Abstract
Arabidopsis root development is orchestrated by signaling pathways that consist of different CLAVATA3/EMBRYO SURROUNDING REGION (CLE) peptide ligands and their cognate CLAVATA (CLV) and BARELY ANY MERISTEM (BAM) receptors. How and where different CLE peptides trigger specific morphological or physiological changes in the root is poorly understood. Here, we report that the receptor‐like protein CLAVATA 2 (CLV2) and the pseudokinase CORYNE (CRN) are necessary to fully sense root‐active CLE peptides. We uncover BAM3 as the CLE45 receptor in the root and biochemically map its peptide binding surface. In contrast to other plant peptide receptors, we found no evidence that SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) proteins act as co‐receptor kinases in CLE45 perception. CRN stabilizes BAM3 expression and thus is required for BAM3‐mediated CLE45 signaling. Moreover, protophloem‐specific CRN expression complements resistance of the crn mutant to root‐active CLE peptides, suggesting that protophloem is their principal site of action. Our work defines a genetic framework for dissecting CLE peptide signaling and CLV/BAM receptor activation in the root.
Keywords: CLAVATA, CLE45, MAKR5, receptor kinase, SERK
Subject Categories: Development & Differentiation, Plant Biology, Signal Transduction
Introduction
Receptor kinases (RKs) are key regulators of growth and development in higher plants such as the model organism Arabidopsis thaliana (Arabidopsis). There are ~180 Arabidopsis RKs with extracellular leucine‐rich repeat (LRR) domains, many of which can perceive peptide ligands, including members of the CLAVATA3/EMBRYO SURROUNDING REGION (CLE) peptide family 1, 2. CLE peptides are encoded endogenously and translated as prepropeptides, which are secreted and processed to yield mature 12–13 amino acid, bioactive peptides 2, 3, 4. In some cases, their activity is amplified by post‐translational modifications, such as proline hydroxylation and additional arabinosylation 5, 6. Arabidopsis contains 32 CLE genes, some of which encode redundant peptides, thereby giving rise to 27 distinct CLE peptides 3, 7, 8. Several of these peptides have been shown to play roles in root development, and chemically synthesized versions of many of them suppress Arabidopsis root growth in tissue culture when applied at nM to low μM concentrations (called root‐active CLEs in the following) 9, 10, 11. However, the perception mechanism for most CLE peptides in the root, including their receptors and co‐receptors, remains unknown to date.
Genetic and biochemical studies have identified several LRR‐RKs involved in the perception of individual CLE peptides. The outstanding, classic example is CLAVATA 1 (CLV1), which directly binds the CLV3 peptide to regulate stem cell homeostasis in the shoot apical meristem 4, 12, 13, 14, 15, 16, 17. The CLV1‐related LRR‐RK BARELY ANY MERISTEM 3 (BAM3) is required to mediate the suppression of protophloem sieve element differentiation in the root meristem by CLE45 application 9, 18, 19. PHLOEM INTERCALATED WITH XYLEM (PXY; a.k.a. TDIF RECEPTOR [TDR]) senses the redundant CLE41/44 peptides (a.k.a. TRACHEARY ELEMENT DIFFERENTIATION INHIBITORY FACTOR [TDIF]) to regulate vascular stem cell proliferation in secondary growth 20, 21, 22, 23, 24, 25.
High‐affinity ligand sensing and receptor activation of plant LRR‐RKs relies on their interaction with shape‐complementary co‐receptor kinases 1, 26, 27, 28. For instance, the LRR‐RK BRASSINOSTEROID INSENSITIVE 1 (BRI1) employs the SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) family co‐receptors SERK1 and SERK3 to transmit the signal triggered by the small molecule ligand brassinosteroid 29. The LRR‐RK HAESA also relies on SERK proteins to transduce the signal triggered by the peptide ligand IDA, which is related to CLE peptides in structure 30, 31. Consistently, it was recently suggested that SERK1 also plays a role in PXY‐mediated CLE41/44 signal transduction 25. Thus, Arabidopsis SERKs have been implicated in multiple signaling pathways, comprising CLE as well as other peptide signals, hormonal cues, and pathogen‐derived ligands 32, 33. Beyond PXY, however, it remains unclear to what degree SERKs could be involved in the perception of CLE peptides.
For CLV1, different types of potential, context‐dependent co‐receptors have been described. In one scenario, CLV3 is perceived in association with CLV2, a receptor‐like protein (RLP) that is comprised of extracellular LRRs and a transmembrane domain but lacks a kinase domain 12, 34. CLV2 in turn dimerizes with CORYNE (CRN), which consists of a transmembrane domain and an intracellular pseudo‐kinase domain 35, 36. Other findings point to a CLV1‐independent role of CLV2‐CRN in CLV3 perception, possibly in conjunction with the CLV1‐related LRR‐RKs BAM1 and BAM2 35, 37, 38. Moreover, it was found that CLV2‐CRN is required for the perception of many if not all root‐active CLE peptides 11, 35, 39, 40. Finally, CLV1 has been implicated in stem cell homeostasis in the root meristem, where it presumably perceives CLE40 together with the non‐LRR‐RK ARABIDOPSIS CRINKLY 4 (ACR4) 41, 42. Likewise, BAM1 and RECEPTOR‐LIKE PROTEIN KINASE 2 are also thought to play a role in CLE perception in the root 43. In this study, we show that BAM3 is a bona fide CLE45 receptor, which appears to operate independent of SERK proteins. Moreover, we demonstrate that phloem‐specific CRN expression is not only required for perception of CLE45, but of all root‐active CLE peptides tested in this study, possibly by stabilizing expression of their receptors.
Results
BAM3 is a CLE45 receptor
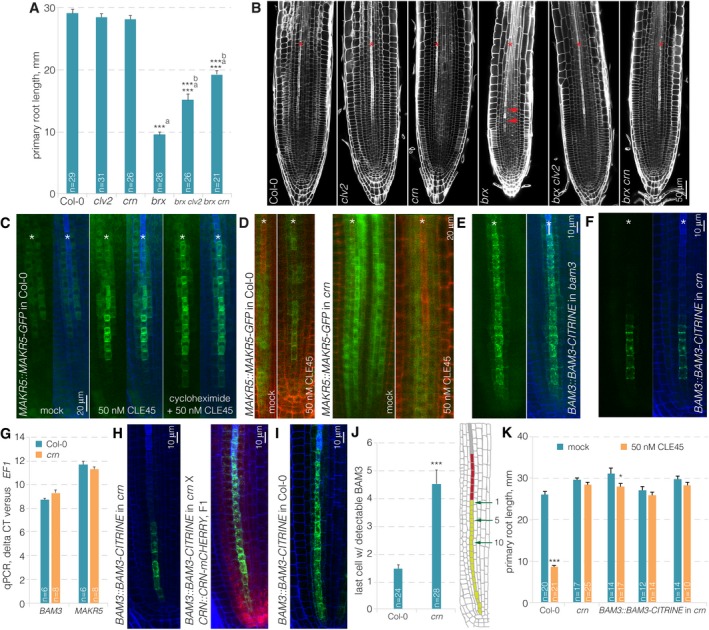
Originally, we isolated bam3 as a second‐site suppressor of loss of function in BREVIS RADIX (BRX), which encodes a positive regulator of root protophloem sieve element differentiation, suggesting that BRX antagonizes the CLE45‐BAM3 pathway 9, 19. Root protophloem differentiation (and thereby root growth) of bam3 loss‐of‐function mutants is not impaired by exogenously applied CLE45 levels that suppress this process in wild‐type plants, suggesting that BAM3 could act as a CLE45 receptor 9. Subsequent results strengthened this notion 19, 44 and also ruled out proposed alternative CLE45 receptors in the root 18. However, direct evidence for CLE45‐BAM3 interaction is still missing. We isolated additional CLE45‐insensitive bam3 loss‐of‐function alleles from our genetic screen 9 (Fig EV1A), including non‐synonymous mutations leading to amino acid changes in the ligand‐binding LRR and the cytoplasmic kinase domains (Fig 1A). Mutation of threonine 150 to isoleucine presumably interferes with proper folding of the BAM3 LRR domain, as does mutation of glycine 364 to arginine. Serine 303, however, maps to the inner face of the BAM3 LRR domain and is located in a surface region, which forms the peptide binding sites in the structurally related IDA receptor HAESA 30 and in the CLE41/44 receptor PXY 25 (Fig 1A and B). Two missense mutations in the BAM3 kinase domain (P883S, G901E) map to the core of the kinase C‐lobe and may interfere with the proper folding or activity of the BAM3 kinase module. Together, our genetic analysis suggests that both the extracellular and cytoplasmic portions of BAM3 are important for the function of the receptor.
Figure EV1. BAM3‐CLE45 control experiments and bam3 alleles.
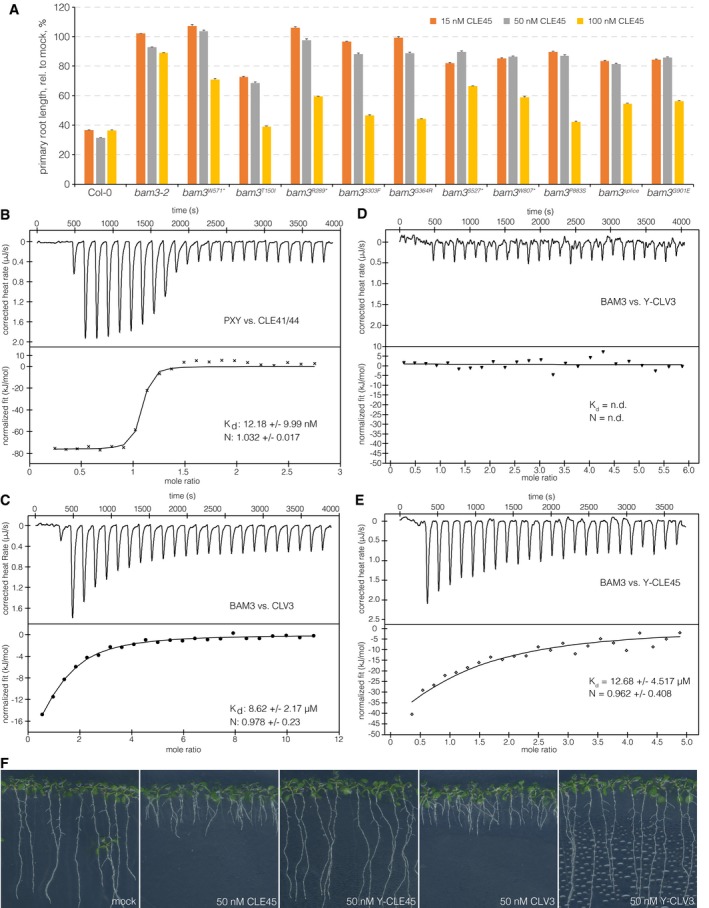
- Relative primary root length of indicated genotypes at 9 dag, in response to increasing amounts of CLE45 in the media. n = 12 for each genotype, mean ± s.e.m. All differences as compared to wild type were statistically significant (Student's t‐test) with P < 0.001 for 15 and 50 nM, and P < 0.05 for 100 nM.
- ITC of purified PXY extracellular domain vs. CLV41/44 peptide. n.d.: not detectable. N: stoichiometry, K d dissociation constant. Shown are experimental values ± fitting errors (95% confidence interval).
- ITC of purified BAM3 extracellular domain vs. CLV3 peptide.
- ITC of purified BAM3 extracellular domain vs. an N‐terminally tyrosine‐modified CLV3 peptide.
- ITC of purified BAM3 extracellular domain vs. an N‐terminally tyrosine‐modified CLE45 peptide.
