Abstract
This study was designed to investigate the effect of single-dose radiation therapy (RT) in combination with evofosfamide (TH-302), a hypoxia-activated prodrug, in a pre-clinical model of pancreatic cancer. AsPC1 tumors were implanted orthotopically in the pancreas of nude mice. Tumors were treated with 15 Gy of RT, using a 1 cm diameter field, and delivered as a continuous arc. Image-guidance to center the field on the tumor was based on CT imaging with intraperitoneal contrast. Evofosfamide (100 mg/kg, i.p.) was administered 3 hours before RT. Tumor volumes were measured using ultrasound, and regrowth curves were plotted. Tumor hypoxia and cell proliferation were measured using pimonidazole and the thymidine analog EdU, respectively. In vitro clonogenic assays were performed. Tumors were shown to contain substantial areas of hypoxia, as calculated by percent pimonidazole staining. Evofosfamide was active in these tumors, as demonstrated by a significant reduction in uptake of the thymidine analog EdU. This effect was visible in oxygenated tissue, consistent with the previously reported bystander effects of evofosfamide. RT produced significant regrowth delay, as did evofosfamide. The combination of both agents produced a growth delay that was at least equal to the sum of the two treatments given separately. The improvement in tumor response when evofosfamide is combined with RT supports the hypothesis that hypoxia is a cause of radioresistance in high dose RT for pancreatic cancer. Assessing the efficacy and safety of stereotactic radiation treatment and evofosfamide is warranted in patients with locally advanced pancreatic cancer.
Introduction
Tumor hypoxia is a prominent feature of pancreatic tumors. Using Eppendorf electrode measurements in seven patients, Koong et al. found hypoxic fractions (defined as pO2< 2.5 mm Hg) ranging from 24–95% [1]. A recent study using pimonidazole in 10 patients also reported significant levels of hypoxia [2], though the pimonidazole positive fraction ranged from 1–40%. Hypoxia PET imaging has also been employed to assess the hypoxic phenotype of pancreatic cancer. Using the hypoxia-imaging agent, [18F]-fluoroazomycin arabinoside, ([18F]-FAZA), Metran-Nascente et al. found substantial hypoxia in a group of 20 patients with pancreatic tumors. However, this group also contained a subset of patients who showed no evidence of tumor hypoxia [3].
Evofosfamide, also known as TH-302, is a hypoxia-activated prodrug, consisting of a 2-nitroimidazole moiety linked to bromo-isophosphoramide mustard (Br-IPM). The nitroimidazole undergoes one electron reduction, and under low oxygen concentration (hypoxic) conditions, the prodrug fragments releasing the Br-IPM effector to react with and cross-link DNA [4]. Evofosfamide has been extensively studied in preclinical studies [5], [6], [7], [8], [9], [10], [11], [12]. The efficacy and safety of the evofosfamide and gemcitabine combination has been tested in xenograft models of pancreatic cancer. Using tumor growth inhibition and tumor growth delay analysis, the combination exhibited superior efficacy compared to the monotherapies in three of four models tested [3]. The efficacy and safety of the evofosfamide gemcitabine, and nab-paclitaxel triplet combination has also been tested in xenograft models of pancreatic cancer. Using tumor growth inhibition and Kaplan–Meier analysis, the triplet combination exhibited superior efficacy at the expense of more toxicity [11].
Several clinical trials have evaluated the safety and efficacy of evofosfamide as monotherapy or in combination with various chemotherapeutics. Adding evofosfamide to gemcitabine has been shown in a phase 2 randomized trial to improve progression-free survival compared with gemcitabine alone in patients with previously untreated advanced-stage pancreatic cancer (6.0 months versus 3.6 months; P = .008) [13]. The phase 3 MAESTRO trial tested this doublet in previously untreated subjects with metastatic or locally advanced unresectable pancreatic adenocarcinoma (NCT01746979), where evofosfamide failed to significantly improve overall survival when added to gemcitabine. However, there was a trend toward an improvement in overall survival – (P = .06), but the trial design did not identify or exclude patients with minimal tumor hypoxia.
