Abstract
Exosomes are nanometer-sized vesicles that are released by cells in a controlled fashion and mediate a plethora of extra- and intercellular activities. Some key functions of exosomes include cell-cell communication, immune modulation, extracellular matrix turnover, stem cell division/differentiation, neovascularization and cellular waste removal. While much is known about their role in cancer, exosome function in the many specialized tissues of the eye is just beginning to undergo rigorous study. Here we review current knowledge of exosome function in the visual system in the context of larger bodies of data from other fields, in both health and disease. Additionally, we discuss recent advances in the exosome field including use of exosomes as a therapeutic vehicle, exosomes as a source of biomarkers for disease, plus current standards for isolation and validation of exosome populations. Finally, we use this foundational information about exosomes in the eye as a platform to identify areas of opportunity for future research studies.
Keywords: exosome, extracellular vesicle, age-related macular degeneration, proteome, glaucoma, biomarker
1. Exosomes: a brief overview
The endocytic pathway consists of compartments involved in the internalization of extracellular ligands or cellular components, recycling of those components to the plasma membrane, and/or their degradation (Gould & Lippincott-Schwartz 2009, Klumperman & Raposo 2014). During the maturation process of early endosomes into late endosomes (Stoorvogel et al. 1991), intraluminal vesicle (ILVs) accumulate. Because of their appearance, these late endosomes are generally referred to as multivesicular endosomes (MVE) or multivesicular bodies (MVBs). Loading of proteins, lipids, and cytosol into these ILVs is achieved by inward budding of the early endosomal membrane and specific sorting. MVEs fuse with lysosomes in most cases, leading to the breakdown of their content. MVEs carrying the lysosomal-associated membrane proteins LAMP1 and LAMP2, the tetraspanin CD63, and other molecules in late endosomes, can however also fuse with the plasma membrane and disseminate their content into the extracellular space (Jaiswal et al. 2002, Raposo et al. 1996; Lo Ciero et al). The most well-known mechanism for formation of MVEs and ILVs is carried out by “the endosomal sorting complex required for transport” (ESCRT). Approximately thirty proteins that assemble into four complexes make up ESCRT (ESCRT-0, -I, -II and -III) and several conserved proteins from yeast to mammals (VPS4, VTA1, ALIX also called PDCD6IP) associate with ESCRT (Hanson & Cashikar 2012). In the early 1980s, small (30–100 nm) vesicles of endosomal origin secreted by reticulocytes, were described for the first time using the term, “exosome” (Harding et al., 1983; Pan et al., 1985). The definition of bona fide exosomes is at present somewhat fluid but in this review, we adhere to the recent definition put forth by Kowal and colleagues. According their recent paper, small vesicles (diameter ≈ 30–150 nm) with a low density by gradient ultracentrifugation and carrying CD81, CD63, CD9, Syntenin-1 and TSG101 proteins, qualify as bona fide exosomes (Kowal et al., 2016).
In this review, we will use the terms “exosome” and “exosomes” interchangeably to denote vesicles released extracellularly from late endosomes, and the use of this terminology should not be confused with the term “exosome complex” (often just called “the exosome”), which is a multi-protein intracellular complex capable of degrading various types of RNA (Kilchert et al., 2016). For a more detailed description of exosome biogenesis and an overview of the diversity of different cargoes (e.g. lipids, proteins, DNA, RNA) found in exosomes, please see the excellent recent review by Colombo and colleagues (Colombo et al., 2014).
It has become increasingly clear that exosomes have specialized functions and play a key role in intercellular signaling, and cellular waste management (van der Pol et al., 2012). Based on these known roles of exosomes released from cells in other organs, exosomes and other extracellular vesicles (EVs) released from different cell types in the eye are likely involved in similar pathways. However, currently very little is known about exosomes and other EVs released from cells in the eye. Exosomes make up the smallest sized subset (diameter ≈ 30–150 nm) of EVs (diameter ≈ 30–1,000 nm). Their biogenesis and extracellular release is distinct from other EVs such as larger microvesicles (diameter ≈ 150–1,000 nm) that bud directly from the plasma membrane (Raposo and Stoorvogel, 2013), blebs (diameter ≈ 400–800 nm) (Marin-Castano et al., 2005), and apoptotic bodies (diameter ≈ 800–5,000 nm) (Hristov et al., 2004). Other terms used for EVs, sometimes interchangeably, are ectosomes, shedding vesicles, and microparticles (Carver and Yang, 2016; Cocucci et al., 2009; Crescitelli et al., 2013; Gyorgy et al., 2011; Hess et al., 1999; Holme et al., 1994). Exosomes and microvesicles are also functionally distinct in many respects; therefore, to avoid confusion and to narrow the focus, this review will only discuss the current state of exosome(s) and small EV research in the eye and their potential role in ocular health and disease (Kowal et al., 2016; Lotvall et al., 2014).
The field of exosome and small EV research has figuratively exploded in recent years. A recent search with the keyword “exosomes” in NCBI’s PubMed database of scientific publications returned 4,584 articles (Dec. 14, 2016), 1,205 of which were published in 2016 and 36 slated to be published in 2017, at the time this review was written (Fig. 1). The cancer field in particular has taken the lead investigating exosomes and other EVs for novel approaches to therapy, new mechanistic understanding of tumorigenesis, tumor signaling, novel biomarkers, and modulation of metastasis (Dhondt et al., 2016; Lopatina et al., 2016; Srivastava et al., 2016). For example, modulation of the extracellular matrix (ECM) by matrix metalloproteinases (MMPs), annexins and proteoglycans on exosomes have been shown to increase metastasis (Hakulinen et al., 2008; Sakwe et al., 2011; Stepp et al., 2015; You et al., 2015). This ECM-modulating exosomal activity may also play an important role in eye diseases where pathological ECM remodeling is an integral part of the disease mechanism, such as in glaucoma and AMD (Bowes Rickman et al., 2013; Roy Chowdhury et al., 2015).
Figure 1. Publications with the keyword “exosomes” in the NCBI PubMed database as of December 2016.
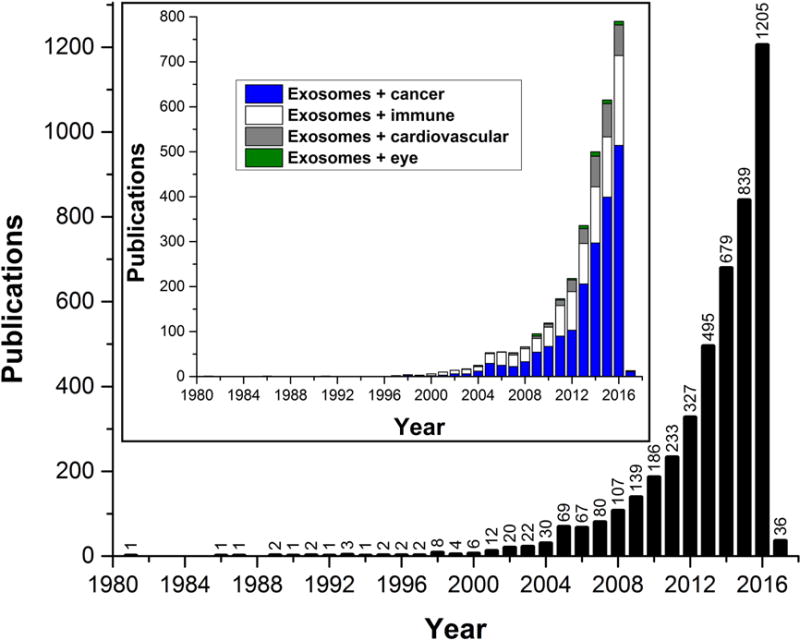
Note the exponential growth in the number of published articles, in particular in the last decade. (Inset) Publications with the indicated search terms in the NCBI PubMed database show a significant increase of exosome publications in the cancer (blue), immune (white), and more recently cardiovascular (gray) research fields; while the eye research field (green) is trailing far behind with only 8 articles in each of the last two years.
2. Methods for exosome isolation – pros and cons
A number of methods can be used for isolating exosomes and small EVs. However, the EV preparations resulting from the different methods span a wide range of purities and properties. Thus, it is very important to choose the isolation method that is appropriate for the downstream analysis methods that will be used or the experiments that will be done with the isolated EVs. In particular, complex biological fluids such as plasma, serum and urine pose difficulties for EV isolation.
Following is a brief discussion of the most common exosome isolation methods with regard to their pros and cons:
2.1. Differential ultracentrifugation
Differential ultracentrifugation is considered to be the gold standard for exosome and small EV isolation (Thery et al., 2006). This technique is based on a scheme of sequential centrifugations of the exosome-containing fluid and the resulting supernatants: 200 g, 2,000 g, 10,000 g, and 100,000 g. The resulting 100,000 g pellet, which contains exosomes, other small to medium-sized EVs, lipoprotein particles and large protein aggregates, is resuspended in PBS and centrifuged again at 100,000 g to control for artifactual trapping of materials. The final pellet is resuspended or lysed depending on the downstream analysis method. It is a relatively simple methodology; however, the resulting pellet, although enriched for, does not purely consist of exosomes. Other drawbacks include the need for access to an ultracentrifuge and a relatively low yield compared to PEG precipitation as discussed below.
2.2. Polyethylene glycol (PEG) precipitation
Precipitation of viruses by PEG solutions is a method that has been used for over 40 years (Adams, 1973; Lewis and Metcalf, 1988; Yamamoto et al., 1970). Recent adaptations of PEG precipitation for isolation of EVs have shown that the most efficient protocols for exosome precipitation utilize PEG polymers with average molecular weights of 6,000 or 8,000 Da (PEG-6000 and PEG-8000) (Antes et al., 2013; Vlassov et al., 2013). PEG precipitation-based kits such as ExoQuick (System Biosciences) and Total Exosome Isolation reagent (TEI; ThermoFisher Scientific) have become increasingly popular due to their ease of use and high yield. Unfortunately, PEG precipitation will not only isolate exosomes but also larger EVs, large protein aggregates, lipoprotein particles (HDL, LDL, VLDL and Chylomicrons), and viruses (Adams, 1973; Iverius and Laurent, 1967; Lewis and Metcalf, 1988; Vikari, 1976; Yamamoto et al., 1970). Thus, PEG precipitation should only be used for exosome and small EV isolation if there is an additional isolation method used on the precipitated EVs and/or if the downstream analysis methods can distinguish exosomes from the other components of the precipitate. In addition, if using commercial kits, the cost of precipitating large volumes of EV-containing solutions is quite high. However, several EV PEG precipitation protocols have been published (Rider et al., 2016; Weng et al., 2016), making the procedure simple and inexpensive if in-house PEG solutions are prepared.
2.3. Sucrose and Iodixanol density ultracentrifugation
Density ultracentrifugation is perhaps the best compromise of methods for EV isolation. It provides purity and specificity sufficient for a highly exosome-enriched preparation, but does not require the method optimization needed for immunoaffinity capture (IAC), as discussed below. Iodixanol (OptiPrep™) rather than sucrose has become the preferred density media due to its superior osmotic characteristics and better separation of EV subpopulations (Kowal et al., 2016). Yields from density ultracentrifugation are much lower than that from PEG precipitation and even standard differential centrifugation, but purity is much higher. A recent study from Théry’s laboratory used proteomic analysis to investigate in detail the identity and characteristics of EVs isolated by density ultracentrifugation and IAC (Kowal et al., 2016). The study highlighted the importance of using preparations of relatively high enrichment for small EVs (100,000 g pellet) to load into the density ultracentrifugation to be able to generate highly pure exosome preparations.
2.4. Immunoaffinity (IAC) capture
For defining bona fide exosomes, small EVs, and other EV populations, IAC is the preferred method (Kowal et al., 2016). IAC provides higher yield than density ultracentrifugation but lower than differential ultracentrifugation (Greening et al., 2015). It is a useful method if robust profiling has been done to validate that the antibody target chosen is present on the majority of EVs in question. The success of the method is dependent on the specificity of the antibody or antibodies used for capture and optimization of capture conditions. Of the three canonical small EV tetraspanin markers (CD9, CD63, and CD81) that have traditionally been used, a recent in-depth study suggests that CD81 may be the best target in most situations for isolation of the majority of the bona fide exosome population of small EVs, see (Kowal et al., 2016) and Fig.2. Due to elution conditions of EVs from IAC columns and/or beads, this method may not be the optimal for EV isolation if biological activity or physical integrity is to be retained.
Figure 2. Extensive subfractionation of EV preparations identified highly enriched and/or specific markers for different subsets of EVs.
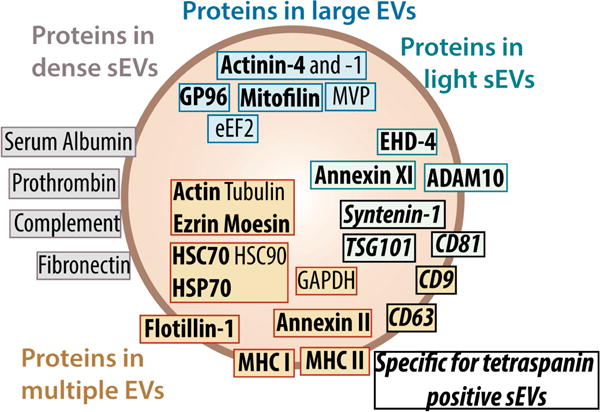
Exosomes make up a subset of tetraspanin positive small EVs (sEVs), here identified by Syntenin-1, TSG101 and CD81. Figure from (Kowal et al., 2016); with permission and copyright PNAS. Permission Pending.
2.5. Size exclusion chromatography
Size exclusion chromatography of EVs has been performed in both fast-protein liquid chromatography (FPLC; (Vickers et al., 2011)) and gravity flow settings (Hong et al., 2016; Lobb et al., 2015). It has not been the preferred method for exosome isolation due to a relatively low yield and, at least previously, a lack of careful characterization of the resulting EV preparations. However, a renewed interest in the method for direct EV isolation from viscous and complex biological fluids such as plasma, serum and urine; has resulted in a number of reports showing its utility (de Menezes-Neto et al., 2015; Lozano-Ramos et al., 2015; Muller et al., 2014). Size exclusion chromatography is in our opinion the preferred method if retaining the biological activity of exosomes is the primary goal.
In conclusion, the choice of method for EV isolation is dependent on scheduled downstream analyses. In the case of protein and proteomics-based analyses, and most likely lipidomic analyses, methods with relatively high yield are necessary; nucleic acid-based detection methods, which contain amplification steps, can work well on preparations with much smaller yields or amounts. To aid in choosing the optimal exosome isolation method for a particular experiment, we have summarized the most relevant characteristics of each method in Table 1. Recent studies have carried out in-depth analyses and extensive subfractionations of EVs to be able to identify bona fide exosome-specific protein markers (Keerthikumar et al., 2015; Kowal et al., 2016) as shown in Fig. 2. The results show that very few proteins are specific for exosomes, in fact only two proteins hold up to scrutiny across different cell types at present: Syntenin-1 and TSG101.
Table 1.
A summary of the most relevant characteristics of common exosome and small EV isolation methods.
| Method | Yield | Exo purity | Ease of use | Cost | Biological activity* | Preferred analysis† |
|---|---|---|---|---|---|---|
| DUC | ++ | ++ | ++ | Low | ++ | EM, NTA, WB |
| PEG | +++ | + | +++ | Low - High | + | 2nd isolation method required |
| DGUC | + | +++ | + | Low | ++ | EM, WB, NTA, MS, NGS, LA |
| IAC | ++ | ++/+++ | +/++ | Medium to High | + | WB, MS, NGS, LA |
| SEC | +/++ | ++ | ++ | Medium | +++ | EM, NTA, WB, MS, NGS, LA |
Biological activity is impacted by different isolation methods by altering exosome integrity. Examples of biological activity includes exosome binding and uptake, enzyme activity, and other protein and nucleic acid-induced activity measured in target cells.
