Abstract
Fibromyalgia (FM) is a chronic pain condition often resulting in functional impairments. Nonrestorative sleep is a prominent symptom of FM that is related to disability, but the day-to-day mechanisms relating the prior night’s sleep quality to next day reports of disability have not been examined. The current study examined the within-day relations among early-morning reports of sleep quality last night, late-morning reports of pain and positive and negative affect, and end-of-day reports of activity interference. Specifically, we tested whether pain, positive affect, and negative affect mediated the association between sleep quality and subsequent activity interference. Data were drawn from electronic diary reports, collected from 220 FM patients for 21 consecutive days. The direct and mediated effects at the within-person level were estimated with Multilevel Structural Equation Modeling. Results showed that pain and positive affect mediated the relation between sleep quality and activity interference. Early-morning reports of poor sleep quality last night predicted elevated levels of pain and lower levels of positive affect at late-morning, which, in turn, predicted elevated end-of-day activity interference. Of note, positive affect was a stronger mediator than pain, and negative affect was not a significant mediator. In summary, the findings identify two parallel mechanisms, pain and positive affect, through which the prior night’s sleep quality predicts disability the next day in FM patients. Further, results highlight the potential utility of boosting positive affect following a poor night’s sleep as one means of preserving daily function in FM.
Keywords: Fibromyalgia, Sleep Disturbance, Pain, Positive Affect, Negative Affect, Activity Interference
Fibromyalgia (FM) is characterized by chronic widespread pain that often results in poor functional health [36] and a high rate of disability [38,33]. A prevalent FM symptom that has implications for disability is nonrestorative sleep, that is, sleep that is poor in quality even though the duration may appear normal [18]. Among FM patients, 60–80% report experiencing nonrestorative sleep [37,17]. In fact, sleep quality is a key predictor of health outcomes in FM; individuals who report more versus less sleep disturbance also report more pain and fatigue, and greater activity limitations [31,1,3]. In an effort to elaborate the dynamic link between sleep quality and activity limitations on a daily basis, Affleck and colleagues collected morning reports of prior nights’ sleep quality and evening reports of goal accomplishment for the day for 30 consecutive days in 50 FM patients [1]. They found that poor sleep one night predicted decreased effort toward accomplishing personal goals the next day.
One mechanism through which nonrestorative sleep may limit day-to-day activities among chronic pain patients is via heightened pain. O’Brien and colleagues measured daily variability in sleep and pain in a sample of chronic pain patients [23]. Not only did increases in pain predict subsequent sleep disturbance, but also increases in sleep disturbance predicted subsequent pain. A review examining the relation between sleep and pain, however, found stronger support for the effects of sleep on pain than for the effects of pain on sleep [7].
A second potential mechanism linking daily sleep quality and activity limitations is affective changes. Much attention has focused on the influence of sleep on negative affective experiences (e.g., negative affect, depression; [11,16]); however, the effects of sleep extend to include positive affect [10]. For instance, in healthy individuals, restricted sleep for 12 days decreased positive affect relative to usual sleep [9]. Of note, FM patients appear to have deficits in the regulation of positive affect [8]. Specifically, daily diary reports suggest that FM patients demonstrate lower levels of positive affect overall, and diminished ability to sustain positive affect in the face of aversive experiences compared to osteoarthritis patients [8]. Sleep disturbance, which is especially prominent in FM [19,18,37], may contribute to their positive affect deficits and ultimately to their increased disability relative to other pain patients [36,38,33].
To our knowledge, no study has examined the within-day links between sleep quality and activity limitations in FM. Here we employed within-day electronic diary reports completed by patients for 21 days to examine the sleep‒activity interference relation (see Figure 1). Three hypotheses were tested: 1) early-morning reports of sleep disturbance last night will predict late-morning reports of elevated pain and negative affect and lower positive affect; 2) elevated late-morning pain and negative affect, and lower positive affect, will predict elevated end-of-day activity interference; and 3) reports of late-morning pain, positive affect, and negative affect will mediate the relation between sleep quality and end-of-day activity interference.
Figure 1.
