Abstract
Skeletal muscle has been identified as an endocrine organ owing to its capacity to produce and secrete a variety of cytokines (myokines) and other proteins. To date, myokines have primarily been studied in response to exercise or metabolic challenges; however, numerous observations suggest that skeletal muscle may also release myokines in response to certain categories of internal or external stress exposure. Internal stress signals include oxidative or nitrosative stress, damaged or unfolded proteins, hyperthermia or energy imbalance. External stress signals, which act as indicators of organismal stress or injury in other cells, employ mediators such as catecholamines, endotoxin, alarmins, ATP and pro-inflammatory cytokines, such as tumour necrosis factor-α and interleukin-1β. External stress signals generally induce cellular responses through membrane receptor systems. In this review, we focus on the regulation of interleukin-6 (IL-6) as a prototypical stress response myokine and highlight evidence that IL-6 gene regulation in muscle is inherently organized to respond to a wide variety of internal and external stressors. Given that IL-6 can initiate protective, anti-inflammatory or restorative processes throughout the organism during life-threatening conditions, we present the argument that skeletal muscle has a physiological function as a sensor and responder to stress. Furthermore, we hypothesize that it may comprise a fundamental component of the organism’s acute stress response.
In this review, we discuss emerging evidence in support of the hypothesis that skeletal muscle plays an important physiological role as a whole-organism ‘stress sensor’ and that muscle endocrine responses to stress can contribute, and perhaps be essential to, the ability of an organism to survive life-threatening conditions. The endocrine response of skeletal muscle is still poorly understood. Much of what we do know has centred on the ability of muscles to generate and secrete cytokines in response to exercise or metabolic challenges (Pedersen & Febbraio, 2008, 2012). These cytokines have more recently been termed ‘myokines’, defined as ‘cytokines and other peptides that are produced, expressed and released by muscle fibres and exert paracrine, autocrine or endocrine effects’ (Pedersen & Febbraio, 2008). The idea that stress may also be an important stimulus for myokine production was inspired by a recent observation that heat stress stimulates interleukin-6 (IL-6) production in skeletal muscle and that IL-6 transcription can be blocked by application of heat shock factor (HSF) inhibitors (Welc et al. 2012). The heat shock response, mediated by HSFs, is the quintessential evolutionarily conserved stress response used by cells to cope with environmental and physiological stresses.
This review focuses on IL-6 as a model signal that represents a potential efferent arm of the ability of skeletal muscle to contribute to the stress response of the organism. We explore experimental evidence of how various stimuli known to accompany acute internal and external stress, injury, infection or trauma can be linked to IL-6 production in muscle and in other cell types. During the discussion, we evaluate the promoter region of the IL-6 gene to outline the relationships between known signalling pathways and their links to stress signals that can be detected by muscle. Finally, we briefly review the potential physiological significance of IL-6 in stressed tissue and in the whole organism.
An overview of cytokine responses to stress: comparison with exercise
In many forms of stress there are striking changes in circulating cytokines that have historically been attributed to the response of immune cells to endotoxin. Endotoxin is thought to arise from the breakdown of the intestinal barrier and leakage of bacterial wall fragments into the circulation. Stressors such as haemorrhagic shock (Fink, 2003), hyperthermia (Pedersen & Febbraio, 2008), burns (Deitch, 1990) and trauma (Ding et al. 2004) have all been shown to induce intestinal barrier dysfunction and to produce cytokine responses. It is, however, unknown whether endotoxin is the primary stimulus and whether immune cells are the chief effector cells.
Similar issues have historically surrounded the cytokine responses to endurance exercise. In prolonged exercise there are often elevations of circulating endotoxin, also presumably arising from intestinal barrier dysfunction (Bosenberg et al. 1988). One of the arguments against endotoxin as the stimulus for exercise-induced cytokine expression is that the time line of the release pattern and the relative concentrations of specific cytokines are distinctly different in exercise compared with endotoxin exposure (Pedersen & Febbraio, 2008). For example, the endotoxin response is characterized by an early rise in tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) and a later elevation of IL-6, interleukin-10 (IL-10) and interleukin-1 receptor antagonist (IL-1RA), whereas in exercise, pro-inflammatory cytokines (TNF-α and IL-1β) are blunted or absent, but there are marked elevations in circulating IL-6, IL-1RA and IL-10 (Pedersen & Febbraio, 2008).
Interleukin-6 has been one of the more predictable and dominant cytokines expressed following endurance exercise (Drenth et al. 1995). The cellular source of circulating IL-6 accompanying exercise was not known for many years; however, experiments sampling across the vasculature of the human leg during exercise demonstrated a net production of IL-6 secreted into the circulation. These findings were the first to show that muscle can be a significant source of circulating IL-6 (Steensberg et al. 2000). Additional experiments that demonstrated elevations in IL-6 protein within muscle cells following exercise and IL-6 protein released in high concentrations into the muscle interstitium during exercise provided important supporting data (reviewed by Pedersen & Febbraio, 2008). These experiments resulted in a paradigm shift in muscle biology, beyond our basic understanding of muscle as a motor to a realization that it may have unrecognized functions as an endocrine and immune organ. Besides IL-6, many other myokines have since been identified in various muscle preparations; these include the interleukins IL-1β, IL-8, IL-10, IL-15 and IL-18, as well as TNF-α, high-mobility group box-1 protein (HMGB1) and fibronectin type III domain containing 5 (FNDC5)-Irisin among many others, as recently reviewed by Pedersen & Febbraio (2012). Based on these observations, the source of the circulating cytokine profile seen in intense exercise cannot be attributed solely to a response to endotoxin alone. Though endotoxin cannot be excluded as a co-stimulus, it is clear that it is not a necessary prerequisite for a cytokine response to intense exercise.
Like exercise, the cytokine response to some stress exposures differs from endotoxin exposure. For example, in hyperthermia the circulating TNF-α and IL-1β responses are also either blunted or are absent, but very strong IL-6, IL-1RA and IL-10 signals occur, either during or shortly after heat exposure (Robins et al. 1995). In haemorrhagic shock, IL-6 and TNF-α elevations tend to coincide, but with a predominant IL-6 response (Mok & Moore, 2008). These patently different cytokine profiles following stress more closely resemble those seen following endurance exercise than those seen after endotoxin exposure.
Interleukin-6 is a dominant signal seen in the circulation during and following stress conditions such as hyperthermia (Welc et al. 2012). For example, in a mouse model of heat stroke, plasma IL-6 can exceed 1000 pg ml−1, measured 2 h after heat exposure (Welc et al. 2012). Equally high levels of IL-6 are seen 2 h following haemorrhagic shock in mice (Isayama et al. 2012). However, although there is ample evidence that skeletal muscles upregulate IL-6 and other myokines in stress, as we shall discuss in the following sections, the link between circulating levels observed and muscle production has not yet been established in stress, as it has in exercising muscle.
The capacity for skeletal muscles to respond to stress signals
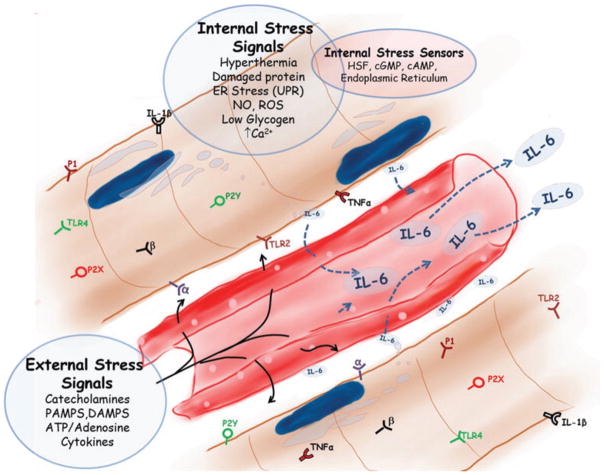
Skeletal muscles are exposed to a variety of ‘internal’ and ‘external’ stress stimuli, as illustrated in Fig. 1. Muscles appear to be able to withstand internal stress intensities that would severely damage most other tissues. Some of these internal stresses include hypoxia, mechanical stress, thermal stress, osmotic stress and oxidative stress (as reviewed by Wright et al. 2009), as well as nitrosative stress (Vassilakopoulos et al. 2003) and energy imbalance (Gleeson, 2000). However, muscle is also exposed and is sensitive, via membrane receptor systems, to blood-borne mediators of stress, such as catecholamines, bacterial wall products, ATP and its metabolites, oxidized lipids, alarmins and pro-inflammatory cytokines commonly produced in many life-threatening conditions. These might be considered ‘external stressors’ that indicate that the entire organism or surrounding tissues are being threatened (Fig. 1). In this review, we consider the evidence that skeletal muscle is equipped to function as an acute sensor of both external and internal stress signals and how these signals are linked to IL-6 expression at the genomic level.
