Abstract
The gene encoding the aquaporin-2 water channel is regulated transcriptionally in response to vasopressin. In the renal collecting duct, vasopressin stimulates the nuclear translocation and phosphorylation (at Ser552) of β-catenin, a multifunctional protein that acts as a transcriptional coregulator in the nucleus. The purpose of this study was to identify β-catenin-interacting proteins that might be involved in transcriptional regulation in rat inner medullary collecting duct (IMCD) cells, using experimental and computational approaches. We used a standard chromatin immunoprecipitation procedure coupled to mass spectrometry (ChIP-MS) in a nuclear fraction isolated from rat IMCD suspensions. Over four biological replicates, we reproducibly identified 43 β-catenin-binding proteins, including several known β-catenin-binding partners as well as novel interacting proteins. Multiple proteins involved in transcriptional regulation were identified (Taf1, Jup, Tdrd3, Cdh1, Cenpj, and several histones). Many of the identified β-catenin-binding partners were found in prior studies to translocate to the nucleus in response to vasopressin. There was only one DNA-binding transcription factor (TF), specifically Taf1, part of the RNA-polymerase II preinitiation complex. To identify sequence-specific TFs that might interact with β-catenin, Bayes’ theorem was used to integrate data from several information sources. The analysis identified several TFs with potential binding sites in the Aqp2 gene promoter that could interact with β-catenin in the regulation of Aqp2 gene transcription, specifically Jun, Junb, Jund, Atf1, Atf2, Mef2d, Usf1, Max, Pou2f1, and Rxra. The findings provide information necessary for modeling the transcriptional response to vasopressin.
Keywords: aquaporin-2, transcription, transcriptional coregulator, kidney
the peptide hormone vasopressin regulates solute and water transport across the epithelium of the renal collecting duct. It controls water transport, in part through regulation of the water channel aquaporin-2 (AQP2) in at least two ways (33, 52): 1) vasopressin stimulates membrane trafficking events that redistribute AQP2 from the endosomal compartment to the apical plasma membrane of collecting duct cells; and 2) vasopressin increases AQP2 protein abundance, in part via regulation of Aqp2 gene expression (60). This paper addresses the latter mechanism.
The multifunctional protein β-catenin can function in the nucleus as a transcriptional coregulator that binds to and modulates the actions of DNA-binding transcription factors (6). It also has roles in the cytoplasm as part of the adherens junctions and as an element of the Wnt signaling complex (72). Recent studies in collecting duct cells have shown that vasopressin markedly increases the phosphorylation of β-catenin at Ser552, a basophilic site that is a putative target of protein kinase A (3, 5, 58, 62), which differs from the sites phosphorylated by the protein kinase GSK3β in Wnt signaling (77). Furthermore, in mpkCCD cells, vasopressin-induced phosphorylation of β-catenin at Ser552 was associated with translocation of β-catenin into the nucleus (62). In addition, a siRNA-mediated knockdown of β-catenin in mpkCCD cells markedly impaired the ability of vasopressin to increase AQP2 protein abundance, consistent with a role in the regulation of Aqp2 gene transcription (32). Also, it has been demonstrated that the soluble prorenin receptor can induce or enhance antidiuresis through β-catenin-dependent effects on Aqp2 gene expression (44). Thus, β-catenin is a prime candidate for a role in vasopressin-mediated transcriptional regulation.
One way to investigate β-catenin-mediated transcriptional regulation in the collecting duct is to identify proteins that bind to it in nuclear fractions in the absence and/or presence of vasopressin. To accomplish this in the rat inner medullary collecting duct (IMCD) in the present study, we carried out chromatin immunoprecipitation (ChIP) of β-catenin followed by identification of coprecipitated proteins by use of protein mass spectrometry (ChIP-MS). We reproducibly identified 43 β-catenin-binding proteins, which included a number of known β-catenin-binding partners as well as novel interacting proteins. We extended the analysis computationally through use of large-scale data integration techniques to identify sequence-specific transcription factors that are known to interact with β-catenin, that are expressed in IMCD cells, and that possess potential binding sites (conserved transcription factor-binding motifs) in the promoter of the Aqp2 gene.
METHODS
Preparation of Rat IMCD Suspensions and Nuclear Fractions
The study design was approved by the Animal Care and Use Committee of the Division of Intramural Research of the National Heart, Lung, and Blood Institute (Animal Study Protocol No. H-0110R3). In each of four experiments, 12 male Sprague-Dawley rats weighing 250–275 g were euthanized by rapid decapitation. Inner medullas from both kidneys were minced and rapidly subjected to treatment with a digestion solution (250 mM sucrose, 10 mM triethanolamine, pH 7.6) containing collagenase B (3 mg/ml) and hyaluronidase (3 mg/ml) for 75–90 min, as previously described, to produce IMCD suspensions (50). The resulting inner medullary suspension (whole IM) was subjected to three low-speed centrifugations (at 70 g, 20s) to separate the IMCD-enriched fraction in the pellet from the non-IMCD fraction in the supernatant. The IMCD-enriched pellet was resuspended in IMCD suspension fluid (in mM: 118 NaCl, 25 NaHCO3, 5 KCl, 4 Na2HPO4, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, and 5 Na acetate, 290 mosmol/kgH2O) and divided into two equal-volume samples. One was exposed to the V2 receptor-selective vasopressin analog dDAVP (1 nM, Bachem) and the other to its vehicle for 30 min at 37°C under 95% air-5% carbon dioxide.
Nuclear pellets were isolated using a commercial kit (truChIP Chromatin Shearing Reagent Kit, Covaris, no. 520154) following the manufacturer’s protocol. Briefly, cells are fixed and cross-linked with formaldehyde, plasma membranes are lysed, allowing cytoplasmic elements to be washed out, and the nuclei left behind are harvested by centrifugation. The nuclear pellets were resuspended in 1 ml of shearing buffer provided in the kit and were sheared by ultrasound to break up DNA. The quality of sheared chromatin was confirmed by DNA gel electrophoresis on 2% agarose gel (E-Gel EX gels 2% agarose, Invitrogen). Protein amounts of the sheared chromatin samples were determined by BCA assay.
Immunoprecipitation
We used a standard ChIP protocol to immunoprecipitate β-catenin, using a commercial ChIP kit (SimpleChIP Enzymatic Chromatin IP Kits; Cell Signaling, no. 9003) following the manufacturer’s protocol. We used a Ctnnb1 antibody (Cell Signaling, no. 9562, 3.4 μg IgG per 700 μg protein at 4°C overnight with gentle rotation). For IP controls, we substituted an equal amount of rabbit preimmune IgG. The immunoprecipitated material consisted of protein plus DNA and is usually used for deep sequencing of DNA. However, in the present study, it was used instead for protein mass spectrometric identification of immunoprecipitated proteins.
In-Gel Digestion
Aliquots (50 μl) of immunoprecipitated material were loaded into individual lanes of a 12-well 7.5% Criterion TGX polyacrylamide gel (Bio-Rad) for SDS-PAGE. The gel was washed and stained with Coomassie blue dye for 10 min. Each lane was sliced into 2 mm × 9 mm rectangles above the nominal molecular mass of β-catenin (above 80 kDa) by using GridCutter (Gel Company). Each of four experiments, then, yielded 32 separate samples for LC-MS/MS analysis (8 minced gel-slice samples × 2 treatments × 2 IP conditions). The gel pieces were washed in 100 µl of 25 mM NH4HCO3 and then dehydrated with 25 mM NH4HCO3-50% ACN. These samples underwent reduction with DTT, alkylation with iodoacetamide, and trypsinization using 12.5 ng/μl Trypsin Gold (Promega).
After digestion, the peptides were extracted from the gel pieces by four successive washes in 50% ACN-0.5% formic acid. The extracted peptides were desalted using ZipTips (Millipore, ZTC18S096, tip size: P10) before MS analysis.
LC-MS/MS
All samples were analyzed on a nanoflow LC system (Eksigent, Dublin, CA) coupled to a tandem mass spectrometer (Orbitrap Elite; Thermo Scientific, San Jose, CA). The sample loading onto a peptide trap cartridge (Agilent Technologies, Palo Alto, CA) occurred at a flow rate of 6 μl/min. The trapped peptides were then fractionated with a reversed-phase PicoFrit column (New Objective, Woburn, MA) using a linear gradient of 5–35% ACN in 0.1% FA. The gradient time was 45 min at a flow rate of 0.25 μl/min. Precursor mass spectra (MS1) were acquired in the Orbitrap at ×60,000 resolution, and product mass spectra (MS2) were acquired with the ion trap.
Peptide/Protein Identification
To maximize the number of peptide identifications, three algorithms were used to match spectra to peptides, specifically those coded by Mascot, SEQUEST, and InsPecT. The posttranslational modifications allowed were a fixed carbamidomethyl modification on cysteine, variable deamination modifications on asparagine and glutamine, and a variable oxidation modification on methionine. False discovery rate (FDR) at a peptide level was set to 0.01 based on target-decoy analysis (13). To identify ambiguous identifications, we used an in-house program (coded in Java) called ProMatch (http://esbl.nhlbi.nih.gov/Bioinformatic%20Tools.htm). The SEQUEST and Mascot searches were executed within Proteome Discoverer and peptides with FDR < 0.01 and peptide rank = 1 were retained for further analyses. The InsPecT search was carried out on the Biowulf Linux Cluster at the National Institutes of Health (https://hpc.nih.gov/). For InsPecT, the P value cut-off for the peptide identification was set at 0.01 (68). Peptides that only matched to one gene symbol were extracted as a “unique” identification. The peptides that matched to more than one gene symbol were separated as “multiple” identifications. To reconcile the multiple identifications, transcriptomic data from Affymetrix array profiling of rat renal IMCD transcripts was used (database at: https://esbl.nhlbi.nih.gov/IMCD-transcriptome/). Peptides that matched to more than one gene symbol were assigned to the gene symbol with the most abundant transcript in the IMCD on the basis of prior data (55).
Raw files, search results, and all spectra have been uploaded to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD005792. These data are accessible at http://www.ebi.ac.uk/pride/archive/.
Spectral Counting
The number of peptide matches for a given gene symbol were counted and designated as the “spectral count” for that gene symbol. This was the sum of all spectra matching to the gene symbol in all gel slices. The spectral counts were normalized by the total number of spectral counts mapped to all proteins. Relative abundance of a given protein was estimated by dividing the normalized spectral counts by the number of amino acids in the mapped protein. Normalized spectral counts from specific-antibody IP vs. preimmune IgG IP were expressed as a ratio of the two values. Vehicle samples and the dDAVP samples were compared using the Fisher exact test with the contingency table consisting of the control count, dDAVP count, total count of all peptides in control, and total count of all peptides in dDAVP.
Semiquantitative Immunoblotting
Sample proteins were resolved by SDS-PAGE on 7.5% Criterion TGX polyacrylamide gel (Bio-Rad) and transferred electrophoretically onto nitrocellulose membranes. Membranes were blocked for 1 h with Odyssey blocking buffer (Li-Cor), and probed overnight at 4°C with primary antibody against β-catenin (from Cell Signaling) at 1:1,000 dilution in Odyssey blocking buffer containing 0.1% Tween 20. After 1 h of incubation with secondary antibody (Li-Cor no. 926-68071, IRDye680RD Goat anti-Rabbit IgG) at 1:5,000 dilution in blocking buffer with 0.1% Tween 20, sites of antigen-antibody reaction were detected using Odyssey's infrared imager (Li-Cor).
Bioinformatics and Statistics
For analysis of the ChIP-MS data, several software tools were used. ABE (Automated Bioinformatics Extractor; Systems Biology Center, NHLBI, Bethesda, MD) was used to extract Gene Ontology terms (http://helixweb.nih.gov/ESBL/ABE/). Nuclear translocation data were mined from a publicly accessible web page: https://hpcwebapps.cit.nih.gov/ESBL/Database/QuantNucProteomics/ (temporary login: clp; temporary password: Esbl!@#$). Random selection of genes from a list was done in Microsoft Excel, using a random number generator RAN() to create an arbitrary order and by selecting those with the lowest random number values. Statistical analysis of mass spectrometry data used Minimum Bayes’ Factors in lieu of conventional P values to summarize strength of evidence (17, 18). Conventional Chi-square analysis (a web-based Chi-Square calculator http://www.socscistatistics.com/tests/chisquare/) was used to test whether a set of proteins with a given property is found more frequently in the interactome list than it would be in a list of randomly chosen proteins. Nuclear localization signal sequences (K-(R/K)-X-(R/K) in β-catenin-interacting proteins were identified using an in-house program ProMatch, which utilizes the Java Pattern Class using “Regular Expressions” (https://hpcwebapps.cit.nih.gov/ESBL/ProMatch/). Annotated domains of β-catenin-interacting proteins were identified from domain information extracted with ABE (https://hpcwebapps.cit.nih.gov/ESBL/ABE/). An interactive network of domain-protein was generated by Medusa (3.0) and Cytoscape (http://www.cytoscape.org/). Data regarding vasopressin responses in renal collecting duct cells were mined from prior publications using Biological Information Gatherer (BIG) (87) (https://big.nhlbi.nih.gov/index.jsp).