The field of radiation therapy is moving in the direction of hypofractionation or single fraction high dose protocols. The well-documented effects of hypoxia on radiotherapy [14], [15] become especially pertinent when radiation is delivered as high dose per fraction, since the impact of hypoxia is believed to be mitigated through reoxygenation when radiation is delivered in conventional 2 Gy/day fractions. Direct evidence for early mid-treatment re-oxygenation has been provided by serial PET imaging using the hypoxia specific radiotracer fluoromisonidazole [16]. High-dose, single-fraction image-guided intensity modulated radiation treatment has been shown to achieve local control rates up to 90% [17]. Nonetheless, preclinical and clinical studies have shown that hypofractionation can result in reduced tumor cell kill compared to conventionally fractionated radiation treatment in tumors with regions of hypoxia [18]. Hypoxia-activated cytotoxins such as evofosfamide provide an opportunity to overcome hypoxic radioresistance. Evofosfamide has been shown both to target hypoxic tumor areas and exert bystander effects on well-oxygenated surrounding tumoral cells [12]. Therefore, we hypothesize that combining evofosfamide with high-dose, single-fraction radiotherapy could increase rates of tumor control. This study is designed to investigate the effect of single-dose radiation treatment and evofosfamide in a pre-clinical model of pancreatic cancer.
Materials and Methods
Cell Culture
AsPC1 cells were originally obtained from the American Type Culture Collection (ATCC, Manassas, VA), and cultured in RPMI with 10% fetal bovine serum and sodium pyruvate (1 mM) in a 5% CO2 atmosphere at 37 °C.
Clonogenic Assay of Cells In Vitro
Cells were trypsinized to produce a single cell suspension, seeded out, and allowed to attach overnight prior to treatment. Visual inspection showed that cells were predominantly in a single-cell state at time of treatment. Cells were seeded out in 25cm2 flasks in the presence of a lethally irradiated feeder layer (105 cells/flask 50 Gy; the single-cell status was determined on flasks containing 105 viable cells alone.) Irradiation was delivered using a 137Cs source, (Shepherd Mark I, JL Shepherd, San Fernando, CA) at a dose rate of approximately 1.7 Gy/minute. For evofosfamide treatment, experiments were performed at 0.1% O2 in an InVivo400 hypoxia workstation (Baker Ruskinn, Sanford, ME). Cells were seeded and allowed to attach in air as above, transferred to the workstation, where their medium was replaced with medium pre-equilibrated for 4 hours in 15 ml aliquots, and drug added. Drug exposure was for one hour. Evofosfamide was dissolved in DMSO (100 mg/ml) and diluted to a maximum of 20 μg/ml. (The final DMSO concentration was not more than 0.02%.) Colonies were allowed to form in air over 10–14 days. For counting, the definition of a colony was taken as >50 cells. Surviving fractions were calculated [19]; radiation survival was fitted to the linear quadratic equation, ln(SF) = −(αD + βD2).
Mouse and Tumor Models
All animal studies were conducted under approved guidelines set forth by the Institutional Animal Care and Use Committee in a protocol approved by the MSKCC Animal Research Center. Female Balb/c nu/nu mice of 6–8 weeks of age were purchased from Harlan Laboratories (Indianapolis, IN). Tumor xenografts were implanted into animals using standard protocols [20]. Briefly, animals were anesthetized (isofluorane) and placed on their right side under surgical drape, within a bioguard safety hood. Carprofen (5 mg/kg subcutaneously) was given for pre-emptive analgesia. Following a standard aseptic prep, a left flank incision was made into the peritoneal cavity. The spleen was gently retracted to expose the pancreas, and the tumor graft secured to the native pancreas with a single, simple interrupted ligature (4–0 Vicryl, polyglactin 910; Ethicon, Bridgewater, NJ). The pancreas and spleen were replaced in the abdomen. Routine two-layer closure was performed with the same absorbable suture as above in the muscle. Stainless steel wound clips were used to appose skin edges. Tumor grafts were obtained from AsPC1 tumors, grown subcutaneously in nude mice. Tumor pieces were stored in liquid nitrogen (with 10% DMSO and 10% fetal bovine serum).
Drug and Tracer Administration
Evofosfamide (Merck, Darmstadt, Germany) was dissolved in DMSO and added to saline at a final concentration of 10 mg/ml drug, 0.2% DMSO. Drug was injected i.p. at 100 mg/kg, three hours prior to radiation treatment. Animals were weighed to assure consistent dosage. To measure hypoxia, pimonidazole (H&I Inc., Burlington MA.) was given at 100 mg/kg i.p. Cell proliferation was visualized with 5-ethynyl-2-deoxyuridine 5 mg/kg i.p. (EdU; InVitrogen, Eugene, OR). In experiments relating evofosfamide toxicity to hypoxia, pimonidazole and evofosfamide were co-injected, and EdU was given 24 hours after evofosfamide. Animals were sacrificed 2 hours after EdU administration. Autoradiography was performed using [imidazole-214C] Evofosfamide (specific activity 2 GBq/mmol, generously supplied by Merck). Animals were injected ip with 370 kBq 14C–evofosfamide, along with unlabeled evofosfamide (100 mg/kg).
