Abstract
Purpose
To determine, in a multicenter double-blinded placebo-controlled trial, whether maximal hepatic arterial phase breath-holding duration is affected by gadoxetate disodium administration.
Materials and Methods
Institutional review board approval was obtained for this prospective multi-institutional HIPAA-compliant study; written informed consent was obtained from all subjects. At three sites, a total of 44 volunteers underwent a magnetic resonance (MR) imaging examination in which images were acquired before and dynamically after bolus injection of gadoxetate disodium, normal saline, and gadoterate meglumine, administered in random order in a single session. The technologist and volunteer were blinded to the agent. Arterial phase breath-holding duration was timed after each injection, and volunteers reported subjective symptoms. Heart rate (HR) and oxygen saturation were monitored. Images were independently analyzed for motion artifacts by three radiologists. Arterial phase breath-holding duration and motion artifacts after each agent were compared by using the Mann-Whitney U test and the McNemar test. Factors affecting the above outcomes were assessed by using a univariate, multivariable model.
Results
Arterial phase breath holds were shorter after gadoxetate disodium (mean, 32 seconds ± 19) than after saline (mean, 40 seconds ± 17; P <.001) or gadoterate meglumine (43 seconds ± 21, P < .001) administration. In 80% (35 of 44) of subjects, arterial phase breath holds were shorter after gadoxetate disodium than after both saline and gadoterate meglumine. Three (7%) of 44 volunteers had severe arterial phase motion artifacts after gadoxetate disodium administration, one (2%; P = .62) had them after gadoterate meglumine administration, and none (P = .25) had them after saline administration. HR and oxygen saturation changes were not significantly associated with contrast agent.
Conclusion
Maximal hepatic arterial phase breath-holding duration is reduced after gadoxetate disodium administration in healthy volunteers, and reduced breath-holding duration is associated with motion artifacts.
Gadoxetate disodium is a gadolinium-based contrast agent that is used worldwide for liver magnetic resonance (MR) imaging (1–7). The key advantage of gadoxetate disodium over other contrast agents is that it enables both dynamic contrast material–enhanced imaging and hepatobiliary phase imaging within a clinically feasible examination of approximately 25–30 minutes. However, recent reports describe frequent suboptimal or nondiagnostic image quality in the hepatic arterial phase with gadoxetate disodium (8–13). This phenomenon has been termed “transient severe motion,” or TSM, as it has been attributed to motion artifact.
Although the cause of TSM remains unknown, its occurrence has been associated with several risk factors, including prior episodes of TSM, chronic obstructive pulmonary disease, and the administered dose of gadoxetate disodium (11,12). Most studies describing TSM have been retrospective and observational and have used indirect measures such as motion artifacts on MR images, subjective reports of symptoms, or vital sign changes (10–13). These measures do not directly assess patient breath-holding duration, and it is likely that the true effect of gadoxetate disodium administration on breath-holding duration has been underestimated by these indirect measures.
The purpose of this study was to determine, in a prospective multicenter randomized double-blinded placebo-controlled trial, whether maximal hepatic arterial phase breath-holding duration is affected by gadoxetate disodium administration.
Materials and Methods
This prospective multi-site study was approved by the institutional review boards of all sites and was compliant with the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all subjects prior to study activities. The study is registered in the ClinicalTrials.gov database (registration number, NCT-02431598). Three of the authors (S.B.R., C.B.S., M.R.B.) have previously been consultants for Bayer Healthcare, the makers of gadoxetate disodium. Guerbet Group, the makers of gadoterate meglumine, provided support and supplied the gadoterate meglumine used in this study. The authors who have not previously been consultants or grant awardees had control of the data and all information that might represent a conflict of interest, as well as all data and information submitted for publication.
Study Population
The study population consisted of healthy volunteers recruited prospectively at three large academic health systems. Inclusion criteria included age of 18 years or older and the ability to provide informed consent. Exclusion criteria included the following: subject unable to undergo MR imaging (eg, because of a metal implant or claustrophobia), impaired renal function (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2), and pregnancy. At sites 1 (Duke University) and 3 (UC-San Diego), renal function was evaluated with serum eGFR measurement, while at site 2 (University of Wisconsin), renal function was evaluated as part of an MR imaging safety screening questionnaire, with serum eGFR testing only in subjects who had risk factors for renal dysfunction.
We estimated that 46 volunteers would be required on the basis of a power analysis, which included the following assumptions: breath-hold capacity reduction of 15%, standard deviation of breath-hold capacities of 10 seconds with each contrast agent, paired data with correlation assumption ρ = 0.75, desired power of 0.8 (1-β), and a type I error rate (α) of .05. Forty-eight volunteers consented to be included in the study and were enrolled. Four volunteers were excluded for the following reasons: an eGFR of 52 mL/min/1.73 m2 (n = 1), incomplete questionnaire data collection (n = 1), MR imaging system malfunction preventing image data acquisition (n = 1), and protocol deviation in contrast agent bolus injection rate (n = 1). Ultimately, the study population comprised 44 subjects (mean age, 32.0 years ± 7.5; mean body mass index [BMI], 27.2 kg/m2 ± 5.6), including 20 men (mean age, 31.1 years ± 6.2; mean BMI, 26.0 kg/m2 ± 3.4) and 24 women (mean age, 32.8 years ± 8.4; mean BMI, 28.2 kg/m2 ± 6.8), including 16 subjects at site 1, 14 at site 2, and 14 at site 3.
