Abstract
Ion channels represent the molecular entities that give rise to the cardiac action potential, the fundamental cellular electrical event in the heart. The concerted function of these channels leads to normal cyclical excitation and resultant contraction of cardiac muscle. Research into cardiac ion channel regulation and mutations that underlie disease pathogenesis has greatly enhanced our knowledge of the causes and clinical management of cardiac arrhythmia. Here we review the molecular determinants, pathogenesis, and pharmacology of congenital Long QT Syndrome. We examine mechanisms of dysfunction associated with three critical cardiac currents that comprise the majority of congenital Long QT Syndrome cases: 1) IKs, the slow delayed rectifier current; 2) IKr, the rapid delayed rectifier current; and 3) INa, the voltage-dependent sodium current. Less common subtypes of congenital Long QT Syndrome affect other cardiac ionic currents that contribute to the dynamic nature of cardiac electrophysiology. Through the study of mutations that cause congenital Long QT Syndrome, the scientific community has advanced understanding of ion channel structure-function relationships, physiology, and pharmacological response to clinically employed and experimental pharmacological agents. Our understanding of congenital Long QT Syndrome continues to evolve rapidly and with great benefits: genotype-driven clinical management of the disease has improved patient care as precision medicine becomes even more a reality.
I. INTRODUCTION
A genetic disorder disrupting electrical activity in the heart, congenital Long QT Syndrome (LQTS) can lead to life-threatening arrhythmias and sudden cardiac death. In the first cases of congenital LQTS, described in 1957, several children in one family presented with prolongation of the QT interval on the electrocardiogram (ECG) and congenital deafness (189). This came to be known as Jervell and Lange-Nielsen syndrome (JLNS), the autosomal recessive form of LQTS. The more common, autosomal dominant form of congenital LQTS that presents without deafness was first described in 1963 and 1964 in two separate cases and became known as Romano-Ward syndrome (346, 459). Since these initial patient descriptions, advances in our understanding of the mechanisms of cardiac electrical excitability at the tissue, cellular, and molecular level have yielded much insight into the pathophysiology of congenital LQTS.
Clinically, congenital LQTS patients often first present after episodes of syncope and/or seizure, and the ECG reveals a prolonged QT interval. The ECG measures electrical activity of the heart over time, at the patient's body surface. The primary electrical signals observed include the P wave, which signifies atrial depolarization; the QRS complex, which arises from ventricular depolarization; and the T wave, due to ventricular repolarization (Figure 1A). The QT interval, therefore, reflects the time elapsed from the initiation of ventricular depolarization to the end of ventricular repolarization. The QT interval shortens with increasing heart rate thus requiring a normalization, or “correction,” for heart rate. For a diagnosis of LQTS, this rate-corrected QT (QTc) interval prolongation on a 12-lead ECG generally is referenced as >470 ms for males and >480 ms for females. QTc also varies with age, and thus, an age-appropriate prolonged QTc interval in a patient aids the diagnosis of LQTS (209). However, diagnosis of LQTS based on absolute QT interval cutoffs can be challenging, since there is considerable overlap in the QTc distribution of affected patients and otherwise healthy individuals (193). Asymptomatic patients can have intervals beyond these cutoff and develop no arrhythmias; similarly, QTc intervals below this cutoff can be seen in patients with established LQTS (with clinical arrhythmias and positive genetic testing) (16, 17, 338). Clinical scoring systems (368), as well as genetic testing, can be helpful to assist with the diagnosis of congenital LQTS (193), particularly when QT intervals are on the borderline (within 20 ms of these cutoffs) or when clinical history is equivocal. This review will focus primarily on the various forms of congenital LQTS.
FIGURE 1.

ECG to cellular ionic currents. A: membrane depolarization and the rapid upstroke of the ventricular action potential give rise to the QRS complex. The duration of the QT-interval corresponds to the time to ventricular repolarization. The relatively stable membrane potential during the plateau phase of the action potential gives rise to a brief isoelectric period. Ventricular repolarization gives rise to the T-wave. B: time course of several ionic currents that underlie ventricular action potential morphology (currents not to scale). The rapidly activating and inactivating INa drives membrane depolarization. Two K+ currents, IKs and IKr, contribute most to the repolarizing current necessary to drive membrane potential back to rest.
Ion channels are the molecular entities underlying most ionic currents in the heart, allowing passive diffusion of ions across the cell membrane's electrochemical gradients (Figure 2). A selectivity filter in the channel pore, determined by distinct atomic components (152, 315), endows selective permeation of ions, such as Na+, K+, and Ca2+ (246). Some ion channels exhibit voltage-dependent gating, where voltage-sensing domains respond to changes in membrane potential to cause channel opening or closing (247).
FIGURE 2.

Schematic of a generic K+ and Na+ ion channel. Ion channels allow for selective permeation of ions through the plasma membrane down their electrochemical gradient. The classic K+ channel consists of four identical pore-forming subunits, whereas each Na+ channel is formed by a single polypeptide with four homologous domains.
The ventricular cellular action potential results from the summation of a large number of ion channel currents and electrogenic pumps that control the cellular membrane potential (see Nerbonne and Kass review, Ref. 299), but in this review we will focus on three key ion channels that are well-established to be linked to LQTS and that are illustrated in Figure 1B. The cellular resting membrane potential is approximately −85 mV, determined largely by inwardly rectifying K+ channels. Inward rectification is a property that hinders the outward flow of K+ as membrane potentials become positive, but passes K+ more efficiently at potentials negative to the K+ equilibrium potential where the flow of K+ would be inward. Hence, the term “inward rectification” is used to describe these channels (304). Activation of Nav1.5, the primary voltage-gated sodium channel in the heart, leads to sodium influx (INa) and membrane depolarization. As the cell reaches approximately −40 mV, voltage-gated L-type calcium channels begin to open, leading to calcium influx (ICa) (299). Concurrently during the upstroke of the action potential, potassium channels, including those carrying the IKs and IKr delayed rectifier currents, begin to activate slowly. As the cell reaches +30 mV, INa inactivates almost completely. At this time, a brief and small repolarization of the membrane potential occurs via fast activation of the transient outward K+ current, Ito. In the plateau phase that follows, influx of Ca2+ through voltage-gated calcium channels is balanced mainly by IKs and IKr. The plateau phase ceases as calcium channels inactivate and outward potassium efflux persists, leading to a net outward membrane current, and cell repolarization back to the cellular resting potential. During this process, the Na+-K+-ATPase helps maintain intracellular concentrations of these key ions (304).
In LQTS patients, the QT is prolonged presumably due to prolongation of underlying action potential durations (APDs), most often caused by decreased repolarizing IKs or IKr activity, or persistent sodium influx that extends through the plateau phase. A loss of IKs or IKr function, or a gain of INa function, predisposes ventricular myocytes to early afterdepolarizations (EADs), and in some cases to delayed afterdepolarizations (DADs) which may underlie degeneration into a characteristic sinusoidal wave pattern on the ECG, referred to as torsades de pointes, which may further regress into ventricular fibrillation and sudden cardiac death. EADs are driven in large part by calcium entry via L-type calcium channels during prolonged action potential plateau phases, whereas DADs, which occur over the diastolic range of potentials after action potential repolarization, are caused by intracellular calcium overload, also a consequence of action potential prolongation (126). Additionally, arrhythmic activity may result from altered refractoriness and impulse block, also putative consequences of prior APD prolongation. Thus treatment in LQTS aims to prevent malignant ventricular arrhythmia by shortening the QTc interval to minimize cardiac event rates.
Ion channels may interact with a variety of molecular entities that contribute to their trafficking, stabilization, signaling, and function (299). Coordination among different ion channel types facilitates the ionic balance necessary for the generation of an action potential and normal electrical propagation through the heart. LQTS mutations may cause an increase or decrease in ion channel function, disrupting normal ionic balance leading to pathological electrical activity in the heart.
There are 15 subtypes of congenital LQTS, each associated with mutations on a different gene (15) (Table 1). The most common subtypes, LQT1, LQT2, and LQT3, account for the vast majority of congenital LQTS. LQT1 and LQT2 are associated with mutations in KCNQ1 and KCNH2 (which encodes hERG), respectively, while LQT3 is associated with mutations in SCN5A, the gene coding for the Nav1.5 sodium channel alpha subunit. Disease association for variants in these three proteins is supported by genome-wide association studies (300) and functional electrophysiological characterization of mutant channels. In addition, LQTS-associated mutations exist less frequently in other ion channels, modulatory channel subunits, and signaling- or cytoskeleton-associated proteins. Understanding the molecular mechanisms that cause LQTS allows for optimization of genotype-specific treatments. In this review, we discuss the molecular physiology, biology, and pathophysiology underlying congenital LQTS, and the cellular and molecular underpinnings of genotype-driven clinical management of LQTS.
Table 1.
Subtypes of congenital LQTS and their associated genes, proteins, and effects on cardiac currents
| LQT Subtype | Gene | Protein | Current |
|---|---|---|---|
| LQT1 | KCNQ1 | KCNQ1 (Kv7.1) | ↓IKs |
| LQT2 | KCNH2 | hERG (Kv11.1) | ↓IKr |
| LQT3 | SCN5A | Nav1.5 | ↑INa |
| LQT4 (ankyrin-B syndrome) | ANK2 | Ankyrin-B | Multichannel interactions |
| LQT5 | KCNE1 | KCNE1 (minK) | ↓IKs |
| LQT6 | KCNE2 | KCNE2 (MiRP1) | ↓IKr |
| LQT7 (Andersen-Tawil syndrome type 1) | KCNJ2 | Kir2.1 | ↓IK1 |
| LQT8 (Timothy syndrome) | CACNA1C | Cav1.2 | ↑ICa |
| LQT9 | CAV3 | Caveolin 3 | ↑INa |
| LQT10 | SCN4B | Nav1.5 β4 | ↑INa |
| LQT11 | AKAP9 | AKAP-9 (yotiao) | ↓IKs |
| LQT12 | SNTA1 | α1-Syntrophin | ↑INa |
| LQT13 | KCNJ5 | Kir3.4 (GIRK4) | ↓IKACh |
| LQT14 | CALM1 | Calmodulin | Multichannel interactions |
| LQT15 | CALM2 | Calmodulin | Multichannel interactions |
II. IKs DYSFUNCTION IN CONGENITAL LQTS
A. Introduction
Of the different subtypes of inherited LQTS, subtypes 1, 5, and 11 are associated with mutations in proteins that participate in the macromolecular complex which generates and modulates the slow delayed rectifier potassium current (IKs), which plays a critical role in the repolarization of the cardiac action potential. Among all LQTS subtypes, LQT1 is the most common, representing 30-35% of all congenital LQTS (8). Upregulation of IKs during β-adrenergic stimulation is critical to normal physiology by shortening ventricular APD and allowing for adequate diastolic filling in the context of an elevated heart rate (354). Cardiac events in patients with IKs-associated LQTS are often triggered by stress and exercise, consistent with the role of adrenergic stimulation in the regulation of IKs (350, 371). Insight into the molecular mechanisms of disease mutations have greatly improved our understanding of LQTS pathophysiology and helped provide a first step to the future development of targeted therapies.
B. Physiology
IKs is an outward potassium current with unique kinetics and voltage dependence and plays a key role in the repolarization of the cardiac action potential (299). In 1969, the delayed rectifier potassium current in sheep Purkinje fibers was studied and shown to comprise two kinetically distinct components (305). These currents were later subjected to pharmacological dissection in guinea pig ventricular myocytes and identified as IKs and IKr (360).
IKs is slowly activating and most prominent during the plateau and repolarizing phases of the cardiac action potential, where it contributes to counterbalancing calcium influx and repolarization (Figure 1B). Expression of IKs has been demonstrated in both human atrial and ventricular myocytes (198, 235, 457). In addition, it has also been measured in cardiomyocytes from a variety of non-human mammalian species including dogs (241, 406, 437, 442, 493) and rabbits (352). On the other hand, IKs is expressed at very low levels or absent in mouse hearts (478), most likely because at very high heart rates in mouse heart (∼500 beats/min) this channel would have little or no time to activate and not affect cardiac electrophysiology in the mouse.
Importantly, IKs is subject to upregulation by β-adrenergic stimulation to control APD in the face of sympathetic nerve activity (210, 444). During sympathetic activation, adrenergic stimulation increases the outward IKs current, which counterbalances the concomitant increase in inward calcium current, prevents prolongation of the cardiac APD, and allows for adequate diastolic filling times between heart beats (386). However, insufficient IKs activation such as that seen in LQT1 results in failure to counterbalance the calcium influx, prolonging the action potential and increasing susceptibility to arrhythmia. This is consistent with exercise being a key trigger of cardiac events seen in LQT1 patients (371).
C. Molecular Biology
The first known subtypes of inherited LQTS, LQT1-3, were distinguished by mapping to distinct chromosomes, with LQT1 mapping to chromosome 11 (191, 212, 213). Eventually it was found that the KCNQ1 (KvLQT1) gene is responsible for LQT1 and encodes a potassium channel (451). The current conducted by this channel was rapidly activating and minimally inactivating, unlike any previously known current in the heart, but soon it was shown that KCNQ1 together with the accessory protein KCNE1 (minK) generates IKs (36, 358). While KCNE1 had previously been thought to be an independent potassium channel (167, 412), it was confirmed that KCNQ1 is actually the α- or pore-forming subunit of IKs while KCNE1 is a critical β- or modulatory subunit. Coexpression of KCNQ1 and KCNE1 generates the hallmark IKs current with slow activation. KCNQ1 and KCNE1 have been shown to be expressed in all four chambers in the heart (45), as well as the inner ear (301, 351), where IKs is thought to play a role in K+ secretion into the endolymph (68). This explains the observation that congenital deafness is a key feature of JLNS. In addition, KCNQ1 and KCNE1 are expressed elsewhere in the body, including the pancreas, the kidneys, and the brain (1).
1. KCNQ1, the pore-forming subunit
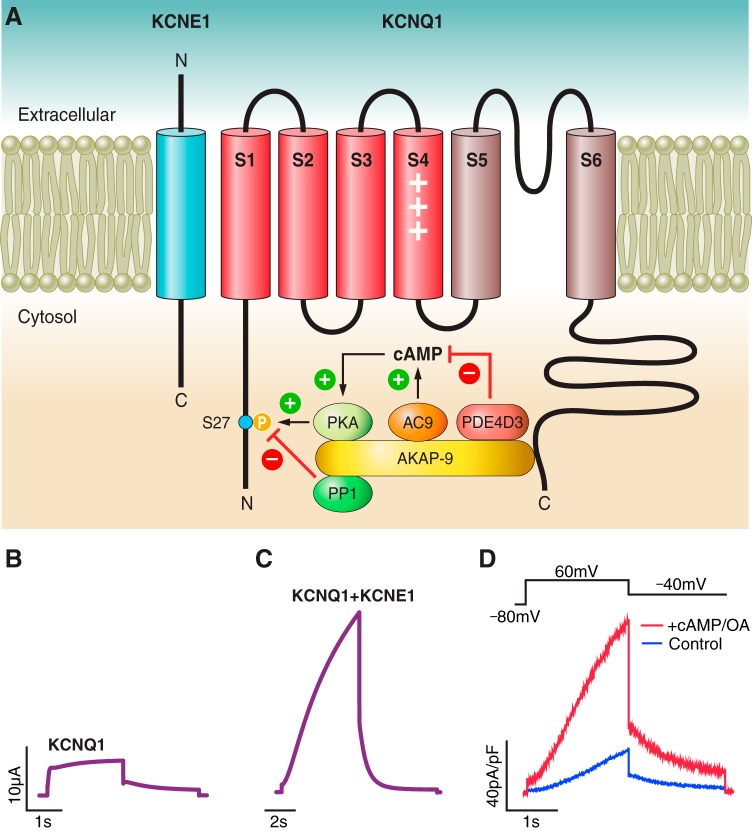
KCNQ1, like most voltage-gated potassium channels, consists of six transmembrane helices (451) (Figure 3A). Four subunits of KCNQ1 come together to form a channel that is capable of voltage-dependent gating (Figure 3B). On each subunit, the helices S1–S4 comprise the voltage-sensing domain, where the S4 helix, with its positively charged arginine residues, senses changes in membrane potential (310). Following the voltage-sensing domain is the pore domain, which comprises the pore-loop, an extracellular segment containing the selectivity filter, and helices that line the pore, S5 and S6. Furthermore, the cytoplasmic loop between S4 and S5 plays important roles in the voltage sensor-to-pore coupling and in voltage-dependent gating (82, 229, 498), which has been demonstrated in other voltage-gated channels as well (75, 122, 355). The cytoplasmic loop between S2 and S3 also plays a role in channel gating (498). The COOH-terminal domain (CTD) of KCNQ1 is large and contains four intracellular α-helices referred to as A-D. A wide range of functions has been attributed to the CTD including calmodulin binding, interaction with β-subunits and scaffolding proteins, as well as channel assembly and trafficking (159, 469).
FIGURE 3.
Molecular biology of IKs and regulation by PKA-mediated signaling. A: the IKs macromolecular complex, including KCNQ1, KCNE1, and associated scaffolding and signaling proteins. B and C: single pulse voltage-clamp recordings of KCNQ1 expressed alone or coexpressed with KCNE1 in Xenopus oocytes. D: dialysis with 200 μM cAMP and 0.2 μM okadaic acid (OA) increases IKs amplitude and slows deactivation when heterologously expressed in CHO cells. [From Chen et al. (78).]
2. KCNE1, the β-subunit
Coexpression of KCNE1 with KCNQ1 leads to a drastic change in channel function to generate IKs. Most prominently, assembly with KCNE1 leads to a delay in the onset of activation, an increase in channel amplitude (Figure 3C), as well as a depolarizing shift in the current-voltage relationship (not illustrated) (36, 358). This results in a channel that, compared with most other voltage-gated potassium channels, activates at more positive voltages and with slower kinetics. KCNE1 is a 129-amino acid protein that consists of a single transmembrane helix, with an extracellular NH2 terminus and intracellular COOH terminus (412). It is thought to have extensive contact with KCNQ1 including the voltage-sensing domain (37, 84, 203, 309, 378, 479), the pore domain (84, 262, 311), as well as the CTD (161, 510). Previous crosslinking studies suggest that KCNE1 is located in a cleft between the voltage-sensing domain and pore domain of different KCNQ1 subunits (84, 479), underlying its ability to modulate KCNQ1 gating. With respect to the stoichiometry of KCNE1 to KCNQ1, some studies suggest a fixed 2:4 ratio (278, 326), while others suggest a flexibility in stoichiometry (293, 456) that allows for modulation of kinetics of assembled channels to provide another level of flexibility in channel function. Although three other members of the KCNE family, KCNE2-KCNE4, also are expressed in the heart (45) and are capable of modulating KCNQ1 activity (45, 154, 367, 422), whether they associate with KCNQ1 in vivo to contribute to potassium currents in the heart remains to be explored.
3. Molecular components of adrenergic stimulation
There have been considerable efforts to elucidate the molecular pathway for the β-adrenergic regulation of IKs. In 1988 it was shown that stimulation of PKA activity by a cAMP analog can upregulate the delayed rectifier current (444). Later it was shown that the scaffolding protein A-kinase anchoring protein 9 (AKAP-9), also known as yotiao, plays a central role in adrenergic regulation of IKs by compartmentalizing key elements of the PKA signaling pathway, allowing for spatiotemporal control. AKAP-9 binds to the CTD of KCNQ1 and recruits signaling proteins including protein kinase A (PKA), protein phosphatase 1 (PP1) (255), adenylyl cyclase 9 (AC9) (238), and the phosphodiesterase PDE4D3 (419) (Figure 3A). Together these proteins form the IKs macromolecular complex that can tightly control the phosphorylation state of the channel in response to adrenergic stimulation. PKA phosphorylates KCNQ1 at the S27 residue, adding a phosphate group and hence a change in charge to this residue, which leads to increased channel activation and slower deactivation (226, 255) (Figure 3D). In addition, phosphorylation of AKAP-9 itself contributes to the PKA-mediated upregulation of IKs (78).
4. Role of PIP2 and calmodulin in IKs function
The lipid molecule phosphatidylinositol 4,5-bisphosphate (PIP2) is critical for KCNQ1 function. PIP2 is found in the inner leaflet of plasma membranes (260) and can regulate a variety of ion channels (51, 83, 172, 179, 250, 475). PIP2 mainly binds to KCNQ1 at its cytoplasmic loops and the COOH-terminal region near S6 (80, 208, 498), which are thought to form the interface between the voltage-sensing domain and the pore domain (82, 497). PIP2 binding is mediated by electrostatic interactions between the anionic lipid headgroup and positively charged channel residues (418). Rundown of PIP2 in the membrane leads to a drastically reduced IKs amplitude and accelerated deactivation, suggesting that PIP2 stabilizes the open state of IKs (250). Furthermore, PIP2 rundown reduces the current amplitude of KCNQ1 in the absence of KCNE1 (239). In addition, PIP2 plays a critical role in the coupling between voltage sensor movement and pore opening/closing (498). Interestingly, one modulatory effect of KCNE1 on KCNQ1 is a large (100-fold) increase in channel sensitivity for PIP2, which contributes to augmentation of KCNQ1 current amplitude (239). Whether PIP2 level is under control by physiological mechanisms to modulate IKs in cardiomyocytes remains to be demonstrated.
Calmodulin is a calcium-binding protein that serves in myriad calcium signaling pathways, some of which regulate ion channels (399). Calmodulin has been linked to IKs function (141, 379). Multiple studies have shown that calmodulin binding to the CTD of KCNQ1 is important for normal channel trafficking and folding (141, 379).
5. Channel trafficking
A variety of molecular entities are involved in the trafficking of KCNQ1KCNE1. The small GTPase-RAB11 plays an important role in the exocytosis of KCNQ1-KCNE1 to the plasma membrane, while RAB5 mediates endocytosis of KCNQ1-KCNE1 from the plasma membrane into endosomes (374). In a physiological stress response, serum and glucocorticoid-regulated kinase 1 (SGK1) upregulates KCNQ1-KCNE1 expression by increasing RAB-11-dependent channel exocytosis (374). There is also evidence to suggest that KCNQ1-KCNE1 can be internalized from the plasma membrane via clathrin-mediated endocytosis, a process facilitated by KCNE1 (480). Furthermore, KCNQ1 expression is regulated by ubiquitination, which can label membrane proteins for internalization and degradation. For example, the ubiquitin ligase Nedd42 can ubiquitinate KCNQ1 (190), while ubiquitin-specific protease 2 (USP2) can prevent channel ubiquitination (221). Both processes allow for control of KCNQ1 expression.
D. Molecular Pathophysiology
There are more than 530 disease-causing mutations associated with the IKs complex (15). While most of these are missense mutations, they also include nonsense mutations, splice site mutations, frameshifts, as well as deletions. The vast majority are mutations in KCNQ1, which cause LQT1, but a number of LQTS-causing mutations have also been found in KCNE1 and AKAP-9, which are classified as LQT5 and LQT11, respectively. JLNS, an autosomal recessive form of LQTS with severe bilateral sensorineural deafness, has so far only been associated with mutations in KCNQ1 or KCNE1 (423). Nonetheless, the majority of LQTS arising from mutations in IKs are autosomal dominant in a form known as Romano-Ward syndrome, which is not associated with deafness.
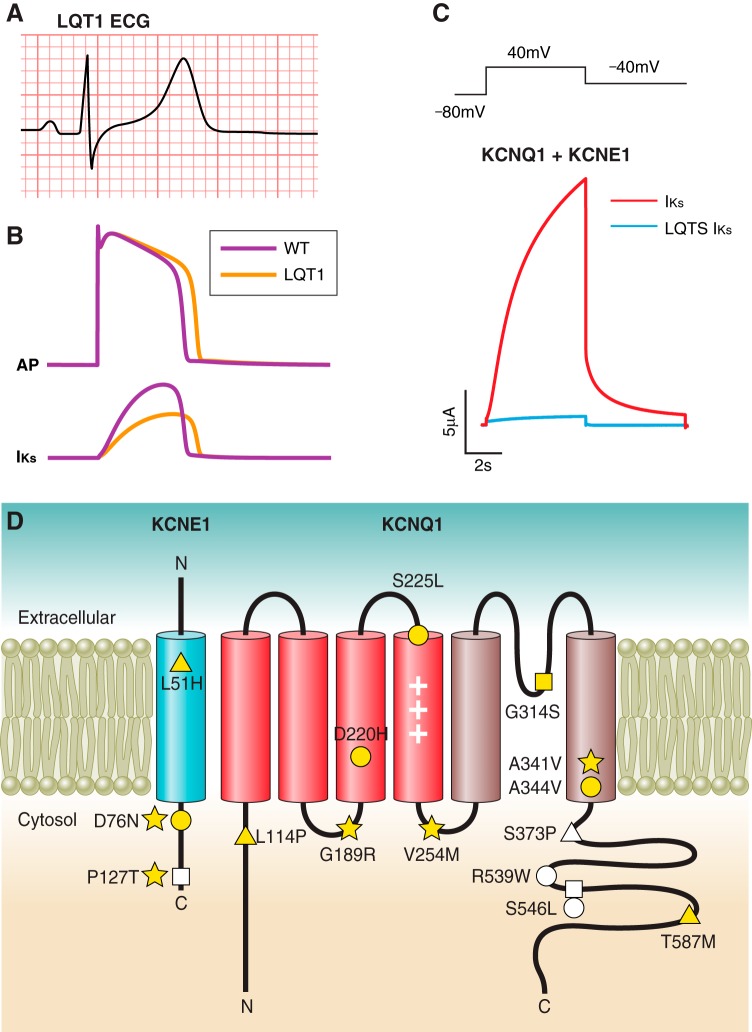
Congenital LQTS patients with dysfunction in IKs present with prolongation of the QT interval on ECG. An ECG characteristic associated with LQT1 patients more specifically is a broad based T wave (Figure 4A). Dysfunction of IKs reduces repolarizing current during the plateau phase, thereby leading to APD prolongation (Figure 4B). With a prolonged APD, the cardiomyocyte is vulnerable to EADs (441, 500), where a second depolarization occurs prior to the complete repolarization of the first due to recovery of voltage-gated Na+ or Ca2+ channels from inactivation, triggering life-threatening arrhythmia such as torsades de pointes (26, 464). As previously mentioned, during adrenergic stimulation IKs plays an especially important role in controlling the cardiac APD. This manifests clinically in LQT1 patients as a susceptibility to arrhythmia during exercise (371).
FIGURE 4.
IKs dysfunction leading to congenital LQTS. A: ECG from a LQT1 patient demonstrates a characteristic broad-based T wave (unpublished data). B: simulated action potential (top) and IKs (bottom) in WT (black) and heterozygous LQT1 (gray) conditions, demonstrating the effect of 50% reduction in IKs. C: single pulse voltage-clamp IKs recording in Xenopus oocytes demonstrating loss of channel function in an IKs-associated LQTS mutation. D: topology of KCNQ1 and KCNE1 in the plasma membrane. Several examples of LQT1- and LQT5-associated mutations are highlighted in blue and teal, respectively. These mutations represent a variety of mechanisms of loss-of-function including disruption of permeation (yellow-filled square), gating (yellow-filled circle), trafficking (yellow-filled triangle), PKA-mediated signaling (yellow-filled star), KCNQ1-KCNE1 interaction (white-filled square), PIP2 affinity (white-filled circle), and calmodulin affinity (white-filled triangle).
1. LQT1
Missense mutations are responsible for the majority of LQT1 cases and can cause channel loss of function through a variety of molecular mechanisms, including defects in ion permeation (altering the pathway through which ions flow through open channels), channel gating (mechanisms that regulate the opening and/or closing or channels), trafficking, KCNQ1-KCNE1 interaction, PKA-mediated signaling pathway, PIP2 binding, and calmodulin binding (Figure 4, C AND D) (Table 2). Non-missense mutations can also cause LQT1. Mutations belonging to certain groups may bear implications on patient phenotype, severity of arrhythmia, as well as response to therapy.
Table 2.
Representative LQT1-associated mutations classified by mechanism
| Mechanism | Mutations | Reference Nos. |
|---|---|---|
| K+ permeation | G314S, Y315C | 52, 237 |
| T322A, T322M, G325R | 13, 61 | |
| Gating | D202H | 113, 114 |
| S225L | 52, 170 | |
| R231C | 41 | |
| A344V | 391 | |
| Trafficking | Y111C, L114P, P117L | 98, 375 |
| T587M, R591H, R594Q | 205 | |
| PKA-mediated signaling | G189R, R190Q, R243C, V254M | 39 |
| A341V | 169 | |
| G589D | 255 | |
| KCNQ1-KCNE1 interaction | S546L, K557E | 110 |
| PIP2 affinity | R539W, R555C | 312 |
| S546L, K557E | 110 | |
| Calmodulin affinity | S373P, W392R | 379 |
| Large-scale defects | ||
| Nonsense | R518X, Q530X | 181 |
| Deletion-insertion, frameshift | Δ544 | 81 |
| Splice site | 1032G>A | 286 |
A) PERMEATION DEFECT.
A number of LQT1 mutations that lead to defects in permeation are found in the pore region of KCNQ1. Pore mutations are thought in general to carry greater risk of cardiac events than other mutations. One study shows that mutations in highly conserved residues, many of which are located in the pore-loop, are associated with higher risk of cardiac events (61, 197). Three LQT1 mutations near the selectivity filter, T322M, T322A, and G325R, have been shown to abolish channel conductance (13, 61). They exert dominant negative effects on wild-type (WT) IKs current in heterologous expression systems (61), suggesting that mutant subunits coassemble with WT subunits to form disrupted channels. Molecular dynamic simulations suggest that these mutations disrupt the conformation of the selectivity filter, leading to diminished K+ permeation. A pair of adjacent LQT1 pore mutations, G314S and Y315C, located in the selectivity filter, dramatically reduce IKs current amplitude and exert dominant negative effects on WT currents (52, 237). Immunofluorescence studies show that Y315C traffics to the membrane normally, suggesting that the mutation results in trafficking of non-conducting channels.
B) GATING DEFECT.
LQT1 mutations that cause defects in KCNQ1 gating can be found in the pore domain (S5–S6). Similar to other voltage-gated potassium channels, the COOH-terminal region of the S6 helix plays a key role in KCNQ1 gating (55, 157, 247). Scanning mutagenesis and heterologous expression studies show that a number of LQT1-linked residues in this region, such as F351 and L353, control KCNQ1 gating properties. For example, F351A leads to a drastic slowing of channel activation and a depolarizing shift in voltage dependence of activation, while L353K leads to a constitutively open channel (55). To further elucidate the mechanism of F351A, a technique known as voltage clamp fluorometry (VCF), which utilizes fluorophore labeling to allow simultaneous measurement of voltage sensor movement and channel current, has been used (37, 292, 308, 309, 348, 498). It was shown using VCF that F351A alters the coupling between voltage sensor and the pore (309), resulting in a slowly activating channel that partially resembles IKs. In addition, a LQT1 mutation in on the S6 helix, A344V, also affects channel gating by shifting the current-voltage relationship of IKs in the depolarizing direction by 30 mV, thereby destabilizing channel opening (391).