- Representative 9‐day‐old Col‐0 seedlings grown on mock or in presence of indicated peptides.
Figure 1. BAM3 is a CLE45 receptor.
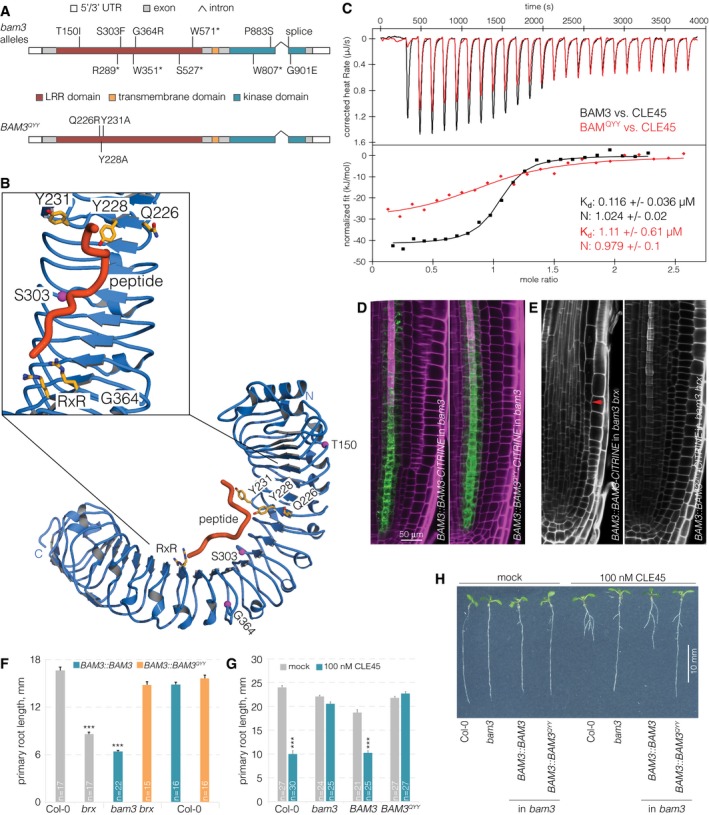
- Schematic overview of the BAM3 gene structure. bam3 loss‐of‐function mutations that were isolated as second‐site suppressors of brx loss of function (top); amino acid point mutations predicted to disrupt BAM3‐CLE45 interaction (bottom).
- Ribbon diagram of a homology model of the BAM3 LRR ectodomain (in blue) based on the HAESA ectodomain (PDB‐ID 5IXO). Magenta spheres indicate the position of genetic BAM3 missense mutations, and residues forming part of the predicted CLE45 binding site are shown in bonds representation (in yellow). The position of CLE45 has been inferred from an IDA‐HAESA complex (PDB‐ID 5IXQ).
- Isothermal titration calorimetry (ITC) of purified BAM3 wild‐type (black) or mutant BAM3QYY (red) extracellular domains vs. CLE45 peptide. N: stoichiometry, K d dissociation constant. Shown are experimental values ± fitting errors (95% confidence interval).
- Expression of BAM3‐CITRINE wild‐type or mutant BAM3QYY fusion protein (green fluorescence) under control of the native BAM3 promoter in root meristems of bam3 mutants (magenta fluorescence: calcofluor white cell wall staining) (confocal microscopy).
- Primary root meristems of bam3 brx double mutants carrying the indicated transgenes. Red arrow on the left panel indicates the approximate position of the final dividing cortex cell, which is out of range in the right panel.
- Primary root length of 5‐day‐old seedlings of the indicated genotypes.
- Primary root length of 7‐day‐old seedlings of the indicated genotypes in mock or CLE45 condition.
- Representative 7‐day‐old seedlings of the indicated genotypes grown on standard mock or CLE45‐containing media.
We produced the BAM3 LRR domain by secreted expression in insect cells and tested if the purified ectodomain interacts with a synthetic CLE45 peptide in isothermal titration calorimetry (ITC) assays. We found that BAM3 bound CLE45 with a K d of ~120 nM and with 1:1 stoichiometry (Fig 1C). The binding affinity for CLE45 to BAM3 was about 10‐fold lower than CLE41/44 binding to the LRR ectodomain of PXY (Fig EV1B) 25. BAM3 showed specific CLE45 binding, as the sequence‐related CLV3 peptide, which is not expressed in the root 40, bound with much lower affinity (K d ~10 μM) (Fig EV1C). Importantly, N‐terminal extension of CLE45 or CLV3 by a tyrosine residue (initially used to quantify the peptide concentrations) rendered the engineered peptides non‐bioactive and drastically reduced binding to the BAM3 ectodomain (Fig EV1D–F). Prompted by our recent finding that the peptide hormone IDA is structurally related to CLE peptides, we created a BAM3 homology model based on the HAESA‐IDA complex structure 30 to predict the CLE45 binding surface in BAM3 (Fig 1B). In our homology model, BAM3 residues Q226, Y228, and Y231 from the LRR domain form a part of the CLE45 binding surface. Consistently, binding of CLE45 to a purified BAM3 ectodomain in which Q226, Y228, and Y231 were mutated to alanines (BAM3QYY) was ~8 times weaker when compared to the wild‐type LRR domain (Fig 1C).
To test the relevance of these mutations in planta, we re‐created them in a full‐length BAM3 coding sequence to express the mutant protein as a CITRINE fusion (BAM3::BAM3 QYY ‐CITRINE). First, we checked the subcellular localization of BAM3QYY‐CITRINE and wild‐type BAM3‐CITRINE (BAM3::BAM3‐CITRINE) in transient transformation of tobacco (Nicotiana benthamiana) leaf cells. In this system, both fusion proteins showed similar plasma membrane localization as well as some internal, likely endoplasmic reticulum signals (Fig EV2A). In Arabidopsis, BAM3QYY‐CITRINE was specifically expressed in the protophloem, with a similar profile of subcellular (plasma membrane and internal) localization and abundance as wild‐type BAM3‐CITRINE (Figs 1D and EV2B). However, unlike BAM3‐CITRINE, BAM3QYY‐CITRINE was neither able to restore the brx phenotype when introduced into a bam3 brx double mutant (Fig 1E and F), nor able to restore CLE45 sensitivity when introduced into a bam3 single mutant (Fig 1G and H). In summary, these data reinforce the view that BAM3 is the genuine CLE45 receptor in the context of the root protophloem 18.
Figure EV2. BAM3 localization and biochemical control experiments.
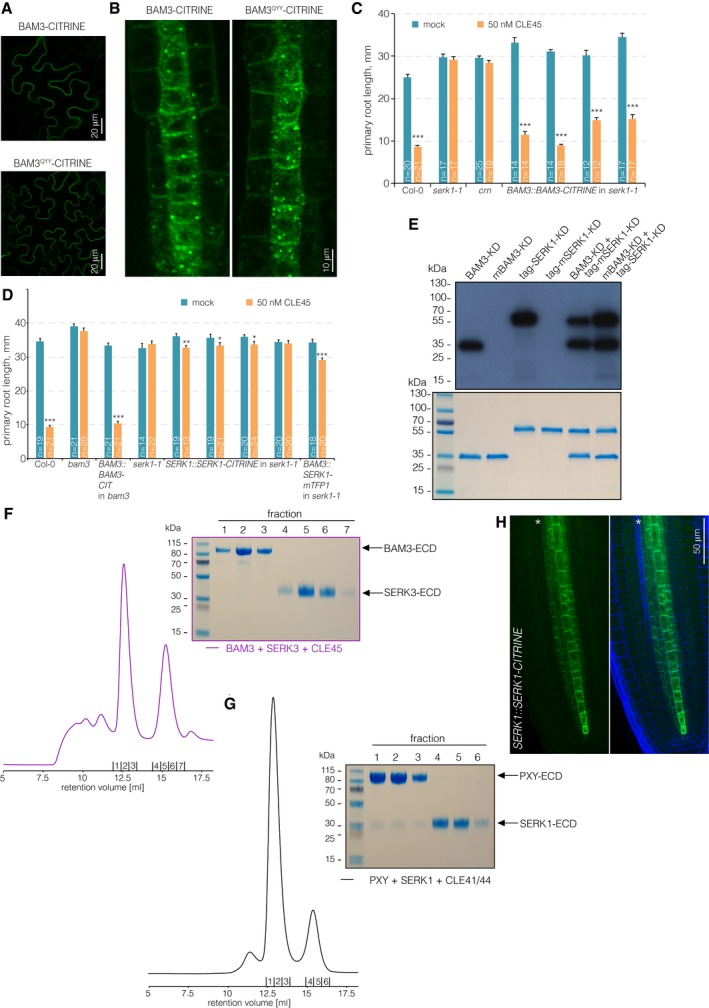
-
ATransient expression of BAM3 wild‐type or mutant BAM3QYY CITRINE fusion proteins (green fluorescence) in tobacco leaf epidermal cells, under control of a constitutive promoter (confocal microscopy).
-
BClose‐up of developing protophloem sieve element cell files expressing BAM3 wild‐type or mutant BAM3QYY CITRINE fusion proteins (green fluorescence).
-
C, DPrimary root length of 7‐day‐old seedlings of indicated genotypes on mock or CLE45 media, several independent lines per transgene construct are shown. Differences as compared to mock are not statistically significant unless indicated (Student's t‐test); *P < 0.05; **P < 0.01; ***P < 0.001; mean ± s.e.m.
-
ETransphosphorylation kinase assays with purified BAM3 kinase domain (BAM3‐KD) or SERK1 kinase domain (SERK1‐KD) as well as kinase dead point mutant versions (mBAM3‐KD & mSERK1‐KD) alone and in combination.
-
FAnalytical size‐exclusion chromatography of purified BAM3 and SERK3 extracellular domains in the presence of CLE45 peptide reveals no ligand‐induced complex formation between BAM3 and SERK3.
-
GAnalytical size‐exclusion chromatography of purified PXY and SERK1 extracellular domains in the presence of CLE41/44 peptide reveals CLE41/44‐induced binding of SERK1 to the PXY ectodomain.
-
HExpression of SERK1‐CITRINE fusion protein (green fluorescence) under control of the native SERK1 promoter (blue fluorescence: calcofluor white cell wall staining). Green channel is shown separately (left) and in overlay with blue channel (right). Asterisk indicates the developing sieve element cell file.
Individual SERKs are not necessary to perceive root‐active CLE peptides
SERK proteins have recently been shown to act as co‐receptors for the CLE41/44 receptor PXY 25, 45 and for many other LRR‐RKs 46. In the case of the peptide receptor HAESA, complex formation with SERK1 allows for the specific and high‐affinity sensing of IDA, and HAESA and SERK1 form stable, IDA‐dependent heteromeric complexes in vitro 30. To test whether SERK proteins could be involved in the sensing of other CLE peptides in the root, we surveyed the response of serk mutants to 14 root‐active CLE peptides (CLV3, CLE8, CLE9/10, CLE11, CLE13, CLE14, CLE16, CLE18, CLE20, CLE21, CLE25, CLE26, CLE40, CLE45), which were selected for their significant, reproducible impact on root growth at 50 nM concentration. In general, the response of representative serk loss‐of‐function mutants (alleles serk1‐3, serk2‐1, serk3‐1, serk4‐1, and serk5‐1) was largely similar to wild type (Fig 2A). Interestingly, another serk1 allele, serk1‐1, showed resistance to CLE45 application and was as insensitive as bam3 (Fig EV2C). However, CLE45 resistance and the serk1‐1 mutation segregated freely in outcrosses to a brx mutant (i.e., only six out of 24 genotyped plants that were CLE45‐resistant were also homozygous for serk1‐1), suggesting that the CLE45 resistance resulted from an unlinked background mutation. Whole‐genome sequencing of the serk1‐1 plants revealed a homozygous 28‐bp deletion in BAM3, which would lead to a frameshift after amino acid 699 and a premature stop codon six amino acids later, thereby deleting the kinase domain. Moreover, introduction of a transgenic BAM3 copy restored CLE45 sensitivity of serk1‐1 (Fig EV2C). No complementation was observed with SERK1 constructs that were reported to rescue the serk1‐1 serk2‐1 double‐mutant shoot phenotype 47 (Fig EV2D). Therefore, the serk1‐1 line should be considered a bam3 serk1 double mutant.