Image Guided X-ray Radiotherapy
The procedure for CT imaging and arc radiotherapy has been described previously [21]. Both imaging and treatment are carried out on a Precision X-ray 225CX small animal micro-irradiator (Precision X-Ray, North Branford, CT). Radiation dosimetry was performed by the dosimetry staff at MSKCC. Animals were anesthetized under isoflurane (2% in air), injected i.p. with 4 ml 5% Iohexol (GE Healthcare) and imaged (40 kVp, 2.5 mA, 2 mm Al). Tumors were identified as anomalous masses usually found near the spleen. (Implantation was in the tail of the pancreas.) An example of a tumor imaged thus is shown in Figure E1. The animal was mechanically moved so that the tumor was positioned at the treatment isocenter. Radiation (225 kVp, 13 mA, 3 mm Cu) was given at a dose rate of approximately 3 Gy/minute, delivered through a circular collimator of 1 cm diameter as a continuous 360o arc, using the rotatable gantry of the micro-irradiator. The dose chosen (15Gy) was previously shown to be consistent with long term survival of the mice for the field size and rotating field.
Ultrasound Imaging
Tumors were imaged using the VeVo 2100 (VisualSonics, Toronto ON). Animals were anesthetized with isoflurane and imaged with a 550 s transducer using the manufacturer's general imaging and abdominal presets. Tumor volume was approximated from an image of the tumor at its maximum cross section, by measuring the diameters (d1, anterior posterior; d2 left right), and applying the formula V = 0.5 × d12 × d2, which correlated well with volumes obtained from the VeVo 3D scan protocol.
Immunohistochemistry
Tumors were removed and frozen in Tissue-Tek embedding medium (Sakura Finetek USA, Torrance CA) and sectioned to a 10 μm thickness on a cryotome. Sections were fixed for 10 minutes in 4% paraformaldehyde in PBS, rinsed three times in PBS, and blocked in 10% goat serum, 10% bovine serum albumin (BSA) in PBS for 1 hour. Sections were stained with FITC-conjugated anti-pimonidazole antibody (H&I Inc) diluted 1:20 in blocking solution for 1 hour. EdU was visualized with AlexaFluor 555 azide (Invitrogen), according to manufacturer's instructions. Immunofluorescent microscopic images were obtained using an Olympus BX60 microscope and Microsuite Biological Suite imaging software (Olympus America, Center Valley, PA). The fraction of the tumor staining positive for pimonidazole was calculated by applying Otsu thresholding in ImageJ. Necrotic tissue was readily identifiable on the sections based on its fragmented appearance in immunohistochemical images and was excluded from the analysis.
Autoradiography
Autoradiography was performed using phosphor screens exposed for 7 days at -20o C. Images were read with the Typhoon FLA 7000 laser scanner (GE Healthcare Life Sciences, Pittsburgh, PA). Prior to exposure, slides were marked with nail polish containing 14C and crystal violet to serve as fiduciary markers for registration of autoradiographic and microscopic images [22].
Statistics
Tumor growth delay was calculated as the median time for tumors to reach 3× their starting volume. Tumors which failed to regrow were assigned an arbitrarily large value. P values were calculated using the Mann-Whitney U test.
Results
In Vitro Sensitivity of AsPC1 Cells to Evofosfamide and Radiation
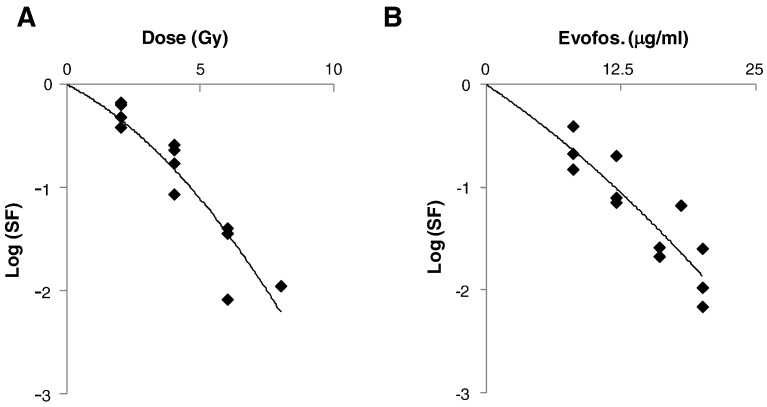
Clonogenic survival of AsPC1 cells after aerobic radiation is illustrated in Figure 1A. The line represents a linear-quadratic fit of the data with α and β values of 0.306 Gy−1 and 0.041 Gy−2 respectively. This data suggests that AsPC1 cells are relatively radioresistant, with a surviving fraction at 2 Gy (SF2) of 0.54 (95% confidence intervals 0.41–0.69), similar to cell lines derived from gliomas [23]. 1 log of cell kill was achieved by 12.5 μg/ml evofosfamide (1 hour exposure at 0.1% O2), which is comparable to the toxicity reported for these conditions in H460 cells [7].