Analyses that the resulting EV preparation is most suited/optimal for based on purity and physical integrity of the exosomes and small EVs. DUC, differential ultracentrifugation; PEG, polyethylene glycol precipitation; DGUC, density gradient ultracentrifugation; IAC, immunoaffinity capture; SEC, size exclusion chromatography; EM, electron microscopy; NTA, nanoparticle tracking analysis; WB, western blotting; MS, mass spectrometry; NGS, next-generation sequencing; LA, lipidomic analysis.
Both of these proteins, and many of the other highly enriched proteins in exosomes and small EVs such as Alix and clathrin for example, are involved in the formation of exosomes by budding from the lumenal side of the membrane in multivesicular endosomes (Baietti et al., 2012). Thus, these markers do not only serve to identify exosomes but also demonstrate the distinct intracellular origin of exosomes compared to other microvesicles that are released directly from the cell surface (Colombo et al., 2014). Going forward, it would therefore be prudent to analyze proteomic datasets and characterize EV preparations with regard to their syntenin-1 and TSG101 content to assess purity and composition in a meaningful way. Since the composition of exosomes reflect the cell-type specific origin (Colombo et al., 2014), unique cell- and tissue-specific exosomal markers can be identified by analyzing the proteins that co-enrich with the pan-cell/tissue exosome markers syntenin-1 and TSG101. Thus, these validated protein markers are extremely valuable to verify purity of exosomal preparations when characterizing protein content in fractions from density gradients in combination with size and quantity determination by nanoparticle tracking analysis and/or transmission electron microscopy.
3. Exosomes and their role in immune regulation
Much of the early seminal work describing, defining and characterizing exosomes was done with exosomes released from immune cells (Blanchard et al., 2002; Escola et al., 1998; Raposo et al., 1996; Skokos et al., 2001; Thery et al., 2006). Over the past decade, the role of exosomes in the regulation and maintenance of immune function has become an area of intense research, displaying substantial promise for novel diagnostic and therapeutic approaches (Robbins and Morelli, 2014). We focus on two areas that appear to have the most potential for future investigations into exosome-mediated immune regulation in the eye.
3.1. Immunomodulation
Considerable work has been aimed at elucidating and harnessing the immunoregulatory function of exosomes in diseases spanning conditions as diverse as cancer (Whiteside, 2016a), infectious diseases (Cheng and Schorey, 2013; Hosseini et al., 2013), inflammatory diseases and autoimmunity (Tran et al., 2015), organ transplant tolerance (Kaipe et al., 2014; Monguio-Tortajada et al., 2014), and neurodegeneration (Hajivalili et al., 2016), to name a few. For example, dendritic cells (DCs), which are professional antigen presenting cells, secrete exosomes expressing functional Major Histocompatibility Complex class I and class II, and T cell co-stimulatory molecules. A groundbreaking study demonstrated that exosomes from DCs loaded with tumor-derived peptides prime specific cytotoxic T lymphocytes in vivo and eradicate or suppress growth of tumors in a T cell-dependent manner (Zitvogel et al., 1998). A recent study showed increased efficacy of vaccines based on exosomes from DCs that were treated with TLR agonists while being loaded with tumor antigens (Damo et al., 2015). In addition, it was recently shown that T cell responses are independent of exosomal MHC/peptide complexes if whole antigen is present (Hiltbrunner et al., 2016). This study suggests that exosomes stripped of personal antigens may be used in designing therapeutic approaches, greatly increasing the feasibility of future trials in humans. Taken together these studies show the powerful immunomodulatory potential of exosomes and pave the way for the use of exosome-based cell-free vaccines as an alternative to adoptive transfer of immune cells for cancer treatment and infectious diseases.
To date no work has explored the immunomodulatory potential of exosomes to treat eye diseases. However, a recent in vitro study found that small EVs released from cultures of the spontaneously immortalized retinal pigmented epithelium (RPE) cell line ARPE-19, promoted an immunoregulatory phenotype in monocytes (Knickelbein et al., 2016). Interestingly, when ARPE-19 cultures were stimulated with inflammatory cytokines, they released EVs that induced monocyte death. Thus, under inflammatory conditions, EVs released from RPE cells may diminish a potentially harmful inflammatory response via monocyte-killing. However, under non-stimulated homeostatic conditions, EVs from ARPE-19 cells induced monocytes to switch to a non-inflammatory phenotype. These findings suggest that RPE-derived EVs under homeo-static conditions downregulate immune activity in the immediate vicinity of the cells. Consequently, an exosome-based approach to immunomodulation in the eye might be to target and kill infiltrating monocytes or to reprogram their phenotype. Infiltrating and/or local monocytes have been implicated in a wide range of eye diseases such as choroidal neovascularization (CNV) (Espinosa-Heidmann et al., 2003), uveitis (Lee et al., 2014), corneal inflammation (Cursiefen et al., 2004; Cursiefen et al., 2011; Koch et al., 1992), diabetic retinopathy (McLeod et al., 1995; Schroder et al., 1991; Serra et al., 2012), and glaucoma (Alvarado et al., 2010; Howell et al., 2012). To elucidate how exosomes modulate ocular immune functions in health and disease, similar studies need to be carried out in bona fide RPE cell culture models (e.g. primary cultures of polarized and pigmented human or porcine RPE monolayers) with exosome-specific isolation methods. The importance of choosing an EV isolation method that is exosome-specific and retains biological function was discussed in detail in Section 2 above.
Human RPE cells release αB-crystallin, a chaperone protein, in association with exosomes from their apical side (Gangalum et al., 2011; Sreekumar et al., 2010). This is noteworthy because αB-crystallin has been implicated as a negative regulator of both innate (Shao et al., 2013) and cellular immunity (Ousman et al., 2007) in the CNS, thus suggesting a potential role in maintaining immune homeostasis in the outer retina. Further studies are needed to clarify whether αB-crystallin is a negative regulator of the immune system in the outer retina.
Finally, a recent report suggests that EVs and possibly apoptotic blebs are responsible for cell surface removal of complement immune regulators including CD46, CD55 and CD59 from RPE (using the ARPE-19 cell model) under conditions of oxidative stress, making them more vulnerable to complement attack (Ebrahimi et al., 2013, 2014). This is particularly relevant to the AMD disease process since genetic, immunohistochemical, and proteomic studies have identified dysregulation of the alternative complement pathway as an important driver of AMD (please see (Anderson et al., 2010), for review). However, the EV isolation methods used in these two studies were not exosome-specific and the methods of analysis did not distinguish exosomes from other EVs. Although intriguing, these studies need validation with differentiated RPE cells and exosome-specific isolation and analysis methods in order to clarify a potential role for RPE-derived exosomes in the modulation of AMD-related complement immune processes.
3.2. Immune privilege
Interesting recent work investigated exosome-mediated immune privilege of the fetus during pregnancy (Mincheva-Nilsson and Baranov, 2014; Stenqvist et al., 2013). Parenchymal cells of immune-privileged tissues secrete CD95L (Fas ligand; FasL) via extracellular vesicles as a mechanism of immune escape (Andreola et al., 2002). Thus, the placental expression of FasL has been implicated as the basis of placental immune privilege, triggering local deletion of activated maternal lymphocytes that recognize placental paternal antigens and express the FasL receptor (Fas, CD95) (Kauma et al., 1999). In vitro studies of cultured trophoblast cells showed that FasL is secreted in association with exosomes (Stenqvist et al., 2013), suggesting that one of the mechanisms by which the placenta promotes a state of immune privilege may therefore be by secretion of the exosome-associated form of FasL (Frangsmyr et al., 2005). However, the topographical location in exosomes was not investigated in this study, thus it is not clear if FasL was on the external face or inside the exosomes. Similarly, exosomes released from the two components of the blood-retinal barrier (retinal vascular endothelium and retinal pigmented epithelium) may play an important role in regulating immune privilege in the eye, which is considered an immune-privileged site akin to the placenta and CNS (Perez and Caspi, 2015). There has been very little research focused on the potential role of exosome-mediated immune privilege in the eye, but one study reported RPE-released exosomes carrying FasL in vitro (McKechnie et al., 2006), supporting this notion. In order to expand on and solidify these findings, polarized RPE cell and retinal vascular endothelial monolayers will be required in future experiments in combination with mechanistic studies of the potential immunotolerogenic effect of FasL-carrying exosomes. We see this area as particularly significant for further inquiry as it may hold considerable potential for novel therapeutic targets and approaches in eye diseases.
4. Exosomes and extracellular matrix (ECM)
4.1. Invadosomes in the trabecular meshwork (TM) and lamina cribrosa (LC)
Exosomes have recently been shown to facilitate interactions between the cell and ECM by acting as key components of cellular structures called invadosomes (Hoshino et al., 2013; Mu et al., 2013). This term encompasses specialized cell structures that range from podosomes to invadosomes where focal turnover of ECM takes place (Saltel et al., 2011). A specialized subpopulation of exosomes is likely released into the pericellular space at or near invadosomes where active ECM remodeling is taking place (Hoshino et al., 2013). The precise role that exosomes play in the function of invadosomes is unclear; however, inhibition of exosome genesis blocks the formation of invadosomes and subsequent matrix degradation (Hoshino et al., 2013). This condition can be rescued by addition of exogenous exosomes (Hoshino et al., 2013). Similarly, exosome secretion is critical for cell migration via podosomes. When exosome formation is inhibited, podosomal protrusions decrease, total cell migration slows and migration directionality is disturbed (Sung et al., 2015). Again, these effects were reversed by application of exogenous exosomes. Taken together, these results demonstrate an essential role for exosomes in the pericellular space where they facilitate proper cell matrix interactions that ultimately control cell behavior. Importantly, invadosome activity has been implicated in normal and pathological remodeling in glaucoma (Aga et al., 2008; Han et al., 2013).
Mechanistically, exosomes contribute to mediating these cell-ECM interactions by binding to ECM components and/or expressing proteases on their surface that cleave ECM proteins. Exosomes bind ECM components via a number of surface proteins. For example, exosomal α4β1 integrins bind fibronectin (Rieu et al., 2000). Non-integrin binding of fibronectin to the exosome surface has also been reported, utilizing fibronectin affinity for heparin/heparan sulfate (Balaj et al., 2015). In these cases myeloma exosomes expressed heparan sulfate proteoglycans of the syndecan family (Purushothaman et al., 2016) or TM-derived exosomes used a surface heparin/heparan sulfate receptor to bind heparan sulfate bound fibronectin (Dismuke et al., 2016). These observations suggest that exosomes bind heparan sulfate proteoglycans or possibly other proteoglycans in the ECM. The TM is enriched in proteoglycans (Keller et al., 2011; Tanihara et al., 2002; Tawara et al., 1989) and such binding may be important in TM physiology, but needs to be explored further. Interestingly, TM exosomes released from glucocorticoid-treated TM tissues display changes in expression of the heparin/heparan sulfate binding protein annexin A2 (Dismuke et al., 2016; Shao et al., 2006). This change correlates with a decreased affinity for fibronectin binding (Fig. 3) via a heparan sulfate bridge and may account for the aberrant accumulation of ECM material in patients with steroid-induced glaucoma.
Figure 3. The corticosteroid, dexamethasone (Dex) treatment of human trabecular meshwork explants increases fibronectin secretion but decreases exosome affinity for fibronectin (Fn) surface.
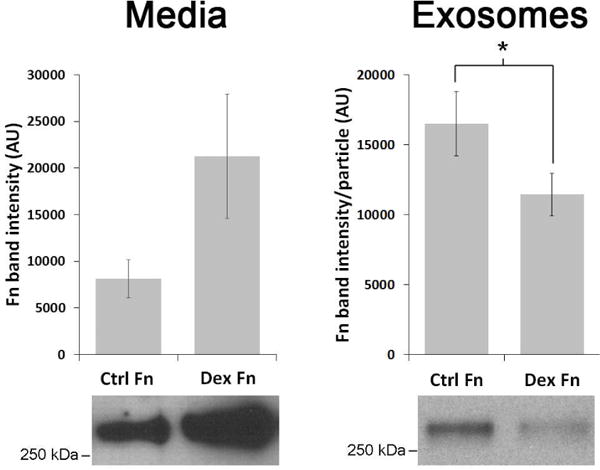
Western blot analysis of exosome-depleted conditioned media and exosomes isolated from conditioned media atop cultured human trabecular meshwork explants in the presence or absence of 100 nM dexamethasone for 72 hrs. Fibronectin content in exosome preparations was normalized to particle number using nanoparticle tracking analysis and quantified via densitometry (n=6) (Dismuke et al., 2016). Permission pending.
Exosomes also mediate extracellular protease activity. For example, exosomal Hsp90α is reported to activate plasminogen (McCready et al., 2010), which when converted to plasmin activates multiple other proteases such as MMPs (Santala et al., 1999). A large number of MMPs, ADAMs and ADAMTSs have been identified on exosomes and have been shown to be catalytically active (Shimoda and Khokha, 2013). Thus far in the eye, only two studies have identified MMPs as exosomal proteins, MMP-14 with corneal fibroblast exosomes (Han et al., 2015) and MMP-2 with trabecular meshwork exosomes (Stamer et al., 2011).
Together, these examples of ECM protein binding and ECM degrading enzyme activity on exosomes likely explain their requirement for invadosome function. TM cells in vivo and in vitro form invadosomes to turn over their local ECM (Aga et al., 2008). Formation/activity of TM invadosomes has also shown to be affected by TFGβ2 (Ebrahimi et al., 2013), a cytokine prominently implicated in the pathogenesis of open angle glaucoma (Prendes et al., 2013; Wordinger et al., 2007). The data showing dexamethasone-treated TM tissue release exosomes with a reduced affinity for fibronectin may also connect exosomes with the TM fibrosis, increased outflow resistance and high intraocular pressure in patients with steroid-induced glaucoma (Dismuke et al., 2016). However, to date, the study of the functional role of exosomes in TM ECM homeostasis is sparse. Further, while invadosomes are thought to mediate the majority of cell-matrix interactions (Saltel et al., 2011), no study to date has looked for this cellular structure in LC cells. Exosomes are a key component of invadosomes (Hoshino et al., 2013) and future studies are needed to determine their precise role in ECM homeostasis in the healthy TM and LC and to determine whether exosomes are involved in the ECM changes in these tissues in glaucoma patients.
Study of the ECM in the TM and LC of the eye is important because in many forms of glaucoma the structure of these two cribriform tissues are preferentially affected. Both of these tissues display signs of fibrosis and/or changes in biochemical, morphological and mechanical properties of the ECM (Hernandez et al., 1990; Overby et al., 2014; Paula et al., 2016; Sigal and Ethier, 2009; Tektas and Lutjen-Drecoll, 2009; Vranka et al., 2015). For example, patients with glaucoma often have changes in the architecture of the LC seen as a posterior displacement of the LC or “cupping” that is due to changes in the mechanical properties of the ECM (Downs, 2015; Roberts et al., 2010; Yang et al., 2011). This posterior displacement reduces the empty spaces between the laminar beams where the RGC axons are located (Yang et al., 2011). Such remodeling appears to compress the unmyelinated RGC axons resulting in RGC stress, death and vision loss (Howell et al., 2013; Nuschke et al., 2015). The cause of this posterior displacement of the LC is not clear, however, elevated IOP is the most common risk factor for developing glaucoma and IOP is likely the mechanical force responsible for the posterior displacement of the LC (Burgoyne et al., 2005; Yang et al., 2011). Thus, artificial elevation of IOP in animal models, including non-human primates, results in posterior displacement of the LC, RGC axon loss and blindness.
