Abstract
Objective:
Despite the enormous expansion of HIV testing services (HTS), an estimated 40% of people with HIV infection remain undiagnosed. To enhance the efficiency of HTS, new approaches are needed. The WHO conducted a systematic review on the effectiveness of assisted partner notification in improving HIV test uptake and diagnosis, and the occurrence of adverse events, to inform the development of normative guidelines.
Methods:
We systematically searched five electronic databases through June 2016. We also contacted experts in the field and study authors for additional information where needed. Eligible studies compared assisted HIV partner notification services to passive or no notification. Where multiple studies reported comparable outcomes, meta-analysis was conducted using a random-effects model to produce relative risks (RRs) or risk ratios and 95% confidence intervals (CIs).
Results:
Of 1742 citations identified, four randomized controlled trials and six observational studies totalling 5150 index patients from eight countries were included. Meta-analysis of three individually randomized trials showed that assisted partner notification services resulted in a 1.5-fold increase in HTS uptake among partners compared with passive referral (RR = 1.46; 95% CI: 1.22–1.75; I2 = 0%). The proportion of HIV-positive partners was 1.5 times higher with assisted partner notification than with passive referral (RR = 1.47; 95% CI: 1.12–1.92; I2 = 0%). Few instances of violence or harm occurred.
Conclusion:
Assisted partner notification improved partner testing and diagnosis of HIV-positive partners, with few reports of harm. WHO strongly recommends voluntary assisted HIV partner notification services to be offered as part of a comprehensive package of testing and care.
Keywords: contact tracing, couples, HIV, notification, partner
Introduction
HIV testing and counselling services (HTS) and the availability of antiretroviral therapy have expanded enormously over the past three decades. Starting with diagnostic testing offered to people with symptoms suggestive of HIV infection and antenatal testing, HTS now encompasses a range of approaches such as community, home-based, and mobile testing to reach larger and more varied populations earlier in their course of infection. As a result, by the end of 2015, 17 million people with HIV infection were receiving antiretroviral treatment [1]. Yet it is currently estimated that over 14.5 million people living with HIV worldwide remain undiagnosed [2]. To address this gap – in particular, the first of the UN 90-90-90 goals to diagnose 90% of people with HIV infection by 2020 [2] – new approaches that enhance the efficiency of testing and increase the coverage of treatment are needed. HIV partner notification is an approach that has the potential to particularly identify people with undiagnosed HIV infection who remain unlinked to prevention, treatment and care services, and continue to be at risk of transmitting HIV vertically or through sexual and drug-injecting partners.
Assisted partner notification, or contact tracing has been an important public health approach in communicable disease management for decades, including in programmes for sexually transmitted infections (STIs) and tuberculosis (TB). The tracing of contacts and the voluntary screening of household members of patients with pulmonary TB is an effective and standard approach [3,4]. A 2013 Cochrane review found that expedited partner therapy was more successful than simple patient referral in preventing recurrent STIs causing urethritis or cervicitis [5]. Although it is well known that the sexual and drug-injecting partners of people diagnosed with HIV infection have an increased probability of also being HIV-positive [6–12], partner notification services for people diagnosed with HIV have not been routinely included in HTS policies internationally [13].
To inform a 2016 WHO HTS guidelines update, we conducted a systematic review and meta-analysis of partner notification services to determine their effectiveness in the uptake of HTS, diagnosing partners and linking them to care, and also to assess the occurrence of adverse events or harm following partner notification.
Methods
We followed the methods described in the PRISMA statement for the reporting of systematic reviews and meta-analyses.
Search strategy and inclusion criteria
Through 1 June 2016, we searched five electronic databases (PubMed, EMBASE, Cumulative Index to Nursing and Allied Health Literature CINAHL, PsycINFO, and Sociological Abstracts), and websites of major HIV-related conferences for relevant abstracts [International AIDS Conference (IAC), Conference on HIV Pathogenesis, Treatment, and Prevention (IAS), and Conference on Retroviruses and Opportunistic Infections (CROI)]. The IAC and IAS conference abstracts were searched for all available years; for CROI, only the most recent conferences (2014, 2015, and 2016) were searched as past conference abstracts were inaccessible online. In addition, selected experts in the field were contacted, and secondary reference searching was conducted on all included studies as well as on relevant review articles [5,14,15] to identify additional articles and abstracts. We also searched for ongoing randomized controlled trials (RCTs) through clinicaltrials.gov, the WHO International Clinical Trials Registry Platform (www.who.int/ictrp/), and the Pan African Clinical Trials Registry (www.pactr.org). We contacted study authors when additional information was needed.
A comprehensive PubMed search strategy was adapted for entry into all computer databases and included terms for HIV and partner notification and was not limited by study design: (HIV [tiab] OR ‘human immunodeficiency virus’ [tiab]) AND (‘contact examination’ [tiab] OR ‘contact detection’ [tiab] OR ‘contact tracing’ [tiab] OR ‘partner notification’ [tiab] OR ‘partner notifications’ [tiab] OR ‘partner tracing’ [tiab] OR ‘partner services’ OR ‘partner counseling and referral services’ [tiab]). No language or geographic limitations were placed on the search.
To be included, an article had to meet the following criteria: a study design that compared persons who received HTS and were diagnosed HIV-positive and who were offered partner notification services using assistance (such as contract or provider referral) to such persons who received HTS with passive referral or no partner notification intervention; measured one or more of the primary or secondary outcomes; and was published in a peer-reviewed journal or conference abstract.