Figure 1. The internal and external stress-related signals that skeletal muscle is equipped to detect via receptors or second messengers and that are genomically linked to regulation of the interleukin-6 (IL-6) gene.
Abbreviations: P2X, Purinergic receptor type 2 X; P2Y, Purinergic receptor type 2 Y; P1, Purinergic receptor type 1; IL-6, Interleukin-6; TNF-alpha, Tumor Necrosis Factor-alpha; IL-1Beta, Interleukin-1Beta; UPR, Unfolded Protein Response; ER, Endoplasmic Reticulum; NO, Nitric Oxide; ROS, Reactive Oxygen Species; Ca2+, Calcium; TLR 2, Toll-Like Receptor 2; TLR 4, Toll-Like Receptor 4; beta, Beta-adrenergic receptor; alpha, Alpha-adrenergic receptor; HSF, Heat Shock Factor; cGMP, cyclic guanosine monophosphate; cAMP, cyclic adenosine monophosphate; PAMPS, Pathogen associated molecular patterns; DAMPs, Damage associated molecular patterns; ATP, Adenosine Triphosphate.
External’ stress signals and IL-6 regulation
Toll-like receptors: pathogen-associated molecular patterns and damage-associated molecular patterns
The early response of the innate immune system to endotoxin or other foreign pathogens is through the initiation of an inflammatory cascade by way of a family of Toll-like receptors (TLRs; Takeda, 2005). Toll-like receptors are evolutionarily conserved receptors that were originally identified to function in recognizing microbial structures such as endotoxin, now referred to in general as pathogen-associated molecular patterns (PAMPs; Takeda, 2005). The ability of the innate immune system to mount an immune response to a pathogen, without prior exposure to an antigen, is mediated largely through TLRs. Toll-like receptors are often associated with immune cells, but can also be highly expressed on other cell phenotypes, such as skeletal muscle. Skeletal muscles express multiple TLRs that make them responsive to a wide range of different microbial components and related substances (Lemaitre et al. 1996). Frost et al. (2002) have characterized the interactions between bacterial endotoxin, lipopolysaccharide (LPS), TLR4 and cytokine production in skeletal muscle. They found muscle to be highly responsive to LPS and that the response is independent of immune cell activation. Injection of a bolus of LPS into the gastrocnemius strongly induced IL-6 mRNA in the injected leg, but not in the contralateral leg, suggesting that LPS acts directly on the muscle and not via a secondary systemic immune response (Frost et al. 2006).
Skeletal muscle is potentially exposed to multiple types of PAMPs. As mentioned, in addition to infection, many acute stress events, even strenuous exercise, can result in release of endotoxins or inflammatory mediators from the intestine (Bosenberg et al. 1988). Given that muscle represents a high percentage of body mass, approximately 40% in man, it has an enormous potential for sensing blood-borne changes, particularly in high metabolic states, such as in exercise, where both capillary cross-sectional area and turnover of interstitial fluid are elevated manyfold. In a classic experiment by Mathison et al. (1980) the accumulation of LPS in tissues of rabbits and primates at rest was characterized following an LPS injection. They found that the liver accounts for the most tissue-bound LPS (~40%) and muscle accounts for the second highest tissue-bound LPS (~5%), largely because of the large relative mass of muscle.
In addition to detecting the presence of invading micro-organisms, TLRs function in ‘sterile inflammation’ by detecting ligands derived from damaged cells in the extracellular environment. These are sometimes referred to as ‘damage-associated molecular patterns (DAMPs)’, ‘alarmins’ or ‘danger signals’ and include extracellular heat shock proteins (eHSPs), β-defensins, HMGB1, free nucleic acids from released DNA or RNA, mitochondrial membranes (which resemble bacterial membranes) and oxidized lipids (Ulevitch, 2004). They all initiate signalling events via subsets of TLRs. For example, although HSPs are well known as molecular chaperones inside the cell, some HSPs, such as Hsp72, are released into the extracellular space, particularly during times of stress, and can exhibit potent innate and acquired immune responses (Johnson & Fleshner, 2006), most probably via TLR activation (Fang et al. 2011). β-Defensins are another type of TLR ligand (Funderburg et al. 2011) that consists of antimicrobial peptides produced by skeletal muscle and other tissues (García et al. 2001). The HMGB1 protein was one of the first identified DAMPs, and though normally a constitutive nuclear protein, it is considered a cytokine, released only from damaged or dying cells (Bustin, 2002). Finally, lipids are oxidized in the presence of reactive oxygen species (ROS) or reactive nitrogen species, which are often elevated in stress, producing a variety of oxidized membrane-like products, some of which can signal via TLRs (Zmijewski et al. 2005).
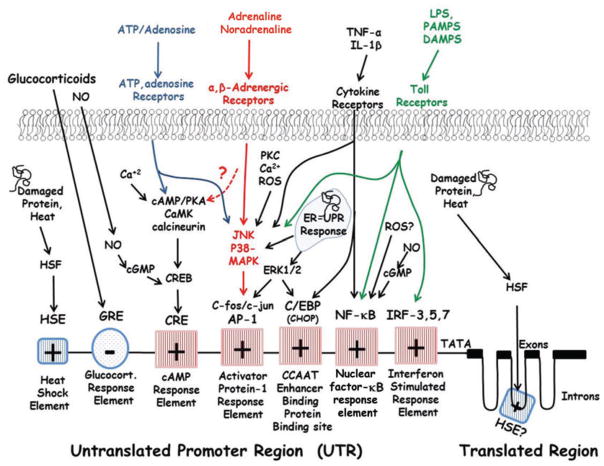
Both PAMPs and DAMPs bind to TLRs and signal through a number of proteins, such as myeloid differentiation primary response gene 88, TRI-domain-containing adapter molecule interferon-β and interleukin-1 receptor-associated kinase, ultimately leading to the activation of downstream transcription factors. Figure 2 shows the primary regulatory sites in the promoter regions for IL-6 gene transcription that communicate with TLRs and other stress signals (the basic elements reviewed by Broderick et al. 2007). Although TLRs can signal through a variety of pathways, all TLRs signal via mobilization of the P60/P65 elements of the nuclear factor-κB (NF-κB) protein, which translocate into the nucleus and activate the NF-κB response element on the promoter region of IL-6 (Patel et al. 2012). Another pathway that appears to be critical and perhaps obligatory for IL-6 signalling from some TLRs, such as TLR3 and TLR4, is via ‘interferon regulatory factor’ (IRF) activation, particularly IRF-3, -5 or -7 (Patel et al. 2012). Finally, nearly all TLRs can activate the IL-6 promoter region via mitogen-activated protein kinases (MAPKs), specifically c-Jun N-terminal kinase (JNK) and p38 MAPK (Patel et al. 2012).
Figure 2. Acute stress stimuli and their relationship to the interleukin-6 promoter region.