MR Imaging Technique and Contrast Media
Subjects were positioned supine and feet first with a torso phased-array coil centered over the upper abdomen. A pulse oximeter was attached to an index finger; heart rate (HR) and peripheral capillary blood oxygenation (Spo2) were monitored continuously. All subjects underwent three “contrast medium” injections with pre- and post-contrast dynamic imaging for each injection. MR imaging systems included a 3.0-T Magnetom TIMTrio system (Siemens Healthcare, Erlangen, Germany) at site 1, a 1.5-T Signa HDxt system (GE Healthcare, Waukesha, Wis) at site 2, and a 3.0-T Signa HDxt system (GE Healthcare) at site 3. Examinations consisted of localizers followed by dynamic contrast-enhanced imaging, including precontrast, arterial, portal venous (approximately 90 seconds after injection), and late dynamic (approximately 3 minutes after injection) phases. Dynamic contrast-enhanced imaging was performed by using a three-dimensional fat-suppressed T1-weighted pulse sequence with the following parameters: repetition time msec/echo time msec, 3.8–4.1/1.6–1.8; flip angle, 9–15°; field of view, 380–420 × 380–420 mm; matrix, 256–320 × 192–224; section thickness, 3–5 mm; parallel acceleration factor, two; and acquisition time, 20 seconds.
For each of the three injections, each volunteer received either 0.025 mmol per kilogram of body weight of gadoxetate disodium (0.1 mL/kg, Eovist; Bayer Healthcare, Wayne, NJ), 0.1 mmol/kg gadoterate meglumine (0.2 mL/kg, Dotarem; Guerbet Group, Princeton, NJ), or 0.2 mL/kg normal saline in random order; all volunteers received all three agents during the study. The gadoterate meglumine and normal saline injections served as control and placebo injections, respectively. Injection rates were 2 mL/sec for all agents, followed by a 40-mL saline flush injected at 2 mL/sec. Both the volunteers and the MR imaging technologist performing the examination were blinded to the injected agent. Real-time bolus-tracking software was used to obtain late hepatic arterial phase timing for the arterial phase acquisitions. During normal saline injection, a separate member of the study team instructed the technologist to start the arterial phase acquisition approximately 15–20 seconds after the start of the injection. The unblinded study team member was instructed not to interact directly with the volunteer during the study.
Between injections, each subject was removed from the MR imaging system bore for approximately 5 minutes and was then placed back in the bore for the subsequent precontrast imaging, contrast agent injection, and dynamic postcontrast imaging.
Breath-hold Timing
For the precontrast, portal venous, and late dynamic phases, the volunteers were instructed to hold their breath in expiration at the beginning of each acquisition, then to breathe after the acquisition was completed. For the arterial phase acquisition, volunteers were instructed to hold their breath in expiration as long as possible, and when they could no longer hold their breath, to breathe and simultaneously squeeze the indicator bulb. A study team member, blinded to the injected agent, tracked the time from the end of the breath-hold instruction to the time the volunteer squeezed the indicator for each injection.
Questionnaires and HR and Oxygen Saturation Measurements
After each breath hold, the technologist asked the volunteer through the imaging unit microphone the following two questions, with responses based on a five-point scale: (a) “How difficult was it to hold your breath?” (where a score of 1 indicated not at all and score of 5, very difficult) and (b) “Do you feel short of breath now?” (where a score of 1 indicated not at all and a score of 5, very short of breath). Responses were recorded for each breath hold.
HR and Spo2 values were recorded every 5 seconds. For each volunteer, the first three values obtained before dynamic contrast-enhanced imaging were averaged and considered to represent the volunteer’s baseline values. Then, for each phase, maximum and minimum HR and Spo2 were recorded for the period of time beginning at the start of breath holding and ending at the time of the start of the next breath hold. In the case of the late dynamic phase, this period was considered to end 15 seconds after the end of the breath hold.
Image Evaluation
Each series (precontrast, arterial, portal venous, and late dynamic phases) from the dynamic contrast-enhanced studies was assigned a random code number by a study coordinator. The series order was then scrambled so that the series were no longer grouped by patient, nor could they be viewed in the order in which they were acquired. Each series was reviewed independently by three readers (B.C.A., C.M.M., and T.A.J.) who were blinded to all demographic, clinical, and laboratory data, as well as to the timing and contrast agent used for the particular series. All readers were board-certified abdominal imaging faculty members with 4, 10, and 13 years post-fellowship experience, respectively. All readers were knowledgeable about differentiating motion artifact from other common image artifacts, particularly ringing, having received training and participated in studies in which motion artifacts are graded.
