Abstract
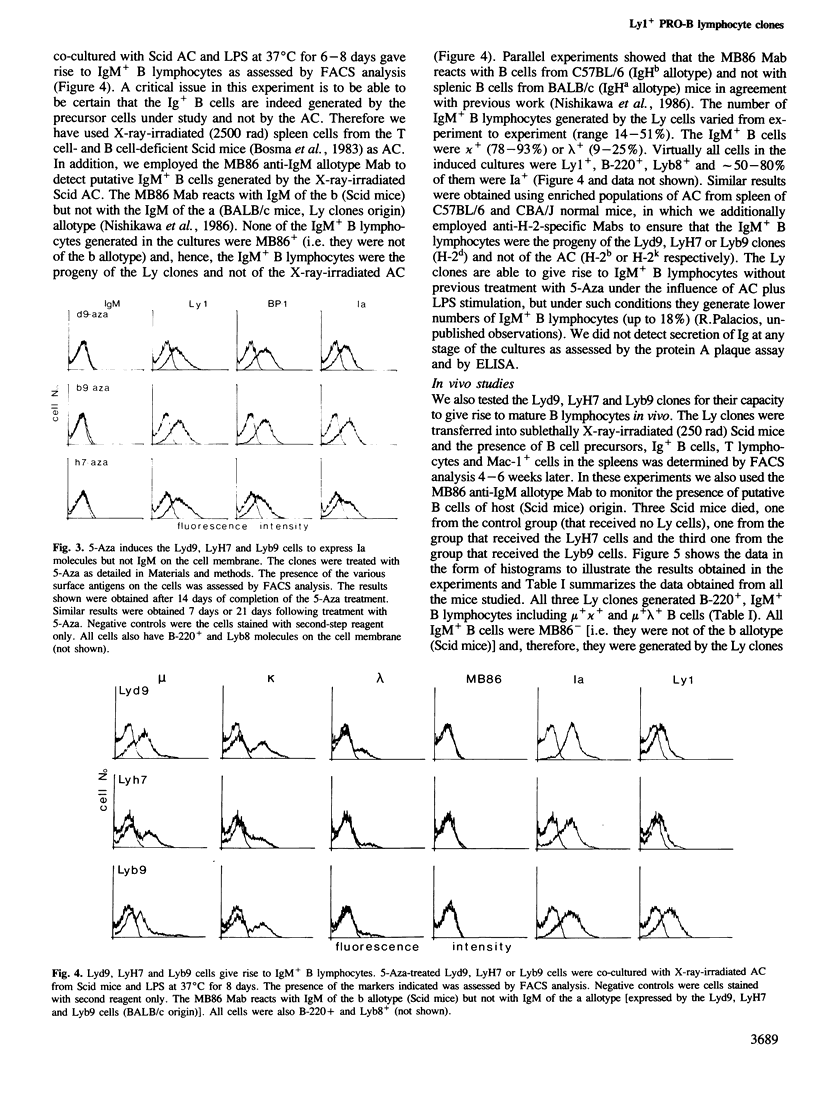
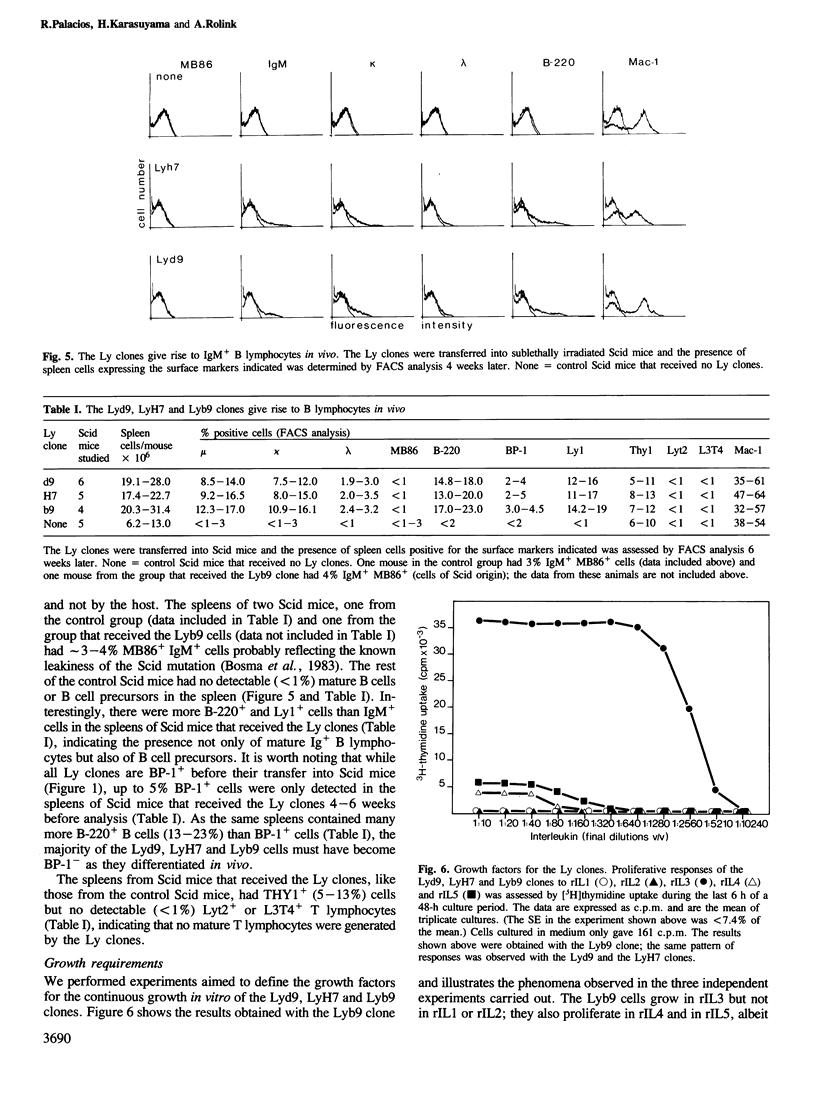
Several clones obtained from the bone marrow of a BALB/c mouse were found to contain the heavy and light chain Ig genes in the germline configuration, to express Ly1 and to carry the B cell lineage markers B-220, Lyb8 and BP-1; these clones are Pgp-1+, LFA-1+, J11d+, Mac-1+ and Thy1-, Lyt2-, L3T4-, GM1.2- and Ia-. Three clones analyzed in detail (Lyd9, LyH7 and Lyb9) have receptors for interleukin (IL) 2 and IL3 as assessed with the 7D4 and CC11 monoclonal antibodies respectively. They grow in rIL3 but not in rIL2 or rIL1; both rIL4 and rIL5 also promote their proliferation, albeit to a much lesser extent than rIL3. None of the interleukins tested alone or in various combinations promoted the clones to differentiate in vitro along the B cell pathway. Treatment with 5-Azacytidine (5-Aza) induced cell surface Ia expression but not rearrangement or expression of Ig genes. However, 5-Aza-treated Lyd9, LyH7 and Lyb9 cells co-cultured with X-ray irradiated accessory cells and LPS gave rise to Ly1+, IgM+ B lymphocytes (range 14-51%) including mu + kappa + (78-93%), and mu + lambda + (9-25%) B lymphocytes. In vivo, the Lyd9, LyH7 and Lyb9 clones gave rise to IgM+ B lymphocytes (8.5-17%) including mu + kappa +, and mu + lambda +, but not to Lyt2+ or L3T4+ T lymphocytes after 4-6 weeks of transfer into Scid mice. Our results indicate that Ly1+ IgM+ cells comprise a subpopulation of B lymphocytes that is derived from IL3-responsive Ly1+ PRO-B lymphocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Braun J., Citri Y., Baltimore D., Forouzanpour F., King L., Teheranizadeh K., Bray M., Kliewer S. B-Ly1 cells: immortal Ly-1+ B lymphocyte cell lines spontaneously arising in murine splenic cultures. Immunol Rev. 1986 Oct;93:5–21. doi: 10.1111/j.1600-065x.1986.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Mulvaney D., Coutinho A., Cazenave P. A. A novel cell surface molecule on early B-lineage cells. Nature. 1986 Jun 5;321(6070):616–618. doi: 10.1038/321616a0. [DOI] [PubMed] [Google Scholar]
- Corbel C., Melchers F. The synergism of accessory cells and of soluble alpha-factors derived from them in the activation of B cells to proliferation. Immunol Rev. 1984 Apr;78:51–74. doi: 10.1111/j.1600-065x.1984.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M., Kemp D. J. Somatic rearrangements forming active immunoglobulin mu genes in B and T lymphoid cell lines. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4943–4947. doi: 10.1073/pnas.77.8.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasch J. R., Jones P. P. Independent regulation of IgM, IgD, and Ia antigen expression in cultured immature B lymphocytes. J Exp Med. 1986 Apr 1;163(4):938–951. doi: 10.1084/jem.163.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W. F., Fredrickson T. N., Rudikoff E. K., Coffman R. L., Hartley J. W., Morse H. C., 3rd A unique series of lymphomas related to the Ly-1+ lineage of B lymphocyte differentiation. J Immunol. 1984 Aug;133(2):744–753. [PubMed] [Google Scholar]