In addition to those in the pore domain, a number of LQT1 mutations that alter channel gating are found on the voltage-sensing S4 helix of KCNQ1. For example, the mutation S225L exerts dominant negative suppression of WT IKs current (52). This mutation alters channel gating, shifting the current-voltage relationship of IKs toward more depolarized membrane potentials (170). The effect of this mutation on voltage sensor movement remains to be determined, but given its location it is possible that it disrupts the movement of the S4 helix in response to changes in voltage. Another LQT1-associated mutation on S4, R231C, decreases peak IKs amplitude, but it also leads to constitutive activation at the same time (41). Interestingly, in one family, this mutation causes familial atrial fibrillation, which is more consistent with action potential shortening than prolongation (41, 296). To explain this finding, the study has used a computational model to show that the atrial action potential is more susceptible to shortening than ventricular action potential when IKs is made constitutively active (41). Structural and mutational studies suggest that R231 forms a salt-bridge interaction with E160 in S2 that stabilizes the channel in its closed state (340, 393, 472). Mutating R231 may disrupt this interaction and cause a defect in channel gating, resulting in constitutive activation. One tool that can be used to elucidate the effects of these S4 mutations on voltage sensor movement is VCF. The technique is capable of providing insights on IKs channel gating not possible with current measurement alone.
LQT1 gating mutations are also present in other transmembrane regions (S1–S3) of the voltage-sensing domain. For example, the mutation D202H leads to biophysical defects in IKs, shifting the current-voltage relationship to more positive potentials, slowing activation, and accelerating deactivation, all leading to reduced channel opening (114). Single-channel recordings of IKs utilized as a tool to study effects of select mutations (113) shows that D202H causes a decrease in channel open probability, a decrease in open states dwell time, and an increase in closed states dwell time, while maintaining single-channel conductance.
C) TRAFFICKING DEFECT.
In addition to altering channel gating, LQT1 mutations can also disrupt channel trafficking. Several LQT1 mutations in helix D of the CTD, including T587M, R591H, and R594Q, have been found to diminish channel trafficking to the membrane (205). These mutations are thought to disrupt a coiled-coil motif in helix D that plays an important role in channel trafficking (205). Yet trafficking mutations are not limited to the CTD of KCNQ1. LQT1 mutations in the NH2-terminal region of KCNQ1, including Y111C, L114P, and P117L, have also been found to reduce surface expression of KCNQ1 and increase retention in the endoplasmic reticulum (98). This region appears to be a conserved trafficking determinant across the KCNQ family. In particular, Y111C and L114P disrupt SKG1's ability to increase channel trafficking to the plasma membrane, suggesting that the NH2 terminus may be important in RAB-mediated exocytosis of channels (375).
D) DEFECT IN PKA-MEDIATED SIGNALING.
Given the critical importance of adrenergic stimulation and PKA-mediated signaling in the regulation of IKs and repolarization of the cardiac action potential during stress, disruption of this stimulation is expected to prolong the QT interval. For example, the mutation G589D is thought to disrupt a leucine zipper motif to which AKAP-9 binds, resulting in failure of IKs to be stimulated by cAMP (255). A mutation on S6, A341V, exhibits dominant suppression of IKs that fails to respond to adrenergic stimulation with cAMP (169). This effect is mediated by a reduction in the phosphorylation S27 on KCNQ1 and is not due to disruption in AKAP-9's interaction with KCNQ1. This result implicates a role of S6 in the regulation of the phosphorylation state of KCNQ1.
Mutations that lead to defective adrenergic stimulation of IKs in LQT1 patients are suggested to be associated with higher risk of cardiac events during exercise and greater response to β-blocker therapy. One study has identified missense mutations in the cytoplasmic loops between S2/S3 and S4/S5 of KCNQ1 to be associated with an elevated risk of aborted cardiac arrest and sudden cardiac death (39). Four mutations in these cytoplasmic loops, G189R, R190Q, R243C, and V254M, all diminish IKs upregulation in response to forskolin, an activator of the PKA signaling pathway, suggesting that patients with these mutations are expected to be especially susceptible to arrhythmic events during stress. This study bears implications on better risk-stratification for LQT1 patients and predicting response to β-blocker therapy. In addition, it suggests that the cytoplasmic loops are involved in adrenergic stimulation of KCNQ1.
E) DISRUPTED KCNQ1-KCNE1 INTERACTION.
A number of LQT1 mutations disrupt the KCNQ1-KCNE1 interaction. Two such mutations, S546L and K557E, are located in the helix C of the CTD of KCNQ1 and disrupt its interaction with the COOH terminus of KCNE1, as demonstrated by GST pulldown assays (110). The same mutations also decrease channel affinity for PIP2, leading to decreased current amplitude and a depolarizing shift in the current-voltage relationship. The decreased PIP2 affinity may be due to disruption of a potential PIP2 binding site on helix C.
F) DECREASED PIP2 AFFINITY.
Some LQT1 mutations have been shown to decrease channel PIP2 affinity. In addition to the mutations described above, two LQT1 mutations on helix C of the CTD, R539W and R555C, both lead to decreased PIP2 affinity, decreased IKs amplitude, and a depolarizing shift in the current-voltage relationship, underscoring the importance of the CTD in PIP2 binding (312). The cytoplasmic loops of KCNQ1 between S2/S3 and S4/S5 also play important roles in PIP2 binding and channel gating (208, 498). Several LQT1-linked residues in these cytoplasmic loops, including residues 195, 258, and 259, are thought to form a binding site for PIP2. Disease mutations in this binding site may result in decreased PIP2 affinity, leading to altered channel function (80, 498).
G) DECREASED CALMODULIN AFFINITY.
A number of LQT1 mutations have been found to weaken calmodulin binding to KCNQ1 as the underlying mechanism of disease. For example, the mutations S373P and W392R, located in the CTD, reduce calmodulin binding both in the absence and presence of KCNE1 (379). Both of these mutations cause decrease in surface channel expression and dramatic reduction in IKs amplitude. Overexpression of calmodulin is able to increase S373P mutant channel expression in the membrane. These results are consistent with the role calmodulin plays in channel assembly and trafficking (141, 379).
H) NON-MISSENSE MUTATIONS.
Non-missense mutations can also cause LQT1. For example, nonsense LQT1 mutations such as R518X and Q530X introduce a stop codon, leading to early termination of channel transcription and loss of IKs function (181). These mutations are mostly associated with autosomal recessive LQTS, although autosomal dominant cases have also been reported for R518X. One study shows that these mutant channels only mildly affect WT IKs current, consistent with their mostly recessive mode of inheritance (349). It is thought that the nonsense transcripts are selectively degraded and do not interfere with WT channel production. Interestingly, the same study suggests that LQT1 patients with nonsense mutations have reduced risk of cardiac events compared with patients with missense noncytoplasmic loop mutations, although the explanation remains to be determined.
Another non-missense LQT1 mutation is the deletion-insertion at residue 544, denoted Δ544, which leads to a frameshift that alters subsequent 107 amino acids and introduces an early stop codon (81). It is an autosomal recessive mutation occurring in the CTD. The mutation has been shown to disrupt channel assembly in vitro (364), underscoring the role of the CTD of KCNQ1 in channel assembly.
LQT1 mutations can also disrupt transcript splicing. A base substitution at a consensus splice donor site the end of exon 7, 1032G>A, can lead to a dropped exon 7 or exons 7 and 8 (286). In terms of channel regions affected, exon 7 encodes parts of the pore-loop and S6, while exon 8 encodes the rest of S6 and part of the CTD. Transcripts lacking one or both of these exons therefore are not expected to produce functioning channels. A study shows that this splicing mutation exerts a dominant-negative suppression of WT IKs, likely through a direct interaction between mutant and WT channels preventing trafficking to the membrane (428). In addition to splice donor sites, splice acceptor sites can also be mutated in LQT1, such as the base substitution 922-1 G→C at the end of intron 6, which leads to the loss of exons 7 and 8 (286).
2. LQT5
Coassembly of KCNQ1 with the KCNE1 subunit is key to the generation of the IKs current. Thus it follows that KCNE1 mutations can alter the physiologically critical current of the assembled channel and lead to LQT5. Similar to LQT1, the mode of inheritance for LQT5 can be autosomal dominant (RW) or recessive (JLNS) (107, 430). Mutations in KCNE1 can lead to defects in gating, trafficking, KCNQ1-KCNE1 interaction, as well as adrenergic stimulation (Figure 4D) (Table 3).
Table 3.
Representative LQT5-associated mutations classified by mechanism
A) GATING DEFECT.
One LQT5 mutation that affects channel gating is D76N. It is an autosomal dominant mutation in the COOH terminus of KCNE1 that has been shown to drastically suppress IKs amplitude, accelerate deactivation, and cause a depolarizing shift in the voltage dependence of IKs activation when expressed in Xenopus oocytes and CHO cells (76, 405). Overexpression of the mutant KCNE1 in guinea pig ventricular myocytes leads to APD prolongation and early afterdepolarizations (176). The mutant appears to traffic normally to the cell surface (53) and does not disrupt KCNE1 binding to the COOH terminus of KCNQ1 (510). However, it reduces IKs upregulation secondary to stimulation of the PKA signaling pathway, suggesting a role for the COOH terminus of KCNE1 in adrenergic stimulation of IKs (226).
B) TRAFFICKING DEFECT.
In addition to channel gating, LQT5 mutations can lead to defects in channel trafficking. The JLNS mutation L51H, located in the transmembrane helix of KCNE1, results in diminished KCNE1 trafficking to the cell surface (53). Furthermore, when coexpressed with KCNQ1 in HEK cells, the mutant KCNE1 decreases KCNQ1 trafficking to the surface (53, 220). In addition, coexpression of the mutant KCNE1 with KCNQ1 in CHO cells leads to a diminished current amplitude and channel biophysical properties that resemble KCNQ1 alone rather than IKs, consistent with a drastic reduction of functional KCNE1 in the membrane. These results together suggest that the mutant KCNE1 interacts with KCNQ1 to disrupt the trafficking of both proteins to the membrane surface, leading to a reduction in current. The transmembrane region of KCNE1 may therefore be important to channel trafficking.
C) DISRUPTED KCNQ1-KCNE1 INTERACTION.
LQT5 mutations can disrupt the interaction between KCNE1 and KCNQ1 required to generate IKs. For example, the double mutation T58P/L59P, located in the transmembrane region of KCNE1, results in near-complete loss of IKs amplitude but has minimal effect when coexpressed with WT IKs in Xenopus oocytes (181). The mutation leads to a diminished ability for KCNE1 to associate with KCNQ1 in coimmunoprecipitation studies, suggesting that the transmembrane region of KCNE1 is important for the KCNQ1-KCNE1 interaction (166). Furthermore, the mutation P127T, located in the COOH-terminal region of KCNE1, appears to disrupt the interaction of KCNE1 with helix C in the CTD of KCNQ1 (110). Interestingly, the mutation was also found to diminish PKA-stimulated upregulation of IKs by decreasing phosphorylation at the S27 residue. Since PKA phosphorylation at this site was previously shown to be independent of KCNE1 (226), it is possible that the disruption in adrenergic stimulation by P127T is independent of the mutation's disruption of KCNQ1-KCNE1 interaction (110).
3. LQT11
AKAP-9 is a scaffolding protein and part of the IKs macromolecular complex that plays a critical role in the compartmentalization of adrenergic-stimulated PKA signaling pathway leading to IKs upregulation. That a mutation in AKAP-9, S1570L, can cause LQTS is a testament to the critical role of PKA signaling in the regulation of IKs macromolecular complex (79). This mutation is located near the COOH-terminal binding domain of AKAP-9, disrupting its interaction with KCNQ1, reducing cAMP-stimulated phosphorylation of KCNQ1, and abolishing IKs upregulation in response to cAMP-mediated stimulation. Computational modeling suggests that a disruption in the basal phosphorylation state of IKs alone can alter IKs function sufficiently to prolong APD (79).
E. Molecular Pharmacology
1. β-Blockers
β-Blockers have been demonstrated as a particularly effective form of therapy for LQT1 patients, who are more sensitive to stress- and exercise-induced arrhythmia than other LQT subtypes (282, 371). Insufficient upregulation of IKs by adrenergic stimulation to counterbalance concomitant rise in inward calcium current is thought to underlie APD prolongation in LQT1 during sympathetic activation (386). β-Blockers antagonize adrenergic receptors and helps prevent this imbalance between potassium and calcium currents, decreasing predisposition for cardiac arrhythmic events.
2. Channel activators
While IKs plays a critical role in cardiac repolarization, it is not a direct target of drugs currently used to treat LQTS. However, conceptually, IKs activators could allow for more precise rescue of disease phenotype. In this section we briefly review IKs activators and refer readers to other studies in which activators of the ATP-sensitive K+ channel, such as nicorandil, have been used in LQTS patients and model systems (14, 388). Currently there are no IKs activators being used in clinical trials or therapy, but a number of small molecules that activate IKs have been identified at the benchside and may guide future development of therapeutic agents. For example, the compounds DIDS and mefenamic acid both increase IKs current amplitude (5). In addition, DIDS has been shown to drastically slow IKs deactivation. Interestingly, the effects of these drugs appear to be dependent on the presence of KCNE1. Compared with KCNQ1 alone, current augmentation by these compounds is much greater in the presence of KCNE1, suggesting their effects are mediated by the β-subunit. Indeed, deletion of the residues 39–43 of KCNE1 leads to a diminished response to these compounds. To demonstrate the potential for activators as a class of therapeutic agents for LQTS in in vitro studies, DIDS and mefenamic acid have been shown to rescue IKs function in a LQT5 mutation, D76N. Future studies will be required to better understand their mechanisms of action and to develop IKs-specific activators that can be effective and used safely in patients.
Another KCNQ1 activator, ML277, has been shown to augment current for KCNQ1 alone more effectively than KCNQ1 with KCNE1 (492). In fact, its activating effect diminishes with progressive increase in KCNE1:KCNQ1 stoichiometry, suggesting that the β-subunit may act to preclude the drug from binding to the channel (491). While a drug with such properties is expected to have minimal effectiveness in human cardiomyocytes, surprisingly the same study showed that the drug can augment IKs and shorten action potential in human iPSC-derived cardiomyocytes from a healthy control. The mechanism underlying this effect requires further elucidation.
3. Channel blockers
IKs-specific blockers are not useful as therapeutic agents for LQT1, but serve as useful tools for research by allowing for pharmacological dissection of IKs currents. Chromanol 293B is the first known IKs-specific blocker, with an IC50 of 6.9 μM in Xenopus oocytes (62, 139). A more effective blocker, HMR1556, with an IC50 of 0.12 μM, was developed using Chromanol 293B as the lead compound (139). These drugs inhibit KCNQ1 with KCNE1 more effectively than KCNQ1 alone. Blockers of IKs are speculated to serve useful roles in the treatment of disease conditions resulting from IKs gain of function, such as familial atrial fibrillation.
III. IKR DYSFUNCTION IN CONGENITAL LQTS
A. Introduction
Alterations in IKr, the rapid component of the delayed rectifier current in the cardiomyocyte action potential (Figure 1), underlies congenital Long QT (LQT) syndromes type 2 and 6, which arise from mutations in the KCNH2 and KCNE2 genes, respectively. LQT2 is the second most common cause of congenital LQTS. KCNH2 mutations lead to defective hERG protein, resulting in a decrease in IKr. Mutations in KCNE2 cause defects in the KCNE2 (or MiRP1) protein leading to LQT6, which also results in a decrease in IKr. Irrespective of the underlying cause, a decrease in IKr delays repolarization of the cardiac action potential prolongs the QT interval on the ECG, and predisposes patients to lethal arrhythmia. This section reviews IKr dysfunction leading to congenital and IKr-mediated drug-induced LQTS. [For a detailed summary of hERG channel structure, molecular biology, and basic electrophysiology, see Vandenberg et al. (436).]
B. Physiology
Heterologous expression of hERG reveals a strong, near identical resemblance to IKr in cardiomyocytes (359, 427). IKr is distinguished based on its relatively slow activation and deactivation kinetics, combined with rapid inactivation and recovery from inactivation (Figure 5). Inactivation refers to a conformation of the channel protein in which the channels cannot conduct ions even if the activating machinery is in a conformation that would promote conduction of ions. Channels will not conduct ions when in an inactivated state, but will conduct ions after the channels recover from the inactivated state, a recovery that takes place at negative voltages (174). Deactivation refers to the transition to a conformation in which channels return to a closed, nonconducting state, a transition that also occurs at negative (diastolic) voltages. hERG channels undergo voltage-dependent and C-type inactivation. Because channel activation is slow relative to the rapidly occurring inactivation process, the hERG I–V curve takes on a bell-shaped relationship, as shown in Figure 5C. Upon repolarization of the membrane, channels recover from inactivation much faster than they deactivate. This crucial channel property results in a marked outward K+ current during the repolarization phase of the cardiomyocyte action potential. By this time, a large percentage of hERG channels have recovered from inactivation, such that outward potassium efflux helps return the cell to its resting potential, despite the fact that the electrochemical gradient for potassium efflux decreases as repolarization progresses (Figure 1B).
FIGURE 5.
hERG structure and electrophysiology. A: schematic of the IKr channel complex. Four hERG1 subunits tetramerize to comprise the pore-forming alpha subunit of IKr. hERG1 contains a voltage-sensing domain (purple), including the S4 helix which contains positively charged gating residues, and a pore domain (gray). KCNE2, an accessory subunit of the IKr channel complex, consists of a single transmembrane helix (blue). B: voltage-clamp protocol (top panel) and heterologously expressed hERG1 ionic currents (bottom panel) recorded from a Xenopus oocyte. Currents were recorded at potentials that ranged from −70 to +50 mV; deactivating (“tail”) currents were measured at −70 mV. C: current-voltage (I-V) relationship for hERG1 currents measured at the end of test pulses, as indicated by red circle in B. D: voltage dependence of hERG1 current activation. The peak of tail currents measured at −70 mV (indicated by blue square in B) were normalized to the largest value and plotted as a function of the test potential. E: voltage dependence of hERG1 inactivation. Channel availability is decreased at positive potentials, resulting in a decreased magnitude of peak outward currents and the bell-shaped I-V relationship depicted in C. [B–E from Sanguinetti (356), with permission of Springer.]
Since hERG inactivation and recovery from inactivation proceed more rapidly than activation or deactivation of hERG (365, 394, 401), IKr contributes to prolongation of the plateau phase duration and thus cardiomyocyte contraction, in addition to cardiac repolarization (361, 394). As hERG recovers from inactivation during cell repolarization, the repolarization itself promotes greater hERG recovery from inactivation due to the voltage dependence of hERG inactivation gating (359, 394, 427). As the cell continues to repolarize and return to its resting membrane potential, the slower process of channel deactivation progresses, leading to closure of hERG (436). The fraction of channels remaining open near the resting potential (see Figure 5E) acts to oppose cell depolarization (242, 394), which helps prevent premature heart beats from leading to an early action potential and a tachyarrhythmia. Loss of hERG function thus predisposes to arrhythmia in the setting of premature beats(46).
C. Molecular Biology
The KCNH2 gene, located on chromosome 7q35-36, encoding the human ether a go-go-related K+ channel protein (hERG), was first discovered in 1994 (460). Mutations in KCNH2 associated with congenital LQTS were discovered a year later in 1995 (97), and soon after, it was determined that hERG represents the α-subunits of the K+ channel responsible for IKr (359, 427). hERG may be referred to as “hERG1,” since other hERG proteins (hERG2 and hERG3) have since been discovered (385). hERG1a, the main hERG isoform present in cardiomyocytes, is 1,159 amino acids in length, with a predicted molecular weight of 127 kDa. As with most voltage-gated K+ channels, functional hERG channels are composed of four hERG α-subunits, forming either a hERG1a homomeric channel, or a hERG1a/hERG1b heteromeric channel that contributes to native cardiac IKr activity. The heteromeric assembly of hERG isoforms 1a and 1b has distinctively altered kinetics compared with hERG1a monomeric channels, including faster activation and deactivation. hERG1b is expressed in smaller amounts at the mRNA level in the heart compared with hERG1a, but nevertheless adds to the complexity of hERG channel regulation and potential therapeutic modalities (195, 196, 231, 323, 353, 426).
Each of the four hERG α-subunits consists of six transmembrane helices, S1–S6, as shown in Figure 5A. The voltage-sensing domain spans from S1 to S4; the S4 transmembrane segment of hERG contains the primary positively charged amino acids required for voltage-sensing and opening of the activation gate (325, 408, 505). The pore domain of the channel, spanning from S5–S6, harbors the K+ permeation pathway necessary for K+ conduction (Figure 5A) (105). The long cytoplasmic NH2 terminus contains the PAS domain with a PAS-cap, and together these sequences make up the “EAG” domain that is conserved among EAG-related voltage-gated potassium channels(274). The long cytoplasmic COOH terminus of the channel contains the cyclic nucleotide-binding domain (cNBD) (56), as well as a more distal RXR endoplasmic reticulum retention signal (224), and a coiled-coil domain (188).
The NH2-terminal PAS domain accelerates deactivation of hERG and plays a role in channel trafficking (77, 274, 436). Slow deactivation of hERG may involve interaction of the PAS domain in the NH2 terminus of the channel, with the S4–S5 linker (450), which couples movement of the transmembrane voltage sensor to activation gate movement (247). Experimental hERG mutants with a deleted PAS domain have faster deactivation kinetics (274, 401). Proper folding of the PAS domain also leads to trafficking of hERG from the endoplasmic reticulum to the plasma membrane (314). The cNBD contributes to channel trafficking and gating as well, while cAMP binding to the cNBD domain results in changes in the gating kinetics of the channel, which are altered in the presence of KCNE2, an accessory hERG subunit (see below) (94). Furthermore, the PAS domain and cNBD appear to bind, working in concert to modulate hERG gating by positioning the NH2-terminal residues in close proximity to the cytosolic side of S6 (23, 100, 160, 287).
hERG can coassemble with two different β-subunits in heterologous systems: KCNE1, encoded for by the KCNE1 gene; and KCNE2 (or the MiRP1 protein), encoded for by the KCNE2 gene (Figure 5A) (2, 259). KCNE1 and KCNE2 are single-pass transmembrane subunits that can interact with the hERG channel (2, 3, 20, 259). While the precise physiological role of the KCNEs in regulating hERG and IKr remains unclear (21, 257, 462), mutations in KCNE1 and KCNE2 lead to LQT5 and LQT6, respectively, and mutation of either KCNE1 or KCNE2 can predispose patients to drug-induced LQT syndrome, possibly via modulation of hERG activity (2, 9, 53, 295, 376, 402).
KCNE1 and hERG associate in heterologous systems and may contribute to regulation of IKr; however, KCNE1 is better characterized physiologically as the β-subunit of the KCNQ1 complex that produces IKs (36, 53, 259, 358). KCNE2 was reported to alter gating properties and drug responses of hERG channels(2), and mutations in KCNE2 leading to LQT6 likely result from changes in hERG and thus IKr current activity, producing a pro-arrhythmic state, further supported by the KCNE2 T10M mutation that confers an arrhythmogenic substrate to auditory stimuli, a known trigger of LQT2-associated arrhythmia (see Figure 7) (2, 151, 182, 251). It is possible that physiologically relevant KCNE2 expression exists only in the Purkinje fibers and pacemaker cells of the human heart (297, 330), which further confounds the exact role of KCNE2 as a β-subunit of hERG in vivo. Moreover, KCNE2 coassembles with KCNQ1, which decreases IKs current(192), adding to the complexity and diversity of IKs and IKr channel subunit interactions.
FIGURE 7.
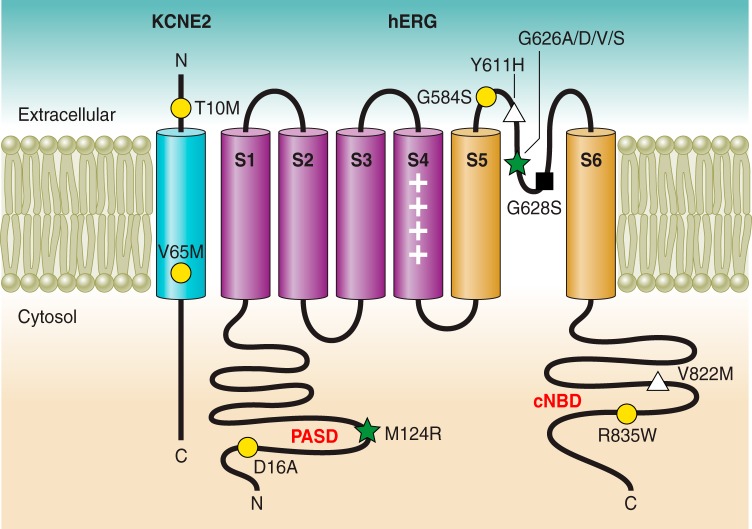
Topology of hERG and KCNE1 in the plasma membrane, representative LQT2- and LQT6-associated mutations highlighted. Different mechanisms of loss of function in hERG or KCNE2, including gating (yellow-filled circle), K+ permeation (black-filled square), trafficking (white-filled triangle), or combined defects (green-filled star) are categorized.
D. Molecular Pathophysiology
A decrease in IKr results in LQT2 via mutations in the KCNH2 gene that encodes hERG, and LQT6 via mutations in the KCNE2 gene that encodes KCNE2 (or MiRP1) protein. The mechanisms underlying disease pathogenesis are described below.
1. LQT2
Patients with congenital LQT2 often present clinically after syncope or seizures not explained by a known medical condition. Prolongation of the QT interval on the ECG supports the diagnosis, and in LQT2 in particular, the T-wave may appear as a classic notched, or “bifid” T-wave (Figure 6A) (503). Syncope occurs following a common pathological process in the LQT syndromes, wherein delayed ventricular repolarization (and resultant QT interval prolongation on the ECG) precipitates an EAD, in some cases DADs, and ventricular tachycardia. EADs trigger the torsades de pointes sinusoidal waveform on the ECG, which may progress to ventricular fibrillation and sudden cardiac death (356).
FIGURE 6.

IKr dysfunction leading to congenital LQTS. A: ECG from a LQT2 patient demonstrates a characteristic “notched,” or bifid, T wave with QTc prolongation (unpublished data). B: simulated action potential (top) and IKr (bottom) in WT (black) and heterozygous LQT2 (gray) conditions, demonstrating the effect of 50% reduction in IKr.
Homozygous mutations in KCNH2 leading to LQT2 are extremely rare in humans, resulting in either death in utero, or very severe prolongation of the QT interval upon birth (175, 194). Heterozygous mutation leading to one defective hERG copy is far more common and may result in significant prolongation of the cardiac action potential duration (see Figure 6B). hERG mutations leading to LQT2 occur by a variety of genetic mechanisms. To date, ∼500 mutations in KCNH2 have been identified in association with LQT2 (23). A study analyzing 226 different LQT mutations in genotype-confirmed LQT2 patients reported that 62% of mutations were missense, 24% were frameshift, while 14% were a combination of nonsense mutations, inframe insertions/deletions, or splice site mutants in the KCNH2 gene. Of the combined 226 mutations, 32% resided in transmembrane and pore-pore domains; 29% in the NH2 terminus, including 8% in the PAS/PAC domains; and 31% in the COOH terminus, including 8% in the c-NBD (207). In terms of molecular mechanism, a small proportion of LQT2 mutations result in nonsense-mediated mRNA decay (150). More commonly, decreased protein trafficking to the cell surface leads to eventual endoplasmic reticulum-associated degradation (ERAD) of mutant hERG (22, 149, 443, 512).
Several important general characteristics of KCNH2 mutations associated with congenital LQT2 were recently highlighted in a large-scale functional study of 167 different LQT2-associated missense mutations (23). Functional analysis was performed by voltage clamp after coexpression of WT and mutant subunits in HEK293 cells. Anderson et al. (23) found that 1) hERG trafficking defects comprised the most common (88%) mechanism of loss of function overall, derived from mutations in the PAS domain, pore domain, and C-linker/cyclic nucleotide-binding domain. Only the distal COOH-terminal region did not yield trafficking defects as the primary mechanism of loss-of-function. 2) Greater than 70% of pore mutations led to dominant negative suppression of hERG, whereas the other intracellular domain mutation locations did not yield dominant negative suppression of current. 3) All mutant channels, regardless of mutation location within the channel, were rescued pharmacologically by E4031, a hERG pore blocker that stabilizes channel structure and folding, thus promoting channel retention at the cell surface (148). Coexpression of WT with pore mutant channel rendered heteromeric pore mutant-WT channels especially responsive to pharmacological correction compared with coexpression of WT with non-pore mutant channels. Overall, these data suggest that pharmacological recovery of hERG channel function is feasible for a large proportion of LQT2 mutations(345) and raise the possibility of this approach as a therapeutic strategy for LQT-2 patients, but to our knowledge this has not been implemented in the clinic.
A) MUTATION SEVERITY: HAPLOTYPE INSUFFICIENCY VS. DOMINANT-NEGATIVE LOSS OF FUNCTION.
Heterozygous mutation resulting in defective KCNH2 gene expression or hERG protein that does not impact normal functioning of the WT hERG protein that remains yields haplotype insufficiency. Only the gene product from the mutant KCNH2 allele is negatively affected, while WT hERG subunits still homomerize to form functional channels. In contrast, some hERG mutations have increased pathogenicity by exerting a dominant-negative loss of channel function, wherein the mutant and defective KCNH2 gene product reduces function of the WT hERG protein encoded in the patient's genome via heteromerization of mutant with WT channel, rendering the healthy hERG subunits nonfunctional when combined with mutant hERG, either by decreasing forward trafficking of mutant WT channels, or decreasing function of the heteromers at the cell surface. In a study of LQT2 patients with 44 unique mutations in different regions of the hERG channel, it was discovered that those patients harboring mutations in the pore region of the channel were more susceptible to cardiac events than patients with non-pore region hERG mutations, likely due to a greater dominant-negative suppression of IKr current exerted by pore vs. non-pore mutations (187, 283).
Perhaps counterintuitively, more harmful mutations in one KCNH2 allele, resulting in a premature stop codon for instance, prevents formation of a hERG protein product from the mutant KCNH2 allele, leading to haplotype insufficiency and the possibility of a less severe phenotype compared with some dominant-negative mutations, as WT hERG remains unaffected(356).
Studies of specific hERG mutants have greatly enhanced our understanding of the underlying mechanisms of loss of function leading to LQT2. Figure 7 provides a schematic of representative LQT-associated hERG and KCNE2 mutations, categorized by mechanism of loss of function (see Table 4). Representative LQT-associated hERG mutations are divided into four general classes: I) decreased hERG synthesis, II) trafficking defect, III) gating defect, and IV) decreased K+ permeability (436).
Table 4.