Note that these regulatory sites are not accurately placed in their respective location along the gene. Abbreviations: HSF, Heat Shock Factor; HSE, Heat Shock Element; GRE, Glucocorticoid Response Element; NO, Nitric Oxide; cGMP, Cyclic guanosine monophosphate; ATP, Adenosine Triphosphate; Ca2+, Calcium; cAMP, Cyclic adenosine monophosphate; PKA, Protein Kinase A; CaMK, Calcium/Calmodulin-dependent Protein Kinase; CREB, cyclic adenosine monophosphate response element-binding protein CRE, cyclic adenosine monophosphate response element; PKC, Protein Kinase C; ROS, Reactive Oxygen Species; JNK, c-Jun N-terminal kinases; p38 MAPK, p38 mitogen-activated protein kinase; AP-1, Activator Protein-1; ERK, Extracellular signal-regulated kinases; C/EBP, Ccaat-enhancer-binding proteins; CHOP, Ccaat-enhancer-binding protein homologous protein; ER, Endoplasmic reticulum; UPR, Unfolded protein response; TNF-alpha, Tumour Necrosis Factor-alpha; IL-1Beta, Interleukin-1Beta; NF-KB, Nuclear Factor Kappa Beta; PAMPS, Pathogen associated molecular patterns; DAMPS, Damage associated molecular patterns; LPS, Lipopolysaccharide; IRF, Interferon regulator factor.
The p38 and JNK pathways are often grouped together and are referred to as ‘stress-activated’ protein kinases. They induce downstream phosphorylation of c-Fos/c-Jun and the activation of activator protein 1 (AP-1). Activator protein 1 can then bind to its transcription site within the promoter region to stimulate IL-6 transcription (Matsuzawa & Ichijo, 2005; Fig. 2). This pathway is probably the most common stress-activated transcriptional pathway for IL-6. Besides TLRs, it is activated by oxidative stress, toxins, hyperosmolarity, ischaemia–reperfusion, ceramide, catecholamine activation and endoplasmic reticular (ER) stress (i.e. the unfolded protein response, UPR; Nishina et al. 2004). Importantly, our most recent understanding of contraction-induced IL-6 regulation has been shown to be highly dependent on the activation of the AP-1 response element via JNK phosphorylation and AP-1 (Whitham et al. 2012).
Catecholamines
Stressful conditions initiate a well-coordinated physiological response marked by increased secretion of the catecholamines adrenaline and noradrenaline from the sympathetic nervous system and adrenal medulla. In classic work, Walter Cannon coined the term ‘fight or flight’ to describe this response to physical or psychological stress. Later, Hans Selye suggested a consistent integrative multi-organ response to stress across a wide variety of threatening stimuli. Adrenaline acts through α- and β-receptors to elicit mainly cardiovascular and metabolic effects, but less well known is the fact that it can activate components of the innate immune system. For example, it promotes an anti-inflammatory cytokine milieu by stimulating IL-1RA, IL-10 (van der Poll et al. 1996) and pleiotropic IL-6 (Steensberg et al. 2001b). Additionally, catecholamines inhibit pro-inflammatory cytokines, such as TNF-α and IL-1β (van der Poll et al. 1996).
Both α- and β-adrenergic receptors are expressed on skeletal muscle cells (van Alphen et al. 1963). Adrenaline predominantly binds β1- and β2-adrenergic receptors and is a strong stimulant for IL-6 synthesis in skeletal muscle (Frost et al. 2004). Although less studied, noradrenaline, which binds α-receptors, also stimulates IL-6 formation in C2C12 muscle cells, over a similar dosage range to adrenaline (Frost et al. 2004). The β-receptor density on muscle is proportional to the percentage of slow-twitch fibres, and the affinity of the receptors for their antagonists is higher in fast- than in slow-twitch or mixed fibres (Martin et al. 1989). In rats, infusion of adrenaline, at a dosage sufficient to achieve plasma concentrations comparable to sepsis, yielded a 40-fold and 15-fold increase of IL-6 mRNA and IL-6 protein, respectively, in skeletal muscle (Frost et al. 2004). Others have found that only supraphysiological doses of adrenaline (>100 nM) stimulate IL-6 gene expression in skeletal muscle (Holmes et al. 2004); however, that study exposed intact soleus muscles to adrenaline for only 1 h and did not test noradrenaline. Considering the time required for diffusion, transcriptional and translational responses to occur, this may not be sufficient to see protein production in an isolated intact muscle setting. Another problem with isolated muscle experiments that we have observed is that muscle isolation is its own strong stimulus for IL-6 transcription (Welc et al. 2012), which tends to minimize the true physiological responses when treated samples are compared against sham controls.
As the most common second messenger system for catecholamine stimulation in most cell systems is cyclic AMP (cAMP), which is upstream of cyclic adenosine monophosphate response element (CRE) signalling on the IL-6 promoter region (Fig. 2), one would think that this would comprise the most likely signalling pathway for catecholamines; however, there is no evidence for this at this time. The primary pathway appears to be through the MAPK signalling described in the previous subsection for TLR activation, i.e. JNK/AP-1 (Frost et al. 2004; Fig. 2).
ATP and adenosine
Several lines of evidence have shown that extracellular ATP, ADP or adenosine causes stimulation of IL-6 formation and/or release in skeletal muscle (Buvinic et al. 2009) or in other cell phenotypes. Both ATP and its metabolites are released into the extracellular medium after pathophysiological events including hypoxia, cell swelling, shear stress or inflammation (Schwiebert & Zsembery, 2003). The fact that cellular damage is a source of extracellular ATP (Ryten et al. 2004) has resulted in ATP sometimes being included in the list of ‘alarmins’, although purinergic receptors for ATP or adenosine do not signal via the same mechanisms as TLRs (Rao & Pober, 2008).
Muscle also releases considerable quantities of ATP, AMP and adenosine during contraction (Buvinic et al. 2009). Most of the extracellular adenosine arises from the degradation of AMP or other adenine nucleotides within the extracellular space via an extracellular ecto-5′-nucleotidase. The adenosine is then resorbed for preservation of adenonucleotide concentrations in the muscle (Lynge et al. 2001). Purinergic P1 receptors are stimulated by adenosine, whereas P2 receptors are stimulated by ATP and/or ADP (Ralevic & Burnstock, 1998), and skeletal muscle sarcolemma is generally equipped with both categories of receptors (Lynge & Hellsten, 2000).
The roles of P1 adenosine receptors in IL-6 regulation in adult skeletal muscle are not well understood. In human and rat primary cultured muscle cells, the effects of adenosine on IL-6 can be inhibited by a general MAPK inhibitor, but which MAPK pathway is involved is not clear (Høier et al. 2010). In cardic fibroblasts exposure to adenosine stimulates IL-6 mRNA via the p38/AP-1 pathway and via protein kinase C-δ, which is upstream of p38 (Fig. 2; Feng et al. 2010). In cultured myotubes and in rat muscle cells extracellular adenosine stimulates G-protein-coupled cAMP activity, but the complete link with IL-6 regulation and P1 receptor activity has not been clearly established (Henning et al. 1993).
The P2 receptors can activate a number of physiological responses in muscle cells. The P2X receptors mediate rapid and selective permeability to several cations (Ralevic & Burnstock, 1998), whereas P2Y receptors reduce Cl− conductance (Voss, 2009) and stimulate G-protein-coupled activation of phospholipase C and protein kinase C (Ralevic & Burnstock, 1998). Via these multiple pathways, P2 stimulation mobilizes ‘slow-release’ intracellular Ca2+ stores, which are then linked in several ways to IL-6 transcription (Buvinic et al. 2009). In several non-skeletal muscle phenotypes there are also links between P2-ATP receptor stimulation through activation of the cAMP/PKA/CRE pathway that is synergistic with elevations in cytosolic Ca2+ (Fig. 2; Shigemoto-Mogami et al. 2001).