- Dorshkind K., Schouest L., Fletcher W. H. Morphologic analysis of long-term bone marrow cultures that support B-lymphopoiesis or myelopoiesis. Cell Tissue Res. 1985;239(2):375–382. doi: 10.1007/BF00218018. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Yodoi J., Nikaido T., Tagaya Y., Taniguchi Y., Honjo T., Yahara I. Expression of interleukin 2 receptors on interleukin 3-dependent cell lines. J Immunol. 1986 Feb 1;136(3):984–987. [PubMed] [Google Scholar]
- Le Gros G. S., Gillis S., Watson J. D. Induction of IL 2 responsiveness in a murine IL 3-dependent cell line. J Immunol. 1985 Dec;135(6):4009–4014. [PubMed] [Google Scholar]
- Manohar V., Brown E., Leiserson W. M., Chused T. M. Expression of Lyt-1 by a subset of B lymphocytes. J Immunol. 1982 Aug;129(2):532–538. [PubMed] [Google Scholar]
- McKearn J. P., McCubrey J., Fagg B. Enrichment of hematopoietic precursor cells and cloning of multipotential B-lymphocyte precursors. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7414–7418. doi: 10.1073/pnas.82.21.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Sieburg C. E., Whitlock C. A., Weissman I. L. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986 Feb 28;44(4):653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Sasaki Y., Kina T., Amagai T., Katsura Y. A monoclonal antibody against Igh6-4 determinant. Immunogenetics. 1986;23(2):137–139. doi: 10.1007/BF00377976. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Paige C. J. Surface immunoglobulin-negative B-cell precursors detected by formation of antibody-secreting colonies in agar. Nature. 1983 Apr 21;302(5910):711–713. doi: 10.1038/302711a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Kiefer M., Brockhaus M., Karjalainen K., Dembić Z., Kisielow P., von Boehmer H. Molecular, cellular, and functional properties of bone marrow T lymphocyte progenitor clones. J Exp Med. 1987 Jul 1;166(1):12–32. doi: 10.1084/jem.166.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Leu T. Both cloned interleukin 2 and purified interleukin 1 are required for optimal growth of purified L3T4+ and Lyt 2+ lymphocytes initiated by concanavalin A. Cell Immunol. 1985 Sep;94(2):369–382. doi: 10.1016/0008-8749(85)90261-8. [DOI] [PubMed] [Google Scholar]
- Palacios R., Leu T. CC11: a monoclonal antibody specific for interleukin 3-sensitive mouse cells defines two major populations of B cell precursors in the bone marrow. Immunol Rev. 1986 Oct;93:125–146. doi: 10.1111/j.1600-065x.1986.tb01505.x. [DOI] [PubMed] [Google Scholar]
