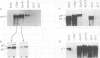Abstract
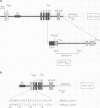
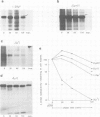
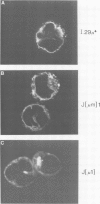
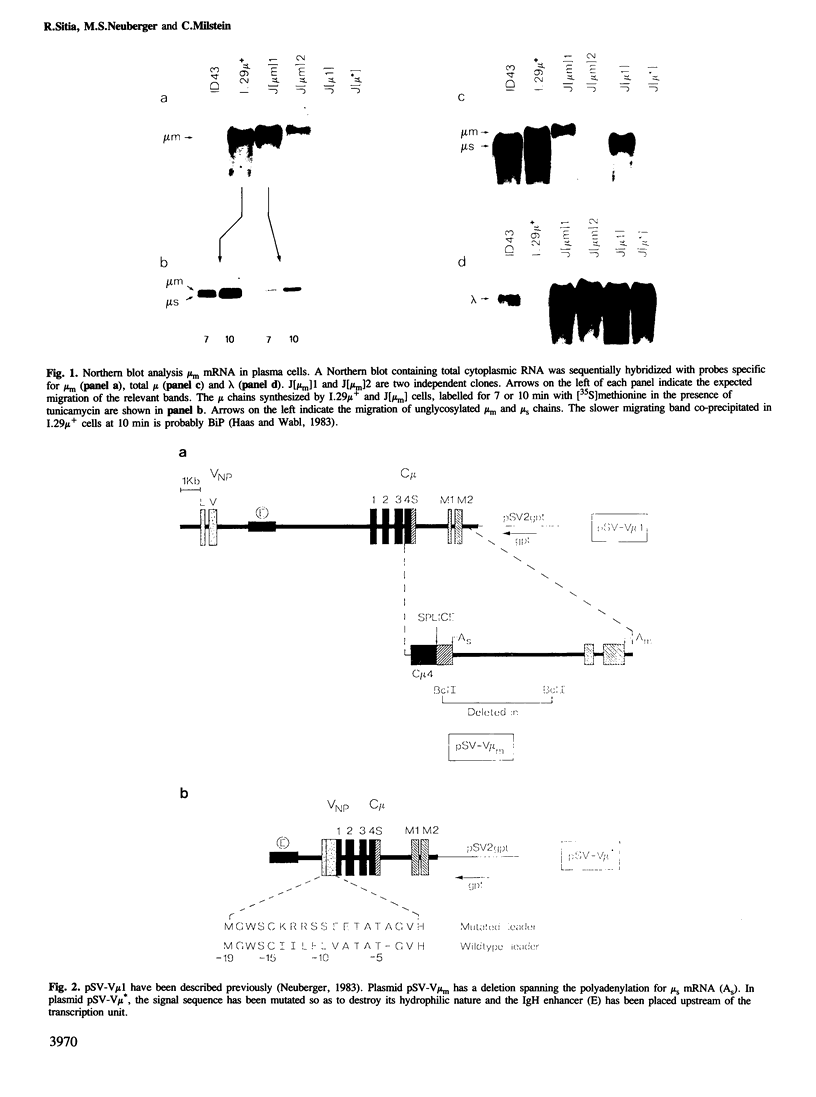
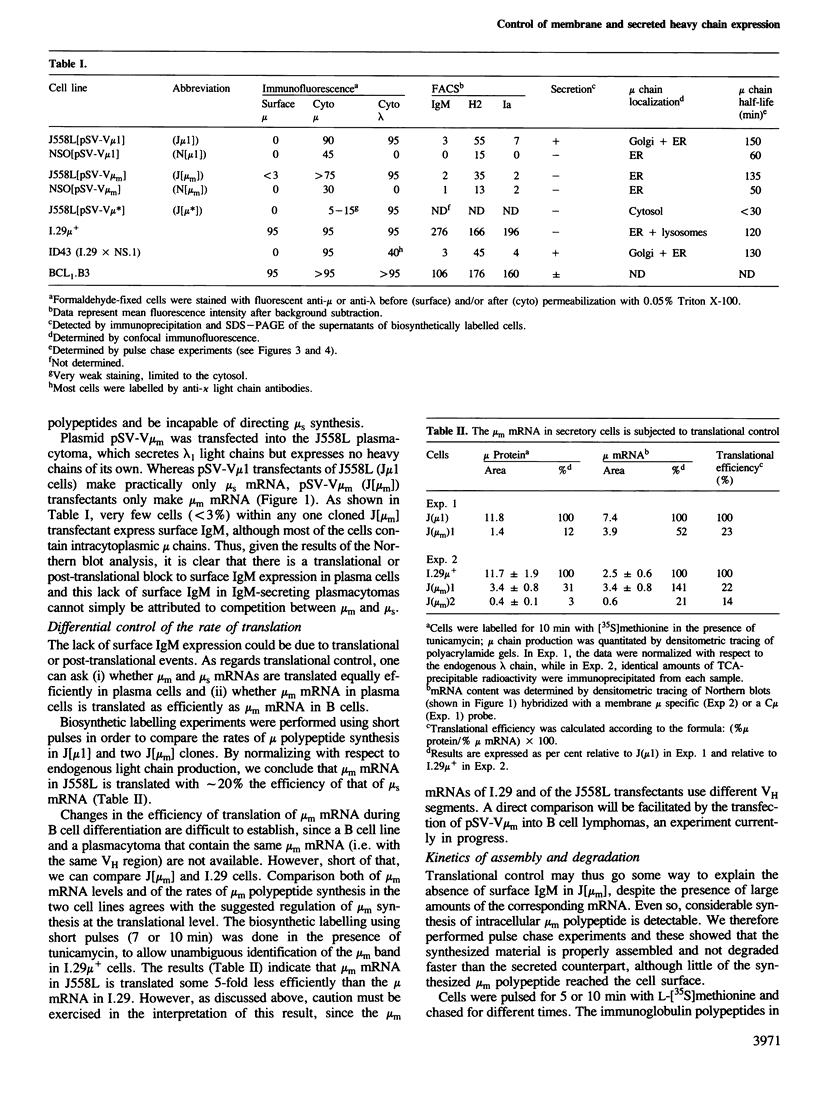
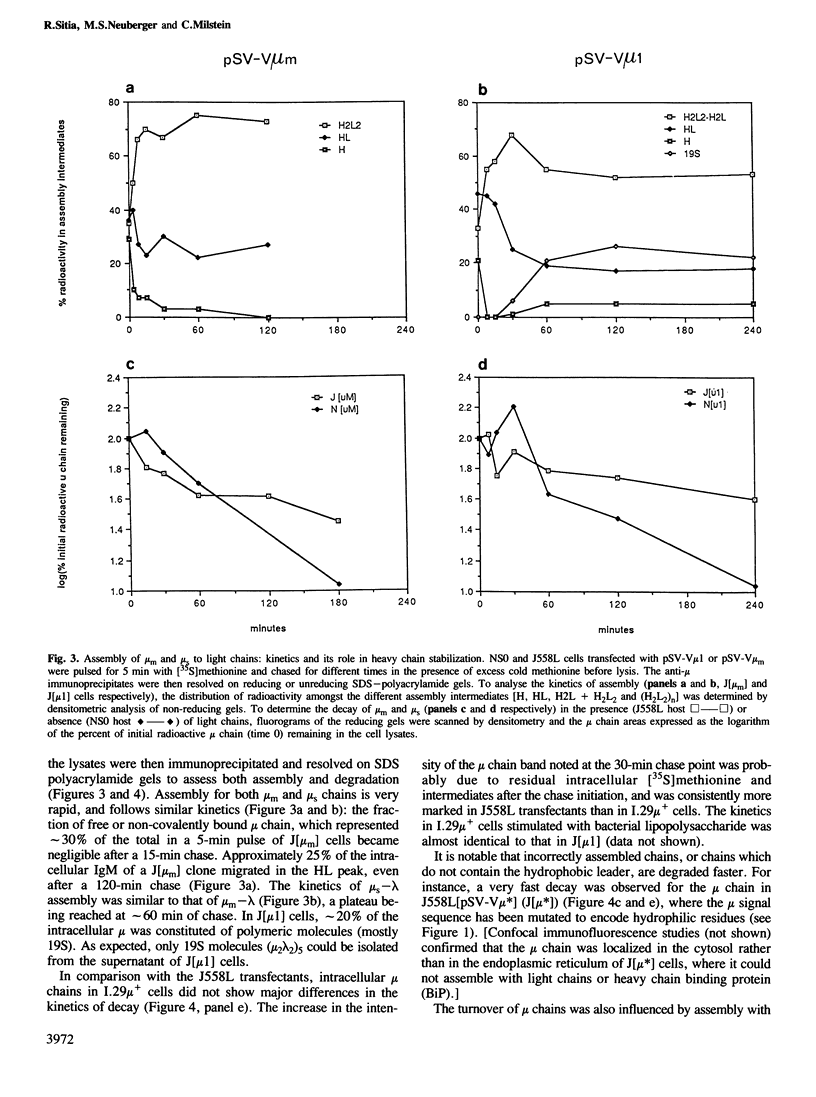
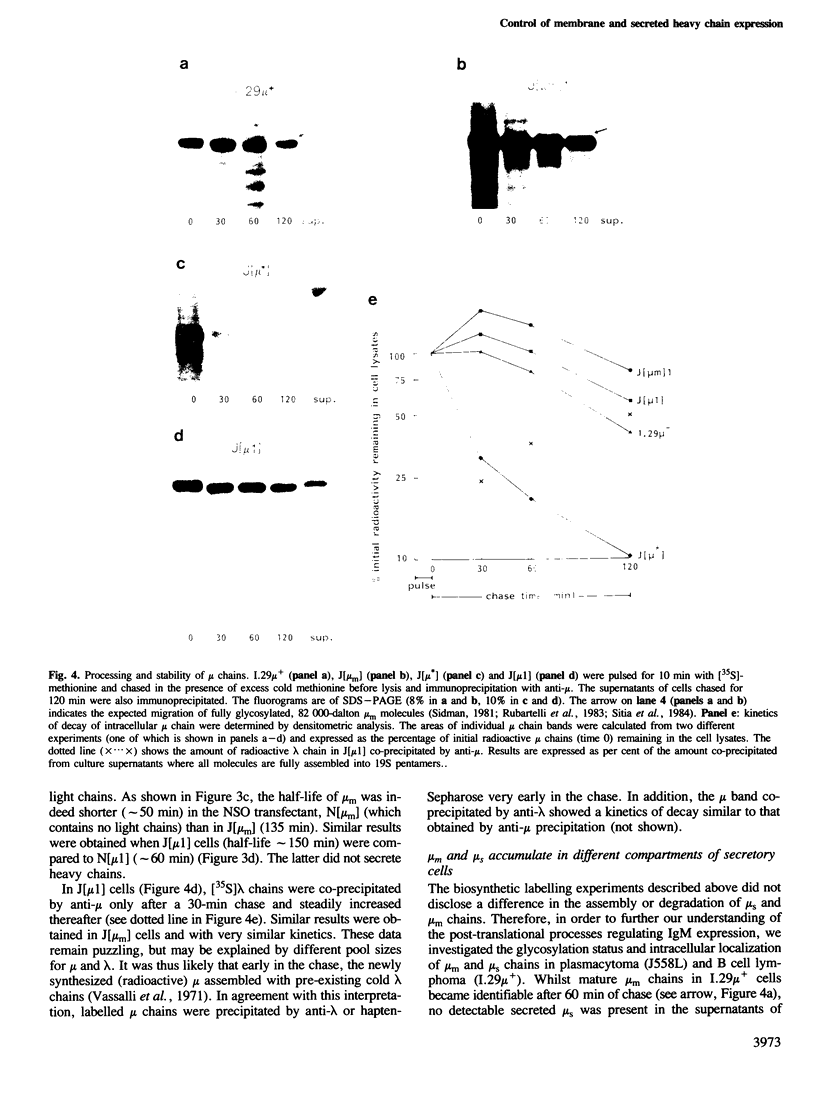
IgM secreting cells express little or no membrane IgM. This is not always due to absence of the relevant mRNA. To investigate the synthesis and processing of membrane (micron) and secreted (microseconds) polypeptides in secretory B cells, myeloma cells were transfected either with a plasmid containing an intact mu gene or with one only capable of directing micron (not microseconds) mRNA synthesis. Although myeloma transfectants could make abundant levels of micron mRNA, they did not express IgM on the cell surface. In the myeloma host, micron mRNA is translated some 5-fold less efficiently than microseconds mRNA. However, this translational control does not totally preclude micron synthesis, indicating post-translational regulatory events. No difference between micron and microseconds chains could be detected in their rate of assembly with light chains or in their stability, although both types of heavy chain were degraded more rapidly when synthesized in the absence of light chain, or when the hydrophobic nature of the leader sequence was destroyed by site-directed mutagenesis. However, whereas intracellular microseconds chains in IgM-secreting plasmacytoma were found to be concentrated in the Golgi, the micron chains were mainly located in the endoplasmic reticulum. Retention in the endoplasmic reticulum is also observed for both micron and microseconds when synthesized in the absence of light chain. We propose that it is the expansion of the endoplasmic reticulum that accompanies B cell to plasma cell differentiation which is in part responsible for the down-regulation of surface IgM expression. Such a mechanism may also affect the expression of other surface proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberini C., Biassoni R., DeAmbrosis S., Vismara D., Sitia R. Differentiation in the murine B cell lymphoma I.29: individual mu + clones may be induced by lipopolysaccharide to both IgM secretion and isotype switching. Eur J Immunol. 