −2 [−7 to 2] | 0.0; 0.89 | 4 [−2 to 10] | 1.80; 0.20 |
| MCSa | 55 ± 6 | 51 ± 14 | −4 [−12 to 5] | 46 ± 6 | 51 ± 8 | 5 [−2 to 12] | 0.1; 0.80 | −8 [−18 to 2] | 3.2; 0.10 |
| Physical functioninga | 67 ± 14 | 74 ± 18 | 7 [−4 to 18] | 52 ± 24 | 55 ± 23 | 3 [−8 to 13] | 2.1; 0.16 | 5 [−9 to 19] | 0.5; 0.49 |
| Role physicala | 72 ± 23 | 69 ± 38 | −3 [−22 to 17] | 44 ± 14 | 44 ± 17 | 0 [−16 to 16] | 0.1; 0.81 | −3 [−27 to 21] | 0.1; 0.81 |
| Bodily paina | 62 ± 12 | 60 ± 26 | −2 [−26 to 22] | 63 ± 23 | 56 ± 33 | −8 [−34 to 19] | 0.4; 0.54 | 6 [−27 to 38] | 0.1; 0.71 |
| General healtha | 72 ± 19 | 72 ± 26 | 0 [−15 to 14] | 63 ± 19 | 65 ± 21 | 3 [−13 to 18] | 0.1; 0.80 | −3 [−22 to 16] | 0.1; 0.73 |
| Vitalitya | 67 ± 18 | 72 ± 30 | −5 [−10 to 20] | 52 ± 17 | 54 ± 17 | 2 [−7 to 10] | 0.7; 0.41 | 3 [−13 to 20] | 0.2; 0.67 |
| Social functioninga | 78 ± 22 | 74 ± 35 | −4 [−21 to 13] | 69 ± 22 | 73 ± 29 | 5 [−15 to 25] | 0.0; 0.96 | −9 [−33 to 15] | 0.6; 0.45 |
| Role emotionala | 88 ± 17 | 80 ± 29 | −8 [−31 to 14] | 57 ± 20 | 68 ± 22 | 10 [−10 to 31] | 0.0; 0.88 | −19 [−47 to 9] | 2.0; 0.18 |
| Mental healtha | 80 ± 14 | 73 ± 24 | −7 [−23 to 9] | 70 ± 18 | 79 ± 17 | 9 [−3 to 20] | 0.1; 0.81 | −15 [−34 to 3] | 3.2; 0.09 |
| HRQoL (FACT-L) | |||||||||
| Physical well-beinga | 25 ± 2 | 24 ± 5 | −1 [−4 to 3] | 22 ± 5 | 21 ± 7 | −1 [−4 to 2] | 0.9; 0.36 | 0 [−4 to 4] | 0.0; 0.91 |
| Social/family well-beinga | 20 ± 8 | 21 ± 7 | 0 [−4 to 4] | 15 ± 9 | 19 ± 6 | 4 [1 to 8] | 3.7; 0.07 | −4 [−9 to 1] | 3.3; 0.09 |
| Emotional well-beinga | 21 ± 2 | 19 ± 6 | −2 [−5 to 1] | 18 ± 5 | 20 ± 4 | 2 [−1 to 5] | 0.0; 0.95 | −4 [−8 to 1] | 3.6; 0.08 |
| Functional well-beinga | 20 ± 5 | 21 ± 9 | 2 [−3 to 6] | 13 ± 7 | 17 ± 8 | 4 [−5 to 12] | 1.8; 0.20 | −2 [−11 to 6] | 0.3; 0.59 |
| Lung cancer subscalea | 20 ± 3 | 22 ± 4 | 3 [0 to 5] | 16 ± 2 | 20 ± 3 | 5 [1 to 8] | 16.3; 0.01 | −2 [−6 to 2] | 1.0; 0.33 |
| Totala | 106 ± 16 | 107 ± 25 | 2 [−10 to 14] | 83 ± 22 | 97 ± 21 | 13 [−1 to 28] | 3.6; 0.08 | −12 [−29 to 5] | 2.2; 0.16 |
| HRQoL (EORTC QLQ-C30) | |||||||||
| Global health statusa | 74 ± 16 | 75 ± 25 | 1 [−22 to 24] | 66 ± 24 | 64 ± 22 | −2 [−10 to 6] | 0.0; 0.91 | 3 [−20 to 27] | 0.1; 0.79 |
| Functional scalesa | 85 ± 9 | 85 ± 17 | 0 [−10 to 11] | 73 ± 16 | 76 ± 12 | 3 [−3 to 9] | 0.3; 0.57 | −3 [−14 to 9] | 0.2; 0.63 |
| Symptoms scalesb | 20 ± 9 | 17 ± 13 | −3 [−15 to 8] | 22 ± 11 | 23 ± 15 | 1 [−8 to 10] | 0.1; 0.77 | −4 [−18 to 9] | 0.4; 0.52 |
| EORTC LC13b | 16 ± 8 | 14 ± 8 | −2 [−10 to 6] | 16 ± 9 | 25 ± 19 | 9 [−1 to 19] | 1.5; 0.23 | −11 [−23 to 1] | 3.8; 0.07 |
CI, confidence interval; EORTC QLQ-C30, The European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire Core 30; EORTC LC13, Lung Cancer subscale of the EORTC QLQ-C30; FACT-L, The Functional Assessment of Cancer Therapy – Lung scale; MCS, mental component score; MD, mean difference; PCS, physical component score; SD, standard deviation; SF-36, Medical Outcomes Study Short-Form 36 general health survey.