Figure 1.
Clonogenic survival of AsPC1 cells exposed to (A) 137Cs γ-radiation in air and (B) evofosfamide in 0.1% O2 for 1 hour. Symbols represent survival from three independent experiments. Plating efficiency of untreated cells was 30%.
Tumor Hypoxia and Reduction in Proliferation in Response to Evofosfamide Treatment
Pimonidazole immunohistochemistry revealed the presence of hypoxia in AsPC1 tumors (Figure E2). Hypoxic fractions were calculated by applying Otsu thresholds to viable tumor tissue. In Otsu thresholding, the thresholds are set so that the combined intra-class variance is minimized. A total of 22 sections were analyzed. The mean hypoxic fraction of all sections was 0.086 (range 0.005–0.19). Three tumors were sectioned at four different depths and a fourth at 3, each level being separated by 1 mm, to allow estimation of inter-tumor variation [24]: pimonidazole positivity ranged from 1.2–11.3%. Overall AsPc1 tumors were within the range found in clinical specimens [2].
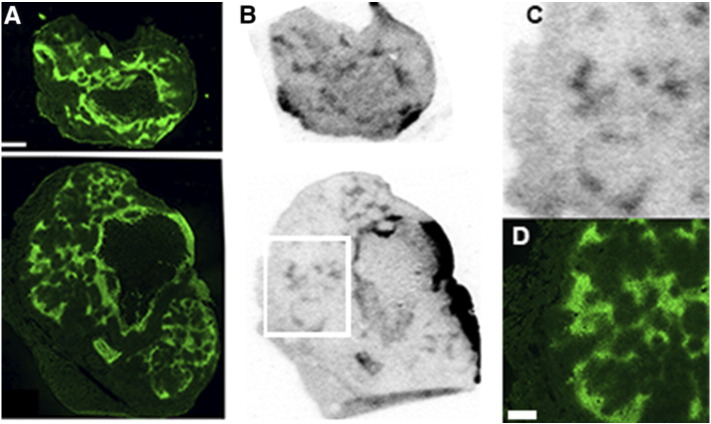
The association of pimonidazole and evofosfamide was investigated by autoradiography (Figure 2). The 14C label is present on the imidazole moiety, and signal thus represents either the intact prodrug or the cleaved imidazole trigger. The images represent tracer distribution five hours after administration. Although visually there is good concordance between pimonidazole and evofosfamide throughout the body of the tumor, there is a striking mismatch on the tumor boundary, where heavy concentrations of evofosfamide are not matched by pimonidazole staining. One explanation is that this may reflect the presence of tumor blood supply, as large vessels are commonly observed round the tumor boundary.
Figure 2.
Distribution of evofosfamide in tumors relative to pimonidazole (green). Animals were treated with pimonidazole and 14C labeled evofosfamide. The 14C label resides on the imidazole moiety which is cleaved when the prodrug is activated. (A) Pimonidazole binding (B) 14C autoradiography obtained from the same section Scale bar = 1 mm The white square in (B) denotes the area expanded in (C) autoradiograph and (D) pimonidazole image. Scale bar = 0.5 mm.
The behavior of the free Br-IPM effector is not revealed by these images. However, it is reported to diffuse away from the site of activation and exert effects on some or perhaps all of the non-hypoxic tissue via a “bystander” effect [12]. To visualize this, we imaged cell proliferation through uptake of the thymidine analog EdU. Edu was injected 24 hours after drug treatment. Although cell proliferation was present throughout untreated tumors except for hypoxic tissue (Figure 3A), tumors treated with evofosfamide showed an almost complete absence of EdU uptake (Figure 3B). In this experiment we saw no evidence of any tumor tissue that was unaffected by drug treatment, though we would note that EdU suppression could reflect a short-term response, and does not necessarily correlate with clonogenic cell kill.
Figure 3.