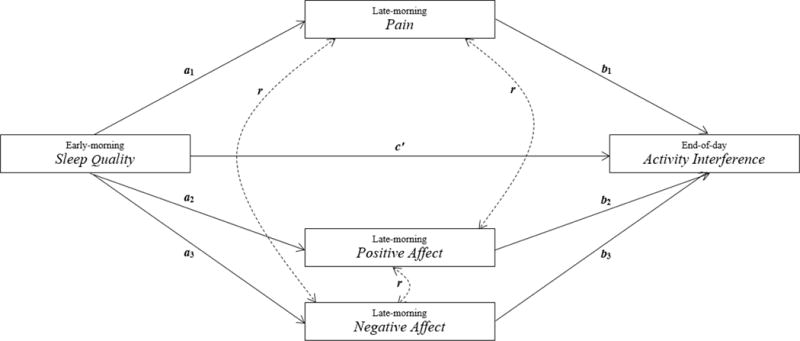
Late-morning pain and positive and negative affect as mediators of the relation between sleep quality and end-of-day activity interference.
Method
Participants
The participants were 220 individuals with FM who agreed to participate in an intervention trial of behavioral treatments for FM and completed the pre-intervention diary assessment (described below). Participants were recruited from the Phoenix, Arizona metropolitan area through physician referrals, print and online advertisements, fliers posted in medical clinics, and fibromyalgia support groups. To be eligible for participation, individuals were required to: 1) be 18 years of age or older; 2) speak English; 3) meet the American College of Rheumatology (ACR) diagnostic criteria for FM [39]; and 4) agree to be randomized into a treatment condition. Participants were excluded from participation if they: 1) reported comorbid medical or psychological conditions that would interfere with their involvement in the study procedures; 2) were involved in litigation related to their pain; and/or 3) were currently involved in a psychosocial treatment related to their pain or mood disturbance.
Procedure
Interested respondents were first screened for eligibility by telephone. Individuals who were screened eligible gave their informed consent and underwent a tender point examination to confirm the presence of widespread pain [39]. As part of the pre-intervention assessment, participants completed a 21-day daily diary that assessed physical, psychological, and social experiences in their day-to-day lives. The present study focuses on these pre-intervention daily diaries.
Electronic diary reports were collected for 21 days through the use of a cellular phone provided to participants for the diary period. A research staff member met with participants to provide instructions regarding the diary data collection. An automated phone system called each participant four times per day: Time 1) 20 minutes following a wake-up time specified by the participant, which was consistent across days, for an early-morning interview; Time 2) at 11:00 a.m. for a late-morning interview; Time 3) at 4:00 p.m. for an afternoon interview; and Time 4) at 7:00 p.m. for an end-of-day interview. Participants who missed the call were instructed to call the system within two and a half hours to complete the interview. If any problem occurred with the phone system, participants could contact the laboratory staff for assistance. To enhance the quality of the data, participants were monitored throughout the 21-day diary assessment. They were contacted if they failed to complete two consecutive days of diaries. Also, participants were compensated $3 a day for each of the 21 days they completed. To examine the hypotheses of the current study, sleep quality assessed at Time 1, pain, positive affect, and negative affect assessed at Time 2, and activity interference assessed at Time 4 were used in the model testing.
Measures
Sleep Quality
Sleep on the previous night was assessed by four items drawn from the Pittsburgh Sleep Quality Index, which has been shown to have strong internal consistency, test-retest reliability, and diagnostic validity [4]. Participants were asked to indicate the total number of hours and minutes they slept; whether they had trouble staying asleep on a 4-point scale (1 = “not at all”; 4 = “quite a bit”); the quality of their sleep on a 101-point scale (0 = “extremely poor sleep”; 100 = “extremely good sleep”); and how refreshed they felt upon awakening on a 101-point scale (0 = “not at all refreshed”; 100 = “extremely refreshed”). Because the present study focused on the experience of nonrestorative sleep each night specifically, only the last two items were used to measure sleep quality for the within-day analyses. Both items were rescaled from a 0 to 100 scale to a 0 to 5 scale by dividing each score by 20. The two items were rescaled (i.e., linearly transformed) to be comparable to the scaling of other variables in the model to ease interpretation of findings. Linear transformations are a way of approaching standardization of variables that does not affect correlations, the proportion of variance explained, or the significance of results [5]. The within-person correlation for the two items was r = .70. A composite of sleep quality was formed by computing a mean of the responses to the two rescaled items on each day.