Greater scores reflect better outcome.
Lower scores reflect better outcome.
No between-group differences were observed in fatigue (FACIT-Fatigue median [interquartile range]; EG, baseline 43 [43–49], post-intervention 47 [38–52]; CG, baseline 39 [26–40], post-intervention 38 [28–42]; p = 0.82 for between-group difference) or feelings of anxiety (HADS: mean ± SD anxiety score; EG, baseline 3 ± 2, post-intervention 5 ± 4; CG, baseline 2 ± 2, post-intervention 4 ± 5; p = 0.17 for between-group difference) and depression (HADS: mean ± SD depression score; EG, baseline 2 ± 2, post-intervention 4 ± 5; CG, baseline 3 ± 3, post-intervention 4 ± 3; p = 0.40 for between-group difference).
No between-group differences were observed in any measure of lung function (mean difference, 95% CI for between-group difference in change from baseline to post-intervention: FEV1 −0.09 [−0.22 to 0.03] L; FVC −0.12 [−0.43 to 0.18] L; total lung capacity −0.03 [−0.61 to 0.54] L and single breath diffusing capacity for carbon monoxide −1.4 [−3.3 to 0.6]).
Discussion
This RCT evaluated the effect of supervised exercise training in people following curative intent treatment for NSCLC. In this population, an 8-week program of supervised exercise training improved exercise capacity over and above any change seen in the CG. Our finding that supervised exercise training improved exercise capacity extends the findings of a Cochrane systematic review35 and a recently published RCT36 by demonstrating improvements in both field- and laboratory-based tests of exercise capacity. Regarding physical activity and sedentary behavior, peripheral muscle force, HRQoL, fatigue, feelings of anxiety and depression and lung function, no between-group differences were observed. The use of concealed computer generated randomization sequence together with blinding of the outcome assessor and the analysis of data according to intention-to-treat principle were some of the strengths of this study.
Effects of the supervised exercise training program
Exercise capacity
This study demonstrated between-group differences in favor of the EG in three cardiopulmonary variables collected during the CPET and the 6MWD. We acknowledge that the between-group difference in VO2peak, expressed as L min−1, was the result of both a modest increase in the EG and a small decrease in the CG. Nevertheless, the between-group difference in VO2peak following completion of the exercise training program is of particular importance given that VO2peak is a predictor of mortality in people undergoing treatment for NSCLC.37 Although this study did not aim to elucidate the mechanisms underpinning this improvement in exercise capacity, the lack of change in lung function suggests that changes in exercise capacity were most likely mediated by conditioning of the cardiovascular system or peripheral muscles. Our data demonstrating between-group differences in favor of the EG, in O2 pulse, and AT supports this contention. Improvements in O2 pulse and AT suggest that exercise training improved exercise capacity by increasing stroke volume and enhancing the oxidative capacity of the exercising muscles.31
A between-group difference in favor of the EG was also demonstrated in 6MWD. The magnitude of this difference was 52 m, which exceeds the minimal important difference of the 6MWD recently reported for people with lung cancer (22–42 m).38 In people with NSCLC, an increase in 6MWD following exercise training is an important finding because this measure appears to be a valuable prognostic indicator in this population.39 Maintenance of these improvements in exercise capacity was not investigated in this RCT. As benefits of exercise training diminish over time,40 future studies should investigate strategies to maintain the improvements.