Partner notification approaches included: first, passive referral, where HIV-positive clients are encouraged to disclose their status and suggest HIV testing to their partner(s) on their own; and assisted approaches: second, contract referral, where HIV-positive clients enter into a contract with a provider to refer their partner(s) to HTS within an agreed time period, after that the provider contacts the partner(s) directly and offers HTS, while maintaining the anonymity of the index patient. Third Provider referral, where providers directly contact partners of index patients to offer HTS, and fourth, dual referral where the provider accompanies the index patient when they disclose their status and offers HTS to their partner(s).
Outcomes
Outcomes were: uptake of HTS among partner(s) of HIV-positive index patients; proportion of partners who tested for HIV and were diagnosed HIV-positive; any experience of social harm/adverse events among HIV-positive patients and/or their partners; measurement of CD4+ cell count or viral load among partners; linkage to clinical assessment or ART among partners following HIV-positive diagnosis; and linkage to a prevention visit among partners after an HIV-negative test result.
Screening and data extraction
Screening was conducted in a two-stage process. First, titles, abstracts, and citation information identified through the search strategy were screened to remove clearly nonrelevant articles. Full-text articles for all selected abstracts were then screened by two independent reviewers for eligibility. Differences were resolved through consensus. Data were extracted into standardized coding forms.
Statistical analysis
Data were analysed according to partner notification approach and outcome. Where multiple RCTs reported the same or comparable outcomes and were considered methodologically and clinically appropriate to combine, meta-analysis was conducted using a random-effects model to produce relative risks (RRs) (or rate ratios where applicable) and 95% confidence intervals (CIs) for dichotomous data using REVMAN version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). We conducted analyses using either all identified partners or all locatable partners as the denominator. For uptake of partner testing and linkage to care, we also analysed the rate ratio of partners tested to index patients to address the attrition of partners between those identified by index patients to those located and notified.
Quality and Grading of Recommendations, Assessment, Development and Evaluation assessments
For individual RCTs, the risk of bias was evaluated using the Cochrane Collaboration's tool for assessing risk of bias [16]. We used Grading of Recommendations, Assessment, Development and Evaluation (GRADE) to determine the overall quality of evidence for each outcome measured in the RCTs. GRADE includes an appraisal of the risk of bias, imprecision, indirectness, inconsistency, and publication bias across included trials to inform an overall grading of high, moderate, low, or very low quality of evidence [17].
Role of the funding source
The funders had no role in the development of this study. The authors alone were responsible for the study design, data collection, analysis, interpretation, and writing of the article. The corresponding author had the final responsibility for the decision to submit for publication.
Results
The searches yielded 1742 citations; four RCTs (three individually randomized trials and one cluster-randomized) met our eligibility criteria (Fig. 1). We included observational studies that compared types of partner notification services but either did not randomize index patients or did not have a nonintervention control arm, in order to provide an indication of broader geographic and population types for the main outcomes; these were not included in meta-analyses. For one cluster RCT [11] and observational study [18], we included results from a conference abstract in addition to results subsequently published in a peer-reviewed article [19,20], respectively, that were made available after the cut-off date for our initial search.
Fig. 1.
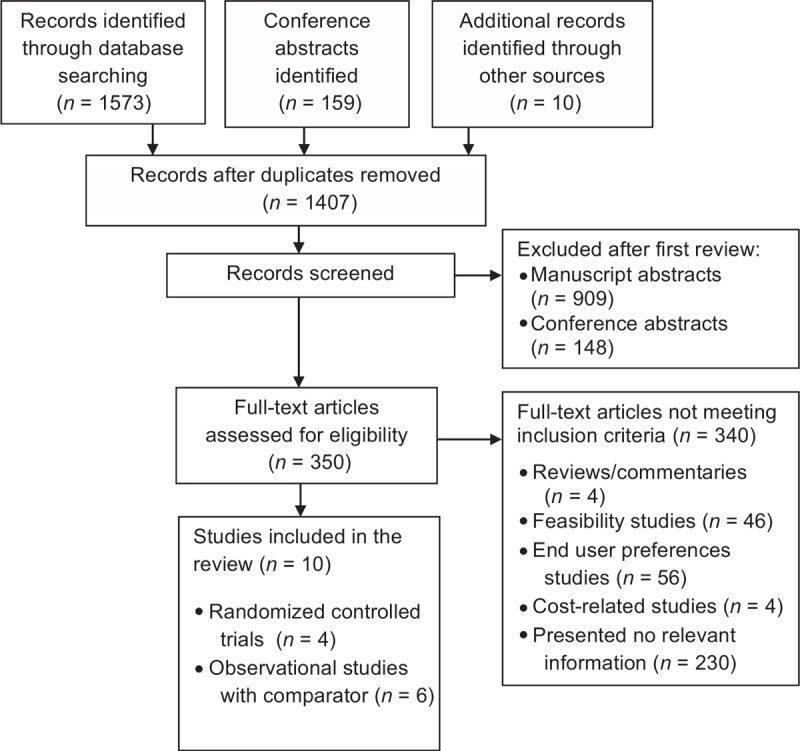
Study selection.
RCTs were conducted in the United States [8], Malawi [7,9], and Kenya [19]; the largest and most recent were in sub-Saharan Africa. Three RCTs compared assisted partner notification services (provider or contract referral) with passive approaches, and the fourth cluster RCT compared immediate assisted notification with a passive referral group that received delayed assisted partner notification after outcomes were assessed. The study populations included pregnant women attending antenatal care [9], patients from STI clinics [7], clients from an HIV testing centre [19], and patients in a United States county health department that included women, men who have sex with men (MSM), and people who inject drugs [8]. Six observational studies were conducted among the general population in Cameroon [21], Mozambique [20], Spain [22], the United Republic of Tanzania [23], and the United States [24], across a variety of healthcare settings and HIV testing sites (Table 1). All studies utilized multiple methods to contact and notify partners, including telephone calls, messages, and in-person visits.
