Abstract
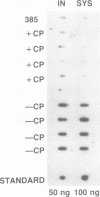
A chimeric gene encoding the alfalfa mosaic virus (AlMV) coat protein was constructed and introduced into tobacco and tomato plants using Ti plasmid-derived plant transformation vectors. The progeny of the self-fertilized transgenic plants were significantly delayed in symptom development and in some cases completely escaped infection after inoculated with AlMV. The inoculated leaves of the transgenic plants had significantly reduced numbers of lesions and accumulated substantially lower amounts of coat protein due to virus replication than the control plants. These results show that high level expression of the chimeric viral coat protein gene confers protection against AlMV, which differs from other plant viruses in morphology, genome structure, gene expression strategy and early steps in viral replication. Based on our results with AlMV and those reported earlier for tobacco mosaic virus, it appears that genetically engineered cross-protection may be a general method for preventing viral disease in plants.
Keywords: genetic engineering, cross-protection, alfalfa mosaic virus, transgenic plants
Full text
PDF
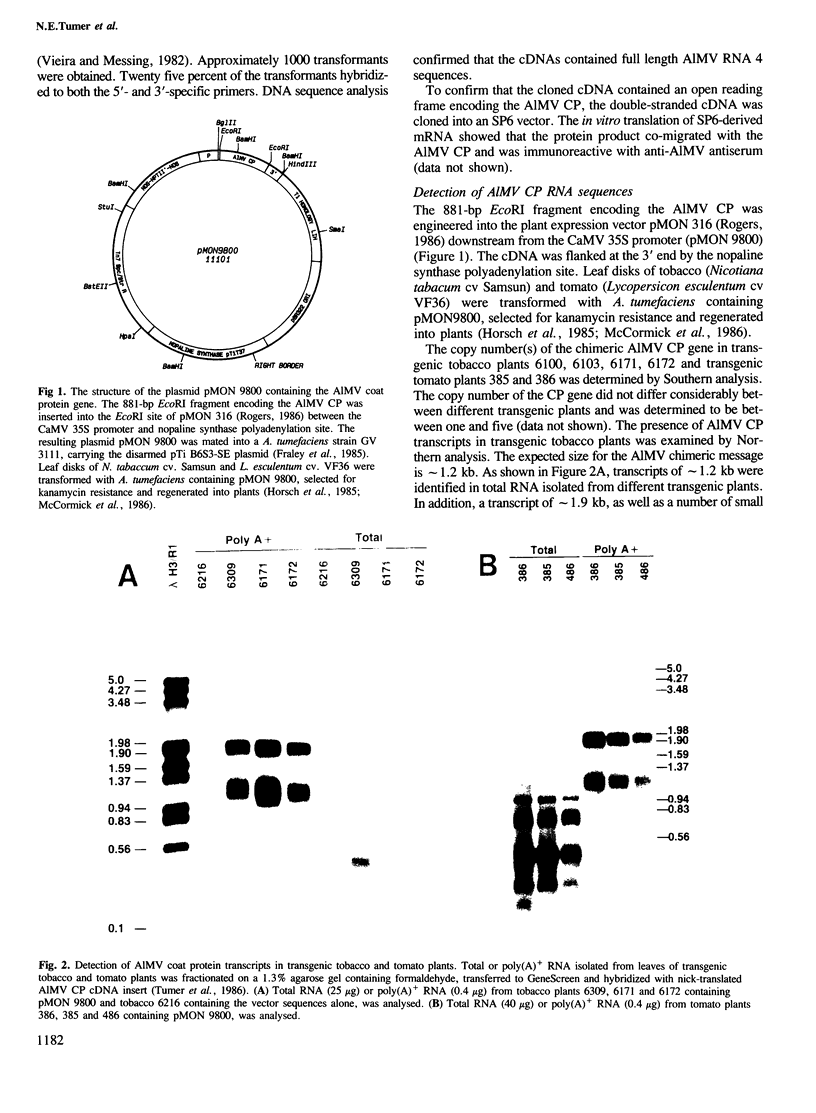
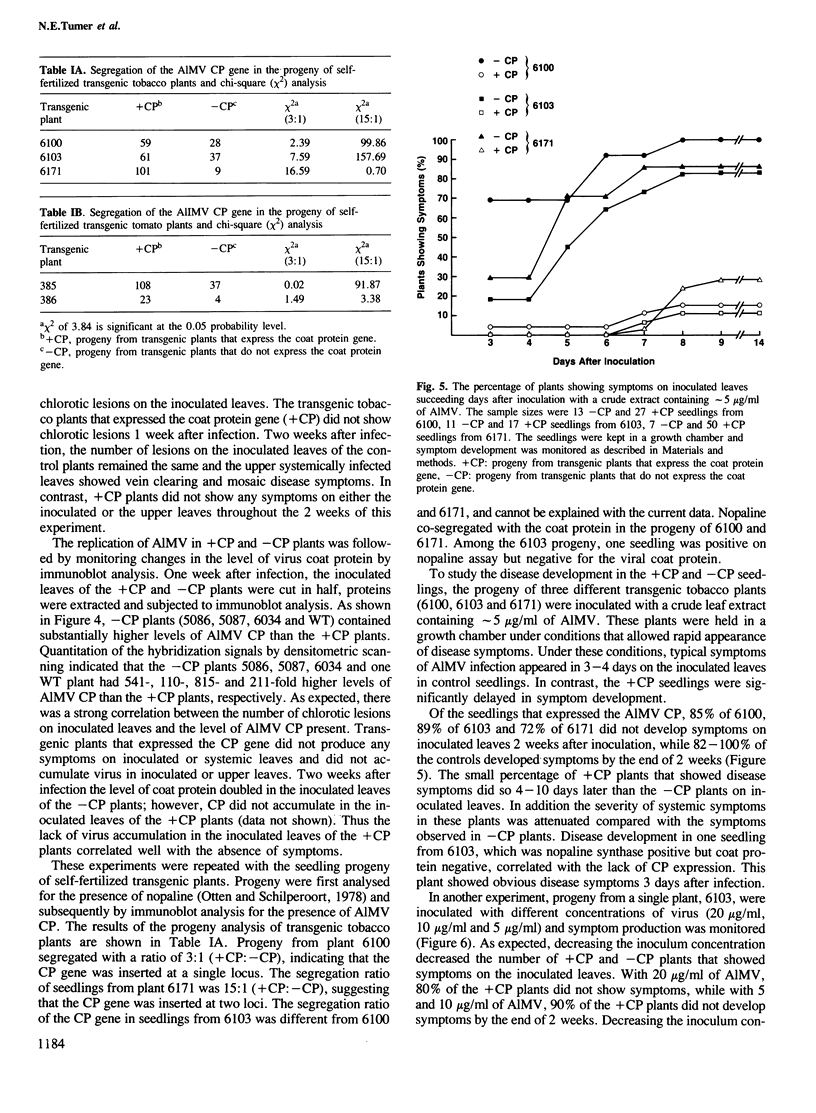
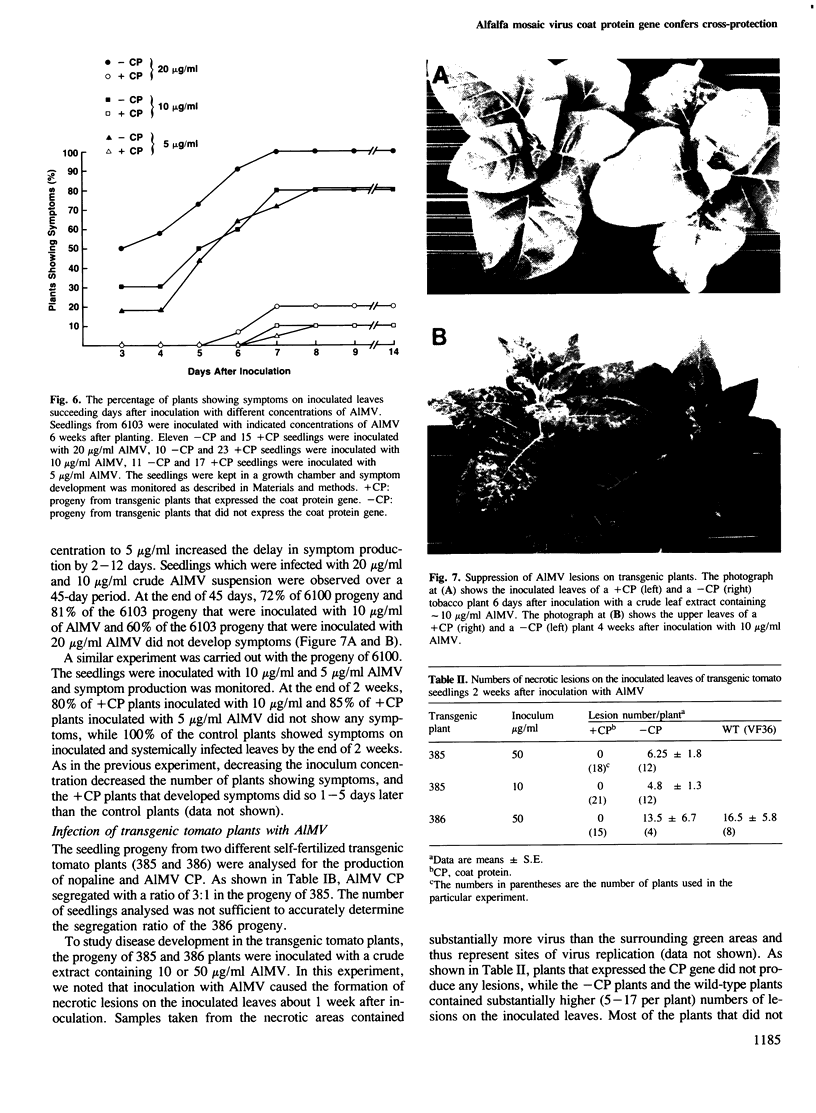