A scoring system that has been shown to have a high interrater repeatability in prior studies was used. With this system, a score of 1 indicated no motion artifact; a score of 2, minimal motion artifact, no effect on diagnostic quality; a score of 3, moderate motion artifact with some, but not a severe, effect on diagnostic quality; a score of 4, severe motion artifact, images degraded but interpretable; and a score of 5, extensive motion artifact, images nondiagnostic (8). Motion scores for each imaging sequence were averaged across the three readers to produce a mean motion score for each phase. TSM was considered to be present in examinations with an arterial phase with an average motion score of 4 or greater and average motion scores of 2 or lower on the precontrast image set and either the portal venous or late dynamic image sets, as has been previously described (10).
Statistical Analysis
For arterial phase breath-holding durations, descriptive statistics were generated. Breath-holding durations were compared between contrast agent groups by using the paired-sample Wilcoxon signed rank test. The proportions of subjects whose arterial phase breath-holding durations were shorter after gadoxetate disodium and gadoterate meglumine injection than after saline injection were calculated. A univariate, multivariable model was used to assess the effect of various predictors on arterial phase breath-holding durations, including age, sex, injection volume, injected agent, imaging site, and order of injection. All potential predictors were included in the model. The predictors with the weakest significance levels were eliminated in a stepwise fashion until only statistically significant (P < .05) predictors remained.
For the motion assessments, average-measure two-way random-effects intraclass correlation coefficients (ICCs) were calculated for all motion scores by phase at the study sites and were interpreted as poor (<0.40), fair to good (0.40–0.75), or excellent (>0.75). Then, motion scores were averaged across all three readers, and descriptive statistics were generated. Then, TSM rates were calculated and compared between groups by using the McNemar test. Breath-holding durations for the gadoxetate disodium–enhanced arterial phases were compared between subjects who experienced TSM and those who did not experience TSM by using the Mann-Whitney U test. Mean motion scores for subjects with breath-hold times less than or equal to the acquisition time (20 seconds) were presented.
For the questionnaire data, change in score in each postcontrast phase was calculated as the score for the postcontrast phase minus the score for precontrast phase, yielding a change in score for arterial, portal venous, and equilibrium phases. Then, a univariate, multivariable model similar to that described for breath-holding durations was used to assess relationships between predictive factors (including age, sex, injection volume, injected agent, imaging site, and order of injection) and changes in scores from baseline.
For HR and Spo2, the percentage change from baseline values was calculated for each dynamic phase as (value during dynamic phase minus baseline value)/(baseline value). Changes were calculated separately for both maximum and minimum values in each phase. Then, a univariate, multivariable model similar to that used for breath-hold times was used to assess relationships between the predictive factors (age, sex, injection volume, injected agent, imaging site, and order of injection) and changes in vital maximum and minimum from baseline.
Statistical analyses were performed by using SPSS, version 22 (IBM, Chicago, Ill). For all tests, P < .05 was considered to indicate a significant difference.
Results
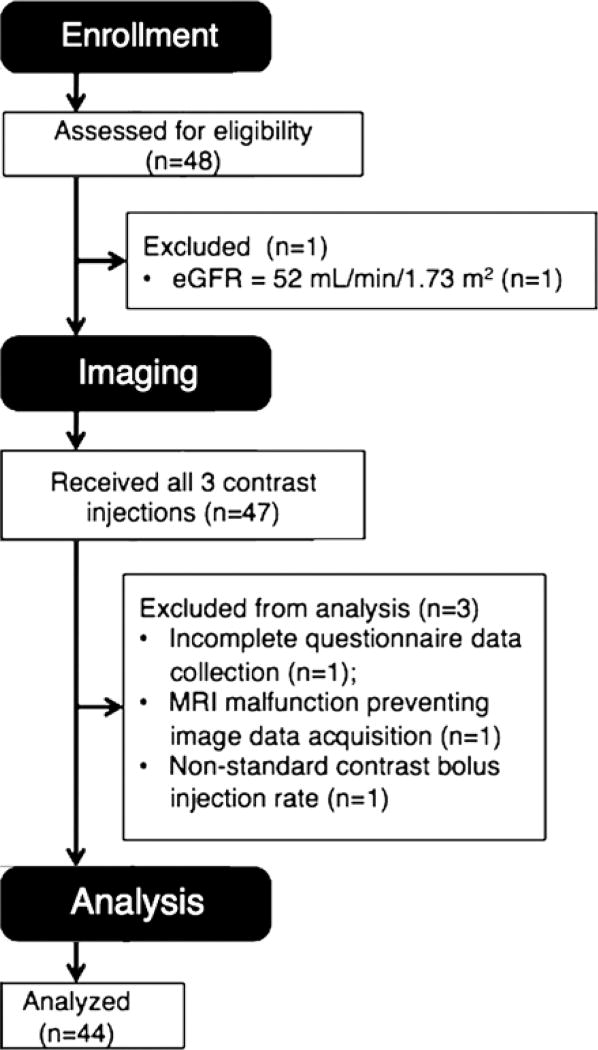
Volunteer enrollment and exclusions are summarized in Figure 1.