Pain
Participants rated their level of pain (i.e., “What was your overall level of pain?”) on a 101-point scale (0 = “no pain”; 100 = “pain as bad as it can be”) during the preceding two- to three-hour period. The 1-item scale has been shown to be equal or superior to other rating scales assessing pain [12]. Responses to the item were rescaled from a 0 to 100 scale to a 0 to 5 scale by dividing each score by 20.
Affect
Items drawn from the Positive and Negative Affect Schedule – Expanded Form (PANAS-X; [35]) were used to assess positive and negative affect. Positive affect was assessed by two items (i.e., “cheerful” and “energetic”) drawn from the Joviality subscale and one item (i.e., calm) drawn from the Serenity subscale; negative affect was assessed by one item (i.e., “sad”) drawn from the Sadness subscale, one item (i.e., “afraid”) drawn from the Fear subscale, and one item (i.e., “angry”) drawn from the Hostility subscale. In the current study, participants rated the extent to which they felt each affect during the preceding two- to three-hour period using a 5-point scale (1 = “not at all”; 5 = “completely”). The within-person correlation for the three positive items was r = .57, and for the three negative items was r = .59. Composites of positive and negative affect were formed by computing a mean from the responses of the three items for each day.
Activity interference
Activity interference was assessed by four items drawn from the Role Physical subscale of the SF-36 health survey [34]. Specifically, participants responded to the following questions regarding their days’ activities: 1) “Did you cut down on the amount of time spent on work or other activities?”; 2) “Today did you accomplish less than you would have liked?”; 3) “Were you limited in the kind of work or other activities you did?”; and 4) “Did you have difficulty performing work or other activities?” Each question was rated on a 3-point scale (1 = “no”; 3 = “yes very much”). The within-person correlation for the four items was r = .74. A composite of activity interference was formed by computing a mean from the responses of the four items for each day.
Data Analytic Plan
Modeling strategy
The data for this study are organized at two levels: the first level is days (within-person), which are nested within the second level, individuals (between-person). Thus, we employed a multilevel data analytic strategy. Mplus version 7 statistical software [21] was used to perform Multilevel Structural Equation Modeling (MSEM; [24]) to test the hypothesized mediated model (see Figure 1). In an Mplus two-level random model, each measured variable is partitioned into a within-person (level-1) latent score and a between-person (level-2) latent score [22]. The creation of the two latent variables produces orthogonal variance components at the within- and between-person levels, which account for the random effects in clustered data [20]. In MSEM, level-1 predictors are subject to “implicit model-based group mean centering,” in which the latent scores are deviated from the latent cluster means (p. 210, [24]). Thus, the relations among the variables in our hypothesized model were tested by using the level-1 model-based centered latent variables rather than the measured centered variables that are typically used in multilevel modeling (MLM). (In MLM, group mean centering is accomplished by deviating the raw scores of the measured variables from the cluster means of those variables [25]). The matrix expressions of a prototypical multilevel structural equation model with a single mediator that detail the partitioning of the within- and between-person variance components can be found in Preacher et al., p. 231 [24].
Study hypotheses were focused at the within-person level, examining covariation within a person throughout a day (e.g., deviations from an individual’s usual late-morning pain to deviations from his/her usual end-of-day activity interference, on the same day). As noted above, Mplus uses “implicit, model-based group mean centering,” an approach that prevents biases in parameter estimates that can derive from clustering in the data by estimating relations among variables at the within-person and between-person levels simultaneously. Because the hypotheses of the current study are all at the within-person level, only the within-person parameter estimates are reported in the results.
