Abstract
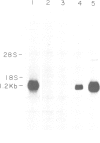
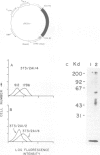
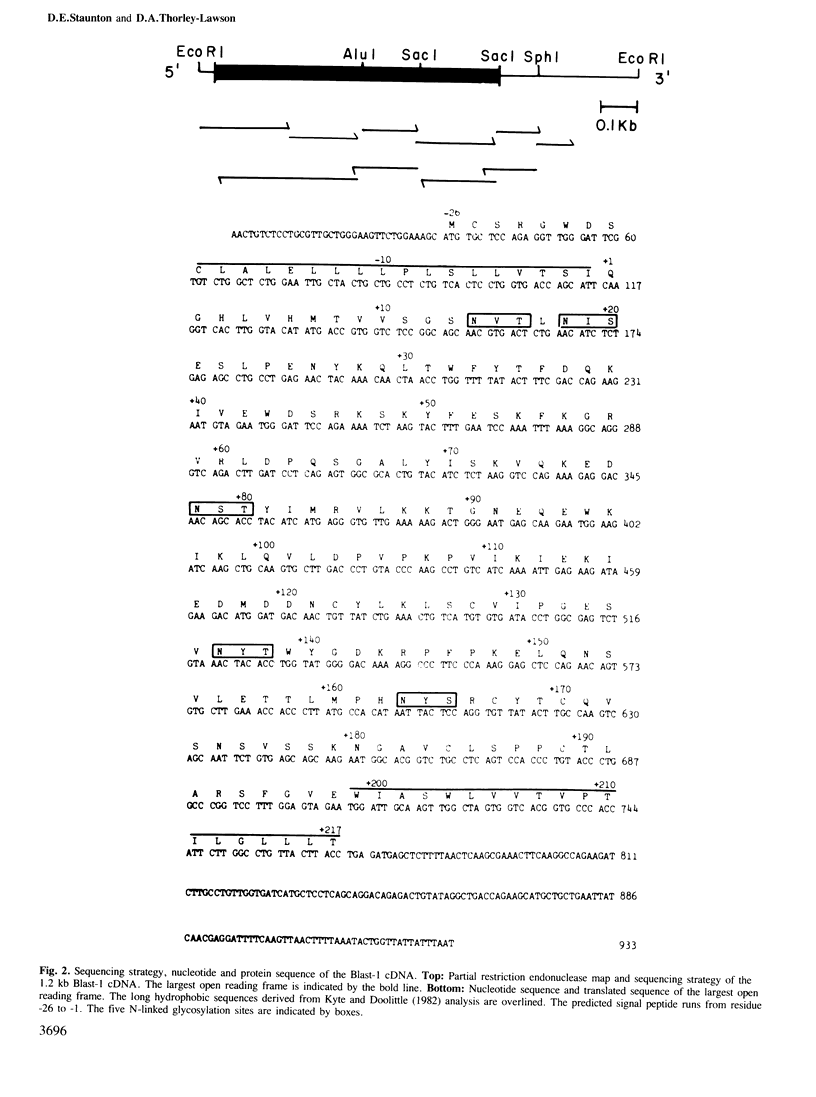
Blast-1 is an early activation-associated glycoprotein expressed on the surface of human lymphocytes. Here we report the isolation and analysis of a cDNA encoding Blast-1. The translated sequence of the Blast-1 cDNA contains a hydrophobic putative signal peptide and a hydrophobic carboxyl terminus devoid of charged residues. The sequence also contains five N-linked glycosylation sites, the utilization of which was confirmed by the shift in relative mol. wt of Blast-1 upon digestion with N-glycosidase F. The translated sequence reveals that Blast-1 is related to members of the immunoglobulin superfamily, especially to CD4 and MHC class II molecules. The homology to these proteins is greatest in their amino termini where they demonstrate 30-32% identity. This region of Blast-1 also demonstrated 25% identity to a V kappa sequence. Considering conservative amino acid substitutions this homology to CD4, MHC class II and V kappa becomes 60, 49 and 48%, respectively.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Lillie J. W., Arnot D., Grossberger D., Kappes D., Strominger J. L. Isotypic and allotypic variation of human class II histocompatibility antigen alpha-chain genes. Nature. 1984 Mar 22;308(5957):327–333. doi: 10.1038/308327a0. [DOI] [PubMed] [Google Scholar]
- Bank I., Chess L. Perturbation of the T4 molecule transmits a negative signal to T cells. J Exp Med. 1985 Oct 1;162(4):1294–1303. doi: 10.1084/jem.162.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. L., Falkoff R. J., Fauci A. S. Development of a human T-cell hybridoma secreting separate B-cell growth and differentiation factors. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2475–2478. doi: 10.1073/pnas.81.8.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate R. L., Mattaliano R. J., Hession C., Tizard R., Farber N. M., Cheung A., Ninfa E. G., Frey A. Z., Gash D. J., Chow E. P. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986 Jun 6;45(5):685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Geoghegan T., Bergmann I., Brawerman G. Studies on the efficiency of translation and on the stability of actin messenger ribonucleic acid in mouse sarcoma ascites cells. Biochemistry. 1979 Jul 10;18(14):3153–3159. doi: 10.1021/bi00581a037. [DOI] [PubMed] [Google Scholar]
- Chen Z. Z., Coggeshall K. M., Cambier J. C. Translocation of protein kinase C during membrane immunoglobulin-mediated transmembrane signaling in B lymphocytes. J Immunol. 1986 Mar 15;136(6):2300–2304. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Coggeshall K. M., Cambier J. C. B cell activation. VIII. Membrane immunoglobulins transduce signals via activation of phosphatidylinositol hydrolysis. J Immunol. 1984 Dec;133(6):3382–3386. [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Edson C. M., Hosler B. A., Respess R. A., Waters D. J., Thorley-Lawson D. A. Cross-reactivity between herpes simplex virus glycoprotein B and a 63,000-dalton varicella-zoster virus envelope glycoprotein. J Virol. 1985 Oct;56(1):333–336. doi: 10.1128/jvi.56.1.333-336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Rollini P., Long E., Mach B. Molecular organization of the HLA-SB region of the human major histocompatibility complex and evidence for two SB beta-chain genes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3934–3938. doi: 10.1073/pnas.81.13.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hood L., Kronenberg M., Hunkapiller T. T cell antigen receptors and the immunoglobulin supergene family. Cell. 1985 Feb;40(2):225–229. doi: 10.1016/0092-8674(85)90133-3. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Auffray C., Korman A. J., Shackelford D. A., Strominger J. The class II molecules of the human and murine major histocompatibility complex. Cell. 1984 Jan;36(1):1–13. doi: 10.1016/0092-8674(84)90068-0. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Differential expression of cell activation markers after stimulation of resting human B lymphocytes. J Immunol. 1984 Jun;132(6):2857–2861. [PubMed] [Google Scholar]