Large-Scale Data Integration using Bayes’ Theorem
We carried out a large-scale data integration analysis using Bayes’ Theorem to answer the question, “Which among the 1,375 DNA-binding transcription factors annotated in the rat genome are most likely to regulate Aqp2 gene expression in a β-catenin-dependent manner?” The method, as applied to physiological questions, was described previously in detail (40, 79). Briefly, to address the question, the method begins with a set of unbiased prior probabilities for each transcription factor (each 1/1,375). It then calculates new posterior probabilities for each transcription factor using likelihood estimates derived from experimentally determined data from relevant data sources. Posterior probabilities after integration of one data set become prior probabilities for the next step. The steps in the calculation, the data sources and the rationale for the use of the data are summarized in Table 7. A novel aspect of the analysis was the prediction of which transcription factors were most likely to bind to the promoter of the Aqp2 gene (defined as the 1,000 bp immediately upstream from the transcription start site). For transcription factor binding site (TFBS) prediction analysis, we used a web-based tool PROMO 3.0 (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) to identify binding motifs from the TRANSFAC database (http://gene-regulation.com/pub/databases.html#transfac). This identified sites by using specific rat transcription factors as exemplars. We mapped the transcription factors expressed in rat IMCD cells to these exemplars using the BLAST algorithm (criterion: E value < 0.001) (https://blast.ncbi.nlm.nih.gov/Blast.cgi) using the sequence corresponding to the DNA-binding region as inputs. As described in Table 8, the likelihood values for each transcription factor used in the Bayes’ analysis were the values from PROMO, giving the probability of finding the indicated motif in the Aqp2 promoter vs. the probability of finding the motif in random sequences of bases of the same length (values in parentheses in Fig. 6). The mapped transcription factors that are known to bind β-catenin were identified through application of STRING analysis (http://string-db.org/), BioGrid (https://thebiogrid.org/), the previously published β-catenin interactome in mouse embryonic stem cells (80), and PubMed searches.
Table 7.
Data sets used in Bayes’ analysis to address the question, “What transcription factors (TFs) are most likely among all known TFs to be involved in regulation of Aqp2 gene transcription?”
| Data Set | Rationale | Assignment of Likelihood Values: P(B|A) | URL |
|---|---|---|---|
| All transcription factors (TFs) in rat | TFs that regulate AQP2 must include one or more of the TFs on this list. | 1/1,375 of total transcription factors as initial probability of each transcription factor | http://www.bioguo.org/AnimalTFDB/ |
| Rat IMCD transcriptome | The ability of a transcription factor to regulate transcription of Aqp2 gene depends on whether or not it is expressed in IMCD cells. | Complements of minimum Bayes’ factors (Z* = signal value/noise value where intrinsic noise is 0.4) | https://esbl.nhlbi.nih.gov/IMCD-transcriptome |
| Rat IMCD proteome | Ability of a transcription factor to regulate transcription of Aqp2 gene depends on whether or not it is expressed in IMCD cells. | Complements of minimum Bayes’ factors from χ2 test using number of data sources among 20 IMCD proteome databases published previously (null hypothesis: 0/20) | https://hpcwebapps.cit.nih.gov/ESBL/Database/IMCD_Proteome_Database/ |
| Rat IMCD nuclear proteome | Ability of a transcription factor to regulate transcription of Aqp2 gene depends on whether or not it is present in nuclei of IMCD cells. | If found in both nuclear extract and nuclear pellet fractions, P = 0.95; if found one of two fractions, P = 0.8; if not found, P = 0.4 | https://helixweb.nih.gov/ESBL/Database/IMCD_Nucleus/NE_NP_Webpage_TF.html |
| Transcription factor binding sites in promoter of rat Aqp2 gene | Ability of a transcription factor to regulate transcription of Aqp2 gene depends on whether or not it binds to specific DNA sites in the promoter of rat Aqp2 gene | Complements of minimum Bayes’ factors from the nucleotide probability of random expectation (expected occurrences of the match in a random sequence of the same length as the query sequence according to the dissimilarity index) | http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3 |
See Supplemental Table S1 (online) for full details of calculation.
Table 8.
Top-ranking TFs answer the question, “What TFs are most likely among all known TFs to be involved in regulation of Aqp2 gene transcription?”
| Gene Symbol | Protein Name | TF Family | Predicted Factor | Initial Probability | IMCD Transcriptome | IMCD Proteome | IMCD Nuclear Proteome | TFBS at Rat AQP2 Promoter |
|---|---|---|---|---|---|---|---|---|
| Jund | Jun D proto-oncogene | TF_bZIP | T00031/T00164 | 0.0007 | 0.0048 | 0.0100 | 0.0106 | 0.0284 |
| Mef2d | Myocyte enhancer factor 2D | SRF | T00766 | 0.0007 | 0.0042 | 0.0087 | 0.0093 | 0.0247 |
| Crem | Camp responsive element modulator | TF_bZIP | T00164 | 0.0007 | 0.0048 | 0.0073 | 0.0092 | 0.0246 |
| Nr1h2 | Oxysterols receptor LXR-beta | THR-like | T00042/T00333 | 0.0007 | 0.0048 | 0.0073 | 0.0092 | 0.0246 |
| Fosl2 | Fos-like antigen 2 | TF_bZIP | T00031/T00124 | 0.0007 | 0.0048 | 0.0073 | 0.0078 | 0.0207 |
| Rxra | Retinoid X receptor alpha | RXR-like | T00042/T00333 | 0.0007 | 0.0048 | 0.0073 | 0.0078 | 0.0207 |
| Nr2f6 | Nuclear receptor subfamily 2, group F, member 6 | RXR-like | T00042/T00333 | 0.0007 | 0.0048 | 0.0073 | 0.0078 | 0.0207 |
| Usf2 | Upstream transcription factor 2 | bHLH | T00875/T02115 | 0.0007 | 0.0048 | 0.0073 | 0.0078 | 0.0207 |
| Foxp1 | Forkhead box P1 | Fork head | T00371/T01049 | 0.0007 | 0.0033 | 0.0051 | 0.0065 | 0.0172 |
| Hoxb8 | Homeo box B8 | Homeobox | T03458 | 0.0007 | 0.0031 | 0.0048 | 0.0060 | 0.0160 |
| Esrra | Estrogen related receptor, alpha | ESR-like | T00042/T00333 | 0.0007 | 0.0030 | 0.0046 | 0.0058 | 0.0154 |
| Atf3 | Activating transcription factor 3 | TF_bZIP | T00031/T00124 | 0.0007 | 0.0048 | 0.0045 | 0.0057 | 0.0152 |
| Ddit3 | DNA-damage inducible transcript 3 | TF_bZIP | T00108/T00459/T00109 | 0.0007 | 0.0048 | 0.0045 | 0.0057 | 0.0152 |
| Nr3c2 | Mineralocorticoid receptor | ESR-like | T00042/T00333 | 0.0007 | 0.0048 | 0.0045 | 0.0057 | 0.0152 |
| Usf1 | Upstream transcription factor 1 | bHLH | T00875/T02115 | 0.0007 | 0.0047 | 0.0044 | 0.0056 | 0.0148 |
| Pou2f1 | POU class 2 homeobox 1 | Pou | T03458 | 0.0007 | 0.0031 | 0.0048 | 0.0051 | 0.0135 |
| Max | MYC associated factor X | bHLH | USF-1/T02115 | 0.0007 | 0.0042 | 0.0040 | 0.0050 | 0.0134 |
| Jun | Jun proto-oncogene | TF_bZIP | T00031/T00164 | 0.0007 | 0.0048 | 0.0090 | 0.0048 | 0.0128 |
| Junb | Jun B proto-oncogene | TF_bZIP | T00031/T00164/T00124 | 0.0007 | 0.0048 | 0.0045 | 0.0048 | 0.0128 |
| Pou3f3 | POU class 3 homeobox 3 | Pou | T03458 | 0.0007 | 0.0048 | 0.0045 | 0.0048 | 0.0128 |
| Srebf1 | Sterol regulatory element binding transcription factor 1 | bHLH | T00875/T02115 | 0.0007 | 0.0048 | 0.0045 | 0.0048 | 0.0128 |
| Mitf | Microphthalmia-associated transcription factor | bHLH | T00875/T02115 | 0.0007 | 0.0047 | 0.0044 | 0.0047 | 0.0125 |
| Arntl | Aryl hydrocarbon receptor nuclear translocator-like | bHLH | T00875/T02115 | 0.0007 | 0.0038 | 0.0036 | 0.0045 | 0.0120 |
Predicted Factor: TF considered as the most potential protein binding to specific region of the query sequence as predicted by ‘PROMO 3.0’ (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3). TFs theoretically capable of binding to these sites were identified by BLAST search against all TFs. Final values after integrating all data sets are highlighted in boldface (last column).
Fig. 6.
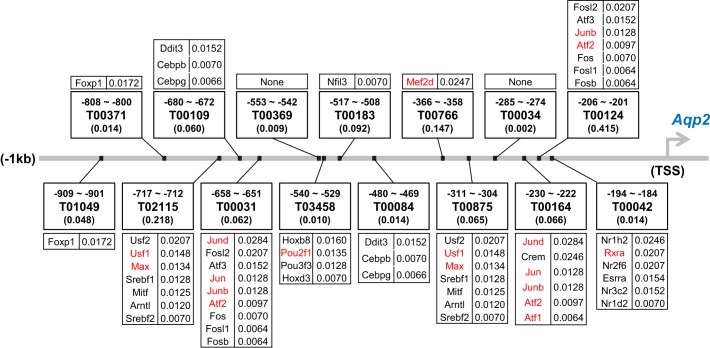
Map of potential binding sites for sequence-specific transcription factors in the promoter of the Aqp2 gene. Designators (Txxxx format) refer to motifs found by PROMO (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) corresponding to TRANSFAC motifs. Values in parentheses refer to the probability of finding the indicated motif in a randomly ordered sequence of the same length and base composition as in the Aqp2 promoter. Assigned likelihood values in Bayes’ analysis are given at the right of gene symbols for specific transcription factors. Transcription factors shown in red are known to bind β-catenin.
RESULTS
To provide information needed for modeling of transcriptional regulation of the Aqp2 gene in the renal collecting duct, we used two approaches: 1) ChIP-MS analysis to identify proteins that complex with β-catenin in the nucleus of native rat renal collecting duct cells, and 2) large-scale data integration using Bayes’ theorem to identify sequence-specific transcription factors likely to bind β-catenin in the promoter of the Aqp2 gene.
ChIP-MS Analysis
We used an antibody to β-catenin and a standard ChIP protocol to isolate β-catenin and its interacting proteins in nuclear fractions of native rat IMCD cells. We combined this with protein mass spectrometry (LC-MS/MS) to identify and quantify the bound proteins. Figure 1 shows the characterization of the antibody with regard to its ability to recognize β-catenin in Western blotting and IP. Western blotting of the “Input” to the IP procedure identified a unitary band of molecular mass of ~90 kDa, which is similar to the nominal molecular mass of β-catenin, namely 85.4 kDa. The middle two lanes of Fig. 1 show that β-catenin was successfully immunoprecipitated with the β-catenin-specific antibody but not with preimmune IgG.
Fig. 1.
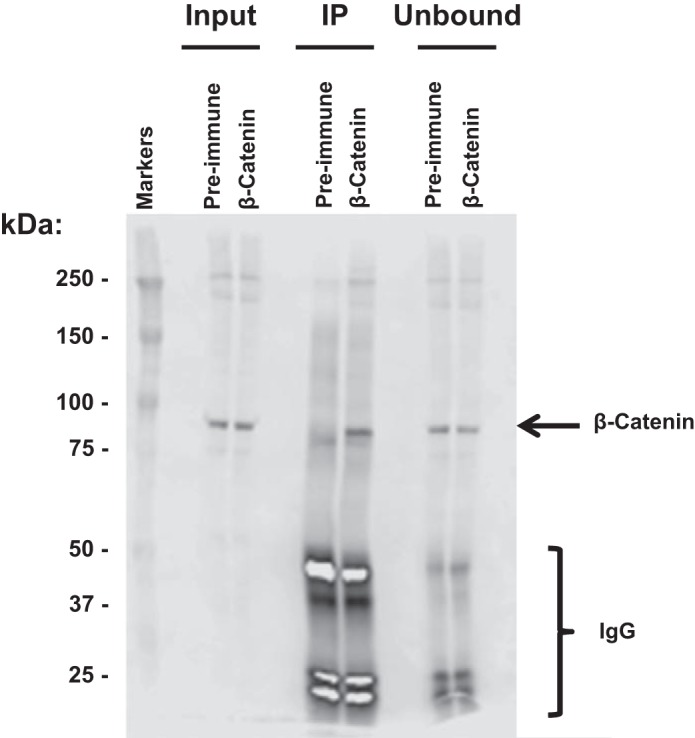
The β-catenin antibody recognizes β-catenin in immunoprecipitation (IP) samples from nuclear fraction of rat inner medullary collecting duct (IMCD) cells. “Input” is pre-IP sample; “IP” is bound material eluted from protein G beads after IP using β-catenin antibody or preimmune IgG as control. An equal amount of IgG was used for preimmune and β-catenin IPs. “Unbound” is material that did not attach to the protein G beads. Loading was on an equal-volume basis (15 µl per lane).