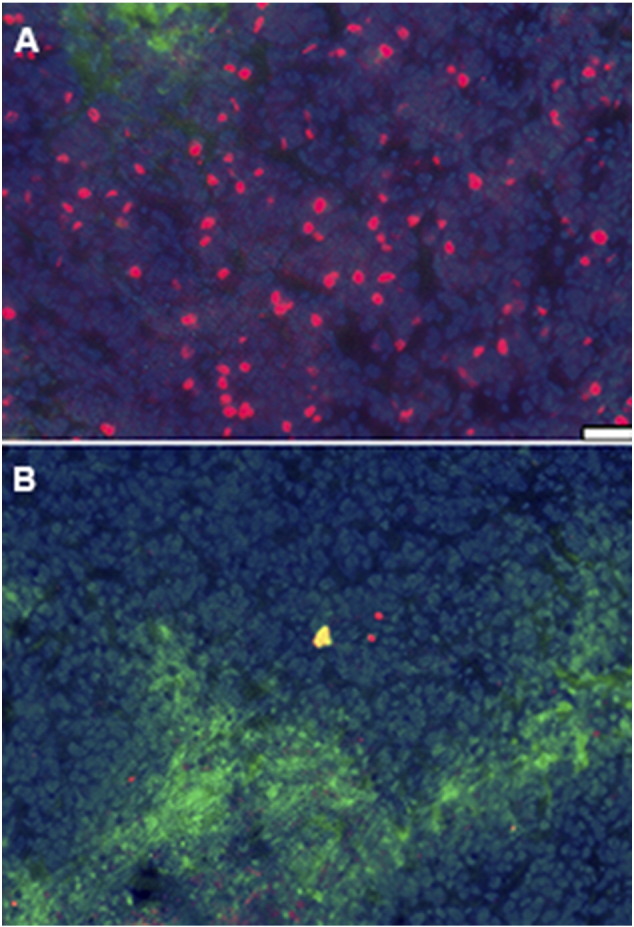
EdU (red) uptake in tumors of mice treated with evofosfamide (100 mg/kg). Evofosfamide and pimonidazole (green) were co-administered; EdU was given 22 hours later, and animals were sacrificed 2 hours after EdU administration. Slides were counterstained with Hoechst 33,342 (blue). (A) Control, (B) Treated. Scale bar = 200 μm.
Tumor Growth Delay After Evofosfamide and Radiation
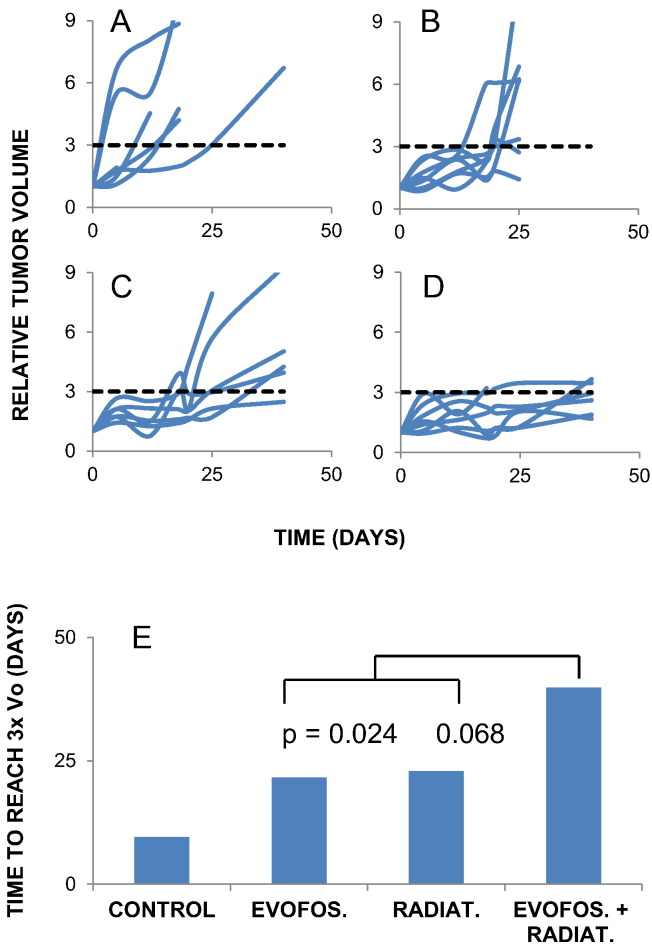
Tumors were irradiated with 15 Gy, delivered via a continuously rotating gantry through a 1 cm diameter collimator. This treatment has been previously shown to be well tolerated: mice may survive for 60 days post treatment with no weight loss [21]. Tumor growth curves for control animals and those treated with either evofosfamide or radiation alone or in combination are shown in Figure 4, A–D. The data is summarized in Figure 4E, as the median time to reach 3× the original volume. (Three was chosen as it was the largest multiple that could be applied to all the data sets). For control tumors, the median growth delay was 10 days, though within a large range. The difference between the controls and each of the three treated groups was highly significant. Evofosfamide produced an additional 12 days growth delay, proving that its toxicity must extend beyond the hypoxic compartment, as even complete ablation of a minority subpopulation would not produce significant growth delay. 15 Gy was almost equally effective, so that simple addition would predict a treatment induced growth delay of 25 days for the combined therapy. The measured growth delay of the combination was somewhat in excess of that (33 days), consistent with the combination being at least additive in effect. Tumor growth delay in the combination group expanded from their starting volume, but never entered exponential growth. Consequently, although five out of the eight tumors in this group at some point achieved a volume greater than three times their original size, no tumor maintained this ratio.