Accordingly, lowering IOP is the only therapeutic intervention shown to slow or prevent vision loss in glaucoma patients (The AGIS Investigators, 2000). High IOP is due to idiopathic reductions in the drainage of aqueous humor through the conventional drainage pathway which consists of the TM and Schlemm’s canal (Grant, 1951). One of the hallmarks of open-angle and steroid-induced glaucoma observed in post mortem eyes is changes in the morphology and ultrastructure of the ECM in the TM (Tektas and Lutjen-Drecoll, 2009). These changes are thought to affect 1) the mechanical properties of the TM, reducing the ability of TM cells to modulate aqueous flow pathways and 2) accumulation of/alterations in ECM materials resulting in reduced outflow facility (Vranka et al., 2015; Wang et al., 2016). Taken together, changes in the ECM of the LC and TM are fundamental to the permanent vision loss in glaucoma. Understanding the defects in matrix homeostasis possibly involving exosomes in these two tissues will give us insights into the pathogenesis of glaucoma.
4.2. Myocilin glaucoma
The first gene variant to be linked to glaucoma was MYOC which codes for the protein myocilin (Stone et al., 1997). Currently there are over 70 amino acid substitutions in the myocilin protein that are linked to high pressure glaucoma and many of these mutations result in early onset glaucoma (Resch and Fautsch, 2009). However, the function of myocilin is still unclear. Myocilin is found as a soluble dimer intra- and extracellularly, in a membrane-associated protein complex and associated with the TM ECM (Dismuke et al., 2012; Hardy et al., 2005; Stamer et al., 2006; Ueda et al., 2002).
Initially myocilin was thought to be secreted in the classical sense, via a novel amino terminal signal sequence (Mertts et al., 1999). However, a large proportion of intracellular myocilin is not associated with secretory vesicles, its “secretion” did not involve trafficking through the golgi (Hardy et al., 2005) and its proposed N-terminal signal sequence fused to GFP failed to be secreted (Stamer et al., 2006). These data suggest that myocilin leaves the cell not by secretion but by an alternative route. A rational explanation for these data was that myocilin exits cells in association with vesicles identified as exosomes (Hardy et al., 2005). Myocilin interacts with the exterior surface of exosomes, as evidenced by sensitivity to protease digestion (Hardy et al., 2005). Myocilin is abundant in aqueous humor and bound to exosomes demonstrating that its association is not a cell culture artifact (Perkumas et al., 2007), (Fig. 4). In other studies, myocilin was released from the RPE in situ associated with exosomes and this release was under the control of a G-protein-coupled receptor (Locke et al., 2014). Finally, proteomic analysis of exosomes released from primary cultures of human TM cell monolayers contained an abundance of myocilin (Stamer et al., 2011).
Figure 4. Comparison of myocilin-associated exosomes in fresh aqueous humor samples from normal and glaucoma patients undergoing surgery.
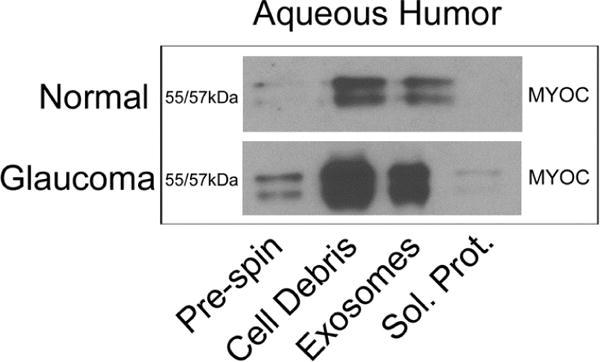
Shown are representative Western blots of volume-equivalent samples taken from aqueous humor subjected to differential centrifugation steps. Pre-spin shows whole aqueous humor; cell debris shows resuspended pellet of whole aqueous humor subjected to 10,000 g; supernatant from pre-spin was subjected to centrifugation at 100,000 g giving exosomes in pellet and soluble proteins (sol. Prot.) in supernatant (Perkumas et al., 2007). Permission pending.
The majority of myocilin mutations are located in the C-terminal olfactomedin domain; a 5-blade, beta-propeller (Donegan et al., 2015). However, this domain does not mediate myocilin’s dimerization or membrane association (Dismuke et al., 2012; Stamer et al., 2006). Thus, myocilin mutations likely do not affect the proteins ability to associate with exosomal membranes but may disrupt other protein-protein interactions, such as those required for biogenesis, targeting or release. Consistent with this idea, most disease-causing forms of myocilin protein are not secreted (Gobeil et al., 2006; Jacobson et al., 2001). Clearly, myocilin’s role in the exosome pathway requires further study.
4.3. Bruch’s Membrane and RPE basal deposits
Bruch’s membrane (BrM) is a pentalaminar basement membrane complex abutting the basal side of the RPE that includes its basolateral cell membrane. It separates the RPE cell monolayer from a fenestrated capillary bed of the systemic blood circulation (choriocapillaris) and thus plays a crucial role in mediating influx of oxygen, electrolytes, nutrients, and cytokines destined for the RPE and photoreceptors, and efflux of waste products and signaling molecules (Curcio, 2013). The pathogenesis of early age-related macular degeneration (AMD) is characterized by thickening of BrM due to lipid and protein accumulation that lead to formation of sub-RPE deposits that occur as discrete accumulations, called drusen; which can be hard or soft, or as continuous accumulations of basal laminar deposits. The lipid buildup is thought to primarily interfere with the fluid, and likely exosome efflux from the RPE across BrM, thereby inflicting stress on the RPE (Curcio, 2013). Cells under stress are known to increase the release of exosomes (Atienzar-Aroca et al., 2016b; King et al., 2012), and it is possible that this process is in part responsible for the deposits in the sub-RPE region.
One of the more common AMD risk-associated single nucleotide polymorphisms, which was identified in genome-wide association studies, is in the promoter region of a gene coding for High-Temperature Requirement A Serine Peptidase 1 (HTRA1) (Yang et al., 2006). The risk-associated nucleotide change correlates with increased expression of HTRA1, which is a secreted serine protease involved in ECM remodeling (Tiaden and Richards, 2013).
Experimental studies that over-expressed (Nakayama et al., 2014; Vierkotten et al., 2011) or deleted (Hasan et al., 2015) HTRA1 in mice, suggest that ECM remodeling in BrM plays an important role in the AMD disease process. Supporting this notion, we recently identified A Disintegrin and Metalloproteinase Domain-Containing Protein 10, also known as ADAM10, as a major component in highly purified exosomes released basolaterally from polarized RPE cultures (Klingeborn et al., 2017). Members of the ADAM family are transmembrane proteinases with a unique structure possessing both adhesion and catalytic domains. Although ADAM MMPs function primarily to cleave membrane proteins at the cellular surface they have also been shown to remodel ECM (White, 2003). Further studies of basolaterally released exosomes from stressed RPE cells may identify additional proteases involved in pathogenic ECM changes.
The source/process of protein and lipid deposition in the sub-RPE region in Bruch’s Membrane (BrM) and subsequent pathognomonic drusen formation in AMD, remain unclear. Progress toward understanding deposit formation, accumulation and biophysical properties of protein plus lipid aggregates may provide novel targets for therapeutic intervention. Interestingly, several proteins found in drusen and sub-RPE deposits, such as annexins and CD63, are also found in exosomes and other EVs (Hageman and Mullins, 1999; Hageman et al., 1999; Mullins et al., 2000; Wang et al., 2009a, b). Furthermore, a recent study revealed an interesting role for Apolipoprotein E (ApoE) and exosomes in regulating pigment granule formation and processing in pigmented cells (van Niel et al., 2015). Perturbation of this pathway in RPE cells may be relevant for AMD since ApoE is one of the major components found in drusen and sub-RPE deposits (Li et al., 2006).
Exosomes released from RPE cells under normal conditions are likely involved in cell-cell communication (on both the apical and basal sides), and lipid homeostasis. Cells under stress are known to increase the release of membranous vesicles including exosomes (King et al., 2012), and this has also been suggested to be the case in RPE cells (Atienzar-Aroca et al., 2016a). Studies have shown that exosomes released by stressed RPE exhibit changes in signaling phosphoproteins (Biasutto et al., 2013), and are coated with complement components (Wang et al., 2009a, b), including the terminal membrane attack complex, C5b-9 (Pilzer et al., 2005), suggesting exosomes play a role in modulating complement activation in the immediate extracellular milieu of the RPE cells. Moreover, severe oxidative stress of polarized primary human RPE cultures resulted in barrier breakdown and a loss of the apical-specific release of exosome-associated αB-Crystallin (Sreekumar et al., 2010). Furthermore, drusen proteins such as enolase and ATP synthase have been found in exosomes (Olver and Vidal, 2007), supporting an exosomal origin for some drusen components. Although these studies support a role for RPE-derived exosomes in the AMD disease process, they were limited by several factors: (i) the use of ARPE-19 cells (ARPE-19 is a spontaneously immortalized RPE-like cell line, lacking many important hallmarks of bona fide RPE cells, discussed in detail in a recent publications (Beebe, 2013; Rizzolo, 2014)), (ii) superficial exosome characterization severely lacking in detail, and most important, (iii) cells were grown on plastic, not on permeable supports (i.e. Transwell, ThinCert, Millicell etc.) to facilitate proper epithelial polarization. Several different RPE culture models on permeable supports have been described previously, e.g. (Maminishkis et al., 2006; Toops et al., 2014).
Culture of primary RPE cells on permeable supports is essential to study basolaterally released exosomes, which could be involved in basal deposit formation, intercellular and systemic signaling and to distinguish them from apically released exosomes. An exhaustive characterization of EVs released both apically and basolaterally from RPE cells under normal and pathophysiological conditions is critical to elucidating the potential role of exosomes in the AMD disease process. Interestingly, a recent study showed that under serum-free conditions of exosome collection from polarized ARPE-19 cultures, the total exosome release was about twofold higher on the apical vs basolateral side (Gangalum et al., 2016). Our own studies using polarized primary RPE cultures show similar results under FBS-supplemented culture conditions. However, under culture conditions using a serum supplement instead of FBS, exosome release is shifted to roughly equal apical and basolateral release (Klingeborn et al., 2017), suggesting that serum components also mediate polarized release of exosomes.
In addition to exosome abundance in these studies, we performed a mass spectrometry-based proteomic analysis of apically and basolaterally RPE-derived EVs by quantitatively profiling hundreds of proteins in EV preparations of increasing purity. This approach, termed protein correlation profiling (PCP) (Andersen et al., 2003; Skiba et al., 2013), permits the analysis of any sub- or extracellular components/complexes that can be enriched by fractionation but not purified to homogeneity. PCP provides a powerful approach to both identify bona fide resident proteins and to exclude contaminating proteins from a proteome dataset. We found that the vast majority of RPE-derived exosomal proteins that were highly co-enriched with the exosome-specific marker Syntenin-1 differed between the apical and basolateral side (Klingeborn et al., 2017), (Fig. 5), which was not unexpected if exosomes contribute to different apical versus basolateral signaling and pathways in these polarized cells. See supplementary tables S1 and S2 for the identity of the apically and basolaterally released RPE-derived exosomal proteins. These findings emphasize the importance of studying exosomes released from both sides of polarized cell types since an apical only approach risks missing important basolaterally released exosomal proteins. Perhaps even more troubling is the risk of mistakenly using findings in the apical RPE exosome proteome to guide research into basolateral-specific biological processes such as lipoprotein particle flux, waste disposal, and nutrient transport, all of which may play a role in sub-RPE deposit and drusen formation under pathological conditions. The next step in these studies will be to study potential changes in the protein cargo in exosomes derived from RPE cells under conditions of stress relevant to the AMD disease process, such as photo-oxidative stress and dysregulation of lipid metabolism, to mention a few. Identified proteomic changes may result in novel targets for therapy.
Figure 5. Exosomal proteins highly co-enriched with the exosome-specific marker Syntenin-1, differ markedly between apically and basolaterally released exosomes in a polarized RPE cell culture model.
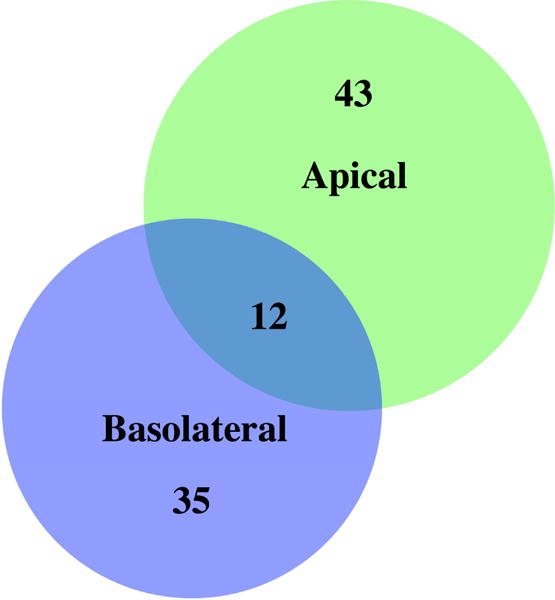
Note that in these highly stringent exosome-specific proteome datasets, only 12 proteins are released in exosomes from both sides, emphasizing the exceptional directionality in EV release from polarized RPE monolayers (Klingeborn et al., 2017). Permission pending.
5. Exosomes in ocular angiogenesis
5.1. Angiogenesis in Cancer
The involvement of exosomes and small EVs in angiogenesis in cancer has been the focus of intense research in the last couple of years (Kalluri, 2016; Whiteside, 2016b). Exosomes and small EVs have been shown to modulate angiogenesis in tumors by both pro- and anti-angiogenic pathways (Ribeiro et al., 2013). For example, exosome release is increased by hypoxia that often occurs in tumors, and these exosomes stimulate angiogenesis, when taken up by endothelial cells (Hong et al., 2009; Park et al., 2010; Skog et al., 2008; Umezu et al., 2013). The vascular remodeling induced by tumor-derived exosomes likely affects not only tumor growth, but also metastasis. One of the ways tumor cell-derived exosomes may impair the structural integrity of endothelial cells and cause leakiness, is via the exosomal microRNA, miR-105, which downregulates the expression of the tight junction associated protein, ZO-1. Downregulation of ZO-1 enhances vascular permeability and thereby metastatic dissemination (Zhou et al., 2014). Two recent studies determined the miRNA content of exosomes derived from uveal melanoma (UM; an ocular cancer) (Eldh et al., 2014; Ragusa et al., 2015). Ragusa and colleagues showed that an exosome-associated miRNA (miR-146a) was upregulated in the vitreous humor of UM patients compared to controls. They also demonstrated that miR-146a was upregulated in serum exosomes from those same patients. Interestingly, the study by Eldh et al. found that UM-derived exosomes from UM metastases in the liver could be recovered from the liver circulation (liver perfusates). Both of these studies exemplify approaches to recover eye-specific exosomal diagnostic markers from tissues and/or fluids that are peripheral to the eye, and thus more easily accessible. It represents encouraging proof-of-concept for identifying eye disease-specific biomarkers outside the eye.
In the eye, both the cornea and the retina maintain very strict homeostatic control over angiogenesis, when left unchecked neovascularization severely affects vision (Abdelfattah et al., 2016; Eichler et al., 2004). Exosomes may play an important role in maintaining this homeostasis under normal conditions.