Representative LQT2- and LQT6-associated mutations classified by mechanism
| Mechanism | Mutations | Reference Nos. |
|---|---|---|
| hERG LQT2 mutations | ||
| K+ permeation | G628S | 59, 116, 357 |
| Gating | D16A, G584S, T613A, I711V, R835W | 23, 329, 509 |
| Trafficking | Y611H, V822M | 512 |
| Decreased hERG synthesis | R1014X, W1001X, Y652X | 50, 150, 356, 409 |
| Multiple mechanisms (e.g., gating and trafficking) | F29L, M124R | 23, 206, 390 |
| KCNE2 LQT6 mutations | ||
| Gating | T10M, V65M | 151, 182 |
I) Decreased hERG synthesis. Defective biogenesis of hERG may occur via mRNA processing abnormalities or mRNA instability (150, 504). The hERG R1014X mutant mRNA transcript is degraded by “nonsense-mediated mRNA decay,” a cellular damage-control mechanism that destroys mRNA transcripts harboring nonsense mutations (premature stop codons), which prevents translation of a shortened hERG peptide (150, 356). Other nonsense-mediated decay mutants include W1001X (150) and Y652X (409). These mutations would result in haplotype insufficiency, as there would be 50% loss of function while the remaining WT hERG channels function normally. Nevertheless, a 50% reduction in IKr can lead to clinically significant LQTS.
II) hERG trafficking defects. Defective hERG trafficking is the most common mechanism of loss of hERG function (22, 23, 436), and most KCNH2 missense mutations cause trafficking defects in hERG (22). Trafficking mutants may result in either dominant negative loss of hERG function (22, 201) or haploinsufficiency (131). If WT hERG associates with trafficking-defective mutants to form heteromeric channels in the endoplasmic reticulum (ER) or Golgi apparatus, dominant negative suppression of IKr results: 93% reduction in IKr was observed by this mechanism (123).
Defects in trafficking may be further subdivided by the underlying mechanism, protein trafficking, or protein misfolding (22, 124, 512); hERG protein becomes core glycosylated in the ER, with modifications made in the Golgi apparatus. When the mature hERG protein traffics to the plasma membrane, it weighs 155 kDa, versus 135 kDa for the core-glycosylated-only hERG protein. This difference in molecular weight aids in determining whether hERG possesses a trafficking and/or protein folding defect (512). The hERG Y611H and hERG V822M LQT2 associated mutations were found to have a molecular weight of 135 kDa, and thus had decreased trafficking to the plasma membrane due to protein misfolding. These mutant hERG channels were retained in the ER, and subsequently ubiquitinated and degraded in proteasomes (512).
III) hERG gating defects. Alteration in activation, deactivation, and/or inactivation kinetics can result in loss of function of hERG and a decrease in IKr at physiologically relevant membrane potentials. Any hERG mutation that, for instance, enhances the speed of inactivation, or causes a depolarizing shift in channel activation, ultimately leads to loss of hERG function at voltages ranging from the resting potential to the plateau phase of the action potential, depending on the nature of the gating defect (48, 77, 290, 357, 363, 509). Several hERG gating defect mutations have been described in mammalian cell lines (48, 363, 509).
Both the I711V and R835W mutations represent examples of c-NBD region LQT2 mutations in the COOH terminus of the channel, with altered channel gating. These hERG mutants deactivate faster at −50 mV compared with WT hERG. In addition, the R835W mutation confers a rightward, depolarizing shift (+16 mV) in the V0.5 of activation, while inactivation measured at 0 mV remains unchanged (23). Within the NH2 terminus of hERG, the LQT2 hERG D16A mutation exhibits a +13 mV rightward shift in the V0.5 of activation, with a minor slowing of inactivation at 0 mV and a slowing of deactivation at −50 mV (23, 302).
The hERG G584S pore mutation increases the rate of channel inactivation (509). G584S represents a pore mutation with a milder loss-of-function phenotype directly and solely attributed to its effect on inactivation. This finding adds further to the complexity of hERG mutant phenotypes as most other pore mutations cause increased arrhythmic events compared with non-pore hERG mutations (283). Recently, the hERG T613A mutation in the outer region of the pore helix, a regulatory site for C-type inactivation, was found to cause greater than 80% inhibition of maximal hERG current via a hyperpolarizing shift in channel inactivation when expressed in Xenopus oocytes alone; coexpression of WT and mutant channel resulted in an intermediate loss of channel function, without dominant-negative suppression of current (329).
Gating defects can arise from mutations across a wide range of locations in hERG, and predispose individuals to LQT2 of varying severity dependent on the functional impact of the mutation.
IV) hERG K+ permeation defect. The potassium selectivity filter in the pore of the hERG channel allows for potassium permeation with great specificity. Conserved amino acid residues comprise the selectivity filter, and mutation of any of these residues or nearby amino acids may greatly alter potassium permeation, even if only one of four hERG α-subunits within the mature channel bears the mutation (i.e., dominant negative suppression of IKr). The hERG G628S mutation, for instance, traffics to the cell surface and gates normally, which was confirmed by voltage-clamp fluorometry experimentation, but the mutant hERG channels do not conduct potassium under physiological conditions due to blockade of potassium permeation by intracellular potassium (116). The G628S mutation was also found to be cause lethal arrhythmia in transgenic rabbits with the mutation, as these rabbits possessed greater than 50% incidence of sudden cardiac death after one year (59).
B) COMBINED MECHANISMS OF LOSS-OF-FUNCTION IN LQT2.
Many mutations leading to LQT2 confer a loss-of-function hERG phenotype by multiple molecular mechanisms. Kanters et al. (206) recently reported the mechanistic basis of channel dysfunction of the hERG F29L mutation in a founder population of Danish families, which results in a malignant form of LQT2. The mutation causes clinically significant prolonged QT, with a penetrance of 73%. The F29L mutation was found to 1) reduce trafficking of hERG to the cell surface, posited to occur based on decreased glycosylation of the hERG F29L channel; and 2) reduce steady-state inactivation current density, positively shift the voltage dependence of inactivation, and increase the rate of deactivation. Similarly, the M124R mutation in the NH2 terminus residing close to the hydrophobic surface of hERG, results in faster channel deactivation, in addition to reduced trafficking to the cell surface (23, 390). Furthermore, the exact amino acid substitution can impact the mechanism of loss of function of the hERG channel: the LQT2-associated G626A mutation in the conserved K+ selectivity filter in the channel pore traffics to the cell surface normally, while the LQT2-associated G626D, G626V, and G626S mutations had trafficking defects. The exact amino acid substitution has potential therapeutic implications: of the three G626 trafficking mutants, only hERG G626S had its trafficking defect corrected by E4031, an experimental pharmacological agent that increases forward trafficking of hERG channels in addition to its pore blocking effects (23).
2. LQT6
KCNE2 (or MiRP1) protein, encoded for by the KCNE2 gene, modulates hERG activity as an accessory β-subunit (Figure 5A). Mutations in KCNE2 cause LQT6 due to a decrease in IKr (see Figure 7 and Table 4) (2). The V65M mutation in KCNE2, for example, induces faster inactivation of colocalized hERG/mutant KCNE2 channel complexes (182). The KCNE2 T10M was found linked to patients with cardiac arrhythmia and LQTS, with particular susceptibility to arrhythmia onset in the setting of auditory stimuli and hypokalemia, both common triggers of arrhythmia in LQT2 patients as well. Coexpression of KCNE2 T10M with hERG decreases tail currents and causes a hyperpolarizing shift in steady-state inactivation, along with slowed recovery from inactivation compared with WT channels in CHO cells. The impact of the KCNE2 T10M mutation in the setting of known LQT2 arrhythmia triggers supports the hypothesis that KCNE2 associates with hERG in the human heart to regulate IKr (151). As KCNE2 is known to interact with other K+ α-subunits in addition to hERG, defective KCNE2 may lead to varying phenotypes. Indeed, the KCNE2 M54T mutation leads clinically to LQT6 in addition to sinus bradycardia, the latter clinical finding mediated by reduction of the HCN4 pacemaker channel activity (297). The complexity of interactions of KCNE2 with a variety of potassium channels may inform differential phenotypes observed clinically in those harboring a KCNE2 mutation.
3. Autoimmune-associated LQT syndrome
Recently, Yue et al. (494) discovered that anti-Ro antibodies block the hERG channel, resulting in an autoimmune-associated LQTS. The anti-Ro antibodies, present in the serum of patients with a variety of common autoimmune illnesses, were found to inhibit IKr in HEK293 cells, and a guinea pig autoimmune model with circulating anti-Ro antibodies had a prolonged QTc interval, caused by inhibition of native IKr. Anti-Ro likely blocks hERG by binding within the pore region of the channel. Patients with anti-Ro-positive autoimmune illness may be well advised to take particular caution to avoid medications that prolong the QT interval (494).
E. Molecular Pharmacology
hERG channel modulation by pharmacological agents has become an increasingly studied research area because it is now well established that a wide variety of clinically used drugs block hERG and thus inhibit IKr, leading to drug-induced LQTS. The section below details mechanisms and the biomedical relevance of drug-induced LQTS, followed by a discussion of various pharmacological tools that hold promise as novel agents for the treatment of LQT2 (and LQT6) in the future.
1. Drug-induced LQTS
Most drugs that block IKr conduction bind to the open state of the hERG channel, binding the inner pore thereby preventing permeation of potassium (67, 215, 267, 400, 483, 513). The anti-arrhythmic pharmacological agent MK-499 interacts mostly with polar and/or aromatic residues within the channel pore, including Thr623, Tyr652, and Phe656, to induce hERG block (266). Different hERG-blocking drugs may interact with different combinations of these four residues (266, 319). For instance, the anti-arrhythmic agent dofetilide interacts with Phe656 to mediate hERG binding (233). While the aromatic and polar residues above are integral to hERG's supreme sensitivity to block by pharmacological agents, C-type inactivation may increase affinity of drugs to hERG's pore residues and enhance drug block (125, 171).
Some drugs, including pentamidine (90, 228) and probucol (156), cause a reduction in IKr by reducing trafficking of hERG to the plasma membrane, leading to prolongation of the QT interval. The widely clinically employed drugs fluoxetine and ketoconazole block hERG directly and decrease hERG channel density at the cell surface (337, 411). Several other pharmacological agents precipitate drug-induced LQTS in susceptible patients, via hERG blockade or modulation of other important ionic currents (e.g., INa, IKs) (439, 482).
KCR1, a 12-pass transmembrane segment regulatory protein, interacts with hERG and mitigates the proarrhythmic effects of hERG-blocking agents (178, 223). In heterologous conditions, KCR1 decreases the sensitivity of hERG to a variety of well-established hERG channel blockers, such as sotalol, quinidine, and dofetilide (223). Loss of function of KCR1 is associated with greater risk of arrhythmia: the KCR1 E33D mutant found in a patient with ventricular fibrillation and QT-interval prolongation conferred reduced protection against drug-induced hERG blockade (168). The KCR1 I447V variant is associated with lower frequency of drug-induced arrhythmia, and reduced sensitivity hERG block by dofetilide when coexpressed heterologously (322). These results suggest that functional KCR1 provides protection against a drug-induced decrease in IKr, with a mechanism of action thought to entail modulation of KCR1 α-1,2-glucosyltransferase activity (291).
2. Drug screening for hERG toxicity
Given the propensity for clinically relevant pharmacological agents to block hERG, leading to drug-induced arrhythmia, it is now a required practice for drugs early in development to undergo hERG toxicity screens. High-throughput screening techniques, including radioligand binding, ion flux and fluorescence assays, and hERG-Lite which measures cell surface density of hERG, detect candidate drugs with strong hERG-blocking and/or traffic-altering properties (436). The development of automated patch-clamp electrophysiology enhances the throughput of data acquisition tremendously, allowing for numerous hERG recordings from different cells, applying different drugs and at different concentrations, to be collected simultaneously. Automated patch-clamp technology is the best initial high-throughput hERG toxicity drug screening method to date (163). A focus on hERG toxicity screening early in the drug development process may save time, and prevent or minimize adverse clinical outcomes due to inadvertent IKr block.
3. Pharmacological treatment in LQT2
β-Blocker treatment represents first-line pharmacological therapy in LQT2 patients. For a more detailed discussion of β-blocker treatment in congenital LQTS, refer to section VI of this review.
A growing interest in hERG activators has developed. Such agents may be especially useful in the setting of congenital LQT2 and/or drug-induced LQT syndromes. hERG activators may be subdivided into type 1 activators type 2 activators, based on mechanism of hERG activation (436).
Type 1 hERG activators include RPR260243, an experimental small molecule compound that slows hERG deactivation in a temperature- and voltage-dependent fashion as its primary means of channel activation (204, 321). RPR260243 may slow deactivation via binding to the S4–S5 linker and the cytoplasmic regions of the S5 and S6 hERG domains (321).
Type 2 hERG activators act primarily by slowing channel inactivation, and/or shifting the voltage dependence of inactivation to more positive membrane voltages (69, 138, 153, 164). Type 2 activators may also have other relatively minor effects on deactivation kinetics and voltage dependence of activation, as well as single-channel open probability (69, 138, 164, 165, 407, 511). Several type 2 hERG activators have been described, including experimental compounds PD118057 (320), ICA105574 (28, 134, 263), and NS1643 (153, 155, 318), and key insights into their mechanism of action alongside their hERG binding location have been found, each having specific binding residues that mediate their mechanism of hERG activation within the “type 2” paradigm.
Other compounds have predominating mechanisms of hERG activation that do not fall into the “type 1” or “type 2” classes. For instance, mallotoxin and KB130015 primarily enhance hERG activation while shifting the voltage dependence of activation to more negative membrane voltages, likely by binding within the channel pore (140, 499). Some compounds, such as A935142 (407), have both “type 1” and “type 2” characteristics. As activators bind hERG at various locations within the channel, efforts to correlate binding location and channel stoichiometry with mechanism of hERG activation by small molecule compounds will aid the design of more selective and potent hERG channel activators by a variety of mechanisms (476).
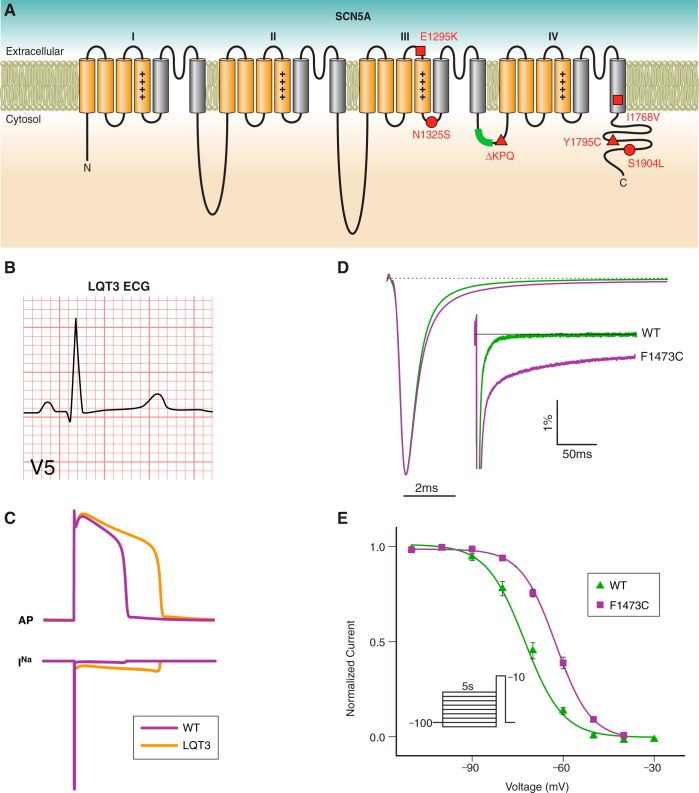
IV. INA DYSFUNCTION IN CONGENITAL LQTS
A. Introduction
LQT3, LQT9, LQT10, and LQT12 account for 5–10% of congenital LQTS (7) and are associated with gain-of-function mutations in the cardiac voltage-gated Na+ channel complex of proteins. As such, patients diagnosed with Na+ channel-associated LQT often, though not exclusively, have a unique risk profile for arrhythmia triggers and require different pharmacological approaches to disease management than patients with LQT1 or LQT2 (370). Electrophysiological experiments have elucidated the connection between aberrant function of single ion channels and the unique clinical presentation of this set of diseases. In each case, congenital mutations result in a failure of the cardiac Na+ current (INa) to properly inactivate. However, even within the subset of Na+ channel-associated LQT, differences in mechanisms of pathological INa generation and risk stratification in some patients makes LQT3 an important case study in the value of patient-specific medicine. Below we will discuss the role of INa in the conduction of electrical impulses in the heart, mechanisms by which congenital mutations may alter these currents, and advances in the discovery of potent and selective pharmacological agents to treat disease.
B. Physiology
In the heart, the majority of voltage-gated Na+ current is carried by the cardiac isoform of the voltage-gated Na+ channel, Nav1.5 (137). Upon membrane depolarization to a threshold around −50 mV, channels activate and allow the influx of Na+ down its electrochemical gradient. The rapid influx of positive charge across the cell membrane drives the membrane potential from its resting voltage to a peak of +50 mV, near the Na+ equilibrium potential (Figure 1). The rapid activation of Nav1.5 is therefore critical to maintaining the speed of cardiac conduction. This rapid depolarization coordinates the stimulation of voltage-gated Ca2+ and K+ channels necessary for excitation-contraction coupling and, eventually, repolarization (299).
In a healthy human heart, the Na+ current is rapidly attenuated in milliseconds as channels inactivate, a process that was first observed and defined in Hodgkin and Huxley's giant squid axon experiments (173). Inactivation is responsible for the refractory period in neurons and myocytes during which the firing of new impulses is not possible. Similar properties are observed in Nav1.5, with inactivation progressing with a time to half peak current on the order of 1 ms and a mean open channel duration of roughly 0.5 ms (49) (Figure 8, A AND B).
FIGURE 8.
Physiology and molecular biology of INa. A: consecutive recordings of single Na+ channels recorded under cell-attached conditions in HEK293 cells transfected with Nav1.5 cDNA (unpublished data). B: WT Na+ currents recorded under whole cell patch-clamp conditions and obtained by depolarizations from −120 to −30 mV (unpublished data). At high gain (inset), whole cell currents return nearly to 0 nA. C: voltage dependence of activation (red-filled square) and inactivation (blue-filled circle) (unpublished data). D: topology of Nav1.5 in the plasma membrane with accessory proteins implicated in LQTS.
Na+ channel inactivation is a complex multistep process that involves multiple channel components and is itself highly voltage dependent. The voltage dependence of inactivation is independent of the voltage dependence of channel activation, as inactivation may occur from either closed or opened states (66). Closed-state inactivation can be measured with subactivation threshold depolarizing pulses. When these pulses are sufficiently long to approach equilibrium, these experiments are referred to as “steady-state inactivation” experiments. As the holding potential becomes more depolarized, channels enter a closed-inactivated state and are unavailable to open upon supra-activation threshold depolarization with a midpoint near −70 mV (Figure 8C). Because this midpoint is in the range of the resting membrane potential in a ventricular myocyte (−85 mV), small changes in the V0.5 of steady-state inactivation caused by mutations or drugs, or small changes in resting membrane potential, can have a large impact on channel availability, Na+ current density, and conduction velocity (86).
The voltage dependence of inactivation from the open state has been more difficult to dissect, as channel fast-inactivation is a nonequilibrium process. The rate of decay of Na+ currents increases with increasing voltage over the physiologically relevant range, reaching half-inactivation in 1.5 ms at −40 mV and 0.5 ms at +20 mV (34). Recovery from inactivation has the opposite voltage dependence of the onset of inactivation: channels recover more rapidly at hyperpolarized potentials, and very slowly at potentials more positive than activation threshold (approximately −45 mV) (508). The presence of multiple inactivation states is evident in the time course of recovery from inactivation. Following an initial depolarizing pulse to inactivate channels, channel recovery during a period at resting membrane potential follows a biexponential time course, suggesting the existence of two distinct populations of channels (508): one in the fast-inactivated state and one in the slow-inactivated state.
C. Molecular Biology
Nav1.5 is encoded by the gene SCN5A (452). Unlike the canonical voltage-gated K+ channels, Nav1.5 is composed of a single polypeptide chain (137). The protein folds into four homologous asymmetric domains, each containing six transmembrane helices (S1–S6) (70) (Figure 8D). S1 through S4 form a voltage-sensing unit in each domain. S4 spans the membrane and contains several charged arginine residues capable of “sensing” changes in membrane potential. Depolarization drives a conformational change in S4 that is transmitted to the pore-forming helices, S5 and S6. Along with the extracellular reentrant loop that connects them, S5 and S6 from each domain form the central ion conduction pathway (70).
The intracellular segments of Nav1.5 have been shown to be critical to proper channel function. The linker between domains III and IV contains the three amino acid isoleucine, phenylalanine, methionine (“IFM”) motif, shown to be a key molecular determinant of fast inactivation (313, 465). Upon channel opening, this motif has been proposed to bind to a hydrophobic cluster in the intracellular linkers between S4 and S5 of domains III and IV (268, 415). Consistent with these findings, disruption of the structure of the DIII/DIV linker by proteolytic cleavage (434) or mutation (47) can impair inactivation, and coexpression of a cytosolic peptide containing the IFM motif can potentiate inactivation (111).
The intracellular COOH-terminal domain has been shown to play an important role in channel function. The COOH terminus has a structured proximal domain and unstructured distal region (91). It has been proposed that interactions between the COOH terminus and DIII/DIV linker are critical in the stabilization of the inactivated state (285), and furthermore that this interaction may be facilitated by calmodulin (435). In fact, several LQT3-causing mutations have been identified in the COOH terminus (34, 343).
Multiple techniques have been used to study the role of voltage-sensing units in channel function. The asymmetric nature of eukaryotic voltage-gated Na+ channels allows the voltage sensors to modulate different aspects of channel gating. Mutagenesis (66, 384), cysteine accessibility (380, 381, 383), and voltage-clamp fluorometry (71, 72) have revealed distinct properties of each voltage sensor. Movements of the DIV voltage sensor have been shown to correlate with the voltage dependence and kinetics of inactivation (71, 147), and DIV-S4 activation is necessary and sufficient for inactivation gating (66). The putative docking site for the inactivation gate resides in the intracellular NH2-terminal loops of S4 in DIII and DIV, potentially bridging our understanding of voltage-sensing domains and voltage-dependent gating transitions (268, 415).
1. Accessory proteins
In the heart, Nav1.5 is associated with several accessory proteins that modulate its trafficking and biophysical properties. Four genes that encode β-subunits of the voltage-gated Na+ channel have been identified, SCN1B through 4B, and all are expressed in the heart (253, 342). These β-subunits have an Ig (immunoglobulin)-like NH2-terminal domain, a single transmembrane helix, and a short cytoplasmic COOH-terminal domain. While they are similar in structure, the β-subunits interact with the α-subunit through different mechanisms. β1 and β3 interact noncovalently with the α-subunit, and β2 and β4 interact via covalent disulfide bond (490).
Immunohistochemical studies suggest that β-subunits play an impo rtant role in subcellular localization of different channel isoforms (253). Nav1.5 predominantly colocalizes with the β2 and β4 subunits, encoded by the gene SCN2B and SCN4B, in the intercalated disks. β4 has no significant effect on Nav1.5 activation or inactivation gating (490). Small amounts of β1 subunit were also found to colocalize with Nav1.5 (101, 211, 253). Unlike β4, β1 displays a dramatic functional effect on inactivation of Nav1.5 currents expressed in tsA cells. β1, but not β2, caused a significant positive shift in the V0.5 of steady-state inactivation (101, 211, 342).
Nav1.5 is also associated with several other structural and signaling complexes in the cardiomyocyte. Caveolin and syntrophin are two such proteins that have been implicated in functional perturbation of INa and Na+ channel-associated LQT. The six known caveolin proteins are encoded by three caveolin genes: CAV1, CAV2, and CAV3 (30). Cav-3 is the most abundant isoform in cardiac tissue where it is associated with cytoplasmic signaling factors (396), is responsible for the formation of lipid raft microdomains and caveolae (218), and potentially plays a role in t-tubule organization (133). Cav-3 null mice lacked caveolae and had abnormal t-tubule development in skeletal muscle. Biochemical and microscopic techniques have revealed that Nav1.5 colocalizes with Cav-3 in lipid rafts, which facilitate the increase in peak INa density in response to β-adrenergic stimulation (486).
Caveolin and Nav1.5 also both associate with the cytoskeletal dystrophin complex (106). Syntrophin-α1 is a member of the dystrophin-associated protein complex and is the most abundantly expressed syntrophin in the heart. Syntrophins mediate the interaction of dystrophin with the PDZ-binding domain on the COOH terminus of Nav1.5 (135). Isolated cardiomyocytes from dystrophin knockout mice showed markedly decreased INa density, and ECGs showed significant QRS prolongation, consistent with an interaction between the dystrophin-associated protein complex and ion channels in the heart.
D. Molecular Pathophysiology
1. LQT3
Like other forms of LQT, the hallmark of LQT3 is a prolongation of the QT interval on the ECG arising from a prolongation of the ventricular action potential and delayed ventricular repolarization. The discovery that mutations in multiple genes can give rise to LQT has allowed for an improved understanding of the risk stratification for cardiac events in this set of patients (Figure 9, A–C). While patients with K+ channel-associated LQT are generally at higher risk or cardiac events during exercise, patients with LQT3 have elevated risk at rest (370, 371). However, even within the umbrella of LQT3 there is a range of triggers for arrhythmia that is not easily explained by a “one size fits all” model, suggesting patient-specific differences in disease mechanism (244).
FIGURE 9.
INa dysfunction leading to congenital LQTS. A: topology of Nav1.5 in the plasma membrane. Several representative LQT3-associated mutations were selected because there is strong evidence that they induce INaL by channel bursting (red-filled triangle) or by late reopening (red-filled circle), or prolong the action potential without INaL (red-filled square). B: ECG from a LQT3 patient (unpublished data). C: simulated action potential (top) and INa (bottom) in WT (green) and heterozygous LQT3 (purple) conditions. INa peak current was truncated to enhance view of INaL. D: representative current recordings from WT (purple) and F1473C (orange) Nav1.5 expressed in HEK293 cells at low and high gain (inset) elicited by depolarization to −10 mV (35). E: steady-state channel availability for WT (green triangle) and F1473C (purple square). [D and E from Bankston et al. (35).]
LQT3 is a disease of impaired Nav1.5 inactivation. Failure of the channel to inactivate or remain inactivated during the plateau or repolarization phases of the ventricular action potential allows for a depolarizing current sufficient to prolong the action potential, leaving patients susceptible to asynchronous EADs or DADs (184, 240). In most cases, impaired inactivation is manifest as a sustained noninactivating inward current, or late current (INaL) (Figure 9D). Rather than inactivating completely, many disease-associated mutations cause a persistent INaL during prolonged depolarizations. It should be noted that INaL of smaller magnitude has also been detected in tissue isolated from normal hearts (432); thus in pathology some INaL may result from the failure to control or modulate normal activity. Nevertheless, while currents through these channels still provide the rapid influx of Na+ necessary for cardiac conduction, at high gain one can observe INaL that amounts to as little as 1% of the magnitude of the peak. Because the membrane impedance during the plateau phase of the action potential is high, this small depolarizing current has a substantial effect on AP duration.
The mechanisms by which gain-of-function Na+ currents may arise have been studied in depth, and recent computational work suggests that these divergent mechanisms may underlie tissue-specific phenotypes (185), as well as arrhythmia risk stratification (32). Two mechanisms have been established for the generation of persistent INaL, and an additional set of mechanisms has been proposed for aberrant Na+ conduction in the absence of INaL. Table 5 lists a set of Nav1.5 mutations known to perturb channel function by specific mechanisms.
Table 5.
Representative LQT3-associated mutations classified by mechanism of gain of function
A) CHANNEL BURSTING.
Channel bursting is best exemplified by the ΔKPQ mutation, a deletion of amino acids 1505–1507 in the DIII/DIV linker near the IFM motif. ΔKPQ was first identified in 1995 (453) and characterized that same year (47). Like WT currents, currents passed by single ΔKPQ channels typically activate and inactivate quickly. However, during a small fraction of depolarizing pulses, channels enter a slow gating mode, or “burst” mode, characterized by a persistent noninactivating current.
Modal transitions have been shown to be highly dependent on inter-pulse duration and resting membrane potential. INaL from ΔKPQ channels in heterologous expression systems (289) is highly rate dependent, decreasing dramatically with increased pulse frequency. In isolated transgenic ΔKPQ mouse cardiomyocytes, changes in pacing rate caused pause-induced spontaneous depolarizations (127). This is consistent with a decrease in EAD frequency and polymorphic VT during increased pacing in intact mouse hearts (120), and explains the elevated risk of arrhythmia and sudden cardiac death at rest in human patients with LQT3 (370, 371).
The inverse dependence on interpulse duration suggests that transitions between background and burst gating modes occur predominantly at rest. At higher resting potentials, a larger fraction of channels exist in closed-inactivated states, leaving fewer channels available to transition into bursting states. Indeed, INaL is smaller during test pulses from more depolarized holding potentials (185). Taken together, Clancy and Rudy (85) were able to develop a computational model of ΔKPQ function by allowing transitions between background and burst modes only when channels exist in the closed state. This model was able to reproduce single-channel and whole cell gating properties, as well as prolongation of the ventricular action potential and the occurrence of EADs.
B) LATE REOPENING.
Following the identification of modal transitions in ΔKPQ channels, other SCN5a mutations were identified in patients with LQT3 that cause INaL by a different mechanism. At the whole cell level, the N1325S and R1644H mutations had markedly different inactivation (109) and recovery from inactivation time courses (448). Single-channel records revealed that these gating defects were in fact not due to bursting channels, but to dispersed channel reopening that occurred during prolonged depolarizations (109). Additional studies revealed that these late reopenings contribute to a fraction of the INaL from ΔKPQ channels, as well (73).
An additional mutation, S1904L, was later shown to also induce late reopenings and INaL (34). Interestingly, this mutation does not reside in the DIII/DIV linker, but rather exists in the distal COOH-terminal domain. Transition metal ion FRET showed that this mutation destabilized a critical intramolecular interaction within the COOH-terminal domain (144), contributing to our understanding of the inactivation gate as a macromolecular complex of different channel components including the DIII/DIV linker and COOH terminus (285).
A computational model has been constructed to simulate late reopening currents at the single-channel and whole cell level (34). Function of the S1904L mutation could be simulated by reducing the rate of transition into the slow inactivated states, allowing channels to reside for a prolonged time in fast inactivated states from which they may reopen. An interesting prediction of this model is that the amplitude of INaL will not display the inverse rate dependence present in bursting channels, and in fact the patient experienced arrhythmic events that were not limited to periods of rest.
C) WINDOW CURRENT.
In WT channels, the voltage dependence of channel availability (V0.5 = −71 mV) and channel activation (V0.5 = −25 mV) overlap only slightly. Therefore, there is a very small range, or “window,” of voltages in which channels may activate but not completely inactivate. This range may provide a small noninactivating current known as “window current.” Typically, this window current is very small and only exists at voltages at which repolarizing currents through delayed rectifier K+ channels are strong, and therefore contribute little to net membrane current.
Changes in steady-state properties of the cardiac sodium current have been proposed as mechanisms for AP prolongation and LQT, even in the absence of sustained INaL during prolonged depolarizing steps (Figure 9E) (6, 344, 395, 461). These mutations cause depolarizing shifts in channel availability such that the window current is either larger, or exists at more depolarized voltages where rectification of K+ currents increases the relative contribution of INa to total membrane current. These patients are therefore more susceptible to drug-induced LQT, most commonly arising from block of hERG channels, less commonly from block of other K+ channels and drugs that potentiate inward currents, including INa (439, 482). Additionally, several disease-causing mutations have been shown to induce depolarizing shifts in channel availability in addition to canonical INaL during prolonged depolarization (35, 448, 463).