Inflammatory cytokines, TNF-α and IL-1β
The innate immune system responds to acute invasion of micro-organisms, as in sepsis, with a rapid increase in circulating plasma TNF-α and somewhat later with elevations in IL-1β. Skeletal muscle contains both IL-1β and TNF-α receptors, which are present in normal conditions, but are generally upregulated only in diseased or damaged muscle (Zhang et al. 2000). The response of cultured muscle cells and intact skeletal muscle (Frost et al. 2003) to inflammatory cytokines emulates the response seen in the circulation, in that marked increases in IL-6 are produced.
As shown in Fig. 2, the skeletal muscle IL-6 response to TNF-α and IL-1β is mediated in part through the JNK/AP-1 signalling network discussed previously (Frost et al. 2003). However, in addition, CCAAT enhancer binding protein C/EBP binding site (Hungness et al. 2002) and NF-κB signalling (Li et al. 1998) have been implicated as important transcriptional mediators in inflammatory cytokine-induced IL-6 formation.
Of some interest in the context of an integrated response to inflammation is the influence of anti-inflammatory signalling via glucocorticoids. As shown in Fig. 2, glucocorticoids inhibit IL-6 via the intracellular glucocorticoid receptor and glucocorticoid-responsive elements (GREs) on the promoter region (Barnes, 1998); therefore, IL-6 works in a global feedback loop where stress/inflammatory signals stimulate IL-6 production and anti-inflammatory signals from steroid release feed back to suppress IL-6 release.
‘Internal’ stress signals and IL-6 regulation
Reactive oxygen species
Interleukin-6 is elevated in cultured myotubes but not myoblasts in response to exposure to ROS (Kosmidou et al. 2002), and in humans IL-6 release from contracting skeletal muscle is inhibited by supplementation with the antioxidants vitamins C and E (Fischer et al. 2004). In vivo, short-term vitamin E supplementation reduces IL-6 mRNA and IL-6 protein production in skeletal muscle and attenuates LPS-induced increases in NF-κB activation (Huey et al. 2008). Also, reduced oxidative stress with N-acetylcysteine decreases extracellular signal-regulated kinase 1/2, p38 and NF-κB signalling proteins and also reduces IL-6 formation (Sigala et al. 2011). These data suggest that ROS are an active component of internal stress-induced IL-6 signalling.
Reactive oxygen species are generated in a variety of different stress-like conditions in skeletal muscle (as reviewed by Wright et al. 2009). For example, increased ROS are produced during both exercise and periods of inactivity, as well as during exposure to hypoxia, hyperthermia and sepsis, among many other examples. Reactive oxygen species do not have to be formed endogenously within the cell; they can also come from external sources, but nevertheless most ROS are short-lived, local signals, ideal for early signalling of local, intracellular stress. One potential stress signalling pathway that can be activated by ROS is the stress MAPKs pathway discussed above. In skeletal muscle cell culture IL-6 has been shown to be upregulated via ROS exposure through AP-1/JNK/p38 signalling (Kosmidou et al. 2002; Fig. 2). Recent studies of intact muscle have also suggested that the primary redox-sensitive pathway is via p38 MAPK (Sigala et al. 2011).
Another redox regulated signalling pathway potentially involved in IL-6 transcriptional regulation is the NF-κB family of proteins. There is considerable support for the concept that one of the chief ways in which ROS influence most transcriptional targets in oxidative stress is via NF-κB signalling (Ji et al. 2004). In addition, a number of cell culture studies using different phenotypes have shown that NF-κB activation can stimulate IL-6 transcription (Craig et al. 2000); however, most recent studies have ruled out NF-κB as the predominant signalling link between ROS and IL-6 both in intact skeletal muscles (Sigala et al. 2011) and in cardiac fibroblasts, in favour of a p38/AP-1 mechanism (Sano et al. 2001).
Reactive nitrogen species
Nitric oxide and other reactive nitrogen species may also play roles in the ability of muscle to respond to acute stress. All three NO synthases (NOSs) have been detected in skeletal muscle in various conditions (Buchwalow et al. 2005), but the neuronal isoform (nNOS) is abundantly expressed in in fast fibres and is most prevalent along the sarcolemma of fast-twitch fibres (Kobzik et al. 1994). Production of NO is significantly elevated in muscle during contraction (Kobzik et al. 1994; Pye et al. 2007), hypoxia (Javeshghani et al. 2000), endotoxin exposure (Frost et al. 2004), ischaemia–reperfusion (Thiemermann et al. 1997), crush injury (Rubinstein et al. 1998) and by exposure to inflammatory cytokines (Williams et al. 1994). Nitric oxide may mediate redox-sensitive transcriptional control via several pathways (Fig. 2) (Steensberg et al. 2007), as well as by modification of proteins within the cytoplasm or nucleus. Pharmacological inhibition of NO during exercise attenuates increases in IL-6 mRNA (Steensberg et al. 2007).
The gene regulatory link between NO and IL-6 transcription has not been entirely determined in skeletal muscle, but results from other cellular phenotypes have pointed to multiple pathways. In mononuclear cells, NO stimulates guanylyl cyclase to form cGMP, which can then activate NF-κB (Siednienko et al. 2011; Fig. 2). Interestingly, the influence of NO is not linear. High concentrations of NO, in the pathophysiological or pharmacological range, inhibit IL-6 formation, whereas low concentrations (<10 μM), common to normal physiological signalling, stimulate IL-6 (Siednienko et al. 2011). Recent reports in osteoblasts have also demonstrated the importance of cyclic adenosine monophosphate response element-binding protein (CREB)/cyclic adenosine monophosphate response element (CRE) signalling via cGMP for NO-stimulated IL-6 formation (Broderick et al. 2007; Fig. 2).
Another possible link for both reactive nitrogen species and ROS with IL-6 is through their influence on Ca2+ signalling and cytosolic Ca2+ concentrations. There are many well-known effects of ROS and reactive nitrogen species on Ca2+ regulation that are dose dependent. In general, both reactants, at low concentrations, stimulate Ca2+ release from the sarcoplasmic reticulum or activate pathways responsible for elevating cytosolic Ca2+ via intracellular stores (Favero et al. 1995). A demonstration of the influence of cellular Ca2+ on IL-6 release is shown in the application of Ca2+ ionophores, which elevate intracellular Ca2+ and stimulate IL-6 production in skeletal muscle (Holmes et al. 2004).
Hyperthermia
During intense exercise in hot environments, muscle operates about 1°C above core temperature, often reaching as high as 41°C in humans (Saltin et al. 1972), and can reach ~44°C in rats in hyperthermic exercise (Brooks et al. 1971). Muscle therefore has excellent potential as a heat sensor because of the wider dynamic range of exposure during physiological conditions. We have recently identified hyperthermia to be an independent stimulus of IL-6 in skeletal muscle (Welc et al. 2012). Interleukin-6 mRNA was upregulated at temperatures ≥41°C in a variety of experimental settings. Interestingly, the response is proportional to the intensity of heat exposure and parallels the expression of Hsp72 mRNA.
Hasselgren and colleagues have shown a link between hyperthermia, HSPs and IL-6 formation in intestinal epithelial cells (Parikh et al. 1998). Through the use of indirect activators of the heat shock transcription factors HSF-1 and HSF-2, the IL-6 mRNA and protein expression were increased in the absence of hyperthermia (Pritts et al. 2002). The stimulus is therefore likely to be via HSF interaction with the IL-6 gene. We have also provided supportive data showing that HSF inhibitor (KNK437) blocks IL-6 transcriptional responses to heat in skeletal muscle cells (Welc et al. 2012).
As a transcriptional regulator, HSF-1 is an important cofactor that functions to partly open the chromatin structure of the IL-6 promoter region, allowing other activators and repressors to bind to it more efficiently (Inouye et al. 2007). Additionally, HSF-1 affects the gene regulation of other cytokines, such as TNF-α and IL-1β. Heat shock factor-1 also has an apparent direct interaction with the C/EBP (sometimes called ‘nuclear factor (NF) of IL-6’), an essential regulator of IL-1β transcription (Xie et al. 2002) and of IL-6 transcription (Fig. 2). However, in contrast to the observed physiological effects of heat on IL-6, HSF-1 inhibits C/EBP binding to its promoter region and would therefore potentially suppress IL-6 (Fig. 2).