Table 1.
Study descriptions and HIV partner notification outcomes for studies included in this review.
| Passive/control groupsa | Assisted groups (includes provider, contract, and dual referral) | |||||||||||||
| Author, year | Country | Study design | Population | Intervention | Number index cases | Number partners identified | Partners tested (%) | Partners HIV positive (%) | Ratio of partners tested to index case | Number index cases | Number partners identified | Partners tested (%) | Partners HIV positive (%) | Ratio of partners tested to index case |
| Landis et al., 1992 | USA | RCT | County health dept women, MSM, PWID | Passive referral vs. mix of provider and contract referral | 35 | 153 | 5 (3) | 1 (20) | 0.14 | 39 | 157 | 36 (23) | 9 (25) | 0.92 |
| Brown et al., 2011 | Malawi | RCT | STI clinic patients | Passive, vs. contract vs. provider referral | 77 | 82 | 20 (24) | 12 (60) | 0.26 | 163 | 170 | 87 (51) | 42 (48) | 0.53 |
| Rosenberg et al., 2015b | Malawi | RCT | Pregnant women | Passive referral vs. contract referral | 100 | NA | 52 (52) | 37 (71) | 0.52 | 100 | NA | 74 (74) | 53 (72) | 0.74 |
| Cherutich et al., 2016 | Kenya | Cluster-RCT | HIV testing centre clients | Immediate vs. delayed PNc | 569 | 959 | 85 (9) | 28 (33) | 0.15 | 550 | 913 | 392 (43) | 136 (35) | 0.71 |
| Udeagu et al., 2012 | USA | Preintervention–postintervention | STI clinic patients | Preinitiative (2005) vs. post-PN program (2008) | 670 | 174 | 4 (2) | 0 | 0.01 | 602 | 562 | 117 (21) | 15 (13) | 0.19 |
| Plotkin et al., 2015 | United Republic of Tanzania | Cross-sectional | 3 hospitals | Offer of passive, provider, or contract referral | 356 | 402 | 241 (60) | 142 (59) | 0.68 | 14 | 16 | 7 (44) | 6 (86) | 0.50 |
| Chiou et al., 2015 | Taiwan | Otherd | MSM | 1 session PN counselling vs. 2 sessions | 42 | 165 | 33 (20) | 9 (27) | 0.79 | 43 | 302 | 78 (26) | 31 (40) | 1.81 |
| Valle et al., 2015 | Spain | Observational | Healthcare settings | Offer of passive or provider referral | 84 | 153 | 100 (65) | 21 (21) | 1.19 | 24 | 46 | 41 (89) | 5 (12) | 1.71 |
| Henley et al., 2015e | Cameroon | Observational | ANC, VCT and inpatients | Offer of passive, provider, and contract referral | 592 | 423 | 191 (45) | – | 0.32 | 870 | 1184 | 709 (60) | – | 0.82 |
| Myers et al., 2016 | Mozambique | Observational | Clinic patients | 4 weeks passive referral followed by 4 weeks contract referral in same index patientsf | 206 | 262 | 82 (31) | 34 (41) | 0.40 | NA | NA | 83 (32) | 43 (52) | – |
ANC, antenatal clinic; Dept, department; NA, not applicable; PN, partner notification; PWID, people who inject drugs; RCT, randomized controlled trial; STI, sexually transmitted infection; VCT, voluntary counselling and testing.
aWe present the data for delayed PN, diagnosis in 2005, patient choice of passive referral, passive phase, and one PN counselling session in the unassisted columns.
bStudy provided one partner invitation card per index client, so the maximum number of partners possible was 100.
cPassive referral group received delayed assisted partner notification after outcomes were assessed.
dStudy used random assignment of participants to either one or two partner notification counselling sessions, and participants in both arms were offered passive, provider, contract, or dual referral for notifying partners. Therefore, it did not meet trial inclusion criteria.
eTotal sample size of index clients = 1462, but data were not available by arm. Data from 107 participants who did not choose a PN type, but where partners were notified and tested, were included in the passive referral group.
fIndex patients first received passive referral for partners for 4 weeks, followed by contract referral for an additional 4 weeks for partners who had not been notified in phase I.
The 10 studies included in our review were published between 1992 and 2016, and included a total of 5150 index patients who identified a total of 6127 partners (one study [9] provided only one partner invitation per index patient). On average, HIV-positive index patients named 2.0 partners, but this varied dramatically between studies (range 0.58–5.58); the largest were among key population groups (defined as MSM, people who inject drugs, sex workers, people in prisons, or transgender people). In the nine studies reporting outcomes by approach, the ratio of partners who tested for HIV per index patient was on average 0.45 (range 0.01–1.19) for passive referral, and 0.85 (range 0.19–1.81) for assisted partner notification (Table 1). A partner notification cascade using five studies which reported each step starting from the ratio of partners identified through to the number of partners newly identified as HIV positive per index patient, shows the progressive loss to follow-up of partners, the largest being between partner identification and notification (Fig. 2). On average, 1.14 (range 0.26–4.37) partners were notified per index patient following passive referral and 1.86 (range 0.93–4.03) with assisted notification.
Fig. 2.