1987 Apr;17(4):555–562. doi: 10.1002/eji.1830170419. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Bothwell A. L., Knapp M., Siden E., Mather E., Koshland M., Baltimore D. Synthesis of secreted and membrane-bound immunoglobulin mu heavy chains is directed by mRNAs that differ at their 3' ends. Cell. 1980 Jun;20(2):293–301. doi: 10.1016/0092-8674(80)90615-7. [DOI] [PubMed] [Google Scholar]
- Anderson K. C., Bates M. P., Slaughenhoupt B., Schlossman S. F., Nadler L. M. A monoclonal antibody with reactivity restricted to normal and neoplastic plasma cells. J Immunol. 1984 Jun;132(6):3172–3179. [PubMed] [Google Scholar]
- Andrew E. M., Parkhouse R. M. Immune induction of Ia antigens in activated T cells and in kidney epithelial cells in mice. Immunology. 1986 Aug;58(4):603–606. [PMC free article] [PubMed] [Google Scholar]
- Argon Y., Milstein C. Intracellular processing of membrane and secreted immunoglobulin delta-chains. J Immunol. 1984 Sep;133(3):1627–1634. [PubMed] [Google Scholar]
- Ballinger D. G., Pardue M. L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983 May;33(1):103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A., Neuberger M. S. Polymeric immunoglobulin M is secreted by transfectants of non-lymphoid cells in the absence of immunoglobulin J chain. EMBO J. 1987 Sep;6(9):2753–2758. doi: 10.1002/j.1460-2075.1987.tb02569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. W., Word C. J., Jones S., Uhr J. W., Tucker P. W., Vitetta E. S. Double isotype production by a neoplastic B cell line. I. Cellular and biochemical characterization of a variant of BCL1 that expresses and secretes both IgM and IgG1. J Exp Med. 1986 Aug 1;164(2):548–561. doi: 10.1084/jem.164.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Espada F., Milstein C., Secher D. The regulation of membrane-bound and secreted immunoglobulins in the human lymphoid cell line LICR-LON and human hybridomas. Mol Immunol. 