Figure 1.
Summary of volunteer enrollment and exclusion for this study.
Arterial Phase Breath-holding Durations
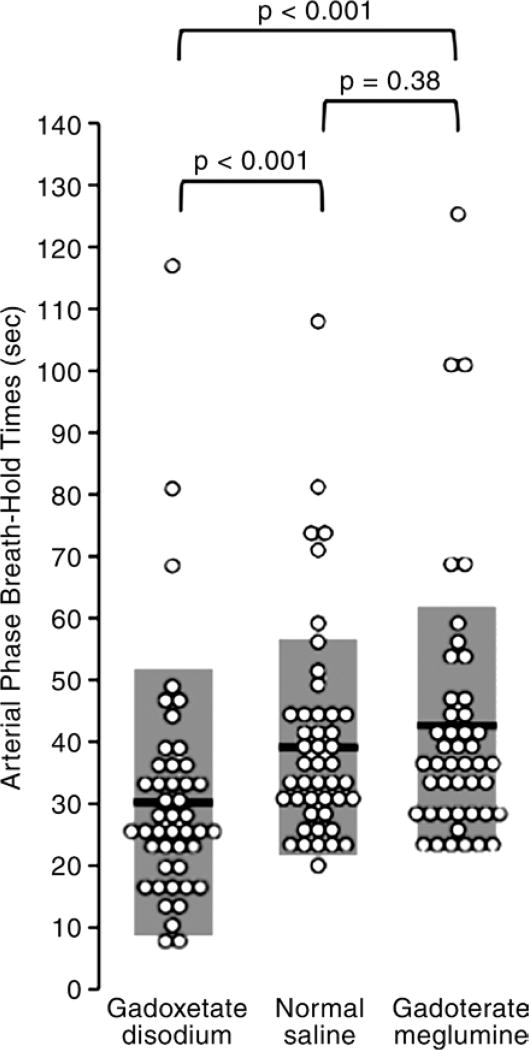
Arterial phase breath-holding durations are summarized in Figure 2. Breath holds after gadoxetate disodium administration were shorter than those after normal saline administration (32 seconds ± 19 vs 40 seconds ± 17, P < .001) or gadoterate meglumine administration (43 seconds ± 21, P < .001). Breath-holding durations after gadoterate meglumine and normal saline administration were similar (P = .38). Notably, all volunteers were able to hold their breath for at least 20 seconds after normal saline or gadoterate meglumine administration, but 12 (27%) were unable to do so after gadoxetate disodium administration.
Figure 2.
Graph shows arterial phase breath-hold times for each of the three administered agents. Breath-hold times after administration of gadoxetate disodium were significantly shorter than breath-hold times after either saline or gadoterate meglumine. Black bars and gray boxes behind the data points = means and standard deviations.
Eighty percent (35 of 44) of subjects had shorter arterial phase breath holds after gadoxetate disodium administration (median, 28 seconds; range, 8–117 seconds) than after both saline (median, 35 seconds; range, 20–108 seconds) and gadoterate meglumine (median, 38 seconds; range, 23–125 seconds). Eighty-nine percent (39 of 44) of subjects had shorter arterial phase breath holds when administered gadoxetate disodium compared with saline. Fortyseven percent (21 of 44) of subjects had shorter breath holds when administered gadoterate meglumine compared with saline. Contrast agent (P < .01), BMI (P < .001), and sex (P < .001) significantly affected breathhold duration.
Image Motion Analysis
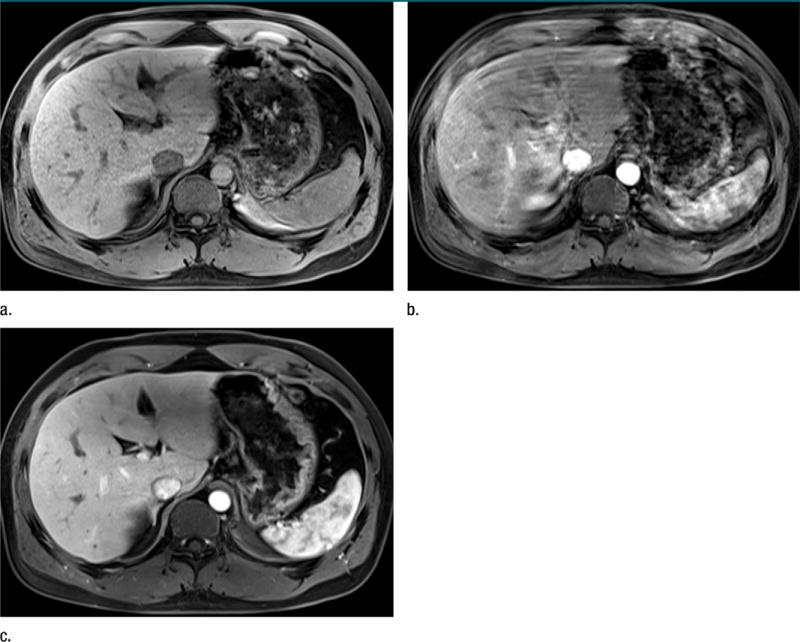
Representative arterial phase images in one of the healthy volunteers are shown in Figure 3.