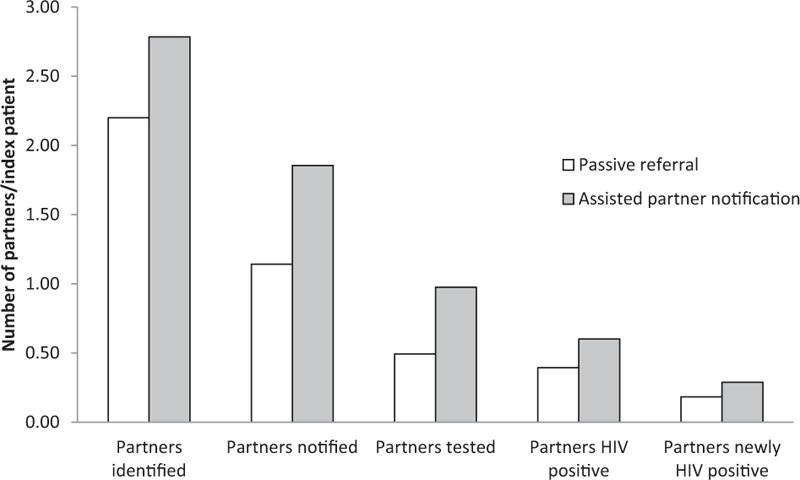
Partner notification cascade from five studies reporting data for each step [8,11,22–24].
Meta-analysis of the three individually randomized trials using all identified partners as the denominator, showed that assisted partner notification services resulted in a 1.5-fold increase in the uptake of HTS among partners compared with passive referral (RR = 1.46; 95% CI: 1.22–1.75; Fig. 3a) [7–9,19]. Meta-analysis restricted to partners who could be located in the denominator found a similar beneficial effect. Statistical heterogeneity was high (RR = 1.39; 95% CI: 0.93; 2.06; Chi2 for heterogeneity = 8.34; df = 2; I2 = 76%) (Appendix Fig. A1). When all four RCTs were included in a meta-analysis of the rate of partner testing and return of partners to the clinic per index case, the rate ratio of the assisted partner notification group was twice that of those in the passive referral group (rate ratio: 2.04; 95% CI: 1.11–3.77; chi2 for heterogeneity = 60.84; df = 3; I2 = 95%) (Fig. 3b). GRADE quality evidence was rated moderate for all analyses of HIV testing uptake due to the lack of blinding across studies, and attrition. In five observational studies, assisted partner notification was associated with increased uptake of HTS among identified partners compared with passive referral [20–22,24].
Fig. 3.
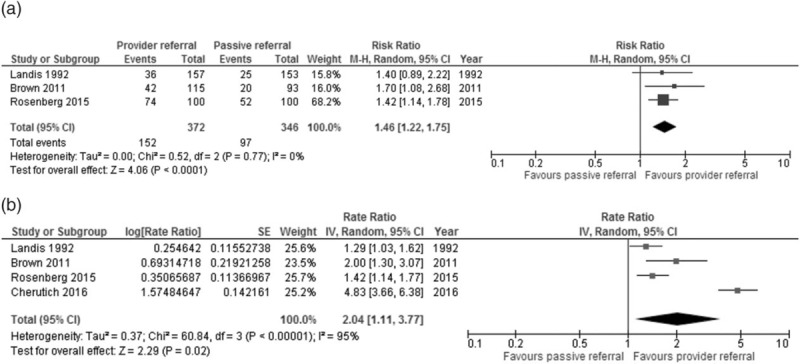
Uptake of HIV testing among partners of index cases assessed with: (a) HIV testing and return to clinic – meta-analysis using all identified partners as the denominator. (b) Rate of partner test or return to clinic of partner per index patient – meta-analysis using generic inverse variance.
The proportion of partners of index patients who tested HIV-positive ranged from 20 to 72% in both passive and assisted arms of the four trials (Table 1) [7–9,19]. Among the observational studies, the highest proportion testing HIV positive was 86%. In the four studies [7,9,20,21] that reported on couples, between 29 and 40% were in serodiscordant partnerships. A meta-analysis of the three individually RCTs found that the proportion of all identified partners who were HIV-positive following testing was 1.5 times higher in the assisted partner notification approach than in the passive approach (RR = 1.47; 95% CI: 1.12–1.92) (Fig. 4). In sensitivity analyses, the results were similar using locatable partners as the denominator (RR = 1.49; 95% CI: 1.14–1.95) (Appendix Fig. A2).
Fig. 4.

Proportion of partners who tested and were diagnosed HIV positive – meta-analysis using all identified partners as the denominator.
The percentage of partners newly diagnosed with HIV among partners who could be located, was higher with provider assisted partner notification (RR = 1.37; 95% CI: 0.98–1.93) (Appendix Fig. A6), and was similar when the cluster RCT was included in analyses with high statistical heterogeneity (Appendix Fig. A3). Across the observational studies, 0 to 86% of partners of HIV-positive individuals were newly diagnosed with HIV [20–24]. An observational study in Mozambique reported a two-fold increase in the percentage of partners diagnosed with HIV when passive approaches were replaced by assisted partner referral [20].
Meta-analysis of the two trials which reported on linkage to care showed that there was a higher rate of linkage to care in HIV-positive partners in the provider referral arm than in the passive arm (rate ratio = 3.76; 95% CI: 2.41–5.86; chi2 for heterogeneity = 1.48; df = 1; I2 = 33%)) (Appendix Fig. A4) [9,19].