5.2. Cornea
The transparency of the cornea is critical for vision. The healthy cornea is an avascular tissue and during normal wound healing, repair occurs within hours without the formation of new blood vessels (Chang et al., 2001). In some cases however, imbalance between pro- and anti-angiogenic factors during wound healing can lead to corneal neovascularization. These blood vessels promote a loss of transparency in the cornea and therefore an impairment of visual clarity. Corneal wound repair is a complex multiphase process that involves apoptosis (Netto et al., 2005), proliferation (Cursiefen et al., 2006), cellular transformation (Mimura et al., 2009), migration (Cursiefen et al., 2006) and ECM remodeling (Mimura et al., 2009). A critical component throughout this process is the transmembrane matrix metalloproteinase-14 (MMP-14). Corneal fibroblasts release exosomes with MMP-14, which are taken up by endothelial cells (Han et al., 2015). Exosomal MMP-14 activity is critical for the accumulation and activation of MMP-2 in the exosomes (Han et al., 2015). This process likely plays a role in the multistep corneal wound healing and represents a therapeutic target to prevent or reverse corneal neovascularization. Thus far, investigation into cornea-derived exosomes has been limited. Additional studies need to determine what role corneal exosomes play in other aspects of neovascularization such as VEGF/PEDF signaling.
5.3. Neovascular AMD and diabetic retinopathy
In contrast to the extensive research studying the involvement of exosomes and small EVs in cancer angiogenesis (see above), very little research has been done aimed at studying the role of exosomes in the development and disease process of neovascular AMD including CNV, or other diseases with aberrant retinal angiogenesis such as diabetic retinopathy. As mentioned above, cancer-cell derived exosomes containing certain microRNAs affect the integrity of endothelial cells in blood vessels by downregulating expression of tight junction-associated protein ZO-1 (Zhou et al., 2014). Thus, there may also be a potential role for exosome-induced ZO-1 downregulation in the RPE monolayer allowing for increased access of nascent choroidal blood vessels into the retina during the CNV disease process. In a pathological state, sources of exosomes inducing increased permeability could include choroidal vasculature and oxidatively stressed and/or hypoxic RPE cells. As the immature leaky blood vessels formed by CNV are responsible for much of the vision loss in patients with exudative AMD (Shao et al., 2016), ways to decrease the infiltration of choroidal vessels into the retina are sorely needed. In the case of diabetic retinopathy, where the disease process is mainly localized to the retinal vasculature, potential impact of exosome-induced downregulation of tight junction proteins in endothelial cells which make up the inner blood-retinal barrier, may be significant (Klaassen et al., 2013). Investigating roles of exosomes in this process may identify novel therapeutic targets. There is a delicate balance of pro- and anti-angiogenic signaling in the retina, RPE and choroid. The role of exosomes in this signaling balance was highlighted by a study demonstrating that exosomes released from retinal astrocytes contain anti-angiogenic components that inhibit laser-induced CNV in a mouse model (Hajrasouliha et al., 2013). Further studies utilizing mass spectrometry and nucleic acid-sequencing to determine the content of exosomes released from astrocytes, Müller cells, photoreceptors and other retinal cells, including apical side exosome release from RPE cells, will be crucial to identify new therapeutic targets to control aberrant retinal neovascularization in a range of retinal diseases.
Exosomes released from cells in the eye in neovascular AMD patients also have the potential to serve as biomarkers. Exosomal biomarkers could be useful not only to stratify neovascular AMD patients according to severity of disease in a way that may be more predictive of disease progression than current methods (e.g. OCT and fluorescein angiography); but they could also provide novel insight to the pathophysiology of the disease. Interestingly, Kang and colleagues recently identified changes in EV proteins found in the aqueous humor from individuals with neovascular AMD compared to individuals without the disease (Kang et al., 2014). This study also compared results to EVs from ARPE-19 cell cultures, which as discussed above lacks many hallmarks of bona fide RPE cell cultures (Rizzolo, 2014) and the methods used for EV isolation were not specific for exosomes or small EVs. Furthermore, no evidence was presented in this study to show that EVs found in aqueous humor originated from RPE cells. Thus, currently it is still unclear if exosomes and/or small EVs in aqueous humor from patients with neovascular AMD can be used as biomarkers of the disease. Interestingly, ongoing studies in our own laboratory recently identified Pigment Epithelium-Derived Factor (PEDF) in highly purified apically released exosomes from polarized RPE cell monolayers, but to a much lesser extent in basolaterally released exosomes (Klingeborn et al., 2017). PEDF is secreted primarily from RPE cells on their apical side to maintain an anti-angiogenic milieu in the outer retina (Ablonczy et al., 2011; Tombran-Tink et al., 1995). Although the majority of secreted PEDF is most likely not released in an exosome-mediated manner, our use of protein correlation profiling (Andersen et al., 2003; Skiba et al., 2013) unequivocally shows that this is a genuine association with exosomes, not due to contamination during the purification procedure. Thus, there may be targeted anti-angiogenic signaling carried out by exosomes that is different from the majority of apically secreted PEDF.
Approaches being developed in the cancer field to use exosomes as carriers for pro and/or anti-angiogenic factors could be adapted to target eye diseases involving dysregulated angiogenesis (Ribeiro et al., 2013; Whiteside, 2016b). For example, high heparanase activity and expression correlate with an aggressive tumor phenotype in many cancers. Heparanase action in cancer results in a large up-regulation of growth factors and increased shedding of syndecan-1, a transmembrane heparan sulfate proteoglycan. A large body of work suggests that syndecan-1 and heparanase together regulate cell behaviors and drive growth factor signaling that enhance tumor growth and angiogenesis. Encouragingly, targeting of the heparanase/syndecan-1 interaction has shown promise in blocking the aggressive behavior of cancer in pre-clinical and clinical studies (Ramani et al., 2013). In addition, both heparanase and syndecan-1 are involved in exosome biogenesis and regulation of exosome release (Thompson et al., 2013). Furthermore, a recent study by Gangalum and colleagues (Gangalum et al., 2016) reported that shRNA knockdown of αB-Crystallin in ARPE-19 cells severely inhibits both apical and basolateral exosome and/or small EV release. Thus, approaches attempting to regulate exosome release in the affected cell types, as well as targeting proteoglycans found on exosomes and in the ECM of the retinal vasculature and BrM at the choriocapillarismay be able to reduce neovascularization in neovascular AMD and diabetic retinopathy.
In conclusion, much research remains to be done to elucidate the role of exosomes in eye diseases with aberrant angiogenesis; nonetheless, the potential for important novel findings is sizeable. Findings may include new drug targets and novel biomarkers for improved diagnostic and prognostic tests. Thus, it appears that there may be significant potential for therapeutic intervention concerning aggressive neovascularization in eye diseases by attempting to regulate exosome release in the affected cell types.
6. Stem cells and exosomes
Cell death of largely post-mitotic cells is part of the disease process in all eye diseases; therefore stem cell-based approaches aimed at cell replacement are being actively studied for therapy and/or intervention. For example, patients with ocular hypertension and glaucoma have fewer TM cells (Alvarado et al., 1984; Gottanka et al., 2006; Rodrigues et al., 1976) and vision loss in glaucoma is due to death of retinal ganglion cells (Quigley, 1993). Patients with late dry AMD known as geographic atrophy have areas of RPE death which then leads to death of photoreceptors (Bonilha, 2008). A number of ocular surface diseases involve loss of cells on the surface and endothelium of the cornea (Ahmad, 2012). For all of these ocular diseases, researchers have proposed that stem cell-based therapy could be used to restore tissue health and function (Abu-Hassan et al., 2015; Al-Shamekh and Goldberg, 2014; Erbani et al., 2016; Mead et al., 2015; Nakamura et al., 2016; Roubeix et al., 2015; Zhu et al., 2016). One strategy for replacing lost cells is to transplant stem cells into the affected areas where they differentiate into the desired cell type and restore tissue/organ function (Blenkinsop et al., 2012). Another approach under investigation for repairing RPE damage is to differentiate stem cells into RPE monolayers and then transplant the differentiated monolayers into the patient (Carr et al., 2013). Therapeutic stem cell strategies to treat the retina tested both strategies, but with limited success. To date, differentiation of stem cells into RGC-like cells has only been accomplished in culture (Phillips et al., 2012). However, it is now widely accepted that a major therapeutic effect of stem cells is due to their secretion of paracrine factors (Tran and Damaser, 2015). In line with this idea, another strategy uses mesenchymal-derived stem cells to secrete neurotrophic factors for neuroprotection or axonal regeneration of retinal cells (Johnson et al., 2010; Johnson et al., 2014; Mead et al., 2013). A recent study demonstrated that intravitreal injections of exosomes from mesenchymal-derived stem cells partially prevents axonal loss and degeneration following mechanical injury (Mead and Tomarev, 2017). Interestingly, the investigators find that RNA exosomal cargo is responsible for these protective effects. Embryonic stem cell (ESC) derived precursors and induced pluripotent stem cells (iPSCs) have also been transplanted to replace degenerated photoreceptors and RPE cells (Gonzalez-Cordero et al., 2013; Meyer et al., 2009). In the trabecular meshwork, iPSCs have been used to repopulate the meshwork and/or provide trophic factors that induce proliferation in endogenous cells (Abu-Hassan et al., 2015; Zhu et al., 2016).
Pluripotent stem cells express a number of transcription factors that contribute to their undifferentiated phenotype. These transcription factors, including HoxB4, Nanog, Oct-4 and Rex-1 have been detected in stem cell derived EVs where they can be transferred to adjacent resident cells (Ratajczak et al., 2006). In addition to transcription factors, stem cells are known to secrete several signaling molecules including WNTs (Clevers et al., 2014), β-catenin (Clevers et al., 2014), TGF-β1 (Watabe and Miyazono, 2009) and VEGF (Gerber et al., 2002), which have also been found to associate with exosomes and other EVs (Gross et al., 2012; Luga et al., 2012). With this in mind, the mechanism behind the therapeutic effect of stem cells on tissue repair is not fully understood. As discussed above the trophic factors released by stem cells release appear to mediate a substantial portion of their biological effect (Ratajczak et al., 2012). Exosomes and other EVs function as delivery vehicles for these trophic factors either by sequestering signaling molecules on their surface or transferring transcription factors and miRNAs to resident cells (Ratajczak et al., 2006). In spite of the promising therapeutic potential of stem cell transplantation another potential problem with this approach is that tumorigenic and immunogenic risks still remain (Mousavinejad et al., 2016). Importantly by harvesting and transplanting stem cell exosomes and other EVs, such risks are alleviated yet much of the therapeutic benefit remains (Kishore and Khan, 2016). This cell-free approach is promising for treatment of many eye diseases, but requires further investigation.
7. Exosome biomarkers for eye diseases
Interest in utilizing exosomes and other EVs to identify biomarkers of disease has increased exponentially in recent years (Gonzalez and Falcon-Perez, 2015). It is easy to understand why the perceived potential for development of exosome-based diagnostic assays is so large. Exosomes and EVs have several unique features that make them ideal as targets for finding new biomarkers: (i) the lipid bilayer provides protection for RNA, DNA, and proteins inside the exosome from nucleases and proteases in the extracellular milieu, (ii) exosomes contain tissue-, cell-, or disease-specific proteins and nucleic acids, and (iii) the relative hardiness of exosomes make it possible to use a wide range of methods for isolation and enrichment from a range of body fluids (i.e. plasma, serum, urine, saliva, semen, breast milk, aqueous humor and cerebrospinal fluid). Studies from the cancer, cardiovascular disease and diabetes research fields report promising findings for the utility of exosome biomarkers for diagnosis, risk assessment, and choice of therapy (Joyce et al., 2016; Lawson et al., 2016).
7.1. Tear fluid
Theoretically, there is a significant potential for identification and characterization of exosomal biomarkers of eye disease in tear fluid. Particularly appealing is the noninvasive nature of collecting tear fluid, but a potential drawback is the relatively small volumes that can be collected. To date, tear fluid as a source for exosomal biomarkers has not been extensively investigated, as evidenced by the fact that we only found one publication investigating exosomes in tear fluid (Grigor’eva et al., 2016). Proteomic biomarker studies of tear fluid have identified a number of proteins (e.g. annexins and heat shock proteins) which are most certainly exosome-associated, although not investigated as such (Aass et al., 2015; Matheis et al., 2015). With the recent advances in exosome isolation techniques, protein identification methods, and nucleic acid sequencing, the diagnostic and therapeutic potential of tear-derived exosomal biomarkers appear to be considerable. Certainly, this is a wide-open area of research.
7.2. Aqueous humor (AH)
Aqueous humor (AH) has been used for protein, nucleic acid, and lipid biomarker analyses in a wide range of eye diseases (Ji et al., 2015; Murthy et al., 2015; Wecker et al., 2016). Some of the most prevalent eye diseases such as glaucoma (Agnifili et al., 2015; Goyal et al., 2014), neovascular AMD (Kang et al., 2014; Liu et al., 2016; Park et al., 2014), diabetes-induced eye diseases (Hillier et al., 2016; Vujosevic et al., 2015, 2016), and uveitis (Haasnoot et al., 2016; Kalinina Ayuso et al., 2013), to name a few, have been investigated for biomarker content in the AH. Although the vast majority of nucleic acid and lipid biomarkers, and some of the protein biomarkers identified in AH were most likely exosome-associated, very little attention has been directed to the exosome-specific biomarkers. The reason for the uncertainty regarding whether identified proteins and nucleic acids are exosome-associated is based on limitations in the studies cited above. The proteins identified in these studies most likely originated from both soluble secreted proteins (and possibly nucleic acid) and proteins, lipids and nucleic acids in and on exosomes and other EVs. The technical reason that there was a mixture of soluble as well as EV-associated proteins and nucleic acids in these preparations is that the methods used for AH sample preparation did not include steps to separate the soluble fraction from the membrane fraction (i.e. exosomes and EVs).
There have been studies where EVs were isolated from AH. For example, Kang and colleagues isolated AH EVs from patients with neovascular AMD (Kang et al., 2014), however the methods used for EV isolation were not specific for exosomes or small EVs, raising doubts about whether the findings truly represent exosomal biomarkers. Specifically, the PEG precipitation procedure used results in a preparation that likely contains a mixture of exosomes, ectosomes, blebs, lipoprotein particles, and protein aggregates, as was discussed in Section 2.2 above. Katome and coworkers reported an increase in exosome-associated peroxisome proliferator-activated receptor gamma (PPARγ) in AH from patients with proliferative diabetic retinopathy compared to controls (Katome et al., 2015). However, the methods used for EV isolation (PEG precipitation) in this study were again not exosome-specific. Thus, it is unclear if these findings represent true exosomal biomarkers of eye disease. Using centrifugation steps to isolate exosomes from mixtures, our laboratory has carried out several studies focused on exosomes in AH and their potential role in glaucoma (Dismuke et al., 2015; Hardy et al., 2005; Hoffman et al., 2009; Perkumas et al., 2007; Stamer et al., 2011), one of which was specifically aimed at generating data which can be used to identify exosomal biomarkers (Dismuke et al., 2015). Finally, it is not currently known if exosomes or other small EVs released from RPE, Müller cells, vascular endothelial cells or other retinal cells can make their way to the AH. To aid in answering this question and potentially identifying novel AH exosomal biomarkers, foundational characterization of the composition of exosomes released from these cell types in vitro is needed. Ongoing studies in our laboratory aimed at careful characterization of exosomes and other EVs released from RPE (Klingeborn et al., 2017), can be used as a resource to identify and validate potential exosomal biomarkers in AH.
In summary, it appears clear that future studies focused on exosomal and small EV biomarkers of eye disease in AH must utilize appropriate exosome- and/or small EV-specific methods for isolation; and that there is a considerable need for characterization of protein and nucleic acid composition of exosomes from several different retinal cell types.
7.3. Vitreous humor
Ample volumes of AH are easily accessible during cataract surgery, however vitreous humor (VH) is much less accessible during most standard procedures. That said, small volumes of VH can be obtained with no more discomfort for the patient than routine anti-VEGF and anti-PDGF injections. Thus, if robust vitreal exosomal biomarkers can be identified and validated, their practical use could be substantial.