Secondary outcomes
On completion of the intervention period, no between-group differences were observed in any of the other measures. The lack of between-group difference in physical activity or sedentary behavior following exercise training in people following lung resection for NSCLC is in agreement with data from a previous RCT41 and is likely to be due to the minimal impairment in these measures at baseline. The baseline values of daily steps reported in the current study is also similar to the results of an earlier work that assessed people within four weeks of hospital discharge following lobectomy for NSCLC (7978 ± 4486 steps/day).42 Further, a recent study published by our group has demonstrated that people following curative intent treatment for NSCLC spend as much time in moderate-to-vigorous intensity physical activity and sedentary behavior as their healthy counterparts.43
Our study did not demonstrate any change in isometric quadriceps muscle force or handgrip force. The reasons for this may relate to: (i) insufficient training load to induce change; (ii) lack of statistical power, especially for changes in quadriceps force as only a small number of participants chose to attend the University for this assessment; and (iii) minimal impairment in these measures at baseline. The lack of improvement in muscle force demonstrated in this study is in agreement with a previous RCT,44 which investigated the effect of a 12-week training program initiated immediately following surgery for NSCLC. Similar to the current study, this earlier study was also likely to be underpowered to demonstrate a between-group difference in quadriceps force. A larger RCT (n = 45) of resistance exercise training for people in stage I to III lung cancer demonstrated significant between-group differences in quadriceps torque on completion of a 12-week training program.45 Of note, this RCT only included people who presented with quadriceps muscle weakness, defined as either a quadriceps muscle force < 70% of their predicted value or a decrement of 10% in quadriceps muscle force following lung cancer treatment.45 Therefore, in people with lung cancer, resistance training may be most effective in those with demonstrated muscle weakness.
Our results showing no difference in HRQoL on completion of exercise training in people following lung resection for NSCLC corroborate findings of earlier studies.14, 44 We used both a generic questionnaire and two disease-specific HRQoL questionnaires, the latter of which were expected to be more responsive than the questionnaires used in an earlier work44; however, we were unable to demonstrate any effect of exercise training on HRQoL. The current study also demonstrated no differences in fatigue or feelings of anxiety and depression on completion of exercise training. Similar to measures of peripheral muscle force, it is possible that the lack of improvement in HRQoL, fatigue and feelings of anxiety and depression may be attributable to near normal baseline scores, suggesting minimal impairment in these domains. Specifically, in the EG, the physical component score (PCS) and the mental component score (PCS) of the SF-36 at baseline were similar to the mean normal scores of 50 ± 10 (PCS) and 53 ± 10 (MCS) reported for the Australian population.46 Likewise, at baseline, participants did not present with fatigue21 and had low scores for feelings of anxiety and depression (HADS anxiety and depression scores ≤7). This suggests that there was little scope for improvement with exercise training.
Study limitations
Recruitment of participants for this study was challenging. Many of the eligible people following curative intent treatment for NSCLC did not consent due to difficulties with traveling to the hospital (if allocated to the EG) or due to other demands on their time. As this was a pilot study, it is possible that the lack of between-group differences in many of the outcomes reflects inadequate statistical power. However, we have provided an estimate of effect of exercise training for each of the study outcomes, which is useful for future sample size calculations. We also acknowledge that adherence to the exercise training was low, which is likely to have compromised the effectiveness of the program.
Conclusions
An 8-week program of supervised exercise training increased exercise capacity in people following curative intent treatment for NSCLC. No changes were observed in physical activity and sedentary behavior, peripheral muscle force, HRQoL, fatigue, feelings of anxiety and depression, and lung function. This study had many strengths in its design. including a concealed, computer-generated randomization sequence, blinding of outcome assessors, and analyzing the data according to the intention-to-treat principle. However, it was a pilot study and the ability to detect changes in outcomes other than exercise capacity is likely to have been influenced by the small sample size and poor adherence to exercise training.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
VC is supported by the Curtin Strategic International Research Scholarship (CSIRS) and Lung Institute of Western Australia (LIWA) PhD Top-up Scholarship. The study received funding from Sir Charles Gairdner Hospital Research Advisory Committee (grant number: 2011/12/013).