Figure 2. Root growth CLE peptide resistance of mutants in potential co‐receptors of CLE signaling pathways.
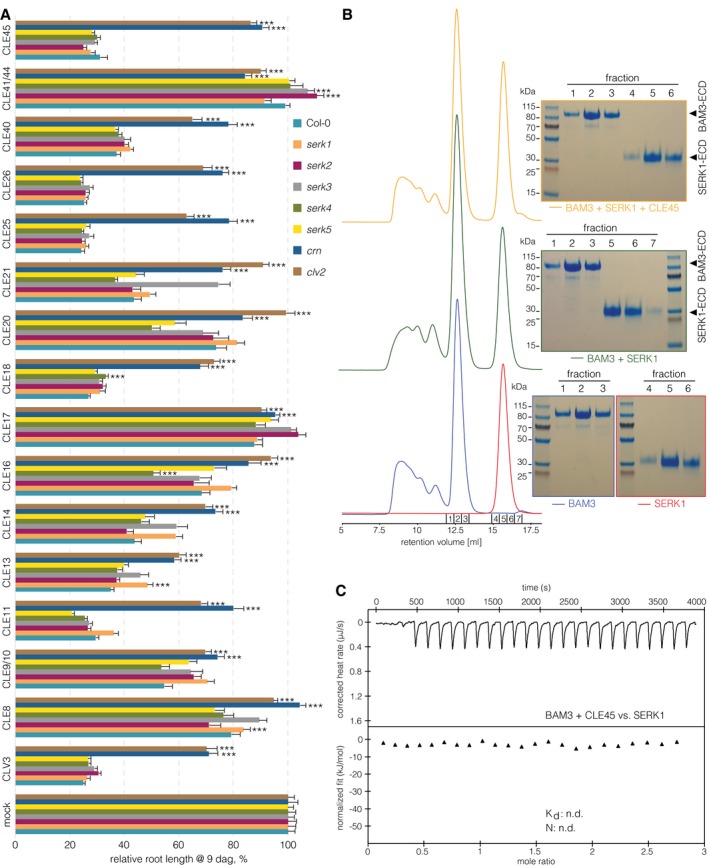
- Primary root length of 9‐day‐old seedlings of indicated genotypes, grown on mock or 50 nM CLE peptide. Fourteen root‐active peptides and two controls (CLE17 and CLE41/44) are shown. n ≥ 12 per column.
- Analytical size‐exclusion chromatography. The BAM3 and SERK1 LRR domains elute as monomers when run stand‐alone (blue and red traces), in combination (green trace), and in the presence of CLE45 (yellow trace). SDS–PAGE gels of the corresponding peak fractions are shown alongside.
- ITC of the purified BAM3 extracellular domain in the presence of CLE45 peptide titrated against the purified SERK1 extracellular domain. n.d.: not detectable.
SERK1 and SERK3 do not act as co‐receptors in BAM3‐mediated CLE45 signaling
In summary, none of the five serk mutants displayed CLE45 resistance. A signaling function of SERK genes in CLE45 perception might be masked by genetic redundancy, and therefore, the notion that SERK1 could be a BAM3 co‐receptor still appeared attractive, especially given recently reported evidence that SERK1 cannot only interact with HAESA, but also with PXY, in a ligand‐dependent manner 25, 30. However, although the BAM3 and SERK1 kinase domains were able to transphosphorylate each other in an in vitro kinase assay (Fig EV2E), neither SERK1 nor SERK3 formed CLE45‐dependent complexes with BAM3 in vitro (Figs 2B and EV2F). In contrast, SERK1 formed CLE41/44‐dependent heterodimers with PXY (Fig EV2G), corroborating earlier results 25, 45. Consistent with our gel filtration experiments, we could not detect binding of the SERK1‐LRR domain to BAM3 in the presence of CLE45 in quantitative ITC assays (Fig 2C), while SERK1 bound to HAESA in the presence of IDA with low nanomolar affinity 30. Finally, although SERK1 has been reported to be expressed throughout the stele 48, 49, closer inspection of a transgene driving expression of a SERK1‐CITRINE fusion protein under control of the SERK1 promoter (SERK1::SERK1‐CITRINE) suggested that SERK1 is not expressed in developing protophloem sieve elements (Fig EV2H). In summary, the results suggest that SERK1 (and based on our biochemical studies also SERK3) is not a co‐receptor for BAM3‐mediated CLE45 signaling.
CLV2 and CRN are necessary for full perception of root‐active CLE peptides
Since our experiments did not support a role for SERK proteins in BAM3 receptor activation and CLE45 signal transduction, we assessed the relative contribution of other known CLV/BAM signaling components, CLV2 and CRN 11, 35, 37, 39, 50. Both CLV2 and CRN are expressed throughout most root tissues including the vascular cylinder 51. We corroborated these findings by creating transgenic lines in which CITRINE fusions of CLV2 or CRN were expressed under control of their native promoters (CLV2::CLV2‐CITRINE and CRN::CRN‐CITRINE). CLV2::CLV2‐CITRINE displayed expression mostly in the stele (Fig EV3A), and CRN::CRN‐CITRINE was expressed in the same domain (Fig EV3B). However, while CLV2 appeared to be evenly expressed throughout the vascular cylinder of the root tip, CRN was apparently enriched in the phloem poles. To investigate CRN mutation in the same background as all other lines used in this study, we obtained a crn loss‐of‐function mutant in the Col‐0 accession. In this CRISPR/Cas9‐generated crn allele, a single nucleotide insertion in front of the 7th codon leads to a frameshift and three subsequent premature stop codons after amino acid 10 52. This crn mutant displayed complete insensitivity to CLE45 concentrations that strongly suppress protophloem differentiation and thus root growth in wild type (Fig 2A). A survey of other root‐active CLE peptides revealed that this crn loss of function also conferred strong resistance to all of them (Fig 2A), corroborating the results for other crn alleles in different parental backgrounds 11. As expected, we obtained very similar results when analyzing clv2 loss‐of‐function mutants (Fig 2A). The CLV2::CLV2‐CITRINE and CRN::CRN‐CITRINE constructs complemented the respective mutants, indicating their functionality (Fig EV3C and D). Taken together, our experiments confirm that CLV2 and CRN are necessary to mediate full sensitivity to all root‐active CLE peptides monitored in this study.
Figure EV3. CLV2 and CRN localizations in Arabidopsis roots.
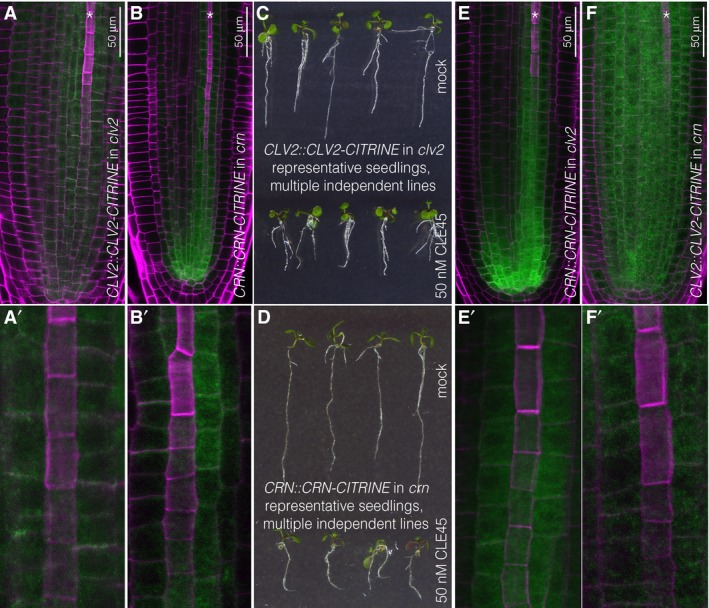
- Expression of CLV2‐CITRINE fusion protein (green fluorescence) under control of the native promoter in clv2 root meristems (magenta fluorescence: calcofluor white cell wall staining) (confocal microscopy). Asterisk marks developing protophloem sieve element strand. Close‐up (A′) with developing protophloem at the center is shown.
- Same as in (A), for CRN‐CITRINE fusion protein under control of the native promoter in crn root meristems.
- Representative 5‐day‐old clv2 seedlings expressing CLV2‐CITRINE fusion protein under control of its native promoter grown on mock or CLE45.
- Representative 5‐day‐old crn seedlings expressing CRN‐CITRINE fusion protein under control of its native promoter grown on mock or CLE45.
- Same as in (A), for CRN‐CITRINE fusion protein under control of the native promoter in clv2 root meristems.
- Same as in (A), for CLV2‐CITRINE fusion protein under control of the native promoter in crn root meristems.
The phloem is a crucial site of action for root‐active CLE peptides
To test whether the CLE45 resistance of crn mutants reflects CRN activity in the developing protophloem, we expressed a transgenic CRN‐CITRINE fusion under control of the BAM3 promoter in crn mutants (Fig 3A–C). Interestingly, in these lines, we not only observed restored sensitivity to CLE45 (Fig 3D), but also to the similarly acting CLE26 44 as well as other strongly root‐active CLE peptides (Fig 3E). To monitor the CLE peptide effects in more detail, we transiently treated transgenic lines that expressed a nuclear localized fluorescent marker under control of the COTYLEDON VASCULAR PATTERN 2 (CVP2) promoter (CVP2::NLS‐VENUS) in wild‐type background. CVP2 is very specifically expressed in the developing sieve elements of the root meristem and is also a specific marker for their differentiation process 44. Investigation of CVP2::NLS‐VENUS seedlings after 24‐h CLE peptide treatment indicated that indeed in practically all cases, protophloem development was perturbed to a large degree after CLE application (Fig 3F). While in some cases, a strong, immediate and specific suppression of protophloem differentiation was observed, in others the sieve element marker faded more gradually. However, in all cases, CVP2 expression eventually disappeared toward the root tip. Together with the rescue of CLE peptide resistance through protophloem‐specific CRN expression, these observations indicate that the developing phloem is a crucial site of action for root‐active CLE peptides, even if some of them are not genuinely expressed in the root meristem and/or vasculature 53, 54.
Figure 3. Protophloem‐specific CRN action in the root meristem.