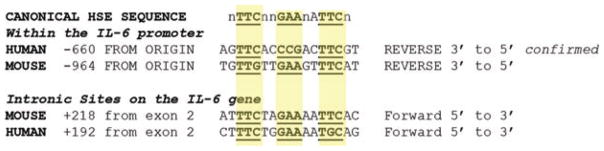
Heat shock factors primarily bind to heat shock elements (HSEs) on the DNA. Heat shock factor monomers normally present in the cytosol form trimers in protein-stress conditions that comprise the necessary configuration for nuclear translocation and DNA interaction. The HSEs on the DNA are identified as a canonical repeat of GAA-TAA nucleotide sequences with preferably two but sometimes seven intermediate nucleotides between the repeats, i.e GAAnnTTCnnGAA on the 5′–3′ strand (Yamamoto et al. 2009; Guertin et al. 2012; Fig. 3). The effectiveness of these HSE sites for binding HSF trimers has been studied in in vivo conditions (Guertin et al. 2012). In both humans and mice, putative HSE sequences have been identified on the IL-6 gene. For example, Pritts et al. (2002) identified three potential HSEs within the IL-6 promoter, although these sequences are still unproven and are atypical of most HSEs (Yamamoto et al. 2009). More classical sequences are present on the human promoter at about −660 nucleotides from the origin (Fig. 3; Inouye et al. 2007). This HSE binding site on IL-6 has been confirmed for the human gene (Inouye et al. 2007). We have identified a similar potential site in the mouse gene at −964 nucleotides from the start site (Fig. 3). Notably, few effective HSEs exactly fit the canonical sequence (Fig. 3), and relaxation of nucleotides at several locations is most often seen for in vivo binding sites (Guertin et al. 2012). Interestingly, we have found sequences outside the 5′ UTR promoter region in the human and mouse genomes that fit closely to the canonical HSE sequence (Fig. 3) and lie within the second intron (Figs 2 and 3), about 200 nucleotides from the end of the second exon. Although these sites have not yet been verified with respect to the physiological function, there are several examples of effective gene regulation by HSFs on intronic promoter regions (Lange et al. 1997; Cooper et al. 2000).
Figure 3.
Potential matches of the canonical heat shock element (HSE) sequence for heat shock factor binding in the human and mouse interleukin-6 gene
Unfolded protein response
A newly described stress signal that is emerging as a regulator of IL-6 and other cytokines, such as IL-8 and monocyte chemotactic protein-1, is the ‘unfolded protein response’ (UPR; Gargalovic et al. 2006). The UPR is initiated when the endoplasmic reticulum, where most proteins are synthesized, is placed under stress such that proteins being produced remain incomplete or unfolded. This sets up a series of signalling pathways through activation of three primary signalling proteins, i.e. protein kinase RNA-like endoplasmic reticulum kinase (PERK) inositol requiring enzyme 1 alpha (IRE1α) Activating transcription factor 6 (ATF6). These proteins release or activate second messengers, such as Activating transcription factor 4 (ATF4) and the X-box-binding protein-1, among others (Wouters & Koritzinsky, 2008). The UPR pathways are upregulated in a variety of muscle disorders and challenges, as well as following chronic loading and unloading of living muscle, and are therefore highly relevant to muscle pathophysiology (Deldicque et al. 2012).
Cytokine regulation in response to UPR is not yet well studied in skeletal muscle, but in endothelial cells and in purified B cells IL-6 transcription is greatly stimulated by UPR via ATF4 and X-box-binding protein-1 (Iwakoshi et al. 2003). The signalling systems for how UPR elevates IL-6 transcription appear to be via JNK/AP-1, NF-κB and/or Ccaat-enhancer-binding proteins (C/EBP; Deldicque et al. 2012); however, the predominant linkage between UPR and IL-6 transcription in skeletal muscle remains unexplored.
Energy imbalance
Pedersen & Febbraio (2008) suggest that the cytokine response of muscle to contraction is not inflammatory, but centred on the ongoing metabolic needs of the muscle, initiating shifts in metabolic pathways in target organs that favour energy availability. Muscle IL-6 gene expression (Keller et al. 2001) and protein release (Steensberg et al. 2001a) are intensified during times of low intramuscular glycogen. Additionally, glucose ingestion during exercise attenuates release of IL-6 protein from leg muscle (Febbraio et al. 2003).
Low glycogen depots are detrimental to moderate and intense exercise performance (Hargreaves & Richter, 1988). The drop in glycogen storage during conditions of continued energy demands could be perceived as an ‘internal stress’, in which the muscle is being asked to function in conditions that are incompatible with normal energy homeostasis. These conditions result in IL-6 upregulation and release. We speculate that when glycogen stores are compromised in conditions of stress or extreme exercise, the functional role of muscle may be switched to include a role as a more sensitive stress sensor/effector for the whole organism. For example, low intramuscular glycogen availability is necessary for contraction-induced Hsp72 gene and protein expression, (Febbraio et al. 2002), suggesting the involvement of HSFs. In addition, low glycogen during contraction leads to the phosphorylation and transfer of p38 MAPK to the nucleus (Chan et al. 2004), which can upregulate IL-6 via the AP-1 site (Fig. 2). Thus, several signalling pathways are likely to be involved with IL-6 responses to energy imbalance.
Significance of skeletal muscle as a stress sensor
This proposed function of skeletal muscle as a stress sensor would presumably provide the whole organism with an ‘early warning’ or ‘preconditioning’ message. What evolutionary advantage would this provide? First, muscle is distributed throughout the body and can sample regionally or globally. Given that it makes up to 40% of the body weight in man, it has an enormous capacity to sample and respond to stress signals coming from anywhere in the circulation. If the circulation is cut off to one organ system in trauma, muscles in other regions are available. Second, as discussed previously, muscle is incredibly resistant to stress, making it an ideal candidate as a resilient tissue capable of sensing stress in harsh environments. Third, muscle has abundant stores of protein as substrate for signalling molecules that can be used without greatly compromising the metabolism of contracting muscle cells.
Although it is likely that an array of signalling proteins could be produced by skeletal muscle in response to stress (Pedersen & Febbraio, 2008, 2012), IL-6 has many important potential functions that may make it a unique prototype. Despite its reputation as a pro-inflammatory cytokine, it inhibits the production of TNF-α, IL-1β and other pro-inflammatory cytokines from inflammatory cells (Xing et al. 1998). It also promotes formation of anti-inflammatory cytokines, such as IL-1RA and IL-10, as well as production of corticosteroids (Febbraio et al. 2003). As the primary mediator initiating the acute phase response arising from the liver and other tissues, it has a critical role in responding to infection, haemorrhage, heat stroke, malignancy or severe trauma (Heinrich et al. 1990).
Interleukin-6 also has many protective and restorative roles throughout the body, particularly in acute stress settings. For example, in haemorrhagic shock, IL-6 supplementation protects the lung from injury, protects the intestine from ischaemia and prevents circulatory collapse and cell apoptosis (Meng et al. 2000; Alten et al. 2008; Moran et al. 2009). In heart muscle, IL-6 and associated Signal transducer and activator of transcription 3 (STAT3) signalling pathways are critical for preconditioning and postconditioning stimuli that protect the ischaemic or damaged heart (Dawn et al. 2004). In heat stroke, IL-6-deficient mice have much higher mortality (Leon, 2007). Interleukin-6 has neuroprotective functions in the brain during hypoxia (Biber et al. 2008) and it promotes wound healing in a variety of tissues (McFarland-Mancini et al. 2010). Its strong effects on mobilization of energy stores in the liver, muscle and other organs during intense exercise (Pedersen & Febbraio, 2012) are also likely to play a significant role in orchestrating energy storage and release in life-threatening conditions when energy supply and delivery are compromised. Like other more classic stress hormones, such as adrenaline and noradrenaline, chronic or excessive exposure to IL-6 during inappropriate times can promote and even cause serious illnesses. As a stress hormone, therefore, IL-6 has its place and time, and it functions in a delicate balance between its life-saving and life-threatening impacts on whole-body homeostasis.
