Abstract
Background
It is currently unknown whether intensive blood pressure (BP) lowering beyond that recommended would lead to more lowering of the risk of Left ventricular hypertrophy (LVH) in patients with hypertension, and whether reducing the risk of LVH explains the reported cardiovascular disease (CVD) benefits of intensive BP lowering in this population.
Methods
This analysis included 8,164 participants (mean age 67.9 years, 35.3% women, 31.2% blacks) with hypertension but no diabetes from the Systolic Blood Pressure Intervention (SPRINT) Trial; 4,086 randomly assigned to intensive BP lowering (target systolic BP<120mmHg) and 4,078 assigned to standard BP lowering (target systolic BP <140mmHg). Progression and regression of LVH as defined by Cornell voltage criteria derived from standard 12-lead electrocardiograms recorded at baseline and biannually were compared between treatment arms during a median follow-up of 3.81 years. The effect of intensive (vs. standard) BP lowering on the SPRINT primary CVD outcome (a composite of myocardial infarction, acute coronary syndrome, stroke, heart failure, and CVD death) was compared before and after adjusting for LVH as a time-varying covariate.
Results
Among SPRINT participants without baseline LVH (n=7,559), intensive (vs. standard) BP lowering was associated with a 46% lower risk of developing LVH (HR=0.54, 95%CI: 0.43 to 0.68). Similarly, among SPRINT participants with baseline LVH (n=605, 7.4%), those assigned to the intensive (vs. standard) BP lowering were 66% more likely to regress/improve their LVH (HR=1.66, 95%CI: 1.31 to 2.11). Adjustment for LVH as a time-varying covariate did not substantially attenuate the effect of intensive BP therapy on CVD events (HR (95%CI) of intensive vs. standard BP lowering on CVD: 0.76(0.64,0.90) and 0.77(0.65,0.91) before and after adjusting for LVH as a time-varying covariate, respectively).
Conclusions
Among patients with hypertension but no diabetes, intensive BP lowering (target systolic BP<120 mmHg), compared with standard BP lowering (target systolic BP<140 mmHg), resulted in lower rates of developing new LVH in those without LVH, and higher rates of regression of LVH in those with existing LVH. This favorable effect on LVH did not explain most of the reduction in CVD events associated with intensive BP lowering in the SPRINT trial.
Keywords: Intensive Blood Pressure Lowering, Left Ventricular Hypertrophy, SPRINT
INTRODUCTION
Left ventricular hypertrophy (LVH), a common finding in patients with hypertension, is a maladaptive response to chronic pressure overload. (1) Successful management of high blood pressure (BP) modifies this response and produces regression of LVH, and selection of individual antihypertensive drugs appears to be less important than the management of blood pressure itself.(2) In patients with both hypertension and diabetes, we have recently shown that more intensive lowering of BP (target systolic BP (SBP) <120 mmHg) leads to more reduction in the risk of LVH.(3) Similar results were reported from a small clinical trial in which SBP lowering to <130 mmHg was compared to a goal of <140 mmHg in adults 55 years of age or older without diabetes.(4) However, it is yet to be established whether a more intensive lowering (target SBP <120 mmHg) in a diverse population with hypertension without diabetes will result in a lower risk of LVH, compared to standard BP lowering (target SBP<140 mm Hg).
Development of LVH is known to be associated with a greater risk of cardiovascular disease (CVD) morbidity and mortality, and this risk could be reversed by regression of LVH. (5–11) In the Framingham Heart Study, regression in the electrocardiographic Cornell voltage LVH criteria was associated with a lower risk of clinical CVD, whereas progression in Cornell voltage identified individuals at increased risk of CVD.(5) Similar conclusions were reported from the Multiple Risk-Factor Intervention Trial (MRFIT)(6), the Heart Outcomes Prevention Evaluation (HOPE)(7), and the Losartan Intervention For Endpoint reduction in hypertension study.(8–11) In the Systolic Blood Pressure Intervention Trial (SPRINT), which included patients with hypertension but no diabetes, intensive BP lowering targeting a SBP of <120 mmHg, as compared with standard SBP lowering targeting <140 mmHg, resulted in lower rates of CVD events.(12) Whether this effect of intensive BP lowering on reducing CVD events could be explained by its effect on LVH is also currently unknown.
Therefore, we examined the differential impact of intensive BP lowering (target SBP <120 mmHg) vs. standard BP lowering (target SBP<140 mm Hg) on LVH in the SPRINT trial, a randomized, multicenter trial involving middle-aged and older patients with hypertension but no diabetes. We also examined whether the positive effect of intensive BP lowering on the CVD outcomes in SPRINT is explained by its effect on LVH.
METHODS
Study Population and Design
SPRINT was a randomized, controlled, open-label trial that was conducted at 102 clinical sites organized into 5 clinical center networks in the United States. The rationale and design of the SPRINT trial have been published elsewhere.(12, 13) Briefly, SPRINT aimed to test whether reducing SBP to <120 mmHg reduces CVD events defined as a composite of non-fatal myocardial infarction, acute coronary syndrome not resulting in myocardial infarction, non-fatal stroke, non-fatal acute decompensated heart failure, and death from CVD (SPRINT primary outcome). Participants were required to meet all the following criteria: an age of at least 50 years, a systolic blood pressure of 130 to 180 mmHg and an increased risk of CVD defined as presence of one or more of the following: clinical or subclinical CVD, chronic kidney disease, a 10-year risk of CVD ≥15% estimated by the Framingham risk score; or an age ≥75 years. Patients with type 2 diabetes mellitus or prior stroke were excluded.
A total of 9,361 participants were enrolled between November 2010 and March 2013, of whom 4,683 were randomized to a SBP target of <140 mmHg (standard treatment arm) and 4,678 participants were randomized to <120 mm Hg (intensive treatment arm). Randomization was stratified by clinical site. The SPRINT intervention was stopped early (median 3.26 years of follow-up) because of a 25% reduction in the primary composite CVD end point and a 27% reduction in all-cause mortality in the intensive treatment group. The study was approved by the institutional review board at each participating site, and written informed consent was obtained from all participants.
