Abstract
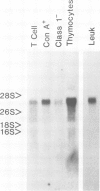
Leukosialin is one of the major glycoproteins of thymocytes and T lymphocytes and is notable for a very high content of O-linked carbohydrate structures. The full protein sequence for rat leukosialin as translated from cDNA clones is now reported. The molecule contains 371 amino acids with 224 residues outside the cell, one transmembrane sequence and 124 cytoplasmic residues. Data from the peptide sequence and carbohydrate composition suggest that one in three of the extracellular amino acids may be O-glycosylated with no N-linked glycosylation sites. The cDNA sequence contained a CpG rich region in the 3' coding sequence and a large 3' non-coding region which included tandem repeats of the sequence GGAT.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson B., Hammarström S., Finne J., Perlmann P. The large sialoglycoprotein of human lymphocytes. II. Biochemical features. Eur J Immunol. 1985 May;15(5):427–433. doi: 10.1002/eji.1830150503. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Jackson D. I., Willis A. C., Williams A. F. Lymphocyte specific heterogeneity in the rat leucocyte common antigen (T200) is due to differences in polypeptide sequences near the NH2-terminus. EMBO J. 1987 May;6(5):1259–1264. doi: 10.1002/j.1460-2075.1987.tb02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. R., Barclay A. N., Sunderland C. A., Williams A. F. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981 Feb 5;289(5797):456–460. doi: 10.1038/289456a0. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Williams A. F. Lymphocyte cell surface glycoproteins which bind to soybean and peanut lectins. Immunology. 1982 Aug;46(4):713–726. [PMC free article] [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. Isolation and characterization of leukosialin, a major sialoglycoprotein on human leukocytes. J Biol Chem. 1986 Sep 25;261(27):12779–12786. [PubMed] [Google Scholar]
- Carlsson S. R., Sasaki H., Fukuda M. Structural variations of O-linked oligosaccharides present in leukosialin isolated from erythroid, myeloid, and T-lymphoid cell lines. J Biol Chem. 1986 Sep 25;261(27):12787–12795. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark S. J., Jefferies W. A., Barclay A. N., Gagnon J., Williams A. F. Peptide and nucleotide sequences of rat CD4 (W3/25) antigen: evidence for derivation from a structure with four immunoglobulin-related domains. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1649–1653. doi: 10.1073/pnas.84.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Pink R., Acuto O., Mach J. P., Dolivo S., Nabholz M. Presence of T 145 on cytolytic T cell lines and their lectin-resistant mutants. Eur J Immunol. 1980 Nov;10(11):860–868. doi: 10.1002/eji.1830101111. [DOI] [PubMed] [Google Scholar]
- De Maio A., Lis H., Gershoni J. M., Sharon N. Identification of glycoproteins that are receptors for peanut agglutinin on immature (cortical) mouse thymocytes. FEBS Lett. 1986 Jan 1;194(1):28–32. doi: 10.1016/0014-5793(86)80045-x. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Häyry P., Andersson L. C. Characterization of surface glycoproteins of mouse lymphoid cells. J Cell Biol. 1976 Mar;68(3):642–653. doi: 10.1083/jcb.68.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner-Garden M., Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987 Jul 20;196(2):261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Gagnon J., Barclay A. N., Williams A. F. Purification, chain separation and sequence of the MRC OX-8 antigen, a marker of rat cytotoxic T lymphocytes. EMBO J. 1985 Oct;4(10):2539–2545. doi: 10.1002/j.1460-2075.1985.tb03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehry M., Ewald S., Douglas R., Sibley C., Raschke W., Fambrough D., Hood L. The immunoglobulin mu chains of membrane-bound and secreted IgM molecules differ in their C-terminal segments. Cell. 1980 Sep;21(2):393–406. doi: 10.1016/0092-8674(80)90476-6. [DOI] [PubMed] [Google Scholar]