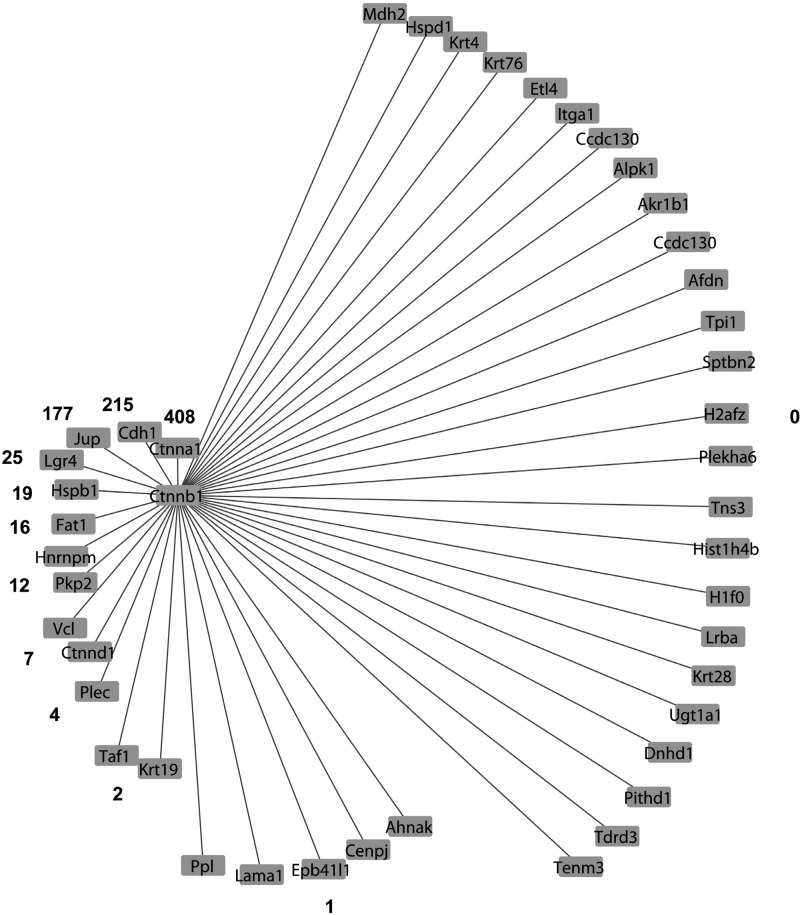
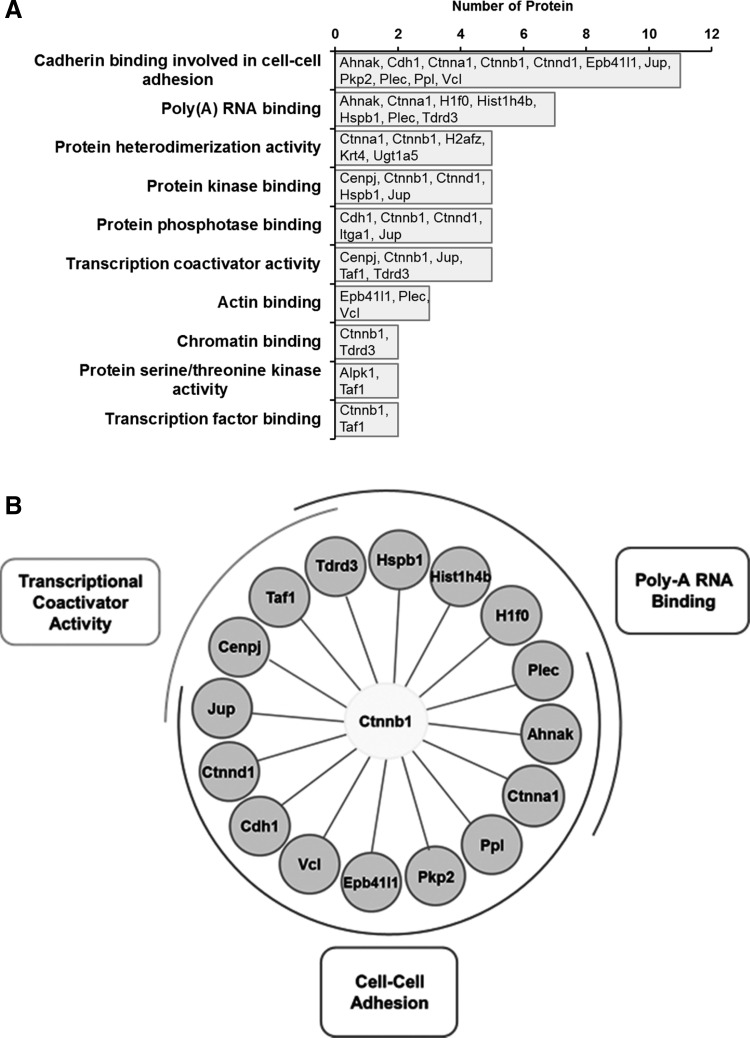
We then used the β-catenin antibody to carry out eight IP experiments consisting of four pairs of samples (vasopressin-treated vs. vehicle-treated native IMCD cells). We first addressed the question, “Among all of the samples, what proteins show significant enrichment in the β-catenin immunoprecipitation compared with the preimmune-IgG immunoprecipitation?” To do this, we used protein mass spectrometry to identify and quantify tryptic peptides from the immunoprecipitated proteins. Figure 2 shows a Venn diagram comparing the numbers of unique peptides that were identified by three search algorithms that are based on different principles, specifically Mascot, SEQUEST, and InsPecT. This figure shows that all three algorithms made unique identifications and thus contributed significantly to the data set of β-catenin-interacting proteins. The quantification of the abundance of a given protein was done by spectral counting, which was normalized by the total number of spectral counts for all proteins obtained for the sample. Table 1 summarizes the results, listing the 43 proteins identified as β-catenin-interacting proteins in all four experimental pairs. Among these, 13 were annotated as adherens junction proteins. Thirty-six were previously found in the nuclei of native rat IMCD cells by Pickering et al. (55). Five (Jup, Ppl, Ahnak, Sptbn2, and Ctnnb1) were previously found to translocate into the nucleus in response to vasopressin (62). Twenty-three were found to possess classical nuclear localization signals [motif: K(K/R)x(K/R)] (Table 2). Among the 43 β-catenin-interacting proteins, there was one transcription factor protein, Taf1, which is the largest component of transcription factor TFIID. The TFIID complex is composed of TATA-binding protein (Tbp) and a variety of Tbp-associated factors (78) and is a component of the RNA-polymerase II preinitiation complex. We also found a number of proteins known to be involved in transcriptional regulation (based on Biological Process Gene Ontology terms) including a few histones, Jup, Tdrd3, Lgr4, Cdh1, and Cenpj. The functional annotations (from UniProt) of these five proteins and Taf1 are shown in Table 3. In addition, several of the proteins in Table 1 are intermediate filament proteins, specifically Krt4, Krt19, Krt28, and Krt76, or intermediate filament binding proteins (Plec, Pkp2, Jup). Also there are proteins involved in the organization of the actin cytoskeleton (Vcl, Epb41l1, Plec, Ctnna1, Cdh1, Ahnak, Jup, H1f0, Hspb1, and Fat1) as well as several β-catenin-like proteins (Pkp2, Jup, Ctnna1, and Ctnnd1). β-Catenin-like proteins may be expected, due to the ability of β-catenin to heterodimerize with other catenins (57). In addition, we found β-catenin binding to its classical interactor, Cdh1 (E-cadherin), as well as another cadherin, Fat1. Interestingly, the analysis did not identify either of two other cadherins that are abundant in IMCD cells, specifically Cdh16 (“kidney cadherin”) and Cdh23 (39). Figure 3 shows a general picture of the β-catenin-interacting protein list from this study, indicating the relative number of publications that link β-catenin with each member. The figure includes proteins previously known to interact with β-catenin as well as novel interactors with no reported publications. Figure 4 summarizes the major Molecular Function Gene Ontology terms that mapped to the β-catenin interactome, specifically those related to transcriptional coactivator activity, cell-cell adhesion, and polyA RNA binding. The first two functional terms are consistent with generally recognized roles for β-catenin in all cells and support the validity of the methodology.
Fig. 2.
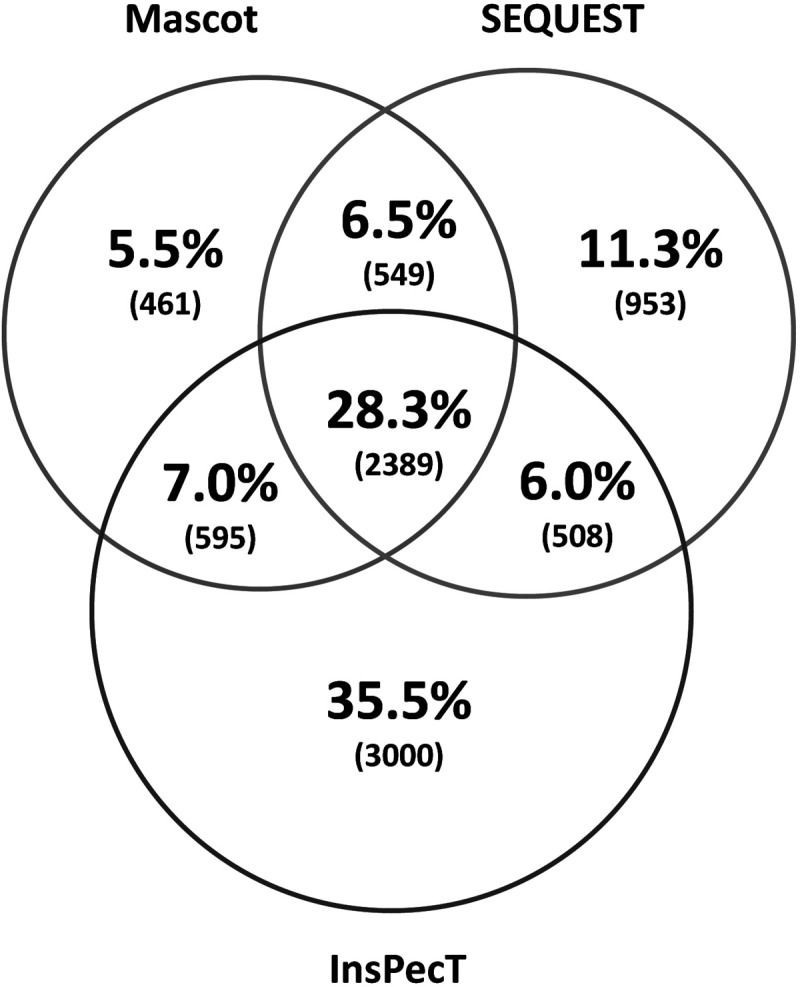
Nominal distribution of unique peptides found from each search algorithm is displayed as a Venn diagram. Mascot and SEQUEST searches were run using Proteome Discoverer 1.4 on desktop computers. The InsPecT search was carried on the Biowulf Linux Cluster at the National Institutes of Health (https://hpc.nih.gov). All three search programs added unique peptide identifications.
Table 1.