For the purpose of this analysis we included SPRINT participants with baseline and at least one follow up ECG. On the other hand, we excluded participants with missing or uninterpretable baseline electrocardiogram (ECG) (n=138) as well as those without any follow up ECG (n= 1,059).
Ascertainment of LVH
LVH was ascertained from standard 12-lead ECGs obtained at baseline, year 2, year 4 and the close-out visit. Digital ECG data were recorded using a GE MAC 1200 electrocardiograph (GE, Milwaukee, Wisconsin) at 10 mm/mV calibration and a speed of 25 mm/s. ECG reading was performed centrally at the Epidemiological Cardiology Research Center (EPICARE), Wake Forest School of Medicine, Winston Salem, North Carolina. All ECG tracings were initially inspected visually for technical errors and inadequate quality before being automatically processed using GE 12-SL Marquette version 2001 (GE, Milwaukee, Wisconsin).
LVH was defined by Cornell voltage criteria (RaVL amplitude + SV3 amplitude) using the following sex-specific cut-off points: ≥2,200 microvolt (μV) in women and ≥2800 μV in men. (14) LVH was considered present or absent (or changed from one status to another) based on crossing these cut-off points up or down (either regression of progression) even by a point. In addition to using LVH as a categorical/binary variable, Cornell voltage was also examined as a continuous variable (referred to in this manuscript as Cornell index). Using Cornell voltage as a continuous variable has the advantage of being not dependent on the cut-off points selected to define LVH, and it is more sensitive to changes during follow up than LVH as a categorical variable. (3) In sensitivity analysis, we also used Cornell voltage product ([RaVL amplitude + SV3 amplitude]*QRS duration) (15), and Sokolow-Lyon (SV1 amplitude + RV5/V6 amplitude) (16) LVH criteria. We also used LVH by Minnesota Code ECG classification which represents LVH criteria with ST/T abnormalities (LVH with strain pattern) in selected analyses that do not requires the use continuous measures (such Cornell index). Minnesota Code LVH is defined as high amplitude R waves (Minnesota Code 3.1: R amplitude > 26 mm in either V5 or V6, or R amplitude > 20.0 mm in any of leads I, II, m, aVF, or R amplitude > 12.0 mm in lead aVL) plus major ST/T abnormalities (Minnesota Codes 4.1, 4.2, 5.1 or 5.2). (17)
Events and Other Study Measurements
Demographic data were collected at baseline before randomization. Clinical and laboratory data were obtained at baseline and every 3 months thereafter. Details of the assessment of BP, the adjustment of medication doses, and antihypertensive drug regimens during the trial are provided elsewhere. (13)
At each visit, trained clinical staff measured blood pressures with an automated BP device (Omron-HEM-907 XL, Omron Healthcare, INC. Bannockburn, Illinois, USA) using standardized procedures. (13) BP measurement requirements included measuring BP early in the visit and not following stressful exam components such as blood draws, proper positioning of the participant in a chair with back support, and proper cuff size determination. The Manual of Procedures (MOP) stated that participants should be resting, not completing questionnaires, and not speaking with study staff during the 5-minute rest period or while BP measurements were being taken. The MOP also stated that staff should leave the room during the 5-minute rest period, and provided a script that staff could use to explain that they would be absent during the 5-minute rest period and would then enter the room and obtain the measurements without speaking to the participant. At 1 year the SBP fell in the intensive treatment group by ~15 mmHg more than in the standard treatment group (mean SBP 121.4 vs. 136.2 mmHg) with administration of an average of 1 more antihypertensive medication.
A structured interview was used in both treatment arms every 3 months to obtain self-reported CVD outcomes. Medical records and ECG data were obtained for documentation of events. Whenever clinical site staff became aware of a death, a standard protocol was used to obtain information on the event. A committee whose members were unaware of the study-group assignments adjudicated the clinical outcomes specified in the protocol. Details on the adjudication of these outcomes including the CVD events have been published elsewhere. (12) The CVD outcomes in this analysis included events through August 20, 2015, similar to the main report from the SPRINT trial but limited to the sample with a good quality baseline ECG and at least one follow up ECG.
Statistical Analyses
We used Cox proportional hazards regression to compare the time to the first occurrence of LVH in those without baseline LVH, and to the first occurrence of regression of LVH (i.e. recovery from LVH) in those with baseline LVH, separately, between the treatment arms. Clinical site at randomization was used as a stratification factor. Follow-up time was censored on the date of last ECG. Interactions between treatment effect and SPRINT pre-specified subgroups (age (<75 vs. ≥75 years), sex, race (black vs. non-black), SBP tertiles (≤132, >132 to <145, ≥145 mmHg), prior CVD, and prior CKD) were assessed with a likelihood-ratio test for the interaction with the use of Hommel-adjusted p-values. (18)
To examine whether the impact of intensive BP lowering on the primary outcome is explained by its impact on LVH, we examined the magnitude of attenuation of the association between intensive (vs. standard) BP lowering with the SPRINT primary CVD outcome after adjusting for LVH as a time-varying covariate. Similar to the main SPRINT results publication (12), Cox proportional-hazards regression with stratification according to clinic was used for this purpose.
Several additional analyses were conducted as follows: 1) We compared the rate of regression of the mean Cornell index during follow-up (as a continuous variable) between the intensive and standard arms. In this analysis we used linear mixed-effects models adjusting for baseline value of the Cornel index. Specifically, we looked at random slope and intercept models to each individual to have a separate intercept and slope for the longitudinal change in Cornell index over time and for there to be population averaged intercepts and slopes; 2) We conducted sensitivity analysis in which we excluded 718 participants with major intraventricular conduction delay. This was done because the ECG diagnosis of LVH in those individuals needs to be interpreted with caution according to the current guidelines for the use of ECG criteria for detection of cardiac chamber enlargement.(19) Major intraventricular conduction included all participants with complete left and right bundle branch blocks, Wolf-Parkinson-White Syndrome, ventricular pacemaker and major non-specific conduction delay (all with QRS duration ≥ 120 ms); and 3) We used Cornell voltage product and Sokolow-Lyon LVH criteria in similar analyses to those used for Cornell voltage to confirm the results as well as Minnesota Code LVH in selected analyses that involve LVH as a categorical variable only (i.e. present vs. absent analysis only).