Estimating mediated effects
The proposed model (see Figure 1) was tested according to the recommendations provided by Preacher and colleagues [24]. Consistent with the MSEM approach, all paths were specified to have random intercepts and fixed slopes with one exception; the relation between ratings of last night’s sleep quality and end-of-day activity interference (the c’ path) was specified to have a random intercept and random slope. In the present study, there were three parallel mediators (i.e., pain, positive affect, and negative affect) and, thus, three mediational chains. The estimated paths at within-person level were: 1) a1, the path from sleep quality last night to late-morning pain; 2) b1, the path from late-morning pain to end-of-day activity interference; 3) a2, the path from sleep quality last night to late-morning positive affect; 4) b2, the path from late-morning positive affect to end-of-day activity interference; 5) a3, the path from sleep quality last night to late-morning negative affect; and 6) b3, the path from late-morning negative affect to end-of-day activity interference. The mediated or indirect effect (i.e., the product of the a and b paths, ab) is asymmetrically distributed and the shape of the distribution depends on the correlation between the a and b paths. Therefore, this correlation must be considered when determining unbiased test statistics of the mediational path [13]. Accordingly, the significance of the mediated effect (i.e., the product ab) was measured by taking into account the correlation between the a and b paths; this was tested through Rmediation, which generated the asymmetric confidence interval for the mediated effect [29]. Further, the 3-mediator model was fully saturated; therefore, no fit indices were available.
Handling missing data
The daily diary assessments were administered consecutively for 21 days, 4 times a day. Because not all participants completed all measures at all time points within a day or across days, missing data resulted in different cluster sizes. The full information maximum likelihood (FIML) estimator employing an accelerated EM algorithm procedure in Mplus version 7 was used. This procedure is robust to missing data, non-normality, and unbalanced cluster sizes in data [24,20].
Results
Sample Characteristics
The mean age of the sample was 51.25 years (SD = 11.02; range = 19 to 72). Most participants were female (88.6%), Caucasian (77.1%), and either married or living with a romantic partner (56.4%). The majority of the sample had completed some level of college education (84.1%); however, only about half of the participants (51.8%) reported being employed. The average yearly income of the sample was between $30,000 and $40,000. With regard to average sleep duration and disturbance across the diary assessment, participants reported sleeping 6 hours and 37 minutes (SD = 1.93) and experiencing some difficulty staying asleep (M = 2.45, SD = 1.11).
Data Completion Rate
During the 21-day assessment period, participants completed an average of 17.84 days (SD = 4.18) of early-morning reports describing sleep quality last night; 17.87 days (SD = 4.43) of late-morning pain; 17.83 days (SD = 4.45) of late-morning positive affect; 17.83 days (SD = 4.47) of late-morning negative affect; and 17.08 days (SD = 4.91) of end-of-day activity interference. Of the 220 participants, 210 (95%) completed at least 10 days of diaries.
Descriptives, Intraclass Correlations (ICCs), and Within-Person Correlations
The descriptive statistics and ICCs of study variables are presented in Table 1. On average, the current sample reported intermediate levels of sleep quality, late-morning pain and positive and negative affect, and end-of-day activity interference. Further the ICC values (range = .303 to .573) indicate that within-person variation was substantial, suggesting that multilevel analysis was warranted for these data.
Table 1.
Descriptives and intraclass correlations (ICCs) of study variables
| Variable | Mean | Standard Deviation | ICC |
|---|---|---|---|
| Sleep Quality (Predictor) | 2.432 | 1.175 | .303 |
| Pain (1st Mediator) | 2.439 | 1.218 | .490 |
| Positive Affect (2nd Mediator) | 2.592 | .903 | .499 |
| Negative Affect (3rd Mediator) | 1.627 | .860 | .573 |
| Activity Interference (Outcome) | 2.003 | .633 | .373 |
Table 2 displays the pooled within-person correlations among sleep quality, late-morning pain and positive and negative affect, and end-of-day activity interference. The correlations indicate that all of the variables are significantly related to one another in the expected direction (e.g., negative correlation between sleep quality and pain).
Table 2.
Within-person correlations among study variables
| Variable | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. Sleep Quality (Predictor) | – | ||||
| 2. Pain (1st Mediator) | −.111** | – | |||
| 3. Positive Affect (2nd Mediator) | .202** | −.326** | – | ||
| 4. Negative Affect (3rd Mediator) | −.128** | .239** | −.397** | – | |
| 5. Activity Interference (Outcome) | −.097** | .184** | −.247** | .096** | – |
Note. N = 4317;
p < .01; Estimated via Maximum Likelihood in a Two-Level Random Coefficient Model in Mplus.