VH has a high potential to contain biomarkers for diseases such as neovascular AMD, geographic atrophy, early-stage AMD, diabetic retinopathy, glaucoma, and a number of other retinopathies. Several recent studies using proteomic, nucleic acid, and lipidomic approaches identified interesting potential vitreal biomarkers in retinal vein occlusion (Reich et al., 2016), neovascular AMD (Menard et al., 2016; Nobl et al., 2016), diabetic retinopathy (Jin et al., 2016), and primary open-angle glaucoma (Agnifili et al., 2015). However, none of these studies explored or discussed the role of exosomes or other EVs in the transport to and presence of biomarkers in the VH. Some of the biomarkers identified in these studies, such as PEDF in the case of neovascular AMD (Nobl et al., 2016) and keratin 1 and PEDF in the case of diabetic retinopathy (Jin et al., 2016) are proteins that have been identified in association with exosomes (discussed in Section 4.3 above). The promise in an exosome-specific approach to identifying relevant biomarkers lies partly in the specificity that can be achieved as opposed to a global approach as was used in the cited studies. Proteomic identification carried out on proteins in the vitreous, which contain a mixture of proteins that are exosome-associated and proteins that are not, risks masking correlations of disease-associated proteins in either fraction. Thus, by isolating EVs specifically, the soluble (non-exosome/EV associated) proteins can be removed and potentially confounding results can be avoided. An inkling of this potential can be seen in the few (three) studies that to date have focused on EVs in vitreous as a source of disease biomarkers (Biasutto et al., 2013; Katome et al., 2015; Ragusa et al., 2015).
7.4. Blood
Blood fractions such as plasma and serum represent perhaps the most promising avenue for identifying exosomal biomarkers in eye diseases. This is in large part due to the facts that (i) it is easier and less invasive to collect than AH and VH, and that (ii) much larger volumes can be collected. A possible disadvantage of using blood as a source of exosomes from the eye is that these are likely to make up a very small fraction of the total exosomes found in the systemic circulation and will therefore be difficult to detect and analyze. For successful identification and isolation of ocular exosomes or small EVs from blood, foundational descriptive characterization of exosomes released from ocular cells under healthy and pathological conditions is essential. Once exosome-associated markers specific for ocular cells have been identified, they can be used to isolate ocular exosomes from the vast excess of non-ocular EVs present in blood. Without an enrichment step targeting ocular exosomes, the task to develop blood-based biomarkers of eye disease may be untenable. Currently, there are immunoaffinity-based commercial kits available aimed at isolating exosomes directly from human plasma and serum (Diagenode, MBL International, and System Biosciences), supporting the feasibility of this approach. However, in many cases there is still a need to develop and validate in-house immunoaffinity methods if samples are from other species than humans (since most commercial kits have only been developed for human samples), or if targets other than those offered in commercial kits are important, as discussed in more detail in Section 2.4 above. Only two published reports address this area of research so far (Eldh et al., 2014; Ragusa et al., 2015). In one of these studies, Ragusa and colleagues showed that an exosome-associated miRNA (miR-146a) was upregulated in the VH of uveal melanoma patients compared to controls. They also demonstrated that miR-146a was upregulated in serum exosomes from those same patients. This study represents an encouraging proof-of-concept for identifying eye disease-specific biomarkers in the systemic circulation, as further outlined in Fig. 6.
Figure 6.
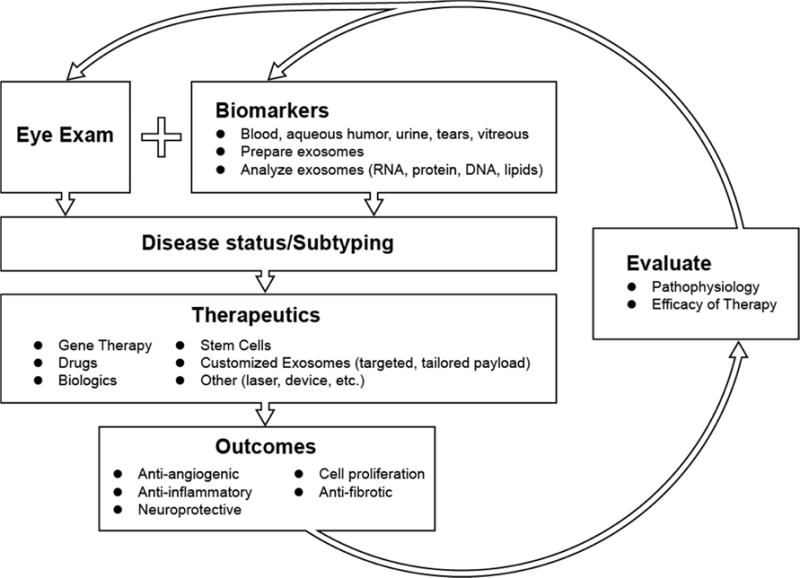
Flow chart outlining an exosomal theragnostic approach to personalized treatment involving development and delivery of therapeutic, followed by diagnostic and prognostic evaluations.
8. Exosomes as therapeutic agents
Today the two leading causes of irreversible blindness in Western societies are AMD and glaucoma. By 2020 it is estimated that 196 million people worldwide will have AMD (Wong et al., 2014) and 79.6 million people worldwide will have glaucoma (Quigley and Broman, 2006). The goal of current therapeutic approaches to treat these late onset diseases is not to reverse the disease course, but only to halt further progression of tissue damage and vision loss. Additionally, many of the therapeutic interventions for these diseases involve monthly administration by a physician in a clinical setting (i.e. injection of anti-VEGF biologics for exudative AMD), repeated daily administration of eye drops by patients where administered doses can vary drastically by individual (glaucoma) or no substantial intervention exists at all (dry AMD). While research has advanced our understanding of the pathogenesis of these diseases and identified targetable pathways that could lead to preservation or even reversal of vision loss, these discoveries have not translated well to the clinic because delivery of drugs, active enzymes/proteins and small RNAs to tissues in the eye remains a steep challenge (Rawas-Qalaji and Williams, 2012). Here we discuss, in detail, the substantial progress made in the use of exosomes for the targeted, effective and safe delivery of these therapeutic molecules.
Exosomes are natural vehicles for the transfer of small RNAs and proteins, as highlighted in previous sections. Cells possess mechanisms to take up exosomes (Mulcahy et al., 2014) and extract the contained microRNAs for use (Zhang et al., 2015). This makes exosomes ideal delivery vehicles for gene therapy involving microRNAs or small interfering RNAs (siRNAs) as they both facilitate uptake (Kooijmans et al., 2012) and protect the RNAs from extracellular degradation (Zhang et al., 2013). Recently exosomes have also been shown to transfer functional proteins to recipient cells. For example, the tumor suppressor, PTEN can be transferred to recipient cells via exosomes where it functioned as a phosphatase (Putz et al., 2012). A number of transmembrane proteins are also transferred from exosomes to recipient cells, including the tyrosine kinase receptors MET (Peinado et al., 2012) and KIT (Atay et al., 2014) as well as αvβ6 integrins (Fedele et al., 2015). Transcription factors are also thought to be transferable between stem cells and recipient cells as discussed above, which has been shown to result in stem cell-like properties such as proliferation in non-proliferating cells (Ratajczak et al., 2006). Exosomes have also been engineered to deliver small molecules, as has been demonstrated for curcumin delivery to activated myeloid cells (Sun et al., 2010). Together, these studies demonstrate that exosomes can function as delivery vehicles for a variety of therapeutic cargos.
A number of studies have also shown that exosomes can target specific cells types and tissues to deliver their cargos. One successful strategy used DCs engineered to express a modified exosomal protein, LAMP2b fused to a peptide from the rabies viral glycoprotein. These exosomes were loaded with siRNA targeting GAPDH and administered intravenously to mice. These engineered exosomes specifically knocked down GAPDH in neurons and microglia in the brain (Alvarez-Erviti et al., 2011). Similar strategies of using modified exosomal proteins to target specific cell subtypes and deliver cargo have also been published (Ohno et al., 2013; Tian et al., 2014). Finally, a recent cancer study examining organ specific metastasis found that α/β integrin expression patterns on the exosomes resulted in organ specific uptake (Hoshino et al., 2015). This suggest that exosome-based therapies could be designed to target specific tissues in the eye once injected locally and supports the possibility that exosomal therapies targeting eye tissues could be administered intravenously, significantly reducing the cost of treating patients.
Exosome-based therapies have a number of potential applications in the eye. As already discussed, neovascularization underlies a number of eye diseases including neovascular AMD, diabetic retinopathy, macular edema, neovascular glaucoma and corneal neovascularization (Neely and Gardner, 1998). A number of groups have demonstrated anti-angiogenic properties of exosomes. For example, exosomes from retinal astrocytes have anti-angiogenic components that were able to suppress vessel leakage and inhibit choroidal neovascularization in a mouse laser CNV model (Hajrasouliha et al., 2013). Exosomes containing the membrane-bound Notch ligand Dll4 suppress vascular sprouting, a fundamental part of angiogenesis (Sharghi-Namini et al., 2014). Anti-VEGF therapies are effective in many of these ocular neovascularization diseases and exosomes derived from mesenchymal stem cells can suppress angiogenesis by down regulating the expression of VEGF, partly due to the microRNA miR-16 (Lee et al., 2013).
Inflammation and fibrosis in the retina, leading to macular degeneration, and in the cornea, leading to dry eye disease are hypothesized to be mediated by activation of immune cells (Cousins et al., 2004; Ishikawa et al., 2016; Pflugfelder, 2004). The immunomodulatory effects of exosomes may be used to address these pathologies. For example, mesenchymal stem cell-derived exosomes possess anti-inflammatory properties that may be applicable to inflammatory eye diseases (Blazquez et al., 2014; Zhang et al., 2014). As mentioned previously, RPE cells are thought to use exosomes to modulate local immune responses by killing monocytes (Knickelbein et al., 2016). Exosomes also appear to deliver anti-inflammatory drugs to microglial cells to suppress neuroinflammation (Zhuang et al., 2011) or αB-crystallin to the neural retina, which could act as neuroprotection to photoreceptors (Sreekumar et al., 2010). Exosomes may also be able to facilitate neural repair. For example, MiR-133b containing exosomes transferred this microRNA to astrocytes and neurons in rats resulting in changes to gene expression that led to neurite remodeling and recovery from stroke (Xin et al., 2013). These neuroprotective effects of exosomes from mesenchymal cells have recently been shown useful in supporting retinal ganglion cells in a model of glaucoma (Mead and Tomarev, 2017). Finally, exosomes can induce proliferation in a number cell types (Deregibus et al., 2007; Jeong et al., 2014; Raimondo et al., 2015). Proliferation of TM cells can restore IOP homeostasis in animal models of glaucoma (Zhu et al., 2016).
We speculate that future studies will determine the minimal essential components of exosomes that mediate the anti-angiogenic, anti-inflammatory, neuroprotective and proliferative effects mentioned above. In combination with targeted delivery methods, engineered exosomes will likely be a viable therapy for the treatment of numerous eye diseases. Further, the heterogeneous nature of many eye diseases means biomarkers will help guide the design of exosomal therapies to provide a personalized, highly effective treatment as outlined in Fig. 6.
9. Conclusions and future directions
The etiology of a number of eye diseases involve activation of immune cells, inflammation, degeneration of neurons, neovascularization and fibrosis (Cousins et al., 2004; Hernandez et al., 1990; Howell et al., 2013; Ishikawa et al., 2016; Neely and Gardner, 1998; Pflugfelder, 2004; Tektas and Lutjen-Drecoll, 2009; Vranka et al., 2015). As discussed in this review, exosomes are likely mediating some, if not all of these effects. More importantly, the use of exosomes has been experimentally shown to predict or combat these processes. While the eye field is significantly trailing other fields in exosome research (Fig. 1, inset), the framework created by these fields will allow for rapid acceleration of exosome research in the eye.
To aid in this endeavor, in Fig. 7 we have indicated a select subset of the published exosome and small EV studies in different parts of the eye. In addition, in supplementary table S3 we have listed all eye-related exosome studies to date (excluding review articles) with brief descriptions of the exosome tissue/cell origin, isolation methods, analysis methods, and main findings in each study. Using exosomes as biomarkers or therapeutic vehicles hold the potential to lead to better, personalized treatments for patients with eye diseases, as outlined in Fig. 6. This summary emphasizes the immense research opportunities that exist to understand the physiological role and clinical potential of exosomes in ocular health and disease.
Figure 7. Select exosome studies in the eye.
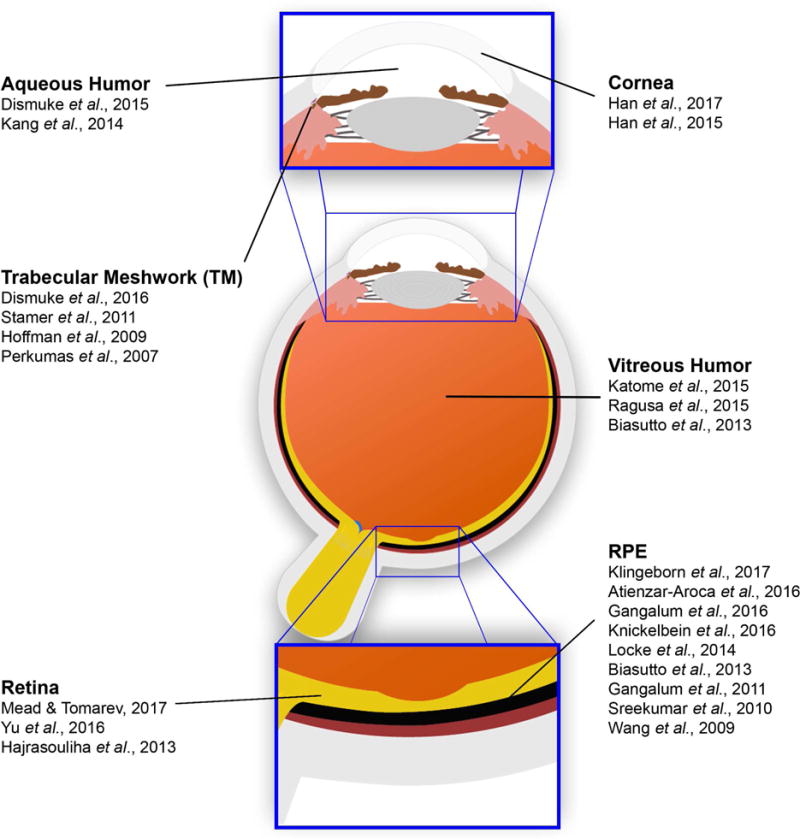
Exosome and small EV research in the eye has to date been limited. Exosome studies in different parts of the eye are indicated. Corresponding citations are shown in the reference list at the end of the article. TM, trabecular meshwork; RPE, retinal pigmented epithelium.
Supplementary Material
Acknowledgments
The authors thank Dr. Nikolai Skiba for mass spectrometric analyses. This study was supported by NIH EY023468 (WMD), EY 026161 (CBR), EY023287 (WDS), EY022359 (WDS), EY019696 (WDS), P30 EY005722 (Core grant), the BrightFocus Foundation M2015221 (MK), a Glaucoma Research Foundation Shaffer Grant (WMD, WDS), and the Foundation Fighting Blindness (CBR). In addition, Duke University Department of Ophthalmology is supported by an unrestricted grant to the Duke Eye Center from Research to Prevent Blindness (RPB).