D) NONEQUILIBRIUM GATING DEFECTS.
In addition to changes in steady-state parameters of channel activity, time-dependent changes in gating kinetics have also been posited as a mechanism for AP prolongation. As membrane potential declines into the repolarization phase of the AP, WT channels recover slowly from inactivation. As membrane repolarization is more rapid than recovery from inactivation, WT channels never recover at potentials that facilitate channel activation. Some LQT3 mutant channels, such as the I1768V mutation, have enhanced recovery from inactivation and are able to recover at voltages above the channel activation threshold (87). Importantly, this nonequilibrium reopening is different than window current because it occurs at potentials at which activation and steady-state channel availability do not overlap.
E) PHOSPHORYLATION-DEPENDENT INAL.
There exists one known mutation, D1790G, for which in vitro studies found INaL only arises after PKA-dependent phosphorylation (416). D1790G channels showed marked INaL in response to cAMP-dependent PKA stimulation and lacked INaL under basal conditions. Furthermore, the D1790E mutation showed no response to cAMP application, suggesting that the negative charge at residue 1790 is important in modulating PKA phosphorylation. Interestingly, alanine mutagenesis of consensus PKA phosphorylation sites showed that S38 and S525 are important in mediating INaL in D1790G channels, despite residing in disparate parts of the channel. These effects were found despite the fact that most spontaneous arrhythmic events in LQT3 patients occur during rest or sleep, suggesting perhaps a role of basal phosphorylation of the Nav1.5 channel in D1790G patients (371).
2. LQT associated with mutations to accessory proteins
In addition to mutations in SCN5A, mutations in the accessory proteins important in cardiac INa have been associated with LQT (11), although there are few documented cases of such diseases.
3. LQT10
Mutations in the β4 subunit give rise to LQT10. The L179F mutation was identified in a 21-mo-old girl with bradycardia and severe QT prolongation (261). When coexpressed with Nav1.5 in HEK cells, this mutation caused both a dramatic INaL and a depolarizing shift in the V0.5 of steady-state inactivation that increased the window current. Interestingly, this β-subunit mutation induced a larger INaL than the ΔKPQ deletion in the α-subunit. Mutations in SCN4B have also been associated with Sudden Infant Death Syndrome (SIDS) (413). The SIDS-associated S206L caused a depolarizing shift in stead-state inactivation and a dramatic increase in INaL. Recently, a mutation was reported in the β1b subunit, a splice variant of β1, that caused a speeding of recovery from inactivation, significant INaL, APD prolongation in HL-1 cells, and QT prolongation and stress-induced syncope in the patient (342). This was the first report of a mutation in β1b associated with LQT.
4. LQT9 and LQT12
LQT9 is a Na+ channel-associated arrhythmia arising from mutations in Cav3, the gene encoding Cav-3, a cardiac isoform of caveolin (30). Four Cav-3 mutations were identified in a sample of 905 LQTS patients (438). Sixty-seven percent of patients identified with mutations in Cav3 exhibited syncope or cardiac arrest during periods of rest, consistent with the risk profile for patients with mutations directly in the α-subunit, Nav1.5. Two mutations, F97C and S141R, were studied further by coexpression in HEK-293 cells with Nav1.5. The S141R mutation sped recovery from slow inactivation, and both mutations caused a significant INaL. Consistent with the functional importance of the Cav-3/Nav1.5 interaction, three additional mutations in Cav3 have been shown to induce INaL and are associated with SIDS (92).
In mouse skeletal muscle, knockout of Cav-3 caused a disruption of the dystrophin complex in lipid rafts (133). Perhaps not surprisingly, mutations in syntrophin-α, a member of the dystrophin protein complex, are also associated with LQTS and give rise to variant 12 of the disease (473). Two mutations in SNTA1, the gene encoding syntrophin-α1 have been identified. The A257G mutation nearly doubled the peak current density, increased window current, and sped recovery from fast inactivation, although it did not induce INaL relative to peak current density in isolated neonatal rat cardiomyocytes (473). It is likely, however, that absolute INaL was greater in A257G myocytes because of the increase in channel expression. The A390V mutation does cause a persistent INaL and a depolarizing shift in steady-state inactivation in a nitric oxide synthase-dependent manner (431).
E. Molecular Pharmacology
The pharmacology of Na+ channel-associated LQT has been difficult to navigate because the diversity in patient phenotypes (392, 397) and risk of on- and off-target effects of standard antiarrhythmic therapies (276) has limited their use clinically. As a result, genetically identified LQT3 patients are the most likely to receive implantable cardioverter-defibrillators (ICDs) as a precautionary measure despite previously having been asymptomatic (372). There is therefore a large unmet medical need for effective pharmacological intervention, and our understanding of Na+ channel blockade and β-adrenergic receptor blockade in LQT3 continues to evolve.
1. Local anesthetics
The utility of local anesthetics in the treatment of LQT3 is rooted in their ability to inhibit the voltage-gated Na+ channel with preference for INaL (Figure 10). Soon after the discovery of the ΔKPQ mutation and its role in LQT3, it was reported that the commonly prescribed local anesthetic and class 1b antiarrhythmic agent lidocaine inhibited INaL more effectively than peak current (19). This phenomenon has been observed in block of several mutations by other clinically relevant local anesthetic-like drugs including mexiletine (35, 258), flecainide (288), and ranolazine (127), as well as recent experimental drugs GS967 (43) and eleclazine (128, 200), making them useful in normalizing the QT-interval (130, 281, 284, 370) and preventing arrhythmic events in some patients (347). This approach has recently been supported by a study of patients with a wide range of LQT3 mutations in which a robust improvement in QT interval and a reduction in arrhythmic events was induced by oral mexiletine (258). Because of the efficacy of these drugs in treating LQT3 patients and because of a common underlying mechanism, preferential inhibition of late versus peak Na+ channel current, development of more selective and effective blockers of INaL is underway by the pharmaceutical community. Nevertheless, it is important to recognize and understand the mechanisms underlying inhibition of this current.
FIGURE 10.
Representative recordings at low and high (insets) gain of F1473C inhibition by 50 μM mexiletine (A), 50 μM ranolazine (B), and 10 μM flecainide (C). D: mexiletine, ranolazine, and flecainide inhibit INa with preference for INaL. [From Bankston et al. (35).]
Local anesthetic-like drugs bind to a conserved phenylalanine (127, 336) in the central cavity of the channel in a highly state-specific manner. This specificity is imparted both by state-dependent changes in drug access to the binding site (243, 336) and by allosteric modulation of channel gating (162). Clinically relevant channel blockers have been shown to bind preferentially either to inactivated states, as is the case for lidocaine, flecainide, and mexiletine, and, in the case of ranolazine, to open channels (275).
The state dependence of drug binding has been assayed by electrophysiological as well as biochemical experiments. Lidocaine induces a hyperpolarizing shift in steady-state inactivation(202), slows recovery from inactivation, and stabilizes voltage-sensing units shown to provide the rate-limiting step in fast inactivation (27, 66, 382). Lidocaine has also been studied at the single-channel level. In cell-attached patches, lidocaine reduced the propensity of ΔKPQ channels to burst, increased the number of depolarizing sweeps with no channel openings, and had no effect on the mean open time of background or burst openings (31, 108). Taken together, these findings are consistent with stabilization or promotion of inactivated channels. Ranolazine, on the other hand, has no effect on steady-state inactivation and has therefore been interpreted as an open-channel blocker (275).
It is therefore not surprising that mutations that alter channel gating by different mechanisms will produce unique allosteric effects on drug binding. For example, flecainide (243) and mexiletine (347) have been shown to more potently inhibit some mutations than others when expressed in heterologous systems. Mutation-specific variability is therefore a major hurdle in disease management (88), as heightened sensitivity to Na+ channel blocking drugs can be pro-arrhythmic in certain settings (112, 276). In fact, flecainide is a useful diagnostic agent in patients suspected to have loss-of-function Na+ channel mutations in Brugada Syndrome because of its ability to reduce peak INa and unmask latent disease phenotypes in a clinical setting (58). It has therefore been proposed that an understanding of channel pathology and drug properties may help guide a pharmacogenomic approach to disease management (88).
2. β-Blockers
There remains some debate over the clinical utility of β-blockers in treatment of LQT3. A mainstay therapy for patients with QT prolongation, clinical data suggest that prophylactic β-blocker therapy in patients with LQT3 may be less effective at preventing cardiac events as it is in K+ channel-associated LQT (282, 331). Interestingly, however, β-blocker therapy did not show evidence of arrhythmia induction, as might be expected from the heart rate-slowing effects of β-blockade in LQT3 patients with elevated risk of arrhythmia at rest. This has prompted further investigation into the mechanism of β-blocker activity in models of LQT3.
Experiments in animal models of LQT3 (64, 333) showed a protective effect of sympathetic stimulation against APD prolongation and torsades de pointes (387), suggesting a deleterious effect of β-blockade. However, experiments in heterologous expression systems have shown that β-blockers have a direct effect on Na+ channels (33). β-Blockers exhibit preferential inhibition of INaL and bind to the local anesthetic binding site, and at high doses have a similar pharmaceutical profile as local anesthetics.
Ahrens-Nicklas et al. (12) constructed a computational model of ΔKPQ-expressing ventricular myocytes stimulated by the β-agonist isoproterenol and inhibited by the β-blocker propranolol. Isoproterenol shortened APD and EADs, consistent with the inverse-rate dependence of INaL and animal models of LQT3. At low doses, propranolol mitigated this effect and prolonged APD, but at higher doses where propranolol inhibits Na+ currents, it provided a beneficial reduction in APD. Taken together, the experimental and computational data suggest a potential benefit of β-blockade in treatment of LQT3, but more robust blinded studies must be performed to better understand the clinical relevance.
V. OTHER SUBTYPES OF CONGENITAL LQTS
A. LQT4: Ankyrin-B Syndrome
The correct targeting of ion channels and transporters to their respective subcellular domains is critical for normal physiological function. It was discovered that autosomal dominant loss-of-function mutations in ANK2, which encodes ankyrin-B, can cause LQTS (273, 366). Ankyrin-B is an adaptor protein that functions in the cytoskeletal network to ensure proper localization and stabilization of ion channels and transporters. Although ankyrin-B was originally characterized as a 220-kDa protein, multiple splice variants exist (474). The protein is composed of a membrane-binding domain, a spectrin-binding domain, and a regulatory domain (271). It has been demonstrated to play important roles in the normal localization of the Na+/Ca2+ exchanger, the Na+-K+-ATPase, as well as the IP3 receptor (IP3R) which mediates intracellular calcium release (95, 269, 270, 273, 429). In addition, ankyrin-B has been shown to regulate voltage-gated Na+ channels (74), ATP-gated K+ channels (236), as well as ryanodine receptors (65) in the heart.
While a loss-of-function ankyrin-B mutation was initially described as LQT4, it was later discovered that mutations in ankyrin-B are not always associated with QT prolongation and may present with a variety of cardiac phenotypes including bradycardia, sinus arrhythmia, as well as atrial fibrillation (96, 232, 271). Most of these mutations, such as E1425G, are located in or near the regulatory domain of ankyrin-B (271). These mutations in general lead to disruption of normal targeting and regulation of transporters as well as ion channels, leading to disrupted calcium handling that contributes to arrhythmia (272, 273). However, variation in severity of cardiac phenotypes exists between different mutations and can be correlated to the degree of loss of function observed in vitro (271).
B. LQT7: Andersen-Tawil Syndrome Type 1
Andersen-Tawil syndrome type 1 arises from LQT7 mutations, which are loss-of-function mutations in the KCNJ2 gene (327). This gene encodes Kir2.1, an inward rectifier potassium channel that underlies the cardiac IK1 current (104, 248, 303, 496), which contributes to setting the myocardial resting potential and aids terminal repolarization of the action potential (264). Because the membrane potential of cardiomyocytes is more positive than the potassium equilibrium potential, the channel actually conducts an outward current in physiological conductions. Kir2.1 expression has been demonstrated in both human atria and ventricles (458). The protein consists of two transmembrane helices, a pore-loop, as well as an NH2- and COOH-terminal region. Four Kir2.1 subunits may come together to form a functional channel. It has been shown that PIP2 binding to Kir2.1 plays an important role in channel activation (179, 410).
Andersen-Tawil syndrome is an autosomal dominant, multisystemic disorder characterized by periodic paralysis, ventricular arrhythmias, as well as dysmorphic features including low-set ears, hypertelorism, and hypoplastic mandible (362, 417). Early reports of patients with Andersen-Tawil syndrome described QT prolongation on ECG; however, most ECGs of patients with this disorder are characterized by prominent U waves that merge with the T wave, and a characteristic prolonged “Q-T-U” interval. The physiological basis for the U wave is not well understood, but the large U waves in Andersen-Tawil syndrome may be the result of delayed after depolarization or tissue-dependent differences in repolarization including the mid myocardium and His-Purkinje system (317, 328, 502). Andersen-Tawil syndrome type 1 results from KCNJ2 mutations, almost all of which are dominant negative (424, 425). Many of these mutations, such as R218W and G300D, located in the COOH-terminal region, either decrease PIP2 affinity or affect residues involved in PIP2 binding (103, 249, 398, 501). Some disease mutations, such as Δ312–314, lead to diminished channel trafficking to the cell surface (44). Disease-associated mutations can also be found in the NH2-terminal region as well as the channel pore (424).
C. LQT8: Timothy Syndrome
Timothy syndrome is caused by gain-of-function mutations in the CACNA1C gene, which encodes Cav1.2, the α1C (pore-forming) subunit of a cardiac voltage-gated calcium channel. The channel generates the L-type calcium current, which allows calcium influx during the plateau phase of the action potential, sustaining membrane depolarization and leading to calcium release from intracellular stores which triggers excitation-contraction coupling in cardiomyocytes. The channel undergoes both calcium-dependent and voltage-dependent inactivation. Inactivation of calcium channels during the action potential causes an imbalance between the inward calcium and outward potassium currents, resulting in repolarization. The α1C subunit consists of four homologous domains of six transmembrane helices each (S1–S6), including a voltage-sensing domain (S1–S4) and a pore domain (S5–S6), similar to voltage-gated sodium channels. Different splice variants of the α1C subunit may be expressed in the heart (445).
Timothy syndrome is an extremely rare genetic multisystemic disorder that includes QT prolongation, syndactyly, craniofacial abnormalities, congenital heart defects, as well as autism. All known Timothy syndrome mutations to date are located at the COOH-terminal end of S6 helices in different domains of α1C (54, 142, 403, 404). Most commonly, Timothy syndrome arises from the de novo missense mutation G406R on exon 8a, which is present in a minor cardiac splice variant of CACNA1C (404). This mutation is located at the COOH-terminal end of the first S6 helix of the α1C subunit and dramatically disrupts voltage-dependent inactivation of Cav1.2, leading to increased calcium influx that can result in APD prolongation. Timothy syndrome resulting from this mutation came to be known as the “classic” variant. Two patients were later found to have the mutations G406R and G402S, respectively, on exon 8, the major splice variant in the heart (403). Similar to the classic Timothy syndrome mutation, these two “atypical” Timothy syndrome mutations also disrupt channel inactivation. Interestingly, neither of the two atypical Timothy syndrome patients had syndactyly, as opposed to 100% of patients with classic Timothy syndrome, suggesting differential expression patterns of the α1C splice variants. One Timothy syndrome mutation, I1166T, shows minimal effect on Cav1.2 inactivation, but is thought to cause disease by increasing window current, which occurs in voltage ranges where channels activate but do not completely inactivate (54). Efforts are underway to develop mechanism-based therapies. In a landmark study, it was found that roscovitine, a cyclin-dependent kinase inhibitor, partially recovers inactivation of mutant Cav1.2 channels (487, 488) and reduces APD in ventricular-like cardiomyocytes derived from iPSC reprogrammed from the skin cells of a Timothy syndrome patient (489). The study provides a model for future development of therapies using human iPSC-derived cardiomyocytes.
D. LQT13
Mutations in the KCNJ5 gene can also cause LQTS. KCNJ5 encodes a G protein-coupled inwardly rectifying potassium channel, Kir3.4 (or GIRK4). Kir3.4 and another GIRK isoform Kir3.1(GIRK1) may form homo- and heterotetramers to generate the IKACh current in the heart (99, 219, 222), a potassium current that is stimulated by acetylcholine through a GPCR-mediated pathway which has been demonstrated to slow heart rate during parasympathetic stimulation (136, 467). Similar to other inward rectifier channels, Kir3.4 contains two transmembrane helices with a pore-loop in-between as well as an NH2- and COOH-terminal region. Activation of GPCR receptors by acetylcholine causes the Gβγ subunit to activate Kir3.4, resulting in an outward K+ current and hyperpolarization of the membrane potential, which in pacemaker cells results in slowing of heart rate (214, 341, 468).
The presence of IKACh has been best demonstrated in sinoatrial node, atrioventricular node, and atrial cells of the heart (225). While it has also been shown to be expressed in the ventricles (42, 183, 217, 466, 485), its role there is not well understood. Identification of LQTS mutations on KCNJ5, however, suggests a role for this current in controlling ventricular action potential (484). For example, the disease mutation G387R, a conserved residue located at the COOH-terminal region of Kir3.4, has been shown to cause autosomal dominant LQTS. The mutation has been shown to reduce trafficking of both Kir3.4 as well as Kir3.1 to the plasma membrane and decrease current amplitude. Interestingly, a number of these LQTS patients also have atrial fibrillation, suggesting a role for Kir3.4 in electrical conduction in both atria and ventricles.
E. LQT14 and LQT15
Calmodulin is a ubiquitous calcium-binding protein that critically mediates cellular calcium signaling. Calmodulin is important not only for the calcium-dependent inactivation of L-type calcium channels and the inactivation of Nav1.5 channels (435, 514), but also in the trafficking and assembly of KCNQ1 (379). Three genes, CALM1-3, all encode the identical calmodulin protein. For example, a recent study suggests that de novo mutations in CALM1 or CALM2 are associated with severely prolonged QT interval, ventricular arrhythmias, as well as atrioventricular block in infants (93). Located in calmodulin EF-motifs that directly bind calcium, these mutations result in a reduction in calcium affinity that may lead to abnormalities in sodium currents, potassium currents, as well as calcium dynamics in the heart. In addition to exhibiting prolonged QT, these patients show neurodevelopmental delay, consistent with widespread calmodulin expression in different tissues (316).
VI. GENOTYPE-DRIVEN CLINICAL MANAGEMENT OF CONGENITAL LQTS
A. Introduction
Clinical management of congenital LQTS aims to minimize symptomatic arrhythmia and prevent life-threatening cardiac events. As described in detail in the preceding sections, the three most common congenital Long QT syndromes (LQT1 due to mutation in KCNQ1; LQT2 due to mutation in hERG; and LQT3 due to mutation in Nav1.5) result in lengthening of the APD in cardiomyocytes, leading to a prolonged QT interval on the ECG.
By consensus guidelines, management strategies in congenital LQTS today are mostly uniform, regardless of genotype. However, given the remarkable strides made in understanding the molecular identity of LQTS subtypes, as well as the specific impact of a wide range of LQT-associated mutations, the ultimate hope is that understanding a patient's genotype and specific LQT mutation can lead to rational treatment considerations. This section reviews the features of molecular physiology that may influence genotype-specific strategies for clinical management.
B. Risk Factors for Arrhythmogenesis
1. Mutation location, type, and cardiac risk
In LQT1, mutations in the transmembrane regions including cytoplasmic loop (C-loop) domains of KCNQ1 confers a twofold greater risk of cardiac events [defined as syncope, aborted cardiac arrest (ACA), or sudden cardiac death (SCD)] compared with patients harboring mutations in the COOH-terminal domain of the KCNQ1 protein (280). The C-loops are involved in sympathetic regulation of KCNQ1 (256), and mutations in this region may underlie an increased risk of cardiac events in these patients compared with mutations in other regions of the channel due to the substantial regulation of KCNQ1, and therefore IKs activity, by sympathetic stimulation (39).
In LQT2, Shimizu et al. (389) reported that pore region missense mutations, located in the S5-loop-S6 portion of the hERG channel, confer greater risk of cardiac events than non-pore mutations in hERG (389). Specific triggers of cardiac events were further subcategorized in LQT2 based on mutation location and age at the time of the cardiac event. A study of 634 LQT2 patients revealed that while patients with pore mutations in hERG confer a >2-fold risk of cardiac events compared with individuals with non-pore mutations overall, non-pore transmembrane domain mutations specifically led to a 6.8-fold increase in exercise-induced cardiac events (216), suggesting that adrenergic pathways interact with hERG non-pore transmembrane domains either directly or indirectly. Thus the location of the hERG mutation variably impacts susceptibility to common triggers in LQT2 patients.
In LQT3, rates of cardiac events are high, and risk is likely related to the degree of channel dysfunction or late current. The canonical SCN5A ΔKPQ mutation results in 2.4-fold greater risk of experiencing cardiac events through age 40 compared with the SCN5A D1790G mutation (244). Furthermore, while preliminary data suggest similar risk for mutations in the COOH-terminal versus transmembrane region of SCN5A, a 2.5-fold risk of ACA and SCD is conferred by mutations that cause both sodium late current and window current, compared with mutations causing only one such gain-of-function defect (38).
2. Genotype-specific triggers of arrhythmia
The first recognition of the relevance of genotype in management of LQTS occurred in the context of a seminal study linking molecular identity in LQTS with clinical features, establishing genotype-specific triggers of arrhythmic events (371). Six hundred seventy symptomatic patients with LQT1, LQT2, and LQT3 were followed, and strong differences arose in the trigger of arrhythmic events based on patient genotype (371). LQT1 patients experienced 62% of their cardiac events during exercise, and swimming or diving was found to be a particularly potent trigger of arrhythmia (10, 279). In contrast, LQT2 patients experienced the highest percentage of arrhythmic events in the setting of emotional arousal (43%), including sudden loud noises and startle (e.g., alarm clock awaking the patient from sleep), or other triggers of emotional stress and fear; 13% of LQT2 patient triggers occurred in the setting of exercise. LQT3 patients experienced the highest percentage of arrhythmic events, 39%, at rest or during sleep (371). LQT1 patients, by extension, experience more cardiac events in the setting of sympathetic nervous system stimulation induced by exercise than LQT2 or LQT3 patients. More generally, these findings highlight the varying pathophysiology of the LQT subtypes, and the specific life-style modifications and treatment considerations dependent on genotype (described later in this section).
3. Role of heart rate in LQT variant susceptibility to arrhythmia
The variable triggers of arrhythmia in congenital LQTS can be explained in part by the role of heart rate in arrhythmogenesis. Heart rate contributes greatly to action potential and QT duration (298), and therefore impacts the most common forms of congenital LQT syndrome in a genotype-specific, and even mutation-specific manner.
In LQT1, a higher basal heart rate increases risk of cardiac events, providing some prognostic value, unlike in LQT2 patients (40, 57, 373). Functional IKs current is pivotal to normal QT adaptation, or shortening with heart rate. At increased heart rates (and/or during β-adrenergic stimulation of the heart), IKs facilitates cardiac repolarization and shortening of the QT interval: IKs has slow deactivation kinetics, rendering the channel open for a longer period of time. Residual IKs channel activation from previous cardiac stimulation can enhance repolarization in the setting of short diastolic intervals (119). Indeed, a hallmark of LQT1 is the failure of the QT interval to adapt to increases in heart rate, as often seen during diagnostic exercise stress testing (29, 230, 470). This feature of IKs molecular physiology may also explain the predilection toward arrhythmias during swimming and diving in LQT1, which can produce a complex autonomically-driven “diving reflex” with abrupt changes in heart rate.
In contrast to LQT1 and LQT2, LQT3 patients experience greater risk of arrhythmic events at slower heart rates (332, 370). Bradycardia can produce prime conditions for SCN5A channel dysfunction, in a mutation-dependent manner. For example, the gain-of-function late sodium current generated by channel “burst” gating in the canonical ΔKPQ deletion mutation has been shown to be strongly attenuated with increases in pacing rate (289). The fraction of channels available to enter bursting channel states is larger at slower pacing frequency (371). The observation of strong association of arrhythmic events with bradycardia has led to concerns about the use of β-blockers, which slow heart rate, in the LQT3 subtype (33). However, it is important to note that β-blockers have multiple intracellular targets that may be protective against arrhythmia, and direct effects of β-blockers on sodium channel function have been reported (33). Additionally, some LQT3 mutations can exert their effect via mechanisms other than channel bursting, and therefore can have different features of rate dependence (34). For example, the LQT3 SCN5A S1904L mutation triggers arrhythmia more often at faster heart rates (185). Biophysical characterization alongside paired computational studies demonstrates increased late channel reopenings at faster pacing frequencies with the S1904L mutation, in direct contrast to ΔKPQ and F1473C mutations (35, 185).
4. Genotype-guided exercise testing
Since changes in heart rate can affect propensity for development of arrhythmia, exercise testing in congenital LQTS has evolved into an essential tool in the diagnostic workup of affected patients. Almost 40% of LQTS patients do not have a prolonged QTc interval on an ECG at rest. Exercise stress testing can aid in diagnosis and risk stratification of congenital LQTS, with particular utility in uncovering cases of concealed LQTS that have otherwise gone undiagnosed (177, 339). Exercise ECG during stress and recovery is a particularly helpful tool in uncovering concealed LQT1 syndrome (177).
Given the diagnostic utility of stress testing, attempts at using this test to guide genotype and mutation-specific risk stratification have been made. Laksman et al. (230) tested the hypothesis that exercise testing can predict QT interval response and effect of β-blockers in LQT1 patients with mutations in the C-loops (which, as described above, represent the site of final sympathetic regulation of KCNQ1). In this retrospective analysis, C-loop KCNQ1 missense mutations were not associated with an increased QTc interval during exercise stress testing or response to β-blocker therapy (230). Despite the known limitations of retrospective analysis, this study highlights the need for caution in the use of exercise stress tests for genotype-specific risk stratification.
5. Female gender and pregnancy as risk factors for arrhythmogenesis
Female gender is a risk factor for developing torsades de pointes in LQTS (186, 234), and the baseline QTc interval in healthy females is prolonged compared with males. Furthermore, women are at increased risk of developing drug-induced torsades de pointes as a consequence of hERG-blocking medications (254). In men, testosterone levels correlate with QTc interval duration (507). In a large study of 1,166 LQT2 syndrome patients, followed through age 40, ACA and SCD occurred significantly more frequently in females (26%) than in males (14%). With regard to location of the mutation, males with pore mutations had a greater than twofold risk of a first ACA or SCD compared with males with non-pore mutations. Pore mutations did not increase risk of these life-threatening cardiac events in females (265).
Women with LQTS are particularly prone to developing arrhythmias in the peripartum period. Seth et al. (377) demonstrated that the postpartum state (defined as 9 mo postdelivery) confers a 2.7-fold increased risk of a cardiac event and a 4.1-fold increased risk of a life-threatening cardiac event. The majority of these events did not occur during labor and delivery. This risk further appears to be genotype specific; the LQT2 genotype, in particular, conferred greater risk of cardiac events postpartum, compared with females with LQT1 and LQT3 genotypes (377). Changes in sex hormones associated with menopause may also affect risk of arrhythmia. Within five years leading to the onset of menopause, the odds ratio for a syncopal event for LQT2 females is 3.4 compared with LQT1 females, and after menopause, this increases to 8.1 (60). In LQT2, females older than 15 years of age have a 3.7-fold increased risk of suffering cardiac events compared with males, while there is no significant difference in risk of cardiac events between males and females younger than 15 years of age (495). A study of 634 LQT2 patients revealed that females >13 years of age were nine times more susceptible specifically to “arousal” triggers compared with males >13 years of age (216).
These clinical observations suggest that sex hormones may impact propensity for arrhythmia formation in LQTS in a genotype-specific manner. Sex hormones are known to play a role in cardiac ion channel regulation and arrhythmia. Estrogen has been shown to decrease IKr activity and prolong the QTc interval (227), and β-estradiol inhibits hERG channel expression in cultured HEK293 cells (24). Progesterone modulates both IKs and L-type calcium channels, leading to a shortened QTc interval (294).
Animal models, including canine, mouse, rat, rabbit, and guinea pig, have been extensively employed to better understand gender differences in cardiac electrophysiology (186), and strongly support the role for male sex hormones shortening the QT interval via enhanced IKr activity (129, 245, 477). In a LQT2 rabbit model, estradiol enhances risk of sudden cardiac death, while progesterone is protective (307). In canines, females have prolonged APD50, APD70, and APD90 values compared with males. Dofetilide, a hERG channel blocker, prolonged the APD90 of Purkinje fibers to a greater extent in female canines compared with males with rate dependence, and the canine studies demonstrate consistency of sex-related differences in cardiac repolarization between humans and canines (4). Further research is needed to elucidate the mechanisms underlying sex hormone and arrhythmia formation, and to possibly uncover therapeutic avenues through sex hormone sensitive pathways.
C. Genotype-Specific Management of Congenital LQTS
Congenital LQTS represents a potentially lethal condition, yet advances in device therapy, medical options, and surgical techniques have aided in the clinical management of the disorder (421). Today, most clinical management of LQTS is uniform across subtypes and consists of targeting of the sympathetic nervous system, through β-blocker therapy and left cervicothoracic sympathetic denervation (LCSD) in selected refractory cases. Primary and secondary prevention of cardiac arrest with an implantable cardioverter-defibrillator (ICD), alongside antiarrhythmic therapy, is considered after weighing risks and benefits for an individual patient.
As more is uncovered regarding the molecular physiology of these conditions, there is growing potential for tailored therapy to a given patient's genotype and specific LQT-causing mutation.
1. Lifestyle modification
Given the striking association of cardiac events with genotype-specific triggers, counseling should be given regarding lifestyle modification. As several examples, LQT1 patients are counseled about exercise limitations, most specifically swimming and possibly competitive athletics. LQT2 patients would be well advised to limit loud startling noises in their surroundings, including alarm clocks and telephones (38).
2. β-Blockers and targeting of the sympathetic nervous system
According to consensus guidelines (335), β-blocker treatment carries a class I recommendation in the setting of a clinical diagnosis of LQTS. β-Blockers reduce overall risk of cardiac events in children and adults, and treatment represents a class IIa indication in all individuals confirmed as carriers of LQTS mutations (118).