Within-day Analyses of Relations from Prior Night’s Sleep Quality to Late-Morning Pain and Positive and Negative Affect to End-of-day Activity Interference
MSEM was performed to examine the relations among sleep quality, late-morning pain and positive and negative affect, and end-of-day activity interference in a single model. At the within-person level, pain significantly mediated the relation between sleep quality and activity interference (see Figure 2 and Table 3). Early-morning reports of higher than usual sleep disturbance last night predicted higher than usual levels of late-morning pain, a1 path (p < .001). Increased late-morning pain, in turn, predicted higher than usual activity interference at the end of that day, b1 path (p < .001). The a1 and b1 paths were positively correlated (r = .005). Further, the asymmetric confidence interval, which accounts for the a1 b1 correlation, indicated that the mediated effect of pain was significant [−.010, −.004].
Figure 2.
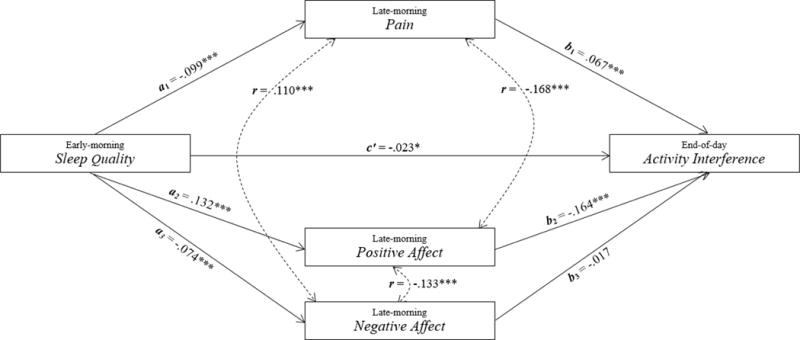
Within-person results of the relation from sleep quality to late-morning pain and positive and negative affect to end-of-day activity interference (* p < .05; *** p < .001).
Table 3.
Within-person mediation model examining the roles of late-morning pain and positive and negative affect in mediating the relation between sleep quality and end-of-day activity interference
| Mediator | a path B (SE B) | b path B (SE B) | ab path B (SE B) | Correlation of a and b | Asymmetric Confidence interval |
|---|---|---|---|---|---|
| Pain | −.099(.019)*** | .067(.012)*** | −.007(.002)*** | .005 | [−.010, −.004] |
| Positive Affect | .132(.015)*** | −.164(.019)*** | −.022(.004)*** | −.067 | [−.029, −.015] |
| Negative Affect | −.074(.013)*** | −.017(.019) | −.001(.001) | −.089 | [−.002, .004] |
p < .001.
The second mediator, positive affect, also significantly mediated the relation between sleep quality and activity interference (see Figure 2 and Table 3). Early-morning reports of higher than usual sleep disturbance last night predicted lower than usual levels of positive affect during the late-morning time period, a2 path (p < .001). Decreased late-morning positive affect, in turn, predicted higher than usual levels of activity interference at the end of the day, b2 path (p < .001). The a2 and b2 paths were negatively correlated (r = −.067). The asymmetric confidence interval, which accounts for the a2 b2 correlation, indicated that positive affect was a significant mediator [−.029, −.015]. Further, a contrast between the mediated paths of pain and positive affect demonstrated that positive affect was a stronger mediator than pain (Est. = −.015, SE = .004, p < .001).
The third mediator, negative affect, did not mediate the relation between sleep quality and activity interference (see Figure 2 and Table 3). Early-morning reports of higher than usual sleep disturbance last night predicted higher than usual levels of negative affect during the late-morning time period, a3 path (p < .001). However, increased late-morning negative affect was not related to end-of-day activity interference, b3 path (p = .366). Lastly, sleep disturbance was directly related to activity interference, c’ path (p = .025); early-morning reports of higher than usual sleep disturbance last night predicted higher than usual levels of activity interference at the end of that day even when accounted for the effects of pain and positive and negative affect (see Figure 2).
In summary, the relation between sleep quality and end-of-day activity interference was partially mediated by both late-morning pain and positive affect. Importantly, negative affect was not a significant mediator of the sleep‒activity interference link, and the mediated path of positive affect was stronger in magnitude than that of pain.