Figure 4.
Regrowth of tumors after drug and/or radiation treatment. Tumor volumes are normalized to volume at the start of the experiment. (A) Untreated tumors (n = 6) (B) Treated with evofosfamide (1 × 100 mg/kg) (n = 7) (C) Treated with 15 Gy (n = 6) (D) Evofosfamide administered 3 hours prior to 15 Gy. (n = 7) Dashed lines represent a threefold increase in tumor volume, which was selected as the endpoint to generate (E) P values calculated from the Mann Whitney test. The controls were significantly different from all the treated groups (vs Rad, 0008; Evofos, 0.012; Combined, 0.0013). The combined therapy was significantly more effective than evofosfamide alone.
Discussion
In these experiments, we showed that for AsPC1 tumors the combination of RT and evofosfamide led to a significant enhancement of tumor growth delay. The growth delay after dual treatment appeared to be more than an additive response. Moreover, the regrowth kinetics of the two-treatment group suggests that the tumors did not enter exponential growth at any time after treatment and failed to progress beyond a threefold increase in volume. Thus growth delay values were ascribed to tumors that failed to show consistent regrowth. By analyzing the data in this way, we likely reached a conservative estimate of the effect of combined treatment on the tumors. Based on the expected actions of evofosfamide and radiation – one targeting hypoxic tissue, with the other primarily killing oxygenated cells, a greater than additive outcome would be expected. However, we believe the results presented here do not require such an explanation.
When combined therapy results in greater killing than would be predicted based on single agent addition, two explanations are possible. (1) Evofosfamide acts as a radiation sensitizer. The “warhead” of evofosfamide is Br-IPM, closely related to the activated form of ifosfamide. We could only identify two reports in the literature reporting radiosensitization by ifosfamide. Tonkin et al. showed that it sensitized human cervical cancer xenografts to radiation, but only at low dose rates [25], and Latz et al. found in vitro radiosensitization, but only in S phase cells [26]. (2) RT and evofosfamide target two different subpopulations within the tumor. (If one agent is uniformly toxic throughout the tumor, it is simple to show that the combined effect of both treatments should be additive.) Hypoxic cells are known to be selectively spared by radiation. However, it is not clear from the data presented here that evofosfamide toxicity is limited to any subset of the tumor. While evofosfamide is activated in hypoxic tissue, the released Br-IPM effector kills not only hypoxic tumor cells, but also cells located within the diffusion distance of the mustard. Evofosfamide's effects certainly extend beyond the hypoxic regions, based on the global suppression of EdU uptake (Figure 3) and the significant tumor growth delay caused by the drug alone (Figure 4). However, it should be noted that EdU labeling reflects a short-term response to insult, and may not be tightly tied to clonogenic inactivation. Also, Saggar and Tannock have reported reduced evofosfamide efficacy in perivascular regions of MCF-7 and PC-3 tumors [9].
A possibility that is more consistent with our data relies on inter-tumor heterogeneity with regard to hypoxia. The spatial relationship between 14C–evofosfamide and pimonidazole revealed in Figure 2 predicts a correlation between the levels of tumor hypoxia and activated drug. Consequently, evofosfamide efficacy should depend on the level of tumor hypoxia, with relatively non-hypoxic tumors being spared and highly hypoxic, radioresistant tumors receiving the greatest impact from evofosfamide. This would have the effect of raising the minimum growth delay in the combined treatment group, without positing any interaction between drug and radiation in any individual tumor.