However, the efficacy of β-blockers is strongly genotype-specific. β-Blockers produce greatest symptom relief and cardiac event reduction in LQT1 patients, followed by LQT2 patients, and are least effective in LQT3 patients (334, 440). High-risk LQTS patient populations, including LQT1 males and LQT2 females, experienced 67 and 71% risk reduction with β-blocker treatment, respectively (145). Along with genotypic considerations, mutation-specific benefits exist based on location of the mutation in the ion channel. For instance, β-blocker treatment resulted in 88% risk reduction of life-threatening cardiac events in LQT1 patients harboring cytoplasmic-loop missense mutations compared with LQT1 patients harboring missense mutations in regions outside of the cytoplasmic loop (39). β-Blockers confer trigger-specific benefits as well, reducing the risk of exercise-induced cardiac events by 78 and 71% in LQT1 and LQT2 patients, respectively. However, β-blockers were not protective against events triggered by arousal or rest/sleep in LQT1 and LQT2 (146, 216). These findings are in line with the molecular physiology of these mutations. In nondiseased conditions, upregulation of repolarizing currents by β-adrenergic stimulation can enhance repolarization and favorably adapt QT interval for the concomitant increase in heart rate. However, in the setting of increased heart rates and LQT1 (and in some cases LQT2) disease, delayed rectifier current is not available for cardiac repolarization. One possible mechanism for β-blocker effect could be prevention of sudden and sustained increase in heart rates, which in itself is associated with arrhythmia formation in these subtypes.
In LQT2 events associated with sudden noises and startle, both adrenergic and other neural pathways may be triggered. As such, β-adrenergic blockade in LQT2 may not fully mitigate the risks. Although β-blockers remain first-line medical treatment in LQT2, a life-threatening cardiac event rate of 6–7% while on therapy remains a serious concern (334).
It is important to note that β-adrenergic stimulation affects multiple intracellular targets, including the pathways for intracellular calcium cycling that regulate L-type calcium current, the ryanodine receptor, and sarcoplasmic reticulum calcium uptake (18, 506). In LQTS, with impaired repolarizing currents (or enhanced depolarizing currents), β-adrenergic stimulation can be pro-arrhythmic, regardless of the genotype producing the impaired repolarization. β-Blockers therefore are commonly prescribed to all LQTS patients regardless of genotype.
In selected cases, left cervicothoracic sympathetic denervation (LCSD), which surgically interrupts adrenergic input to the heart, can be considered. In a study of 147 high-risk LQTS patients, cardiac event rate dropped by 91% per patient post-LCSD (369). When carried out at experienced centers, this treatment modality can be very effective in refractory cases.
3. Other pharmacological treatments in LQT2
Treatment of LQT2 syndrome by increasing serum K+ concentration has been explored, as hERG conductance is modulated by changes in extracellular K+, and LQT2 patients can develop low body and/or serum potassium levels (436). IKr activity decreases in the setting of hypokalemia (359) and increases in the setting of hyperkalemia, due mainly to extracellular K+ effects on inactivation gating (446, 454, 455).
As such, potassium supplementation and therapy with spironolactone, a potassium-sparing diuretic, were tested as treatment options to enhance IKr activity, and each treatment significantly shortened the QTc interval (89, 117). However, there was no evidence of a decrease in cardiac events. Safety dilemmas have been raised, including hyperkalemia, with the use of spironolactone in LQT2 treatment (38). Potential adverse effects of spironolactone may be explained by the fact that its metabolism into canrenoic acid was actually shown to induce hERG blockade and reduce channel activity (63).
4. Other pharmacological treatments in LQT3
The efficacy of local anesthetic drugs in LQT3 treatment is due to preferential inhibition of late sodium current (more than peak current). Limitation of the use of late sodium current blockers is due, in part, to off-target drug effects as well as to the relative selectivity of block of late versus peak sodium channel currents. The introduction of newer compounds with significantly higher selectivity for INaL block offers great potential for more effective clinical management of LQT3. The need for improved pharmacotherapy is supported by a high breakthrough cardiac event rate for LQT3 patients on β-blocker treatment, affecting as many as 15% of patients, generating a growing interest in INa-specific blocking agents (143). The use of the local anesthetic class of sodium channel blockers lidocaine (during acute settings and arrhythmic storm) and mexiletine (for suppressive therapy) are commonly clinically employed. Mexiletine administration shortens the QT interval in LQT3 patients, and not in LQT2 patients (370). Mexiletine blocks mutant Nav1.5 channel function, diminishing the late sodium current more than the peak sodium current by stabilizing the inactivated state of the Nav1.5 channel (449). Ranolazine, on the other hand, is thought to block Nav1.5 current via open channel block (275). The utility of local anesthetic-like drugs is weighed against their off-target undesirable side effects. For example, flecainide inhibits peak INa and has been reported to unmask Brugada syndrome-type patterns on surface ECG (indeed, this class of agents is clinically utilized as a provocative challenge in the diagnosis of Brugada syndrome) (58). Various mechanisms of drug block of Nav1.5 underlie, in part, the mutation-specific efficacy of LQT3 pharmacological agents (see sect. IV).
Pharmacological sodium channel blockade appears on the surface to be a specific and targeted strategy against gain-of-function mutations in the sodium channel, and in individual cases does provide remarkable reduction in arrhythmia burden. However, a mortality benefit for these agents in LQT3 has yet to be established. Anti-arrhythmic therapy in LQT3, as with all LQT subtypes, must be considered carefully after thorough review of all patient-specific factors, including mutation or genotype-specific risk, clinical variables (e.g., family history, age, gender), and overall treatment plan (including role of ICD placement and/or LCSD) (143). Given the complexities of drug effect, these agents are often employed empirically in LQTS, with close attention to clinical response.
D. Emerging Translational Strategies in Congenital LQTS
1. Cellular models of LQTS
The physiological significance of cardiac channelopathies can be difficult to model at the cellular level because of the complex interplay between several ionic currents in the cardiomyocyte. While heterologous expression systems and animal models are invaluable tools in our understanding of disease phenotypes, they lack the genetic background and molecular biology of human cells. Induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) represent a useful platform for studying human cardiac ion channel function in health and disease in a cell system with human molecular and physiological components, acquired from unaffected patients and/or those carrying mutations.
In 2010, iPSC-CMs were generated from skin cells taken from two LQT1 patients with the autosomal dominant KCNQ1 R190Q mutation (277). These cardiomyocytes were spontaneously beating and classified as ventricular-, atrial-, or nodal-like based on cellular electrophysiology and morphology. Compared with WT controls, LQT1 cardiomyocytes demonstrate decreased IKs amplitude and prolonged APD, consistent with LQT1 pathogenesis. More recently, Jouni et al. (199) derived iPSC-CMs from a LQT2 patient harboring a hERG A561P mutation, to study the electrophysiological consequences of hERG loss-of-function in a patient-specific genetic background. Ventricular-, atrial-, and nodal-like action potentials were recorded, and a trafficking defect was characterized as the mechanism of loss of IKr, leading to a prolonged APD. Furthermore, a study by Jones et al. (195) reported the use of mRNA silencing in human iPSC-CMs to show that the hERG1b isoform is a significant component of cardiac repolarization, and that loss of hERG1b function provides a substrate for arrhythmogenesis. Several additional studies have investigated LQT3 in the context of iPSC-CMs (121, 252, 420).
In the context of drug development, iPSC-derived cardiomyocytes provide a means for testing compounds in patient-specific genetic backgrounds, allowing for a targeted approach in optimizing therapy. Terrenoire et al. (420) studied human iPSC-CMs from a patient harboring a LQTS mutation in SCN5A and a polymorphism in hERG. Upon electrophysiological characterization of ionic currents, it was determined that the SCN5A F1473C mutation was responsible for the LQTS clinical phenotype via increased INaL, while the hERG K897T variant was a benign polymorphism. Experiments in the iPSC-CMs revealed key rate-dependent properties of INaL and its inhibition by mexiletine; these results provided insight into control of arrhythmias in the proband. This study offers an example of how iPSC-CMs can elucidate mechanisms of LQTS pathogenesis to inform patient-specific therapy.
Use of iPSC-CMs is not without limitations. Discrepancies may still exist in the expression profile of cardiac ion channels between iPSC-derived cardiomyocytes and native human cardiac cells due in part to differences in cell maturity and growth environment. Cardiomyocyte differentiation can produce a mix of atrial-like and ventricular-like cells of varying morphology. Furthermore, recent publications using iPSC-CMs report a resting membrane potential depolarized to near −60 mV (252, 420), which can alter properties of important cardiac channels such as Nav1.5 and affect state-dependent pharmacological inhibition. Efforts are underway to improve iPSC-CMs by using physiological systems to promote cellular maturity such that they more closely resemble native mature cardiomyocytes. As efficiency and specificity of cell differentiation improve, iPSC-CMs hold great promise as a model cell system for the study of LQTS disease and patient-specific pharmacology (115).
2. Pharmacological and medical approach to LQTS
More specific targeting of the mechanisms underlying ion channel defects that predispose to arrhythmia guides the advancement and rational drug design of pharmacological agents in the treatment of congenital LQTS. The experimental drug GS-967 preferentially inhibits late INa over peak INa with considerable selectivity, and does so more potently and efficaciously than flecainide and ranolazine, while also reducing arrhythmia burden in experimental rabbit cardiomyocytes (43). Computational modeling of GS-967 suggests therapeutic benefit of late INa block by decreasing arrhythmogenesis in the setting of LQTS (481). Another experimental compound, F15845, selectively blocks late INa potently, preventing cardiac angina and arrhythmia in animal models (324, 433). The benefits of any selective late INa blocker must be weighed against the relative effects on peak INa, in addition to off-target effects that include block of peak INa and IKr. Studies to date suggest that selective late INa blockers are not proarrhythmic; clinical trials of these and related drugs are currently underway, and it is expected that, in the near future, these studies will inform whether this drug class represents a viable treatment option in congenital LQTS (25).
Along with more selective targeting of LQT-associated mutant ion channels, growing interest in both mutation-specific and tissue-specific treatment strategies may become fruitful. For instance, ectopic rhythms leading to ventricular fibrillation in LQT patients has been shown to originate 50% of the time from the Purkinje fiber cells in the cardiac specialized conduction system (158, 306, 414). The Purkinje system is especially prone to arrhythmia formation, owing to differences in membrane current density, APD and morphology, and calcium cycling (102, 132, 158). In a computational model of the His-Purkinje system, LQT3-associated mutations were shown to confer a more severe phenotype in Purkinje fibers compared with LQT1 and LQT2 mutations (185), predictions that were confirmed in isolated murine Purkinje fiber cells expressing ΔKPQ mutant Na+ channels (175). Continued research into the functional implications of mutations in different cell types may uncover further therapies directed at tissues most prone to arrhythmia formation. For example, targeting the insertion of these Purkinje fibers in the ventricular endocardium via catheter ablation may prove to be an effective strategy in reducing arrhythmia burden (471).
VII. CONCLUSIONS
The growing body of mechanistic insights into the pathophysiology of channelopathies and other arrhythmia-causing mutations has helped elucidate the molecular underpinnings of congenital LQTS and genotype-phenotype correlation in disease. Although insights from benchside to bedside have facilitated progress toward better therapeutic strategies, there remains a need for tailoring management toward individuals in a genotype-driven and mechanism-specific manner to optimize care. Techniques such as iPSC-derived cardiomyocytes and high-throughput patch clamping that allow for screening of compounds in patient-specific contexts may help pave the way for future development of therapeutics. In addition, continued progress toward fundamental understanding of mechanisms of ion channel function and drug-channel interaction will guide the development of more effective, mechanism-based molecular agents in the treatment of LQTS.
GRANTS
This work was supported by the American Heart Association and National Heart, Lung, and Blood Institute Grants F30HL129656, 5K08HL116790, and 5R01HL123483.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
M. S. Bohnen, G. Peng, and S. H. Robey contributed equally to this work.
Present address of C. Terrenoire: The New York Stem Cell Foundation Research Institute, New York, NY 10032.
Address for reprint requests and other correspondence: R. S. Kass, Dept. of Pharmacology, Columbia University Medical Center, 630 W. 168th St., New York, NY 10032 (e-mail: rsk20@cumc.columbia.edu).
REFERENCES
- 1.Abbott GW. Biology of the KCNQ1 potassium channel. New J Sci 2014: 1–26, 2014. [Google Scholar]
- 2.Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell 97: 175–187, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Abbott GW, Xu X, Roepke TK. Impact of ancillary subunits on ventricular repolarization. J Electrocardiol 40: S42–46, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abi-Gerges N, Small BG, Lawrence CL, Hammond TG, Valentin JP, Pollard CE. Evidence for gender differences in electrophysiological properties of canine Purkinje fibres. Br J Pharmacol 142: 1255–1264, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abitbol I, Peretz A, Lerche C, Busch AE, Attali B. Stilbenes and fenamates rescue the loss of I(KS) channel function induced by an LQT5 mutation and other IsK mutants. EMBO J 18: 4137–4148, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abriel H, Cabo C, Wehrens XH, Rivolta I, Motoike HK, Memmi M, Napolitano C, Priori SG, Kass RS. Novel arrhythmogenic mechanism revealed by a long-QT syndrome mutation in the cardiac Na+ channel. Circ Res 88: 740–745, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 8: 1308–1339, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP, Heart Rhythm S, European Heart Rhythm Association. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace 13: 1077–1109, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Ackerman MJ, Tester DJ, Jones GS, Will ML, Burrow CR, Curran ME. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc 78: 1479–1487, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Ackerman MJ, Tester DJ, Porter CJ. Swimming, a gene-specific arrhythmogenic trigger for inherited long QT syndrome. Mayo Clin Proc 74: 1088–1094, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Adsit GS, Vaidyanathan R, Galler CM, Kyle JW, Makielski JC. Channelopathies from mutations in the cardiac sodium channel protein complex. J Mol Cell Cardiol 61: 34–43, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahrens-Nicklas RC, Clancy CE, Christini DJ. Re-evaluating the efficacy of β-adrenergic agonists and antagonists in long QT-3 syndrome through computational modelling. Cardiovasc Res 82: 439–447, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aidery P, Kisselbach J, Schweizer PA, Becker R, Katus HA, Thomas D. Impaired ion channel function related to a common KCNQ1 mutation: implications for risk stratification in long QT syndrome 1. Gene 511: 26–33, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Aizawa Y, Uchiyama H, Yamaura M, Nakayama T, Arita M. Effects of the ATP-sensitive K channel opener nicorandil on the QT interval and the effective refractory period in patients with congenital long QT syndrome. Investigator Group for K-Channel Openers and Arrhythmias. J Electrocardiol 31: 117–123, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Alders M, Christiaans I. Long QT syndrome. In: Gene Reviews, edited by Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K. Seattle, WA: Univ. of Washington, 1993. [Google Scholar]
- 16.Allan WC, Timothy K, Vincent GM, Palomaki GE, Neveux LM, Haddow JE. Long QT syndrome in children: the value of rate corrected QT interval and DNA analysis as screening tests in the general population. J Med Screen 8: 173–177, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Allan WC, Timothy K, Vincent GM, Palomaki GE, Neveux LM, Haddow JE. Long QT syndrome in children: the value of the rate corrected QT interval in children who present with fainting. J Med Screen 8: 178–182, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Amanfu RK, Saucerman JJ. Modeling the effects of beta1-adrenergic receptor blockers and polymorphisms on cardiac myocyte Ca2+ handling. Mol Pharmacol 86: 222–230, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An RH, Bangalore R, Rosero SZ, Kass RS. Lidocaine block of LQT-3 mutant human Na+ channels. Circ Res 79: 103–108, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Anantharam A, Abbott GW. Does hERG coassemble with a beta subunit? Evidence for roles of MinK and MiRP1. Novartis Found Symp 266: 100–112, 2005. [PubMed] [Google Scholar]
- 21.Anantharam A, Lewis A, Panaghie G, Gordon E, McCrossan ZA, Lerner DJ, Abbott GW. RNA interference reveals that endogenous Xenopus MinK-related peptides govern mammalian K+ channel function in oocyte expression studies. J Biol Chem 278: 11739–11745, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113: 365–373, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Anderson CL, Kuzmicki CE, Childs RR, Hintz CJ, Delisle BP, January CT. Large-scale mutational analysis of Kv11.1 reveals molecular insights into type 2 long QT syndrome. Nat Commun 5: 5535, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ando F, Kuruma A, Kawano S. Synergic effects of beta-estradiol and erythromycin on hERG currents. J Membr Biol 241: 31–38, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Antzelevitch C, Nesterenko V, Shryock JC, Rajamani S, Song Y, Belardinelli L. The role of late I Na in development of cardiac arrhythmias. Handb Exp Pharmacol 221: 137–168, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antzelevitch C, Sicouri S. Clinical relevance of cardiac arrhythmias generated by afterdepolarizations. Role of M cells in the generation of U waves, triggered activity and torsade de pointes. J Am Coll Cardiol 23: 259–277, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Arcisio-Miranda M, Muroi Y, Chowdhury S, Chanda B. Molecular mechanism of allosteric modification of voltage-dependent sodium channels by local anesthetics. J Gen Physiol 136: 541–554, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asayama M, Kurokawa J, Shirakawa K, Okuyama H, Kagawa T, Okada J, Sugiura S, Hisada T, Furukawa T. Effects of an hERG activator, ICA-105574, on electrophysiological properties of canine hearts. J Pharmacol Sci 121: 1–8, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Aziz PF, Wieand TS, Ganley J, Henderson J, Patel AR, Iyer VR, Vogel RL, McBride M, Vetter VL, Shah MJ. Genotype- and mutation site-specific QT adaptation during exercise, recovery, and postural changes in children with long-QT syndrome. Circ Arrhythm Electrophysiol 4: 867–873, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol 98: 149–160, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balser JR, Nuss HB, Orias DW, Johns DC, Marban E, Tomaselli GF, Lawrence JH. Local anesthetics as effectors of allosteric gating. Lidocaine effects on inactivation-deficient rat skeletal muscle Na channels. J Clin Invest 98: 2874–2886, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bankston JR, Kass RS. Fading sodium channels in failing hearts. Circ Res 101: 1073–1074, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Bankston JR, Kass RS. Molecular determinants of local anesthetic action of beta-blocking drugs: Implications for therapeutic management of long QT syndrome variant 3. J Mol Cell Cardiol 48: 246–253, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bankston JR, Sampson KJ, Kateriya S, Glaaser IW, Malito DL, Chung WK, Kass RS. A novel LQT-3 mutation disrupts an inactivation gate complex with distinct rate-dependent phenotypic consequences. Channels 1: 273–280, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Bankston JR, Yue M, Chung W, Spyres M, Pass RH, Silver E, Sampson KJ, Kass RS. A novel and lethal de novo LQT-3 mutation in a newborn with distinct molecular pharmacology and therapeutic response. PLoS One 2: e1258, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384: 78–80, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Barro-Soria R, Rebolledo S, Liin SI, Perez ME, Sampson KJ, Kass RS, Larsson HP. KCNE1 divides the voltage sensor movement in KCNQ1/KCNE1 channels into two steps. Nat Commun 5: 3750, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barsheshet A, Dotsenko O, Goldenberg I. Genotype-specific risk stratification and management of patients with long QT syndrome. Ann Noninvasive Electrocardiol 18: 499–509, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barsheshet A, Goldenberg I, JOU, Moss AJ, Jons C, Shimizu W, Wilde AA, McNitt S, Peterson DR, Zareba W, Robinson JL, Ackerman MJ, Cypress M, Gray DA, Hofman N, Kanters JK, Kaufman ES, Platonov PG, Qi M, Towbin JA, Vincent GM, Lopes CM. Mutations in cytoplasmic loops of the KCNQ1 channel and the risk of life-threatening events: implications for mutation-specific response to beta-blocker therapy in type 1 long-QT syndrome. Circulation 125: 1988–1996, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barsheshet A, Peterson DR, Moss AJ, Schwartz PJ, Kaufman ES, McNitt S, Polonsky S, Buber J, Zareba W, Robinson JL, Ackerman MJ, Benhorin J, Towbin JA, Vincent GM, Zhang L, Goldenberg I. Genotype-specific QT correction for heart rate and the risk of life threatening cardiac events in adolescents with the congenital long-QT syndrome. Heart Rhythm 8: 1207–1213, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartos DC, Duchatelet S, Burgess DE, Klug D, Denjoy I, Peat R, Lupoglazoff JM, Fressart V, Berthet M, Ackerman MJ, January CT, Guicheney P, Delisle BP. R231C mutation in KCNQ1 causes long QT syndrome type 1 and familial atrial fibrillation. Heart Rhythm 8: 48–55, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beckmann C, Rinne A, Littwitz C, Mintert E, Bosche LI, Kienitz MC, Pott L, Bender K. G protein-activated (GIRK) current in rat ventricular myocytes is masked by constitutive inward rectifier current [I(K1)]. Cell Physiol Biochem 21: 259–268, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Belardinelli L, Liu G, Smith-Maxwell C, Wang WQ, El-Bizri N, Hirakawa R, Karpinski S, Li CH, Hu L, Li XJ, Crumb W, Wu L, Koltun D, Zablocki J, Yao L, Dhalla AK, Rajamani S, Shryock JC. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J Pharmacol Exp Ther 344: 23–32, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Bendahhou S, Donaldson MR, Plaster NM, Tristani-Firouzi M, Fu YH, Ptacek LJ. Defective potassium channel Kir2.1 trafficking underlies Andersen-Tawil syndrome. J Biol Chem 278: 51779–51785, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Bendahhou S, Marionneau C, Haurogne K, Larroque MM, Derand R, Szuts V, Escande D, Demolombe S, Barhanin J. In vitro molecular interactions and distribution of KCNE family with KCNQ1 in the human heart. Cardiovasc Res 67: 529–538, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Benhorin J, Medina A. Images in clinical medicine. Congenital long-QT syndrome. N Engl J Med 336: 1568, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Bennett PB, Yazawa K, Makita N, George AL Jr. Molecular mechanism for an inherited cardiac arrhythmia. Nature 376: 683–685, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Berecki G, Zegers JG, Verkerk AO, Bhuiyan ZA, de Jonge B, Veldkamp MW, Wilders R, van Ginneken AC. HERG channel (dys)function revealed by dynamic action potential clamp technique. Biophys J 88: 566–578, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berman MF, Camardo JS, Robinson RB, Siegelbaum SA. Single sodium channels from canine ventricular myocytes: voltage dependence and relative rates of activation and inactivation. J Physiol 415: 503–531, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhuiyan ZA, Momenah TS, Gong Q, Amin AS, Ghamdi SA, Carvalho JS, Homfray T, Mannens MM, Zhou Z, Wilde AA. Recurrent intrauterine fetal loss due to near absence of HERG: clinical and functional characterization of a homozygous nonsense HERG Q1070X mutation. Heart Rhythm 5: 553–561, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bian J, Cui J, McDonald T. VHERG K+ channel activity is regulated by changes in phosphatidyl inositol 4,5-bisphosphate. Circ Res 89: 1168–1176, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Bianchi L, Priori SG, Napolitano C, Surewicz KA, Dennis AT, Memmi M, Schwartz PJ, Brown AM. Mechanisms of I(Ks) suppression in LQT1 mutants. Am J Physiol Heart Circ Physiol 279: H3003–H3011, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Bianchi L, Shen Z, Dennis AT, Priori SG, Napolitano C, Ronchetti E, Bryskin R, Schwartz PJ, Brown AM. Cellular dysfunction of LQT5-minK mutants: abnormalities of IKs, IKr and trafficking in long QT syndrome. Hum Mol Genet 8: 1499–1507, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Boczek NJ, Miller EM, Ye D, Nesterenko VV, Tester DJ, Antzelevitch C, Czosek RJ, Ackerman MJ, Ware SM. Novel Timothy syndrome mutation leading to increase in CACNA1C window current. Heart Rhythm 12: 211–219, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulet IR, Labro AJ, Raes AL, Snyders DJ. Role of the S6 C-terminus in KCNQ1 channel gating. J Physiol 585: 325–337, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brelidze TI, Carlson AE, Sankaran B, Zagotta WN. Structure of the carboxy-terminal region of a KCNH channel. Nature 481: 530–533, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brink PA, Crotti L, Corfield V, Goosen A, Durrheim G, Hedley P, Heradien M, Geldenhuys G, Vanoli E, Bacchini S, Spazzolini C, Lundquist AL, Roden DM, George AL Jr, Schwartz PJ. Phenotypic variability and unusual clinical severity of congenital long-QT syndrome in a founder population. Circulation 112: 2602–2610, 2005. [DOI] [PubMed] [Google Scholar]
- 58.Brugada R, Brugada J, Antzelevitch C, Kirsch GE, Potenza D, Towbin JA, Brugada P. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation 101: 510–515, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, Mathur R, Hajjiri M, Odening KE, Steinberg E, Folco EJ, Pringa E, Centracchio J, Macharzina RR, Donahay T, Schofield L, Rana N, Kirk M, Mitchell GF, Poppas A, Zehender M, Koren G. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest 118: 2246–2259, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buber J, Mathew J, Moss AJ, Hall WJ, Barsheshet A, McNitt S, Robinson JL, Zareba W, Ackerman MJ, Kaufman ES, Luria D, Eldar M, Towbin JA, Vincent M, Goldenberg I. Risk of recurrent cardiac events after onset of menopause in women with congenital long-QT syndrome types 1 and 2. Circulation 123: 2784–2791, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burgess DE, Bartos DC, Reloj AR, Campbell KS, Johnson JN, Tester DJ, Ackerman MJ, Fressart V, Denjoy I, Guicheney P, Moss AJ, Ohno S, Horie M, Delisle BP. High-risk long QT syndrome mutations in the Kv7.1 (KCNQ1) pore disrupt the molecular basis for rapid K+ permeation. Biochemistry 51: 9076–9085, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Busch AE, Suessbrich H, Waldegger S, Sailer E, Greger R, Lang H, Lang F, Gibson KJ, Maylie JG. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflügers Arch 432: 1094–1096, 1996. [DOI] [PubMed] [Google Scholar]
- 63.Caballero R, Moreno I, Gonzalez T, Arias C, Valenzuela C, Delpon E, Tamargo J. Spironolactone and its main metabolite, canrenoic acid, block human ether-a-go-go-related gene channels. Circulation 107: 889–895, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Calvillo L, Spazzolini C, Vullo E, Insolia R, Crotti L, Schwartz PJ. Propranolol prevents life-threatening arrhythmias in LQT3 transgenic mice: implications for the clinical management of LQT3 patients. Heart Rhythm 11: 126–132, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camors E, Mohler PJ, Bers DM, Despa S. Ankyrin-B reduction enhances Ca spark-mediated SR Ca release promoting cardiac myocyte arrhythmic activity. J Mol Cell Cardiol 52: 1240–1248, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Capes DL, Goldschen-Ohm MP, Arcisio-Miranda M, Bezanilla F, Chanda B. Domain IV voltage-sensor movement is both sufficient and rate limiting for fast inactivation in sodium channels. J Gen Physiol 142: 101–112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J Pharmacol Exp Ther 262: 809–817, 1992. [PubMed] [Google Scholar]