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Abel P. P., Nelson R. S., De B., Hoffmann N., Rogers S. G., Fraley R. T., Beachy R. N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986 May 9;232(4751):738–743. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- Barker R. F., Jarvis N. P., Thompson D. V., Loesch-Fries L. S., Hall T. C. Complete nucleotide sequence of alfalfa mosaic virus RNA3. Nucleic Acids Res. 1983 May 11;11(9):2881–2891. doi: 10.1093/nar/11.9.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol J. F., van Vloten-Doting L., Jaspars E. M. A functional equivalence of top component a RNA and coat protein in the initiation of infection by alfalfa mosaic virus. Virology. 1971 Oct;46(1):73–85. doi: 10.1016/0042-6822(71)90007-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brederode F. T., Koper-Zwarthoff E. C., Bol J. F. Complete nucleotide sequence of alfalfa mosaic virus RNA 4. Nucleic Acids Res. 1980 May 24;8(10):2213–2223. doi: 10.1093/nar/8.10.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Dhaese P., De Greve H., Gielen J., Seurinck L., Van Montagu M., Schell J. Identification of sequences involved in the polyadenylation of higher plant nuclear transcripts using Agrobacterium T-DNA genes as models. EMBO J. 1983;2(3):419–426. doi: 10.1002/j.1460-2075.1983.tb01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth L., Richards K. E. Tobacco mosaic virus: model for structure and function of a simple virus. Adv Virus Res. 1981;26:145–199. doi: 10.1016/s0065-3527(08)60423-6. [DOI] [PubMed] [Google Scholar]
- Houwing C. J., Jaspars E. M. Protein binding sites in nucleation complexes of alfalfa mosaic virus RNA 4. Biochemistry. 1982 Jul 6;21(14):3408–3414. doi: 10.1021/bi00257a025. [DOI] [PubMed] [Google Scholar]