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). For Cox regression models, the proportional hazards assumption was checked and was met. All p-values reported were 2-sided, and statistical significance threshold was chosen as 5%.
RESULTS
A total of 8,164 participants (mean age 67.9 years, 35.3% women, 31.2% blacks) were included in the analysis (4,086 from the intensive BP lowering and 4,078 from the standard BP lowering). About 7.4% (n=605) of the participants had LVH at baseline with similar prevalence in both arms (302 in the intensive BP lowering arm and 303 in the standard BP arm). The baseline characteristics of the study participants did not differ by treatment arms in the study overall or in subgroups stratified by LVH status. Table 1 shows the baseline characteristics of the overall sample while Supplemental Table 1 and Supplemental Table 2 show the baseline characteristics in those without and with baseline LVH, respectively.
Table 1.
Baseline characteristics
| Characteristics * | ALL (N=8,164) | Intensive Arm (N=4,086) | Standard Arm (N=4,078) | P-value † |
|---|---|---|---|---|
| Age (years) | 67.9 ± 9.3 | 67.9 ± 9.2 | 67.8 ± 9.3 | 0.87 |
| Age≥75 years | 2249 (27.5) | 1131 (27.7) | 1118 (27.4) | 0.79 |
| Sex (women) | 2879 (35.3) | 1455 (35.6) | 1424 (34.9) | 0.51 |
| Black | 2551 (31.2) | 1253 (30.7) | 1298 (31.8) | 0.26 |
| Smoking | 0.71 | |||
| Former smoker | 3482 (42.7) | 1730 (42.3) | 1752 (43.0) | |
| Current smoker | 1050 (12.9) | 539 (13.2) | 511 (12.5) | |
| Body mass index (kg/m2) | 29.9 ± 5.7 | 30.0 ± 5.8 | 29.8 ± 5.6 | 0.16 |
| Systolic BP (mmHg) | 139.5 ± 15.5 | 139.4 ± 15.7 | 139.6 ± 15.3 | 0.56 |
| Diastolic BP (mmHg) | 78.1 ± 11.8 | 78.2 ± 11.8 | 78.1 ± 11.9 | 0.66 |
| Systolic BP tertiles | 0.24 | |||
| ≤ 132 mm Hg | 2747 (33.6) | 1402 (34.3) | 1345 (33.0) | |
| >132 to< 145 mm Hg | 2669 (32.7) | 1302 (31.9) | 1367 (33.5) | |
| ≥ 145 mm Hg | 2748 (33.7) | 1382 (33.8) | 1366 (33.5) | |
| Number of BP medications | 1.8 (1.0) | 1.8 (1.0) | 1.8 (1.0) | 0.21 |
| Not using BP medications | 778 (9.5) | 376 (9.2) | 402 (9.9) | 0.31 |
| Serum creatinine (mg/dL) | 1.07 ± 0.33 | 1.07 ± 0.34 | 1.07 ± 0.33 | 0.88 |
| Urine albumin/creatinine (mg/g) | 38.9 ± 138.2 | 40.3 ± 147.5 | 37.5 ± 128.2 | 0.37 |
| Chronic kidney disease ‡ | 2259 (27.7) | 1157 (28.3) | 1102 (27.0) | 0.19 |
| Total cholesterol (mg/dL) | 190.1 ± 41.2 | 190.2 ± 41.6 | 190.0 ± 40.8 | 0.84 |
| HDL-cholesterol (mg/dL) | 52.8 ± 14.4 | 52.8 ± 14.3 | 52.8 ± 14.4 | 0.98 |
| Triglycerides (mg/dL) | 126.0 ± 91.0 | 126.8 ± 88.7 | 126.2 ± 93.2 | 0.75 |
| Fasting plasma glucose (mg/dL) | 98.9 ± 13.4 | 99.0 ± 13.7 | 98.7 ± 13.1 | 0.44 |
| History of prior CVD | 1618 (19.8) | 814 (19.9) | 804 (19.7) | 0.82 |
| Cornell voltage LVH | 605 (7.4) | 302 (7.4) | 303 (7.4) | 0.95 |
BP= blood pressure; HDL= high density lipoprotein; CVD= cardiovascular disease; LVH= left ventricular hypertrophy
Data are presented as number (%) or mean ± standard deviation
p-value comparing participants’ characteristics in the standard vs. intensive blood pressure-lowering arms
Defined as baseline estimated glomerular filtration rate< 60 ml/min/1.73m2
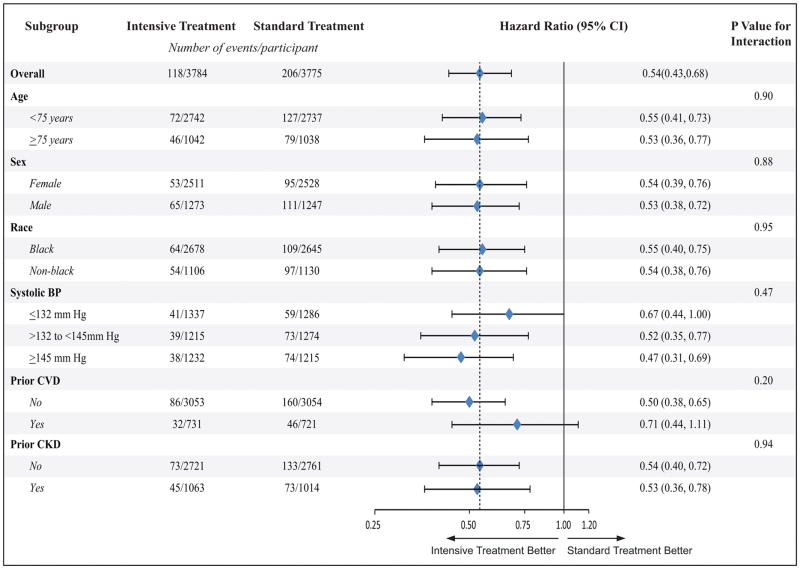
Among SPRINT participants without LVH at baseline (n= 7,559) and during a median follow up of 3.81 years, 324 new LVH occurred (118 in the intensive BP lowering arm and 206 in the standard BP arm). Intensive (compared to standard) BP lowering was associated with a 46% (p-value<0.001) lower risk of developing LVH (Table 2). These results were consistent across subgroups of age, sex, race, systolic blood pressure levels, prior CVD, and prior CKD (Figure 1).