New findings.
What is the Topic of this review?
This review discusses the regulation of the interleukin-6 (IL-6) gene and how it is uniquely set up to respond to stress signals in skeletal muscle. We propose that skeletal muscle is a ‘stress sensor’ that responds by releasing highly active endocrine and paracrine proteins, such as IL-6.
What advances does this highlight?
Recent discoveries on the regulation of the IL-6 gene in various stress exposures have resulted in the emergence of a new potential role for skeletal muscle in life-threatening stress conditions. Understanding the relationship between IL-6 secretion and stress may provide new insights into normal adaptive responses to disease, deconditioning, inflammation and ageing.
Acknowledgments
Authors were supported by the BK and Betty Stevens Endowment and the American Heart Association Grant in Aid.
Footnotes
Call for comments
Readers are invited to give their opinion on this article. To submit a comment, go to: http://ep.physoc.org/letters/submit/expphysiol;98/2/359
References
- Alten JA, Moran A, Tsimelzon AI, Mastrangelo M-AA, Hilsenbeck SG, Poli V, Tweardy DJ. Prevention of hypovolemic circulatory collapse by IL-6 activated Stat3. PLoS One. 2008;3:e1605. doi: 10.1371/journal.pone.0001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Biber K, Pinto-Duarte A, Wittendorp MC, Dolga AM, Fernandes CC, Von Frijtag Drabbe Künzel J, Keijser JN, de Vries R, Ijzerman AP, Ribeiro JA, Eisel U, Sebastião AM, Boddeke HWGM. Interleukin-6 upregulates neuronal adenosine A1 receptors: implications for neuromodulation and neuroprotection. Neuropsychopharmacology. 2008;33:2237–2250. doi: 10.1038/sj.npp.1301612. [DOI] [PubMed] [Google Scholar]
- Bosenberg AT, Brock-Utne JG, Gaffin SL, Wells MT, Blake GT. Strenuous exercise causes systemic endotoxemia. J Appl Physiol. 1988;65:106–108. doi: 10.1152/jappl.1988.65.1.106. [DOI] [PubMed] [Google Scholar]
- Broderick KE, Zhang T, Rangaswami H, Zeng Y, Zhao X, Boss GR, Pilz RB. Guanosine 3′,5′-cyclic monophosphate (cGMP)/cGMP-dependent protein kinase induce interleukin-6 transcription in osteoblasts. Mol Endocrinol. 2007;21:1148–1162. doi: 10.1210/me.2005-0389. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE. Tissue temperatures and whole-animal oxygen consumption after exercise. Am J Physiol. 1971;221:427–431. doi: 10.1152/ajplegacy.1971.221.2.427. [DOI] [PubMed] [Google Scholar]
- Buchwalow IB, Minin EA, Samoilova VE, Boecker W, Wellner M, Schmitz W, Neumann J, Punkt K. Compartmentalization of NO signaling cascade in skeletal muscles. Biochem Biophys Res Commun. 2005;330:615–621. doi: 10.1016/j.bbrc.2005.02.182. [DOI] [PubMed] [Google Scholar]
- Bustin M. At the crossroads of necrosis and apoptosis: signaling to multiple cellular targets by HMGB1. Sci STKE. 2002;2002:pe39. doi: 10.1126/stke.2002.151.pe39. [DOI] [PubMed] [Google Scholar]
- Buvinic S, Almarza G, Bustamante M, Casas M, López J, Riquelme M, Sáez JC, Huidobro-Toro JP, Jaimovich E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J Biol Chem. 2009;284:34490–34505. doi: 10.1074/jbc.M109.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MHS, McGee SL, Watt MJ, Hargreaves M, Febbraio MA. Altering dietary nutrient intake that reduces glycogen content leads to phosphorylation of nuclear p38 MAP kinase in human skeletal muscle: association with IL-6 gene transcription during contraction. FASEB J. 2004;18:1785–1787. doi: 10.1096/fj.03-1039fje. [DOI] [PubMed] [Google Scholar]
- Cooper LF, Uoshima K, Guo Z. Transcriptional regulation involving the intronic heat shock element of the rat hsp27 gene. Biochim Biophys Acta. 2000;1490:348–354. doi: 10.1016/s0167-4781(00)00005-1. [DOI] [PubMed] [Google Scholar]
- Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, McDonough PM, Glembotski CC. p38 MAPK and NF-κB collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J Biol Chem. 2000;275:23814–23824. doi: 10.1074/jbc.M909695199. [DOI] [PubMed] [Google Scholar]
- Dawn B, Xuan Y-T, Guo Y, Rezazadeh A, Stein AB, Hunt G, Wu W-J, Tan W, Bolli R. IL-6 plays an obligatory role in late preconditioning via JAK–STAT signaling and upregulation of iNOS and COX-2. Cardiovasc Res. 2004;64:61–71. doi: 10.1016/j.cardiores.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107:411–416. [PubMed] [Google Scholar]
- Deldicque L, Hespel P, Francaux M. Endoplasmic reticulum stress in skeletal muscle: origin and metabolic consequences. Exerc Sport Sci Rev. 2012;40:43–49. doi: 10.1097/JES.0b013e3182355e8c. [DOI] [PubMed] [Google Scholar]
- Ding L-A, Li J-S, Li Y-S, Zhu N-T, Liu F-N, Tan L. Intestinal barrier damage caused by trauma and lipopolysaccharide. World J Gastroenterol. 2004;10:2373–2378. doi: 10.3748/wjg.v10.i16.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth JP, Van Uum SH, Van Deuren M, Pesman GJ, Van der Ven-Jongekrijg J, Van der Meer JW. Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-alpha and IL-1 beta production. J Appl Physiol. 1995;79:1497–1503. doi: 10.1152/jappl.1995.79.5.1497. [DOI] [PubMed] [Google Scholar]
- Fang H, Wu Y, Huang X, Wang W, Ang B, Cao X, Wan T. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J Biol Chem. 2011;286:30393–30400. doi: 10.1074/jbc.M111.266528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero TG, Zable AC, Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Keller C, Starkie RL, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen BK. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol. 2003;549:607–612. doi: 10.1113/jphysiol.2003.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Walsh R, Koukoulas I, van Hall G, Saltin B, Pedersen BK. Reduced glycogen availability is associated with an elevation in HSP72 in contracting human skeletal muscle. J Physiol. 2002;538:911–917. doi: 10.1113/jphysiol.2001.013145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Song Y, Chen C, Lu ZZ, Zhang Y. Stimulation of adenosine A2B receptors induces interleukin-6 secretion in cardiac fibroblasts via the PKC-δ–P38 signalling pathway. Br J Pharmacol. 2010;159:1598–1607. doi: 10.1111/j.1476-5381.2009.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink MP. Intestinal epithelial hyperpermeability: update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care. 2003;9:143–151. doi: 10.1097/00075198-200304000-00011. [DOI] [PubMed] [Google Scholar]
- Fischer CP, Hiscock NJ, Penkowa M, Basu S, Vessby B, Kallner A, Sjöberg L-B, Pedersen BK. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J Physiol. 2004;558:633–645. doi: 10.1113/jphysiol.2004.066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2002;283:R698–R709. doi: 10.1152/ajpregu.00039.2002. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide and proinflammatory cytokines stimulate interleukin-6 expression in C2C12 myoblasts: role of the Jun NH2-terminal kinase. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1153–R1164. doi: 10.1152/ajpregu.00164.2003. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Epinephrine stimulates IL-6 expression in skeletal muscle and C2C12 myoblasts: role of c-Jun NH2-terminal kinase and histone deacetylase activity. Am J Physiol Endocrinol Metab. 2004;286:E809–E817. doi: 10.1152/ajpendo.00560.2003. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom GJ, Lang CH. Multiple Toll-like receptor ligands induce an IL-6 transcriptional response in skeletal myocytes. Am J Physiol Regul Integr Comp Physiol. 2006;290:R773–R784. doi: 10.1152/ajpregu.00490.2005. [DOI] [PubMed] [Google Scholar]