A recent study in multiple patient derived pancreatic xenograft tumors convincingly showed pronounced benefit from combining evofosfamide and radiation [27], and our results similarly support the combination of evofosfamide and radiation in high-dose radiotherapy regimes, and indicate a potential benefit of combining these two therapeutic agents in a clinical trial.
The following are the supplementary data related to this article.
CT scans of normal and tumor bearing mice, obtained using IP injection of lohexol (4 ml).
PIMO staining in AsPC1 tumors.
Funding
This work was supported by NIH award 1 R01 CA194321-01A1. Technical services provided by the Memorial Sloan Kettering Small-Animal Imaging Core Facility were supported by National Institutes of Health grant P30-CA08748 (to C. Thompson). A Geoffrey Beene Research Foundation Shared Resources grant (to J. Deasy and P.B. Zanzonico) provided funding support for the purchase of the XRAD225Cx.
Footnotes
Classification: Biological Sciences; Medical Sciences.
Conflict of Interest: Charles P. Hart is employed by and a stockholder of Threshold Pharmaceuticals.
References
- 1.Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919–922. doi: 10.1016/s0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 2.Dhani NC, Serra S, Pintilie M, Schwock J, Xu J, Gallinger S, Hill RP, Hedley DW. Analysis of the intra- and intertumoral heterogeneity of hypoxia in pancreatic cancer patients receiving the nitroimidazole tracer pimonidazole. Br J Cancer. 2015;113:864–871. doi: 10.1038/bjc.2015.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metran-Nascente C, Yeung I, Vines DC, Metser U, Dhani NC, Green D, Milosevic M, Jaffray D, Hedley DW. Measurement of Tumor Hypoxia in Patients with Advanced Pancreatic Cancer Based on 18F-Fluoroazomyin Arabinoside Uptake. J Nucl Med. 2016;57:361–366. doi: 10.2967/jnumed.115.167650. [DOI] [PubMed] [Google Scholar]
- 4.Duan JX, Jiao H, Kaizerman J, Stanton T, Evans JW, Lan L, Lorente G, Banica M, Jung D, Wang J. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J Med Chem. 2008;51:2412–2420. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- 5.Jung D, Lin L, Jiao H, Cai X, Duan JX, Matteucci M. Pharmacokinetics of TH-302: a hypoxically activated prodrug of bromo-isophosphoramide mustard in mice, rats, dogs and monkeys. Cancer Chemother Pharmacol. 2012;69:643–654. doi: 10.1007/s00280-011-1741-6. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Sun JD, Wang J, Ahluwalia D, Baker AF, Cranmer LD, Ferraro D, Wang Y, Duan JX, Ammons WS. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer Chemother Pharmacol. 2012;69:1487–1498. doi: 10.1007/s00280-012-1852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng F, Evans JW, Bhupathi D, Banica M, Lan L, Lorente G, Duan JX, Cai X, Mowday AM, Guise CP. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther. 2012;11:740–751. doi: 10.1158/1535-7163.MCT-11-0634. [DOI] [PubMed] [Google Scholar]
- 8.Peeters SG, Zegers CM, Biemans R, Lieuwes NG, van Stiphout RG, Yaromina A, Sun JD, Hart CP, Windhorst AD, van Elmpt W. TH-302 in Combination with Radiotherapy Enhances the Therapeutic Outcome and Is Associated with Pretreatment [18F]HX4 Hypoxia PET Imaging. Clin Cancer Res. 2015;21:2984–2992. doi: 10.1158/1078-0432.CCR-15-0018. [DOI] [PubMed] [Google Scholar]
- 9.Saggar JK, Tannock IF. Activity of the hypoxia-activated pro-drug TH-302 in hypoxic and perivascular regions of solid tumors and its potential to enhance therapeutic effects of chemotherapy. Int J Cancer. 2014;134:2726–2734. doi: 10.1002/ijc.28595. [DOI] [PubMed] [Google Scholar]
- 10.Saggar JK, Tannock IF. Chemotherapy Rescues Hypoxic Tumor Cells and Induces Their Reoxygenation and Repopulation-An Effect That Is Inhibited by the Hypoxia-Activated Prodrug TH-302. Clin Cancer Res. 2015;21:2107–2114. doi: 10.1158/1078-0432.CCR-14-2298. [DOI] [PubMed] [Google Scholar]