Table 2.
Effect of intensive blood pressure lowering on the risk of developing new LVH in SPRINT participants without baseline LVH
| Treatment Arm | Participants (n) | Events (n) | Event rate %/year (95%CI) | Hazard ratio (95% CI) | p-value |
|---|---|---|---|---|---|
| Intensive BP lowering | 3784 | 118 | 0.89 (0.74, 1.06) | 0.54 (0.43–0.68) | <.001 |
| Standard BP lowering | 3775 | 206 | 1.57 (1.37, 1.80) |
LVH= left ventricular hypertrophy; BP= blood pressure; CI= confidence interval
Figure 1.
Effect of intensive vs. standard blood pressure lowering on the risk of developing new incident LVH during follow up in SPRINT participants without LVH at baseline in pre-specified subgroups.
BP= blood pressure CKD= chronic kideny disease; CVD= cardiovascualr disease; CI= confidence interval
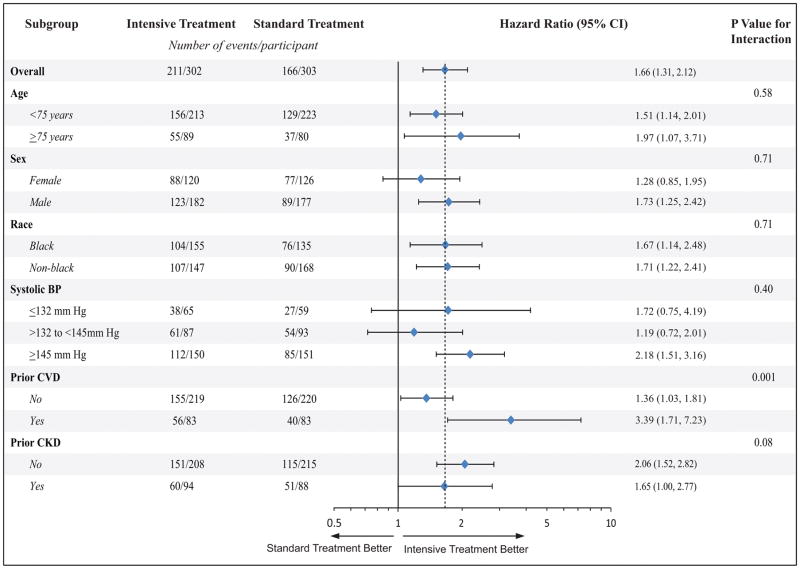
Similarly, regression (improvement) of LVH was more common in the intensive vs. standard BP lowering arm, but improvement occurred in both arms. Among SPRINT participants with baseline LVH (n=605), 62% (n=377) showed regression of (recovery from) their LVH (211 (70%) in the intensive BP lowering arm vs. 166 (55%) in the standard BP arm). Participants assigned to the intensive (compared to standard) BP lowering were 66% (p-value<0.001) more likely to regress their LVH (Table 3). These results were consistent among subgroups of SPRINT participants stratified by age, sex, race, systolic blood pressure levels and prior CKD, but the effect was stronger in those with prior CVD compared to those without prior CVD (interaction p-value =0.001) (Figure 2).
Table 3.
Effect of intensive blood pressure lowering on regression (improvement) of LVH during follow up in SPRINT participants with baseline LVH
| Treatment Arm | Participants (n) | Events (n) | Event rate %/year (95%CI) | Hazard ratio (95% CI) | P-value |
|---|---|---|---|---|---|
| Intensive BP lowering | 302 | 211 | 25.88 (22.61, 29.62) | 1.66 (1.31, 2.11) | <.001 |
| Standard BP lowering | 303 | 166 | 18.68 (16.05, 21.75) |
LVH= left ventricular hypertrophy; BP= blood pressure; CI= confidence interval
Figure 2.
Effect of intensive vs. standard blood pressure lowering on regression of LVH during follow up in SPRINT participants with LVH at baseline in pre-specified subgroups
BP= blood pressure CKD= chronic kideny disease; CVD= cardiovascualr disease; CI= confidence interval
Using random coefficient models in all participants with and without baseline LVH, the rate of regression of Cornell voltage index (the sum of the amplitude of RaVL +SV3) was faster in the intensive BP lowering arm than that in the standard arm by −33.7 μV/year (95% CI: −39.6 to −27.8) (p<.001) (Table 4).
Table 4.
Effect of intensive blood pressure lowering on the rate of regression of mean Cornell index during SPRINT follow up
| Treatment arm | Participants (n) | Regression rate per year (95%CI) | Difference (intensive - standard) (95% CI) | p-value |
|---|---|---|---|---|
| Intensive BP lowering | 4078 | −39.0(−43.5, −35.2) μV | −33.7 (−39.6, −27.8) μV | <.001 |
| Standard BP lowering | 4086 | −5.6 (−9.8, −1.4) μV |
LVH= left ventricular hypertrophy; BP= blood pressure; CI= confidence interval
Cornell index is defined as the sum of the R amplitude in aVL and S amplitude in V3 in microvolt (μV)
Model adjusted for adjusted for baseline Cornell index value
Similar effects of intensive BP lowering on LVH were observed with LVH criteria other than Cornell voltage criteria we used in the main analysis. As shown in Supplemental Table 3, intensive BP lowering was associated with a lower incidence of developing new LVH by Cornell voltage product (HR=0.59, 95% CI: 0.49 to 0.71), Sokolow-Lyon (HR=0.50, 95% CI: 0.36 to 0.70) and Minnesota Code (HR= 0.65, 95%CI: 0.46, 0.90) in SPRINT participants without LVH by these criteria at baseline. Also, intensive BP lowering was associated with more regression (improvement) of LVH by Cornell voltage product (HR=1.22, 95% CI: 1.02 to 1.45), Sokolow-Lyon (HR=1.78, 95% CI: 1.34 to 2.39) and Minnesota Code (HR= 1.57, 95% CI: 1.03, 2.42) in SPRINT participants with LVH using these criteria at baseline as shown in Supplemental Table 4.