- Kenney D., Cairns L., Remold-O'Donnell E., Peterson J., Rosen F. S., Parkman R. Morphological abnormalities in the lymphocytes of patients with the Wiskott-Aldrich syndrome. Blood. 1986 Dec;68(6):1329–1332. [PubMed] [Google Scholar]
- Kitajima K., Inoue Y., Inoue S. Polysialoglycoproteins of Salmonidae fish eggs. Complete structure of 200-kDa polysialoglycoprotein from the unfertilized eggs of rainbow trout (Salmo gairdneri). J Biol Chem. 1986 Apr 25;261(12):5262–5269. [PubMed] [Google Scholar]
- Mentzer S. J., Remold-O'Donnell E., Crimmins M. A., Bierer B. E., Rosen F. S., Burakoff S. J. Sialophorin, a surface sialoglycoprotein defective in the Wiskott-Aldrich syndrome, is involved in human T lymphocyte proliferation. J Exp Med. 1987 May 1;165(5):1383–1392. doi: 10.1084/jem.165.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Ralph S. J., Thomas M. L., Morton C. C., Trowbridge I. S. Structural variants of human T200 glycoprotein (leukocyte-common antigen). EMBO J. 1987 May;6(5):1251–1257. doi: 10.1002/j.1460-2075.1987.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Davis A. E., 3rd, Kenney D., Bhaskar K. R., Rosen F. S. Purification and chemical composition of gpL115, the human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Biol Chem. 1986 Jun 5;261(16):7526–7530. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D. M., Parkman R., Cairns L., Savage B., Rosen F. S. Characterization of a human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J Exp Med. 1984 Jun 1;159(6):1705–1723. doi: 10.1084/jem.159.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remold-O'Donnell E., Kenney D., Rosen F. S. Biosynthesis of human sialophorins and analysis of the polypeptide core. Biochemistry. 1987 Jun 30;26(13):3908–3913. doi: 10.1021/bi00387a025. [DOI] [PubMed] [Google Scholar]
- Saito M., Osawa T. The major sialoglycoprotein of human T-lymphocytes. Carbohydr Res. 1980 Jan 15;78(2):341–348. doi: 10.1016/0008-6215(80)90014-2. [DOI] [PubMed] [Google Scholar]
- Shibuya K., Noguchi S., Yamaki M., Nishimura S., Sekiya T. Characterization of a rat tRNA gene cluster: comparison of nucleotide sequences of the gene for tRNALeu newly found in repeating units. J Biochem. 1985 Jun;97(6):1719–1725. doi: 10.1093/oxfordjournals.jbchem.a135230. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Staden R. The current status and portability of our sequence handling software. Nucleic Acids Res. 1986 Jan 10;14(1):217–231. doi: 10.1093/nar/14.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring R., McMaster W. R., Sunderland C. A., Williams A. F. The predominant heavily glycosylated glycoproteins at the surface of rat lymphoid cells are differentiation antigens. Eur J Immunol. 1978 Dec;8(12):832–839. doi: 10.1002/eji.1830081203. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Swallow D. M., Gendler S., Griffiths B., Corney G., Taylor-Papadimitriou J., Bramwell M. E. The human tumour-associated epithelial mucins are coded by an expressed hypervariable gene locus PUM. Nature. 1987 Jul 2;328(6125):82–84. doi: 10.1038/328082a0. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Barclay A. N., Gagnon J., Williams A. F. Evidence from cDNA clones that the rat leukocyte-common antigen (T200) spans the lipid bilayer and contains a cytoplasmic domain of 80,000 Mr. Cell. 1985 May;41(1):83–93. doi: 10.1016/0092-8674(85)90063-7. [DOI] [PubMed] [Google Scholar]
- Weller P., Jeffreys A. J., Wilson V., Blanchetot A. Organization of the human myoglobin gene. EMBO J. 1984 Feb;3(2):439–446. doi: 10.1002/j.1460-2075.1984.tb01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- de Petris S. Lectin-binding and spontaneous capping characteristics of the thymocyte glycophorin-like glycoprotein. Exp Cell Res. 1984 Jun;152(2):510–519. doi: 10.1016/0014-4827(84)90653-0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]