All four trials and two observational studies reported few (0–3%) instances of harm resulting from partner notification [7–9,19–21]. A meta-analysis of two individually randomized and one cluster-randomized trial, showed no difference in social harm or adverse events comparing assisted and passive partner notification (RR = 1.86; 95% CI: 0.37–9.50) (Appendix Fig. A5). Reported incidents of harm in RCTs in Kenya and Malawi appeared not to be associated with HIV partner notification services, as they occurred prior to the intervention [9,19].
A single RCT [7] compared contract referral with passive referral. The quality of evidence was graded as very low for all outcomes as it was a single study, there was a lack of blinding of staff and participants, and results were imprecise. Results showed that assisted partner notification services using contract referral resulted in a two-fold increase in test uptake among the partners of HIV-positive individuals compared with passive referral (RR = 2.08; 95% CI: 1.33–3.25). The proportion of identifiable partners who tested for HIV and were diagnosed HIV positive was higher for contract referral than passive referral (RR = 1.91; 95% CI: 1.07; 3.40). Sensitivity analyses were comparable (data not shown).
Discussion
When HIV positive index patients were offered assistance in notifying their sexual and drug-injecting partners of their exposure to HIV infection, our analyses show that it resulted in higher uptake of partner HIV testing, identified higher proportions of HIV-infected persons, and increased linkage to care through the referral of newly identified HIV-infected partners to ART services. Although there were few RCTs in our meta-analyses, the results are consistent towards favouring assisted approaches, as are the results from observational studies with control groups. Across all studies, high proportions of partners returned for HIV testing when contacted by a provider, whichever method was used. Overall, index patients identified an average of two partners each and this resulted in 0.44 (range 0.01–1.8) partners per index patient eventually testing, following attrition between identified and notified partners, and acceptance of testing. The proportion of partners who tested HIV positive following assisted notification across all studies ranged from 12 to 86%, and between 29 and 40% of couples were serodiscordant.
Although all the studies that were reviewed showed improved outcomes with assisted partner notification approaches in both RCTs and observational studies, passive referral also resulted in uptake of HIV testing among partners (range 2–65%) [7–9,20,22,23]. In some studies, HIV test uptake in the passive group was seen at a similar or higher level to that of assisted approaches from other studies. Two studies with very low HTS uptake in the passive referral groups were conducted in the United States before triple therapy was available (3% testing uptake) [8], and when implementation of partner notification reporting regulations appeared to be low (2% test uptake) [24]. The remaining studies presenting this information reported HIV test uptake between 24 and 65% [7,9,19,20,22,23,25]. Furthermore, observational data from Cameroon demonstrate the scalability of partner notification with the offer of multiple notification approaches to index patients in a programmatic setting, resulting in high partner test uptake overall (67%) [21]. Thus, the simple act of encouraging partner notification and offering services to a person who is HIV positive, whether verbally during counselling, or through written invitation letters or referral cards, is beneficial, and could be considered while assisted approaches are being brought to scale. Linkage to care for partners who test HIV positive was higher with assisted partner notification methods than with passive methods in both RCTs [9,19] and observational studies [21,23].
The ratio of partners tested per index patient varies, but in all studies, a substantial drop occurs between identified and notified partners and may be due to the difficulty in contacting partners. One of the challenges to partner notification has been that key populations [26] and people with casual partners [7,27] may be less able or willing to identify partners; spouses and steady partners have been more likely to be notified than other partners [7,21,28–30]. Recall of, and contact information for, partners was reported to be better among heterosexual women than among MSM or people who inject drugs in one study [31]. Yet, as was found in studies conducted among the general population, assisted partner notification services among key populations resulted in higher uptake of HTS, and particularly among MSM and people who inject drugs, also identified a high proportion of HIV-positive partners (5–80%) [32–54]. A recently published observational study found that 36% of newly diagnosed partners had acute or early HIV infection, and among partners with genetic sequences, 61% were genetically linked to the index patient, emphasizing the importance of reaching partners to prevent transmission in discordant partnerships [55]. Providing partner notification to key populations and those with casual partners may require more intensive efforts to locate partners, including the assurance of confidentiality and anonymity for HIV-positive clients.
Reported social harm and other adverse events following HIV partner notification, using passive or assisted approaches, have been rare. Fears about social harm following disclosure or partner notification are of particular concern in situations where certain behaviours associated with HIV infection are criminalized, such as among people who inject drugs, or where one partner is economically dependent on the other and fears losing social or financial support. However, although issues around confidentiality [56], and mostly hypothetical concerns about potential harm [57,58] have been raised in the literature, when adverse events have actually been measured, very few have occurred [7–9,19]. Moreover, studies from the United States showed no differences in partnership dissolution following HIV partner notification when compared with a high-risk HIV-negative control group [59], or to syphilis partner notification [28]. Some studies screened for intimate partner violence (IPV) and excluded those persons with a history of IPV, which would put them at risk of harm following disclosure. Reported incidents of harm in RCTs in Kenya and Malawi appeared not to be associated with HIV partner notification services, as they occurred prior to the intervention [9,19]. Although programme implementers should be sensitive to the potential for harm arising from disclosure of HIV status and assisted partner notification, this should be balanced against the benefit of diagnosing HIV infection and linking people to treatment. These results were obtained from a limited number of studies undertaken in the United States and Africa; studies from other world regions are needed.