Figure 3.
Representative MR images in subject 12, who experienced TSM after gadoxetate disodium administration. (a) Arterial phase image obtained after saline administration (mean motion score = 1.7, breath-holding duration = 24 seconds). (b) Arterial phase image obtained after gadoxetate disodium administration (mean motion score = 4.7, breath-holding duration = 18 seconds). (c) Arterial phase image obtained after gadoterate meglumine administration (mean motion score = 1.7, breath-holding duration = 41 seconds).
Reader agreement for motion score assignment was excellent overall (ICC = 0.83) and for each dynamic phase (precontrast ICC = 0.85, arterial phase ICC = 0.89, portal venous ICC = 0.76, late dynamic ICC = 0.79).
After gadoxetate disodium administration, 7% (three of 44) of subjects experienced TSM, on the basis of image motion scores. After gadoterate meglumine administration, 2% (one of 44, P = .63) had TSM, while none (P = .25) had TSM after saline administration. No subjects had TSM related to both gadoxetate disodium and gadoterate meglumine.
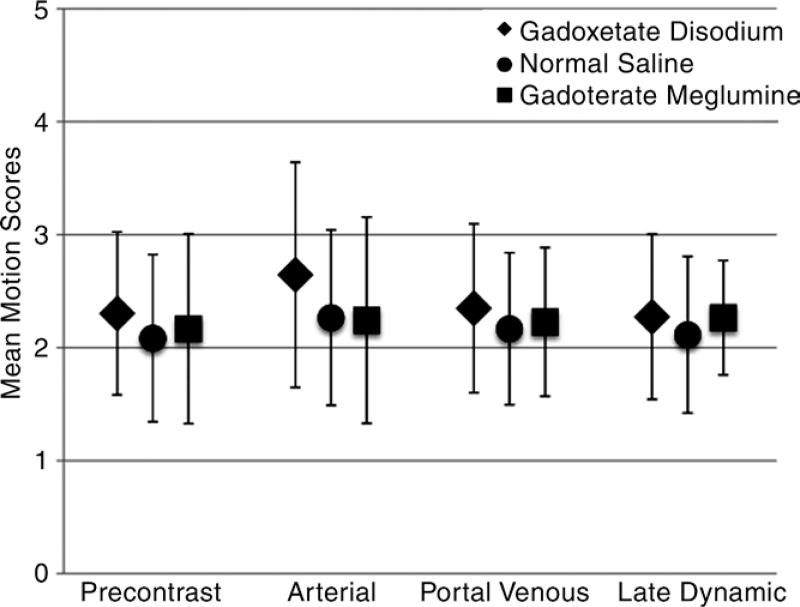
Mean motion scores are summarized in Figure 4, and motion scores for subjects with breath-holding durations of less than 20 seconds after administration of gadoxetate disodium are presented in Table 1; all volunteers were able to hold their breaths for at least 20 seconds after the administration of saline and gadoterate meglumine. These nine subjects had arterial phase breath holds that ranged from 8 to 18 seconds. All nine were able to sustain longer arterial phase breath holds after gadoterate meglumine (range, 23–41 seconds) and saline (range, 24–36 seconds). All three of the subjects who experienced TSM were in this group and had mean arterial phase breath-hold times of 13 seconds ± 4 after gadoxetate disodium, 31 seconds ± 7 after gadoterate meglumine, and 32 seconds ± 6 after saline, which were lower than the breath-hold times for the rest of the volunteers (34 seconds ± 19 for gadoxetate disodium, 44 seconds ± 21 for gadoterate meglumine, and 40 seconds ± 19 for saline; P < .01 for all). Additionally, three other subjects in this group were considered to have baseline motion, but all three of those had severe motion (motion scores of 4.7–5) in the arterial phase after gadoxetate disodium administration.
Figure 4.
Graph shows mean motion scores according to contrast agent and dynamic phase. Note the significant increase in mean motion scores in the arterial phase that occurred only after gadoxetate disodium administration.
Table 1.