- Funderburg NT, Jadlowsky JK, Lederman MM, Feng Z, Weinberg A, Sieg SF. The Toll-like receptor 1/2 agonists Pam3CSK4 and human β-defensin-3 differentially induce interleukin-10 and nuclear factor-κB signalling patterns in human monocytes. Immunology. 2011;134:151–160. doi: 10.1111/j.1365-2567.2011.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García JR, Jaumann F, Schulz S, Krause A, Rodríguez-Jiménez J, Forssmann U, Adermann K, Klüver E, Vogelmeier C, Becker D, Hedrich R, Forssmann WG, Bals R. Identification of a novel, multifunctional β-defensin (human β-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001;306:257–264. doi: 10.1007/s004410100433. [DOI] [PubMed] [Google Scholar]
- Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang W-P, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- Gleeson M. Interleukins and exercise. J Physiol. 2000;529:1. doi: 10.1111/j.1469-7793.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin MJ, Martins AL, Siepel A, Lis JT. Accurate prediction of inducible transcription factor binding intensities in vivo. PLoS Genet. 2012;8:e1002610. doi: 10.1371/journal.pgen.1002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves M, Richter EA. Regulation of skeletal muscle glycogenolysis during exercise. Can J Sport Sci. 1988;13:197–203. [PubMed] [Google Scholar]
- Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning RH, Duin M, den Hertog A, Nelemans A. Activation of the phospholipase C pathway by ATP is mediated exclusively through nucleotide type P2-purinoceptors in C2C12 myotubes. Br J Pharmacol. 1993;110:747–752. doi: 10.1111/j.1476-5381.1993.tb13875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høier B, Olsen K, Nyberg M, Bangsbo J, Hellsten Y. Contraction-induced secretion of VEGF from skeletal muscle cells is mediated by adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H857–H862. doi: 10.1152/ajpheart.00082.2010. [DOI] [PubMed] [Google Scholar]
- Holmes AG, Watt MJ, Carey AL, Febbraio MA. Ionomycin, but not physiologic doses of epinephrine, stimulates skeletal muscle interleukin-6 mRNA expression and protein release. Metab Clin Exp. 2004;53:1492–1495. doi: 10.1016/j.metabol.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Huey KA, Fiscus G, Richwine AF, Johnson RW, Meador BM. In vivo vitamin E administration attenuates interleukin-6 and interleukin-1β responses to an acute inflammatory insult in mouse skeletal and cardiac muscle. Exp Physiol. 2008;93:1263–1272. doi: 10.1113/expphysiol.2008.043190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungness ES, Luo G, Pritts TA, Sun X, Robb BW, Hershko D, Hasselgren P-O. Transcription factors C/EBP-β and -δ regulate IL-6 production in IL-1β-stimulated human enterocytes. J Cell Physiol. 2002;192:64–70. doi: 10.1002/jcp.10116. [DOI] [PubMed] [Google Scholar]
- Inouye S, Fujimoto M, Nakamura T, Takaki E, Hayashida N, Hai T, Nakai A. Heat shock transcription factor 1 opens chromatin structure of interleukin-6 promoter to facilitate binding of an activator or a repressor. J Biol Chem. 2007;282:33210–33217. doi: 10.1074/jbc.M704471200. [DOI] [PubMed] [Google Scholar]
- Isayama K, Murao Y, Saito F, Hirakawa A, Nakatani T. Effects of hypertonic saline on CD4+CD25+Foxp3+ regulatory T cells after hemorrhagic shock in relation to iNOS and cytokines. J Surg Res. 2012;172:137–145. doi: 10.1016/j.jss.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Iwakoshi NN, Lee A-H, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- Javeshghani D, Sakkal D, Mori M, Hussain SN. Regulation of diaphragmatic nitric oxide synthase expression during hypobaric hypoxia. Am J Physiol Lung Cell Mol Physiol. 2000;279:L520–L527. doi: 10.1152/ajplung.2000.279.3.L520. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera M-C, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-κB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol. 2006;79:425–434. doi: 10.1189/jlb.0905523. [DOI] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Kosmidou I, Vassilakopoulos T, Xagorari A, Zakynthinos S, Papapetropoulos A, Roussos C. Production of interleukin-6 by skeletal myotubes: role of reactive oxygen species. Am J Respir Cell Mol Biol. 2002;26:587–593. doi: 10.1165/ajrcmb.26.5.4598. [DOI] [PubMed] [Google Scholar]
- Lange P, Victor M, Benecke BJ. Basal level transcription of the human hsp86 gene is directed by intron-based elements. Genes Cells. 1997;2:185–194. doi: 10.1046/j.1365-2443.1997.d01-309.x. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Leon LR. Heat stroke and cytokines. Prog Brain Res. 2007;162:481–524. doi: 10.1016/S0079-6123(06)62024-4. [DOI] [PubMed] [Google Scholar]
- Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-κB activation in response to tumor necrosis factor α. FASEB J. 1998;12:871–880. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- Lynge J, Hellsten Y. Distribution of adenosine A1, A2A and A2B receptors in human skeletal muscle. Acta Physiol Scand. 2000;169:283–290. doi: 10.1046/j.1365-201x.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- Lynge J, Juel C, Hellsten Y. Extracellular formation and uptake of adenosine during skeletal muscle contraction in the rat: role of adenosine transporters. J Physiol. 2001;537:597–605. doi: 10.1111/j.1469-7793.2001.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland-Mancini MM, Funk HM, Paluch AM, Zhou M, Giridhar PV, Mercer CA, Kozma SC, Drew AF. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184:7219–7228. doi: 10.4049/jimmunol.0901929. [DOI] [PubMed] [Google Scholar]
- Martin WH, Murphree SS, Saffitz JE. β-Adrenergic receptor distribution among muscle fiber types and resistance arterioles of white, red, and intermediate skeletal muscle. Circ Res. 1989;64:1096–1105. doi: 10.1161/01.res.64.6.1096. [DOI] [PubMed] [Google Scholar]
- Mathison JC, Ulevitch RJ, Fletcher JR, Cochrane CG. The distribution of lipopolysaccharide in normocomplementemic and C3-depleted rabbits and rhesus monkeys. Am J Pathol. 1980;101:245–264. [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal. 2005;7:472–481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- Meng ZH, Dyer K, Billiar TR, Tweardy DJ. Distinct effects of systemic infusion of G-CSF vs. IL-6 on lung and liver inflammation and injury in hemorrhagic shock. Shock. 2000;14:41–48. doi: 10.1097/00024382-200014010-00008. [DOI] [PubMed] [Google Scholar]
- Mok Y-YP, Moore PK. Hydrogen sulphide is pro-inflammatory in haemorrhagic shock. Inflamm Res. 2008;57:512–518. doi: 10.1007/s00011-008-7231-6. [DOI] [PubMed] [Google Scholar]
- Moran A, Tsimelzon AI, Mastrangelo M-AA, Wu Y, Yu B, Hilsenbeck SG, Poli V, Tweardy DJ. Prevention of trauma/hemorrhagic shock-induced lung apoptosis by IL-6-mediated activation of Stat3. Clin Transl Sci. 2009;2:41–49. doi: 10.1111/j.1752-8062.2008.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina H, Wada T, Katada T. Physiological roles of SAPK/JNK signaling pathway. J Biochem. 2004;136:123–126. doi: 10.1093/jb/mvh117. [DOI] [PubMed] [Google Scholar]
- Parikh AA, Moon MR, Kane CD, Salzman AL, Fischer JE, Hasselgren PO. Interleukin-6 production in human intestinal epithelial cells increases in association with the heat shock response. J Surg Res. 1998;77:40–44. doi: 10.1006/jsre.1998.5332. [DOI] [PubMed] [Google Scholar]