- 68.Casimiro MC, Knollmann BC, Ebert SN, Vary JC Jr, Greene AE, Franz MR, Grinberg A, Huang SP, Pfeifer K. Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc Natl Acad Sci USA 98: 2526–2531, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Casis O, Olesen SP, Sanguinetti MC. Mechanism of action of a novel human ether-a-go-go-related gene channel activator. Mol Pharmacol 69: 658–665, 2006. [DOI] [PubMed] [Google Scholar]
- 70.Catterall WA. Voltage-gated sodium channels at 60: structure, function, and pathophysiology. J Physiol 590: 2577–2589, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cha A, Ruben PC, George AL Jr, Fujimoto E, Bezanilla F. Voltage sensors in domains III and IV, but not I and II, are immobilized by Na+ channel fast inactivation. Neuron 22: 73–87, 1999. [DOI] [PubMed] [Google Scholar]
- 72.Chanda B, Bezanilla F. Tracking voltage-dependent conformational changes in skeletal muscle sodium channel during activation. J Gen Physiol 120: 629–645, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chandra R, Starmer CF, Grant AO. Multiple effects of KPQ deletion mutation on gating of human cardiac Na+ channels expressed in mammalian cells. Am J Physiol Heart Circ Physiol 274: H1643–H1654, 1998. [DOI] [PubMed] [Google Scholar]
- 74.Chauhan VS, Tuvia S, Buhusi M, Bennett V, Grant AO. Abnormal cardiac Na+ channel properties and QT heart rate adaptation in neonatal ankyrin(B) knockout mice. Circ Res 86: 441–447, 2000. [DOI] [PubMed] [Google Scholar]
- 75.Chen J, Mitcheson JS, Tristani-Firouzi M, Lin M, Sanguinetti MC. The S4–S5 linker couples voltage sensing and activation of pacemaker channels. Proc Natl Acad Sci USA 98: 11277–11282, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen J, Zheng R, Melman YF, McDonald TV. Functional interactions between KCNE1 C-terminus and the KCNQ1 channel. PLoS One 4: e5143, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen J, Zou A, Splawski I, Keating MT, Sanguinetti MC. Long QT syndrome-associated mutations in the Per-Arnt-Sim (PAS) domain of HERG potassium channels accelerate channel deactivation. J Biol Chem 274: 10113–10118, 1999. [DOI] [PubMed] [Google Scholar]
- 78.Chen L, Kurokawa J, Kass RS. Phosphorylation of the A-kinase-anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J Biol Chem 280: 31347–31352, 2005. [DOI] [PubMed] [Google Scholar]
- 79.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci USA 104: 20990–20995, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen L, Zhang Q, Qiu Y, Li Z, Chen Z, Jiang H, Li Y, Yang H. Migration of PIP2 lipids on voltage-gated potassium channel surface influences channel deactivation. Scientific Reports 5: 15079, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chouabe C, Neyroud N, Guicheney P, Lazdunski M, Romey G, Barhanin J. Properties of KvLQT1 K+ channel mutations in Romano-Ward and Jervell and Lange-Nielsen inherited cardiac arrhythmias. EMBO J 16: 5472–5479, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choveau FS, Rodriguez N, Abderemane Ali F, Labro AJ, Rose T, Dahimene S, Boudin H, Le Henaff C, Escande D, Snyders DJ, Charpentier F, Merot J, Baro I, Loussouarn G. KCNQ1 channels voltage dependence through a voltage-dependent binding of the S4–S5 linker to the pore domain. J Biol Chem 286: 707–716, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411: 957–962, 2001. [DOI] [PubMed] [Google Scholar]
- 84.Chung DY, Chan PJ, Bankston JR, Yang L, Liu G, Marx SO, Karlin A, Kass RS. Location of KCNE1 relative to KCNQ1 in the I(KS) potassium channel by disulfide cross-linking of substituted cysteines. Proc Natl Acad Sci USA 106: 743–748, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clancy CE, Rudy Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature 400: 566–569, 1999. [DOI] [PubMed] [Google Scholar]
- 86. Clancy CE, Rudy Y. Na+ channel mutation that causes both Brugada and long-QT syndrome phenotypes: a simulation study of mechanism. Circulation 105: 1208–1213, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clancy CE, Tateyama M, Liu H, Wehrens XH, Kass RS. Non-equilibrium gating in cardiac Na+ channels: an original mechanism of arrhythmia. Circulation 107: 2233–2237, 2003. [DOI] [PubMed] [Google Scholar]
- 88.Clancy CE, Zhu ZI, Rudy Y. Pharmacogenetics and anti-arrhythmic drug therapy: a theoretical investigation. Am J Physiol Heart Circ Physiol 292: H66–H75, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Compton SJ, Lux RL, Ramsey MR, Strelich KR, Sanguinetti MC, Green LS, Keating MT, Mason JW. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation 94: 1018–1022, 1996. [DOI] [PubMed] [Google Scholar]
- 90.Cordes JS, Sun Z, Lloyd DB, Bradley JA, Opsahl AC, Tengowski MW, Chen X, Zhou J. Pentamidine reduces hERG expression to prolong the QT interval. Br J Pharmacol 145: 15–23, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cormier JW, Rivolta I, Tateyama M, Yang AS, Kass RS. Secondary structure of the human cardiac Na+ channel C terminus: evidence for a role of helical structures in modulation of channel inactivation. J Biol Chem 277: 9233–9241, 2002. [DOI] [PubMed] [Google Scholar]
- 92.Cronk LB, Ye B, Kaku T, Tester DJ, Vatta M, Makielski JC, Ackerman MJ. Novel mechanism for sudden infant death syndrome: persistent late sodium current secondary to mutations in caveolin-3. Heart Rhythm 4: 161–166, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crotti L, Johnson CN, Graf E, De Ferrari GM, Cuneo BF, Ovadia M, Papagiannis J, Feldkamp MD, Rathi SG, Kunic JD, Pedrazzini M, Wieland T, Lichtner P, Beckmann BM, Clark T, Shaffer C, Benson DW, Kaab S, Meitinger T, Strom TM, Chazin WJ, Schwartz PJ, George AL Jr. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation 127: 1009–1017, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cui J, Melman Y, Palma E, Fishman GI, McDonald TV. Cyclic AMP regulates the HERG K+ channel by dual pathways. Curr Biol 10: 671–674, 2000. [DOI] [PubMed] [Google Scholar]
- 95.Cunha SR, Bhasin N, Mohler PJ. Targeting and stability of Na/Ca exchanger 1 in cardiomyocytes requires direct interaction with the membrane adaptor ankyrin-B. J Biol Chem 282: 4875–4883, 2007. [DOI] [PubMed] [Google Scholar]
- 96.Cunha SR, Hund TJ, Hashemi S, Voigt N, Li N, Wright P, Koval O, Li J, Gudmundsson H, Gumina RJ, Karck M, Schott JJ, Probst V, Le Marec H, Anderson ME, Dobrev D, Wehrens XH, Mohler PJ. Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation 124: 1212–1222, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 80: 795–803, 1995. [DOI] [PubMed] [Google Scholar]
- 98.Dahimene S, Alcolea S, Naud P, Jourdon P, Escande D, Brasseur R, Thomas A, Baro I, Merot J. The N-terminal juxtamembranous domain of KCNQ1 is critical for channel surface expression: implications in the Romano-Ward LQT1 syndrome. Circ Res 99: 1076–1083, 2006. [DOI] [PubMed] [Google Scholar]
- 99.Dascal N, Schreibmayer W, Lim NF, Wang W, Chavkin C, DiMagno L, Labarca C, Kieffer BL, Gaveriaux-Ruff C, Trollinger D. Atrial G protein-activated K+ channel: expression cloning and molecular properties. Proc Natl Acad Sci USA 90: 10235–10239, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De la Pena P, Machin A, Fernandez-Trillo J, Dominguez P, Barros F. Mapping of interactions between the N- and C-termini and the channel core in HERG K+ channels. Biochem J 451: 463–474, 2013. [DOI] [PubMed] [Google Scholar]
- 101.Dhar Malhotra J, Chen C, Rivolta I, Abriel H, Malhotra R, Mattei LN, Brosius FC, Kass RS, Isom LL. Characterization of sodium channel alpha- and beta-subunits in rat and mouse cardiac myocytes. Circulation 103: 1303–1310, 2001. [DOI] [PubMed] [Google Scholar]
- 102.Dobrzynski H, Anderson RH, Atkinson A, Borbas Z, D'Souza A, Fraser JF, Inada S, Logantha SJ, Monfredi O, Morris GM, Moorman AF, Nikolaidou T, Schneider H, Szuts V, Temple IP, Yanni J, Boyett MR. Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacol Ther 139: 260–288, 2013. [DOI] [PubMed] [Google Scholar]
- 103.Donaldson MR, Jensen JL, Tristani-Firouzi M, Tawil R, Bendahhou S, Suarez WA, Cobo AM, Poza JJ, Behr E, Wagstaff J, Szepetowski P, Pereira S, Mozaffar T, Escolar DM, Fu YH, Ptacek LJ. PIP2 binding residues of Kir2.1 are common targets of mutations causing Andersen syndrome. Neurology 60: 1811–1816, 2003. [DOI] [PubMed] [Google Scholar]
- 104.Doupnik CA, Davidson N, Lester HA. The inward rectifier potassium channel family. Curr Opin Neurobiol 5: 268–277, 1995. [DOI] [PubMed] [Google Scholar]
- 105.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280: 69–77, 1998. [DOI] [PubMed] [Google Scholar]
- 106.Doyle DD, Goings G, Upshaw-Earley J, Ambler SK, Mondul A, Palfrey HC, Page E. Dystrophin associates with caveolae of rat cardiac myocytes: relationship to dystroglycan. Circ Res 87: 480–488, 2000. [DOI] [PubMed] [Google Scholar]
- 107.Duggal P, Vesely MR, Wattanasirichaigoon D, Villafane J, Kaushik V, Beggs AH. Mutation of the gene for IsK associated with both Jervell and Lange-Nielsen and Romano-Ward forms of Long-QT syndrome. Circ J 97: 142–146, 1998. [DOI] [PubMed] [Google Scholar]
- 108.Dumaine R, Kirsch GE. Mechanism of lidocaine block of late current in long Q-T mutant Na+ channels. Am J Physiol Heart Circ Physiol 274: H477–H487, 1998. [DOI] [PubMed] [Google Scholar]
- 109.Dumaine R, Wang Q , Keating MT, Hartmann HA, Schwartz PJ, Brown AM, Kirsch GE. Multiple mechanisms of Na+ channel-linked long-QT syndrome. Circ Res 78: 916–924, 1996. [DOI] [PubMed] [Google Scholar]
- 110.Dvir M, Strulovich R, Sachyani D, Ben-Tal Cohen I, Haitin Y, Dessauer C, Pongs O, Kass R, Hirsch JA, Attali B. Long QT mutations at the interface between KCNQ1 helix C and KCNE1 disrupt I(KS) regulation by PKA and PIP2. J Cell Sci 127: 3943–3955, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eaholtz G, Scheuer T, Catterall WA. Restoration of inactivation and block of open sodium channels by an inactivation gate peptide. Neuron 12: 1041–1048, 1994. [DOI] [PubMed] [Google Scholar]
- 112.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo The Cardiac Arrhythmia Suppression Trial. N Engl J Med 324: 781–788, 1991. [DOI] [PubMed] [Google Scholar]
- 113.Eldstrom J, Wang Z, Werry D, Wong N, Fedida D. Microscopic mechanisms for long QT syndrome type 1 revealed by single-channel analysis of I(Ks) with S3 domain mutations in KCNQ1. Heart Rhythm 12: 386–394, 2015. [DOI] [PubMed] [Google Scholar]
- 114.Eldstrom J, Xu H, Werry D, Kang C, Loewen ME, Degenhardt A, Sanatani S, Tibbits GF, Sanders C, Fedida D. Mechanistic basis for LQT1 caused by S3 mutations in the KCNQ1 subunit of IKs. J Gen Physiol 135: 433–448, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eng G, Lee BW, Protas L, Gagliardi M, Brown K, Kass RS, Keller G, Robinson RB, Vunjak-Novakovic G. Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nat Commun 7: 10312, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Es-Salah-Lamoureux Z, Xiong PY, Goodchild SJ, Ahern CA, Fedida D. Blockade of permeation by potassium but normal gating of the G628S nonconducting hERG channel mutant. Biophys J 101: 662–670, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Etheridge SP, Compton SJ, Tristani-Firouzi M, Mason JW. A new oral therapy for long QT syndrome: long-term oral potassium improves repolarization in patients with HERG mutations. J Am Coll Cardiol 42: 1777–1782, 2003. [DOI] [PubMed] [Google Scholar]
- 118.European. Heart Rhythm Association, Heart Rhythm Society, Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology, American Heart Association Task Force, European Society of Cardiology Committee for Practice Guidelines. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 48: e247–346, 2006. [DOI] [PubMed] [Google Scholar]
- 119.Faber GM, Rudy Y. Action potential and contractility changes in [Na+]i overloaded cardiac myocytes: a simulation study. Biophys J 78: 2392–2404, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fabritz L, Kirchhof P, Franz MR, Nuyens D, Rossenbacker T, Ottenhof A, Haverkamp W, Breithardt G, Carmeliet E, Carmeliet P. Effect of pacing and mexiletine on dispersion of repolarisation and arrhythmias in DeltaKPQ SCN5A (long QT3) mice. Cardiovasc Res 57: 1085–1093, 2003. [DOI] [PubMed] [Google Scholar]
- 121.Fatima A, Kaifeng S, Dittmann S, Xu G, Gupta MK, Linke M, Zechner U, Nguemo F, Milting H, Farr M, Hescheler J, Saric T. The disease-specific phenotype in cardiomyocytes derived from induced pluripotent stem cells of two long QT syndrome type 3 patients. PLoS One 8: e83005, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferrer T, Rupp J, Piper DR, Tristani-Firouzi M. The S4–S5 linker directly couples voltage sensor movement to the activation gate in the human ether-a-go-go-related gene (hERG) K+ channel. J Biol Chem 281: 12858–12864, 2006. [DOI] [PubMed] [Google Scholar]
- 123.Ficker E, Dennis AT, Obejero-Paz CA, Castaldo P, Taglialatela M, Brown AM. Retention in the endoplasmic reticulum as a mechanism of dominant-negative current suppression in human long QT syndrome. J Mol Cell Cardiol 32: 2327–2337, 2000. [DOI] [PubMed] [Google Scholar]
- 124.Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ Res 92: e87–100, 2003. [DOI] [PubMed] [Google Scholar]
- 125.Ficker E, Jarolimek W, Brown AM. Molecular determinants of inactivation and dofetilide block in ether a-go-go (EAG) channels and EAG-related K+ channels. Mol Pharmacol 60: 1343–1348, 2001. [DOI] [PubMed] [Google Scholar]
- 126.Fozzard HA. Afterdepolarizations and triggered activity. Basic Res Cardiol 87 Suppl 2: 105–113, 1992. [DOI] [PubMed] [Google Scholar]
- 127.Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol 148: 16–24, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fuller H, Justo F, Nearing BD, Kahlig KM, Rajamani S, Belardinelli L, Verrier RL. Eleclazine, a new selective cardiac late sodium current inhibitor, confers concurrent protection against autonomically induced atrial premature beats, repolarization alternans and heterogeneity, and atrial fibrillation in an intact porcine model. Heart Rhythm 13: 1679–1686, 2016. [DOI] [PubMed] [Google Scholar]
- 129.Fulop L, Banyasz T, Szabo G, Toth IB, Biro T, Lorincz I, Balogh A, Peto K, Miko I, Nanasi PP. Effects of sex hormones on ECG parameters and expression of cardiac ion channels in dogs. Acta Physiol 188: 163–171, 2006. [DOI] [PubMed] [Google Scholar]
- 130.Funasako M, Aiba T, Ishibashi K, Nakajima I, Miyamoto K, Inoue Y, Okamura H, Noda T, Kamakura S, Anzai T, Noguchi T, Yasuda S, Miyamoto Y, Fukushima Kusano K, Ogawa H, Shimizu W. Pronounced shortening of QT interval with mexiletine infusion test in patients with type 3 congenital long QT syndrome. Circ J 80: 340–345, 2016. [DOI] [PubMed] [Google Scholar]
- 131.Furutani M, Trudeau MC, Hagiwara N, Seki A, Gong Q, Zhou Z, Imamura S, Nagashima H, Kasanuki H, Takao A, Momma K, January CT, Robertson GA, Matsuoka R. Novel mechanism associated with an inherited cardiac arrhythmia: defective protein trafficking by the mutant HERG (G601S) potassium channel. Circulation 99: 2290–2294, 1999. [DOI] [PubMed] [Google Scholar]
- 132.Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol 582: 675–693, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem 276: 21425–21433, 2001. [DOI] [PubMed] [Google Scholar]
- 134.Garg V, Stary-Weinzinger A, Sanguinetti MC. ICA-105574 interacts with a common binding site to elicit opposite effects on inactivation gating of EAG and ERG potassium channels. Mol Pharmacol 83: 805–813, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gavillet B, Rougier JS, Domenighetti AA, Behar R, Boixel C, Ruchat P, Lehr HA, Pedrazzini T, Abriel H. Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res 99: 407–414, 2006. [DOI] [PubMed] [Google Scholar]
- 136.Gehrmann J, Meister M, Maguire CT, Martins DC, Hammer PE, Neer EJ, Berul CI, Mende U. Impaired parasympathetic heart rate control in mice with a reduction of functional G protein betagamma-subunits. Am J Physiol Heart Circ Physiol 282: H445–H456, 2002. [DOI] [PubMed] [Google Scholar]
- 137.Gellens ME, George AL Jr, Chen LQ, Chahine M, Horn R, Barchi RL, Kallen RG. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc Natl Acad Sci USA 89: 554–558, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gerlach AC, Stoehr SJ, Castle NA. Pharmacological removal of human ether-a-go-go-related gene potassium channel inactivation by 3-nitro-N-(4-phenoxyphenyl) benzamide (ICA-105574). Mol Pharmacol 77: 58–68, 2010. [DOI] [PubMed] [Google Scholar]
- 139.Gerlach U, Brendel J, Lang HJ, Paulus EF, Weidmann K, Bruggemann A, Busch AE, Suessbrich H, Bleich M, Greger R. Synthesis and activity of novel and selective I(Ks)-channel blockers. J Med Chem 44: 3831–3837, 2001. [DOI] [PubMed] [Google Scholar]
- 140.Gessner G, Macianskiene R, Starkus JG, Schonherr R, Heinemann SH. The amiodarone derivative KB130015 activates hERG1 potassium channels via a novel mechanism. Eur J Pharmacol 632: 52–59, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ghosh S, Nunziato DA, Pitt GS. KCNQ1 assembly and function is blocked by long-QT syndrome mutations that disrupt interaction with calmodulin. Circ Res 98: 1048–1054, 2006. [DOI] [PubMed] [Google Scholar]
- 142.Gillis J, Burashnikov E, Antzelevitch C, Blaser S, Gross G, Turner L, Babul-Hirji R, Chitayat D. Long QT, syndactyly, joint contractures, stroke and novel CACNA1C mutation: expanding the spectrum of Timothy syndrome. Am J Med Genet 158: 182–187, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Giudicessi JR, Ackerman MJ. Genotype- and phenotype-guided management of congenital long QT syndrome. Curr Probl Cardiol 38: 417–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Glaaser IW, Osteen JD, Puckerin A, Sampson KJ, Jin X, Kass RS. Perturbation of sodium channel structure by an inherited Long QT Syndrome mutation. Nat Commun 3: 706, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Goldenberg I, Bradley J, Moss A, McNitt S, Polonsky S, Robinson JL, Andrews M, Zareba W. Beta-blocker efficacy in high-risk patients with the congenital long-QT syndrome types 1 and 2: implications for patient management. J Cardiovasc Electrophysiol 21: 893–901, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Goldenberg I, Thottathil P, Lopes CM, Moss AJ, McNitt S, JOU, Robinson JL, Zareba W, Ackerman MJ, Kaufman ES, Towbin JA, Vincent M, Barsheshet A. Trigger-specific ion-channel mechanisms, risk factors, and response to therapy in type 1 long QT syndrome. Heart Rhythm 9: 49–56, 2012. [DOI] [PubMed] [Google Scholar]
- 147.Goldschen-Ohm MP, Capes DL, Oelstrom KM, Chanda B. Multiple pore conformations driven by asynchronous movements of voltage sensors in a eukaryotic sodium channel. Nat Commun 4: 1350, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gong Q, Jones MA, Zhou Z. Mechanisms of pharmacological rescue of trafficking-defective hERG mutant channels in human long QT syndrome. J Biol Chem 281: 4069–4074, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gong Q, Keeney DR, Molinari M, Zhou Z. Degradation of trafficking-defective long QT syndrome type II mutant channels by the ubiquitin-proteasome pathway. J Biol Chem 280: 19419–19425, 2005. [DOI] [PubMed] [Google Scholar]
- 150.Gong Q, Zhang L, Vincent GM, Horne BD, Zhou Z. Nonsense mutations in hERG cause a decrease in mutant mRNA transcripts by nonsense-mediated mRNA decay in human long-QT syndrome. Circulation 116: 17–24, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gordon E, Panaghie G, Deng L, Bee KJ, Roepke TK, Krogh-Madsen T, Christini DJ, Ostrer H, Basson CT, Chung W, Abbott GW. A KCNE2 mutation in a patient with cardiac arrhythmia induced by auditory stimuli and serum electrolyte imbalance. Cardiovasc Res 77: 98–106, 2008. [DOI] [PubMed] [Google Scholar]
- 152.Gouaux E, Mackinnon R. Principles of selective ion transport in channels and pumps. Science 310: 1461–1465, 2005. [DOI] [PubMed] [Google Scholar]
- 153.Grunnet M, Abbruzzese J, Sachse FB, Sanguinetti MC. Molecular determinants of human ether-a-go-go-related gene 1 (hERG1) K+ channel activation by NS1643. Mol Pharmacol 79: 1–9, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grunnet M, Jespersen T, Rasmussen HB, Ljungstrom T, Jorgensen NK, Olesen SP, Klaerke DA. KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol 542: 119–130, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guo J, Cheng YM, Lees-Miller JP, Perissinotti LL, Claydon TW, Hull CM, Thouta S, Roach DE, Durdagi S, Noskov SY, Duff HJ. NS1643 interacts around L529 of hERG to alter voltage sensor movement on the path to activation. Biophys J 108: 1400–1413, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guo J, Massaeli H, Li W, Xu J, Luo T, Shaw J, Kirshenbaum LA, Zhang S. Identification of IKr and its trafficking disruption induced by probucol in cultured neonatal rat cardiomyocytes. J Pharmacol Exp Ther 321: 911–920, 2007. [DOI] [PubMed] [Google Scholar]
- 157.Hackos DH, Chang TH, Swartz KJ. Scanning the intracellular S6 activation gate in the shaker K+ channel. J Gen Physiol 119: 521–532, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Haissaguerre M, Vigmond E, Stuyvers B, Hocini M, Bernus O. Ventricular arrhythmias and the His-Purkinje system. Nat Rev Cardiol 13: 155–166, 2016. [DOI] [PubMed] [Google Scholar]
- 159.Haitin Y, Attali B. The C-terminus of Kv7 channels: a multifunctional module. J Physiol 586: 1803–1810, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Haitin Y, Carlson AE, Zagotta WN. The structural mechanism of KCNH-channel regulation by the eag domain. Nature 501: 444–448, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Haitin Y, Wiener R, Shaham D, Peretz A, Cohen EB, Shamgar L, Pongs O, Hirsch JA, Attali B. Intracellular domains interactions and gated motions of I(KS) potassium channel subunits. EMBO J 28: 1994–2005, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hanck DA, Nikitina E, McNulty MM, Fozzard HA, Lipkind GM, Sheets MF. Using lidocaine and benzocaine to link sodium channel molecular conformations to state-dependent antiarrhythmic drug affinity. Circ Res 105: 492–499, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hancox JC, McPate MJ, El Harchi A, Zhang YH. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol Ther 119: 118–132, 2008. [DOI] [PubMed] [Google Scholar]
- 164.Hansen RS, Diness TG, Christ T, Demnitz J, Ravens U, Olesen SP, Grunnet M. Activation of human ether-a-go-go-related gene potassium channels by the diphenylurea 1,3-bis-(2-hydroxy-5-trifluoromethyl-phenyl)-urea (NS1643). Mol Pharmacol 69: 266–277, 2006. [DOI] [PubMed] [Google Scholar]
- 165.Hansen RS, Diness TG, Christ T, Wettwer E, Ravens U, Olesen SP, Grunnet M. Biophysical characterization of the new human ether-a-go-go-related gene channel opener NS3623 [N-(4-bromo-2-(1H-tetrazol-5-yl)-phenyl)-N'-(3'-trifluoromethylphenyl)urea]. Mol Pharmacol 70: 1319–1329, 2006. [DOI] [PubMed] [Google Scholar]
- 166.Harmer SC, Wilson AJ, Aldridge R, Tinker A. Mechanisms of disease pathogenesis in long QT syndrome type 5. Am J Physiol Cell Physiol 298: C263–C273, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hausdorff SF, Goldstein SA, Rushin EE, Miller C. Functional characterization of a minimal K+ channel expressed from a synthetic gene. Biochemistry 30: 3341–3346, 1991. [DOI] [PubMed] [Google Scholar]
- 168.Hayashi K, Fujino N, Ino H, Uchiyama K, Sakata K, Konno T, Masuta E, Funada A, Sakamoto Y, Tsubokawa T, Hodatsu A, Yasuda T, Kanaya H, Kim MY, Kupershmidt S, Higashida H, Yamagishi M. A KCR1 variant implicated in susceptibility to the long QT syndrome. J Mol Cell Cardiol 50: 50–57, 2011. [DOI] [PubMed] [Google Scholar]
- 169.Heijman J, Spatjens RL, Seyen SR, Lentink V, Kuijpers HJ, Boulet IR, de Windt LJ, David M, Volders PG. Dominant-negative control of cAMP-dependent IKs upregulation in human long-QT syndrome type 1. Circ Res 110: 211–219, 2012. [DOI] [PubMed] [Google Scholar]
- 170.Henrion U, Strutz-Seebohm N, Duszenko M, Lang F, Seebohm G. Long QT syndrome-associated mutations in the voltage sensor of I(Ks) channels. Cell Physiol Biochem 24: 11–16, 2009. [DOI] [PubMed] [Google Scholar]
- 171.Herzberg IM, Trudeau MC, Robertson GA. Transfer of rapid inactivation and sensitivity to the class III antiarrhythmic drug E-4031 from HERG to M-eag channels. J Physiol 511: 3–14, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hilgemann DW, Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science 273: 956–959, 1996. [DOI] [PubMed] [Google Scholar]
- 173.Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol 116: 449–472, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hodgkin AL, Huxley AF. A quantitive description of membrane current and its application to conduction and excitation in nerve. J Physiol 117: 500–544, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hoorntje T, Alders M, van Tintelen P, van der Lip K, Sreeram N, van der Wal A, Mannens M, Wilde A. Homozygous premature truncation of the HERG protein: the human HERG knockout. Circulation 100: 1264–1267, 1999. [DOI] [PubMed] [Google Scholar]
- 176.Hoppe UC, Marban E, Johns DC. Distinct gene-specific mechanisms of arrhythmia revealed by cardiac gene transfer of two long QT disease genes, HERG and KCNE1. Proc Natl Acad Sci USA 98: 5335–5340, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Horner JM, Horner MM, Ackerman MJ. The diagnostic utility of recovery phase QTc during treadmill exercise stress testing in the evaluation of long QT syndrome. Heart Rhythm 8: 1698–1704, 2011. [DOI] [PubMed] [Google Scholar]
- 178.Hoshi N, Takahashi H, Shahidullah M, Yokoyama S, Higashida H. KCR1, a membrane protein that facilitates functional expression of non-inactivating K+ currents associates with rat EAG voltage-dependent K+ channels. J Biol Chem 273: 23080–23085, 1998. [DOI] [PubMed] [Google Scholar]
- 179.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature 391: 803–806, 1998. [DOI] [PubMed] [Google Scholar]
- 180.Huang FD, Chen J, Lin M, Keating MT, Sanguinetti MC. Long-QT syndrome-associated missense mutations in the pore helix of the HERG potassium channel. Circulation 104: 1071–1075, 2001. [DOI] [PubMed] [Google Scholar]
- 181.Huang L, Bitner-Glindzicz M, Tranebjaerg L, Tinker A. A spectrum of functional effects for disease causing mutations in the Jervell and Lange-Nielsen syndrome. Cardiovasc Res 51: 670–680, 2001. [DOI] [PubMed] [Google Scholar]
- 182.Isbrandt D, Friederich P, Solth A, Haverkamp W, Ebneth A, Borggrefe M, Funke H, Sauter K, Breithardt G, Pongs O, Schulze-Bahr E. Identification and functional characterization of a novel KCNE2 (MiRP1) mutation that alters HERG channel kinetics. J Mol Med 80: 524–532, 2002. [DOI] [PubMed] [Google Scholar]
- 183.Ito H, Hosoya Y, Inanobe A, Tomoike H, Endoh M. Acetylcholine and adenosine activate the G protein-gated muscarinic K+ channel in ferret ventricular myocytes. Naunyn-Schmiedebergs Arch Pharmacol 351: 610–617, 1995. [DOI] [PubMed] [Google Scholar]
- 184.Iyer V, Roman-Campos D, Sampson KJ, Kang G, Fishman GI, Kass RS. Purkinje cells as sources of arrhythmias in long QT syndrome type 3. Scientific Reports 5: 13287, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Iyer V, Sampson KJ, Kass RS. Modeling tissue- and mutation-specific electrophysiological effects in the long QT syndrome: role of the Purkinje fiber. PLoS One 9: e97720, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.James AF, Choisy SC, Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol 94: 265–319, 2007. [DOI] [PubMed] [Google Scholar]
- 187.January CT, Gong Q, Zhou Z. Long QT syndrome: cellular basis and arrhythmia mechanism in LQT2. J Cardiovasc Electrophysiol 11: 1413–1418, 2000. [DOI] [PubMed] [Google Scholar]
- 188.Jenke M, Sanchez A, Monje F, Stuhmer W, Weseloh RM, Pardo LA. C-terminal domains implicated in the functional surface expression of potassium channels. EMBO J 22: 395–403, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J 54: 59–68, 1957. [DOI] [PubMed] [Google Scholar]
- 190.Jespersen T, Membrez M, Nicolas CS, Pitard B, Staub O, Olesen SP, Baro I, Abriel H. The KCNQ1 potassium channel is down-regulated by ubiquitylating enzymes of the Nedd4/Nedd4-like family. Cardiovasc Res 74: 64–74, 2007. [DOI] [PubMed] [Google Scholar]
- 191.Jiang C, Atkinson D, Towbin JA, Splawski I, Lehmann MH, Li H, Timothy K, Taggart RT, Schwartz PJ, Vincent GM. Two long QT syndrome loci map to chromosomes 3 and 7 with evidence for further heterogeneity. Nat Genet 8: 141–147, 1994. [DOI] [PubMed] [Google Scholar]
- 192.Jiang M, Xu X, Wang Y, Toyoda F, Liu XS, Zhang M, Robinson RB, Tseng GN. Dynamic partnership between KCNQ1 and KCNE1 and influence on cardiac IKs current amplitude by KCNE2. J Biol Chem 284: 16452–16462, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Johnson JN, Ackerman MJ. QTc: how long is too long? Br J Sports Med 43: 657–662, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Johnson WH Jr, Yang P, Yang T, Lau YR, Mostella BA, Wolff DJ, Roden DM, Benson DW. Clinical, genetic, and biophysical characterization of a homozygous HERG mutation causing severe neonatal long QT syndrome. Pediatr Res 53: 744–748, 2003. [DOI] [PubMed] [Google Scholar]
- 195.Jones DK, Liu F, Vaidyanathan R, Eckhardt LL, Trudeau MC, Robertson GA. hERG 1b is critical for human cardiac repolarization. Proc Natl Acad Sci USA 111: 18073–18077, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jones EM, Roti Roti EC, Wang J, Delfosse SA, Robertson GA. Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. J Biol Chem 279: 44690–44694, 2004. [DOI] [PubMed] [Google Scholar]
- 197.Jons C, Moss AJ, Lopes CM, McNitt S, Zareba W, Goldenberg I, Qi M, Wilde AA, Shimizu W, Kanters JK, Towbin JA, Ackerman MJ, Robinson JL. Mutations in conserved amino acids in the KCNQ1 channel and risk of cardiac events in type-1 long-QT syndrome. J Cardiovasc Electrophysiol 20: 859–865, 2009. [DOI] [PubMed] [Google Scholar]
- 198.Jost N, Virag L, Bitay M, Takacs J, Lengyel C, Biliczki P, Nagy Z, Bogats G, Lathrop DA, Papp JG, Varro A. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation 112: 1392–1399, 2005. [DOI] [PubMed] [Google Scholar]