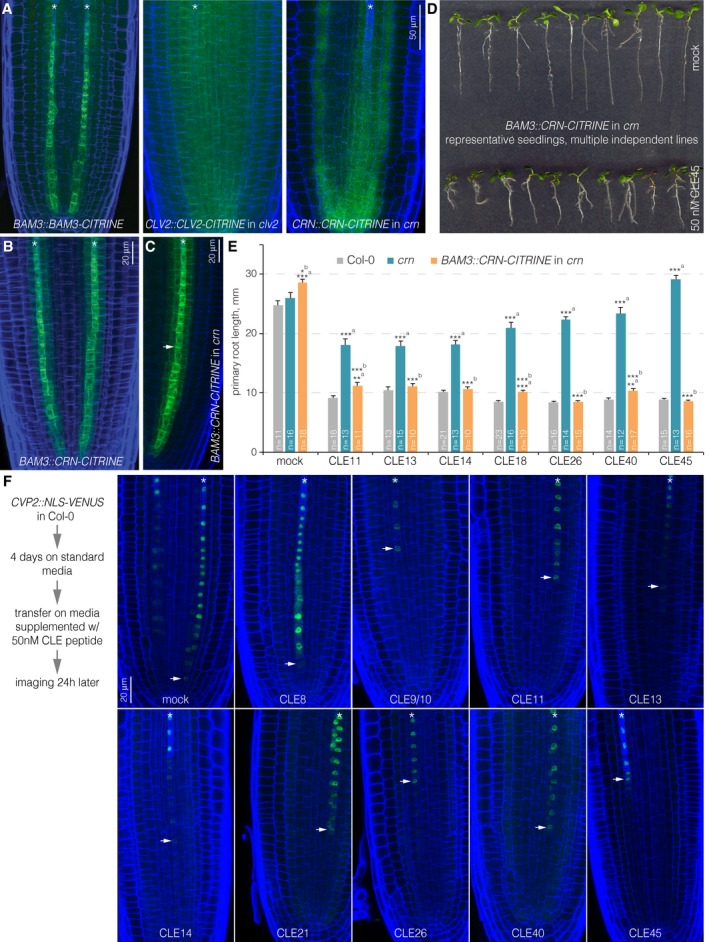
-
AExpression pattern of BAM3, CLV2, and CRN‐CITRINE fusion proteins (green fluorescence) under control of their native promoters (blue fluorescence: calcofluor white cell wall staining) (confocal microscopy). Asterisks mark developing protophloem sieve element strands.
-
B, CExpression pattern of CRN‐CITRINE fusion protein under control of the BAM3 promoter in Col‐0 (B) or crn (C) background. Arrowhead in (C) highlights plasma membrane‐localized CRN.
-
DRepresentative 7‐day‐old crn seedlings expressing CRN‐CITRINE fusion protein under control of the BAM3 promoter grown on mock or CLE45.
-
EPrimary root length of 7‐day‐old seedlings of indicated genotypes, grown on mock or 50 nM of selected CLE peptides. Differences as compared to a: Col‐0 or b: crn are not statistically significant unless indicated (Student's t‐test); *P < 0.05; **P < 0.01; ***P < 0.001; mean ± s.e.m.
-
FExpression of nuclear localized, fluorescent VENUS protein under control of the CVP2 promoter (which specifically marks developing protophloem sieve elements), 24 h after transfer from standard media to 50 nM of selected CLE peptides. Arrowheads indicate the cells closest to the tip in which CVP2 expression was still detectable.
CRN promotes CLE45 sensitivity by enhancing BAM3 expression
To test whether CLV2‐CRN might interact with BAM3, we next investigated these proteins in the cellular setting of the transient tobacco expression system. While BAM3 was mostly plasma membrane‐localized (Fig EV4A), when expressed alone, CLV2 and CRN fusion proteins were mostly found inside cells and co‐localized substantially with an endoplasmic reticulum marker (Figs 4A and B, and EV4B–D), in line with earlier findings 48, 55. Some plasma membrane localization could be observed at variable degrees in replicate experiments, which might be due to endogenous CLV2/CRN proteins, because as previously reported 55, co‐expression of CLV2 and CRN resulted in increased delivery of both fusion proteins to the plasma membrane (Figs 4C and D, and EV4E and F). We confirmed these findings in the root vasculature of stable transgenic lines, by introducing the BAM3::CRN‐CITRINE construct into the clv2 mutant. While in wild type or the crn background CRN‐CITRINE displayed substantial plasma membrane localization, it did not accumulate at the plasma membrane to the same extent in clv2 mutants (Figs 4E and F, and EV3E). Conversely, CLV2‐CITRINE fusion protein expressed under control of the CLV2 promoter displayed some clear plasma membrane localization when expressed in the clv2 mutant background, but mostly diffusive cytoplasmic localization when expressed in the crn mutant (Fig EV3F). Thus, as previously reported for the shoot, plasma membrane localization of CLV2 and CRN is largely interdependent in the root.
Figure EV4. Tobacco co‐localization, additional, and control experiments.
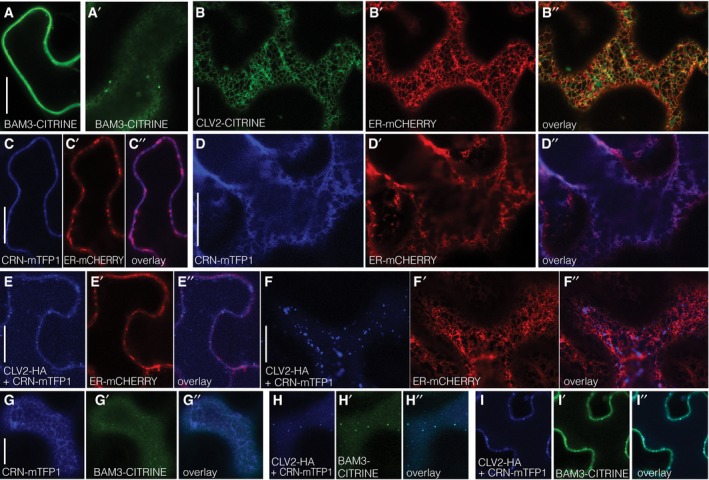
-
ATransient expression of BAM3‐CITRINE fusion protein (green fluorescence) in tobacco (Nicotiana benthamiana) leaf epidermal cells, under control of a constitutive promoter (confocal microscopy), optical section through cell center. Panel (A′): same in cell surface view.
-
BTransient co‐expression of CLV2‐CITRINE fusion protein (green fluorescence) and an endoplasmic reticulum marker (ER‐mCHERRY, red fluorescence).
-
CTransient co‐expression of CRN‐mTFP1 fusion protein (blue fluorescence) and the ER‐mCHERRY marker (red fluorescence), optical section through cell center.
-
DSame as (C), in cell surface view.
-
E, FCorresponding to (C) and (D), in the additional presence of (non‐fluorescent) CLV2‐HA fusion protein.
-
GTransient co‐expression of CRN‐mTFP1 (blue fluorescence) and BAM3‐CITRINE (green fluorescence) fusion proteins, in cell surface view.
-
H, ITransient co‐expression of CRN‐mTFP1 (blue fluorescence) and BAM3‐CITRINE (green fluorescence) fusion proteins, in the additional presence of (non‐fluorescent) CLV2‐HA fusion protein. Panel (H): cell surface view. Panel (I): optical section through cell center.
Figure 4. Co‐localizations and interactions of BAM3, CRN, and CLV2.
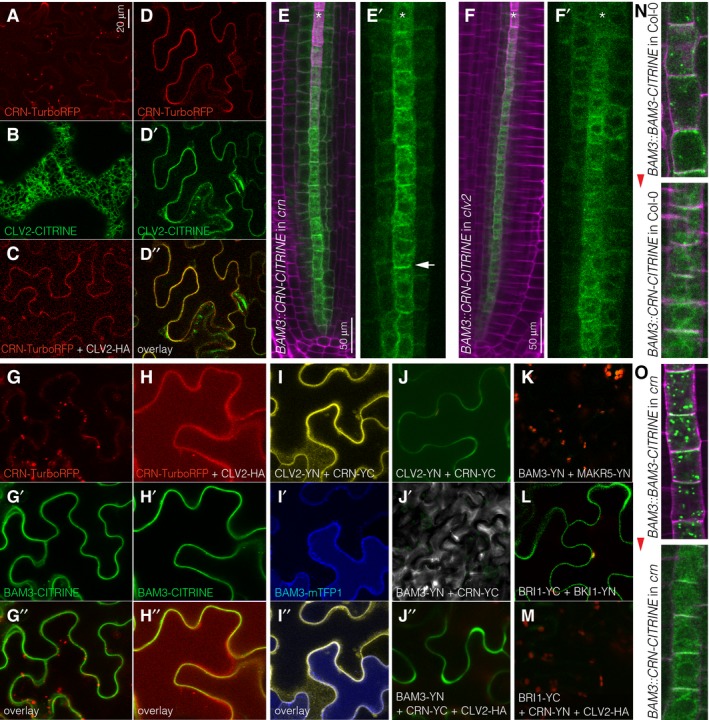
-
ATransient expression of CRN‐TurboRFP fusion protein (red fluorescence) in tobacco (Nicotiana benthamiana) leaf epidermal cells, under control of a constitutive promoter (confocal microscopy).
-
BTransient expression of CLV2‐CITRINE fusion protein (green fluorescence) in tobacco leaf epidermal cells.
-
CTransient co‐expression of CRN‐TurboRFP fusion protein and (non‐fluorescent) CLV2‐HA fusion protein in tobacco leaf epidermal cells.
-
DTransient co‐expression of CRN‐TurboRFP and CLV2‐CITRINE fusion proteins in tobacco leaf epidermal cells. Red fluorescent and green fluorescent channels are shown separately and in overlay.
-
E, FExpression pattern of CRN‐CITRINE fusion protein (green fluorescence) under control of the BAM3 promoter in crn (E) or clv2 mutant (F), with corresponding close‐ups (magenta fluorescence: calcofluor white cell wall staining). Asterisks mark developing protophloem sieve element strands. Arrowhead in (E′) highlights plasma membrane‐localized CRN‐CITRINE.
-
GTransient co‐expression of CRN‐TurboRFP and BAM3‐CITRINE fusion proteins in tobacco leaf epidermal cells. Red fluorescent and green fluorescent channels are shown separately and in overlay.
-
HTransient co‐expression of CRN‐TurboRFP, BAM3‐CITRINE, and (non‐fluorescent) CLV2‐HA fusion proteins in tobacco leaf epidermal cells. Red fluorescent and green fluorescent channels are shown separately and in overlay.
-
IBimolecular fluorescence complementation (BiFC) between transiently co‐expressed CLV2 and CRN proteins fused to one half each of YFP (yellow fluorescence) in the presence of co‐expressed BAM3‐mTFP1 fusion protein (blue fluorescence). Yellow fluorescent and blue fluorescent channels are shown separately and in overlay.
-
JBiFC between transiently co‐expressed BAM3 and CRN proteins fused to one half each of YFP (green fluorescence) in the presence of co‐expressed (non‐fluorescent) CLV2‐HA fusion protein in tobacco leaf epidermal cells (J′′). Parallel control experiments for BiFC between CLV2 and CRN (J) and BAM3 and CRN (J′) are shown.
-
KBiFC between transiently co‐expressed BAM3 and MAKR5 proteins fused to one half each of YFP (red: chloroplast autofluorescence).
-
LBiFC between transiently co‐expressed BRI1 and BKI1 proteins fused to one half each of YFP (green fluorescence).
-
MBiFC between transiently co‐expressed BRI1 and CRN proteins fused to one half each of YFP in the presence of co‐expressed (non‐fluorescent) CLV2‐HA fusion protein in tobacco leaf epidermal cells (red: chloroplast autofluorescence).
-
N, OExpression of BAM3‐CITRINE or CRN‐CITRINE fusion proteins (green fluorescence) under control of the BAM3 promoter in the developing sieve element cells close to the stem cells in Col‐0 (N) or crn (O) background (magenta fluorescence: calcofluor white cell wall staining). Red arrowhead indicates rootward direction.