Our review identified four RCTs with which to assess the effectiveness of HIV partner notification services with the quality of evidence for the primary outcome graded as moderate. For some outcomes, significant statistical heterogeneity was present, mostly driven by the large effects observed in the cluster-randomized trial. We conducted sensitivity analyses to test the robustness of the results, using different denominators and methods of outcome measurement and found consistent results. One trial [8] was conducted before the advent of combination antiretroviral therapy and was the only one which included key populations, and assessed outcomes by two assisted approaches (provider and contract referral); excluding it would have strengthened the impact of partner notification interventions. Although included data were derived from RCTs, evaluation of the quality of the evidence using the GRADE approach identified a high risk of performance and detection bias due to a lack of blinding across trials, attrition, and in some instances, imprecision and data arising from a single study. Despite these limitations, our result for the main outcome of HIV test uptake was clear and consistent, with observational data also indicating that assisted partner notification was beneficial. The pooled synthesis on social harm indicated very few events. The quality of evidence was downgraded due to imprecision and risk of bias, but when considered with similarly few adverse events from observational data, it suggests that the rate of social harm is not likely to differ between assisted and passive partner notification approaches.
In conclusion, our findings show that assisted partner notification increased HIV test uptake and diagnosed high proportions of people with HIV infection, with very few reports of harm. The difficulty in tracing identified partners may have resulted in the low ratio of partners notified per index patient. However, the high proportion of partners who were HIV positive among those who were notified warrants the efforts needed to reach partners for testing. Furthermore, treatment for infected partners is critical to prevent transmission to seronegative partners for those in serodiscordant partnerships. Assisted HIV partner notification should be implemented as a routine part of HTS and should be offered to all newly diagnosed persons, and periodically to all HIV positive persons throughout their care and treatment.
Acknowledgements
We thank Ping Teresa Yeh, Caitlin Payne, and Sophie Morse for their assistance with the literature search and screening process, and the WHO guideline development group members: Kindi Adam, Oliver Anene, Karen Champenois, Kathleen Charters, Martin Choo, Miriam Franchini, Rebecca Guy, Mehdi Karkouri, Dasha Matyushina Ocheret, Getrude Ncube, Sabin Nsanzimana, Bathabile Nyathi, Carla Obermeyer, Niluka Perera, Archana Sarkar, Jennifer Stuart-Dixon, Joseph Tak Fai Lau, Jane Thiomi, Francois Venter, and Vincent Wong. Thanks also to the WHO guideline steering committee members: Wale Ajose, Annabel Baddaley, Michel Beusenberg, Brian Chirombo, Lastone Chitembo, Rosalind Coleman, Meg Doherty, Philippa Easterbrook, Shaffiq Essajee, Haileyesus Getahun, Peter Godfrey-Faussett, Joumana Hermez, Naoko Ishikawa, Lali Khotenashvili, Daniel Low Beer, Frank Lule, Christine Mushanu, Simbarsha Mabaya, Buhle Ncube, Augustine Ntilivamunda, Ishmael Nyasulu, Martina Penazzato, Carmen Perez Casas, Julie Samuelson, Anita Sands, Willy Urassa, Freddy Perez, Razia Pendse, Bharat Rewari, Ying Ru Lo, Mukta Sharma, Nicole Seguy, Annette Verster, and Teodora Wi.
The current systematic review was supported with funding by the Bill and Melinda Gates Foundation and the United States Agency for International Development.
Contributors: R.B. and C.J. conceptualized the study. C.E.K. and V.F. conducted record screening and led data extraction. S.D., N.S., and C.J. extracted additional data. S.D. and N.S. conducted statistical analyses. S.D. drafted the article. C.F. and R.B. provided methodological and contextual input. All authors reviewed and approved the final article.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
References
- 1.Joint United Nations Programme on HIV/AIDS. Global AIDS update. Geneva: Joint United Nations Programme on HIV/AIDS; 2016. [PubMed] [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS. Prevention gap report. Geneva: Joint United Nations Programme on HIV/AIDS; 2016. [Google Scholar]
- 3.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2008; 8:359–368. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 5.Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev 2013. CD002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown L, Miller W, Kamanga G, Kaufman J, Pettifor A, Dominik R, et al. Predicting partner HIV testing and counseling following a partner notification intervention. AIDS Behav 2012; 16:1148–1155. 1148p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr 2011; 56:437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landis SE, Schoenbach VJ, Weber DJ, Mittal M, Krishan B, Lewis K, et al. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. N Engl J Med 1992; 326:101–106. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg NE, Mtande TK, Saidi F, Stanley C, Jere E, Paile L, et al. Recruiting male partners for couple HIV testing and counselling in Malawi's option B+ programme: an unblinded randomised controlled trial. Lancet HIV 2015; 2:e483–e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy JA, Fox SE. The outreach-assisted model of partner notification with IDUs. Public Health Rep 1998; 113:160–169. [PMC free article] [PubMed] [Google Scholar]