Using random coefficient models in all participants with and without baseline LVH, the rate of regression of Cornell voltage product index and Sokolow-Lyon index (i.e. as continuous variables) were also faster in the intensive BP lowering arm than that in the standard arm similar to what was observed in the main analysis with Cornell voltage index (Supplemental Table 5).
When we excluded 718 participants with major intraventricular conduction delay, the impact of intensive (compared with standard) BP lowering on the risk of developing new LVH remained similar to that observed without this exclusion regardless of the ECG LVH criteria used. (HR=0.53, 95% CI: 0.41 to 0.69 for Cornell voltage; HR=0.58 (95% CI: 0.48 to 0.71) for Cornell voltage product; HR=0.47 (95% CI: 0.33 to 0.67) for Sokolow-Lyon; and HR= 0.65, 95%CI: 0.46 to 0.92 for Minnesota Code LVH)-(Supplemental Table 6)
Among SPRINT participants with ECG data who were included in the analysis (n=8,164), a total of 552 CVD events occurred, while 10 events occurred among those excluded from the analysis due to missing or uninterpretable ECG data (n=1,197). Intensive BP lowering was associated with a 24% (p-value= 0.001) lower risk of CVD events which was marginally attenuated to 23% lower risk (p-value =0.003) after adjusting for LVH as a time-varying covariate among those with ECG data. Notably, in the same model, presence (vs. absence) of LVH as a time-varying covariate was associated with almost twice the risk of CVD events compared to those without LVH (HR=1.99, 95%CI: 1.53 to 2.57; p-value <0.001) (Table 5).
Table 5.
Effect of intensive BP lowering on SPRINT primary CVD outcome with and without adjusting for Cornell ECG-LVH and Cornell Index, separately, as time-varying covariates
| Hazard ratio (95%CI) | P-value | |
|---|---|---|
| Intensive vs. standard BP lowering | 0.76 (0.64, 0.90) | 0.001 |
| Intensive vs. standard BP lowering with adjusting for Cornell voltage ECG-LVH (categorical variable) as time varying covariate* | 0.77 (0.65, 0.91) | 0.003 |
| Intensive vs. standard BP lowering with adjusting for Cornell index (continuous variable) as a time varying covariate† | 0.77 (0.65, 0.91) | 0.002 |
LVH= left ventricular hypertrophy; Cornell Index= sum of the R amplitude in aVL and S amplitude in V3 in microvolt (μV); BP= blood pressure; CI= confidence interval; SPRINT primary CVD outcome= first occurrence of myocardial infarction, acute coronary syndrome, stroke, heart failure, or death from cardiovascular causes.
In the model ECG-LVH was associated with almost double the risk of CVD events (HR= 1.99, 95%CI: 1.53 to 2.57; p-value<0.001)
In the same model each 1-standard deviation (669 μV) increase in mean Cornell voltage index was associated with 23% increased risk of CVD events (HR= 1.23, 95%CI: 1.13 to 1.32; p-value <0.001).
Similar magnitude of attenuation was observed when Cornell voltage index (as a continuous variable) was used in the model as a time-varying covariate instead of ECG-LVH. That is to say, the effect of intensive BP on lowering the risk of the primary outcome also was attenuated from 24% (p=0.001) to 23% (p=0.002) as well (Table 5). In the same model each 1-standard deviation (669 μV) increase in mean Cornell voltage index was associated with 23% increased risk of CVD events (HR=1.23, 95% CI: 1.13, 1.32); p-value <0.001).
Using Cornell voltage product or Sokolow-Lyon Criteria either as categorical variables or continuous variables instead of Cornell voltage yielded the same marginal attenuation of the relationship between intensive BP lowering and CVD events (Supplemental Table 7 and Supplemental Table 8). Also, there was no effect modification (i.e. interaction) by baseline ECG-LVH status by any of the criteria on the relationship between intensive BP lowering and the SPRINT primary CVD outcome (interaction p-value= 0.52 for Cornell voltage LVH, 0.57 for Cornell voltage product LVH, and 0.66 for Sokolow-Lyon LVH (Supplemental Table 9).
DISCUSSION
Principal findings
In this post-hoc analysis from the SPRINT trial we examined the effect of intensive BP lowering on the risk of LVH and whether this effect explains the reported cardiovascular benefits of intensive BP lowering in patients with hypertension at high risk for CVD but no diabetes. The key findings are: 1) intensive BP lowering, compared with standard BP lowering, resulted in lower rates of developing new LVH in those without LVH, with these results consistent among several subgroups of SPRINT participants; 2) intensive BP lowering, compared with standard BP lowering, resulted in more regression of LVH in those with existing LVH, with the effect of intensive BP lowering on regression of LVH stronger in those with than in those without prior CVD, but consistent across other subgroups; 3) the benefit of CVD risk reduction associated with intensive BP lowering was not substantially attenuated after adjusting for LVH as a time-varying covariate; and 4) there was no effect modification of the baseline LVH status on the relationship between intensive BP lowering and SPRINT primary CVD outcome.
Taken altogether, intensive BP lowering resulted in lower rates of LVH in the SPRINT trial by reducing the risk of developing new LVH and improving existing LVH. This favorable impact on LVH, however, appears to explain little of the reduction in CVD events associated with intensive BP lowering in SPRINT.
Results in Context
LVH is an adaptive response to the wall stress associated with increased impedance to ventricular emptying due to increased peripheral resistance occurring as a result of high blood pressure.(20) This explains results from several prior reports showing that regression of LVH is possible by interventions aimed at lowering high BP.(21–30) However, none of these studies were designed to examine whether lowering BP beyond a standard goal of BP <140–150/90 mmHg is associated with greater reduction of the risk of LVH. Only two trials, however, tried to answer this question before. In the Cardio-Sis trial (1,111 participants, without diabetes and with at least one CVD risk factor) lowering of SBP to <130 mmHg decreased the likelihood of ECG-LVH by 39%, compared with usual lowering to SBP <140 mmHg.(4) In the ACCORD BP trial (4,331 participants with hypertension with diabetes and at high risk of CVD) intensive BP lowering (target SBP to <120 mmHg) resulted in a similar 39% reduction in LVH risk compared to standard BP lowering (target <140 mmHg).(3) To our knowledge, our results from the SPRINT trial are the first to provide evidence from a randomized clinical trial that includes a large diverse population of patients with hypertension without diabetes to suggest that intensive (SBP <120 mmHg) is associated with a lower risk of LVH compared with standard BP lowering (SBP<140 mmHg).