- 199.Jouni M, Si-Tayeb K, Es-Salah-Lamoureux Z, Latypova X, Champon B, Caillaud A, Rungoat A, Charpentier F, Loussouarn G, Baro I, Zibara K, Lemarchand P, Gaborit N. Toward personalized medicine: using cardiomyocytes differentiated from urine-derived pluripotent stem cells to recapitulate electrophysiological characteristics of type 2 long QT syndrome. J Am Heart Assoc 4: e002159, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Justo F, Fuller H, Nearing BD, Rajamani S, Belardinelli L, Verrier RL. Inhibition of the cardiac late sodium current with eleclazine protects against ischemia-induced vulnerability to atrial fibrillation and reduces atrial and ventricular repolarization abnormalities in the absence and presence of concurrent adrenergic stimulation. Heart Rhythm 13: 1860–1867, 2016. [DOI] [PubMed] [Google Scholar]
- 201.Kagan A, Yu Z, Fishman GI, McDonald TV. The dominant negative LQT2 mutation A561V reduces wild-type HERG expression. J Biol Chem 275: 11241–11248, 2000. [DOI] [PubMed] [Google Scholar]
- 202.Kambouris NG, Nuss HB, Johns DC, Marban E, Tomaselli GF, Balser JR. A revised view of cardiac sodium channel “blockade” in the long-QT syndrome. J Clin Invest 105: 1133–1140, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kang C, Tian C, Sonnichsen FD, Smith JA, Meiler J, George AL Jr, Vanoye CG, Kim HJ, Sanders CR. Structure of KCNE1 and implications for how it modulates the KCNQ1 potassium channel. Biochemistry 47: 7999–8006, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kang J, Chen XL, Wang H, Ji J, Cheng H, Incardona J, Reynolds W, Viviani F, Tabart M, Rampe D. Discovery of a small molecule activator of the human ether-a-go-go-related gene (HERG) cardiac K+ channel. Mol Pharmacol 67: 827–836, 2005. [DOI] [PubMed] [Google Scholar]
- 205.Kanki H, Kupershmidt S, Yang T, Wells S, Roden DM. A structural requirement for processing the cardiac K+ channel KCNQ1. J Biol Chem 279: 33976–33983, 2004. [DOI] [PubMed] [Google Scholar]
- 206.Kanters JK, Skibsbye L, Hedley PL, Dembic M, Liang B, Hagen CM, Eschen O, Grunnet M, Christiansen M, Jespersen T. Combined gating and trafficking defect in Kv11.1 manifests as a malignant long QT syndrome phenotype in a large Danish p F29L founder family. Scand J Clin Lab Invest 75: 699–709, 2015. [DOI] [PubMed] [Google Scholar]
- 207.Kapplinger JD, Tester DJ, Salisbury BA, Carr JL, Harris-Kerr C, Pollevick GD, Wilde AA, Ackerman MJ. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm 6: 1297–1303, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kasimova MA, Zaydman MA, Cui J, Tarek M. PIP2-dependent coupling is prominent in Kv7.1 due to weakened interactions between S4–S5 and S6. Scientific Reports 5: 7474, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kass RS, Moss AJ. Long QT syndrome: novel insights into the mechanisms of cardiac arrhythmias. J Clin Invest 112: 810–815, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kass RS, Wiegers SE. The ionic basis of concentration-related effects of noradrenaline on the action potential of calf cardiac Purkinje fibres. J Physiol 322: 541–558, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kazen-Gillespie KA, Ragsdale DS, D'Andrea MR, Mattei LN, Rogers KE, Isom LL. Cloning, localization, and functional expression of sodium channel beta1A subunits. J Biol Chem 275: 1079–1088, 2000. [DOI] [PubMed] [Google Scholar]
- 212.Keating M, Atkinson D, Dunn C, Timothy K, Vincent GM, Leppert M. Linkage of a cardiac arrhythmia, the long QT syndrome, and the Harvey ras-1 gene. Science 252: 704–706, 1991. [DOI] [PubMed] [Google Scholar]
- 213.Keating M, Dunn C, Atkinson D, Timothy K, Vincent GM, Leppert M. Consistent linkage of the long-QT syndrome to the Harvey ras-1 locus on chromosome 11. Am J Hum Genet 49: 1335–1339, 1991. [PMC free article] [PubMed] [Google Scholar]
- 214.Kennedy ME, Nemec J, Corey S, Wickman K, Clapham DE. GIRK4 confers appropriate processing and cell surface localization to G- protein-gated potassium channels. J Biol Chem 274: 2571–2582, 1999. [DOI] [PubMed] [Google Scholar]
- 215.Kiehn J, Wible B, Lacerda AE, Brown AM. Mapping the block of a cloned human inward rectifier potassium channel by dofetilide. Mol Pharmacol 50: 380–387, 1996. [PubMed] [Google Scholar]
- 216.Kim JA, Lopes CM, Moss AJ, McNitt S, Barsheshet A, Robinson JL, Zareba W, Ackerman MJ, Kaufman ES, Towbin JA, Vincent M, Goldenberg I. Trigger-specific risk factors and response to therapy in long QT syndrome type 2. Heart Rhythm 7: 1797–1805, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Koumi S, Sato R, Nagasawa K, Hayakawa H. Activation of inwardly rectifying potassium channels by muscarinic receptor-linked G protein in isolated human ventricular myocytes. J Membr Biol 157: 71–81, 1997. [DOI] [PubMed] [Google Scholar]
- 218.Kovtun O, Tillu VA, Ariotti N, Parton RG, Collins BM. Cavin family proteins and the assembly of caveolae. J Cell Sci 128: 1269–1278, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature 374: 135–141, 1995. [DOI] [PubMed] [Google Scholar]
- 220.Krumerman A, Gao X, Bian JS, Melman YF, Kagan A, McDonald TV. An LQT mutant minK alters KvLQT1 trafficking. Am J Physiol Cell Physiol 286: C1453–C1463, 2004. [DOI] [PubMed] [Google Scholar]
- 221.Krzystanek K, Rasmussen HB, Grunnet M, Staub O, Olesen SP, Abriel H, Jespersen T. Deubiquitylating enzyme USP2 counteracts Nedd4-2-mediated downregulation of KCNQ1 potassium channels. Heart Rhythm 9: 440–448, 2012. [DOI] [PubMed] [Google Scholar]
- 222.Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan LY. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature 364: 802–806, 1993. [DOI] [PubMed] [Google Scholar]
- 223.Kupershmidt S, Yang IC, Hayashi K, Wei J, Chanthaphaychith S, Petersen CI, Johns DC, George AL Jr, Roden DM, Balser JR. The IKr drug response is modulated by KCR1 in transfected cardiac and noncardiac cell lines. FASEB J 17: 2263–2265, 2003. [DOI] [PubMed] [Google Scholar]
- 224.Kupershmidt S, Yang T, Chanthaphaychith S, Wang Z, Towbin JA, Roden DM. Defective human Ether-a-go-go-related gene trafficking linked to an endoplasmic reticulum retention signal in the C terminus. J Biol Chem 277: 27442–27448, 2002. [DOI] [PubMed] [Google Scholar]
- 225.Kurachi Y, Tung RT, Ito H, Nakajima T. G protein activation of cardiac muscarinic K+ channels. Prog Neurobiol 39: 229–246, 1992. [DOI] [PubMed] [Google Scholar]
- 226.Kurokawa J, Chen L, Kass RS. Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel. Proc Natl Acad Sci USA 100: 2122–2127, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kurokawa J, Tamagawa M, Harada N, Honda S, Bai CX, Nakaya H, Furukawa T. Acute effects of oestrogen on the guinea pig and human IKr channels and drug-induced prolongation of cardiac repolarization. J Physiol 586: 2961–2973, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kuryshev YA, Ficker E, Wang L, Hawryluk P, Dennis AT, Wible BA, Brown AM, Kang J, Chen XL, Sawamura K, Reynolds W, Rampe D. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther 312: 316–323, 2005. [DOI] [PubMed] [Google Scholar]
- 229.Labro AJ, Boulet IR, Choveau FS, Mayeur E, Bruyns T, Loussouarn G, Raes AL, Snyders DJ. The S4–S5 linker of KCNQ1 channels forms a structural scaffold with the S6 segment controlling gate closure. J Biol Chem 286: 717–725, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Laksman ZW, Hamilton RM, Chockalingam P, Ballantyne E, Stephenson EA, Gross GJ, Gula LJ, Klein GJ, Wilde AA, Krahn AD. Mutation location effect on severity of phenotype during exercise testing in type 1 long-QT syndrome: impact of transmembrane and C-loop location. J Cardiovasc Electrophysiol 24: 1015–1020, 2013. [DOI] [PubMed] [Google Scholar]
- 231.Larsen AP, Olesen SP, Grunnet M, Jespersen T. Characterization of hERG1a and hERG1b potassium channels-a possible role for hERG1b in the I (Kr) current. Pflügers Arch 456: 1137–1148, 2008. [DOI] [PubMed] [Google Scholar]
- 232.Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME, Mohler PJ. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci USA 105: 15617–15622, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lees-Miller JP, Duan Y, Teng GQ, Duff HJ. Molecular determinant of high-affinity dofetilide binding to HERG1 expressed in Xenopus oocytes: involvement of S6 sites. Mol Pharmacol 57: 367–374, 2000. [PubMed] [Google Scholar]
- 234.Lehmann MH, Hardy S, Archibald D, quart B, MacNeil DJ. Sex difference in risk of torsade de pointes with d,l-sotalol. Circulation 94: 2535–2541, 1996. [DOI] [PubMed] [Google Scholar]
- 235.Li GR, Feng J, Wang Z, Fermini B, Nattel S. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Circ Res 78: 903–915, 1996. [DOI] [PubMed] [Google Scholar]
- 236.Li J, Kline CF, Hund TJ, Anderson ME, Mohler PJ. Ankyrin-B regulates Kir6.2 membrane expression and function in heart. J Biol Chem 285: 28723–28730, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li W, Du R, Wang QF, Tian L, Yang JG, Song ZF. The G314S KCNQ1 mutation exerts a dominant-negative effect on expression of KCNQ1 channels in oocytes. Biochem Biophys Res Commun 383: 206–209, 2009. [DOI] [PubMed] [Google Scholar]
- 238.Li Y, Chen L, Kass RS, Dessauer CW. The A-kinase anchoring protein Yotiao facilitates complex formation between adenylyl cyclase type 9 and the IKs potassium channel in heart. J Biol Chem 287: 29815–29824, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li Y, Zaydman MA, Wu D, Shi J, Guan M, Virgin-Downey B, Cui J. KCNE1 enhances phosphatidylinositol 4,5-bisphosphate (PIP2) sensitivity of IKs to modulate channel activity. Proc Natl Acad Sci USA 108: 9095–9100, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lindegger N, Hagen BM, Marks AR, Lederer WJ, Kass RS. Diastolic transient inward current in long QT syndrome type 3 is caused by Ca2+ overload and inhibited by ranolazine. J Mol Cell Cardiol 47: 326–334, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circ Res 76: 351–365, 1995. [DOI] [PubMed] [Google Scholar]
- 242.Liu GX, Derst C, Schlichthorl G, Heinen S, Seebohm G, Bruggemann A, Kummer W, Veh RW, Daut J, Preisig-Muller R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol 532: 115–126, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liu H, Tateyama M, Clancy CE, Abriel H, Kass RS. Channel openings are necessary but not sufficient for use-dependent block of cardiac Na+ channels by flecainide: evidence from the analysis of disease-linked mutations. J Gen Physiol 120: 39–51, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Liu JF, Moss AJ, Jons C, Benhorin J, Schwartz PJ, Spazzolini C, Crotti L, Ackerman MJ, McNitt S, Robinson JL, Qi M, Goldenberg I, Zareba W. Mutation-specific risk in two genetic forms of type 3 long QT syndrome. Am J Cardiol 105: 210–213, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Liu XK, Katchman A, Whitfield BH, Wan G, Janowski EM, Woosley RL, Ebert SN. In vivo androgen treatment shortens the QT interval and increases the densities of inward and delayed rectifier potassium currents in orchiectomized male rabbits. Cardiovasc Res 57: 28–36, 2003. [DOI] [PubMed] [Google Scholar]
- 246.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309: 897–903, 2005. [DOI] [PubMed] [Google Scholar]
- 247.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science 309: 903–908, 2005. [DOI] [PubMed] [Google Scholar]
- 248.Lopatin AN, Nichols CG. Inward rectifiers in the heart: an update on I(K1). J Mol Cell Cardiol 33: 625–638, 2001. [DOI] [PubMed] [Google Scholar]
- 249.Lopes CM, Zhang H, Rohacs T, Jin T, Yang J, Logothetis DE. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron 34: 933–944, 2002. [DOI] [PubMed] [Google Scholar]
- 250.Loussouarn G, Park KH, Bellocq C, Baro I, Charpentier F, Escande D. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J 22: 5412–5421, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lu Y, Mahaut-Smith MP, Huang CL, Vandenberg JI. Mutant MiRP1 subunits modulate HERG K+ channel gating: a mechanism for pro-arrhythmia in long QT syndrome type 6. J Physiol 551: 253–262, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ma D, Wei H, Zhao Y, Lu J, Li G, Sahib NB, Tan TH, Wong KY, Shim W, Wong P, Cook SA, Liew R. Modeling type 3 long QT syndrome with cardiomyocytes derived from patient-specific induced pluripotent stem cells. Int J Cardiol 168: 5277–5286, 2013. [DOI] [PubMed] [Google Scholar]
- 253.Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation 109: 1421–1427, 2004. [DOI] [PubMed] [Google Scholar]
- 254.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA 270: 2590–2597, 1993. [DOI] [PubMed] [Google Scholar]
- 255.Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295: 496–499, 2002. [DOI] [PubMed] [Google Scholar]
- 256.Matavel A, Medei E, Lopes CM. PKA and PKC partially rescue long QT type 1 phenotype by restoring channel-PIP2 interactions. Channels 4: 3–11, 2010. [DOI] [PubMed] [Google Scholar]
- 257.Mazhari R, Greenstein JL, Winslow RL, Marban E, Nuss HB. Molecular interactions between two long-QT syndrome gene products, HERG and KCNE2, rationalized by in vitro and in silico analysis. Circ Res 89: 33–38, 2001. [DOI] [PubMed] [Google Scholar]
- 258.Mazzanti A, Maragna R, Faragli A, Monteforte N, Bloise R, Memmi M, Novelli V, Baiardi P, Bagnardi V, Etheridge SP, Napolitano C, Priori SG. Gene-specific therapy with mexiletine reduces arrhythmic events in patients with long QT syndrome type 3. J Am Coll Cardiol 67: 1053–1058, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.McDonald TV, Yu Z, Ming Z, Palma E, Meyers MB, Wang KW, Goldstein SA, Fishman GI. A minK-HERG complex regulates the cardiac potassium current I(Kr). Nature 388: 289–292, 1997. [DOI] [PubMed] [Google Scholar]
- 260.McLaughlin S, Wang J, Gambhir A, Murray D. PIP2 and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct 31: 151–175, 2002. [DOI] [PubMed] [Google Scholar]
- 261.Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, Valdivia C, Ueda K, Canizales-Quinteros S, Tusie-Luna MT, Makielski JC, Ackerman MJ. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation 116: 134–142, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Melman YF, Um SY, Krumerman A, Kagan A, McDonald TV. KCNE1 binds to the KCNQ1 pore to regulate potassium channel activity. Neuron 42: 927–937, 2004. [DOI] [PubMed] [Google Scholar]
- 263.Meng J, Shi C, Li L, Du Y, Xu Y. Compound ICA-105574 prevents arrhythmias induced by cardiac delayed repolarization. Eur J Pharmacol 718: 87–97, 2013. [DOI] [PubMed] [Google Scholar]
- 264.Miake J, Marban E, Nuss HB. Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant-negative suppression. J Clin Invest 111: 1529–1536, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Migdalovich D, Moss AJ, Lopes CM, Costa J, Ouellet G, Barsheshet A, McNitt S, Polonsky S, Robinson JL, Zareba W, Ackerman MJ, Benhorin J, Kaufman ES, Platonov PG, Shimizu W, Towbin JA, Vincent GM, Wilde AA, Goldenberg I. Mutation and gender specific risk in type-2 long QT syndrome: implications for risk stratification for life-threatening cardiac events in patients with long QT syndrome. Heart Rhythm 8: 1537–1543, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA 97: 12329–12333, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mitcheson JS, Chen J, Sanguinetti MC. Trapping of a methanesulfonanilide by closure of the HERG potassium channel activation gate. J Gen Physiol 115: 229–240, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Miyamoto K, Nakagawa T, Kuroda Y. Solution structures of the cytoplasmic linkers between segments S4 and S5 (S4–S5) in domains III and IV of human brain sodium channels in SDS micelles. J Pept Res 58: 193–203, 2001. [DOI] [PubMed] [Google Scholar]
- 269.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol 3: e423, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P, Bennett V. Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J Biol Chem 279: 12980–12987, 2004. [DOI] [PubMed] [Google Scholar]
- 271.Mohler PJ, Le Scouarnec S, Denjoy I, Lowe JS, Guicheney P, Caron L, Driskell IM, Schott JJ, Norris K, Leenhardt A, Kim RB, Escande D, Roden DM. Defining the cellular phenotype of “ankyrin-B syndrome” variants: human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation 115: 432–441, 2007. [DOI] [PubMed] [Google Scholar]
- 272.Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, Bennett V. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci USA 101: 17533–17538, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421: 634–639, 2003. [DOI] [PubMed] [Google Scholar]
- 274.Morais Cabral JH, Lee A, Cohen SL, Chait BT, Li M, Mackinnon R. Crystal structure and functional analysis of the HERG potassium channel N terminus: a eukaryotic PAS domain. Cell 95: 649–655, 1998. [DOI] [PubMed] [Google Scholar]
- 275.Moreno JD, Yang PC, Bankston JR, Grandi E, Bers DM, Kass RS, Clancy CE. Ranolazine for congenital and acquired late INa-linked arrhythmias: in silico pharmacological screening. Circ Res 113: e50–61, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Moreno JD, Zhu ZI, Yang PC, Bankston JR, Jeng MT, Kang C, Wang L, Bayer JD, Christini DJ, Trayanova NA, Ripplinger CM, Kass RS, Clancy CE. A computational model to predict the effects of class I anti-arrhythmic drugs on ventricular rhythms. Sci Transl Med 3: 98ra83, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, Dorn T, Goedel A, Hohnke C, Hofmann F, Seyfarth M, Sinnecker D, Schomig A, Laugwitz KL. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med 363: 1397–1409, 2010. [DOI] [PubMed] [Google Scholar]
- 278.Morin TJ, Kobertz WR. Counting membrane-embedded KCNE beta-subunits in functioning K+ channel complexes. Proc Natl Acad Sci USA 105: 1478–1482, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Moss AJ, Robinson JL, Gessman L, Gillespie R, Zareba W, Schwartz PJ, Vincent GM, Benhorin J, Heilbron EL, Towbin JA, Priori SG, Napolitano C, Zhang L, Medina A, Andrews ML, Timothy K. Comparison of clinical and genetic variables of cardiac events associated with loud noise versus swimming among subjects with the long QT syndrome. Am J Cardiol 84: 876–879, 1999. [DOI] [PubMed] [Google Scholar]
- 280.Moss AJ, Shimizu W, Wilde AA, Towbin JA, Zareba W, Robinson JL, Qi M, Vincent GM, Ackerman MJ, Kaufman ES, Hofman N, Seth R, Kamakura S, Miyamoto Y, Goldenberg I, Andrews ML, McNitt S. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation 115: 2481–2489, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Moss AJ, Windle JR, Hall WJ, Zareba W, Robinson JL, McNitt S, Severski P, Rosero S, Daubert JP, Qi M, Cieciorka M, Manalan AS. Safety and efficacy of flecainide in subjects with Long QT-3 syndrome (DeltaKPQ mutation): a randomized, double-blind, placebo-controlled clinical trial. Ann Noninvasive Electrocardiol 10: 59–66, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, Vincent GM, Locati EH, Priori SG, Napolitano C, Medina A, Zhang L, Robinson JL, Timothy K, Towbin JA, Andrews ML. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation 101: 616–623, 2000. [DOI] [PubMed] [Google Scholar]
- 283.Moss AJ, Zareba W, Kaufman ES, Gartman E, Peterson DR, Benhorin J, Towbin JA, Keating MT, Priori SG, Schwartz PJ, Vincent GM, Robinson JL, Andrews ML, Feng C, Hall WJ, Medina A, Zhang L, Wang Z. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation 105: 794–799, 2002. [DOI] [PubMed] [Google Scholar]
- 284.Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol 19: 1289–1293, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Motoike HK, Liu H, Glaaser IW, Yang AS, Tateyama M, Kass RS. The Na+ channel inactivation gate is a molecular complex: a novel role of the COOH-terminal domain. J Gen Physiol 123: 155–165, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Murray A, Donger C, Fenske C, Spillman I, Richard P, Dong YB, Neyroud N, Chevalier P, Denjoy I, Carter N, Syrris P, Afzal AR, Patton MA, Guicheney P, Jeffery S. Splicing mutations in KCNQ1: a mutation hot spot at codon 344 that produces in frame transcripts. Circulation 100: 1077–1084, 1999. [DOI] [PubMed] [Google Scholar]
- 287.Muskett FW, Thouta S, Thomson SJ, Bowen A, Stansfeld PJ, Mitcheson JS. Mechanistic insight into human ether-a-go-go-related gene (hERG) K+ channel deactivation gating from the solution structure of the EAG domain. J Biol Chem 286: 6184–6191, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Nagatomo T, January CT, Makielski JC. Preferential block of late sodium current in the LQT3 DeltaKPQ mutant by the class I(C) antiarrhythmic flecainide. Mol Pharmacol 57: 101–107, 2000. [PubMed] [Google Scholar]
- 289.Nagatomo T, January CT, Ye B, Abe H, Nakashima Y, Makielski JC. Rate-dependent QT shortening mechanism for the LQT3 deltaKPQ mutant. Cardiovasc Res 54: 624–629, 2002. [DOI] [PubMed] [Google Scholar]
- 290.Nakajima T, Furukawa T, Tanaka T, Katayama Y, Nagai R, Nakamura Y, Hiraoka M. Novel mechanism of HERG current suppression in LQT2: shift in voltage dependence of HERG inactivation. Circ Res 83: 415–422, 1998. [DOI] [PubMed] [Google Scholar]
- 291.Nakajima T, Hayashi K, Viswanathan PC, Kim MY, Anghelescu M, Barksdale KA, Shuai W, Balser JR, Kupershmidt S. HERG is protected from pharmacological block by alpha-1,2-glucosyltransferase function. J Biol Chem 282: 5506–5513, 2007. [DOI] [PubMed] [Google Scholar]
- 292.Nakajo K, Kubo Y. Steric hindrance between S4 and S5 of the KCNQ1/KCNE1 channel hampers pore opening. Nat Commun 5: 4100, 2014. [DOI] [PubMed] [Google Scholar]
- 293.Nakajo K, Ulbrich MH, Kubo Y, Isacoff EY. Stoichiometry of the KCNQ1-KCNE1 ion channel complex. Proc Natl Acad Sci USA 107: 18862–18867, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nakamura H, Kurokawa J, Bai CX, Asada K, Xu J, Oren RV, Zhu ZI, Clancy CE, Isobe M, Furukawa T. Progesterone regulates cardiac repolarization through a nongenomic pathway: an in vitro patch-clamp and computational modeling study. Circulation 116: 2913–2922, 2007. [DOI] [PubMed] [Google Scholar]
- 295.Napolitano C, Priori SG, Schwartz PJ, Bloise R, Ronchetti E, Nastoli J, Bottelli G, Cerrone M, Leonardi S. Genetic testing in the long QT syndrome: development and validation of an efficient approach to genotyping in clinical practice. JAMA 294: 2975–2980, 2005. [DOI] [PubMed] [Google Scholar]
- 296.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol 1: 62–73, 2008. [DOI] [PubMed] [Google Scholar]
- 297.Nawathe PA, Kryukova Y, Oren RV, Milanesi R, Clancy CE, Lu JT, Moss AJ, Difrancesco D, Robinson RB. An LQTS6 MiRP1 mutation suppresses pacemaker current and is associated with sinus bradycardia. J Cardiovasc Electrophysiol 24: 1021–1027, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Nemec J, Buncova M, Shusterman V, Winter B, Shen WK, Ackerman MJ. QT interval variability and adaptation to heart rate changes in patients with long QT syndrome. Pacing Clin Electrophysiol 32: 72–81, 2009. [DOI] [PubMed] [Google Scholar]
- 299.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev 85: 1205–1253, 2005. [DOI] [PubMed] [Google Scholar]
- 300.Newton-Cheh C, Eijgelsheim M, Rice KM, de Bakker PI, Yin X, Estrada K, Bis JC, Marciante K, Rivadeneira F, Noseworthy PA, Sotoodehnia N, Smith NL, Rotter JI, Kors JA, Witteman JC, Hofman A, Heckbert SR, O'Donnell CJ, Uitterlinden AG, Psaty BM, Lumley T, Larson MG, Stricker BH. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat Genet 41: 399–406, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Faure S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet 15: 186–189, 1997. [DOI] [PubMed] [Google Scholar]
- 302.Ng CA, Hunter MJ, Perry MD, Mobli M, Ke Y, Kuchel PW, King GF, Stock D, Vandenberg JI. The N-terminal tail of hERG contains an amphipathic alpha-helix that regulates channel deactivation. PLoS One 6: e16191, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Nichols CG, Makhina EN, Pearson WL, Sha Q, Lopatin AN. Inward rectification and implications for cardiac excitability. Circ Res 78: 1–7, 1996. [DOI] [PubMed] [Google Scholar]
- 304.Noble D. The Initiation of the Heartbeat. Oxford, UK: Clarendon, 1978. [Google Scholar]
- 305.Noble D, Tsien RW. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol 200: 205–231, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Nogami A, Sugiyasu A, Kubota S, Kato K. Mapping and ablation of idiopathic ventricular fibrillation from the Purkinje system. Heart Rhythm 2: 646–649, 2005. [DOI] [PubMed] [Google Scholar]
- 307.Odening KE, Choi BR, Liu GX, Hartmann K, Ziv O, Chaves L, Schofield L, Centracchio J, Zehender M, Peng X, Brunner M, Koren G. Estradiol promotes sudden cardiac death in transgenic long QT type 2 rabbits while progesterone is protective. Heart Rhythm 9: 823–832, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Osteen JD, Barro-Soria R, Robey S, Sampson KJ, Kass RS, Larsson HP. Allosteric gating mechanism underlies the flexible gating of KCNQ1 potassium channels. Proc Natl Acad Sci USA 109: 7103–7108, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Osteen JD, Gonzalez C, Sampson KJ, Iyer V, Rebolledo S, Larsson HP, Kass RS. KCNE1 alters the voltage sensor movements necessary to open the KCNQ1 channel gate. Proc Natl Acad Sci USA 107: 22710–22715, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Panaghie G, Abbott GW. The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes. J Gen Physiol 129: 121–133, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Panaghie G, Tai KK, Abbott GW. Interaction of KCNE subunits with the KCNQ1 K+ channel pore. J Physiol 570: 455–467, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Park KH, Piron J, Dahimene S, Merot J, Baro I, Escande D, Loussouarn G. Impaired KCNQ1-KCNE1 and phosphatidylinositol-4,5-bisphosphate interaction underlies the long QT syndrome. Circ Res 96: 730–739, 2005. [DOI] [PubMed] [Google Scholar]
- 313.Patton DE, West JW, Catterall WA, Goldin AL. Amino acid residues required for fast Na(+)-channel inactivation: charge neutralizations and deletions in the III-IV linker. Proc Natl Acad Sci USA 89: 10905–10909, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Paulussen A, Raes A, Matthijs G, Snyders DJ, Cohen N, Aerssens J. A novel mutation (T65P) in the PAS domain of the human potassium channel HERG results in the long QT syndrome by trafficking deficiency. J Biol Chem 277: 48610–48616, 2002. [DOI] [PubMed] [Google Scholar]
- 315.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature 475: 353–358, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Pearson PJ, Lin PJ, Schaff HV. Production of endothelium-derived contracting factor is enhanced after coronary reperfusion. Ann Thoracic Surg 51: 788–793, 1991. [DOI] [PubMed] [Google Scholar]
- 317.Perez Riera AR, Ferreira C, Filho CF, Ferreira M, Meneghini A, Uchida AH, Schapachnik E, Dubner S, Zhang L. The enigmatic sixth wave of the electrocardiogram: the U wave. Cardiol J 15: 408–421, 2008. [PubMed] [Google Scholar]
- 318.Perissinotti LL, Guo J, De Biase PM, Clancy CE, Duff HJ, Noskov SY. Kinetic model for NS1643 drug activation of WT and L529I variants of Kv11.1 (hERG1) potassium channel. Biophys J 108: 1414–1424, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Perry M, de Groot MJ, Helliwell R, Leishman D, Tristani-Firouzi M, Sanguinetti MC, Mitcheson J. Structural determinants of HERG channel block by clofilium and ibutilide. Mol Pharmacol 66: 240–249, 2004. [DOI] [PubMed] [Google Scholar]
- 320.Perry M, Sachse FB, Abbruzzese J, Sanguinetti MC. PD-118057 contacts the pore helix of hERG1 channels to attenuate inactivation and enhance K+ conductance. Proc Natl Acad Sci USA 106: 20075–20080, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Perry M, Sachse FB, Sanguinetti MC. Structural basis of action for a human ether-a-go-go-related gene 1 potassium channel activator. Proc Natl Acad Sci USA 104: 13827–13832, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Petersen CI, McFarland TR, Stepanovic SZ, Yang P, Reiner DJ, Hayashi K, George AL, Roden DM, Thomas JH, Balser JR. In vivo identification of genes that modify ether-a-go-go-related gene activity in Caenorhabditis elegans may also affect human cardiac arrhythmia. Proc Natl Acad Sci USA 101: 11773–11778, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Phartiyal P, Jones EM, Robertson GA. Heteromeric assembly of human ether-a-go-go-related gene (hERG) 1a/1b channels occurs cotranslationally via N-terminal interactions. J Biol Chem 282: 9874–9882, 2007. [DOI] [PubMed] [Google Scholar]
- 324.Pignier C, Rougier JS, Vie B, Culie C, Verscheure Y, Vacher B, Abriel H, Le Grand B. Selective inhibition of persistent sodium current by F 15845 prevents ischaemia-induced arrhythmias. Br J Pharmacol 161: 79–91, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Piper DR, Sanguinetti MC, Tristani-Firouzi M. Voltage sensor movement in the hERG K+ channel. Novartis Found Symp 266: 46–52, 2005. [DOI] [PubMed] [Google Scholar]
- 326.Plant LD, Xiong D, Dai H, Goldstein SA. Individual IKs channels at the surface of mammalian cells contain two KCNE1 accessory subunits. Proc Natl Acad Sci USA 111: E1438–1446, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir21 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell 105: 511–519, 2001. [DOI] [PubMed] [Google Scholar]
- 328.Postema PG, Ritsema van Eck HJ, Opthof T, van Herpen G, van Dessel PF, Priori SG, Wolpert C, Borggrefe M, Kors JA, Wilde AA. IK1 modulates the U-wave: insights in a 100-year-old enigma. Heart Rhythm 6: 393–400, 2009. [DOI] [PubMed] [Google Scholar]
- 329.Poulsen KL, Hotait M, Calloe K, Klaerke DA, Rebeiz A, Nemer G, Tejada MA, Refaat MM. The mutation PT613a in the pore helix of the Kv 11.1 potassium channel is associated with long QT syndrome. Pacing Clin Electrophysiol 38: 1304–1309, 2015. [DOI] [PubMed] [Google Scholar]
- 330.Pourrier M, Zicha S, Ehrlich J, Han W, Nattel S. Canine ventricular KCNE2 expression resides predominantly in Purkinje fibers. Circ Res 93: 189–191, 2003. [DOI] [PubMed] [Google Scholar]
- 331.Priori SG. From trials to guidelines to clinical practice: the need for improvement. Europace 6: 176–178, 2004. [DOI] [PubMed] [Google Scholar]
- 332.Priori SG, Cantu F, Schwartz PJ. the long QT syndrome–new diagnostic and therapeutic approach in the era of molecular biology. Schweiz Med Wochenschr 126: 1727–1731, 1996. [PubMed] [Google Scholar]
- 333.Priori SG, Napolitano C, Cantu F, Brown AM, Schwartz PJ. Differential response to Na+ channel blockade, beta-adrenergic stimulation and rapid pacing in a cellular model mimicking the SCN5A and HERG defects present in the long-QT syndrome. Circ Res 78: 1009–1015, 1996. [DOI] [PubMed] [Google Scholar]