1987 Jun;24(6):595–603. doi: 10.1016/0161-5890(87)90040-x. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Three types of muscle-specific gene expression in fusion-blocked rat skeletal muscle cells: translational control in EGTA-treated cells. Cell. 1987 May 22;49(4):515–526. doi: 10.1016/0092-8674(87)90454-5. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Ben-Ze'av A., Benecke B. J., Penman S. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell. 1978 Oct;15(2):627–637. doi: 10.1016/0092-8674(78)90031-4. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Haas I. G., Wabl M. Immunoglobulin heavy chain binding protein. Nature. 1983 Nov 24;306(5941):387–389. doi: 10.1038/306387a0. [DOI] [PubMed] [Google Scholar]
- Hendershot L., Bole D., Köhler G., Kearney J. F. Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J Cell Biol. 1987 Mar;104(3):761–767. doi: 10.1083/jcb.104.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. F., Auffray C., Korman A. J., Shackelford D. A., Strominger J. The class II molecules of the human and murine major histocompatibility complex. Cell. 1984 Jan;36(1):1–13. doi: 10.1016/0092-8674(84)90068-0. [DOI] [PubMed] [Google Scholar]
- Klein J., Figueroa F., David C. S. H-2 haplotypes, genes and antigens: second listing. II. The H-2 complex. Immunogenetics. 1983;17(6):553–596. doi: 10.1007/BF00366126. [DOI] [PubMed] [Google Scholar]
- Koch G., Smith M., Macer D., Webster P., Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J Cell Sci. 1986 Dec;86:217–232. doi: 10.1242/jcs.86.1.217. [DOI] [PubMed] [Google Scholar]
- Krüger C., Benecke B. J. In vitro translation of Drosophila heat-shock and non--heat-shock mRNAs in heterologous and homologous cell-free systems. Cell. 1981 Feb;23(2):595–603. doi: 10.1016/0092-8674(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamson G., Koshland M. E. Changes in J chain and mu chain RNA expression as a function of B cell differentiation. J Exp Med. 1984 Sep 1;160(3):877–892. doi: 10.1084/jem.160.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Neuberger M. S. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2(8):1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Rajewsky K. Switch from hapten-specific immunoglobulin M to immunoglobulin D secretion in a hybrid mouse cell line. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1138–1142. doi: 10.1073/pnas.78.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S., Williams G. T., Fox R. O. Recombinant antibodies possessing novel effector functions. Nature. 1984 Dec 13;312(5995):604–608. doi: 10.1038/312604a0. [DOI] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M. Assembly and secretion of immunoglobulin M (IgM) by plasma cells and lymphocytes. Transplant Rev. 1973;14:131–144. doi: 10.1111/j.1600-065x.1973.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Rubartelli A., Sitia R., Zicca A., Grossi C. E., Ferrarini M. Differentiation of chronic lymphocytic leukemia cells: correlation between the synthesis and secretion of immunoglobulins and the ultrastructure of the malignant cells. Blood. 1983 Aug;62(2):495–504. [PubMed] [Google Scholar]
- Sidman C. B lymphocyte differentiation and the control of IgM mu chain expression. Cell. 1981 Feb;23(2):379–389. doi: 10.1016/0092-8674(81)90133-1. [DOI] [PubMed] [Google Scholar]
- Sitia R. Biosynthesis of membrane and secreted epsilon-chains during lipopolysaccharide-induced differentiation of an IgE+ murine B-lymphoma. Mol Immunol. 1985 Nov;22(11):1289–1296. doi: 10.1016/0161-5890(85)90048-3. [DOI] [PubMed] [Google Scholar]
- Sitia R., Rubartelli A., Hämmerling U. The role of glycosylation in secretion and membrane expression of immunoglobulins M and A. Mol Immunol. 1984 Aug;21(8):709–719. doi: 10.1016/0161-5890(84)90023-3. [DOI] [PubMed] [Google Scholar]
- Sitia R., Rubartelli A., Kikutani H., Hammerling U., Stavnezer J. The regulation of membrane-bound and secreted alpha-chain biosynthesis during the differentiation of the B cell lymphoma I.29. J Immunol. 1985 Oct;135(4):2859–2864. [PubMed] [Google Scholar]