- 11.Cherutich P, Golden M, Wamuti B, Richardson B, Asbjörnsdottir K, Otieno F, et al. Effectiveness of partner services for HIV in Kenya: A cluster randomized trial. Conference on Retroviruses and Opportunistic Infections. Boston, MA; 2016. [Google Scholar]
- 12.Conrad C, Bradley H, Broz D, Buddha S, Champman E, Galang R, et al. Community outbreak of HIV Infection linked to injection drug use of oxymorphone – Indiana, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:443–444. [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Global analysis of policies on partner notification. Annex to Guidelines on HIV self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 14.Hogben M, McNally T, McPheeters M, Hutchinson AB. The effectiveness of HIV partner counseling and referral services in increasing identification of HIV-positive individuals a systematic review. Am J Prev Med 2007; 33:S89–100. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy CE, Fonner VA, Armstrong KA, O’Reilly KR, Sweat MD. Increasing HIV serostatus disclosure in low and middle-income countries: a systematic review of intervention evaluations. AIDS 2015; 29 suppl 1:S7–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman D, Antes G, Gøtzsche P, Higgins J, Jüni P, Lewis S, et al. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org. [Google Scholar]
- 17.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldacker C, Myers S, Cesar F, Parades Z, Ferrao C, Citao Citao S, et al. Who benefits from partner services in Mozambique? Results from a pilot programme in a public, urban clinic. J Int AIDS Soc 2015; 18:109–110. [Google Scholar]
- 19.Cherutich P, Golden MR, Wamuti B, Richardson BA, Asbjornsdottir KH, Otieno FA, et al. Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. Lancet HIV 2017; 4:e74–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers R, Feldacker C, Cesar F, Paredes Z, Augusto G, Muluana X, et al. Acceptability and effectiveness of assisted HIV partner services in Mozambique: results from a pilot program in a public, urban clinic. Sex Transm Dis 2016; 43:690–695. [DOI] [PubMed] [Google Scholar]
- 21.Henley C, Forgwei G, Welty T, Golden M, Adimora A, Shields R, et al. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sex Transm Dis 2013; 40:909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valle SM, De Olalla PG, Molas E, Barberá MJ, Knobel H, Díez E, et al. Acceptability and effectiveness of two partners’ notification strategies of new HIV cases. Int J STD AIDS 2015; 26:102–103. [Google Scholar]
- 23.Plotkin M, Kahabuka C, Amuri M, Njozi M, Maokola W, Mlanga E, et al. Effective, high-yield HIV testing for partners of newly diagnosed persons in Tanzania. Conference on Retroviruses and Opportunistic Infections. Boston, MA, USA; 2016. [Google Scholar]
- 24.Udeagu CC, Shah D, Shepard CW, Bocour A, Guiterrez R, Begier EM. Impact of a New York City Health Department initiative to expand HIV partner services outside STD clinics. Public Health Rep 2012; 127:107–114. [PMC free article] [PubMed] [Google Scholar]
- 25.Chiou PY, Lin LC, Chen YM, Wu SC, Lew-Ting CY, Yen HW, et al. The effects of early multiple-time PN counseling on newly HIV-diagnosed men who have sex with men in Taiwan. AIDS Behav 2015; 19:1773–1781. [DOI] [PubMed] [Google Scholar]
- 26.Carballo-Diéguez A, Remien RH, Benson DA, Dolezal C, Decena CU, Blank S. Intention to notify sexual partners about potential HIV exposure among New York City STD clinics’ clients. Sex Transm Dis 2002; 29:465–471. [DOI] [PubMed] [Google Scholar]
- 27.Tsega A, Udeagu CC, Begier EM. A comparison of partner notification effectiveness in African-, Caribbean-, and United States-born HIV-infected Blacks in New York City. AIDS Patient Care STDS 2012; 26:406–410. [DOI] [PubMed] [Google Scholar]
- 28.Kissinger PJ, Niccolai LM, Magnus M, Farley TA, Maher JE, Richardson-Alston G, et al. Partner notification for HIV and syphilis: effects on sexual behaviors and relationship stability. Sex Transm Dis 2003; 30:75–82. [DOI] [PubMed] [Google Scholar]
- 29.Brewer DD. Case-finding effectiveness of partner notification and cluster investigation for sexually transmitted diseases/HIV. Sex Transm Dis 2005; 32:78–83. [DOI] [PubMed] [Google Scholar]
- 30.Toomey KE, Peterman TA, Dicker LW, Zaidi AA, Wroten JE, Carolina J. Human immunodeficiency virus partner notification. Cost and effectiveness data from an attempted randomized controlled trial. Sex Transm Dis 1998; 25:310–316. [DOI] [PubMed] [Google Scholar]
- 31.Jordan WC. Reaching lost-to-care populations. Clin Infect Dis 2007; 45 suppl 4:S275–S280. [DOI] [PubMed] [Google Scholar]
- 32.Ahrens K, Kent CK, Kohn RP, Nieri G, Reynolds A, Philip S, et al. HIV partner notification outcomes for HIV-infected patients by duration of infection, San Francisco to, 2004 to 2006. J Acquir Immune Defic Syndr 2007; 46:479–484. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein KT, Stephens SC, Moss N, Scheer S, Parisi MK, Philip SS. Partner services as targeted HIV screening – changing the paradigm. Public Health Rep 2014; 129 suppl 1:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen MJ, Pipkin S, Marcus JL, Bernstein KT, Scheer S. Using HIV testing history to measure the success of HIV partner services. Sex Transm Dis 2013; 40:419–421. [DOI] [PubMed] [Google Scholar]
- 35.Elliott SA, Ahmad S, Ross JD. Partner notification in newly diagnosed HIV-positive patients. AIDS 1998; 12:1559–1560. [DOI] [PubMed] [Google Scholar]