Abbreviations
- MVE
multivesicular endosome
- EVs
extracellular vesicles
- DCs
dendritic cells
- RPE
retinal pigmented epithelium
- CNV
choroidal neovascularization
- FasL
Fas ligand
- ECM
extracellular matrix
- LC
lamina cribrosa
- TM
trabecular meshwork
- MMPs
matrix metalloproteinases
- AMD
age-related macular degeneration
- ApoE
apolipoprotein E
- PCP
protein correlation profiling
- AH
aqueous humor
- PEG
polyethylene glycol
- VEGF
vascular endothelial growth factor
- PEDF
pigment epithelium-derived factor
- fn
fibronectin
- dex
dexamethasone
Glossary
- Apoptotic bodies or blebs
Large portions (1–5 microns) of cells undergoing apoptosis which are released into the extracellular milieu by blebbing from the plasma membrane
- Ectosome
Sometimes used to indicate neutrophil- or monocyte-derived MVs specifically, sometimes used to indicate MVs from any cell type
- Endosome
Membrane-bound compartment inside eukaryotic cells. It is a compartment of the endocytic membrane transport pathway originating from the trans-Golgi membrane
- ESCRT
Endosomal sorting complexes required for transport (ESCRT). Are made up of cytosolic protein complexes ESCRT-0 through –III
- Exosome
Smallest subset of EVs. Of endolysosomal origin and released to extracellular milieu upon fusion of MVE with plasma membrane
- Extracellular vesicle (EV)
Any lipid bilayer vesicle released from the cell in a controlled manner in an approximate size range of 30–1,000 nm. Does not include apoptotic blebs/bodies
- Invadopodia
Newer, broader term replacing the terms invados ome, podosome and PILS
- Invadosome, podosome, PILS
Any of several actin-rich adhesion structures. Podosome or invadopodia-like structures (PILS)
- Lipoprotein particle
HDL, LDL, VLDL & Chylomicrons. Particles containing lipids, cholesterol and apolipoproteins
- Lysosome
Organelle with an acidic interior containing a large range of digestive enzymes used primarily for digestion and removal of excess or damaged organelles, proteins, and engulfed viruses or bacteria
- Multivesicular endosome (MVE)
ESCRT complexes generate exosomes by a controlled inward budding process from the endosome membraneresulting in an MVE
- Microparticle, microvesicle (MP, MV)
EVs budding directly from the plasma membrane into the extracellular milieu. Approximate size range 150–1,000 nm
- Proteasome
A multisubunit enzyme complex that plays a central role in the degradation of proteins that have been tagged by ubiquitin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aass C, Norheim I, Eriksen EF, Thorsby PM, Pepaj M. Single unit filter-aided method for fast proteomic analysis of tear fluid. Anal Biochem. 2015;480:1–5. doi: 10.1016/j.ab.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Abdelfattah NS, Amgad M, Zayed AA, Hussein H, Abd El-Baky N. Molecular underpinnings of corneal angiogenesis: advances over the past decade. Int J Ophthalmol. 2016;9:768–779. doi: 10.18240/ijo.2016.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablonczy Z, Dahrouj M, Tang PH, Liu Y, Sambamurti K, Marmorstein AD, Crosson CE. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest Ophthalmol Vis Sci. 2011;52:8614–8620. doi: 10.1167/iovs.11-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Hassan DW, Li X, Ryan EI, Acott TS, Kelley MJ. Induced pluripotent stem cells restore function in a human cell loss model of open-angle glaucoma. Stem Cells. 2015;33:751–761. doi: 10.1002/stem.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A. Concentration of Epstein-Barr virus from cell culture fluids with polyethylene glycol. J Gen Virol. 1973;20:391–394. doi: 10.1099/0022-1317-20-3-391. [DOI] [PubMed] [Google Scholar]
- Aga M, Bradley JM, Keller KE, Kelley MJ, Acott TS. Specialized podosome- or invadopodia-like structures (PILS) for focal trabecular meshwork extracellular matrix turnover. Invest Ophthalmol Vis Sci. 2008;49:5353–5365. doi: 10.1167/iovs.07-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnifili L, Pieragostino D, Mastropasqua A, Fasanella V, Brescia L, Tosi GM, Sacchetta P, Mastropasqua L. Molecular biomarkers in primary open-angle glaucoma: from noninvasive to invasive. Prog Brain Res. 2015;221:1–32. doi: 10.1016/bs.pbr.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Ahmad S. Concise review: limbal stem cell deficiency, dysfunction, and distress. Stem Cells Transl Med. 2012;1:110–115. doi: 10.5966/sctm.2011-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shamekh S, Goldberg JL. Retinal repair with induced pluripotent stem cells. Transl Res. 2014;163:377–386. doi: 10.1016/j.trsl.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–579. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- Alvarado JA, Katz LJ, Trivedi S, Shifera AS. Monocyte modulation of aqueous outflow and recruitment to the trabecular meshwork following selective laser trabeculoplasty. Arch Ophthalmol. 2010;128:731–737. doi: 10.1001/archophthalmol.2010.85. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis revisited. Progress in retinal and eye research. 2010;29:95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G, Fais S. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antes TJ, Kwei K, Wu F. Methods for microvesicle isolation and selective removal. System Biosciences. 2013 https://patents.google.com/patent/US9005888B9005882.
- Atay S, Banskota S, Crow J, Sethi G, Rink L, Godwin AK. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A. 2014;111:711–716. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienzar-Aroca S, Flores-Bellver M, Serrano-Heras G, Martinez-Gil N, Barcia JM, Aparicio S, Perez-Cremades D, Garcia-Verdugo JM, Diaz-Llopis M, Romero FJ, Sancho-Pelluz J. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J Cell Mol Med. 2016a doi: 10.1111/jcmm.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienzar-Aroca S, Flores-Bellver M, Serrano-Heras G, Martinez-Gil N, Barcia JM, Aparicio S, Perez-Cremades D, Garcia-Verdugo JM, Diaz-Llopis M, Romero FJ, Sancho-Pelluz J. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J Cell Mol Med. 2016b;20:1457–1466. doi: 10.1111/jcmm.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- Balaj L, Atai NA, Chen W, Mu D, Tannous BA, Breakefield XO, Skog J, Maguire CA. Heparin affinity purification of extracellular vesicles. Sci Rep. 2015;5:10266. doi: 10.1038/srep10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC. The use of cell lines to “model” ocular tissues: cautionary tales. Invest Ophthalmol Vis Sci. 2013;54:5720. doi: 10.1167/iovs.13-12873. [DOI] [PubMed] [Google Scholar]
- Biasutto L, Chiechi A, Couch R, Liotta LA, Espina V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp Cell Res. 2013;319:2113–2123. doi: 10.1016/j.yexcr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, Casado JG. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front Immunol. 2014;5:556. doi: 10.3389/fimmu.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkinsop TA, Corneo B, Temple S, Stern JH. Ophthalmologic stem cell transplantation therapies. Regen Med. 2012;7:32–39. doi: 10.2217/rme.12.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha VL. Age and disease-related structural changes in the retinal pigment epithelium. Clin Ophthalmol. 2008;2:413–424. doi: 10.2147/opth.s2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes Rickman C, Farsiu S, Toth CA, Klingeborn M. Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci. 2013;54:ORSF68–80. doi: 10.1167/iovs.13-12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Carr AJ, Smart MJ, Ramsden CM, Powner MB, da Cruz L, Coffey PJ. Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci. 2013;36:385–395. doi: 10.1016/j.tins.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Carver KA, Yang D. N-Acetylcysteine Amide Protects Against Oxidative Stress-Induced Microparticle Release From Human Retinal Pigment Epithelial Cells. Invest Ophthalmol Vis Sci. 2016;57:360–371. doi: 10.1167/iovs.15-17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Schorey JS. Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur J Immunol. 2013;43:3279–3290. doi: 10.1002/eji.201343727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004;122:1013–1018. doi: 10.1001/archopht.122.7.1013. [DOI] [PubMed] [Google Scholar]
- Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, Buzas EI, Lotvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Johnson M. Structure, Function, and Pathology of Bruch’s Membrane. In: Ryan SJ, editor. Retina. 5th. Elsevier Inc; 2013. pp. 465–481. [Google Scholar]
- Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, Dana R, Masli S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208:1083–1092. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25:443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- Damo M, Wilson DS, Simeoni E, Hubbell JA. TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma. Sci Rep. 2015;5:17622. doi: 10.1038/srep17622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes-Neto A, Saez MJ, Lozano-Ramos I, Segui-Barber J, Martin-Jaular L, Ullate JM, Fernandez-Becerra C, Borras FE, Del Portillo HA. Size-exclusion chromatography as a standalone methodology identifies novel markers in mass spectrometry analyses of plasma-derived vesicles from healthy individuals. J Extracell Vesicles. 2015;4:27378. doi: 10.3402/jev.v4.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- Dhondt B, Rousseau Q, De Wever O, Hendrix A. Function of extracellular vesicle-associated miRNAs in metastasis. Cell Tissue Res. 2016 doi: 10.1007/s00441-016-2430-x. [DOI] [PubMed] [Google Scholar]
- Dismuke WM, Challa P, Navarro I, Stamer WD, Liu Y. Human aqueous humor exosomes. Exp Eye Res. 2015;132:73–77. doi: 10.1016/j.exer.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismuke WM, Klingeborn M, Stamer WD. Mechanism of Fibronectin Binding to Human Trabecular Meshwork Exosomes and Its Modulation by Dexamethasone. PLoS One. 2016;11:e0165326. doi: 10.1371/journal.pone.0165326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismuke WM, McKay BS, Stamer WD. Myocilin, a component of a membrane-associated protein complex driven by a homologous Q-SNARE domain. Biochemistry. 2012;51:3606–3613. doi: 10.1021/bi300073r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan RK, Hill SE, Freeman DM, Nguyen E, Orwig SD, Turnage KC, Lieberman RL. Structural basis for misfolding in myocilin-associated glaucoma. Hum Mol Genet. 2015;24:2111–2124. doi: 10.1093/hmg/ddu730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC. Optic nerve head biomechanics in aging and disease. Exp Eye Res. 2015;133:19–29. doi: 10.1016/j.exer.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Decreased membrane complement regulators in the retinal pigmented epithelium contributes to age-related macular degeneration. J Pathol. 2013;229:729–742. doi: 10.1002/path.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi KB, Fijalkowski N, Cano M, Handa JT. Oxidized low-density-lipoprotein-induced injury in retinal pigment epithelium alters expression of the membrane complement regulatory factors CD46 and CD59 through exosomal and apoptotic bleb release. Adv Exp Med Biol. 2014;801:259–265. doi: 10.1007/978-1-4614-3209-8_33. [DOI] [PubMed] [Google Scholar]
- Eichler W, Yafai Y, Keller T, Wiedemann P, Reichenbach A. PEDF derived from glial Muller cells: a possible regulator of retinal angiogenesis. Exp Cell Res. 2004;299:68–78. doi: 10.1016/j.yexcr.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Eldh M, Olofsson Bagge R, Lasser C, Svanvik J, Sjostrand M, Mattsson J, Lindner P, Choi DS, Gho YS, Lotvall J. MicroRNA in exosomes isolated directly from the liver circulation in patients with metastatic uveal melanoma. BMC Cancer. 2014;14:962. doi: 10.1186/1471-2407-14-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbani J, Aberdam D, Larghero J, Vanneaux V. Pluripotent Stem Cells and Other Innovative Strategies for the Treatment of Ocular Surface Diseases. Stem Cell Rev. 2016;12:171–178. doi: 10.1007/s12015-016-9643-y. [DOI] [PubMed] [Google Scholar]
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The alphavbeta6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290:4545–4551. doi: 10.1074/jbc.C114.617662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangsmyr L, Baranov V, Nagaeva O, Stendahl U, Kjellberg L, Mincheva-Nilsson L. Cytoplasmic microvesicular form of Fas ligand in human early placenta: switching the tissue immune privilege hypothesis from cellular to vesicular level. Mol Hum Reprod. 2005;11:35–41. doi: 10.1093/molehr/gah129. [DOI] [PubMed] [Google Scholar]
- Gangalum RK, Atanasov IC, Zhou ZH, Bhat SP. AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem. 2011;286:3261–3269. doi: 10.1074/jbc.M110.160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangalum RK, Bhat AM, Kohan SA, Bhat SP. Inhibition of the Expression of the Small Heat Shock Protein alphaB-Crystallin Inhibits Exosome Secretion in Human Retinal Pigment Epithelial Cells in Culture. J Biol Chem. 2016;291:12930–12942. doi: 10.1074/jbc.M115.698530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- Gobeil S, Letartre L, Raymond V. Functional analysis of the glaucoma-causing TIGR/myocilin protein: integrity of amino-terminal coiled-coil regions and olfactomedin homology domain is essential for extracellular adhesion and secretion. Exp Eye Res. 2006;82:1017–1029. doi: 10.1016/j.exer.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Falcon-Perez JM. Cell-derived extracellular vesicles as a platform to identify low-invasive disease biomarkers. Expert Rev Mol Diagn. 2015;15:907–923. doi: 10.1586/14737159.2015.1043272. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, Naeem A, Blackford SJ, Georgiadis A, Lakowski J, Hubank M, Smith AJ, Bainbridge JW, Sowden JC, Ali RR. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31:741–747. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottanka J, Johnson DH, Grehn F, Lutjen-Drecoll E. Histologic findings in pigment dispersion syndrome and pigmentary glaucoma. J Glaucoma. 2006;15:142–151. doi: 10.1097/00061198-200604000-00011. [DOI] [PubMed] [Google Scholar]
- Goyal A, Srivastava A, Sihota R, Kaur J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr Eye Res. 2014;39:823–829. doi: 10.3109/02713683.2011.556299. [DOI] [PubMed] [Google Scholar]
- Grant WM. Clinical measurements of aqueous outflow. AMA Arch Ophthalmol. 1951;46:113–131. doi: 10.1001/archopht.1951.01700020119001. [DOI] [PubMed] [Google Scholar]
- Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- Grigor’eva AE, Tamkovich SN, Eremina AV, Tupikin AE, Kabilov MR, Chernykh VV, Vlassov VV, Laktionov PP, Ryabchikova EI. Characteristics of exosomes andmicroparticles discovered in human tears. Biomed Khim. 2016;62:99–106. doi: 10.18097/PBMC20166201099. [DOI] [PubMed] [Google Scholar]
- Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasnoot AM, Kuiper JJ, Hiddingh S, Schellekens PA, de Jager W, Imhof SM, Radstake TR, de Boer JH. Ocular Fluid Analysis in Children Reveals Interleukin-29/Interferon-lambda1 as a Biomarker for Juvenile Idiopathic Arthritis-Associated Uveitis. Arthritis Rheumatol. 2016;68:1769–1779. doi: 10.1002/art.39621. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Mullins RF. Molecular composition of drusen as related to substructural phenotype. Mol Vis. 1999;5:28. [PubMed] [Google Scholar]
- Hageman GS, Mullins RF, Russell SR, Johnson LV, Anderson DH. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB J. 1999;13:477–484. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- Hajivalili M, Pourgholi F, Kafil HS, Jadidi-Niaragh F, Yousefi M. Mesenchymal Stem Cells in the Treatment of Amyotrophic Lateral Sclerosis. Curr Stem Cell Res Ther. 2016;11:41–50. doi: 10.2174/1574888x10666150902095031. [DOI] [PubMed] [Google Scholar]
- Hajrasouliha AR, Jiang G, Lu Q, Lu H, Kaplan HJ, Zhang HG, Shao H. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J Biol Chem. 2013;288:28058–28067. doi: 10.1074/jbc.M113.470765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakulinen J, Sankkila L, Sugiyama N, Lehti K, Keski-Oja J. Secretion of active membrane type 1 matrix metalloproteinase (MMP-14) into extracellular space in microvesicular exosomes. J Cell Biochem. 2008;105:1211–1218. doi: 10.1002/jcb.21923. [DOI] [PubMed] [Google Scholar]