Although mechanical stress due to pressure overload is the major driver for LVH in patients with hypertension, it is currently recognized that neuro-hormonal abnormalities play an important role as well. Neuro-hormonal substances such as angiotensin II, aldosterone, norepinephrine, and insulin can directly promote myocyte hypertrophy and matrix deposition independent of their effects on systemic arterial pressure. (31) This could explain why although successful lowering of SBP in our study caused regression of LVH in a large proportion of SPRINT participants with baseline LVH (62% total; 70% in intensive arm, and 55% in standard arm), still some patients remained with LVH. It also has been reported that LVH can lead to irreversible fibrosis and scars in the myocardium that may not be responsive to antihypertensive treatment (32), which could also explain why successful BP lowering did not improve all LVH and also suggest that prevention of development of LVH rather than treating it may be a better strategy.
We also found that the benefit of intensive BP lowering on the risk of CVD events was not meaningfully influenced by its favorable effect on LVH. This suggests that the effect of intensive BP lowering on CVD may be through different mechanisms and LVH is just one of many mediating factors. Another possible explanation is that LVH perhaps mediate the effect of intensive BP lowering on certain CVD outcomes but not others. Notably, intensive BP lowering was associated with a lower risk of heart failure but not myocardial infarction or acute coronary syndromes in SPRINT; all are SPRINT secondary outcomes (12). Compared to a composite of CVD events (33) or coronary heart disease (34), LVH is an established predictor heart failure and is a component of the Framingham heart failure risk prediction score. (35) This may explain why intensive BP lowering selectively reduced the risk of heart failure more than other SPRINT secondary CVD outcomes. Due to the relatively small number of the individual SPRINT secondary CVD outcomes (heart failure, stoke, myocardial infarction, death from any cause), we could not usefully examine the associations between intensive BP lowering, individual CVD outcomes and LVH i.e. a statistical power limitation.
On the basis of our results in SPRINT that included patients with hypertension but no diabetes and taking into account our prior results from the ACCORD BP trial that included patients with hypertension and diabetes (3), it could be suggested that intensive treatment to a target SBP of < 120 mm Hg in hypertensive patients at high risk of CVD will reduce the risk of LVH. Nevertheless, there could be variations in the response to the effect of intensive BP lowering on LVH among certain groups. In our subgroup analysis, those with prior CVD showed more benefit for regression of LVH although they did not show more benefit for developing new incident LVH during the follow up period.
Limitations and Strengths
Our results should be read in the context of certain limitations and methodological considerations. By design, the SPRINT trial had an open-label design which could lead to bias the identification of certain types of endpoints. However, it is unlikely that the open label design had an impact on the ascertainment of LVH, which was measured from ECGs that were read centrally at an ECG core laboratory blinded to the treatment assignment. Since SPRINT was a treatment strategy trial in the sense that it examined the effect of different levels of SBP rather than the effect of individual drugs, we could not separate the impact of lowering BP from the impact of individual medications. Another limitation is that it may not be appropriate to generalize our findings to other types of hypertension patients not included in SPRINT such as those with lower CVD risk, prior stroke, younger than 50 years or with diabetes. Nevertheless, some of these groups such as those with hypertension and diabetes (3) have been examined before, which actually makes our study unique.
We defined LVH using ECG not imaging (echocardiography or cardiac magnetic resonance imaging). Although imaging provides a more accurate assessment of LVH than does the ECG, any misclassification should have impacted both arms equally and hence the effect should be balanced. Nevertheless, significant non-differential misclassification of LVH could impact our ability to estimate the true mediation of LVH, which could explain the marginal attenuation of the CVD risk associated with intensive BP after adjusting for LVH as a time-varying covariate. More importantly, LVH detected by ECG has been shown to be predictive of poor outcomes in a similar way to LVH detected by imaging. (36–39) These findings along with its wide availability and low-cost have made the ECG the ideal tool for initial evaluation of patients with hypertension to detect LVH. (40) In a related point, we decided to use Cornell voltage to define LVH because of its simple calculation that incorporates sex specific cut-off points. As one of the most commonly used LVH criteria, it has had good diagnostic performance in multi-ethnic settings compared to other LVH criteria as well as high prognostic significance as a predictor for CVD events (41), and is not impacted by obesity. (42) Since there are several other LVH criteria, it could be argued that our results should only be applied to Cornell voltage LVH. However, we did sensitivity analyses using two other commonly used LVH criteria (Cornell voltage product and Sokolow-Lyon) and we observed similar results. Further, the current recommendations for the use of ECG criteria for detection of cardiac chamber enlargement (18) do not favor or recommend one set of LVH criteria over the other (i.e. any LVH criteria could be used as long as specifically named). Therefore, using Cornell voltage or another should serve the purpose and accord with these recommendations.
Despite these limitations, this analysis is the first report from a well-designed large clinical trial in which the effect of intensive BP lowering on LVH in patients with hypertension without diabetes is examined. The strengths of our study include large sample size, racially diverse population with representation of both sexes and inclusion of a large proportion of patients over 75 years old, the random assignment of participants to treatment arms resulting in balanced groups at baseline, standardized data collection including ECG data that were centrally read, and achievement and maintenance of the intended differences in SBP between arms throughout the study.
Conclusions
In patients with hypertension but no diabetes intensive BP lowering (target <120 mmHg) reduces the risk of LVH by preventing development of new LVH in those without LVH and causing regression of LVH in those with existing LVH. This favorable impact on LVH, however, does not explain most of the reduction in CVD events associated with intensive BP lowering in SPRINT. These findings add further evidence of the benefits of the intensive BP lowering in patients with hypertension, and suggest that these benefits go beyond reducing the hemodynamic stress on the cardiac structure. Understanding the mediating factors and the mechanisms by which intensive BP lowering impacts the cardiovascular system would help in better selection of those who may benefit with least harm.