- Palacios R., Neri T., Brockhaus M. Monoclonal antibodies specific for interleukin 3-sensitive murine cells. J Exp Med. 1986 Feb 1;163(2):369–382. doi: 10.1084/jem.163.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Sideras P., von Boehmer H. Recombinant interleukin 4/BSF-1 promotes growth and differentiation of intrathymic T cell precursors from fetal mice in vitro. EMBO J. 1987 Jan;6(1):91–95. doi: 10.1002/j.1460-2075.1987.tb04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Megson M., Owen J. J., Cooper M. D. Early production of intracellular IgM by B-lymphocyte precursors in mouse. Nature. 1976 Jan 22;259(5540):224–226. doi: 10.1038/259224a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolink A. G., Radaszkiewicz T., Melchers F. The autoantigen-binding B cell repertoires of normal and of chronically graft-versus-host-diseased mice. J Exp Med. 1987 Jun 1;165(6):1675–1687. doi: 10.1084/jem.165.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sideras P., Bergstedt-Lindqvist S., MacDonald H. R., Severinson E. Secretion of IgG1 induction factor by T cell clones and hybridomas. Eur J Immunol. 1985 Jun;15(6):586–593. doi: 10.1002/eji.1830150611. [DOI] [PubMed] [Google Scholar]
- Sideras P., Palacios R. Bone marrow pro-T and pro-B lymphocyte clones express functional receptors for interleukin (IL) 3 and IL 4/BSF-1 and nonfunctional receptors for IL 2. Eur J Immunol. 1987 Feb;17(2):217–221. doi: 10.1002/eji.1830170211. [DOI] [PubMed] [Google Scholar]
- Spalding D. M., Griffin J. A. Different pathways of differentiation of pre-B cell lines are induced by dendritic cells and T cells from different lymphoid tissues. Cell. 1986 Feb 14;44(3):507–515. doi: 10.1016/0092-8674(86)90472-1. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Zachau H. G. Two rearranged immunoglobulin kappa light chain genes in one mouse myeloma. Nucleic Acids Res. 1980 Apr 25;8(8):1693–1707. doi: 10.1093/nar/8.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U., Arp B. Methylation patterns of immunoglobulin genes in lymphoid cells: correlation of expression and differentiation with undermethylation. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6642–6646. doi: 10.1073/pnas.80.21.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori T., Rajewsky K. Lambda chain expression at different stages of ontogeny in C57BL/6, BALB/c and SJL mice. Eur J Immunol. 1981 Aug;11(8):618–625. doi: 10.1002/eji.1830110806. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Tidmarsh G. F., Muller-Sieburg C., Weissman I. L. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell. 1987 Mar 27;48(6):1009–1021. doi: 10.1016/0092-8674(87)90709-4. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]