- Han H, Kampik D, Grehn F, Schlunck G. TGF-beta2-induced invadosomes in human trabecular meshwork cells. PLoS One. 2013;8:e70595. doi: 10.1371/journal.pone.0070595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KY, Dugas-Ford J, Seiki M, Chang JH, Azar DT. Evidence for the Involvement of MMP14 in MMP2 Processing and Recruitment in Exosomes of Corneal Fibroblasts. Invest Ophthalmol Vis Sci. 2015;56:5323–5329. doi: 10.1167/iovs.14-14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy KM, Hoffman EA, Gonzalez P, McKay BS, Stamer WD. Extracellular trafficking of myocilin in human trabecular meshwork cells. J Biol Chem. 2005;280:28917–28926. doi: 10.1074/jbc.M504803200. [DOI] [PubMed] [Google Scholar]
- Hasan MZ, Ikawati M, Tocharus J, Kawaichi M, Oka C. Abnormal development of placenta in HtrA1-deficient mice. Dev Biol. 2015;397:89–102. doi: 10.1016/j.ydbio.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Andrzejewska WM, Neufeld AH. Changes in the extracellular matrix of the human optic nerve head in primary open-angle glaucoma. Am J Ophthalmol. 1990;109:180–188. doi: 10.1016/s0002-9394(14)75984-7. [DOI] [PubMed] [Google Scholar]
- Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA. Ectosomes released by human neutrophils are specialized functional units. J Immunol. 1999;163:4564–4573. [PubMed] [Google Scholar]
- Hillier RJ, Ojaimi E, Wong DT, Mak MY, Berger AR, Kohly RP, Kertes PJ, Forooghian F, Boyd SR, Eng K, Altomare F, Giavedoni LR, Nisenbaum R, Muni RH. Aqueous Humor Cytokine Levels as Biomarkers of Disease Severity in Diabetic Macular Edema. Retina. 2016 Jul 28; doi: 10.1097/IAE.0000000000001210. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hiltbrunner S, Larssen P, Eldh M, Martinez-Bravo MJ, Wagner AK, Karlsson MC, Gabrielsson S. Exosomal cancer immunotherapy is independent of MHC molecules on exosomes. Oncotarget. 2016;7:38707–38717. doi: 10.18632/oncotarget.9585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EA, Perkumas KM, Highstrom LM, Stamer WD. Regulation of myocilin-associated exosome release from human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2009;50:1313–1318. doi: 10.1167/iovs.08-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme PA, Solum NO, Brosstad F, Roger M, Abdelnoor M. Demonstration of platelet-derived microvesicles in blood from patients with activated coagulation and fibrinolysis using a filtration technique and western blotting. Thromb Haemost. 1994;72:666–671. [PubMed] [Google Scholar]
- Hong BS, Cho JH, Kim H, Choi EJ, Rho S, Kim J, Kim JH, Choi DS, Kim YK, Hwang D, Gho YS. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CS, Funk S, Muller L, Boyiadzis M, Whiteside TL. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J Extracell Vesicles. 2016;5:29289. doi: 10.3402/jev.v5.29289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini HM, Fooladi AA, Nourani MR, Ghanezadeh F. The role of exosomes in infectious diseases. Inflamm Allergy Drug Targets. 2013;12:29–37. doi: 10.2174/1871528111312010005. [DOI] [PubMed] [Google Scholar]
- Howell GR, Soto I, Libby RT, John SW. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp Neurol. 2013;246:54–61. doi: 10.1016/j.expneurol.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, MacNicoll KH, Barbay JM, Porciatti V, Anderson MG, Smith RS, Clark AF, Libby RT, John SW. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest. 2012;122:1246–1261. doi: 10.1172/JCI61135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Kannan R, Hinton DR. Molecular mechanisms of subretinal fibrosis in age-related macular degeneration. Exp Eye Res. 2016;142:19–25. doi: 10.1016/j.exer.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverius PH, Laurent TC. Precipitation of some plasma proteins by the addition of dextran or polyethylene glycol. Biochim Biophys Acta. 1967;133:371–373. doi: 10.1016/0005-2795(67)90079-7. [DOI] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, Alward WL, Stone EM, Clark AF, Sheffield VC. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- Jeong D, Jo W, Yoon J, Kim J, Gianchandani S, Gho YS, Park J. Nanovesicles engineered from ES cells for enhanced cell proliferation. Biomaterials. 2014;35:9302–9310. doi: 10.1016/j.biomaterials.2014.07.047. [DOI] [PubMed] [Google Scholar]
- Ji Y, Rong X, Ye H, Zhang K, Lu Y. Proteomic analysis of aqueous humor proteins associated with cataract development. Clin Biochem. 2015;48:1304–1309. doi: 10.1016/j.clinbiochem.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Jin J, Min H, Kim SJ, Oh S, Kim K, Yu HG, Park T, Kim Y. Development of Diagnostic Biomarkers for Detecting Diabetic Retinopathy at Early Stages Using Quantitative Proteomics. J Diabetes Res. 2016;2016:6571976. doi: 10.1155/2016/6571976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, DeKorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, Heller JP, Villasmil R, Bull ND, Martin KR, Tomarev SI. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014;137:503–519. doi: 10.1093/brain/awt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce DP, Kerin MJ, Dwyer RM. Exosome-encapsulated microRNAs as circulating biomarkers for breast cancer. Int J Cancer. 2016;139:1443–1448. doi: 10.1002/ijc.30179. [DOI] [PubMed] [Google Scholar]
- Kaipe H, Erkers T, Sadeghi B, Ringden O. Stromal cells-are they really useful for GVHD? Bone Marrow Transplant. 2014;49:737–743. doi: 10.1038/bmt.2013.237. [DOI] [PubMed] [Google Scholar]
- Kalinina Ayuso V, de Boer JH, Byers HL, Coulton GR, Dekkers J, de Visser L, van Loon AM, Schellekens PA, Rothova A, de Groot-Mijnes JD. Intraocular biomarker identification in uveitis associated with juvenile idiopathic arthritis. Invest Ophthalmol Vis Sci. 2013;54:3709–3720. doi: 10.1167/iovs.12-10865. [DOI] [PubMed] [Google Scholar]
- Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang GY, Bang JY, Choi AJ, Yoon J, Lee WC, Choi S, Yoon S, Kim HC, Baek JH, Park HS, Lim HJ, Chung H. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. Journal of proteome research. 2014;13:581–595. doi: 10.1021/pr400751k. [DOI] [PubMed] [Google Scholar]
- Katome T, Namekata K, Mitamura Y, Semba K, Egawa M, Naito T, Harada C, Harada T. Expression of intraocular peroxisome proliferator-activated receptor gamma in patients with proliferative diabetic retinopathy. J Diabetes Complications. 2015;29:275–281. doi: 10.1016/j.jdiacomp.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Kauma SW, Huff TF, Hayes N, Nilkaeo A. Placental Fas ligand expression is a mechanism for maternal immune tolerance to the fetus. J Clin Endocrinol Metab. 1999;84:2188–2194. doi: 10.1210/jcem.84.6.5730. [DOI] [PubMed] [Google Scholar]
- Keerthikumar S, Gangoda L, Liem M, Fonseka P, Atukorala I, Ozcitti C, Mechler A, Adda CG, Ang CS, Mathivanan S. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget. 2015;6:15375–15396. doi: 10.18632/oncotarget.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Vranka JA, Acott TS. Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Invest Ophthalmol Vis Sci. 2011;52:5049–5057. doi: 10.1167/iovs.10-6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilchert C, Wittmann S, Vasiljeva L. The regulation and functions of the nuclear RNA exosome complex. Nat Rev Mol Cell Biol. 2016;17:227–239. doi: 10.1038/nrm.2015.15. [DOI] [PubMed] [Google Scholar]
- King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore R, Khan M. More Than Tiny Sacks: Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. Circ Res. 2016;118:330–343. doi: 10.1161/CIRCRESAHA.115.307654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. doi: 10.1016/j.preteyeres.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Klingeborn M, Dismuke WM, Skiba NP, Kelly U, Stamer WD, Bowes Rickman C. Directional Extracellular Vesicle Proteomes Reflect Polarity-Specific Functions in Retinal Pigmented Epithelium Monolayers. Sci Rep. 2017 doi: 10.1038/s41598-017-05102-9. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickelbein JE, Liu B, Arakelyan A, Zicari S, Hannes S, Chen P, Li Z, Grivel JC, Chaigne-Delalande B, Sen HN, Margolis L, Nussenblatt RB. Modulation of Immune Responses by Extracellular Vesicles From Retinal Pigment Epithelium. Invest Ophthalmol Vis Sci. 2016;57:4101–4107. doi: 10.1167/iovs.15-18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol. 2016;228:R57–71. doi: 10.1530/JOE-15-0201. [DOI] [PubMed] [Google Scholar]
- Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, Kim YG, Jang JY, Kim CW. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8:e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RW, Nicholson LB, Sen HN, Chan CC, Wei L, Nussenblatt RB, Dick AD. Autoimmune and autoinflammatory mechanisms in uveitis. Semin Immunopathol. 2014;36:581–594. doi: 10.1007/s00281-014-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GD, Metcalf TG. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol. 1988;54:1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CM, Clark ME, Chimento MF, Curcio CA. Apolipoprotein localization in isolated drusen and retinal apolipoprotein gene expression. Invest Ophthalmol Vis Sci. 2006;47:3119–3128. doi: 10.1167/iovs.05-1446. [DOI] [PubMed] [Google Scholar]
- Liu F, Ding X, Yang Y, Li J, Tang M, Yuan M, Hu A, Zhan Z, Li Z, Lu L. Aqueous humor cytokine profiling in patients with wet AMD. Mol Vis. 2016;22:352–361. [PMC free article] [PubMed] [Google Scholar]
- Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Moller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke CJ, Congrove NR, Dismuke WM, Bowen TJ, Stamer WD, McKay BS. Controlled exosome release from the retinal pigment epithelium in situ. Exp Eye Res. 2014;129:1–4. doi: 10.1016/j.exer.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Lopatina T, Gai C, Deregibus MC, Kholia S, Camussi G. Cross Talk between Cancer and Mesenchymal Stem Cells through Extracellular Vesicles Carrying Nucleic Acids. Front Oncol. 2016;6:125. doi: 10.3389/fonc.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Thery C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Ramos I, Bancu I, Oliveira-Tercero A, Armengol MP, Menezes-Neto A, Del Portillo HA, Lauzurica-Valdemoros R, Borras FE. Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J Extracell Vesicles. 2015;4:27369. doi: 10.3402/jev.v4.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Castano ME, Csaky KG, Cousins SW. Nonlethal oxidant injury to human retinal pigment epithelium cells causes cell membrane blebbing but decreased MMP-2 activity. Invest Ophthalmol Vis Sci. 2005;46:3331–3340. doi: 10.1167/iovs.04-1224. [DOI] [PubMed] [Google Scholar]
- Matheis N, Lantz M, Grus FH, Ponto KA, Wolters D, Brorson H, Planck T, Shahida B, Pitz S, Pfeiffer N, Kahaly GJ. Proteomics of Orbital Tissue in Thyroid-Associated Orbitopathy. J Clin Endocrinol Metab. 2015;100:E1523–1530. doi: 10.1210/jc.2015-2976. [DOI] [PubMed] [Google Scholar]
- McCready J, Sims JD, Chan D, Jay DG. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. doi: 10.1186/1471-2407-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKechnie NM, King BC, Fletcher E, Braun G. Fas-ligand is stored in secretory lysosomes of ocular barrier epithelia and released with microvesicles. Exp Eye Res. 2006;83:304–314. doi: 10.1016/j.exer.2005.11.028. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol. 1995;147:642–653. [PMC free article] [PubMed] [Google Scholar]
- Mead B, Berry M, Logan A, Scott RA, Leadbeater W, Scheven BA. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015;14:243–257. doi: 10.1016/j.scr.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthalmol Vis Sci. 2013;54:7544–7556. doi: 10.1167/iovs.13-13045. [DOI] [PubMed] [Google Scholar]
- Mead B, Tomarev S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl Med. 2017 doi: 10.1002/sctm.16-0428. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Rezende FA, Miloudi K, Wilson A, Tetreault N, Hardy P, SanGiovanni JP, De Guire V, Sapieha P. MicroRNA signatures in vitreous humour and plasma of patients with exudative AMD. Oncotarget. 2016;7:19171–19184. doi: 10.18632/oncotarget.8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertts M, Garfield S, Tanemoto K, Tomarev SI. Identification of the region in the N-terminal domain responsible for the cytoplasmic localization of Myoc/Tigr and its association with microtubules. Lab Invest. 1999;79:1237–1245. [PubMed] [Google Scholar]
- Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Han KY, Onguchi T, Chang JH, Kim TI, Kojima T, Zhou Z, Azar DT. MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res. 2009;46:541–550. doi: 10.1159/000226222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mincheva-Nilsson L, Baranov V. Placenta-derived exosomes and syncytiotrophoblast microparticles and their role in human reproduction: immune modulation for pregnancy success. Am J Reprod Immunol. 2014;72:440–457. doi: 10.1111/aji.12311. [DOI] [PubMed] [Google Scholar]
- Monguio-Tortajada M, Lauzurica-Valdemoros R, Borras FE. Tolerance in organ transplantation: from conventional immunosuppression to extracellular vesicles. Front Immunol. 2014;5:416. doi: 10.3389/fimmu.2014.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavinejad M, Andrews PW, Shoraki EK. Current Biosafety Considerations in Stem Cell Therapy. Cell J. 2016;18:281–287. doi: 10.22074/cellj.2016.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu W, Rana S, Zoller M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15:875–887. doi: 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller L, Hong CS, Stolz DB, Watkins SC, Whiteside TL. Isolation of biologically-active exosomes from human plasma. J Immunol Methods. 2014;411:55–65. doi: 10.1016/j.jim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- Murthy KR, Rajagopalan P, Pinto SM, Advani J, Murthy PR, Goel R, Subbannayya Y, Balakrishnan L, Dash M, Anil AK, Manda SS, Nirujogi RS, Kelkar DS, Sathe GJ, Dey G, Chatterjee A, Gowda H, Chakravarti S, Shankar S, Sahasrabuddhe NA, Nair B, Somani BL, Prasad TS, Pandey A. Proteomics of human aqueous humor. OMICS. 2015;19:283–293. doi: 10.1089/omi.2015.0029. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Inatomi T, Sotozono C, Koizumi N, Kinoshita S. Ocular surface reconstruction using stem cell and tissue engineering. Prog Retin Eye Res. 2016;51:187–207. doi: 10.1016/j.preteyeres.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Iejima D, Akahori M, Kamei J, Goto A, Iwata T. Overexpression of HtrA1 and exposure to mainstream cigarette smoke leads to choroidal neovascularization and subretinal deposits in aged mice. Invest Ophthalmol Vis Sci. 2014;55:6514–6523. doi: 10.1167/iovs.14-14453. [DOI] [PubMed] [Google Scholar]
- Neely KA, Gardner TW. Ocular neovascularization: clarifying complex interactions. Am J Pathol. 1998;153:665–670. doi: 10.1016/S0002-9440(10)65607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Ambrosio R, Jr, Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- Nobl M, Reich M, Dacheva I, Siwy J, Mullen W, Schanstra JP, Choi CY, Kopitz J, Kretz FT, Auffarth GU, Koch F, Koss MJ. Proteomics of vitreous in neovascular age-related macular degeneration. Exp Eye Res. 2016;146:107–117. doi: 10.1016/j.exer.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Nuschke AC, Farrell SR, Levesque JM, Chauhan BC. Assessment of retinal ganglion cell damage in glaucomatous optic neuropathy: Axon transport, injury and soma loss. Exp Eye Res. 2015;141:111–124. doi: 10.1016/j.exer.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olver C, Vidal M. Proteomic analysis of secreted exosomes. Sub-cellular biochemistry. 2007;43:99–131. doi: 10.1007/978-1-4020-5943-8_7. [DOI] [PubMed] [Google Scholar]
- Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O’Connor KC, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- Overby DR, Bertrand J, Tektas OY, Boussommier-Calleja A, Schicht M, Ethier CR, Woodward DF, Stamer WD, Lutjen-Drecoll E. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Invest Ophthalmol Vis Sci. 2014;55:4922–4933. doi: 10.1167/iovs.14-14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Choi AJ, Yoon J, Lim D, Woo SJ, Park SJ, Kim HC, Chung H. Wnt modulators in the aqueous humor are associated with outer retinal damage severity in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:5522–5530. doi: 10.1167/iovs.14-14566. [DOI] [PubMed] [Google Scholar]