Proteins that bind β-catenin in nuclear fractions of rat IMCD cells
| Gene Symbol | Annotation | Mean Relative Abundance | Median Ab/Preimmune Ratio | P (χ2) | Adherens Junction? | Nucleus? | Nuclear Translocation in Response to Vasopressin? |
|---|---|---|---|---|---|---|---|
| Ctnnb1 | Catenin beta-1 | 508 | ∞ | 0.0001 | Yes | Yes | Yes |
| Ctnna1 | Catenin alpha-1 | 337 | ∞ | 0.0001 | Yes | Yes | No |
| Ctnnd1 | Catenin delta-1 | 101 | ∞ | 0.0001 | Yes | Yes | No |
| Cdh1 | Cadherin-1 | 87 | ∞ | 0.0001 | Yes | Yes | No |
| Lama1 | Laminin subunit alpha-1 | 67 | ∞ | 0.0209 | No | Yes | No |
| Hspd1 | 60 kda heat shock protein, mitochondrial | 51 | ∞ | 0.0004 | No | Yes | No |
| Tenm3 | Teneurin-3 | 40 | ∞ | 0.0001 | No | No | No |
| Plekha6 | Pleckstrin homology domain-containing family A member 6 | 36 | ∞ | 0.0001 | No | Yes | No |
| Akr1b1 | Aldose reductase | 34 | ∞ | 0.0004 | No | Yes | No |
| Lrba | Lipopolysaccharide-responsive and beige-like anchor protein | 33 | ∞ | 0.0001 | No | Yes | No |
| Tpi1 | Triosephosphate isomerase | 33 | ∞ | 0.0209 | No | Yes | No |
| Sptbn2 | Spectrin beta chain, non-erythrocytic 2 | 32 | ∞ | 0.0001 | Yes | No | Yes |
| Tdrd3 | Tudor domain-containing protein 3 | 31 | ∞ | 0.0004 | No | Yes | No |
| Taf1 | Transcription initiation factor TFIID subunit 1 | 25 | ∞ | 0.0209 | No | Yes | No |
| Vcl | Vinculin | 20 | ∞ | 0.0019 | Yes | Yes | No |
| Hspb1 | Heat shock protein b-1 | 20 | ∞ | 0.0209 | No | Yes | No |
| Fat1 | Protocadherin Fat 1 | 18 | ∞ | 0.0209 | No | Yes | No |
| Epb41l1 | Band 4.1-like protein 1 | 16 | ∞ | 0.0209 | Yes | Yes | No |
| Afdn | Afadin | 16 | ∞ | 0.0019 | No | No | No |
| Ugt1a5 | UDP-glucuronosyltransferase 1–5 | 15 | ∞ | 0.0209 | No | No | No |
| Pkp2 | Plakophilin-2 | 12 | ∞ | 0.0209 | Yes | Yes | No |
| Pithd1 | PITH domain-containing protein 1 | 11 | ∞ | 0.0209 | No | Yes | No |
| Etl4 | Sickle tail protein homolog | 10 | ∞ | 0.0019 | No | Yes | No |
| Mdh2 | Malate dehydrogenase, mitochondrial | 9 | ∞ | 0.0004 | No | Yes | No |
| Dnhd1 | Dynein heavy chain domain-containing protein 1 | 6 | ∞ | 0.0209 | No | No | No |
| Hnrnpm | Heterogeneous nuclear ribonucleoprotein M | 4 | ∞ | 0.0001 | No | Yes | No |
| Ahnak | Neuroblast differentiation-associated protein AHNAK | 46 | 8.06 | 0.0004 | Yes | Yes | Yes |
| Ccdc130 | Coiled-coil domain-containing protein 130 | 109 | 3.82 | 0.0019 | No | Yes | No |
| Tns3 | Tensin-3 | 136 | 3.78 | 0.0019 | No | Yes | No |
| Lgr4 | Leucine-rich repeat-containing G-protein coupled receptor 4 | 325 | 3.06 | 0.0004 | No | Yes | No |
| Cenpj | Centromere protein J | 61 | 2.82 | 0.0019 | No | Yes | No |
| Jup | Junction plakoglobin | 206 | 2.64 | 0.0001 | Yes | Yes | Yes |
| Plec | Plectin | 30 | 2.19 | 0.0004 | Yes | Yes | No |
| Itga1 | Integrin alpha-1 | 123 | 1.87 | 0.0004 | No | No | No |
| Alpk1 | Alpha-protein kinase 1 | 92 | 1.82 | 0.0019 | No | Yes | No |
| Ppl | Periplakin | 14 | 1.82 | 0.0019 | Yes | Yes | Yes |
| Krt4 | Keratin, type II cytoskeletal 4 | 16 | 1.80 | 0.0209 | No | No | No |
| Krt76 | Keratin, type II cytoskeletal 2 | 75 | 1.76 | 0.0019 | No | Yes | No |
| Hist1h4b | Histone H4 | 408 | 1.70 | 0.0019 | No | Yes | No |
| H1f0 | Histone H1.0 | 313 | 1.69 | 0.0019 | No | Yes | No |
| H2afz | Histone H2A.Z | 294 | 1.65 | 0.0019 | No | Yes | No |
| Krt19 | Keratin, type I cytoskeletal 19 | 382 | 1.64 | 0.0001 | No | Yes | No |
| Krt28 | Keratin, type I cytoskeletal 28 | 149 | 1.61 | 0.0019 | No | Yes | No |
Mean relative abundance is the normalized spectral counts divided by the number of amino acids (in millions) averaged over all positive samples. Ab/IgG ratio is the ratio of normalized spectral counts with the β-catenin-specific antibody divided by the normalized spectral counts with the same concentration of preimmune IgG. χ2 P value is based on a comparison of the number of samples (max = 4) that had Ab/IgG ratio greater than 1 vs. the null hypothesis for vasopressin-treated or vehicle-treated cells. Presence in adherens junction was identified through extraction of Cellular Component GO terms. Presence in nucleus was identified by comparison with nuclear proteome of rat inner medullary collecting duct (IMCD) cells (55) (https://helixweb.nih.gov/ESBL/Database/IMCD_Nucleus/). Nuclear Translocation in Response to Vasopressin is based on data from Schenk et al. (62), with P < 0.05 for nuclear extract fraction or nuclear pellet fraction. (https://hpcwebapps.cit.nih.gov/ESBL/Database/QuantNucProteomics/).
Table 2.
IMCD nuclear β-catenin interacting proteins with nuclear localization signal sequences (K-(K/R)-X-(K/R)
| Gene symbol | Annotation | RefSeq | NLS Location(s) and Sequence(s) |
|---|---|---|---|
| Afdn | Afadin | NP_037349.1 | 163–166:KKEK;166–169:KKEK;169–172:KKKR;171–174:KREK;223–226:KRRR;353–356:KKMK |
| Ahnak | Neuroblast differentiation-associated protein AHNAK | NP_001178880.1 | 2627–2630:KKSR;4550–4553:KKPK;4593–4596:KKSK;5275–5278:KKSK;5341–5344:KKPR;5418–5421:KKSR |
| Alpk1 | Alpha-protein kinase 1 | XP_017446832.1 | 1092–1095:KKGK |
| Ccdc130 | Coiled-coil domain-containing protein 130 | NP_001032733.1 | 137–140:KKEK |
| Cenpj | Centromere protein J | NP_001100735.1 | 941–944:KKER |
| Ctnna1 | Catenin alpha-1 | NP_001007146.1 | 51–54:KRGR;375–378:KKTR;873–876:KREK |
| Ctnnd1 | Catenin delta-1 | NP_001101210.1 | 622–625:KKGK;788–791:KKLR |
| Dnhd1 | Dynein heavy chain domain-containing protein 1 | XP_008758040.1 | 547–550:KKLK |
| Epb41l1 | Band 4.1-like protein 1 isoform L | NP_067713.2 | 92–95:KKFK;1055–1058:KKPR |
| Etl4 | Sickle tail protein homolog | XP_008770135.2 | 608–611:KKLR;690–693:KRVR |
| Fat1 | Protocadherin Fat 1 precursor | NP_114007.1 | 1504–1507:KKFR |
| H1f0 | Histone H1.0 | NP_036710.1 | 14–17:KRAK;108–111:KKTK;136–139:KKPK;144–147:KKAK;155–158:KKAK;158–161:KKPK;174–177:KKAK |
| Hist1h4b | Histone H4 | NP_073177.1 | 17–20:KRHR |
| Hnrnpm | Heterogeneous nuclear ribonucleoprotein M | NP_001103381.1 | 68–71:KRYR;217–220:KKLK |
| Hspd1 | 60 kDa Heat shock protein, mitochondrial | NP_071565.2 | 417–420:KKDR |
| Jup | Junction plakoglobin | NP_112309.2 | 83–86:KRVR |
| Plec | Plectin | NP_001157780.1 | 667–670:KKIK;783–786:KRAK;1130–1133:KKLR;1298–1301:KKPK;1811–1814:KRQR;3859–3862:KRCR;4122–4125:KRER |
| Ppl | Periplakin | NP_001100446.1 | 7–10:KKNK;822–825:KRAR |
| Sptbn2 | Spectrin beta chain, non-erythrocytic 2 | NP_062040.2 | 1258–1261:KRHR;2086–2089:KRKR;2385–2388:KKNK |
| Taf1 | Transcription initiation factor TFIID subunit 1 | NP_001178652.1 | 167–170:KKER;282–285:KRKK;285–288:KKHR;404–407:KKLR;557–560:KKSR;666–669:KKAK;865–868:KRLK;1270–1273:KKPK;1273–1276:KKMK;1374–1377:KKKR;1375–1378:KKRR |
| Tdrd3 | Tudor domain-containing protein 3 | NP_001012043.1 | 439–442:KRGK |
| Tns3 | Tensin-3 | NP_001163930.1 | 247–250:KKFR |
| Vcl | Vinculin | NP_001100718.1 | 975–978:KRIR |
Protein sequences were analyzed using ProMatch (see methods).
Table 3.
UniProt annotations of proteins involved in transcriptional regulation
| Gene Symbol | UniProt Functional Annotation |
|---|---|
| Jup | Common junctional plaque protein. Membrane-associated plaques are architectural elements in an important strategic position to influence the arrangement and function of both the cytoskeleton and the cells within the tissue. The presence of Jup in both the desmosomes and in the intermediate junctions suggests that it plays a central role in the structure and function of submembranous plaques. Acts as a substrate for VE-PTP and is required by it to stimulate VE-cadherin function in endothelial cells. Can replace β-catenin in E-cadherin/catenin adhesion complexes, which are proposed to couple cadherins to the actin cytoskeleton. [Translocates to nucleus in response to vasopressin (62). Jup can activate the Wnt signaling cascade directly without interaction of β-catenin, and has multiple functions as a transcriptional activator and a cell adhesion molecule like β-catenin (45).] |
| Tdrd3 | Scaffolding protein that specifically recognizes and binds dimethyl arginine-containing proteins. In nucleus, acts as a coactivator: recognizes and binds asymmetric dimethylation on the core histone tails associated with transcriptional activation (H3R17me2a and H4R3me2a) and recruits proteins at these arginine-methylated loci. May play a role in the assembly and/or disassembly of mRNA stress granules and in regulation of translation of target mRNAs by binding Arg/Gly-rich motifs (GAR) in dimethyl arginine-containing proteins. |
| Lgr4 | Receptor for R-spondins that potentiates the canonical Wnt signaling pathway and is involved in the formation of various organs. Upon binding to R-spondins (RSPO1, RSPO2, RSPO3, or RSPO4), associates with phosphorylated LRP6 and frizzled receptors that are activated by extracellular Wnt, triggering the canonical Wnt signaling pathway to increase expression of target genes. Its function as activator of the Wnt signaling pathway is required for the development of various organs, including liver, kidney, intestine, bone, reproductive tract, and eye. May also act as a receptor for norrin (NDP); such results, however require additional confirmation in vivo. Involved in kidney development; required for maintaining the ureteric bud in an undifferentiated state. |
| Cdh1 | Cadherins are calcium-dependent cell adhesion proteins. They preferentially interact with themselves in a homophilic manner in connecting cells; cadherins may thus contribute to the sorting of heterogeneous cell types. CDH1 is involved in mechanisms regulating cell-cell adhesions and mobility and proliferation of epithelial cells. It is a ligand for integrin α-E/β-7.1 E-Cad/CTF2 promotes nonamyloidogenic degradation of Aβ precursors. Has a strong inhibitory effect on APP C99 and C83 production. [Cleaved Cdh1 translocates to nucleus to regulate transcription (15).] |
| Cenpj | Plays an important role in cell division and centrosome function by participating in centriole duplication. Inhibits microtubule nucleation from the centrosome. |
| Taf1 | Largest component and core scaffold of the TFIID basal transcription factor complex. Contains novel NH2- and COOH-terminal Ser/Thr kinase domains, which can autophosphorylate or transphosphorylate other transcription factors. Phosphorylates TP53 on Thr55, which leads to MDM2-mediated degradation of TP53. Phosphorylates GTF2A1 and GTF2F1 on Ser residues. Possesses DNA-binding activity. Essential for progression of the G1 phase of the cell cycle. Exhibits histone acetyltransferase activity toward histones H3 and H4. |
Square brackets indicate additional material culled from the literature.
Fig. 3.
β-Catenin interactome includes members that have been previously studied relative to β-catenin and members with novel relationships to β-catenin. The value listed for each interacting protein is the number of publications found via a Pubmed search (http://www.ncbi.nlm.nih.gov/pubmed) of the gene symbol for that protein and the term “β-catenin” or “Ctnnb1”. The lengths of the edges connecting the protein to β-catenin correspond to the reciprocal of the number of publications.
Fig. 4.
Classification of β-catenin-binding proteins based on Molecular Function Gene Ontology terms. A: bar graph representation. B: 3 terms stood out, specifically transcriptional coactivator activity, polyA RNA binding, and cell-cell adhesion.
What proteins show a change in binding to β-catenin in response to vasopressin?
Next, we asked, “Among the interacting proteins found in Table 1, which show changes in binding in response to dDAVP treatment of the cells?” The dDAVP/Vehicle ratio values are shown in Table 4. Focusing only on those with a dDAVP/Vehicle ratio greater than 2 or less than 0.5, five had minimum Bayes’ factors less than 0.01 (increased: Lrba, Sptbn2, Tenm3, and Krt4; decreased: Plec) and nine had minimum Bayes’ factors less than 0.1.
Table 4.
Proteins that show a change in binding to β-catenin in response to vasopressin
| Unique Symbol | Protein Name | Average (dDAVP/Vehicle)* | MBF‡ | Odds Ratio?† |
|---|---|---|---|---|
| Lrba | Lipopolysaccharide-responsive and beige-like anchor | ∞ | 0 | ∞ |
| Sptbn2 | Spectrin beta chain, non-erythrocytic 2 | ∞ | 0 | ∞ |
| Tenm3 | Teneurin-3 | ∞ | 0 | ∞ |
| Krt4 | Keratin, type II cytoskeletal 4 | 16.4 | 3.3E-05 | 31,000 |
| Epb41l1 | Band 4.1-like protein-1 | 13.7 | 0.042 | 24 |
| Ccdc130 | Coiled-coil domain-containing protein 130 | 6.0 | 0.44 | 2.3 |
| Hnrnpm | Heterogeneous nuclear ribonucleoprotein M | 5.4 | 0.54 | 1.8 |
| Tns3 | Tensin-3 | 0.5 | 0.081 | 12 |
| Itga1 | Integrin alpha-1 | 0.4 | 0.092 | 11 |
| Tpi1 | Triosephosphate isomerase | 0.4 | 0.23 | 4.3 |
| Alpk1 | Alpha-protein kinase-1 | 0.4 | 0.14 | 7.2 |
| Plec | Plectin | 0.4 | 8.4E-15 | 1.2E+14 |
| Hspb1 | Heat shock protein beta-1 | 0.4 | 0.51 | 1.9 |
| Lama1 | Laminin subunit alpha-1 | 0.4 | 0.61 | 1.6 |
| Ugt1a5 | UDP-glucuronosyltransferase 1–5 | 0.3 | 0.51 | 1.9 |
| Mdh2 | Malate dehydrogenase, mitochondrial | 0.2 | 0.072 | 14 |
| Pkp2 | Plakophilin-2 | 0.2 | 0.51 | 1.9 |
| Cenpj | Centromere protein J | 0.2 | 0.23 | 4.3 |
dDAVP/Control Ratio is the average ratio of normalized spectral counts (≥2 or ≤0.5) over 4 dDAVP:Vehicle pairs. dDAVP:Vehicle ratios were formed from spectral counts normalized to total counts for the sample but without any correction for spectral counts obtained from the preimmune IgG sample.