Footnotes
Trial registered the Australian New Zealand Clinical Trials Registry (ACTRN12611000864921). https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=343247.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.AIHW and Cancer Australia . AIHW; Canberra: 2011. Lung Cancer in Australia: An Overview. Cat. no. CAN 58. [Google Scholar]
- 3.Sher T., Dy G.K., Adjei A.A. Small cell lung cancer. Mayo Clin Proc. 2008;83(3):355–367. doi: 10.4065/83.3.355. [DOI] [PubMed] [Google Scholar]
- 4.Howington J.A., Blum M.G., Chang A.C., Balekian A.A., Murthy S.C. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 5.Jones L.W. Physical activity and lung cancer survivorship. Recent Results Cancer Res. 2011;186:255–274. doi: 10.1007/978-3-642-04231-7_11. [DOI] [PubMed] [Google Scholar]
- 6.Win T., Groves A.M., Ritchie A.J., Wells F.C., Cafferty F., Laroche C.M. The effect of lung resection on pulmonary function and exercise capacity in lung cancer patients. Respir Care. 2007;52(6):720–726. [PubMed] [Google Scholar]
- 7.Cavalheri V., Jenkins S., Cecins N. Impairments after curative intent treatment for non-small cell lung cancer: a comparison with age and gender-matched healthy controls. Respir Med. 2015;109(10):1332–1339. doi: 10.1016/j.rmed.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Poghosyan H., Sheldon L.K., Leveille S.G., Cooley M.E. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2013;81(1):11–26. doi: 10.1016/j.lungcan.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Balduyck B., Hendriks J., Lauwers P., Sardari Nia P., Van Schil P. Quality of life evolution after lung cancer surgery in septuagenarians: a prospective study. Eur J Cardiothorac Surg. 2009;35(6):1070–1075. doi: 10.1016/j.ejcts.2009.01.050. discussion 1075. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy B., Casey D., Devane D., Murphy K., Murphy E., Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2 doi: 10.1002/14651858.CD003793.pub3. CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowman L., Hill C.J., Holland A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2014;10 doi: 10.1002/14651858.CD006322.pub3. CD006322. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz K.H., Holtzman J., Courneya K.S., Masse L.C., Duval S., Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 13.McNeely M.L., Campbell K.L., Rowe B.H., Klassen T.P., Mackey J.R., Courneya K.S. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalheri V., Tahirah F., Nonoyama M., Jenkins S., Hill K. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev. 2013;7 doi: 10.1002/14651858.CD009955.pub2. CD009955. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins S., Cecins N., Camarri B., Williams C., Thompson P., Eastwood P. Regression equations to predict 6-minute walk distance in middle-aged and elderly adults. Physiother Theory Pract. 2009;25(7):516–522. doi: 10.3109/09593980802664711. [DOI] [PubMed] [Google Scholar]
- 16.Holland A.E., Spruit M.A., Troosters T. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 17.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 18.Cella D.F., Bonomi A.E., Lloyd S.R., Tulsky D.S., Kaplan E., Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12(3):199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 19.Aaronson N.K., Ahmedzai S., Bergman B. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 20.Snaith R.P. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yellen S.B., Cella D.F., Webster K., Blendowski C., Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manag. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 22.Spruit M.A., Sillen M.J., Groenen M.T., Wouters E.F., Franssen F.M. New normative values for handgrip strength: results from the UK Biobank. J Am Med Dir Assoc. 2013;14(10) doi: 10.1016/j.jamda.2013.06.013. 775.e775–711. [DOI] [PubMed] [Google Scholar]
- 23.Hill K., Dolmage T.E., Woon L., Goldstein R., Brooks D. Measurement properties of the SenseWear armband in adults with chronic obstructive pulmonary disease. Thorax. 2010;65(6):486–491. doi: 10.1136/thx.2009.128702. [DOI] [PubMed] [Google Scholar]
- 24.Cavalheri V., Donaria L., Ferreira T. Energy expenditure during daily activities as measured by two motion sensors in patients with COPD. Respir Med. 2011;105(6):922–929. doi: 10.1016/j.rmed.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Cindy Ng L.W., Jenkins S., Hill K. Accuracy and responsiveness of the stepwatch activity monitor and ActivPAL in patients with COPD when walking with and without a rollator. Disabil Rehabil. 2012;34(15):1317–1322. doi: 10.3109/09638288.2011.641666. [DOI] [PubMed] [Google Scholar]