We next tested whether CLV2‐CRN could interact with BAM3. We could not obtain the CLV2 ectodomain in sufficient quantity and quality for in vitro biochemical assays. In bimolecular fluorescence complementation (BiFC) experiments, we could not observe interaction of BAM3 with CRN, with the caveat that in this assay, CRN was supposedly not efficiently delivered to the plasma membrane. Also, in transient co‐expression in tobacco, no co‐localization was observed for CRN and BAM3 fusion proteins (Figs 4G and EV4G). However, substantial co‐localization occurred once a (non‐fluorescent) HA‐tagged CLV2 protein was co‐expressed in addition (Figs 4H and EV4H and I). Co‐localization of the three proteins was also observed when BiFC was performed for CLV2 and CRN in the presence of a fluorescent mTFP1‐tagged BAM3 (Fig 4I). Moreover, a modest but robust BiFC interaction between BAM3 and CRN could be observed when (non‐fluorescent) CLV2‐HA was co‐expressed as well (Fig 4J), as compared to a negative control (Fig 4K). Control experiments with BRI1 did not show such interaction (Fig 4L and M). Thus, it appears that in principle, BAM3 is capable of interaction with the CLV2‐CRN dimer in a cellular setting. Such interaction could actually occur in planta, since both BAM3 and CRN displayed plasma membrane localization in developing sieve elements, which appeared to be mostly shootward for BAM3 (Fig 4N and O).
Despite the possibility for BAM3‐CLV2/CRN interaction, it appears unlikely that CLV2‐CRN acts in a capacity of co‐receptor in CLE45 perception, since CRN is a pseudokinase 36. To further investigate the role of CRN in CLE45 signaling, we thus conducted additional genetic experiments. Second‐site loss of function in BAM3 or in its downstream signaling component MEMBRANE‐ASSOCIATED KINASE REGULATOR 5 (MAKR5) suppresses brx mutant phenotypes 9, 18. Similarly, clv2 or crn second‐site mutation substantially rescued the protophloem differentiation, root meristem size and root growth defects of brx mutants (Fig 5A and B). The phenotype of brx crn double mutants qualified crn as a strong but partial brx suppressor, with overall rescue roughly comparable to brx makr5 double mutants 18, but less penetrant than in bam3 brx double mutants 9. Thus, crn loss of function dampens CLE45 signaling in the brx background, where the CLE45‐BAM3 pathway is apparently hyperactive 9, 18. Another feature of CLE45 perception is the accumulation of MAKR5‐GFP fusion protein in developing protophloem sieve elements upon CLE45 treatment 18. This response, which appears to be triggered by post‐translational events since it still takes place in the presence of cycloheximide (Fig 5C), is abolished in bam3 mutants and was likewise undetectable in crn mutants (Fig 5D). Therefore, CRN is required for CLE45 signaling as judged by all established criteria. To investigate whether crn loss of function can affect the expression or subcellular localization of BAM3, we introduced the BAM3::BAM3‐CITRINE construct into the crn mutant background. Indeed, we observed substantially reduced overall BAM3‐CITRINE abundance in the crn mutant, especially at later stages of protophloem development (Fig 5E and F). Since BAM3 gene expression was not affected in crn root tips (Fig 5G), this apparently reflected post‐translational regulation. We verified this observation by crossing individual BAM3::BAM3‐CITRINE lines in crn background with a crn line that was complemented by a CRN::CRN‐mCHERRY construct. In corresponding F1 plants that thus carried both hemizygous BAM3::BAM3‐CITRINE and CRN::CRN‐mCHERRY transgenes in homozygous crn background, BAM3‐CITRINE expression was restored to its wild‐type levels (Fig 5H and I). This included expression throughout the developing sieve element cell file, which was one feature that could be easily quantified. While BAM3‐CITRINE signal could practically always be observed up to the last cell before the protophloem transition zone in wild type, the signal was already completely absent in crn roots much earlier (Fig 5J). Finally, because increase in BAM3 dosage through BAM3::BAM3‐CITRINE could not overcome the CLE45 resistance of crn (Fig 5K), it appears that CRN is essential for CLE45 perception because it is required for efficient BAM3 protein expression and its maintenance during sieve element development.
Figure 5. Genetic interactions between CRN,BRX, and MAKR5 .
-
APrimary root length of 9‐day‐old seedlings of indicated genotypes.
-
BRoot meristems of indicated genotypes (white fluorescence: calcofluor white cell wall staining) (confocal microscopy). Red arrowheads indicate the “gap” cells in brx.
-
CMAKR5‐GFP fusion protein (green fluorescence) expressed under control of the native MAKR5 promoter in response to CLE45 application in the presence or absence of cycloheximide (blue fluorescence: calcofluor white cell wall staining). Green channel is shown separately (left) and in overlay with blue channel (right).
-
DResponse of MAKR5‐GFP fusion protein to CLE45 application in crn mutant background as compared to Col‐0 control (red fluorescence: propidium iodide cell wall staining).
-
E, FExpression of BAM3‐CITRINE fusion protein under control of the BAM3 promoter in developing protophloem of bam3 (E) or crn (F).
-
GqPCR of BAM3 expression in Col‐0 or crn root tips, with MAKR5 as control, relative to the EF1 housekeeping gene, representing the average for 2–3 technical replicates of three biological replicates, mean ± s.e.m. Differences were not statistically significant between Col‐0 and crn (Student's t‐test; P = 0.096 for BAM3, P = 0.273 for MAKR5).
-
HExpression of BAM3‐CITRINE fusion protein (green fluorescence) under control of the BAM3 promoter in developing protophloem of crn (left panel) and in an F1 plant derived from a cross of the same line to a crn mutant complemented by a CRN::CRN‐mCHERRY (red fluorescence) transgene (right panel).
-
IExpression of BAM3‐CITRINE fusion protein (green fluorescence) in Col‐0, as a parallel control for panel (J).
-
JQuantification of the last proliferating protophloem cell (light green cells in the root schematic) with detectable BAM3‐CITRINE signal, with respect to the beginning of the protophloem transition zone (red cells).
-
KPrimary root length of 7‐day‐old seedlings of indicated genotypes on mock or CLE45 media, and several independent transgenic lines are shown.
Discussion
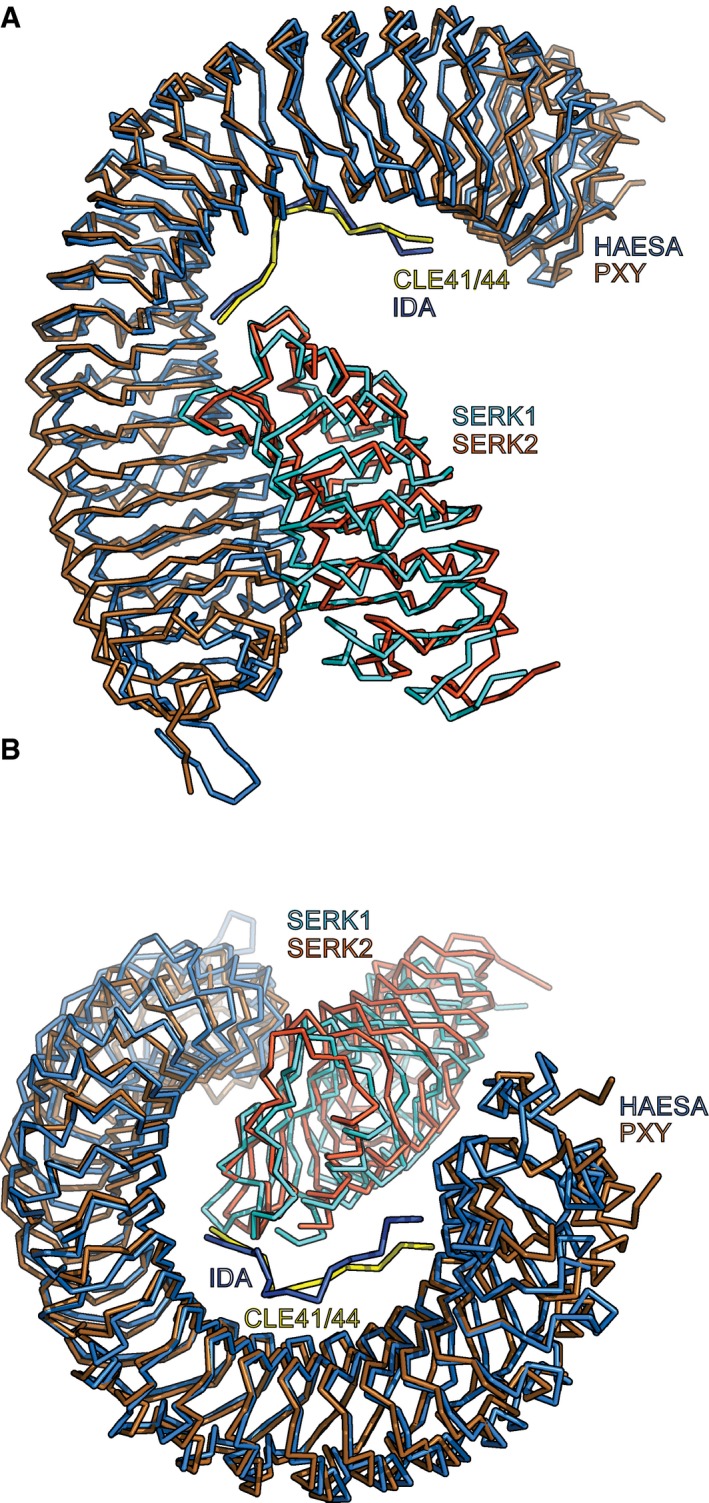
Plant LRR‐RKs are central signaling hubs that can sense diverse ligands to control various facets of the plant life cycle, from plant–pathogen interactions to intrinsic developmental processes. Many of the currently known LRR‐RKs require shape‐complementary co‐receptor kinases for receptor activation 46. For instance, LRR‐RKs of the SERK family can serve as co‐receptors for BRI1 in brassinosteroid sensing 29, for FLAGELLIN INSENSITIVE 2 in innate immunity 56, 57, for ERECTA and related RKs in stomata development 58, and for HAESA in abscission 30. More recently, SERKs have also been implicated in CLE peptide sensing, as co‐receptors of PXY as well as PXY‐LIKE LRR‐RKs 25, 45. In the case of the LRR‐RK HAESA, the presence of SERK1 strongly increases the binding affinity for the IDA peptide (a ~60‐fold increase from 20 μM to 350 nM) 30. Consequently, while there is no detectable binding of the SERK1 ectodomain to HAESA in the absence of ligand, IDA‐bound HAESA senses SERK1 with 75 nM affinity 30. Structural comparison of the HAESA‐IDA‐SERK1 ternary complex with a CLE receptor complex containing PXY‐CLE41/44‐SERK2 revealed that both complexes are highly similar (root‐mean‐square deviation, r.m.s.d., is 2.3 Å comparing 782 Cα atoms) (Fig EV5A and B). However, in contrast to HAESA, the isolated PXY ectodomain binds CLE41/44 with nanomolar (10–30 nM), not micromolar, affinity (Fig EV1B) 25. Interestingly, the SERK1 ectodomain binds relatively weakly to CLE41/44‐bound PXY, suggesting that, despite their structural similarities, the activation mechanisms for HAESA and PXY may differ 30, 45.
Figure EV5. Structure comparison of HAESA‐IDA‐SERK1 and PXY‐CLE41‐SERK2 signaling complexes.
-
A, BStructural superposition of HAESA‐IDA‐SERK1 (PDB‐ID 5IYX, HAESA in light blue, IDA in dark blue, and SERK1 in cyan) (Santiago et al 30) with a PXY‐CLE41/44‐SERK2 complex (PDB‐ID 5GQR, PXY in gold, IDA in yellow, and SERK2 in orange) (Zhang et al 45). The complexes closely align with an r.m.s.d. of 2.3 Å comparing 770 Cα atoms.