Discussion
Experiencing nonrestorative sleep is a prominent feature of FM that has implications for health and well-being [37]. The objective of the current study was to elaborate the within-day relation between nonrestorative sleep and limitations in daily activities among individuals with FM. We hypothesized that self-reports of poor sleep last night would predict late-morning elevations in pain and negative affect and declines in positive affect, which in turn, would predict elevated activity limitations at the end of the day. The findings with regard to pain and positive affect were consistent with the hypotheses in that both factors served as mediators; of note, the mediating role of positive affect was stronger than that of pain. However, although nonrestorative sleep predicted elevated negative affect, elevations in negative affect did not predict activity limitations.
Our findings build on the limited data available regarding the within-day relations between sleep quality and daily activities among individuals with FM. Affleck and colleagues were the first to demonstrate a positive link between good sleep quality last night and accomplishing personal goals the next day [1]. The current study extends the work of Affleck et al. [1] by documenting two specific mechanisms (i.e., changes in pain and positive affect) that account for much of the impact of sleep quality on daily functioning in FM. The role of pain as a mediator in the relation between poor sleep quality and activity interference is in line with existing prospective evidence linking sleep quality with subsequent pain [7,2] and disability [36,38] among chronic pain patients. Perhaps most noteworthy, positive affect was a stronger mediator of the sleep‒activity limitation relation within a day than was pain. A growing body of evidence points to the contribution of positive affective resources to functional health. For example, Steptoe, de Oliveira, Demakakos, and Zaninotto [28] recently found a link between initial levels of positive affective health and physical function eight years later in a sample of older adults. Specifically, adults who reported greater enjoyment of life experienced less impairment in activities of daily living and had a slower decline in gait speed over time [28]. Together, the available evidence suggests that the processes linking sleep, pain and positive affect, and functional health may unfold both within a day and over months and years to impact patients’ lives.
Interestingly, sleep quality predicted both subsequent positive and negative affect, but only positive affect was linked to subsequent limitations in daily activity. In their diary study of FM patients, Hamilton and colleagues [10] similarly found that sleep disturbance prospectively predicted declines in positive and elevations in negative affect; moreover, they reported that restricted sleep interfered with positive and negative affect recovery following stress. Thus, the accruing evidence points to the importance of sleep disturbance in affect regulation in FM, but also highlights the central role of positive rather than negative affect in the sleep‒disability relation.
Several limitations of the present study deserve comment. First, because the diary data are all based on self-report measures, the relations among variables could be accounted for at least in part by shared method variance. The temporal ordering of the assessments and articulation of specific hypotheses in the current study allays this concern to some extent. Nevertheless, inclusion of objective measures would strengthen confidence in our findings. For instance, incorporating actigraphic recordings to measure sleep quality and activity levels would provide additional information to complement the data gleaned from self-reports [14]. Second, the sample included only individuals with FM; thus, the generalizability of the findings to individuals with other chronic pain conditions remains to be determined. Finan et al. suggested that individuals with FM appear to have deficits in the regulation of positive affect, even relative to other pain patients [8]. Thus, the central role of positive affect in the mediational chain linking sleep with functional health may be especially relevant to those with FM and less important for individuals with other pain conditions. Third, the study was based on correlational data, precluding causal interpretations regarding the relations among variables. Only through experimental manipulations of key variables, including sleep quality, pain, and positive affect, can the causal links between sleep disturbance and activity limitations be established. Fourth, a small proportion of early-morning responses (8.94%) were recorded between 10:00 a.m. and 10:30 a.m., which constrained the time lapse between the early-morning and late-morning ratings in those instances. As a result, participant ratings between these two time points may not have significantly varied for those who chose to wake up later in the morning.
Despite its limitations, the current study has yielded findings that highlight some areas for future research on the day-to-day consequences of sleep disturbance in FM. In particular, elaboration of the dimensions of positive and negative affect that are influenced by poor sleep is worthy of attention. For example, high activation positive affect (e.g., cheerfulness, happiness, enthusiasm) may be more profoundly affected by sleep problems than low activation positive affect (e.g., calmness, relaxation, contentedness), and may prove to be more important in preserving daily functioning in FMS [6,30]. Thus, including additional items from the PANAS-X [34], or other similar empirically-validated scales with high reliability that reflect each dimension of positive and negative affect would extend the effects of affect. Additionally, inclusion of objective indicators of sleep quality could help to identify aspects of sleep (e.g., latency, efficiency, architecture) that have the most potent effects on subsequent pain, mood, and functional health in FM (e.g., [14]). For example, findings gleaned from polysomnographic recordings of sleep indicate that disturbance in the phasic pattern of the alpha EEG slow wave sleep (i.e., alpha-delta EEG sleep) is linked to subsequent pain and increased tenderness and stiffness after awakening in FM patients [26], and to decreases in pain threshold and psychological vigor in healthy individuals [15]. Thus, disturbance of slow wave sleep in particular may have deleterious effects on positive affect and functioning in FM. Finally, developing dynamic models that test the reciprocal relations between sleep quality, pain, positive affect, and activity interference over time would provide a more comprehensive picture of the daily life experience of individuals with FM.