- Jobling S. A., Gehrke L. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence. Nature. 1987 Feb 12;325(6105):622–625. doi: 10.1038/325622a0. [DOI] [PubMed] [Google Scholar]
- Otten L. A., Schilperoort R. A. A rapid micro scale method for the detection of lysopine and nopaline dehydrogenase activities. Biochim Biophys Acta. 1978 Dec 8;527(2):497–500. doi: 10.1016/0005-2744(78)90363-7. [DOI] [PubMed] [Google Scholar]
- Ravelonandro M., Pinck M., Pinck L. Complete nucleotide sequence of RNA 3 from alfalfa mosaic virus, strain S. Biochimie. 1984 May;66(5):395–402. doi: 10.1016/0300-9084(84)90023-3. [DOI] [PubMed] [Google Scholar]
- Tumer N. E., Clark W. G., Tabor G. J., Hironaka C. M., Fraley R. T., Shah D. M. The genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase are expressed differentially in petunia leaves. Nucleic Acids Res. 1986 Apr 25;14(8):3325–3342. doi: 10.1093/nar/14.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vloten-Doting L., Jaspars E. M. The uncoating of alfalfa mosaic virus by its own RNA. Virology. 1972 Jun;48(3):699–708. doi: 10.1016/0042-6822(72)90154-7. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Zuidema D., Bierhuizen M. F., Cornelissen B. J., Bol J. F., Jaspars E. M. Coat protein binding sites on RNA 1 of alfalfa mosaic virus. Virology. 1983 Mar;125(2):361–369. doi: 10.1016/0042-6822(83)90208-8. [DOI] [PubMed] [Google Scholar]