Our system‐wide analysis of root‐active CLE peptides in Arabidopsis suggests that only few CLE‐sensing complexes may critically involve SERK co‐receptor kinases or that CLE resistance phenotypes in serk mutants are caused by secondary effects. However, in our study, we did not investigate genetic redundancy between SERK genes. It appears possible that an array of higher order serk mutants will uncover fully redundant, overlapping roles of SERKs in CLE peptide sensing. Such analyses are substantially complicated by the dwarf and short root phenotypes of higher order serk mutants, however 59. Moreover, despite their overall high sequence similarity, SERK proteins have diversified sufficiently to adopt potentially separate signaling roles 60. Thus, their potential genetic requirement in CLE perception might be determined by a combination of differential expression patterns and levels as well as protein structure variation.
Importantly, we could not find biochemical, genetic, or cell biological evidence that would support a role for SERKs in BAM3‐mediated CLE45 sensing and signaling. While we could demonstrate CLE45 binding by BAM3 in vitro and observe matching in planta evidence, SERK1 or SERK3 did neither form CLE45‐dependent or CLE45‐independent complexes with BAM3, nor did serk mutants display CLE45‐resistant phenotypes. Moreover, our finding that the serk1‐1 allele carries a bam3 background mutation that confers CLE45 resistance emphasizes that full‐scale analysis of serk mutant redundancy could only be considered reliable in conjunction with transgenic rescue. Reported phenotypes for serk multiple mutants that involve the serk1‐1 allele should thus be carefully considered in light of CLE45 resistance, especially with respect to vascular phenotypes 45. Indeed, it appears possible that BAM3 also has a role in secondary growth 61.
Compared to SERK genes, the genetic requirement for CLV2 and CRN in the full‐scale sensing of all root‐active CLE peptides that we investigated in this study was absolute. The results point to a generic rate‐limiting role of CLV2‐CRN in CLE receptor activity, for which multiple scenarios could be envisaged. In this respect, a classic role as co‐receptor appears least likely, because CRN does not possess an active kinase domain and CLV2 is very different in size and sequence from SERK proteins 36. Nevertheless, CLV2‐CRN might participate as a component in receptor complexes, for instance to stabilize them or to recruit downstream components. Such a role would not be mutually exclusive with another possibility, a role of CLV2‐CRN in promoting the plasma membrane delivery of LRR‐RKs, or in enhancing their plasma membrane localization indirectly, for instance through molecular crowding. Our observations of reduced BAM3 abundance in crn mutants support the latter ideas, and it remains to be seen whether this will also apply to other, yet to be identified CLE peptide receptors in the root. So far, a conceptually similar role of CRN was not observed in the shoot 62. However, such a function might be masked by the observed compensatory transcriptional cross‐regulation between redundantly acting receptors 63, a scenario that apparently does not exist for BAM3 in the root 9, 18.
Our most surprising finding is the observation that CRN activity in the developing protophloem was sufficient to restore sensitivity to all root‐active CLE peptides investigated in this study. This observation is consistent with the more or less penetrant effect of all of these peptides on protophloem differentiation, irrespective of additional effects on root development that could be observed for individual CLEs. Thus, the results reinforce the emerging notion of the protophloem as a limiting, systemic organizer of overall root meristem development 9, 19, 64. What remains enigmatic, however, is why crn mutants do not display an apparent morphological root phenotype if none of the root‐efficient CLE peptides can be sensed properly? One possibility is that observations obtained from external CLE application might at least in part be misleading with regard to the genuine role of those peptides. Yet the CLV2/CRN module could function in certain conditions that upregulate CLE peptide levels and thereby might be crucial for root growth adaptation. Alternatively, it could also mean that most CLE peptide signaling is not essential for root development, at least in tissue culture conditions, or that compensatory, possibly non‐peptide‐mediated mechanisms exist. It is important to note, however, that crn loss of function does not confer complete CLE peptide resistance, as exemplified by the strong, yet partial CLE45 resistance of crn as compared to bam3. Therefore, it appears possible that partially redundant CRN‐related functions exist. Identification of additional genuine CLE peptide receptors in the root context might enable us to address this topic systematically in future studies.
In summary, we present a genetic framework for CLE peptide sensing in the Arabidopsis root that assigns distinct functions to various known receptor pathway components. Our data suggest that SERK proteins are involved in some, but not in the majority of the root CLE‐sensing membrane signaling complexes, or alternatively act in a highly redundant manner. Instead, we provide evidence that CLV2 and CRN are part of root‐active CLE peptide signaling pathways, possibly by controlling the expression, proper membrane localization, and/or stability of LRR‐RK signaling complexes. Finally, our data suggest that the root protophloem is the crucial site of action of root‐active CLE peptides. Nevertheless, they are apparently perceived by several distinct receptor complexes, many of which remain to be identified. The data presented in this study could serve as a resource to facilitate this task.
Materials and Methods
Plant tissue culture, plant transformation, and common molecular biology procedures such as genomic DNA isolation, genotyping, sequencing, and peptide or inhibitor treatments were performed according to standard procedures as previously described 9, 19, 44, 65.
Sequence analysis of the serk1‐1 mutant
Whole‐genome sequencing and data analysis of serk1‐1 mutants were performed as described 9. The data and experimental details can be retrieved from the NCBI Short Read Archive at https://www.ncbi.nlm.nih.gov/Traces/study/?acc=SRP104341 [accessions: STUDY: PRJNA383544 (SRP104341); SAMPLE: serk1‐1 (SRS2134599); EXPERIMENT: A_ (SRX2748469); RUN: serk1‐1_R1_fused.fastq.gz (SRR5460456)].
Plant materials, growth conditions, and physiological assays
All mutant and transgenic lines were in the Arabidopsis Columbia‐0 (Col‐0) background. The following previously described mutant alleles were used throughout: brx‐2, bam3‐2, brx‐2 bam3, clv2‐1, serk1‐1 and serk1‐3, serk2‐1, serk3‐1, serk4‐1, serk5‐1 9, 47, 59, 66, 67, 68. The crn loss‐of‐function mutant allele (crn‐10) carries a single nucleotide insertion in front of the 7th codon, which leads to a frameshift and three subsequent premature stop codons after amino acid 10 52. Observations with transgene constructs were confirmed in multiple independent transgenic lines.
Transgenic constructs and lines
The BAM3::BAM3‐CITRINE, MAKR5::MAKR5‐GFP, and CVP2::NLS‐VENUS constructs and transgenic lines have been described before 18, 19, 44. The transgenic lines created for this study are summarized in Table EV1. All constructs used in this study were created with the multi‐site GATEWAY cloning system according to standard protocols (Invitrogen). The constructs are listed in Table EV2. Oligonucleotides used in cloning procedures or for genotyping are listed in Table EV3.
qRT–PCR
For qRT–PCR (qPCR), RNA was prepared from root tips of 7‐day‐old Col‐0 and crn seedlings, and BAM3 and MAKR5 expressions were quantified relative to the EF1 housekeeping gene on an Applied Biosystems Quantstudio 3 instrument as previously described 9, 18.
Transient expression and BiFC assays
For transient expression experiments, we used the 4th to 6th leaves of N. benthamiana plants. Infiltrations, co‐localization, and BiFC analyses were essentially performed as previously described 69.
Confocal imaging
Confocal images were obtained with Zeiss 700 or Zeiss 780 inverted confocal microscopes. All dual‐color images were acquired by sequential line switching, allowing the separation of channels by both excitation and emission.
Root counter staining
In some experiments, we used calcofluor white instead of standard propidium iodide (PI) staining for visualizing cell walls. To this end, 4‐ to 6‐day‐old seedlings were fixed in 4% paraformaldehyde in PBS for 45 min. After washing with PBS, seedlings were cleared with ClearSee solution 70 overnight. After incubation in 0.2% calcofluor white in ClearSee solution, seedlings were transferred to fresh ClearSee solution for 2–24 h before imaging.
Peptides
Peptides were obtained at the 1–4 mg scale, > 75% pure (GenScript USA Inc.). The peptide sequences were as follows. CLV3: RTVPSGPDPLHH; CLE8: RRVPTGPNPLHH; CLE9/10: RLVPSGPNPLHN; CLE11: RVVPSGPNPLHH; CLE13: RLVPSGPNPLHH; CLE14: RLVPKGPNPLHN; CLE16: RLVHTGPNPLHN; CLE17: RVVHTGPNPLHN; CLE18: RQIPTGPDPLHN; CLE20: RKVKTGSNPLHN; CLE21: RSIPTGPNPLHN; CLE25: RKVPNGPDPIHN; CLE26: RKVPRGPDPIHN; CLE40: RQVPTGSDPLHH; CLE41/44: HEVPSGPNPISN; CLE45: RRVRRGSDPIHN. N‐terminally tyrosine‐modified CLV3, CLE41/44, and CLE45 peptides were used as standards for quantification. Peptides for biochemical assays were synthesized by Peptide Specialty Laboratories, Heidelberg, Germany (see below).
Protein homology modeling
Structural homologs of the BAM3 LRR (residues 30–651) and kinase (residues 699–903) domains were identified using the program HHpred 71, and homology modeling was done in Modeller 72 using the isolated LRR domain of HAESA (PDB‐ID 5XIO) 30 and the kinase domain of BRI1 (PDB‐ID 5LPB) 73 as templates.
Protein expression and isolation of LRR‐RLK ectodomains
The coding sequences for BAM3 (amino acids 30–651), PXY/TDR (30–647), SERK1 (24–213), and SERK3 (1–220) ectodomains were amplified out of Arabidopsis Col‐0 cDNA using the PfuX7 polymerase (Norholm). The point mutations Q226A, Y228A, and Y231A for the BAM3QYY‐ECD were introduced by site‐directed mutagenesis. All DNA fragments were cloned into a modified pFastBac1 vector (Geneva Biotech), fusing BAM3‐, PXY‐, and SERK1‐ECD coding sequences with an N‐terminal azurocidin signal peptide. To all ectodomain sequences, a C‐terminal StrepII‐9xHIS tandem affinity purification tag was added, and all constructs were confirmed by sequencing. Bacmids were generated by transforming the plasmids into Escherichia coli DH10MultiBac (Geneva Biotech), isolated, transformed into Spodoptera frugiperda Sf9 with Profectin transfection reagent (AB Vector) followed by viral amplification. Secreted protein was expressed by infecting Trichoplusia ni Tnao38 cells or for PXY‐ECD S. frugiperda Sf9 with a viral multiplicity of 1, and incubation for 3 days post‐infection. Cells were separated by centrifugation at 5,000 × g for 30 min, and the supernatant was filtered through 0.45 μm filters. The proteins were isolated from the medium by Ni2+ (HisTrap excel; GE Healthcare) and subsequent StrepII (Strep‐Tactin Superflow high capacity, IBA) affinity chromatography and further purified by size‐exclusion chromatography (Superdex Increase 200) with 20 mM citrate pH 5 and 150 mM NaCl for BAM3 and SERK1/3 as buffer. For PXY and also one preparation of SERK1, the gel filtration was carried out with 10 mM Bis–Tris and 100 mM NaCl 45. Molecular weights of all purified proteins were determined by MALDI‐TOF mass spectroscopy (BAM3‐ECD: 94,586 Da; PXY‐ECD: 92,519 Da; SERK1‐ECD: 27,551 Da; SERK3‐ECD: 29,951 Da), and concentrations were measured via the absorption at 280 nm (corrected with the extinction coefficient for each protein).