A growing body of empirical evidence links nonrestorative sleep with poor outcomes in FM. The current study demonstrated two parallel mechanisms (i.e., changes in pain and positive affect) through which sleep disturbance during the previous night contributes to activity limitations at the end of the next day in individuals with FM. Importantly, positive affect was a stronger mediator than was pain of the sleep–activity interference relation. These findings can inform efforts to develop and implement psychological interventions aimed at improving daily function in those with FM. CBT for sleep disturbance has been effective in improving sleep quality and reducing pain in chronic pain populations (e.g., osteoarthritis; [32,27]). The current findings suggest that focusing efforts on maintaining or boosting positive affect following a poor night’s sleep may also be beneficial in preserving day-to-day function.
Acknowledgments
We would like to thank participants of the treatment study, and members of the research team, including Alex Zautra, Shannon Taylor, Laurie Wolf, and Kirti Thummala, for their contributions to the study.
The study was funded through NIH grant 5R01AR053245.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Affleck G, Tennen H, Urrows S, Higgins P, Abeles M, Hall C, Karoly P, Newton C. Fibromyalgia and women’s pursuit of personal goals: A daily process analysis. Health Psychol. 1998;17:40–7. doi: 10.1037//0278-6133.17.1.40. [DOI] [PubMed] [Google Scholar]
- 2.Alsaadi SM, McAuley JH, Hush JM, Lo S, Lin CC, Williams CM, Maher CG. Poor sleep quality is strongly associated with subsequent pain intensity in patients with acute low back pain. Arthritis Rheumatol. 2014;66:1388–94. doi: 10.1002/art.38329. [DOI] [PubMed] [Google Scholar]
- 3.Bigatti SM, Marie-Hernandez A, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: Relationship to pain and depression. Arthritis Care Res. 2008;59:961–7. doi: 10.1002/art.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buysse DJ, Reynolds CF, Monk TH, Hoch CC. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–38. [PubMed] [Google Scholar]
- 5.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd. Mahwah, NJ: Lawrence Erlbaum; 2003. [Google Scholar]
- 6.Côté KA, Moldofsky H. Sleep, daytime symptoms, and cognitive performance in patients with fibromyalgia. J Rheumatol. 1997;24:2014–23. [PubMed] [Google Scholar]
- 7.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: An update and a path forward. J Pain. 2013;14:1539–52. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med. 2009;71:474–82. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- 9.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton NA, Affleck G, Tennen H, Karlson C, Luxton D, Preacher KJ, Templin JL. Fibromyalgia: The role of sleep in affect and in negative event reactivity and recovery. Health Psychol. 2008;27:490–97. doi: 10.1037/0278-6133.27.4.490. [DOI] [PubMed] [Google Scholar]
- 11.Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CF, Kupfer DJ. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–230. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: A comparison of six methods. Pain. 1986;27:117–26. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 13.Kenny DA, Korchmaros JD, Bolger N. Lower level mediation in multilevel models. Psychol Methods. 2003;8:115–128. doi: 10.1037/1082-989x.8.2.115. [DOI] [PubMed] [Google Scholar]
- 14.Landis CA, Frey CA, Lentz MJ, Rothermel J, Buchwald D, Shaver JLF. Self-reported sleep quality and fatigue correlates with actigraphy in midlife women with fibromyalgia. Nurs Res. 2003;52:140–7. doi: 10.1097/00006199-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lentz MJ, Landis CA, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–92. [PubMed] [Google Scholar]
- 16.McCracken LM, Iverson GL. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res Manag. 2001;7:75–79. doi: 10.1155/2002/579425. [DOI] [PubMed] [Google Scholar]
- 17.Moldofsky H. Management of sleep disorders in fibromyalgia. Rheum Dis Clin North Am. 2002;28:353–65. doi: 10.1016/s0889-857x(01)00012-6. [DOI] [PubMed] [Google Scholar]