Mean Motion Scores for Subjects with Arterial Phase Breath-hold Times of Less than 20 Seconds
| Subject No. |
Arterial Phase Breath-hold Times (sec) |
TSM | Baseline Motion* |
Mean Motion Scores | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gadoxetate Disodium |
Gadoterate Meglumine |
Saline | Precontrast Phase |
Arterial Phase |
Portal Venous Phase |
Late Dynamic Phase |
|||
| 11 | 8 | 37 | 25 | Yes | No | 1.7 | 4.7 | 2.3 | 2 |
| 15 | 9 | 29 | 32 | No | Yes | 2.7 | 5 | 2.7 | 2.3 |
| 5 | 11 | 25 | 25 | No | No | 1.3 | 2.7 | 2 | 1.7 |
| 7 | 14 | 34 | 36 | Yes | No | 2 | 4.7 | 3 | 2 |
| 6 | 15 | 38 | 27 | No | Yes | 2 | 4.7 | 4.3 | 4 |
| 37 | 17 | 23 | 35 | Yes | No | 2 | 4 | 2 | 2 |
| 4 | 18 | 30 | 34 | No | Yes | 4.7 | 4.7 | 4.7 | 4.7 |
| 12 | 18 | 41 | 24 | No | No | 2.3 | 3.3 | 2.3 | 1.7 |
| 30 | 18 | 23 | 24 | No | No | 3.3 | 2.7 | 3 | 2.7 |
Subjects with baseline motion (mean motion score > 2 in the precontrast phase or in both portal venous and late dynamic phases) were not categorized as having TSM, regardless of the arterial-phase motion scores.
Questionnaire Data and HR and Spo2 Changes
Mean precontrast responses were 1.4 ± 0.7 for question A (“How difficult was it to hold your breath?”) and 1.1 ± 0.4 for question B (“Do you feel short of breath now?”). Mean change in response in the arterial phase was 0.5 ± 0.8 for question A and 0.2 ± 0.6 for question B. Administered agent was not a significant predictor of change in response for any postcontrast phase (P = .12–.92). BMI, injection volume, and imaging site were associated with small but statistically significant changes in response to question A in some phases (all responses changed < 0.2 points). Specifically, increasing BMI was associated with higher scores for breath-holding difficulty in the arterial, portal venous, and late dynamic phases, while increasing injection volume was associated with higher scores in the arterial and portal venous phases (question A). There were no significant predictors for change in response to question B. Predictors of changes in question responses are summarized in Table 2.
Table 2.
Statistically Significant Predictors of Changes in Question Responses and Vital Signs
| Change | Precontrast Phase | Arterial Phase | Portal Venous Phase | Late Dynamic Phase |
|---|---|---|---|---|
| Change in response A | NA | BMI (P < .001); injection volume (P < .001) | BMI (P < .05); injection volume (P < .001) | BMI (P < .05); imaging site (P < .05) |
| Change in response B | NA | None | None | None |
| Change in minimum HR | None | None | None | None |
| Change in maximum HR | Imaging site (P < .05) | Age (P < .001); injection volume (P < .05) | None | None |
| Change in minimum Spo2 | None | None | None | None |
| Change in maximum Spo2 | None | Age (P < .01); BMI (P < .05) | Age (P < .005); BMI (P < .05) | Age (P < .001) |
Note.—Predictors are given as predictor (P value). P < .05 was considered to indicate a statistically significant difference. NA = not applicable. Spo2 was measured with continuous pulse oxymetry. Question A: “How difficult was it to hold your breath?” Question B: “Do you feel short of breath now?”
Mean baseline HR was 70 beats per minute ± 12, and mean baseline Spo2 was 98% ± 2. The statistical model showed that the administered agent was not a significant predictor of change in HR or Spo2 for any dynamic phase (P = .10–.99). Volunteer age, imaging site, injection volume, and BMI were associated with small but statistically significant changes in maximum HR and/or maximum Spo2 (all changes in maximum HR or Spo2 < 5%). Specifically, increasing age and injection volume were associated with slightly larger decreases in maximal HR. Increasing age predicted larger reductions in maximum Spo2 in the arterial, portal venous, and late dynamic phases, while increasing BMI predicted larger reductions in maximal Spo2 in the arterial and portal venous phases. No predictors were significantly associated with changes in minimum HR and/or minimum Spo2. Predictors of changes in HR and Spo2 are summarized in Table 2.
Discussion
In this prospective double-blinded placebo-controlled study, we demonstrated that the intravenous injection of gadoxetate disodium is associated with reduced arterial phase breath-holding duration in healthy volunteers. Prior observational research in patients has evaluated rates of TSM or subjective dyspnea, with motion rates of 5%–17% and rates of patient-reported dyspnea of up to 14% (8–13). Our study showed that 80% (35 of 44) of volunteers had reduced maximal breath-holding durations after receiving gadoxetate disodium compared with both saline and gadoterate meglumine, and 27% (12 of 44) were unable to complete the 20-second breath hold required for a standard arterial phase acquisition.