Minimum Bayes’ Factor calculated as exp(−t2/2), where t is the Student’s t statistic for vasopressin vs. vehicle comparison. Boldface indicates MBF < 0.1.
Pr(difference)/Pr(no difference), where Pr is probability.
What protein domains are represented in β-catenin-binding partners?
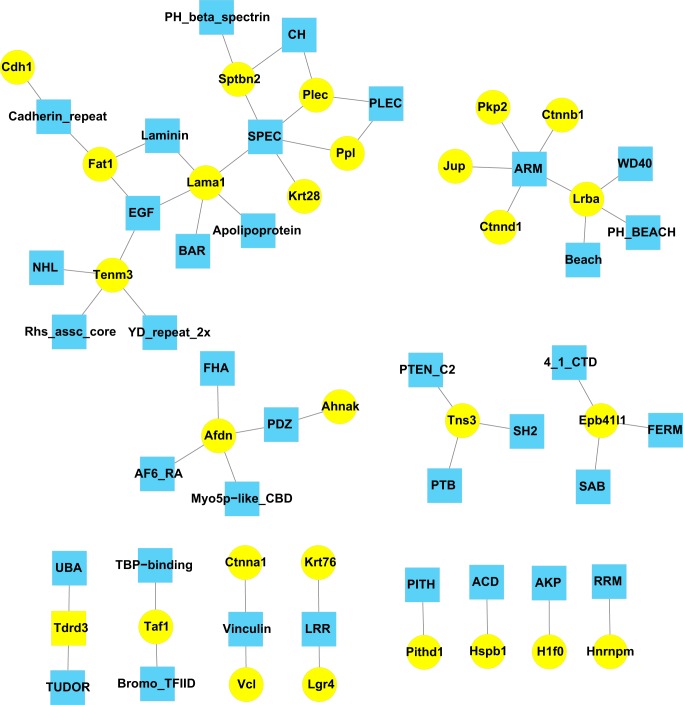
Analysis of protein domains can be informative regarding protein function. Figure 5 summarizes the protein domains represented among proteins coimmunoprecipitated with β-catenin. The most prevalent domain was the armadillo domain (ARM), originally described in the β-catenin homolog in Drosophila (49). This includes sites in β-catenin itself as well as in three other catenins (δ-catenin, plakophilin-2, and Jup) and Lrba (lipopolysaccharide-responsive and beige-like anchor protein). The latter is a member of the LYST family of proteins (9), involved in coupling signal transduction and endosomal trafficking in cells. The ARM domain may play a role in nuclear translocation (16). Another prevalent domain among β-catenin-interacting proteins is the spectrin repeat domain, present in laminin subunit α1, β-spectrin, plectin, periplakin, and the intermediate filament protein keratin type I-28. Several β-catenin-interacting proteins have multiple binding domains consistent with roles in protein scaffolding.
Fig. 5.
Domain map of β-catenin-interacting proteins identify proteins with related domain structure and scaffold proteins. Blue nodes indicate domains; yellow nodes indicate β-catenin interacting proteins. Domains were identified using the Automated Bioinformatics Extractor (ABE) (see methods).
Relationship between membership in the β-catenin interactome and vasopressin-regulated phosphorylation of nuclear proteins.
We mapped the β-catenin interactome to a list of proteins in the nucleus of cultured mpkCCD cells previously identified to undergo changes in phosphorylation in response to vasopressin (5) (Table 6). Remarkably, among the 43 proteins in the IMCD nuclear β-catenin interactome, there were increases in phosphorylation at 11 distinct phosphorylation sites in seven different interacting proteins. In contrast, when 43 proteins were randomly selected from the IMCD nuclear proteome (55), none showed increased phosphorylation in the data from Bolger et al. (5) (Chi-square, P < 0.05). Thus, it appears that there may be a functional connection between vasopressin-mediated phosphorylation in IMCD cells and being a nuclear binding partner for β-catenin.
Table 6.
Several β-catenin binding partners have been shown to undergo increases in phosphorylation in the nucleus of mouse mpkCCD cells in response to the vasopressin analog dDAVP (5)
| Nuclear Fraction | Gene Symbol | Protein Name | RefSeq | Phosphorylation Site | Log2 (dDAVP/Vehicle) Phosphorylation (means ± SE) | P Value |
|---|---|---|---|---|---|---|
| NP | Ahnak | AHNAK nucleoprotein | NP_033773 | S5522 | 0.86 ± 0.17 | 0.000070 |
| NE | Ahnak | AHNAK nucleoprotein | NP_033773 | S5522 | 0.70 ± 0.22 | 0.000785 |
| NP | Ahnak | AHNAK nucleoprotein | NP_033773 | S5525 | 0.79 ± 0.12 | 0.000357 |
| NE | Ahnak | AHNAK nucleoprotein | NP_033773 | S5525 | 0.77 ± 0.27 | 0.001428 |
| NP | Ctnnb1 | Catenin beta-1 | NP_031640 | S552 | 1.79 ± 0.11 | 0.000000 |
| NP | Ctnnb1 | Catenin beta-1 | NP_031640 | S675 | 0.28 ± 0.03 | 0.000004 |
| NP | Ctnnb1 | Catenin beta-1 | NP_031640 | T551 | 1.83 ± 0.39 | 0.000092 |
| NP | Ctnnd1 | Catenin delta-1 | NP_031641 | S862 | 2.23 ± 1.18 | 0.030759 |
| NP | Ctnnd1 | Catenin delta-1 | NP_031641 | S864 | 1.78 ± 0.57 | 0.005715 |
| NP | Jup | Junction plakoglobin | NP_034723 | S665 | 0.60 ± 0.12 | 0.000067 |
| NE | Krt19 | Keratin, type I cytoskeletal 19 | NP_032497 | S67 | 2.00 ± 0.25 | 0.000004 |
| NP | Krt19 | Keratin, type I cytoskeletal 19 | NP_032497 | S67 | 1.50 ± 0.36 | 0.000186 |
| NP | Pkp2 | Plakophilin-2 | NP_080439 | S151 | 0.44 ± 0.19 | 0.004469 |
| NE | Ppl | Periplakin | NP_032935 | S1655 | 0.66 ± 0.50 | 0.087711 |
NE, nuclear extract; NP, nuclear pellet. Data are from phosphoproteomic analysis of mpkCCD collecting duct cells published by Bolger et al. (5).
Large-Scale Data Integration Using Bayes’ Theorem
The ChIP-MS analysis did not identify any sequence-specific transcription factors of the type that bind to regulatory elements in the promoter to activate or repress gene expression. This was likely due to the low abundances of proteins in this class relative to other types of proteins in the nucleus (29, 30), presumably placing them below the detection limit for mass spectrometry in the type of experiment described in this paper. Modeling the transcription network involved in Aqp2 gene regulation, however, requires identification of the sequence-specific transcription factors most likely to interact with the Aqp2 promoter. To identify sequence-specific transcription factors that might interact with β-catenin in the promoter region of the Aqp2 gene, we followed two steps. 1) We utilized large-scale data integration techniques using Bayes’ theorem (40, 79) to identify which of the 1,375 transcription factors annotated in the rat genome were most likely to bind to the Aqp2 promoter; and 2) we looked for prior evidence of interactions between these transcription factors and β-catenin. The algorithm for the calculation is summarized in Table 7, and the 23 top-ranked transcription factors are listed in order in Table 8. The predicted binding sites in the Aqp2 promoter are shown in Fig. 6. The transcription factors labeled in red in Fig. 6 are highly ranked transcription factors that are known to bind β-catenin and include Jun, Junb, Jund, Atf1, Atf2, Mef2d, Usf1, Max, Pou2f1, and Rxra.
DISCUSSION
Regulation of Aqp2 gene expression is a central process in the regulation of water excretion by the kidney, and failures of this process result in disorders of water balance (36). The molecular machine that links the vasopressin receptor to an increase in Aqp2 gene expression is, however, largely unknown. Model building is an essential technique in the analysis of complex systems like the transcriptional regulatory network in collecting duct cells. Modeling occurs in at least two steps: 1) identification of key components of the system, and 2) identification of the causal interactions among the components to create a predictive model. This paper begins to address the first step.
We previously identified an important transcriptional coregulator, β-catenin, that is regulated by vasopressin in two ways. 1) Vasopressin binding to the V2 receptor in collecting duct cells increases the phosphorylation of β-catenin at Ser552; and 2) vasopressin stimulates the translocation of β-catenin into the nuclei of collecting duct cells. These effects are likely to be independent of the role of β-catenin in the canonical Wnt pathway, which is a key component of collecting duct development (63). Thus, we make the assumption that β-catenin may be involved in regulation of Aqp2 gene transcription and ask the question, “What proteins interact with β-catenin in nuclear fractions of collecting duct cells; and how might the interactions affect Aqp2 gene transcription?” To address these, we Semployed both an experimental and a computational approach, yielding potentially complementary information.
Nuclear Proteins That Bind β-Catenin
The experimental approach combined chromatin immunoprecipitation with modern mass spectrometry to identify 43 β-catenin-interacting proteins in the nuclei of IMCDs from rats. We used a standard approach for enrichment of nuclei from IMCDs and ChIP as provided in a commercial kit. The principle of the nuclear isolation is to use gentle detergent treatment to remove the plasma membrane, allowing liberation of cytosol and all membrane structures not tethered to the nucleus. What is left behind is generally nucleus and a good share of the endoplasmic reticulum, which includes the outer nuclear membrane. Since the outer membrane of the nucleus is part of the endoplasmic reticulum, it is likely that some of the identified β-catenin interactions occur within the endoplasmic reticulum rather than on chromatin. However, because the approach identified a number of proteins already known to interact with β-catenin in the nucleus and identified many of the proteins previously seen to translocate to the nucleus in response to vasopressin (62), it is reasonable to surmise that some of the novel β-catenin-interacting proteins also play roles in the nucleus, especially those that are known to be nuclear resident proteins. With regard to junctional proteins that were isolated, it seems possible that the identified interactions occur either in the endoplasmic reticulum or in the nucleus. The literature is replete with examples of junctional proteins that migrate into the nucleus (2, 12, 48, 70), so that the finding of interactions in nuclei cannot be regarded as unexpected.
A previous study also identified β-catenin-interacting proteins using a similar ChIP-MS protocol in mouse embryonic stem cells (80). Among the 43 β-catenin-interacting proteins found in the present study, 25 were identified in the previous study and 18 were unique to our study [These are: Tenm3, Plekha6, Lrba, Sptbn2, Tdrd3, Taf1, Epb41l1, Afdn, Ugt1a5, Pkp2, Pithd1, Etl4, Dnhd1, Ccdc130, Lgr4, Cenpj, Alpk1 and H1f0 (see Table 5 for annotations.)]. Many of the identified β-catenin-interacting proteins had been found in other studies to undergo vasopressin-mediated translocation into the nucleus (62) and/or vasopressin-mediated changes in phosphorylation (5), consistent with an important role for β-catenin in vasopressin-mediated regulation of transcription in renal collecting duct cells.
Table 5.
UniProt annotations for proteins that show a change in binding to β-catenin in response to vasopressin
| Gene Symbol | UniProt Annotation |
|---|---|
| Plec | Interlinks intermediate filaments with microtubules and microfilaments and anchors intermediate filaments to desmosomes or hemidesmosomes. Could also bind muscle proteins such as actin to membrane complexes in muscle. May be involved not only in the filaments network but also in the regulation of their dynamics. Structural component of muscle. Isoform 9 plays a major role in the maintenance of myofiber integrity. |
| Cdh1 | See Table 3. |
| Krt4 | Cytokeratin-4. |
| Lrba | May be involved in coupling signal transduction and vesicle trafficking to enable polarized secretion and/or membrane deposition of immune effector molecules. [Involved in endosomal traffic and may be involved in efficient delivery of cell surface proteins to the lysosome (11).]. |
| Sptbn2 | Probably plays an important role in membrane skeleton; interacts with Epb41l1. [Spectrin is a cytoskeletal protein that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming scaffolding and playing an important role in maintenance of plasma membrane integrity and cytoskeletal structure (27); interacts with Epb41l1]. |
| Tenm3 | Integral membrane protein involved in development. Promotes homophilic cell adhesion. May function as a cellular signal transducer. |
Square brackets indicate additional material culled from the literature.
One particularly interesting interacting protein is Taf1 (TATA-binding protein-associated factor). It is a component of the transcriptional initiation complex TFIID (TFIID subunit 1), and theoretically, regulation of transcriptional initiation could be exerted through its binding to β-catenin. Recently, Sandoval et al. (60) demonstrated that vasopressin causes an almost global increase in transcriptional initiation in mpkCCD collecting duct cells. In previous studies, Taf1 abundance in the nuclear extract fraction of mpkCCD was seen to increase by 68% in response to vasopressin (https://hpcwebapps.cit.nih.gov/ESBL/Database/QuantNucProteomics/), providing a potential explanation for the increase in transcriptional initiation. Whether this translocation event is mediated by Taf1 binding to β-catenin remains unknown. However, another member of the RNA-polymerase II preinitiation complex, Tbp, is known to bind directly to β-catenin, allowing β-catenin to play a role in transcriptional initiation (24).