- Kintner C., Sugden B. Identification of antigenic determinants unique to the surfaces of cells transformed by Epstein-Barr virus. Nature. 1981 Dec 3;294(5840):458–460. doi: 10.1038/294458a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krangel M. S., Orr H. T., Strominger J. L. Assembly and maturation of HLA-A and HLA-B antigens in vivo. Cell. 1979 Dec;18(4):979–991. doi: 10.1016/0092-8674(79)90210-1. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Travers P. J., Carey J., Grosveld F., Jenkins J., Bodmer W. F. Sequence of an HLA-DR alpha-chain cDNA clone and intron-exon organization of the corresponding gene. Nature. 1982 Oct 21;299(5885):750–752. doi: 10.1038/299750a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Release of alkaline phosphatase from membranes by a phosphatidylinositol-specific phospholipase C. Biochem J. 1977 Oct 1;167(1):281–284. doi: 10.1042/bj1670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Malissen M., Hunkapiller T., Hood L. Nucleotide sequence of a light chain gene of the mouse I-A subregion: A beta d. Science. 1983 Aug 19;221(4612):750–754. doi: 10.1126/science.6410508. [DOI] [PubMed] [Google Scholar]
- Mann K. P., Staunton D., Thorley-Lawson D. A. Epstein-Barr virus-encoded protein found in plasma membranes of transformed cells. J Virol. 1985 Sep;55(3):710–720. doi: 10.1128/jvi.55.3.710-720.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J. G., Cambier J. C. B cell activation. III. B cell plasma membrane depolarization and hyper-Ia antigen expression induced by receptor immunoglobulin cross-linking are coupled. J Exp Med. 1983 Nov 1;158(5):1589–1599. doi: 10.1084/jem.158.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Butler J. L., Fauci A. S. Sequential requirements for cell cycle progression of resting human B cells after activation by anti-Ig. J Immunol. 1984 Jan;132(1):176–180. [PubMed] [Google Scholar]
- Nakagawa T., Hirano T., Nakagawa N., Yoshizaki K., Kishimoto T. Effect of recombinant IL 2 and gamma-IFN on proliferation and differentiation of human B cells. J Immunol. 1985 Feb;134(2):959–966. [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom J. T., Cambier J. C. B cell activation. VII. Independent and synergistic effects of mobilized calcium and diacylglycerol on membrane potential and I-A expression. J Immunol. 1986 Jan;136(1):66–72. [PubMed] [Google Scholar]
- Ransom J. T., Harris L. K., Cambier J. C. Anti-Ig induces release of inositol 1,4,5-trisphosphate, which mediates mobilization of intracellular Ca++ stores in B lymphocytes. J Immunol. 1986 Jul 15;137(2):708–714. [PubMed] [Google Scholar]
- Ravetch J. V., Luster A. D., Weinshank R., Kochan J., Pavlovec A., Portnoy D. A., Hulmes J., Pan Y. C., Unkeless J. C. Structural heterogeneity and functional domains of murine immunoglobulin G Fc receptors. Science. 1986 Nov 7;234(4777):718–725. doi: 10.1126/science.2946078. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani S., Mulligan R., Berg P. Expression of the mouse dihydrofolate reductase complementary deoxyribonucleic acid in simian virus 40 vectors. Mol Cell Biol. 1981 Sep;1(9):854–864. doi: 10.1128/mcb.1.9.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Edson C. M. Polypeptides of the Epstein-Barr virus membrane antigen complex. J Virol. 1979 Nov;32(2):458–467. doi: 10.1128/jvi.32.2.458-467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Mann K. P. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985 Jul 1;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Nadler L. M., Bhan A. K., Schooley R. T. BLAST-2 [EBVCS], an early cell surface marker of human B cell activation, is superinduced by Epstein Barr virus. J Immunol. 1985 May;134(5):3007–3012. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Schooley R. T., Bhan A. K., Nadler L. M. Epstein-Barr virus superinduces a new human B cell differentiation antigen (B-LAST 1) expressed on transformed lymphoblasts. Cell. 1982 Sep;30(2):415–425. doi: 10.1016/0092-8674(82)90239-2. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Swendeman S. L., Edson C. M. Biochemical analysis suggests distinct functional roles for the BLAST-1 and BLAST-2 antigens. J Immunol. 1986 Mar 1;136(5):1745–1751. [PubMed] [Google Scholar]
- Williams A. F. The immunoglobulin superfamily takes shape. Nature. 1984 Mar 1;308(5954):12–13. doi: 10.1038/308012a0. [DOI] [PubMed] [Google Scholar]