Several strategies have been described for mitigating the deleterious effects of arterial phase motion. Pietryga et al (10) showed that performing fast, multi–arterial phase imaging in a single breath hold can provide arterial phase image sets with reduced or absent motion artifacts. Inherently motion-resistant techniques, such as those using radial and spiral k-space filling trajectories, may also reduce the effects of motion.
In our study, gadoxetate disodium administration had no significant effect on subject HR or peripheral capillary oxygen saturation, confirming the findings of Motosugi et al (13). Thus, the reduced breath-holding duration associated with gadoxetate disodium administration is unlikely to be a significant acute health risk. Although we did not measure breath-holding duration beyond the arterial phase, our data set and those of many others shows normalization of motion artifacts to baseline levels within a few minutes of gadoxetate disodium injection, confirming the transience of this phenomenon.
Additionally, we found that subjects who experienced TSM in response to gadoxetate disodium administration had shorter breath-holding durations when administered saline, as compared with the rest of the volunteer cohort. This suggests that patients with low baseline breath-holding duration may be the most affected by gadoxetate disodium–associated TSM. Davenport et al (9) previously found that TSM occurred more frequently in patients with chronic obstructive pulmonary disease than in those without, although other studies did not replicate this finding. Bashir et al (11) showed that patients who experienced TSM after gadoxetate disodium administration were more likely to have additional episodes of TSM with subsequent administrations. When selecting imaging protocols for liver MR imaging examinations, a history of conditions that predispose to poor breath-holding duration, as well as prior episodes of TSM, may inform the protocol-ordering radiologist’s choice of contrast agents.
This study had several limitations. First, healthy volunteers were studied, rather than patients with liver disease. Additionally, we did not directly monitor body wall motion by use of respiratory bellows, as has been described, but rather relied on volunteer self-report for the determination of breath-holding duration and image artifacts for significant motion (13). However, our study does show that several previously reported methods for measuring the effect of gadoxetate disodium administration (visible severe motion artifacts, patient self-report, and vital sign changes) are insensitive for detecting reductions in breath-holding duration. Nonetheless, the fact that most volunteers with shortened breath-holding durations were still able to hold their breaths for the entirety of the arterial phase acquisition suggests that the reduced breath-hold capacity may have modest or infrequent impact on image quality in most patients. This may explain the recent report by Luetkens et al (14) that failed to demonstrate TSM using gadoxetate disodium compared with gadobutrol, likely because of the relatively short breath holds (14–15 seconds) used in that study.
In conclusion, maximal hepatic arterial phase breath-holding duration is reduced after gadoxetate disodium administration in healthy volunteers. This reduction in maximal achievable breath-holding duration is associated with motion artifacts in the hepatic arterial phase.
Advances in Knowledge.
-
■
Administration of gadoxetate disodium significantly reduces maximal achievable breath-holding duration (mean, 32 seconds ± 19) in the hepatic arterial phase in healthy volunteers compared with either saline (mean, 40 seconds ± 17; P < .001) or gadoterate meglumine (43 seconds ± 21; P < .001).
-
■
More than one-quarter of volunteers (27%, 12 of 44) failed to hold their breath for the required 20 seconds of a standard arterial phase acquisition after administration of gadoxetate disodium.
Implication for Patient Care.
-
■
Gadoxetate disodium administration significantly reduces maximal breath-holding duration in the arterial phase, and reduced breath holding is associated with motion artifacts.
Acknowledgments
S.B.R., C.B.S., and M.R.B. supported by Guerbet Group.
Abbreviations
- BMI
body mass index
- eGFR
estimated glomerular filtration rate
- HR
heart rate
- ICC
intraclass correlation coefficient
- Spo2
peripheral capillary blood oxygenation
- TSM
transient severe motion
Footnotes
Author contributions:
Guarantors of integrity of entire study, T.R.M., M.S.M., C.B.S., M.R.B.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, T.R.M., U.M., S.B.R., C.B.S., M.R.B.; clinical studies, U.M., M.S.M., B.C.A., T.A.J., C.M.M., C.B.S., M.R.B.; experimental studies, T.R.M., U.M., S.B.R.; statistical analysis, T.R.M., S.B.R., C.B.S., M.R.B.; and manuscript editing, all authors
Disclosures of Conflicts of Interest: T.R.M. disclosed no relevant relationships. U.M. Activities related to the present article: received grant support and personal fees from Guerbet. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. M.S.M. Activities related to the present article: institution received grant support from Guerbet, the makers of gadoterate meglumine. Activities not related to the present article: is an unpaid consultant for Bracco; institution receives support from Bristol-Myers Squibb and Siemens; owns stock in General Electric. Other relationships: disclosed no relevant relationships. B.C.A. disclosed no relevant relationships. T.A.J. disclosed no relevant relationships. C.M.M. disclosed no relevant relationships. S.B.R. Activities related to the present article: institution received grant support from Bracco Diagnostics, GE Healthcare, and Guerbet Group, the makers of gadoterate meglumine; has previously been a paid consultant to Bayer Healthcare, the makers of gadoxetate disodium. Activities not related to the present article: owns stock in Cellectar Biosciences and Elucent Medical; is a consultant for Parexel International. Other relationships: disclosed no relevant relationships. C.B.S. Activities related to the present article: institution received grant support from Guerbet Group, the makers of gadoterate meglumine; has previously been a paid consultant to Bayer Healthcare, the makers of gadoxetate disodium. Activities not related to the present article: institution has received support from Alexion, AstraZeneca, Bioclinica, Biomedical Systems, Bristol-Myers Squibb, Galmed, General Electric, Genzyme, Gilead, Isis, Janssen, NuSirt, Pfizer, Profil, Sanofi, Synageva, Takeda, and Virtualscopics; is a consultant to Bracco, Fibrogen, Merge Healthcare, Tobira, and Virtualscopics; has received grants from General Electric, Guerbet; has a CDA to discuss contracted work through institution with Icon, Intercept Pharmaceuticals, Perspectum, and Shire. Other relationships: disclosed no relevant relationships. M.R.B. Activities related to the present article: institution received grant support from Guerbet Group, the makers of gadoterate meglumine; has previously been a paid consultant to Bayer Healthcare, the makers of gadoxetate disodium. Activities not related to the present article: has received grant support from Bayer Healthcare and Siemens Healthcare. Other relationships: disclosed no relevant relationships.