- Patel H, Shaw SG, Shi-Wen X, Abraham D, Baker DM, Tsui JCS. Toll-like receptors in ischaemia and its potential role in the pathophysiology of muscle damage in critical limb ischaemia. Cardiol Res Pract. 2012;2012:121237. doi: 10.1155/2012/121237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Pritts TA, Hungness ES, Hershko DD, Robb BW, Sun X, Luo G-J, Fischer JE, Wong HR, Hasselgren P-O. Proteasome inhibitors induce heat shock response and increase IL-6 expression in human intestinal epithelial cells. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1016–R1026. doi: 10.1152/ajpregu.00492.2001. [DOI] [PubMed] [Google Scholar]
- Pye D, Palomero J, Kabayo T, Jackson MJ. Real-time measurement of nitric oxide in single mature mouse skeletal muscle fibres during contractions. J Physiol. 2007;581:309–318. doi: 10.1113/jphysiol.2006.125930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rao DA, Pober JS. Endothelial injury, alarmins, and allograft rejection. Crit Rev Immunol. 2008;28:229–248. doi: 10.1615/critrevimmunol.v28.i3.40. [DOI] [PubMed] [Google Scholar]
- Robins HI, Kutz M, Wiedemann GJ, Katschinski DM, Paul D, Grosen E, Tiggelaar CL, Spriggs D, Gillis W, d’Oleire F. Cytokine induction by 41.8 °C whole body hyperthermia. Cancer Lett. 1995;97:195–201. doi: 10.1016/0304-3835(95)03976-4. [DOI] [PubMed] [Google Scholar]
- Rubinstein I, Abassi Z, Coleman R, Milman F, Winaver J, Better OS. Involvement of nitric oxide system in experimental muscle crush injury. J Clin Invest. 1998;101:1325–1333. doi: 10.1172/JCI810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryten M, Yang SY, Dunn PM, Goldspink G, Burnstock G. Purinoceptor expression in regenerating skeletal muscle in the mdx mouse model of muscular dystrophy and in satellite cell cultures. FASEB J. 2004;18:1404–1406. doi: 10.1096/fj.03-1175fje. [DOI] [PubMed] [Google Scholar]
- Saltin B, Gagge AP, Bergh U, Stolwijk JA. Body temperatures and sweating during exhaustive exercise. J Appl Physiol. 1972;32:635–643. doi: 10.1152/jappl.1972.32.5.635. [DOI] [PubMed] [Google Scholar]
- Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S, Koyasu S, Matsui H, Yamauchi-Takihara K, Harada M, Saito Y, Ogawa S. ERK and p38 MAPK, but not NF-κB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res. 2001;89:661–669. doi: 10.1161/hh2001.098873. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem. 2001;78:1339–1349. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- Siednienko J, Nowak J, Moynagh PN, Gorczyca WA. Nitric oxide affects IL-6 expression in human peripheral blood mononuclear cells involving cGMP-dependent modulation of NF-κB activity. Cytokine. 2011;54:282–288. doi: 10.1016/j.cyto.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Sigala I, Zacharatos P, Toumpanakis D, Michailidou T, Noussia O, Theocharis S, Roussos C, Papapetropoulos A, Vassilakopoulos T. MAPKs and NF-κB differentially regulate cytokine expression in the diaphragm in response to resistive breathing: the role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1152–R1162. doi: 10.1152/ajpregu.00376.2010. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001a;537:633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Keller C, Hillig T, Frøsig C, Wojtaszewski JFP, Pedersen BK, Pilegaard H, Sander M. Nitric oxide production is a proximal signaling event controlling exercise-induced mRNA expression in human skeletal muscle. FASEB J. 2007;21:2683–2694. doi: 10.1096/fj.06-7477com. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Toft AD, Schjerling P, Halkjaer-Kristensen J, Pedersen BK. Plasma interleukin-6 during strenuous exercise: role of epinephrine. Am J Physiol Cell Physiol. 2001b;281:C1001–C1004. doi: 10.1152/ajpcell.2001.281.3.C1001. [DOI] [PubMed] [Google Scholar]
- Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K. Evolution and integration of innate immune recognition systems: the Toll-like receptors. J Endotoxin Res. 2005;11:51–55. doi: 10.1179/096805105225006687. [DOI] [PubMed] [Google Scholar]
- Thiemermann C, Bowes J, Myint FP, Vane JR. Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia–reperfusion injury in the heart and skeletal muscle. Proc Natl Acad Sci USA. 1997;94:679–683. doi: 10.1073/pnas.94.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch RJ. Therapeutics targeting the innate immune system. Nat Rev Immunol. 2004;4:512–520. doi: 10.1038/nri1396. [DOI] [PubMed] [Google Scholar]
- van Alphen GWHM, Robinette SL, Macri FJ. The adrenergic receptors of the intraocular muscles of the cat. Int J Neuropharmacol. 1963;2:259–272. doi: 10.1016/0028-3908(63)90001-7. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-α and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996;97:713–719. doi: 10.1172/JCI118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R, Hussain SNA. Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol. 2003;284:L452–L457. doi: 10.1152/ajplung.00270.2002. [DOI] [PubMed] [Google Scholar]
- Voss AA. Extracellular ATP inhibits chloride channels in mature mammalian skeletal muscle by activating P2Y1 receptors. J Physiol. 2009;587:5739–5752. doi: 10.1113/jphysiol.2009.179275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welc SS, Phillips NA, Oca-Cossio J, Wallet SM, Chen DL, Clanton TL. Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am J Physiol Cell Physiol. 2012;303:C455–C466. doi: 10.1152/ajpcell.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham M, Chan MHS, Pal M, Matthews VB, Prelovsek O, Lunke S, El-Osta A, Broenneke H, Alber J, Bruning JC, Wunderlich FT, Lancaster GI, Febbraio MA. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem. 2012;287:10771–10779. doi: 10.1074/jbc.M111.310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G, Brown T, Becker L, Prager M, Giroir BP. Cytokine-induced expression of nitric oxide synthase in C2C12 skeletal muscle myocytes. Am J Physiol. 1994;267:R1020–R1025. doi: 10.1152/ajpregu.1994.267.4.R1020. [DOI] [PubMed] [Google Scholar]
- Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- Wright VP, Reiser PJ, Clanton TL. Redox modulation of global phosphatase activity and protein phosphorylation in intact skeletal muscle. J Physiol. 2009;587:5767–5781. doi: 10.1113/jphysiol.2009.178285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Chen C, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor 1 represses transcription of the IL-1β gene through physical interaction with the nuclear factor of interleukin 6. J Biol Chem. 2002;277:11802–11810. doi: 10.1074/jbc.M109296200. [DOI] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Takemori Y, Sakurai M, Sugiyama K, Sakurai H. Differential recognition of heat shock elements by members of the heat shock transcription factor family. FEBS J. 2009;276:1962–1974. doi: 10.1111/j.1742-4658.2009.06923.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pilon G, Marette A, Baracos VE. Cytokines and endotoxin induce cytokine receptors in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E196–E205. doi: 10.1152/ajpendo.2000.279.1.E196. [DOI] [PubMed] [Google Scholar]
- Zmijewski JW, Landar A, Watanabe N, Dickinson DA, Noguchi N, Darley-Usmar VM. Cell signalling by oxidized lipids and the role of reactive oxygen species in the endothelium. Biochem Soc Trans. 2005;33:1385–1389. doi: 10.1042/BST20051385. [DOI] [PMC free article] [PubMed] [Google Scholar]