- Paula JS, O’Brien C, Stamer WD. Life under pressure: The role of ocular cribriform cells in preventing glaucoma. Exp Eye Res. 2016;151:150–159. doi: 10.1016/j.exer.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VL, Caspi RR. Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol. 2015;36:354–363. doi: 10.1016/j.it.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkumas KM, Hoffman EA, McKay BS, Allingham RR, Stamer WD. Myocilin-associated exosomes in human ocular samples. Exp Eye Res. 2007;84:209–212. doi: 10.1016/j.exer.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137:337–342. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, Wallace KA, Dickerson SJ, Miller MJ, Verhoeven AD, Martin JM, Wright LS, Shen W, Capowski EE, Percin EF, Perez ET, Zhong X, Canto-Soler MV, Gamm DM. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012;53:2007–2019. doi: 10.1167/iovs.11-9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer seminars in immunopathology. 2005;27:375–387. doi: 10.1007/s00281-005-0004-1. [DOI] [PubMed] [Google Scholar]
- Prendes MA, Harris A, Wirostko BM, Gerber AL, Siesky B. The role of transforming growth factor beta in glaucoma and the therapeutic implications. Br J Ophthalmol. 2013;97:680–686. doi: 10.1136/bjophthalmol-2011-301132. [DOI] [PubMed] [Google Scholar]
- Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions. J Biol Chem. 2016;291:1652–1663. doi: 10.1074/jbc.M115.686295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J, Tan SS. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal. 2012;5:ra70. doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328:1097–1106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragusa M, Barbagallo C, Statello L, Caltabiano R, Russo A, Puzzo L, Avitabile T, Longo A, Toro MD, Barbagallo D, Valadi H, Di Pietro C, Purrello M, Reibaldi M. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: Pathological and diagnostic implications. Cancer Biol Ther. 2015;16:1387–1396. doi: 10.1080/15384047.2015.1046021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo S, Saieva L, Corrado C, Fontana S, Flugy A, Rizzo A, De Leo G, Alessandro R. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun Signal. 2015;13:8. doi: 10.1186/s12964-015-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani VC, Purushothaman A, Stewart MD, Thompson CA, Vlodavsky I, Au JL, Sanderson RD. The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. FEBS J. 2013;280:2294–2306. doi: 10.1111/febs.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Kucia M, Jadczyk T, Greco NJ, Wojakowski W, Tendera M, Ratajczak J. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? Leukemia. 2012;26:1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- Rawas-Qalaji M, Williams CA. Advances in ocular drug delivery. Curr Eye Res. 2012;37:345–356. doi: 10.3109/02713683.2011.652286. [DOI] [PubMed] [Google Scholar]
- Reich M, Dacheva I, Nobl M, Siwy J, Schanstra JP, Mullen W, Koch FH, Kopitz J, Kretz FT, Auffarth GU, Koss MJ. Proteomic Analysis of Vitreous Humor in Retinal Vein Occlusion. PLoS One. 2016;11:e0158001. doi: 10.1371/journal.pone.0158001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch ZT, Fautsch MP. Glaucoma-associated myocilin: a better understanding but much more to learn. Exp Eye Res. 2009;88:704–712. doi: 10.1016/j.exer.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MF, Zhu H, Millard RW, Fan GC. Exosomes Function in Pro- and Anti-Angiogenesis. Curr Angiogenes. 2013;2:54–59. doi: 10.2174/22115528113020020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider MA, Hurwitz SN, Meckes DG., Jr ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci Rep. 2016;6:23978. doi: 10.1038/srep23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu S, Geminard C, Rabesandratana H, Sainte-Marie J, Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1. Eur J Biochem. 2000;267:583–590. doi: 10.1046/j.1432-1327.2000.01036.x. [DOI] [PubMed] [Google Scholar]
- Rizzolo LJ. Barrier properties of cultured retinal pigment epithelium. Exp Eye Res. 2014;126:16–26. doi: 10.1016/j.exer.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Liang Y, Sigal IA, Grimm J, Reynaud J, Bellezza A, Burgoyne CF, Downs JC. Correlation between local stress and strain and lamina cribrosa connective tissue volume fraction in normal monkey eyes. Invest Ophthalmol Vis Sci. 2010;51:295–307. doi: 10.1167/iovs.09-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MM, Spaeth GL, Sivalingam E, Weinreb S. Histopathology of 150 trabeculectomy specimens in glaucoma. Trans Ophthalmol Soc U K. 1976;96:245–255. [PubMed] [Google Scholar]
- Roubeix C, Godefroy D, Mias C, Sapienza A, Riancho L, Degardin J, Fradot V, Ivkovic I, Picaud S, Sennlaub F, Denoyer A, Rostene W, Sahel JA, Parsadaniantz SM, Brignole-Baudouin F, Baudouin C. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res Ther. 2015;6:177. doi: 10.1186/s13287-015-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Chowdhury U, Hann CR, Stamer WD, Fautsch MP. Aqueous humor outflow: dynamics and disease. Invest Ophthalmol Vis Sci. 2015;56:2993–3003. doi: 10.1167/iovs.15-16744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakwe AM, Koumangoye R, Guillory B, Ochieng J. Annexin A6 contributes to the invasiveness of breast carcinoma cells by influencing the organization and localization of functional focal adhesions. Exp Cell Res. 2011;317:823–837. doi: 10.1016/j.yexcr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltel F, Daubon T, Juin A, Ganuza IE, Veillat V, Genot E. Invadosomes: intriguing structures with promise. Eur J Cell Biol. 2011;90:100–107. doi: 10.1016/j.ejcb.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Santala A, Saarinen J, Kovanen P, Kuusela P. Activation of interstitial collagenase, MMP-1, by Staphylococcus aureus cells having surface-bound plasmin: a novel role of plasminogen receptors of bacteria. FEBS Lett. 1999;461:153–156. doi: 10.1016/s0014-5793(99)01440-4. [DOI] [PubMed] [Google Scholar]
- Schroder S, Palinski W, Schmid-Schonbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991;139:81–100. [PMC free article] [PubMed] [Google Scholar]
- Serra AM, Waddell J, Manivannan A, Xu H, Cotter M, Forrester JV. CD11b+ bone marrow-derived monocytes are the major leukocyte subset responsible for retinal capillary leukostasis in experimental diabetes in mouse and express high levels of CCR5 in the circulation. Am J Pathol. 2012;181:719–727. doi: 10.1016/j.ajpath.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Shao C, Zhang F, Kemp MM, Linhardt RJ, Waisman DM, Head JF, Seaton BA. Crystallographic analysis of calcium-dependent heparin binding to annexin A2. J Biol Chem. 2006;281:31689–31695. doi: 10.1074/jbc.M604502200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Choudhary MM, Schachat AP. Neovascular Age-Related Macular Degeneration. Dev Ophthalmol. 2016;55:125–136. doi: 10.1159/000438969. [DOI] [PubMed] [Google Scholar]
- Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, Chen T, Li SB, Xu M, Zhou JN, Hu G, Zhou JW. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature. 2013;494:90–94. doi: 10.1038/nature11748. [DOI] [PubMed] [Google Scholar]
- Sharghi-Namini S, Tan E, Ong LL, Ge R, Asada HH. Dll4-containing exosomes induce capillary sprout retraction in a 3D microenvironment. Sci Rep. 2014;4:4031. doi: 10.1038/srep04031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda M, Khokha R. Proteolytic factors in exosomes. Proteomics. 2013;13:1624–1636. doi: 10.1002/pmic.201200458. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Ethier CR. Biomechanics of the optic nerve head. Exp Eye Res. 2009;88:799–807. doi: 10.1016/j.exer.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Skiba NP, Spencer WJ, Salinas RY, Lieu EC, Thompson JW, Arshavsky VY. Proteomic identification of unique photoreceptor disc components reveals the presence of PRCD, a protein linked to retinal degeneration. Journal of proteome research. 2013;12:3010–3018. doi: 10.1021/pr4003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokos D, Le Panse S, Villa I, Rousselle JC, Peronet R, David B, Namane A, Mecheri S. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J Immunol. 2001;166:868–876. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS One. 2010;5:e12578. doi: 10.1371/journal.pone.0012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Babu A, Filant J, Moxley KM, Ruskin R, Dhanasekaran D, Sood AK, McMeekin S, Ramesh R. Exploitation of Exosomes as Nanocarriers for Gene-, Chemo-, and Immune-Therapy of Cancer. J Biomed Nanotechnol. 2016;12:1159–1173. doi: 10.1166/jbn.2016.2205. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Hoffman EA, Luther JM, Hachey DL, Schey KL. Protein profile of exosomes from trabecular meshwork cells. Journal of proteomics. 2011;74:796–804. doi: 10.1016/j.jprot.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Perkumas KM, Hoffman EA, Roberts BC, Epstein DL, McKay BS. Coiled-coil targeting of myocilin to intracellular membranes. Exp Eye Res. 2006;83:1386–1395. doi: 10.1016/j.exer.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol. 2013;191:5515–5523. doi: 10.4049/jimmunol.1301885. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A. Syndecan-1 and Its Expanding List of Contacts. Adv Wound Care (New Rochelle) 2015;4:235–249. doi: 10.1089/wound.2014.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanihara H, Inatani M, Koga T, Yano T, Kimura A. Proteoglycans in the eye. Cornea. 2002;21:S62–69. doi: 10.1097/01.ico.0000263121.45898.d2. [DOI] [PubMed] [Google Scholar]
- Tawara A, Varner HH, Hollyfield JG. Distribution and characterization of sulfated proteoglycans in the human trabecular tissue. Invest Ophthalmol Vis Sci. 1989;30:2215–2231. [PubMed] [Google Scholar]
- Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In: Bonifacino Juan S, et al., editors. Current protocols in cell biology. 2006. Chapter 3, Unit 3 22. [DOI] [PubMed] [Google Scholar]
- Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288:10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiaden AN, Richards PJ. The emerging roles of HTRA1 in musculoskeletal disease. Am J Pathol. 2013;182:1482–1488. doi: 10.1016/j.ajpath.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- Tombran-Tink J, Shivaram SM, Chader GJ, Johnson LV, Bok D. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci. 1995;15:4992–5003. doi: 10.1523/JNEUROSCI.15-07-04992.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toops KA, Tan LX, Lakkaraju A. A detailed three-step protocol for live imaging of intracellular traffic in polarized primary porcine RPE monolayers. Exp Eye Res. 2014;124:74–85. doi: 10.1016/j.exer.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran C, Damaser MS. Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv Drug Deliv Rev. 2015:82–83. doi: 10.1016/j.addr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Mattheolabakis G, Aldawsari H, Amiji M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin Immunol. 2015;160:46–58. doi: 10.1016/j.clim.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002;43:1068–1076. [PubMed] [Google Scholar]
- Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacological reviews. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- van Niel G, Bergam P, Di Cicco A, Hurbain I, Lo Cicero A, Dingli F, Palmulli R, Fort C, Potier MC, Schurgers LJ, Loew D, Levy D, Raposo G. Apolipoprotein E Regulates Amyloid Formation within Endosomes of Pigment Cells. Cell Rep. 2015;13:43–51. doi: 10.1016/j.celrep.2015.08.057. [DOI] [PubMed] [Google Scholar]
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierkotten S, Muether PS, Fauser S. Overexpression of HTRA1 leads to ultrastructural changes in the elastic layer of Bruch’s membrane via cleavage of extracellular matrix components. PLoS One. 2011;6:e22959. doi: 10.1371/journal.pone.0022959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikari J. Precipitation of plasma lipoproteins by PEG-6000 and its evaluation with electrophoresis and ultracentrifugation. Scand J Clin Lab Invest. 1976;36:265–268. doi: 10.1080/00365517609055259. [DOI] [PubMed] [Google Scholar]
- Vlassov VA, Li M, Zeringer E, Conrad R. Methods and compositions for exosome isolation. Life Technologies Corporation. 2013 https://www.google.com/patents/US20130273544.
- Vranka JA, Kelley MJ, Acott TS, Keller KE. Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma. Exp Eye Res. 2015;133:112–125. doi: 10.1016/j.exer.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujosevic S, Micera A, Bini S, Berton M, Esposito G, Midena E. Aqueous Humor Biomarkers of Muller Cell Activation in Diabetic Eyes. Invest Ophthalmol Vis Sci. 2015;56:3913–3918. doi: 10.1167/iovs.15-16554. [DOI] [PubMed] [Google Scholar]
- Vujosevic S, Micera A, Bini S, Berton M, Esposito G, Midena E. Proteome analysis of retinal glia cells-related inflammatory cytokines in the aqueous humour of diabetic patients. Acta Ophthalmol. 2016;94:56–64. doi: 10.1111/aos.12812. [DOI] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009a;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy, exosomes and drusen formation in age-related macular degeneration. Autophagy. 2009b;5:563–564. doi: 10.4161/auto.5.4.8163. [DOI] [PubMed] [Google Scholar]
- Wang K, Read AT, Sulchek T, Ethier CR. Trabecular meshwork stiffness in glaucoma. Exp Eye Res. 2016 Jul;:19. doi: 10.1016/j.exer.2016.07.011. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe T, Miyazono K. Roles of TGF-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- Wecker T, Hoffmeier K, Plotner A, Gruning BA, Horres R, Backofen R, Reinhard T, Schlunck G. MicroRNA Profiling in Aqueous Humor of Individual Human Eyes by Next-Generation Sequencing. Invest Ophthalmol Vis Sci. 2016;57:1706–1713. doi: 10.1167/iovs.15-17828. [DOI] [PubMed] [Google Scholar]
- Weng Y, Sui Z, Shan Y, Hu Y, Chen Y, Zhang L, Zhang Y. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst. 2016;141:4640–4646. doi: 10.1039/c6an00892e. [DOI] [PubMed] [Google Scholar]
- White JM. ADAMs: modulators of cell-cell and cell-matrix interactions. Curr Opin Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016a;126:1216–1223. doi: 10.1172/JCI81136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv Clin Chem. 2016b;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, Zode GS, Fuller JA, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto KR, Alberts BM, Benzinger R, Lawhorne L, Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970;40:734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Yang H, Thompson H, Roberts MD, Sigal IA, Downs JC, Burgoyne CF. Deformation of the early glaucomatous monkey optic nerve head connective tissue after acute IOP elevation in 3-D histomorphometric reconstructions. Invest Ophthalmol Vis Sci. 2011;52:345–363. doi: 10.1167/iovs.09-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, Dewan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- You Y, Shan Y, Chen J, Yue H, You B, Shi S, Li X, Cao X. Matrix metalloproteinase 13-containing exosomes promote nasopharyngeal carcinoma metastasis. Cancer Sci. 2015;106:1669–1677. doi: 10.1111/cas.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23:1233–1244. doi: 10.1089/scd.2013.0479. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release. 2013;172:962–974. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor ST, Chin AR, Yen Y, Wang Y, Marcusson EG, Chu P, Wu J, Wu X, Li AX, Li Z, Gao H, Ren X, Boldin MP, Lin PC, Wang SE. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Gramlich OW, Laboissonniere L, Jain A, Sheffield VC, Trimarchi JM, Tucker BA, Kuehn MH. Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc Natl Acad Sci U S A. 2016;113:E3492–3500. doi: 10.1073/pnas.1604153113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