Supplementary Material
Clinical Perspective.
What is new?
In patients with hypertension but no diabetes enrolled in the SPRINT trial, intensive blood pressure (BP) lowering (target <120 mmHg) reduced the risk of left ventricular hypertrophy (LVH) by preventing development of new LVH in those without LVH and causing regression of LVH in those with existing LVH.
This favorable impact on LVH, however, did not explain most of the reduction in cardiovascular (CVD) events associated with intensive BP lowering.
What are the clinical implications?
These findings add further evidence of the benefits of the intensive BP lowering in patients with hypertension, and suggest that these benefits go beyond reducing the hemodynamic stress on the cardiac structure.
Further research is needed to understand the mediating factors and the mechanisms by which intensive BP lowering impacts the cardiovascular system.
Acknowledgments
Funding Sources:
The Systolic Blood Pressure Intervention Trial is funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. The SPRINT investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. We also acknowledge the support from the following CTSAs funded by NCATS: CWRU: UL1TR000439, OSU: UL1RR025755, U Penn: UL1RR024134& UL1TR000003, Boston: UL1RR025771, Stanford: UL1TR000093, Tufts: UL1RR025752, UL1TR000073 & UL1TR001064, University of Illinois: UL1TR000050, University of Pittsburgh: UL1TR000005, UT Southwestern: 9U54TR000017-06, University of Utah: UL1TR000105-05, Vanderbilt University: UL1 TR000445, George Washington University: UL1TR000075, University of CA, Davis: UL1 TR000002, University of Florida: UL1 TR000064, University of Michigan: UL1TR000433, Tulane University: P30GM103337 COBRE Award NIGMS.
All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the U.S. Department of Veterans Affairs, or the United States Government. For a full list of contributors to SPRINT, please see the supplementary acknowledgement list: https://www.sprinttrial.org/public/dspScience.cfm.
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the United States Department of Health and Human Services or of the Veterans Administration.
Footnotes
Clinical Trial Registration: ClinicalTrials.gov NCT01206062
Conflict of Interest: None
References
- 1.Katholi RE, Couri DM. Left ventricular hypertrophy: major risk factor in patients with hypertension: update and practical clinical applications. Int J Hypertens. 2011;2011:495349. doi: 10.4061/2011/495349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 3.Soliman EZ, Byington RP, Bigger JT, Evans G, Okin PM, Goff DC, Jr, Chen H. Effect of Intensive Blood Pressure Lowering on Left Ventricular Hypertrophy in Patients With Diabetes Mellitus: Action to Control Cardiovascular Risk in Diabetes Blood Pressure Trial. Hypertension. 2015;66:1123–1129. doi: 10.1161/HYPERTENSIONAHA.115.06236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdecchia P, Staessen JA, Angeli F, de Simone G, Achilli A, Ganau A, Mureddu G, Pede S, Maggioni AP, Lucci D, Reboldi G Cardio-Sis investigators. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525–533. doi: 10.1016/S0140-6736(09)61340-4. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Salomon M, D’Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90:1786–1793. doi: 10.1161/01.cir.90.4.1786. [DOI] [PubMed] [Google Scholar]
- 6.Prineas RJ, Rautaharju PM, Grandits G, Crow R MRFIT Research Group. Independent risk for cardiovascular disease predicted by modified continuous score electrocardiographic criteria for 6-year incidence and regression of left ventricular hypertrophy among clinically disease free men: 16-year follow-up for the Multiple Risk-Factor Intervention Trial. J Electrocardiol. 2001;34:91–101. doi: 10.1054/jelc.2001.23360. [DOI] [PubMed] [Google Scholar]
- 7.Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 8.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlöf B LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 9.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 10.Okin PM, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Lindholm LH, Dahlöf B LIFE Study Investigators. In-treatment resolution or absence of electrocardiographic left ventricular hypertrophy is associated with decreased incidence of new-onset diabetes mellitus in hypertensive patients: the Losartan Intervention for Endpoint reduction in hypertension (LIFE) Study. Hypertension. 2007;50:984–990. doi: 10.1161/HYPERTENSIONAHA.107.096818. [DOI] [PubMed] [Google Scholar]
- 11.Okin PM, Devereux RB, Harris KE, Jern S, Kjeldsen SE, Julius S, Edelman JM, Dahlöf B LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med. 2007;147:311–319. doi: 10.7326/0003-4819-147-5-200709040-00006. [DOI] [PubMed] [Google Scholar]
- 12.SPRINT Research Group. Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC, Jr, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT, Jr, Whelton PK SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casale PN, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 15.Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol. 1992;20:1180–1186. doi: 10.1016/0735-1097(92)90376-x. [DOI] [PubMed] [Google Scholar]
- 16.Sokolow M, Lyon TP. Ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 17.Prineas RJ, Crow RS, Zhang ZM. The Minnesota Code Manual of Electrocardiographic Findings. 2. Springer-Verlag Limited; London, New York, USA: 2010. [Google Scholar]
- 18.Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75:383–386. [Google Scholar]
- 19.Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, Bailey JJ, Childers R, Gorgels A, Josephson M, Kors JA, Macfarlane P, Mason JW, Pahlm O, Rautaharju PM, Surawicz B, van Herpen G, Wagner GS, Wellens H American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy. J Am Coll Cardiol. 2009;53:992–1002. doi: 10.1016/j.jacc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Prisant LM. Hypertensive Heart Disease. J Clin Hypertens. 2005;7:231–238. doi: 10.1111/j.1524-6175.2005.04119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebson PR, Grandits GA, Dianzumba S, Prineas RJ, Grimm RH, Jr, Neaton JD, Stamler J. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS) Circulation. 1995;91:698–706. doi: 10.1161/01.cir.91.3.698. [DOI] [PubMed] [Google Scholar]