- 26.Garber C.E., Blissmer B., Deschenes M.R. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 27.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 28.Wanger J., Clausen J.L., Coates A. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 29.Macintyre N., Crapo R.O., Viegi G. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 30.American Thoracic Society/European Respiratory S ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 31.ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 32.Blackie S.P., Fairbarn M.S., McElvaney G.N., Morrison N.J., Wilcox P.G., Pardy R.L. Prediction of maximal oxygen uptake and power during cycle ergometry in subjects older than 55 years of age. Am Rev Respir Dis. 1989;139(6):1424–1429. doi: 10.1164/ajrccm/139.6.1424. [DOI] [PubMed] [Google Scholar]
- 33.Decramer M., Gosselink R., Troosters T., Verschueren M., Evers G. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J. 1997;10(2):417–423. doi: 10.1183/09031936.97.10020417. [DOI] [PubMed] [Google Scholar]
- 34.Jenkins S., Hill K., Cecins N.M. State of the art: how to set up a pulmonary rehabilitation program. Respirology. 2010;15(8):1157–1173. doi: 10.1111/j.1440-1843.2010.01849.x. [DOI] [PubMed] [Google Scholar]
- 35.Cavalheri V., Tahirah F., Nonoyama M., Jenkins S., Hill K. Exercise training for people following lung resection for non-small cell lung cancer – a Cochrane systematic review. Cancer Treat Rev. 2014;40(4):585–594. doi: 10.1016/j.ctrv.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Edvardsen E., Skjonsberg O.H., Holme I., Nordsletten L., Borchsenius F., Anderssen S.A. High-intensity training following lung cancer surgery: a randomised controlled trial. Thorax. 2015;70(3):244–250. doi: 10.1136/thoraxjnl-2014-205944. [DOI] [PubMed] [Google Scholar]
- 37.Jones L.W., Watson D., Herndon J.E., 2nd Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116(20):4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granger C.L., Holland A.E., Gordon I.R., Denehy L. Minimal important difference of the 6-minute walk distance in lung cancer. Chron Respir Dis. 2015;12(2):146–154. doi: 10.1177/1479972315575715. [DOI] [PubMed] [Google Scholar]
- 39.Zarogoulidis P., Kerenidi T., Huang H. Six minute walking test and carbon monoxide diffusing capacity for non-small cell lung cancer: easy performed tests in every day practice. J Thorac Dis. 2012;4(6):569–576. doi: 10.3978/j.issn.2072-1439.2012.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffiths T.L., Burr M.L., Campbell I.A. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355(9201):362–368. doi: 10.1016/s0140-6736(99)07042-7. [DOI] [PubMed] [Google Scholar]
- 41.Arbane G., Douiri A., Hart N. Effect of postoperative physical training on activity after curative surgery for non-small cell lung cancer: a multicentre randomised controlled trial. Physiotherapy. 2014;100(2):100–107. doi: 10.1016/j.physio.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Novoa N., Varela G., Jimenez M.F., Aranda J.L. Influence of major pulmonary resection on postoperative daily ambulatory activity of the patients. Interact Cardiovasc Thorac Surg. 2009;9(6):934–938. doi: 10.1510/icvts.2009.212332. [DOI] [PubMed] [Google Scholar]
- 43.Cavalheri V., Jenkins S., Cecins N., Phillips M., Sanders L.H., Hill K. Patterns of sedentary behaviour and physical activity in people following curative intent treatment for non-small cell lung cancer. Chron Respir Dis. 2016;13(1):82–85. doi: 10.1177/1479972315616931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arbane G., Tropman D., Jackson D., Garrod R. Evaluation of an early exercise intervention after thoracotomy for non-small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: randomised controlled trial. Lung Cancer. 2011;71(2):229–234. doi: 10.1016/j.lungcan.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 45.Salhi B., Huysse W., Van Maele G., Surmont V.F., Derom E., van Meerbeeck J.P. The effect of radical treatment and rehabilitation on muscle mass and strength: a randomized trial in stages I–III lung cancer patients. Lung Cancer. 2014;84(1):56–61. doi: 10.1016/j.lungcan.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Hawthorne G., Osborne R.H., Taylor A., Sansoni J. The SF36 Version 2: critical analyses of population weights, scoring algorithms and population norms. Qual Life Res. 2007;16(4):661–673. doi: 10.1007/s11136-006-9154-4. [DOI] [PubMed] [Google Scholar]