- Stavnezer J., Sirlin S., Abbott J. Induction of immunoglobulin isotype switching in cultured I.29 B lymphoma cells. Characterization of the accompanying rearrangements of heavy chain genes. J Exp Med. 1985 Mar 1;161(3):577–601. doi: 10.1084/jem.161.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada N., Hämmerling U. Secretion of either of a pair of immunoglobulins, IgM or IgX, in somatic hybrid cells derived by fusion of a B-cell lymphoma cell line carrying both immunoglobulin isotypes. Immunogenetics. 1980 Jul;11(1):7–19. doi: 10.1007/BF01567765. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Trimble R. B., Maley F. endo-beta-N-Acetylglucosaminidase from Streptomyces plicatus. Methods Enzymol. 1978;50:574–580. doi: 10.1016/0076-6879(78)50065-7. [DOI] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P. Plasma cell immunoglobulin M molecules. Their biosynthesis, assembly, and intracellular transport. J Cell Biol. 1979 Nov;83(2 Pt 1):284–299. doi: 10.1083/jcb.83.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M., Song J., Wong J. R., Weiss M. J., Chen L. B. Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell. 1984 Aug;38(1):101–108. doi: 10.1016/0092-8674(84)90530-0. [DOI] [PubMed] [Google Scholar]
- Vassalli P., Lisowska-Bernstein B., Lamm M. E. Cell-free synthesis of rat immunoglobulin. 3. Analysis of the cell-free made chains and of their mode of assembly. J Mol Biol. 1971 Feb 28;56(1):1–19. doi: 10.1016/0022-2836(71)90080-5. [DOI] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Effect of lipopolysaccharide stimulation on the transcription and translation of messenger RNA for cell surface immunoglobulin M. J Exp Med. 1982 Oct 1;156(4):962–974. doi: 10.1084/jem.156.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Regulation of IgM and IgD synthesis in B lymphocytes. I. Changes in biosynthesis of mRNA for mu- and delta-chains. J Immunol. 1984 Mar;132(3):1561–1565. [PubMed] [Google Scholar]
- Ziegler A., Hengartner H. Sodium dodecyl sulfate electrophoresis of high molecular weight proteins in N, N'-diallyltartardiamide-cross-linked polyacrylamide slab gels. Eur J Immunol. 1977 Oct;7(10):690–690. doi: 10.1002/eji.1830071007. [DOI] [PubMed] [Google Scholar]