β-Catenin Interacting Proteins with Indirect Roles in Transcriptional Regulation
Several of the 43 β-catenin-interacting proteins in Table 1 have been previously shown to be involved in transcriptional regulation. Aside from Taf1, several other β-catenin-binding partners identified in this study may have indirect roles in transcriptional regulation, namely Tdrd3, Jup, Cdh1, and Cenpj. We discuss these in the following.
Tdrd3 (Tudor domain-containing protein) is a transcriptional coactivator that binds to dimethyl arginine moieties on histone tails and also interacts with the asymmetrically dimethylated COOH-terminal domain of RNA Pol II (65). TDRD3 protein was found to be mainly recruited near the transcriptional start sites of many genes in a recent ChIP-Seq analysis (83) and, along with Taf1, could play a role in the global effect of vasopressin to increase transcriptional initiation (60).
Jup (junction plakoglobin), also known as γ-catenin, is a paralog of β-catenin. Considering it contains the conserved 12 armadillo repeats as β-catenin, Jup may fulfill some of the same roles, including as a transcriptional coregulator. Interestingly, as seen with Ser552 of β-catenin, vasopressin increases phosphorylation of Jup in mouse mpkCCD cells at Ser665 (5), a basophilic site (RKRVS). This sequence is similar to that seen with many protein kinase A substrates (66, 75, 82).
Cdh1 (E-cadherin) is the prototypical epithelial cadherin. It is an integral membrane protein that is the central component of the adherens junction complex, which includes β-catenin. There are two possible explanations for finding E-cadherin in the nuclear fraction. 1) Being an integral membrane protein, β-catenin is translated on the rough endoplasmic reticulum; it may therefore be present in intact form in the nuclear fraction by virtue of the fact that the endoplasmic reticulum is an extension of the outer nuclear membrane, allowing it to be isolated with the nuclei. 2) Cdh1 may be present in the nuclear fraction when cleaved proteolytically to yield a polypeptide that is not membrane bound (43) and can be translocated to the nucleus, where it can alter intranuclear signaling (15).
Cenpj (centromere protein J) plays a key role in cell division as a component of a ternary complex that mediates centriole duplication. In interphase, it has been shown to play a role as a transcriptional coregulator that binds to the transcription factors STAT5 (54) and NF-κB (37). The latter is known to be involved in regulation of Aqp2 gene transcription (21). Cenpj has previously been shown to bind to band 4.1 (Epb41) (28), a paralog of another protein identified here as a β-catenin-interacting protein, specifically band 4.1-like protein-1.
Sequence-Specific Transcription Factors Likely to Bind the Promoter of the Aqp2 Gene and β-Catenin
The computational approach employed in this study used modern large-scale data integration techniques to identify a set of 10 sequence-specific transcription factors that are likely to bind to the promoter of the Aqp2 gene and to bind β-catenin, specifically Jun, Junb, Jund, Atf1, Atf2, Mef2d, Usf1, Max, Pou2f1, and Rxra. The first five of these are b-ZIP family transcription factors that are predicted to bind to one or more of three AP-1/CREB-motifs (T00031, T00124, T00164; Fig. 6). AP-1 components have previously been proposed to be involved in regulation of Aqp2 gene expression (64, 84). Medf2d is predicted to bind to a SRF motif (T00766, Fig. 6) that has been described previously (68, 86). Usf1 and Max bind E-box elements (T00875, T02115; Fig. 6), which may be involved in global amplification of gene expression (42). The canonical heterodimerization partner of Max is Myc, which was found to have extremely low protein and mRNA abundance. Pou2f1 is a homeobox transcription factor that is predicted to bind to a well-described Hox-binding site (T03458; Fig. 6) in the Aqp2 promoter. Finally, Rxra is a ligand activated nuclear receptor predicted to bind to a conserved nuclear receptor binding site (T00042; Fig. 6).
It is worthwhile to point out a couple of interesting aspects of the general analysis of TFs that could regulate Aqp2 gene transcription that are independent of β-catenin. First, Creb1, which is often listed as a likely regulator of Aqp2 gene expression in review articles (22, 46, 76), is ranked very low relative to other b-ZIP family transcription factors, including Crem, Jun family (Jun, Junb, and Jund), Fos family (Fos, Fosb, Fosl1, and Fosl2), and Atf family (Atf1, Atf2, and Atf3). Its low ranking is primarily because Creb1 mRNA was not detected in the transcriptome data set from microarray assay of rat IMCD, in contrast to other Creb proteins (Creb3, Crebl1, and Crebl2). Second, the analysis predicts that the mineralocorticoid receptor Nr3c2 may play a role in regulation of Aqp2 gene expression. This is a ligand-activated transcription factor that is known to regulate expression of the gene coding for the α subunit of the epithelial sodium channel (ENaC) (4). Although several studies on mineralocorticoid-mediated Aqp2 gene expression have been reported (23, 31, 38, 53), addressing the possibility of the importance of the mineralocorticoid receptor relative to other transcription factors has not been established.
Previous studies have established the idea that multiple transcription factors are required to exert precise transcriptional control (19). Aqp2 is a cell type-specific gene that is expressed virtually exclusively in collecting duct principal cells and IMCD cells (52). Moreover, Aqp2 gene expression is also strongly regulated by vasopressin as a result of selective transcriptional elongation (60). Both cell type-specific expression and regulation by vasopressin are likely to occur largely as a consequence of binding of as-yet-unknown combinations of sequence-specific transcription factors to the gene promoter.
Transcription Factors That May Regulate Aqp2 Gene Transcription Independently of β-Catenin
Although the main focus of this paper is on β-catenin-interacting proteins, for completeness it is worth mentioning that several transcription factors may be involved in vasopressin-regulated Aqp2 gene transcription independently of β-catenin. These include some of the AP-1 components (64), nuclear receptors (8, 74), members of the NFAT family (20, 21, 41), GATA family (71, 85), and Ets (E26 transformation-specific) family (68, 86). See supplemental discussion, linked to this paper online, for details.
β-Catenin-Interacting Proteins and Vasopressin-Mediated Regulation of Nuclear Translocation
In a prior study, β-catenin was seen to translocate into the nuclei of mouse collecting duct mpkCCD cells in response to vasopressin in association with an increase in β-catenin phosphorylation at Ser552 (62). It is interesting that several of the identified β-catenin-interacting proteins also translocated into the nucleus in response to vasopressin as determined by quantitative mass spectrometry of nuclear fractions (62) or the 1,000-g fraction from differential centrifugation of mpkCCD cell homogenates (81). These are phakophilin-2 (Pkp2), the multifunctional protein Annak, junction plakoglobin (Jup or γ-catenin), periplakin (Ppl), β-spectrin (Sptbn2), α-catenin, δ-catenin, and E-cadherin (Table 9). β-Catenin, which lacks a classical nuclear localization signal sequence, is believed to move into the nucleus by binding directly to the nuclear pore machinery via its multiple ARM domains (14). Among the proteins that are present in the β-catenin interactome and translocate into the nucleus in response to vasopressin, Jup, Pkp2, and δ-catenin also have ARM domains (Fig. 5). It seems possible that some or all of these components move together into the nucleus bound to β-catenin. Indeed, it has been suggested that binding relationships among different types of catenins that exist at the adherens junction and desmosomes may be conserved in nuclei as part of their coordinate roles in transcriptional regulation (47). β-Catenin contains multiple ARM domains that can interact with the nuclear pore complex, facilitating its entry (despite the absence of a nuclear localization signal) (14), and this interaction may hypothetically facilitate nuclear entry of its binding partners, as has been demonstrated for the transcription factor Lef1 (1). Among the proteins previously found to translocate to the nucleus in response to vasopressin, phakophilin-2 (Pkp2), junction plakoglobin (Jup), and δ-catenin, all possess ARM domains. In mouse mpkCCD cells, vasopressin markedly increases phosphorylation of β-catenin at Ser552 (3, 5, 58, 62), a basophilic site (sequence RRTS) that is believed to be a target for protein kinase A (34). This site is located within the 10th ARM domain, i.e., within the region thought to interact with the nuclear pore complex. Thus, it is possible that vasopressin-induced nuclear translocation of β-catenin is triggered by the increase in Ser552 phosphorylation. In addition, the ARM10-12 domains of β-catenin interact with other cofactors, such as CREBBP and p300 (51), which are involved in transcriptional control through histone acetylation. CREBBP has been found to be translocated into the nucleus in response to vasopressin in cultured collecting duct cells (62), although it was not identified as a β-catenin-binding partner in the present study. β-Catenin-mediated transactivation occurs, in part, through its ability to bind CREBBP (25, 67), which acetylates histone H3 at Lys27, thereby increasing chromatin accessibility locally (69).
Table 9.
β-Catenin-interacting proteins: vasopressin responses in prior studies
| Gene Symbol | Annotation | Regulation by Vasopressin |
|---|---|---|
| Ctnna1 | Catenin alpha-1 | dDAVP at a concentration of 0.1 nM for 1 h increases abundance of Ctnna1 in nuclear pellet of mouse mpkCCD to 150% (62). dDAVP at a concentration of 1 nM for 0–15 min decreases phosphorylation of Ctnna1 protein at S643 protein in rat IMCD to 70% (26). |
| Ctnnd1 | Catenin delta-1 | Ctnnd1 protein undergoes a reciprocal abundance change in the 17,000 g membrane fraction [down] and the 1,000-g pellet fraction [up] in response to vasopressin (81). dDAVP at a concentration of 0.1 nM for 1 h increases phosphorylation of Ctnnd1 protein at S862 in nuclear pellet of mouse mpkCCD to 460% (5). dDAVP at a concentration of 0.1 nM for 1 h increases phosphorylation of Ctnnd1 protein at S864 in nuclear pellet of mouse mpkCCD to 350% (5). dDAVP at a concentration of 1 nM for 0.5 h decreases phosphorylation of Ctnnd1 protein at S899 in mouse mpkCCD to 80% (58). |
| Cdh1 | Cadherin-1 | Cdh1 protein undergoes a reciprocal abundance change in the 200,000-g cytosolic fraction [down] and the 1,000-g pellet fraction [up] in response to vasopressin (81). |
| Lama1 | Laminin subunit alpha-1 | None |
| Hspd1 | 60 kda heat shock protein, mitochondrial | None |
| Tenm3 | Teneurin-3 | None |
| Plekha6 | Pleckstrin homology domain-containing family A member 6 | dDAVP at a concentration of 1 nM for 0–15 min increases phosphorylation of Plekha6 at S970 in rat IMCD to 140% (26). |
| Akr1b1 | Aldose reductase | dDAVP at a concentration of 5 ng/h to rats for 72 h decreases abundance of Akr1b1 protein in rat IMCD to 50% (73). |
| Lrba | Lipopolysaccharide-responsive and beige-like anchor protein | dDAVP at a concentration of 5 ng/h to rats for 72 h decreases abundance of Lrba protein in rat IMCD to 70% (56). dDAVP at a concentration of 0.1 nM for 120 h decreases abundance of Lrba protein in mouse mpkCCD to 80% (35). dDAVP at a concentration of 1 nM for 0–15 min increases phosphorylation of Lrba protein at T1231 protein in rat IMCD to 140% (26). |
| Tpi1 | Triosephosphate isomerase | dDAVP at a concentration of 5 ng/h to rats for 72 h decreases abundance of Tpi1 protein in rat IMCD to 40% (56). |
| Sptbn2 | Spectrin beta chain, non-erythrocytic 2 | Sptbn2 protein undergoes a reciprocal abundance change in the 200,000-g cytosolic fraction [down] and the 1,000-g pellet fraction [up] in response to vasopressin (81). dDAVP at a concentration of 5 ng/h to rats for 72 h increases abundance of Sptbn2 protein in rat IMCD to 160% (56). |
| Tdrd3 | Tudor domain-containing protein 3 | None |
| Taf1 | Transcription initiation factor TFIID subunit 1 | None |
| Vcl | Vinculin | dDAVP at a concentration of 0.1 nM for 120 h decreases abundance of Vcl protein in mouse mpkCCD to 80% (35). |
| Hspb1 | Heat shock protein b-1 | dDAVP at a concentration of 5 ng/h to rats for 72 h increases abundance of Hspb1 protein in rat IMCD to 250% (56). dDAVP at a concentration of 1 nM for 0–15 min increases phosphorylation of Hspb1 protein at S13 protein in rat IMCD to 120% (26). dDAVP at a concentration of 1 nM for 0–15 min increases phosphorylation of Hspb1 protein at S85 protein in rat IMCD to 130% (26). |
| Fat1 | Protocadherin Fat 1 | None |
| Epb41l1 | Band 4.1-like protein 1 isoform S | None |
| Afdn | Afadin | None |
| Ugt1a5 | UDP-glucuronosyltransferase 1–5 | None |