Gel filtration experiments
For each gel filtration experiment, 100 μg of SERK1‐ECD or SERK3‐ECD and equimolar amounts of either BAM3‐ECD or PXY‐ECD as well as 25 μM of CLE45 (RRVRRGSDPIHN), and 25 μM CLE41/44 (HEV‐Hyp‐SG‐Hyp‐NPISN) were used in the combinations indicated in the figures, incubated for 10 min after mixing and then subjected to gel filtration on a Superdex Increase 200 equilibrated with 20 mM citrate pH 5 and 150 mM NaCl, except for the gel filtration with PXY + SERK1 + CLE41/44, for which the column was equilibrated in 10 mM Bis–Tris and 100 mM NaCl 45. The concentration of peptides modified N‐terminally with a tyrosine (Y‐peptides) was determined by their absorbance at 280 nm (corrected by the extinction coefficient). The concentrations for the peptides without tyrosine modification were determined by a quantitative colorimetric peptide assay (Pierce) using the respective Y‐peptides as standards. The elution of proteins from the gel filtration column was monitored by absorption at 280 nm. Fractions indicated in the figures were separated on Bis–Tris polyacrylamide gels.
Isothermal titration calorimetry
Isothermal titration calorimetry assays were run on a Nano ITC (1 ml standard cell; 250 μl syringe; TA Instruments) at 25°C in 20 mM sodium citrate pH 5 and 150 mM NaCl for all ITCs with BAM3 and 10 mM Bis–Tris pH 6 and 100 mM NaCl for the ITC with PXY. CLE41/44 (HEVPSGPNPISN), CLE45 (RRVRRGSDPIHN), CLV3 (RTV‐HYP‐SG‐HYP‐DPLHHH), and Y‐CLV3 (YRTV‐HYP‐SG‐HYP‐DPLHH) were dissolved in the respective ITC buffer (20 mM sodium citrate pH 5 and 150 mM NaCl for CLE45, Y‐CLE45, CLV3, and Y‐CLV3; 10 mM Bis–Tris pH 6 and 100 mM NaCl for CLE41/44), and the following concentrations were used in the assays: BAM3‐ECD vs. CLE45: 10 and 80 μM; BAM3‐ECD vs. Y‐CLE45: 9 and 154.5 μM; BAM3QYY vs. CLE45: 8.6 and 80 μM; BAM3‐ECD vs. CLV3: 10 and 400 μM; BAM3‐ECD vs. Y‐CLV3: 8.6 and 175 μM; PXY‐ECD vs. CLE41/44: 7.5 and 75 μM; BAM3‐ECD + CLE45 vs. SERK1‐ECD: 8.2 + 25 μM and 82 μM, respectively. For each experiment, 10 μl was repetitively injected into the cell in 150 s time intervals. The measured heat rates for the BAM3‐ECD vs. CLE45, BAM3QYY vs. CLE45, BAM3‐ECD vs. Y‐CLE45, BAM3‐ECD vs. CLV3, BAM3‐ECD vs. Y‐CLV3, and PXY‐ECD vs. CLE41/44 were corrected by subtracting the heat rates measured for injecting CLE45, Y‐CLE45, CLV3, Y‐CLV3, or CLE41/44 into the ITC buffer, respectively. The data for the BAM3‐ECD + CLE45 vs. SERK1‐ECD measurement was corrected by subtracting heat rates acquired by injecting SERK1‐ECD into a cell containing CLE45. Data analyses and modeling were carried out using the software supplied by the manufacturer (NanoAnalyze, version 3.5).
Kinase domain protein expression and isolation as well as in vitro kinase assays
Kinase domain protein production and in vitro kinase assays were carried out as previously described 30. In brief, the coding sequence for the cytosolic part of BAM3 (679–992; BAM3‐KD) was amplified from Arabidopsis Col‐0 cDNA. The DNA for the cytosolic region of SERK1 (264–625; SERK1‐KD) was synthesized and codon‐optimized for expression in E. coli. Both were inserted into an expression vector based on pET (Novagen) that gives rise to an N‐terminal tag consisting of 8xHis‐StrepII‐Thioredoxin, which can be cleaved by a TEV‐protease. Per site‐directed mutagenesis point mutations were introduced into both coding sequences, resulting in inactive kinase domains for SERK1‐KD (D447 to N; mSERK1‐KD) and BAM3‐KD (D854 to N; mBAM3‐KD) 73. All constructs were confirmed by sequencing and transformed into E. coli Rosetta 2 (Novagen). The bacteria were grown to an ODA600 = 0.6, and protein expression was induced by adding IPTG to a final concentration of 0.5 mM followed by incubation for 18 h at 16°C. Cells were harvested by centrifugation at 5,000 × g and 4°C for 15 min, resuspended in buffer A (20 mM Tris–HCl pH 8, 500 mM NaCl, 4 mM MgCl2, and 2 mM β‐mercaptoethanol) to which 15 mM imidazole and 0.1% (v/v) Igepal were added, and lysed by sonication. The sample was then centrifuged at 35,000 × g and 4°C for 30 min, and the supernatant was used for a Co2+ affinity purification. Co2+ resin (HIS‐Select Cobalt Affinity Gel, Sigma) was incubated with the cell lysate for 60 min at 4°C, subjected to a gravity flow column (Pierce), and washed twice with buffer A (+15 mM imidazole). Recombinant proteins were eluted in buffer A (+250 mM imidazole) and dialyzed in buffer B (20 mM Tris–HCl pH 8, 250 mM NaCl, 4 mM MgCl2, and 0.5 mM TCEP). To BAM3‐KD and BAM3QYY‐KD, TEV‐protease was added before dialyses and then removed after dialyses by a second Co2+ HIS‐affinity purification (during this step, the cut tag was also removed). The proteins were then subjected to gel filtration on a Superdex Increase 200 column using buffer B, collected, and concentrated in Amicon Ultra devices (10,000 MWCO cutoff).
To perform in vitro kinase assays, 1 μg of each kinase domain, in the combination indicated in the figure, was added in a total volume of 20 μl (buffer B). To start the reaction, 5 μCi of [γ‐32P]‐ATP (Perkin‐Elmer) was added and the reaction was carried out for 45 min at room temperature before being terminated by the addition of 4 μl of 6× SDS‐loading dye and immediate incubation at 95°C for 7 min. SDS–PAGE in 4–15% gradient gels (TGX, Bio‐Rad) separated the proteins, and the gels were subsequently stained with Instant Blue (Expedeon). Gel pictures were taken, and subsequently, an X‐ray film was exposed to the gel in order to detect the radioactive signals of 32P.
Author contributions
OH, BB, MH, and CSH designed the study and wrote the paper together. OH, BB, PC, JS, and AR‐V performed experiments.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Review Process File
Acknowledgements
We would like to thank Dr. Zachary Nimchuk for the crn‐10 mutant allele and Drs. N. Geldner and S. Yalovsky for vector plasmids. This work was supported by Swiss National Science Foundation grants 31003A_166394 (awarded to C.S.H.) and 31003A_156920 (awarded to M.H.), German Research Foundation (DFG) grant CRC 1101‐D01 (awarded to M.H.), a Human Frontier Science Program Career Development Award (M.H.), and the European Molecular Biology Organization (EMBO) Young Investigator program (M.H.). A.R.V., J.S., and B.B. were supported by EMBO long‐term post‐doctoral fellowships.
EMBO Reports (2017) 18: 1367–1381
Contributor Information
Michael Hothorn, Email: michael.hothorn@unige.ch.
Christian S Hardtke, Email: christian.hardtke@unil.ch.
References
- 1. Katsir L, Davies KA, Bergmann DC, Laux T (2011) Peptide signaling in plant development. Curr Biol 21: R356–R364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cock JM, McCormick S (2001) A large family of genes that share homology with CLAVATA3. Plant Physiol 126: 939–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H (2006) Dodeca‐CLE peptides as suppressors of plant stem cell differentiation. Science 313: 842–845 [DOI] [PubMed] [Google Scholar]
- 4. Kondo T, Sawa S, Kinoshita A, Mizuno S, Kakimoto T, Fukuda H, Sakagami Y (2006) A plant peptide encoded by CLV3 identified by in situ MALDI‐TOF MS analysis. Science 313: 845–848 [DOI] [PubMed] [Google Scholar]
- 5. Matsubayashi Y (2011) Small post‐translationally modified peptide signals in Arabidopsis . Arabidopsis Book 9: e0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohyama K, Shinohara H, Ogawa‐Ohnishi M, Matsubayashi Y (2009) A glycopeptide regulating stem cell fate in Arabidopsis thaliana . Nat Chem Biol 5: 578–580 [DOI] [PubMed] [Google Scholar]
- 7. Goad DM, Zhu C, Kellogg EA (2016) Comprehensive identification and clustering of CLV3/ESR‐related (CLE) genes in plants finds groups with potentially shared function. New Phytol 10.1111/nph.14348 [DOI] [PubMed] [Google Scholar]
- 8. Strabala TJ, O'Donnell PJ, Smit AM, Ampomah‐Dwamena C, Martin EJ, Netzler N, Nieuwenhuizen NJ, Quinn BD, Foote HC, Hudson KR (2006) Gain‐of‐function phenotypes of many CLAVATA3/ESR genes, including four new family members, correlate with tandem variations in the conserved CLAVATA3/ESR domain. Plant Physiol 140: 1331–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Depuydt S, Rodriguez‐Villalon A, Santuari L, Wyser‐Rmili C, Ragni L, Hardtke CS (2013) Suppression of Arabidopsis protophloem differentiation and root meristem growth by CLE45 requires the receptor‐like kinase BAM3. Proc Natl Acad Sci USA 110: 7074–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kinoshita A, Nakamura Y, Sasaki E, Kyozuka J, Fukuda H, Sawa S (2007) Gain‐of‐function phenotypes of chemically synthetic CLAVATA3/ESR‐related (CLE) peptides in Arabidopsis thaliana and Oryza sativa . Plant Cell Physiol 48: 1821–1825 [DOI] [PubMed] [Google Scholar]
- 11. Miwa H, Betsuyaku S, Iwamoto K, Kinoshita A, Fukuda H, Sawa S (2008) The receptor‐like kinase SOL2 mediates CLE signaling in Arabidopsis . Plant Cell Physiol 49: 1752–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R (2000) Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289: 617–619 [DOI] [PubMed] [Google Scholar]
- 13. Clark SE, Running MP, Meyerowitz EM (1995) CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1 . Development 121: 2057–2067 [Google Scholar]
- 14. Clark SE, Williams RW, Meyerowitz EM (1997) The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis . Cell 89: 575–585 [DOI] [PubMed] [Google Scholar]
- 15. Ogawa M, Shinohara H, Sakagami Y, Matsubayashi Y (2008) Arabidopsis CLV3 peptide directly binds CLV1 ectodomain. Science 319: 294 [DOI] [PubMed] [Google Scholar]
- 16. Rojo E, Sharma VK, Kovaleva V, Raikhel NV, Fletcher JC (2002) CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. Plant Cell 14: 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283: 1911–1914 [DOI] [PubMed] [Google Scholar]
- 18. Kang YH, Hardtke CS (2016) Arabidopsis MAKR5 is a positive effector of BAM3‐dependent CLE45 signaling. EMBO Rep 17: 1145–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez‐Villalon A, Gujas B, Kang YH, Breda AS, Cattaneo P, Depuydt S, Hardtke CS (2014) Molecular genetic framework for protophloem formation. Proc Natl Acad Sci USA 111: 11551–11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Etchells JP, Turner SR (2010) The PXY‐CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of vascular cell division. Development 137: 767–774 [DOI] [PubMed] [Google Scholar]
- 21. Fisher K, Turner S (2007) PXY, a receptor‐like kinase essential for maintaining polarity during plant vascular‐tissue development. Curr Biol 17: 1061–1066 [DOI] [PubMed] [Google Scholar]