- 11.Sun JD, Liu Q, Ahluwalia D, Li W, Meng F, Wang Y, Bhupathi D, Ruprell AS, Hart CP. Efficacy and safety of the hypoxia-activated prodrug TH-302 in combination with gemcitabine and nab-paclitaxel in human tumor xenograft models of pancreatic cancer. Cancer Biol Ther. 2015;16:438–449. doi: 10.1080/15384047.2014.1003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun JD, Liu Q, Wang J, Ahluwalia D, Ferraro D, Wang Y, Duan JX, Ammons WS, Curd JG, Matteucci MD. Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin Cancer Res. 2012;18:758–770. doi: 10.1158/1078-0432.CCR-11-1980. [DOI] [PubMed] [Google Scholar]
- 13.Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, Schelman WR, Stephenson J, Jr., Chiorean EG, Rosen PJ. Randomized Phase II Trial of Gemcitabine Plus TH-302 Versus Gemcitabine in Patients With Advanced Pancreatic Cancer. J Clin Oncol. 2015;33:1475–1481. doi: 10.1200/JCO.2014.55.7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25:4066–4074. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
- 15.Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck--a systematic review and meta-analysis. Radiother Oncol. 2011;100:22–32. doi: 10.1016/j.radonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Lee N, Schoder H, Beattie B, Lanning R, Riaz N, McBride S, Katabi N, Li D, Yarusi B, Chan S. Strategy of Using Intratreatment Hypoxia Imaging to Selectively and Safely Guide Radiation Dose De-escalation Concurrent With Chemotherapy for Locoregionally Advanced Human Papillomavirus-Related Oropharyngeal Carcinoma. Int J Radiat Oncol Biol Phys. 2016;96:9–17. doi: 10.1016/j.ijrobp.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada Y, Bilsky MH, Lovelock DM, Venkatraman ES, Toner S, Johnson J, Zatcky J, Zelefsky MJ, Fuks Z. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 18.Carlson DJ, Keall PJ, Loo BW, Jr., Chen ZJ, Brown JM. Hypofractionation results in reduced tumor cell kill compared to conventional fractionation for tumors with regions of hypoxia. Int J Radiat Oncol Biol Phys. 2011;79:1188–1195. doi: 10.1016/j.ijrobp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall E, Giaccia A. Lippincott Williams and Wilkins; Philadelphia: 2006. Radiobiology for the Radiologist. [Google Scholar]
- 20.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4:1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorek DL, Kramer RM, Chen Q, Jeong J, Lupu ME, Lee AM, Moynahan ME, Lowery M, Ulmert D, Zanzonico P. Reverse-Contrast Imaging and Targeted Radiation Therapy of Advanced Pancreatic Cancer Models. Int J Radiat Oncol Biol Phys. 2015;93:444–453. doi: 10.1016/j.ijrobp.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Axente M, He J, Bass CP, Hirsch JI, Sundaresan G, Zweit J, Pugachev A. Comprehensive approach to coregistration of autoradiography and microscopy images acquired from a set of sequential tissue sections. J Nucl Med. 2011;52:1621–1629. doi: 10.2967/jnumed.111.091595. [DOI] [PubMed] [Google Scholar]
- 23.Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34:251–266. doi: 10.1016/0360-3016(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 24.Thrall DE, Rosner GL, Azuma C, McEntee MC, Raleigh JA. Hypoxia marker labeling in tumor biopsies: quantification of labeling variation and criteria for biopsy sectioning. Radiother Oncol. 1997;44:171–176. doi: 10.1016/s0167-8140(97)01931-2. [DOI] [PubMed] [Google Scholar]
- 25.Tonkin KS, Kelland LR, Steel GG. Chemotherapy-radiation interactions in human cervix carcinoma xenografts. Br J Cancer. 1988;58:738–741. doi: 10.1038/bjc.1988.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latz D, Schulze T, Manegold C, Schraube P, Flentje M, Weber KJ. Combined effects of ionizing radiation and 4-hydroperoxyfosfamide in vitro. Radiother Oncol. 1998;46:279–283. doi: 10.1016/s0167-8140(97)00194-1. [DOI] [PubMed] [Google Scholar]
- 27.Lohse I, Rasowski J, Cao P, Pintilie M, Do T, Tsao MS, Hill RP, Hedley DW. Targeting hypoxic microenvironment of pancreatic xenografts with the hypoxia-activated prodrug TH-302. Oncotarget. 2016;7:33571–33580. doi: 10.18632/oncotarget.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CT scans of normal and tumor bearing mice, obtained using IP injection of lohexol (4 ml).
PIMO staining in AsPC1 tumors.