- 22.Lièvre M, Guéret P, Gayet C, Roudaut R, Haugh MC, Delair S, Boissel JP. Ramipril-induced regression of left ventricular hypertrophy in treated hypertensive individuals. HYCAR Study Group. Hypertension. 1995;25:92–97. doi: 10.1161/01.hyp.25.1.92. [DOI] [PubMed] [Google Scholar]
- 23.Gottdiener JS, Reda DJ, Massie BM, Materson BJ, Williams DW, Anderson RJ. Effect of single-drug therapy on reduction of left ventricular mass in mild to moderate hypertension: comparison of six antihypertensive agents. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Circulation. 1997;95:2007–2014. doi: 10.1161/01.cir.95.8.2007. [DOI] [PubMed] [Google Scholar]
- 24.Thürmann PA, Kenedi P, Schmidt A, Harder S, Rietbrok N. Influence of the angiotensin II antagonist valsartan on left ventricular hypertrophy in patients with essential hypertension. Circulation. 1998;98:2037–2042. doi: 10.1161/01.cir.98.19.2037. [DOI] [PubMed] [Google Scholar]
- 25.Gerritsen TA, Bak AA, Stolk RP, Jonker JJ, Grobbee DE. Effects of nitrendipine and enalapril on left ventricular mass in patients with non-insulin-dependent diabetes mellitus and hypertension. J Hypertens. 1998;16:689–696. doi: 10.1097/00004872-199816050-00017. [DOI] [PubMed] [Google Scholar]
- 26.Roman MJ, Alderman MH, Pickering TG, Pini R, Keating JO, Sealey JE, Devereux RB. Differential effects of angiotensin converting enzyme inhibition and diuretic therapy on reductions in ambulatory blood pressure, left ventricular mass, and vascular hypertrophy. Am J Hypertens. 1998;11:387–396. doi: 10.1016/s0895-7061(97)00492-5. [DOI] [PubMed] [Google Scholar]
- 27.Gosse P, Sheridan DJ, Zannad F, Dubourg O, Guéret P, Karpov Y, de Leeuw PW, Palma-Gamiz JL, Pessina A, Motz W, Degaute JP, Chastang C. Regression of left ventricular hypertrophy in hypertensive patients treated with indapamide SR 1.5 mg versus enalapril 20 mg : the LIVE study. J Hypertens. 2000;18:1465–1475. doi: 10.1097/00004872-200018100-00015. [DOI] [PubMed] [Google Scholar]
- 28.Terpstra WF, May JF, Smit AJ, de Graeff PA, Havinga TK, van den Veur E, Schuurman FH, Meyboom-de Jong B, Crijns HJ. Long-term effects of amlodipine and lisinopril on left ventricular mass and diastolic function in elderly, previously untreated hypertensive patients: the ELVERA trial. J Hypertens. 2001;19:303–309. doi: 10.1097/00004872-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Devereux RB, Palmieri V, Sharpe N, De Quattro V, Bella JN, de Simone G, Walker JF, Hahn RT, Dahlöf B. Effects of once-daily angiotensin-converting enzyme inhibition and calcium channel blockade-based antihypertensive treatment regimens on left ventricular hypertrophy and diastolic filling in hypertension: the prospective randomized enalapril study evaluating regression of ventricular enlargement (PRESERVE) trial. Circulation. 2001;104:1248–1254. doi: 10.1161/hc3601.095927. [DOI] [PubMed] [Google Scholar]
- 30.Malmqvist K, Kahan T, Edner M, Held C, Hägg A, Lind L, Müller-Brunotte R, Nyström F, Ohman KP, Osbakken MD, Ostergern J. Regression of left ventricular hypertrophy in human hypertension with irbesartan. J Hypertens. 2001;19:1167–1176. doi: 10.1097/00004872-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 31.Johnson DB, Dell’Italia LJ. Cardiac hypertrophy and failure in hypertension. Curr Opin Nephrol Hypertens. 1996;5:186–191. doi: 10.1097/00041552-199603000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Koyanagi S, Eastham C, Marcus ML. Effects of chronic hypertension and left ventricular hypertrophy on the incidence of sudden cardiac death after coronary artery occlusion in conscious dogs. Circulation. 1982;65:1192–1197. doi: 10.1161/01.cir.65.6.1192. [DOI] [PubMed] [Google Scholar]
- 33.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 34.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 35.Kannel WB, D’Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 36.Oseni AO, Qureshi WT, Almahmoud MF, Bertoni AG, Bluemke DA, Hundley WG, Lima JA, Herrington DM, Soliman EZ. Left ventricular hypertrophy by ECG versus cardiac MRI as a predictor for heart failure. Heart. 2016 doi: 10.1136/heartjnl-2016-309516. pii: heartjnl-2016-309516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almahmoud MF, O’Neal WT, Qureshi W, Soliman EZ. Electrocardiographic versus echocardiographic left ventricular hypertrophy in prediction of congestive heart failure in the elderly. Clin Cardiol. 2015;38:365–370. doi: 10.1002/clc.22402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Neal WT, Almahmoud MF, Qureshi WT, Soliman EZ. Electrocardiographic and echocardiographic left ventricular hypertrophy in the prediction of stroke in the elderly. J Stroke Cerebrovasc Dis. 2015;24:1991–1997. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leigh JA, O’Neal WT, Soliman EZ. Electrocardiographic left ventricular hypertrophy as a predictor of cardiovascular disease independent of left ventricular anatomy in subjects aged ≥65 years. Am J Cardiol. 2016;117:1831–1835. doi: 10.1016/j.amjcard.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moser M. Initial workup of the hypertensive patient. In: Izzo JL, Black HR, Goodfriend TL, editors. Hypertensive Primer: The Essentials of High Blood Pressure. Williams and Wilkins; Philadelphia, PA, USA: 1998. pp. 221–223. [Google Scholar]
- 41.Jain A, Tandri H, Dalal D, Chahal H, Soliman EZ, Prineas RJ, Folsom AR, Lima JA, Bluemke DA. Diagnostic and prognostic utility of electrocardiography for left ventricular hypertrophy defined by magnetic resonance imaging in relationship to ethnicity: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2010;159:652–658. doi: 10.1016/j.ahj.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abergel E, Tase M, Menard J, Chatellier G. Influence of obesity on the diagnostic value of electrocardiographic criteria for detecting left ventricular hypertrophy. Am J Cardiol. 1996;77:739–744. doi: 10.1016/s0002-9149(97)89209-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.