- 22. Hirakawa Y, Kondo Y, Fukuda H (2010) TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis . Plant Cell 22: 2618–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hirakawa Y, Shinohara H, Kondo Y, Inoue A, Nakanomyo I, Ogawa M, Sawa S, Ohashi‐Ito K, Matsubayashi Y, Fukuda H (2008) Non‐cell‐autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc Natl Acad Sci USA 105: 15208–15213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morita J, Kato K, Nakane T, Kondo Y, Fukuda H, Nishimasu H, Ishitani R, Nureki O (2016) Crystal structure of the plant receptor‐like kinase TDR in complex with the TDIF peptide. Nat Commun 7: 12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang H, Lin X, Han Z, Qu LJ, Chai J (2016) Crystal structure of PXY‐TDIF complex reveals a conserved recognition mechanism among CLE peptide‐receptor pairs. Cell Res 26: 543–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hazak O, Hardtke CS (2016) CLAVATA 1‐type receptors in plant development. J Exp Bot 67: 4827–4833 [DOI] [PubMed] [Google Scholar]
- 27. Li J, Tax FE (2013) Receptor‐like kinases: key regulators of plant development and defense. J Integr Plant Biol 55: 1184–1187 [DOI] [PubMed] [Google Scholar]
- 28. Brandt B, Hothorn M (2016) SERK co‐receptor kinases. Curr Biol 26: R225–R226 [DOI] [PubMed] [Google Scholar]
- 29. Santiago J, Henzler C, Hothorn M (2013) Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co‐receptor kinases. Science 341: 889–892 [DOI] [PubMed] [Google Scholar]
- 30. Santiago J, Brandt B, Wildhagen M, Hohmann U, Hothorn LA, Butenko MA, Hothorn M (2016) Mechanistic insight into a peptide hormone signaling complex mediating floral organ abscission. Elife 5: e15075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meng X, Zhou J, Tang J, Li B, de Oliveira MV, Chai J, He P, Shan L (2016) Ligand‐induced receptor‐like kinase complex regulates floral organ abscission in Arabidopsis . Cell Rep 14: 1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwessinger B, Rathjen JP (2015) Changing SERKs and priorities during plant life. Trends Plant Sci 20: 531–533 [DOI] [PubMed] [Google Scholar]
- 33. Torii KU (2012) Mix‐and‐match: ligand‐receptor pairs in stomatal development and beyond. Trends Plant Sci 17: 711–719 [DOI] [PubMed] [Google Scholar]
- 34. Jeong S, Trotochaud AE, Clark SE (1999) The Arabidopsis CLAVATA2 gene encodes a receptor‐like protein required for the stability of the CLAVATA1 receptor‐like kinase. Plant Cell 11: 1925–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muller R, Bleckmann A, Simon R (2008) The receptor kinase CORYNE of Arabidopsis transmits the stem cell‐limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 20: 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nimchuk ZL, Tarr PT, Meyerowitz EM (2011) An evolutionarily conserved pseudokinase mediates stem cell production in plants. Plant Cell 23: 851–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo Y, Han L, Hymes M, Denver R, Clark SE (2010) CLAVATA2 forms a distinct CLE‐binding receptor complex regulating Arabidopsis stem cell specification. Plant J 63: 889–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shinohara H, Matsubayashi Y (2015) Reevaluation of the CLV3‐receptor interaction in the shoot apical meristem: dissection of the CLV3 signaling pathway from a direct ligand‐binding point of view. Plant J 82: 328–336 [DOI] [PubMed] [Google Scholar]
- 39. Meng L, Feldman LJ (2010) CLE14/CLE20 peptides may interact with CLAVATA2/CORYNE receptor‐like kinases to irreversibly inhibit cell division in the root meristem of Arabidopsis . Planta 232: 1061–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fiers M, Golemiec E, Xu J, van der Geest L, Heidstra R, Stiekema W, Liu CM (2005) The 14‐amino acid CLV3, CLE19, and CLE40 peptides trigger consumption of the root meristem in Arabidopsis through a CLAVATA2‐dependent pathway. Plant Cell 17: 2542–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. De Smet I, Vassileva V, De Rybel B, Levesque MP, Grunewald W, Van Damme D, Van Noorden G, Naudts M, Van Isterdael G, De Clercq R et al (2008) Receptor‐like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322: 594–597 [DOI] [PubMed] [Google Scholar]
- 42. Stahl Y, Grabowski S, Bleckmann A, Kuhnemuth R, Weidtkamp‐Peters S, Pinto KG, Kirschner GK, Schmid JB, Wink RH, Hulsewede A et al (2013) Moderation of Arabidopsis root stemness by CLAVATA1 and ARABIDOPSIS CRINKLY4 receptor kinase complexes. Curr Biol 23: 362–371 [DOI] [PubMed] [Google Scholar]
- 43. Shimizu N, Ishida T, Yamada M, Shigenobu S, Tabata R, Kinoshita A, Yamaguchi K, Hasebe M, Mitsumasu K, Sawa S (2015) BAM 1 and RECEPTOR‐LIKE PROTEIN KINASE 2 constitute a signaling pathway and modulate CLE peptide‐triggered growth inhibition in Arabidopsis root. New Phytol 208: 1104–1113 [DOI] [PubMed] [Google Scholar]
- 44. Rodriguez‐Villalon A, Gujas B, van Wijk R, Munnik T, Hardtke CS (2015) Primary root protophloem differentiation requires balanced phosphatidylinositol‐4,5‐biphosphate levels and systemically affects root branching. Development 142: 1437–1446 [DOI] [PubMed] [Google Scholar]
- 45. Zhang H, Lin X, Han Z, Wang J, Qu LJ, Chai J (2016) SERK family receptor‐like kinases function as a co‐receptor with PXY for plant vascular development. Mol Plant 10: 1406–1414 [DOI] [PubMed] [Google Scholar]
- 46. Hohmann U, Lau K, Hothorn M (2017) The structural basis of ligand perception and signal activation by receptor kinases. Annu Rev Plant Biol 68: 109–137 [DOI] [PubMed] [Google Scholar]
- 47. Colcombet J, Boisson‐Dernier A, Ros‐Palau R, Vera CE, Schroeder JI (2005) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASES 1 and 2 are essential for tapetum development and microspore maturation. Plant Cell 17: 3350–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Somssich M, Ma Q, Weidtkamp‐Peters S, Stahl Y, Felekyan S, Bleckmann A, Seidel CA, Simon R (2015) Real‐time dynamics of peptide ligand‐dependent receptor complex formation in planta. Sci Signal 8: ra76 [DOI] [PubMed] [Google Scholar]
- 49. Kwaaitaal MA, de Vries SC (2007) The SERK1 gene is expressed in procambium and immature vascular cells. J Exp Bot 58: 2887–2896 [DOI] [PubMed] [Google Scholar]
- 50. Pallakies H, Simon R (2014) The CLE40 and CRN/CLV2 signaling pathways antagonistically control root meristem growth in Arabidopsis . Mol Plant 7: 1619–1636 [DOI] [PubMed] [Google Scholar]
- 51. Somssich M, Bleckmann A, Simon R (2016) Shared and distinct functions of the pseudokinase CORYNE (CRN) in shoot and root stem cell maintenance of Arabidopsis . J Exp Bot 67: 4901–4915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nimchuk ZL (2017) CLAVATA1 controls distinct signaling outputs that buffer shoot stem cell proliferation through a two‐step transcriptional compensation loop. PLoS Genet 13: e1006681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hobe M, Muller R, Grunewald M, Brand U, Simon R (2003) Loss of CLE40, a protein functionally equivalent to the stem cell restricting signal CLV3, enhances root waving in Arabidopsis . Dev Genes Evol 213: 371–381 [DOI] [PubMed] [Google Scholar]
- 54. Jun J, Fiume E, Roeder AH, Meng L, Sharma VK, Osmont KS, Baker C, Ha CM, Meyerowitz EM, Feldman LJ et al (2010) Comprehensive analysis of CLE polypeptide signaling gene expression and overexpression activity in Arabidopsis . Plant Physiol 154: 1721–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bleckmann A, Weidtkamp‐Peters S, Seidel CA, Simon R (2010) Stem cell signaling in Arabidopsis requires CRN to localize CLV2 to the plasma membrane. Plant Physiol 152: 166–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JD, Felix G, Boller T (2007) A flagellin‐induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- 57. Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Mussig C et al (2007) The BRI1‐associated kinase 1, BAK1, has a brassinolide‐independent role in plant cell‐death control. Curr Biol 17: 1116–1122 [DOI] [PubMed] [Google Scholar]
- 58. Meng X, Chen X, Mang H, Liu C, Yu X, Gao X, Torii KU, He P, Shan L (2015) Differential function of Arabidopsis SERK family receptor‐like kinases in stomatal patterning. Curr Biol 25: 2361–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Albrecht C, Russinova E, Kemmerling B, Kwaaitaal M, de Vries SC (2008) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid‐dependent and ‐independent signaling pathways. Plant Physiol 148: 611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aan den Toorn M, Albrecht C, de Vries S (2015) On the origin of SERKs: bioinformatics analysis of the somatic embryogenesis receptor kinases. Mol Plant 8: 762–782 [DOI] [PubMed] [Google Scholar]
- 61. Lehmann F, Hardtke CS (2016) Secondary growth of the Arabidopsis hypocotyl‐vascular development in dimensions. Curr Opin Plant Biol 29: 9–15 [DOI] [PubMed] [Google Scholar]
- 62. Nimchuk ZL, Tarr PT, Ohno C, Qu X, Meyerowitz EM (2011) Plant stem cell signaling involves ligand‐dependent trafficking of the CLAVATA1 receptor kinase. Curr Biol 21: 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nimchuk ZL, Zhou Y, Tarr PT, Peterson BA, Meyerowitz EM (2015) Plant stem cell maintenance by transcriptional cross‐regulation of related receptor kinases. Development 142: 1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kang YH, Breda A, Hardtke CS (2017) Brassinosteroid signaling directs formative cell divisions and protophloem differentiation in Arabidopsis root meristems. Development 144: 272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Scacchi E, Salinas P, Gujas B, Santuari L, Krogan N, Ragni L, Berleth T, Hardtke CS (2010) Spatio‐temporal sequence of cross‐regulatory events in root meristem growth. Proc Natl Acad Sci USA 107: 22734–22739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. DeYoung BJ, Bickle KL, Schrage KJ, Muskett P, Patel K, Clark SE (2006) The CLAVATA1‐related BAM1, BAM2 and BAM3 receptor kinase‐like proteins are required for meristem function in Arabidopsis . Plant J 45: 1–16 [DOI] [PubMed] [Google Scholar]
- 67. Deyoung BJ, Clark SE (2008) BAM receptors regulate stem cell specification and organ development through complex interactions with CLAVATA signaling. Genetics 180: 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rodrigues A, Santiago J, Rubio S, Saez A, Osmont KS, Gadea J, Hardtke CS, Rodriguez PL (2009) The short‐rooted phenotype of the brevis radix mutant partly reflects root abscisic acid hypersensitivity. Plant Physiol 149: 1917–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bracha‐Drori K, Shichrur K, Katz A, Oliva M, Angelovici R, Yalovsky S, Ohad N (2004) Detection of protein‐protein interactions in plants using bimolecular fluorescence complementation. Plant J 40: 419–427 [DOI] [PubMed] [Google Scholar]
- 70. Kurihara D, Mizuta Y, Sato Y, Higashiyama T (2015) ClearSee: a rapid optical clearing reagent for whole‐plant fluorescence imaging. Development 142: 4168–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Soding J, Biegert A, Lupas AN (2005) The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res 33: W244–W248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234: 779–815 [DOI] [PubMed] [Google Scholar]
- 73. Bojar D, Martinez J, Santiago J, Rybin V, Bayliss R, Hothorn M (2014) Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation. Plant J 78: 31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded View Figures PDF
Table EV1
Table EV2
Table EV3
Review Process File