References
- 1.Chen L, Zhang J, Zhang L, et al. Meta-analysis of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging for the detection of liver metastases. PLoS One. 2012;7(11):e48681. doi: 10.1371/journal.pone.0048681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Martino M, Marin D, Guerrisi A, et al. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010;256(3):806–816. doi: 10.1148/radiol.10091334. [DOI] [PubMed] [Google Scholar]
- 3.Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci. 2007;6(1):43–52. doi: 10.2463/mrms.6.43. [DOI] [PubMed] [Google Scholar]
- 4.Bashir MR, Gupta RT, Davenport MS, et al. Hepatocellular carcinoma in a North American population: does hepatobiliary MR imaging with Gd-EOB-DTPA improve sensitivity and confidence for diagnosis? J Magn Reson Imaging. 2013;37(2):398–406. doi: 10.1002/jmri.23818. [DOI] [PubMed] [Google Scholar]
- 5.Grazioli L, Bondioni MP, Haradome H, et al. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012;262(2):520–529. doi: 10.1148/radiol.11101742. [DOI] [PubMed] [Google Scholar]
- 6.Muhi A, Ichikawa T, Motosugi U, et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2011;34(2):326–335. doi: 10.1002/jmri.22613. [DOI] [PubMed] [Google Scholar]
- 7.Bashir MR, Husarik DB, Ziemlewicz TJ, Gupta RT, Boll DT, Merkle EM. Liver MRI in the hepatocyte phase with gadolinium-EOB-DTPA: does increasing the flip angle improve conspicuity and detection rate of hypointense lesions? J Magn Reson Imaging. 2012;35(3):611–616. doi: 10.1002/jmri.22850. [DOI] [PubMed] [Google Scholar]
- 8.Davenport MS, Viglianti BL, Al-Hawary MM, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013;266(2):452–461. doi: 10.1148/radiol.12120826. [DOI] [PubMed] [Google Scholar]
- 9.Davenport MS, Caoili EM, Kaza RK, Hussain HK. Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology. 2014;272(1):123–131. doi: 10.1148/radiol.14132269. [DOI] [PubMed] [Google Scholar]
- 10.Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology. 2014;271(2):426–434. doi: 10.1148/radiol.13131988. [DOI] [PubMed] [Google Scholar]
- 11.Bashir MR, Castelli P, Davenport MS, et al. Respiratory motion artifact affecting hepatic arterial phase MR imaging with gadoxetate disodium is more common in patients with a prior episode of arterial phase motion associated with gadoxetate disodium. Radiology. 2015;274(1):141–148. doi: 10.1148/radiol.14140386. [DOI] [PubMed] [Google Scholar]
- 12.Davenport MS, Bashir MR, Pietryga JA, Weber JT, Khalatbari S, Hussain HK. Dose-toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. AJR Am J Roentgenol. 2014;203(4):796–802. doi: 10.2214/AJR.13.11587. [DOI] [PubMed] [Google Scholar]
- 13.Motosugi U, Bannas P, Bookwalter CA, Sano K, Reeder SB. An investigation of transient severe motion related to gadoxetic acid-enhanced MR imaging. Radiology. 2016;279(1):93–102. doi: 10.1148/radiol.2015150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luetkens JA, Kupczyk PA, Doerner J, et al. Respiratory motion artefacts in dynamic liver MRI: a comparison using gadoxetate disodium and gadobutrol. Eur Radiol. 2015;25(11):3207–3213. doi: 10.1007/s00330-015-3736-x. [DOI] [PubMed] [Google Scholar]