- 36.Fenton KA, French R, Giesecke J, Johnson AM, Trotter S, Petruckevitch A, et al. An evaluation of partner notification for HIV infection in genitourinary medicine clinics in England. AIDS 1998; 12:95–102. [DOI] [PubMed] [Google Scholar]
- 37.Giesecke J, Ramstedt K, Ripa T, Rado G, Scalia-Tomba G, Westrell M. Partner notification for HIV in Sweden. Lancet 1990; 336:508. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Branan L, Hoff GL, Datwyler ML, Bayer WL. Voluntary human immunodeficiency virus testing, recidivism, partner notification, and sero-prevalence in a sexually transmitted disease clinic: a need for mandatory testing. Sex Transm Dis 1990; 17:169–174. [DOI] [PubMed] [Google Scholar]
- 39.Lewis F, Eberhart M, Anschuetz G, Salmon M, Terrell C, Brady K. High yield of new HIV diagnoses and patients with high viral loads from HIV partner services, Philadelphia Department of Public Health STD Control Program (STDCP) and AIDS Activities Coordinating Office (AACO), 2012. Sex Transm Dis 2014; 41:S34. [Google Scholar]
- 40.Lin H, He N, Ding Y, Qiu D, Zhu W, Liu X, et al. Tracing sexual contacts of HIV-infected individuals in a rural prefecture, Eastern China. BMC Public Health 2012; 12:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macke BA, Hennessy M, McFarlane MM, Bliss MJ. Partner notification in the real world: a four site time-allocation study. Sex Transm Dis 1998; 25:561–568. [DOI] [PubMed] [Google Scholar]
- 42.Mir N, Scoular A, Lee K, Taylor A, Bird SM, Hutchinson S, et al. Partner notification in HIV-1 infection: a population based evaluation of process and outcomes in Scotland. Sex Transm Infect 2001; 77:187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muffih PT, Mboh E, Fang E, Wainfen W, Fon H, Welty T, et al. Integrating partner notification services into PMTCT (Option B+) services in the northwest and southwest regions of Cameroon. J Int AIDS Soc 2015; 18:96–97. [Google Scholar]
- 44.Pattman RS, Gould EM. Partner notification for HIV infection in the United Kingdom: a look back on seven years experience in Newcastle upon Tyne. Genitourin Med 1993; 69:94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters PJ, Gay C, Beagle S, Shankar A, Switzer WM, Hightow-Weidman LB. HIV infection among partners of HIV-infected black men who have sex with men – North Carolina, 2011–2013. MMWR Morb Mortal Wkly Rep 2014; 63:90–94. [PMC free article] [PubMed] [Google Scholar]
- 46.Ramstedt K, Hallhagen G, Lundin BI, Hakansson C, Johannisson G, Lowhagen GB, et al. Contact tracing for human immunodeficiency virus (HIV) infection. Sex Transm Dis 1990; 17:37–41. [PubMed] [Google Scholar]
- 47.Renaud TC, Wong MR, Bocour A, Udeagu CC, Pickett L, Alt EN, et al. The effect of HIV field-based testing on the proportion of notified partners who test for HIV in New York City. Am J Public Health 2011; 101:1168–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutherford GW, Woo JM, Neal DP, Rauch KJ, Geoghegan C, McKinney KC, et al. Partner notification and the control of human immunodeficiency virus infection. Two years of experience in San Francisco. Sex Transm Dis 1991; 18:107–110. [DOI] [PubMed] [Google Scholar]
- 49.Schwarcz S, McFarland W, Delgado V, Dilley J, Rinaldi J, Adler B, et al. Partner notification for persons recently infected with HIV: experience in San Francisco. J Acquir Immune Defic Syndr 2001; 28:403–404. [DOI] [PubMed] [Google Scholar]
- 50.Shan D, Duan S, Cui Y, Ye RH, Xiang LF, Yang YC, et al. Survey on contact tracing of newly reported HIV infections in 2009 in Dehong prefecture, Yunnan province. Zhonghua Yu Fang Yi Xue Za Zhi 2011; 45:965–970. [PubMed] [Google Scholar]
- 51.Udeagu CC, Webster-León T, Bocour A, Michel P, Shepard C. Using surveillance data to identify HIV-positive persons out-of-care (OOC) in New York City (NYC) and offer linkage to care and HIV partner services. J Int AIDS Soc 2012; 15:146. [Google Scholar]
- 52.Wykoff RF, Jones JL, Longshore ST, Hollis SL, Quiller CB, Dowda H, et al. Notification of the sex and needle-sharing partners of individuals with human immunodeficiency virus in rural South Carolina: 30-month experience. Sex Transm Dis 1991; 18:217–222. [DOI] [PubMed] [Google Scholar]
- 53.Wykoff RF, Heath CW, Jr, Hollis SL, Leonard ST, Quiller CB, Jones JL, et al. Contact tracing to identify human immunodeficiency virus infection in a rural community. JAMA 1988; 259:3563–3566. [PubMed] [Google Scholar]
- 54.Zhang Y, Yin F, Zhong P, He N. Practice and correlates of partner notification of HIV infection status among 307 HIV-infected individuals of Shanghai. Zhonghua Yu Fang Yi Xue Za Zhi 2015; 49:956–961. [PubMed] [Google Scholar]
- 55.Green N, Hoenigl M, Chaillon A, Anderson CM, Pond SLK, Smith DM, et al. Partner services in adults with acute and early HIV infection. AIDS 2017; 31:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abraham S, Prasad J, Joseph A, Jacob K. Confidentiality, partner notification and HIV infection. Indian J Med Ethics 2002; 10:157–160. [Google Scholar]
- 57.Low N, Broutet N, Adu-Sarkodie Y, Barton P, Hossain M, Hawkes S. Global control of sexually transmitted infections. Lancet 2006; 368:2001–2016. [DOI] [PubMed] [Google Scholar]
- 58.Wamuti BM, Macharia G, Asbjörnsdottir K, Sambai B, Dunbar M, Ng’an’a A, et al. Linkage to care for index clients in the assisted partner services (APS) study, Kenya. In: 21st International AIDS Conference. Durban, South Africa; 2016. [Google Scholar]
- 59.Hoxworth T, Spencer NE, Peterman TA, Craig T, Johnson S, Maher JE. Changes in partnerships and HIV risk behaviors after partner notification. Sex Transm Dis 2003; 30:83–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