- 18.Moldofsky H. The significance, assessment, and management of nonrestorative sleep in fibromyalgia syndrome. Prim Psychiatry. 2008;15:22–6. doi: 10.1017/s1092852900026808. [DOI] [PubMed] [Google Scholar]
- 19.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculoskeletal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37:341–51. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Muthén BO, Asparouhov T. Growth mixture modeling: Analysis with non-Gaussian random effects. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, editors. Longitudinal Data Analysis. Boca Raton, FL: Chapman & Hall/CRC; 2008. pp. 143–165. [Google Scholar]
- 21.Muthé LK, Muthén BO. Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén; 1998–2002. [Google Scholar]
- 22.Muthé LK, Muthén BO. Mplus User’s Guide. 7th. Los Angeles, CA: Muthén & Muthén; 2013. [Google Scholar]
- 23.O’Brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, Robinson ME. Intraindividual variability in daily sleep and pain ratings among chronic pain patients: Bidirectional association and the role of negative mood. Clin J Pain. 2011;27:425–33. doi: 10.1097/AJP.0b013e318208c8e4. [DOI] [PubMed] [Google Scholar]
- 24.Preacher KJ, Zyphur MJ, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods. 2010;15:209–33. doi: 10.1037/a0020141. [DOI] [PubMed] [Google Scholar]
- 25.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd. Newbury Park, CA: Sage; 2002. [Google Scholar]
- 26.Rizzi M, Sarzi-Puttini P, Atzeni F, Capsoni F, Andreoli A, Pecis M, Colombo S, Carrabba M, Sergi M. Cyclic alternating pattern: A new marker of sleep alteration in patients with fibromyalgia? J Rheumatol. 2004;31:1193–99. [PubMed] [Google Scholar]
- 27.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 28.Steptoe A, de Oliveira C, Demakakos P, Zaninotto P. Enjoyment of life and declining physical function at older ages: A longitudinal cohort study. CMAJ. 2014:1–7. doi: 10.1503/cmaj.131155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Totterdell P, Reynolds S, Parkinson B, Briner RB. Associations of sleep with everyday mood, minor symptoms and social interaction experience. Sleep. 1994;17:466–75. doi: 10.1093/sleep/17.5.466. [DOI] [PubMed] [Google Scholar]
- 31.Theadom A, Cropley M, Humphrey KL. Exploring the role of sleep and coping in quality of life in fibromyalgia. J Psychosom Res. 2007;62:145–51. doi: 10.1016/j.jpsychores.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5:355–62. [PMC free article] [PubMed] [Google Scholar]
- 33.Walker EA, Keegan D, Gardner G, Sullivan M, Katon WJ, Bernstein D. Psychosocial factors in fibromyalgia compared with rheumatoid arthritis: I. Psychiatric diagnoses and functional disability. Psychosom Med. 1997;59:565–71. doi: 10.1097/00006842-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 35.Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule – Expanded form. Iowa City: University of Iowa; 1994. [Google Scholar]
- 36.White KP, Speechley M, Harth M, Ostbye T. Comparing self‐reported function and work disability in 100 community cases of fibromyalgia syndrome versus controls in London, Ontario: the London Fibromyalgia Epidemiology Study. Arthritis Rheum. 1999;42:76–83. doi: 10.1002/1529-0131(199901)42:1<76::AID-ANR10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 37.White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: Comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol. 1999;26:1577–85. [PubMed] [Google Scholar]
- 38.Wolfe F, Anderson J, Harkness D, Bennett RM, Caro XJ, Goldenberg DL, Russell IJ, Yunus MB. Work and disability status of persons with fibromyalgia. J Rheumatol. 1997;24:1171–78. [PubMed] [Google Scholar]
- 39.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin M, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 40.Zautra AJ, Fasman R, Reich JW, Harakas P, Johnson LM, Olmsted ME, Davis MC. Fibromyalgia: Evidence for deficits in positive affect regulation. Psychosom Med. 2005;67:147–55. doi: 10.1097/01.psy.0000146328.52009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]