| Pkp2 | Plakophilin-2 | dDAVP at a concentration of 0.1 nM for 1 h increases abundance of Pkp2 in nuclear pellet of mouse mpkCCD to 200% (62). Pkp2 protein undergoes a reciprocal abundance change in the 17,000-g membrane fraction [down] and the 1,000-g pellet fraction [up] in response to vasopressin (81). Pkp2 protein half-life increases from 17.5 to 29.0 h in mouse mpkCCD cells in response to vasopressin (61). |
| Pithd1 | PITH domain-containing protein 1 | None |
| Etl4 | Sickle tail protein homolog | None |
| Mdh2 | Malate dehydrogenase, mitochondrial | None |
| Dnhd1 | Dynein heavy chain domain-containing protein 1 | None |
| Hnrnpm | Heterogeneous nuclear ribonucleoprotein M | None |
| Ahnak | Neuroblast differentiation-associated protein AHNAK | Ahnak protein undergoes a reciprocal abundance change in the 200,000-g cytosolic fraction [down] and the 1,000-g pellet fraction [up] in response to vasopressin (81). dDAVP alters phosphorylation of Ahnak at multiple sites in rat IMCD (26). dDAVP at a concentration of 0.1 nM for 1 h increases abundance of Ahnak in nuclear pellet of mouse mpkCCD to 150% (62). dDAVP aalters phosphorylation of Ahnak protein at multiple sites in nuclei of mouse mpkCCD (5). |
| Ccdc130 | Coiled-coil domain-containing protein 130 | None |
| Tns3 | Tensin-3 | dDAVP at a concentration of 1 nM for 0–15 min increases phosphorylation of Tns3 protein at S864 protein in rat IMCD to 260% (26). |
| Lgr4 | Leucine-rich repeat-containing G-protein coupled receptor 4 | None |
| Cenpj | Centromere protein J | None |
| Jup | Junction plakoglobin | dDAVP at a concentration of 0.1 nM for 1 h increases abundance of Jup in nuclear pellet of mouse mpkCCD to 160% (62). Jup protein undergoes a reciprocal abundance change in the 17,000-g membrane fraction [down] and the 1,000-g pellet fraction [up] in response to vasopressin (81). Jup protein half-life increases from 13.3 to 23.6 h in mouse mpkCCD cells in response to vasopressin (61). dDAVP at a concentration of 0.1 nM for 1 h increases phosphorylation of Jup protein at S665 in nuclear pellet of mouse mpkCCD to 150% (5). dDAVP at a concentration of 0.1 nM for 120 h decreases translation rate of Jup protein in mouse mpkCCD to 50% (61). |
| Plec | Plectin | dDAVP at a concentration of 0.1 nM for 1 h increases abundance of Plec in nuclear pellet of mouse mpkCCD to 150% (62). dDAVP at a concentration of 1 nM for 0–15 min decreases phosphorylation of Plec protein at S21 protein in rat IMCD to 80% (26). dDAVP at 0.5 ng/h for 7 days increases mRNA abundance of Plec in AQP1 KO mouse renal inner medulla to 150% (7). |
| Itga1 | Integrin alpha-1 | Itga1 protein half-life decreases from 52.8 to 10.6 h in mouse mpkCCD cells in response to vasopressin (61). |
| Alpk1 | Alpha-protein kinase 1 | None |
| Ppl | Periplakin | dDAVP at a concentration of 0.1 nM for 1 h increases abundance of Ppl in nuclear extract of mouse mpkCCD to 170% (62). Ppl protein undergoes a reciprocal abundance change in the 200,000-g cytosolic fraction [down] and the 1,000-g pellet fraction [up] in response to vasopressin (81). AVP at 10 nM for 4 h decreases mRNA abundance of Ppl in mouse mpkCCDcl4 (SAGE) to 0% (59). dDAVP at a concentration of 1 nM for 0.5 h decreases phosphorylation of Ppl protein at S14 in mouse mpkCCD to 20% (5). |
| Krt4 | Keratin, type II cytoskeletal 4 | None |
| Krt76 | Keratin, type II cytoskeletal 2 | None |
| Hist1h4b | Histone H4 | None |
| H1f0 | Histone H1.0 | dDAVP at a concentration of 0.1 nM for 120 h decreases abundance of H1f0 protein in mouse mpkCCD to 70% (35). |
| H2afz | Histone H2A.Z | None |
| Krt19 | Keratin, type I cytoskeletal 19 | dDAVP at a concentration of 5 ng/h to rats for 72 h increases abundance of Krt19 protein in rat IMCD to 170% (56). dDAVP at a concentration of 1 nM for 0–15 min increases phosphorylation of Krt19 protein at S67 protein in rat IMCD to 150% (26). dDAVP at a concentration of 0.1 nM for 1 h increases phosphorylation of Krt19 protein at S67 in nuclear pellet of mouse mpkCCD to 400% (5). dDAVP at a concentration of 0.1 nM for 1 h increases phosphorylation of Krt19 protein at S67 in nuclear extract of mouse mpkCCD to 280% (5). |
| Krt28 | Keratin, type I cytoskeletal 28 | None |
Data mined from prior publications using Biological Information Gatherer (BIG) (see methods).
Significance of the Study
Prior studies have identified β-catenin as a target for vasopressin regulation via phosphorylation (3, 5, 58, 62) and nuclear translocation (62). Other studies have shown that vasopressin regulates transcription of Aqp2 and other genes in collecting duct cells (60). Given known roles in transcriptional regulation, it seems likely that β-catenin is involved in the transcriptional response to vasopressin. It is likely that β-catenin interacts with specific proteins in the nucleus that mediate changes in transcription, including the proteins identified in this study. It is appears unlikely that these proteins include any members of the TCF/LEF family of transcription factors that are involved in canonical β-catenin signaling, since there are no obvious TCF/LEF-binding motifs in the promotor of the Aqp2 gene (68, 86), and none of the proteins found in this study are TCF/LEF family members. However, the proteins identified herein provide new candidates for vasopressin-regulated, β-catenin-mediated transcriptional regulation to be pursued in future studies using both computational and experimental approaches.
GRANTS
This work was primarily funded by the Division of Intramural Research, National Heart, Lung, and Blood Institute (Project ZIA HL-001285 and ZIA HL-006129, M.A. Knepper). Protein mass spectrometry was done in the NHLBI Proteomics Core Facility (M. Gucek, Director). J. Hwang was supported by the American Physiological Society Undergraduate Summer Research Fellowships Program (May–August, 2016). H. J. Jung is the recipient of the Korean Visiting Scientist the Training Award, supported by the Korea Health Industry Development Institute of the Korea Ministry of Health and Welfare in cooperation with National Institutes of Health (HI13C1211).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R.H., C.-L.C., and H.J.J. performed experiments; J.R.H., C.-L.C., B.M., M.A.K., and H.J.J. analyzed data; J.R.H., C.-L.C., M.A.K., and H.J.J. interpreted results of experiments; J.R.H., C.-L.C., B.M., and H.J.J. prepared figures; J.R.H., C.-L.C., B.M., M.A.K., and H.J.J. edited and revised manuscript; J.R.H., C.-L.C., B.M., M.A.K., and H.J.J. approved final version of manuscript; C.-L.C., M.A.K., and H.J.J. conceived and designed research; C.-L.C., M.A.K., and H.J.J. drafted manuscript.
REFERENCES
- 1.Asally M, Yoneda Y. Beta-catenin can act as a nuclear import receptor for its partner transcription factor, lymphocyte enhancer factor-1 (lef-1). Exp Cell Res 308: 357–363, 2005. doi: 10.1016/j.yexcr.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta 1788: 761–767, 2009. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Bansal AD, Hoffert JD, Pisitkun T, Hwang S, Chou CL, Boja ES, Wang G, Knepper MA. Phosphoproteomic profiling reveals vasopressin-regulated phosphorylation sites in collecting duct. J Am Soc Nephrol 21: 303–315, 2010. doi: 10.1681/ASN.2009070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol 10: 923–934, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Bolger SJ, Hurtado PA, Hoffert JD, Saeed F, Pisitkun T, Knepper MA. Quantitative phosphoproteomics in nuclei of vasopressin-sensitive renal collecting duct cells. Am J Physiol Cell Physiol 303: C1006–C1020, 2012. doi: 10.1152/ajpcell.00260.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol 4: a007906, 2012. doi: 10.1101/cshperspect.a007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Q, McReynolds MR, Keck M, Greer KA, Hoying JB, Brooks HL. Vasopressin receptor subtype 2 activation increases cell proliferation in the renal medulla of AQP1 null mice. Am J Physiol Renal Physiol 293: F1858–F1864, 2007. doi: 10.1152/ajprenal.00068.2007. [DOI] [PubMed] [Google Scholar]
- 8.Cheema MU, Irsik DL, Wang Y, Miller-Little W, Hyndman KA, Marks ES, Frøkiær J, Boesen EI, Norregaard R. Estradiol regulates AQP2 expression in the collecting duct: a novel inhibitory role for estrogen receptor α. Am J Physiol Renal Physiol 309: F305–F317, 2015. doi: 10.1152/ajprenal.00685.2014. [DOI] [PubMed] [Google Scholar]
- 9.Cullinane AR, Schäffer AA, Huizing M. The BEACH is hot: a LYST of emerging roles for BEACH-domain containing proteins in human disease. Traffic 14: 749–766, 2013. doi: 10.1111/tra.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Souza N, Vallier LG, Fares H, Greenwald I, de SN . SEL-2, the C. elegans neurobeachin/LRBA homolog, is a negative regulator of lin-12/Notch activity and affects endosomal traffic in polarized epithelial cells. Development 134: 691–702, 2007. doi: 10.1242/dev.02767. [DOI] [PubMed] [Google Scholar]
- 12.Du W, Liu X, Fan G, Zhao X, Sun Y, Wang T, Zhao R, Wang G, Zhao C, Zhu Y, Ye F, Jin X, Zhang F, Zhong Z, Li X. From cell membrane to the nucleus: an emerging role of E-cadherin in gene transcriptional regulation. J Cell Mol Med 18: 1712–1719, 2014. doi: 10.1111/jcmm.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4: 207–214, 2007. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 14.Fagotto F, Glück U, Gumbiner BM. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Curr Biol 8: 181–190, 1998. doi: 10.1016/S0960-9822(98)70082-X. [DOI] [PubMed] [Google Scholar]
- 15.Ferber EC, Kajita M, Wadlow A, Tobiansky L, Niessen C, Ariga H, Daniel J, Fujita Y. A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J Biol Chem 283: 12691–12700, 2008. doi: 10.1074/jbc.M708887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galea MA, Eleftheriou A, Henderson BR. ARM domain-dependent nuclear import of adenomatous polyposis coli protein is stimulated by the B56 alpha subunit of protein phosphatase 2A. J Biol Chem 276: 45833–45839, 2001. doi: 10.1074/jbc.M107149200. [DOI] [PubMed] [Google Scholar]
- 17.Goodman SN. Toward evidence-based medical statistics. 1: The P value fallacy. Ann Intern Med 130: 995–1004, 1999. doi: 10.7326/0003-4819-130-12-199906150-00008. [DOI] [PubMed] [Google Scholar]
- 18.Goodman SN. Toward evidence-based medical statistics. 2: The Bayes factor. Ann Intern Med 130: 1005–1013, 1999. doi: 10.7326/0003-4819-130-12-199906150-00019. [DOI] [PubMed] [Google Scholar]
- 19.Halfon MS, Grad Y, Church GM, Michelson AM. Computation-based discovery of related transcriptional regulatory modules and motifs using an experimentally validated combinatorial model. Genome Res 12: 1019–1028, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasler U, Jeon US, Kim JA, Mordasini D, Kwon HM, Féraille E, Martin PY. Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J Am Soc Nephrol 17: 1521–1531, 2006. doi: 10.1681/ASN.2005121317. [DOI] [PubMed] [Google Scholar]
- 21.Hasler U, Leroy V, Jeon US, Bouley R, Dimitrov M, Kim JA, Brown D, Kwon HM, Martin PY, Féraille E. NF-kappaB modulates aquaporin-2 transcription in renal collecting duct principal cells. J Biol Chem 283: 28095–28105, 2008. doi: 10.1074/jbc.M708350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasler U, Leroy V, Martin PY, Féraille E. Aquaporin-2 abundance in the renal collecting duct: new insights from cultured cell models. Am J Physiol Renal Physiol 297: F10–F18, 2009. doi: 10.1152/ajprenal.00053.2009. [DOI] [PubMed] [Google Scholar]
- 23.Hasler U, Mordasini D, Bianchi M, Vandewalle A, Féraille E, Martin PY. Dual influence of aldosterone on AQP2 expression in cultured renal collecting duct principal cells. J Biol Chem 278: 21639–21648, 2003. doi: 10.1074/jbc.M212388200. [DOI] [PubMed] [Google Scholar]
- 24.Hecht A, Litterst CM, Huber O, Kemler R. Functional characterization of multiple transactivating elements in beta-catenin, some of which interact with the TATA-binding protein in vitro. J Biol Chem 274: 18017–18025, 1999. doi: 10.1074/jbc.274.25.18017. [DOI] [PubMed] [Google Scholar]
- 25.Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J 19: 1839–1850, 2000. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffert JD, Pisitkun T, Saeed F, Song JH, Chou CL, Knepper MA. Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics. Mol Cell Proteomics 11: M111.014613, 2012. doi: 10.1074/mcp.M111.014613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh GY, Glantz SB, Je S, Morrow JS, Kim JH. Calpain proteolysis of alpha II-spectrin in the normal adult human brain. Neurosci Lett 316: 41–44, 2001. doi: 10.1016/S0304-3940(01)02371-0. [DOI] [PubMed] [Google Scholar]
- 28.Hung LY, Tang CJ, Tang TK. Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex. Mol Cell Biol 20: 7813–7825, 2000. doi: 10.1128/MCB.20.20.7813-7825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang D, Zhou Y, Moxley RA, Jarrett HW. Purification and identification of positive regulators binding to a novel element in the c-Jun promoter. Biochemistry 47: 9318–9334, 2008. doi: 10.1021/bi800285q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang S, Galindo MR, Jarrett HW. Purification and identification of a transcription factor, USF-2, binding to E-box element in the promoter of human telomerase reverse transcriptase (hTERT). Proteomics 10: 203–211, 2010. doi: 10.1002/pmic.200800693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonassen TE, Promeneur D, Christensen S, Petersen JS, Nielsen S. Decreased vasopressin-mediated renal water reabsorption in rats with chronic aldosterone-receptor blockade. Am J Physiol Renal Physiol 278: F246–F256, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Jung HJ, Kim SY, Choi HJ, Park EJ, Lim JS, Frøkiaer J, Nielsen S, Kwon TH. Tankyrase-mediated β-catenin activity regulates vasopressin-induced AQP2 expression in kidney collecting duct mpkCCDc14 cells. Am J Physiol Renal Physiol 308: F473–F486, 2015. doi: 10.1152/ajprenal.00052.2014. [DOI] [PubMed] [Google Scholar]
- 33.Jung HJ, Kwon TH. Molecular mechanisms regulating aquaporin-2 in kidney collecting duct. Am J Physiol Renal Physiol 311: F1318–F1328, 2016. doi: 10.1152/ajprenal.00485.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemp BE, Pearson RB. Protein kinase recognition sequence motifs. Trends Biochem Sci 15: 342–346, 1990. doi: 10.1016/0968-0004(90)90073-K. [DOI] [PubMed] [Google Scholar]
- 35.Khositseth S, Pisitkun T, Slentz DH, Wang G, Hoffert JD, Knepper MA, Yu MJ. Quantitative protein and mRNA profiling shows selective post-transcriptional control of protein expression by vasopressin in kidney cells. Mol Cell Proteomics 10: M110.004036, 2011. doi: 10.1074/mcp.M110.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knepper MA, Kwon TH, Nielsen S. Molecular physiology of water balance. N Engl J Med 372: 1349–1358, 2015. doi: 10.1056/NEJMra1404726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koyanagi M, Hijikata M, Watashi K, Masui O, Shimotohno K. Centrosomal P4.1-associated protein is a new member of transcriptional coactivators for nuclear factor-kappaB. J Biol Chem 280: 12430–12437, 2005. doi: 10.1074/jbc.M410420200. [DOI] [PubMed] [Google Scholar]
- 38.Kwon TH, Nielsen J, Masilamani S, Hager H, Knepper MA, Frokiaer J, Nielsen S. Regulation of collecting duct AQP3 expression: response to mineralocorticoid. Am J Physiol Renal Physiol 283: F1403–F1421, 2002. doi: 10.1152/ajprenal.00059.2002. [DOI] [PubMed] [Google Scholar]
- 39.Lee JW, Chou CL, Knepper MA. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeMaire SM, Raghuram V, Grady CR, Pickering CM, Chou CL, Umejiego EN, Knepper MA. Serine/threonine phosphatases and aquaporin-2 regulation in renal collecting duct. Am J Physiol Renal Physiol 312: F84–F95, 2017. doi: 10.1152/ajprenal.00455.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li SZ, McDill BW, Kovach PA, Ding L, Go WY, Ho SN, Chen F. Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am J Physiol Cell Physiol 292: C1606–C1616, 2006. doi: 10.1152/ajpcell.00588.2005. [DOI] [PubMed] [Google Scholar]
- 42.Lin CY, Lovén J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 151: 56–67, 2012. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 139: 1861–1872, 1997. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou SF, Gustafsson JA, Yang T. Soluble (pro)renin receptor via β-catenin enhances urine concentration capability as a target of liver X receptor. Proc Natl Acad Sci USA 113: E1898–E1906, 2016. doi: 10.1073/pnas.1602397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maeda O, Usami N, Kondo M, Takahashi M, Goto H, Shimokata K, Kusugami K, Sekido Y. Plakoglobin (gamma-catenin) has TCF/LEF family-dependent transcriptional activity in beta-catenin-deficient cell line. Oncogene 23: 964–972, 2004. doi: 10.1038/sj.onc.1207254. [DOI] [PubMed] [Google Scholar]
- 46.Marples D, Frøkiaer J, Nielsen S. Long-term regulation of aquaporins in the kidney. Am J Physiol Renal Physiol 276: F331–F339, 1999. [DOI] [PubMed] [Google Scholar]
- 47.McCrea PD, Gottardi CJ. Beyond β-catenin: prospects for a larger catenin network in the nucleus. Nat Rev Mol Cell Biol 17: 55–64, 2015. doi: 10.1038/nrm.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCrea PD, Maher MT, Gottardi CJ. Nuclear signaling from cadherin adhesion complexes. Curr Top Dev Biol 112: 129–196, 2015. doi: 10.1016/bs.ctdb.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254: 1359–1361, 1991. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- 50.Miranda CA, Lee JW, Chou CL, Knepper MA. Tolvaptan as a tool in renal physiology. Am J Physiol Renal Physiol 306: F359–F366, 2014. doi: 10.1152/ajprenal.00330.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mosimann C, Hausmann G, Basler K. Beta-catenin hits chromatin: regulation of Wnt target gene activation. Nat Rev Mol Cell Biol 10: 276–286, 2009. doi: 10.1038/nrm2654. [DOI] [PubMed] [Google Scholar]
- 52.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 53.Ohara M, Cadnapaphornchai MA, Summer SN, Falk S, Yang J, Togawa T, Schrier RW. Effect of mineralocorticoid deficiency on ion and urea transporters and aquaporin water channels in the rat. Biochem Biophys Res Commun 299: 285–290, 2002. doi: 10.1016/S0006-291X(02)02634-7. [DOI] [PubMed] [Google Scholar]
- 54.Peng B, Sutherland KD, Sum EY, Olayioye M, Wittlin S, Tang TK, Lindeman GJ, Visvader JE. CPAP is a novel stat5-interacting cofactor that augments stat5-mediated transcriptional activity. Mol Endocrinol 16: 2019–2033, 2002. doi: 10.1210/me.2002-0108. [DOI] [PubMed] [Google Scholar]
- 55.Pickering CM, Grady C, Medvar B, Emamian M, Sandoval PC, Zhao Y, Yang CR, Jung HJ, Chou CL, Knepper MA. Proteomic profiling of nuclear fractions from native renal inner medullary collecting duct cells. Physiol Genomics 48: 154–166, 2016. doi: 10.1152/physiolgenomics.00090.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pisitkun T, Bieniek J, Tchapyjnikov D, Wang G, Wu WW, Shen RF, Knepper MA. High-throughput identification of IMCD proteins using LC-MS/MS. Physiol Genomics 25: 263–276, 2006. doi: 10.1152/physiolgenomics.00214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pokutta S, Weis WI. Structure of the dimerization and beta-catenin-binding region of alpha-catenin. Mol Cell 5: 533–543, 2000. doi: 10.1016/S1097-2765(00)80447-5. [DOI] [PubMed] [Google Scholar]
- 58.Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci USA 107: 3882–3887, 2010. doi: 10.1073/pnas.0910646107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robert-Nicoud M, Flahaut M, Elalouf JM, Nicod M, Salinas M, Bens M, Doucet A, Wincker P, Artiguenave F, Horisberger JD, Vandewalle A, Rossier BC, Firsov D. Transcriptome of a mouse kidney cortical collecting duct cell line: effects of aldosterone and vasopressin. Proc Natl Acad Sci USA 98: 2712–2716, 2001. doi: 10.1073/pnas.051603198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandoval PC, Claxton JS, Lee JW, Saeed F, Hoffert JD, Knepper MA. Systems-level analysis reveals selective regulation of Aqp2 gene expression by vasopressin. Sci Rep 6: 34863, 2016. doi: 10.1038/srep34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandoval PC, Slentz DH, Pisitkun T, Saeed F, Hoffert JD, Knepper MA. Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol 24: 1793–1805, 2013. doi: 10.1681/ASN.2013030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schenk LK, Bolger SJ, Luginbuhl K, Gonzales PA, Rinschen MM, Yu MJ, Hoffert JD, Pisitkun T, Knepper MA. Quantitative proteomics identifies vasopressin-responsive nuclear proteins in collecting duct cells. J Am Soc Nephrol 23: 1008–1018, 2012. doi: 10.1681/ASN.2011070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt-Ott KM, Barasch J. WNT/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int 74: 1004–1008, 2008. doi: 10.1038/ki.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene 20: 2390–2400, 2001. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 65.Sims RJ III, Rojas LA, Beck D, Bonasio R, Schüller R, Drury WJ III, Eick D, Reinberg D. The C-terminal domain of RNA polymerase II is modified by site-specific methylation. Science 332: 99–103, 2011. doi: 10.1126/science.1202663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith FD, Samelson BK, Scott JD. Discovery of cellular substrates for protein kinase A using a peptide array screening protocol. Biochem J 438: 103–110, 2011. doi: 10.1042/BJ20110720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol 149: 249–254, 2000. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tchapyjnikov D, Li Y, Pisitkun T, Hoffert JD, Yu MJ, Knepper MA. Proteomic profiling of nuclei from native renal inner medullary collecting duct cells using LC-MS/MS. Physiol Genomics 40: 167–183, 2010. doi: 10.1152/physiolgenomics.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tie F, Banerjee R, Stratton CA, Prasad-Sinha J, Stepanik V, Zlobin A, Diaz MO, Scacheri PC, Harte PJ. CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing. Development 136: 3131–3141, 2009. doi: 10.1242/dev.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H. The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J Biol Chem 278: 2692–2700, 2003. doi: 10.1074/jbc.M206821200. [DOI] [PubMed] [Google Scholar]
- 71.Uchida S, Matsumura Y, Rai T, Sasaki S, Marumo F. Regulation of aquaporin-2 gene transcription by GATA-3. off. Biochem Biophys Res Commun 232: 65–68, 1997. doi: 10.1006/bbrc.1997.6236. [DOI] [PubMed] [Google Scholar]
- 72.Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J 31: 2714–2736, 2012. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Balkom BW, Hoffert JD, Chou CL, Knepper MA. Proteomic analysis of long-term vasopressin action in the inner medullary collecting duct of the Brattleboro rat. Am J Physiol Renal Physiol 286: F216–F224, 2004. doi: 10.1152/ajprenal.00307.2003. [DOI] [PubMed] [Google Scholar]
- 74.Vanacker JM, Pettersson K, Gustafsson JA, Laudet V. Transcriptional targets shared by estrogen receptor- related receptors (ERRs) and estrogen receptor (ER) alpha, but not by ERbeta. EMBO J 18: 4270–4279, 1999. doi: 10.1093/emboj/18.15.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wickline ED, Du Y, Stolz DB, Kahn M, Monga SP. γ-Catenin at adherens junctions: mechanism and biologic implications in hepatocellular cancer after β-catenin knockdown. Neoplasia 15: 421–434, 2013. doi: 10.1593/neo.122098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson JL, Miranda CA, Knepper MA. Vasopressin and the regulation of aquaporin-2. Clin Exp Nephrol 17: 751–764, 2013. doi: 10.1007/s10157-013-0789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 35: 161–168, 2010. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu SY, Thomas MC, Hou SY, Likhite V, Chiang CM. Isolation of mouse TFIID and functional characterization of TBP and TFIID in mediating estrogen receptor and chromatin transcription. J Biol Chem 274: 23480–23490, 1999. doi: 10.1074/jbc.274.33.23480. [DOI] [PubMed] [Google Scholar]
- 79.Xue Z, Chen JX, Zhao Y, Medvar B, Knepper MA. Data integration in physiology using Bayes’ rule and minimum Bayes’ factors: deubiquitylating enzymes in the renal collecting duct. Physiol Genomics 49: 151–159, 2017. doi: 10.1152/physiolgenomics.00120.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yakulov T, Raggioli A, Franz H, Kemler R. Wnt3a-dependent and -independent protein interaction networks of chromatin-bound β-catenin in mouse embryonic stem cells. Mol Cell Proteomics 12: 1980–1994, 2013. doi: 10.1074/mcp.M112.026914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang CR, Raghuram V, Emamian M, Sandoval PC, Knepper MA. Deep proteomic profiling of vasopressin-sensitive collecting duct cells. II. Bioinformatic analysis of vasopressin signaling. Am J Physiol Cell Physiol 309: C799–C812, 2015. doi: 10.1152/ajpcell.00214.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang SD, Huang TJ. Identification of -R-X-(X)-S/T-X3-S/T- as consensus sequence motif for autophosphorylation-dependent protein kinase. J Biol Chem 269: 29855–29859, 1994. [PubMed] [Google Scholar]
- 83.Yang Y, Lu Y, Espejo A, Wu J, Xu W, Liang S, Bedford MT. TDRD3 is an effector molecule for arginine-methylated histone marks. Mol Cell 40: 1016–1023, 2010. doi: 10.1016/j.molcel.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yasui M, Zelenin SM, Celsi G, Aperia A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol Renal Physiol 272: F443–F450, 1997. [DOI] [PubMed] [Google Scholar]
- 85.Yu L, Moriguchi T, Souma T, Takai J, Satoh H, Morito N, Engel JD, Yamamoto M. GATA2 regulates body water homeostasis through maintaining aquaporin 2 expression in renal collecting ducts. Mol Cell Biol 34: 1929–1941, 2014. doi: 10.1128/MCB.01659-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106: 2441–2446, 2009. doi: 10.1073/pnas.0813002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Y, Yang CR, Raghuram V, Parulekar J, Knepper MA. BIG: a large-scale data integration tool for renal physiology. Am J Physiol Renal Physiol 311: F787–F792, 2016. doi: 10.1152/ajprenal.00249.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]