- 334.Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, Moncalvo C, Tulipani C, Veia A, Bottelli G, Nastoli J. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA 292: 1341–1344, 2004. [DOI] [PubMed] [Google Scholar]
- 335.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 10: 1932–1963, 2013. [DOI] [PubMed] [Google Scholar]
- 336.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 265: 1724–1728, 1994. [DOI] [PubMed] [Google Scholar]
- 337.Rajamani S, Eckhardt LL, Valdivia CR, Klemens CA, Gillman BM, Anderson CL, Holzem KM, Delisle BP, Anson BD, Makielski JC, January CT. Drug-induced long QT syndrome: hERG K+ channel block and disruption of protein trafficking by fluoxetine and norfluoxetine. Br J Pharmacol 149: 481–489, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Ramirez AH, Schildcrout JS, Blakemore DL, Masys DR, Pulley JM, Basford MA, Roden DM, Denny JC. Modulators of normal electrocardiographic intervals identified in a large electronic medical record. Heart Rhythm 8: 271–277, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Refaat MM, Hotait M, Tseng ZH. Utility of the exercise electrocardiogram testing in sudden cardiac death risk stratification. Ann Noninvasive Electrocardiol 19: 311–318, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Restier L, Cheng L, Sanguinetti MC. Mechanisms by which atrial fibrillation-associated mutations in the S1 domain of KCNQ1 slow deactivation of IKs channels. J Physiol 586: 4179–4191, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature 370: 143–146, 1994. [DOI] [PubMed] [Google Scholar]
- 342.Riuro H, Campuzano O, Arbelo E, Iglesias A, Batlle M, Perez-Villa F, Brugada J, Perez GJ, Scornik FS, Brugada R. A missense mutation in the sodium channel beta1b subunit reveals SCN1B as a susceptibility gene underlying long QT syndrome. Heart Rhythm 11: 1202–1209, 2014. [DOI] [PubMed] [Google Scholar]
- 343.Rivolta I, Abriel H, Tateyama M, Liu H, Memmi M, Vardas P, Napolitano C, Priori SG, Kass RS. Inherited Brugada and long QT-3 syndrome mutations of a single residue of the cardiac sodium channel confer distinct channel and clinical phenotypes. J Biol Chem 276: 30623–30630, 2001. [DOI] [PubMed] [Google Scholar]
- 344.Rivolta I, Clancy CE, Tateyama M, Liu H, Priori SG, Kass RS. A novel SCN5A mutation associated with long QT-3: altered inactivation kinetics and channel dysfunction. Physiol Genomics 10: 191–197, 2002. [DOI] [PubMed] [Google Scholar]
- 345.Robertson GA, January CT. HERG trafficking and pharmacological rescue of LQTS-2 mutant channels. Handb Exp Pharmacol 349–355, 2006. [DOI] [PubMed] [Google Scholar]
- 346.Romano C, Gemme G, Pongiglione R. Rare cardiac arrhythmias of the pediatric age. I. Repetitive paroxysmal tachycardia. Minerva Pediatr 15: 1155–1164, 1963. [PubMed] [Google Scholar]
- 347.Ruan Y, Liu N, Bloise R, Napolitano C, Priori SG. Gating properties of SCN5A mutations and the response to mexiletine in long-QT syndrome type 3 patients. Circulation 116: 1137–1144, 2007. [DOI] [PubMed] [Google Scholar]
- 348.Ruscic KJ, Miceli F, Villalba-Galea CA, Dai H, Mishina Y, Bezanilla F, Goldstein SA. IKs channels open slowly because KCNE1 accessory subunits slow the movement of S4 voltage sensors in KCNQ1 pore-forming subunits. Proc Natl Acad Sci USA 110: E559–566, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ruwald MH, Xu Parks X, Moss AJ, Zareba W, Baman J, McNitt S, Kanters JK, Shimizu W, Wilde AA, Jons C, Lopes CM. Stop-codon and C-terminal nonsense mutations are associated with a lower risk of cardiac events in patients with long QT syndrome type 1. Heart Rhythm 13: 122–131, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Ryan JJ, Kalscheur M, Dellefave L, McNally E, Archer SL. A KCNE1 missense variant (V47I) causing exercise-induced long QT syndrome (Romano Ward). Int J Cardiol 156: e33–35, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sakagami M, Fukazawa K, Matsunaga T, Fujita H, Mori N, Takumi T, Ohkubo H, Nakanishi S. Cellular localization of rat Isk protein in the stria vascularis by immunohistochemical observation. Hear Res 56: 168–172, 1991. [DOI] [PubMed] [Google Scholar]
- 352.Salata JJ, Jurkiewicz NK, Jow B, Folander K, Guinosso PJ Jr, Raynor B, Swanson R, Fermini B. IK of rabbit ventricle is composed of two currents: evidence for IKs. Am J Physiol Heart Circ Physiol 271: H2477–H2489, 1996. [DOI] [PubMed] [Google Scholar]
- 353.Sale H, Wang J, O'Hara TJ, Tester DJ, Phartiyal P, He JQ, Rudy Y, Ackerman MJ, Robertson GA. Physiological properties of hERG 1a/1b heteromeric currents and a hERG 1b-specific mutation associated with Long-QT syndrome. Circ Res 103: e81–95, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Sampson KJ, Kass RS. Molecular mechanisms of adrenergic stimulation in the heart. Heart Rhythm 7: 1151–1153, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sanguinetti MC. Dysfunction of delayed rectifier potassium channels in an inherited cardiac arrhythmia. Ann NY Acad Sci 868: 406–413, 1999. [DOI] [PubMed] [Google Scholar]
- 356.Sanguinetti MC. HERG1 channelopathies. Pflügers Arch 460: 265–276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Sanguinetti MC, Curran ME, Spector PS, Keating MT. Spectrum of HERG K+-channel dysfunction in an inherited cardiac arrhythmia. Proc Natl Acad Sci USA 93: 2208–2212, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384: 80–83, 1996. [DOI] [PubMed] [Google Scholar]
- 359.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81: 299–307, 1995. [DOI] [PubMed] [Google Scholar]
- 360.Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol 96: 195–215, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature 440: 463–469, 2006. [DOI] [PubMed] [Google Scholar]
- 362.Sansone V, Griggs RC, Meola G, Ptacek LJ, Barohn R, Iannaccone S, Bryan W, Baker N, Janas SJ, Scott W, Ririe D, Tawil R. Andersen's syndrome: a distinct periodic paralysis. Ann Neurol 42: 305–312, 1997. [DOI] [PubMed] [Google Scholar]
- 363.Sasano T, Ueda K, Orikabe M, Hirano Y, Kawano S, Yasunami M, Isobe M, Kimura A, Hiraoka M. Novel C-terminus frameshift mutation, 1122fs/147, of HERG in LQT2: additional amino acids generated by frameshift cause accelerated inactivation. J Mol Cell Cardiol 37: 1205–1211, 2004. [DOI] [PubMed] [Google Scholar]
- 364.Schmitt N, Schwarz M, Peretz A, Abitbol I, Attali B, Pongs O. A recessive C-terminal Jervell and Lange-Nielsen mutation of the KCNQ1 channel impairs subunit assembly. EMBO J 19: 332–340, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Schonherr R, Heinemann SH. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J Physiol 493: 635–642, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Schott JJ, Charpentier F, Peltier S, Foley P, Drouin E, Bouhour JB, Donnelly P, Vergnaud G, Bachner L, Moisan JP, et al. Mapping of a gene for long QT syndrome to chromosome 4q25-27. Am J Hum Genet 57: 1114–1122, 1995. [PMC free article] [PubMed] [Google Scholar]
- 367.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 403: 196–199, 2000. [DOI] [PubMed] [Google Scholar]
- 368.Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation 88: 782–784, 1993. [DOI] [PubMed] [Google Scholar]
- 369.Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, Bloise R, De Ferrari GM, Klersy C, Moss AJ, Zareba W, Robinson JL, Hall WJ, Brink PA, Toivonen L, Epstein AE, Li C, Hu D. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation 109: 1826–1833, 2004. [DOI] [PubMed] [Google Scholar]
- 370.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantu F, Towbin JA, Keating MT, Hammoude H, Brown AM, Chen LS. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation 92: 3381–3386, 1995. [DOI] [PubMed] [Google Scholar]
- 371.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 103: 89–95, 2001. [DOI] [PubMed] [Google Scholar]
- 372.Schwartz PJ, Spazzolini C, Priori SG, Crotti L, Vicentini A, Landolina M, Gasparini M, Wilde AA, Knops RE, Denjoy I, Toivonen L, Monnig G, Al-Fayyadh M, Jordaens L, Borggrefe M, Holmgren C, Brugada P, De Roy L, Hohnloser SH, Brink PA. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: data from the European Long-QT Syndrome Implantable Cardioverter-Defibrillator (LQTS ICD) Registry. Circulation 122: 1272–1282, 2010. [DOI] [PubMed] [Google Scholar]
- 373.Schwartz PJ, Vanoli E, Crotti L, Spazzolini C, Ferrandi C, Goosen A, Hedley P, Heradien M, Bacchini S, Turco A, La Rovere MT, Bartoli A, George AL Jr, Brink PA. Neural control of heart rate is an arrhythmia risk modifier in long QT syndrome. J Am Coll Cardiol 51: 920–929, 2008. [DOI] [PubMed] [Google Scholar]
- 374.Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, Baltaev R, Mack AF, Korniychuk G, Choudhury A, Marks D, Pagano RE, Attali B, Pfeufer A, Kass RS, Sanguinetti MC, Tavare JM, Lang F. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res 100: 686–692, 2007. [DOI] [PubMed] [Google Scholar]
- 375.Seebohm G, Strutz-Seebohm N, Ureche ON, Henrion U, Baltaev R, Mack AF, Korniychuk G, Steinke K, Tapken D, Pfeufer A, Kaab S, Bucci C, Attali B, Merot J, Tavare JM, Hoppe UC, Sanguinetti MC, Lang F. Long QT syndrome-associated mutations in KCNQ1 and KCNE1 subunits disrupt normal endosomal recycling of IKs channels. Circ Res 103: 1451–1457, 2008. [DOI] [PubMed] [Google Scholar]
- 376.Sesti F, Abbott GW, Wei J, Murray KT, Saksena S, Schwartz PJ, Priori SG, Roden DM, George AL Jr, Goldstein SA. A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc Natl Acad Sci USA 97: 10613–10618, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Seth R, Moss AJ, McNitt S, Zareba W, Andrews ML, Qi M, Robinson JL, Goldenberg I, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Schwartz PJ, Towbin JA, Vincent GM, Zhang L. Long QT syndrome and pregnancy. J Am Coll Cardiol 49: 1092–1098, 2007. [DOI] [PubMed] [Google Scholar]
- 378.Shamgar L, Haitin Y, Yisharel I, Malka E, Schottelndreier H, Peretz A, Paas Y, Attali B. KCNE1 constrains the voltage sensor of Kv7.1 K+ channels. PLoS One 3: e1943, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Shamgar L, Ma L, Schmitt N, Haitin Y, Peretz A, Wiener R, Hirsch J, Pongs O, Attali B. Calmodulin Is Essential for Cardiac IKS Channel Gating and Assembly. Impaired Function in Long-QT Mutations. Circ Res 98: 1055–1063, 2006. [DOI] [PubMed] [Google Scholar]
- 380.Sheets MF, Chen T, Hanck DA. Outward stabilization of the voltage sensor in domain II but not domain I speeds inactivation of voltage-gated sodium channels. Am J Physiol Heart Circ Physiol 305: H1213–H1221, 2013. [DOI] [PubMed] [Google Scholar]
- 381.Sheets MF, Hanck DA. Charge immobilization of the voltage sensor in domain IV is independent of sodium current inactivation. J Physiol 563: 83–93, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Sheets MF, Hanck DA. Molecular action of lidocaine on the voltage sensors of sodium channels. J Gen Physiol 121: 163–175, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Sheets MF, Hanck DA. The outermost lysine in the S4 of domain III contributes little to the gating charge in sodium channels. Biophys J 82: 3048–3055, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Sheets MF, Kyle JW, Kallen RG, Hanck DA. The Na channel voltage sensor associated with inactivation is localized to the external charged residues of domain IV, S4. Biophys J 77: 747–757, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Shi W, Wymore RS, Wang HS, Pan Z, Cohen IS, McKinnon D, Dixon JE. Identification of two nervous system-specific members of the erg potassium channel gene family. J Neurosci 17: 9423–9432, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long-QT syndrome: effects of beta-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation 98: 2314–2322, 1998. [DOI] [PubMed] [Google Scholar]
- 387.Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol 35: 778–786, 2000. [DOI] [PubMed] [Google Scholar]
- 388.Shimizu W, Antzelevitch C. Effects of a K+ channel opener to reduce transmural dispersion of repolarization and prevent torsade de pointes in LQT1, LQT2, and LQT3 models of the long-QT syndrome. Circulation 102: 706–712, 2000. [DOI] [PubMed] [Google Scholar]
- 389.Shimizu W, Moss AJ, Wilde AA, Towbin JA, Ackerman MJ, January CT, Tester DJ, Zareba W, Robinson JL, Qi M, Vincent GM, Kaufman ES, Hofman N, Noda T, Kamakura S, Miyamoto Y, Shah S, Amin V, Goldenberg I, Andrews ML, McNitt S. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol 54: 2052–2062, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Shushi L, Kerem B, Goldmit M, Peretz A, Attali B, Medina A, Towbin JA, Kurokawa J, Kass RS, Benhorin J. Clinical, genetic, and electrophysiologic characteristics of a new PAS-domain HERG mutation (M124R) causing Long QT syndrome. Ann Noninvasive Electrocardiol 10: 334–341, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Siebrands CC, Binder S, Eckhoff U, Schmitt N, Friederich P. Long QT 1 mutation KCNQ1A344V increases local anesthetic sensitivity of the slowly activating delayed rectifier potassium current. Anesthesiology 105: 511–520, 2006. [DOI] [PubMed] [Google Scholar]
- 392.Silver ES, Liberman L, Chung WK, Spotnitz HM, Chen JM, Ackerman MJ, Moir C, Hordof AJ, Pass RH. Long QT syndrome due to a novel mutation in SCN5A: treatment with ICD placement at 1 month and left cardiac sympathetic denervation at 3 months of age. J Interv Card Electrophysiol 26: 41–45, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Smith JA, Vanoye CG, George AL Jr, Meiler J, Sanders CR. Structural models for the KCNQ1 voltage-gated potassium channel. Biochemistry 46: 14141–14152, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature 379: 833–836, 1996. [DOI] [PubMed] [Google Scholar]
- 395.Smits JP, Veldkamp MW, Bezzina CR, Bhuiyan ZA, Wedekind H, Schulze-Bahr E, Wilde AA. Substitution of a conserved alanine in the domain IIIS4–S5 linker of the cardiac sodium channel causes long QT syndrome. Cardiovasc Res 67: 459–466, 2005. [DOI] [PubMed] [Google Scholar]
- 396.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem 271: 15160–15165, 1996. [DOI] [PubMed] [Google Scholar]
- 397.Song W, Xiao Y, Chen H, Ashpole NM, Piekarz AD, Ma P, Hudmon A, Cummins TR, Shou W. The human Nav1.5 F1486 deletion associated with long QT syndrome leads to impaired sodium channel inactivation and reduced lidocaine sensitivity. J Physiol 590: 5123–5139, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Soom M, Schonherr R, Kubo Y, Kirsch C, Klinger R, Heinemann SH. Multiple PIP2 binding sites in Kir2.1 inwardly rectifying potassium channels. FEBS Lett 490: 49–53, 2001. [DOI] [PubMed] [Google Scholar]
- 399.Sorensen AB, Sondergaard MT, Overgaard MT. Calmodulin in a heartbeat. FEBS J 280: 5511–5532, 2013. [DOI] [PubMed] [Google Scholar]
- 400.Spector PS, Curran ME, Keating MT, Sanguinetti MC. Class III antiarrhythmic drugs block HERG, a human cardiac delayed rectifier K+ channel. Open-channel block by methanesulfonanilides. Circ Res 78: 499–503, 1996. [DOI] [PubMed] [Google Scholar]
- 401.Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol 107: 611–619, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 102: 1178–1185, 2000. [DOI] [PubMed] [Google Scholar]
- 403.Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci USA 102: 8089–8096, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119: 19–31, 2004. [DOI] [PubMed] [Google Scholar]
- 405.Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet 17: 338–340, 1997. [DOI] [PubMed] [Google Scholar]
- 406.Stengl M, Ramakers C, Donker DW, Nabar A, Rybin AV, Spatjens RL, van der Nagel T, Wodzig WK, Sipido KR, Antoons G, Moorman AF, Vos MA, Volders PG. Temporal patterns of electrical remodeling in canine ventricular hypertrophy: focus on IKs downregulation and blunted beta-adrenergic activation. Cardiovasc Res 72: 90–100, 2006. [DOI] [PubMed] [Google Scholar]
- 407.Su Z, Limberis J, Souers A, Kym P, Mikhail A, Houseman K, Diaz G, Liu X, Martin RL, Cox BF, Gintant GA. Electrophysiologic characterization of a novel hERG channel activator. Biochem Pharmacol 77: 1383–1390, 2009. [DOI] [PubMed] [Google Scholar]
- 408.Subbiah RN, Clarke CE, Smith DJ, Zhao J, Campbell TJ, Vandenberg JI. Molecular basis of slow activation of the human ether-a-go-go related gene potassium channel. J Physiol 558: 417–431, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Sun Y, Zhang P, Li X, Zhang H, Li J, Liu G, Guo J. A novel nonsense mutation Y652X in the S6/pore region of human ether-go-go gene found in a long QT syndrome family. Scand Cardiovasc J 43: 181–186, 2009. [DOI] [PubMed] [Google Scholar]
- 410.Takano M, Kuratomi S. Regulation of cardiac inwardly rectifying potassium channels by membrane lipid metabolism. Prog Biophys Mol Biol 81: 67–79, 2003. [DOI] [PubMed] [Google Scholar]
- 411.Takemasa H, Nagatomo T, Abe H, Kawakami K, Igarashi T, Tsurugi T, Kabashima N, Tamura M, Okazaki M, Delisle BP, January CT, Otsuji Y. Coexistence of hERG current block and disruption of protein trafficking in ketoconazole-induced long QT syndrome. Br J Pharmacol 153: 439–447, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science 242: 1042–1045, 1988. [DOI] [PubMed] [Google Scholar]
- 413.Tan BH, Pundi KN, Van Norstrand DW, Valdivia CR, Tester DJ, Medeiros-Domingo A, Makielski JC, Ackerman MJ. Sudden infant death syndrome-associated mutations in the sodium channel beta subunits. Heart Rhythm 7: 771–778, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Tan VH, Yap J, Hsu LF, Liew R. Catheter ablation of ventricular fibrillation triggers and electrical storm. Europace 14: 1687–1695, 2012. [DOI] [PubMed] [Google Scholar]
- 415.Tang L, Kallen RG, Horn R. Role of an S4–S5 linker in sodium channel inactivation probed by mutagenesis and a peptide blocker. J Gen Physiol 108: 89–104, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Tateyama M, Rivolta I, Clancy CE, Kass RS. Modulation of cardiac sodium channel gating by protein kinase A can be altered by disease-linked mutation. J Biol Chem 278: 46718–46726, 2003. [DOI] [PubMed] [Google Scholar]
- 417.Tawil R, Ptacek LJ, Pavlakis SG, DeVivo DC, Penn AS, Ozdemir C, Griggs RC. Andersen's syndrome: potassium-sensitive periodic paralysis, ventricular ectopy, and dysmorphic features. Ann Neurol 35: 326–330, 1994. [DOI] [PubMed] [Google Scholar]
- 418.Telezhkin V, Reilly JM, Thomas AM, Tinker A, Brown DA. Structural requirements of membrane phospholipids for M-type potassium channel activation and binding. J Biol Chem 287: 10001–10012, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Terrenoire C, Houslay MD, Baillie GS, Kass RS. The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J Biol Chem 284: 9140–9146, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Terrenoire C, Wang K, Tung KW, Chung WK, Pass RH, Lu JT, Jean JC, Omari A, Sampson KJ, Kotton DN, Keller G, Kass RS. Induced pluripotent stem cells used to reveal drug actions in a long QT syndrome family with complex genetics. J Gen Physiol 141: 61–72, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Tester DJ, Ackerman MJ. Genetic testing for potentially lethal, highly treatable inherited cardiomyopathies/channelopathies in clinical practice. Circulation 123: 1021–1037, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Tinel N, Diochot S, Borsotto M, Lazdunski M, Barhanin J. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J 19: 6326–6330, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Tranebjaerg L, Samson RA, Green GE. Jervell and Lange-Nielsen Syndrome. In: Gene Reviews, edited by Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K. Seattle, WA:Univ. of Washington, 1993. [PubMed] [Google Scholar]
- 424.Tristani-Firouzi M, Etheridge SP. Kir 2.1 channelopathies: the Andersen-Tawil syndrome. Pflügers Arch 460: 289–294, 2010. [DOI] [PubMed] [Google Scholar]
- 425.Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome). J Clin Invest 110: 381–388, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Trudeau MC, Leung LM, Roti ER, Robertson GA. hERG1a N-terminal eag domain-containing polypeptides regulate homomeric hERG1b and heteromeric hERG1a/hERG1b channels: a possible mechanism for long QT syndrome. J Gen Physiol 138: 581–592, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269: 92–95, 1995. [DOI] [PubMed] [Google Scholar]
- 428.Tsuji K, Akao M, Ishii TM, Ohno S, Makiyama T, Takenaka K, Doi T, Haruna Y, Yoshida H, Nakashima T, Kita T, Horie M. Mechanistic basis for the pathogenesis of long QT syndrome associated with a common splicing mutation in KCNQ1 gene. J Mol Cell Cardiol 42: 662–669, 2007. [DOI] [PubMed] [Google Scholar]
- 429.Tuvia S, Buhusi M, Davis L, Reedy M, Bennett V. Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J Cell Biol 147: 995–1008, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Tyson J, Tranebjaerg L, Bellman S, Wren C, Taylor JF, Bathen J, Aslaksen B, Sorland SJ, Lund O, Malcolm S, Pembrey M, Bhattacharya S, Bitner-Glindzicz M. IsK and KvLQT1: mutation in either of the two subunits of the slow component of the delayed rectifier potassium channel can cause Jervell and Lange-Nielsen syndrome. Hum Mol Genet 6: 2179–2185, 1997. [DOI] [PubMed] [Google Scholar]
- 431.Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, Ackerman MJ, Makielski JC. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci USA 105: 9355–9360, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Undrovinas AI, Maltsev VA, Kyle JW, Silverman N, Sabbah HN. Gating of the late Na+ channel in normal and failing human myocardium. J Mol Cell Cardiol 34: 1477–1489, 2002. [DOI] [PubMed] [Google Scholar]
- 433.Vacher B, Pignier C, Letienne R, Verscheure Y, Le Grand B. F 15845 inhibits persistent sodium current in the heart and prevents angina in animal models. Br J Pharmacol 156: 214–225, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Valenzuela C, Bennett PB Jr. Gating of cardiac Na+ channels in excised membrane patches after modification by alpha-chymotrypsin. Biophys J 67: 161–171, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Van Petegem F, Lobo PA, Ahern CA. Seeing the forest through the trees: towards a unified view on physiological calcium regulation of voltage-gated sodium channels. Biophys J 103: 2243–2251, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev 92: 1393–1478, 2012. [DOI] [PubMed] [Google Scholar]
- 437.Varro A, Balati B, Iost N, Takacs J, Virag L, Lathrop DA, Csaba L, Talosi L, Papp JG. The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J Physiol 523: 67–81, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation 114: 2104–2112, 2006. [DOI] [PubMed] [Google Scholar]
- 439.Veerman CC, Verkerk AO, Blom MT, Klemens CA, Langendijk PN, van Ginneken AC, Wilders R, Tan HL. Slow delayed rectifier potassium current blockade contributes importantly to drug-induced long QT syndrome. Circ Arrhythm Electrophysiol 6: 1002–1009, 2013. [DOI] [PubMed] [Google Scholar]
- 440.Vincent GM, Schwartz PJ, Denjoy I, Swan H, Bithell C, Spazzolini C, Crotti L, Piippo K, Lupoglazoff JM, Villain E, Priori SG, Napolitano C, Zhang L. High efficacy of beta-blockers in long-QT syndrome type 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of beta-blocker treatment “failures”. Circulation 119: 215–221, 2009. [DOI] [PubMed] [Google Scholar]
- 441.Viswanathan PC, Rudy Y. Pause induced early afterdepolarizations in the long QT syndrome: a simulation study. Cardiovasc Res 42: 530–542, 1999. [DOI] [PubMed] [Google Scholar]
- 442.Volders PG, Sipido KR, Carmeliet E, Spatjens RL, Wellens HJ, Vos MA. Repolarizing K+ currents ITO1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation 99: 206–210, 1999. [DOI] [PubMed] [Google Scholar]
- 443.Walker VE, Wong MJ, Atanasiu R, Hantouche C, Young JC, Shrier A. Hsp40 chaperones promote degradation of the HERG potassium channel. J Biol Chem 285: 3319–3329, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science 242: 67–69, 1988. [DOI] [PubMed] [Google Scholar]
- 445.Wang D, Papp AC, Binkley PF, Johnson JA, Sadee W. Highly variable mRNA expression and splicing of L-type voltage-dependent calcium channel alpha subunit 1C in human heart tissues. Pharmacogenet Genomics 16: 735–745, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Wang DT, Hill AP, Mann SA, Tan PS, Vandenberg JI. Mapping the sequence of conformational changes underlying selectivity filter gating in the K(v)11.1 potassium channel. Nat Struct Mol Biol 18: 35–41, 2011. [DOI] [PubMed] [Google Scholar]
- 447.Wang DW, Crotti L, Shimizu W, Pedrazzini M, Cantu F, De Filippo P, Kishiki K, Miyazaki A, Ikeda T, Schwartz PJ, George AL Jr. Malignant perinatal variant of long-QT syndrome caused by a profoundly dysfunctional cardiac sodium channel. Circ Arrhythm Electrophysiol 1: 370–378, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Wang DW, Yazawa K, George AL Jr, Bennett PB. Characterization of human cardiac Na+ channel mutations in the congenital long QT syndrome. Proc Natl Acad Sci USA 93: 13200–13205, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Wang HW, Zheng YQ, Yang ZF, Li CZ, Liu YM. Effect of mexiletine on long QT syndrome model. Acta Pharmacol Sin 24: 316–320, 2003. [PubMed] [Google Scholar]
- 450.Wang J, Trudeau MC, Zappia AM, Robertson GA. Regulation of deactivation by an amino terminal domain in human ether-a-go-go-related gene potassium channels. J Gen Physiol 112: 637–647, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet 12: 17–23, 1996. [DOI] [PubMed] [Google Scholar]
- 452.Wang Q, Li Z, Shen J, Keating MT. Genomic organization of the human SCN5A gene encoding the cardiac sodium channel. Genomics 34: 9–16, 1996. [DOI] [PubMed] [Google Scholar]
- 453.Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell 80: 805–811, 1995. [DOI] [PubMed] [Google Scholar]
- 454.Wang S, Liu S, Morales MJ, Strauss HC, Rasmusson RL. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. J Physiol 502: 45–60, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Wang S, Morales MJ, Liu S, Strauss HC, Rasmusson RL. Time, voltage and ionic concentration dependence of rectification of h-erg expressed in Xenopus oocytes. FEBS Lett 389: 167–173, 1996. [DOI] [PubMed] [Google Scholar]
- 456.Wang W, Xia J, Kass RS. MinK-KvLQT1 fusion proteins, evidence for multiple stoichiometries of the assembled IsK channel. J Biol Chem 273: 34069–34074, 1998. [DOI] [PubMed] [Google Scholar]
- 457.Wang Z, Fermini B, Nattel S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovasc Res 28: 1540–1546, 1994. [DOI] [PubMed] [Google Scholar]
- 458.Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation 98: 2422–2428, 1998. [DOI] [PubMed] [Google Scholar]
- 459.Ward OC. A new familial cardiac syndrome in children. J Ir Med Assoc 54: 103–106, 1964. [PubMed] [Google Scholar]
- 460.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA 91: 3438–3442, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Wedekind H, Smits JP, Schulze-Bahr E, Arnold R, Veldkamp MW, Bajanowski T, Borggrefe M, Brinkmann B, Warnecke I, Funke H, Bhuiyan ZA, Wilde AA, Breithardt G, Haverkamp W. De novo mutation in the SCN5A gene associated with early onset of sudden infant death. Circulation 104: 1158–1164, 2001. [DOI] [PubMed] [Google Scholar]
- 462.Weerapura M, Nattel S, Chartier D, Caballero R, Hebert TE. A comparison of currents carried by HERG, with and without coexpression of MiRP1, and the native rapid delayed rectifier current. Is MiRP1 the missing link? J Physiol 540: 15–27, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Wehrens XH, Rossenbacker T, Jongbloed RJ, Gewillig M, Heidbuchel H, Doevendans PA, Vos MA, Wellens HJ, Kass RS. A novel mutation L619F in the cardiac Na+ channel SCN5A associated with long-QT syndrome (LQT3): a role for the I–II linker in inactivation gating. Hum Mutat 21: 552, 2003. [DOI] [PubMed] [Google Scholar]
- 464.Weiss JN, Garfinkel A, Karagueuzian HS, Chen PS, Qu Z. Early afterdepolarizations and cardiac arrhythmias. Heart Rhythm 7: 1891–1899, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. A cluster of hydrophobic amino acid residues required for fast Na+-channel inactivation. Proc Natl Acad Sci USA 89: 10910–10914, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Wickman K, Clapham DE. Ion channel regulation by G proteins. Physiol Rev 75: 865–885, 1995. [DOI] [PubMed] [Google Scholar]
- 467.Wickman K, Nemec J, Gendler SJ, Clapham DE. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron 20: 103–114, 1998. [DOI] [PubMed] [Google Scholar]
- 468.Wickman KD, Iniguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature 368: 255–257, 1994. [DOI] [PubMed] [Google Scholar]
- 469.Wiener R, Haitin Y, Shamgar L, Fernandez-Alonso MC, Martos A, Chomsky-Hecht O, Rivas G, Attali B, Hirsch JA. The KCNQ1 (Kv7.1) COOH terminus, a multitiered scaffold for subunit assembly and protein interaction. J Biol Chem 283: 5815–5830, 2008. [DOI] [PubMed] [Google Scholar]
- 470.Wong JA, Gula LJ, Klein GJ, Yee R, Skanes AC, Krahn AD. Utility of treadmill testing in identification and genotype prediction in long-QT syndrome. Circ Arrhythm Electrophysiol 3: 120–125, 2010. [DOI] [PubMed] [Google Scholar]
- 471.Wright M, Sacher F, Haissaguerre M. Catheter ablation for patients with ventricular fibrillation. Curr Opin Cardiol 24: 56–60, 2009. [DOI] [PubMed] [Google Scholar]
- 472.Wu D, Delaloye K, Zaydman MA, Nekouzadeh A, Rudy Y, Cui J. State-dependent electrostatic interactions of S4 arginines with E1 in S2 during Kv7.1 activation. J Gen Physiol 135: 595–606, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Wu G, Ai T, Kim JJ, Mohapatra B, Xi Y, Li Z, Abbasi S, Purevjav E, Samani K, Ackerman MJ, Qi M, Moss AJ, Shimizu W, Towbin JA, Cheng J, Vatta M. Alpha-1-syntrophin mutation and the long-QT syndrome: a disease of sodium channel disruption. Circ Arrhythm Electrophysiol 1: 193–201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Wu HC, Yamankurt G, Luo J, Subramaniam J, Hashmi SS, Hu H, Cunha SR. Identification and characterization of two ankyrin-B isoforms in mammalian heart. Cardiovasc Res 107: 466–477, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Wu L, Bauer CS, Zhen XG, Xie C, Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature 419: 947–952, 2002. [DOI] [PubMed] [Google Scholar]
- 476.Wu W, Sachse FB, Gardner A, Sanguinetti MC. Stoichiometry of altered hERG1 channel gating by small molecule activators. J Gen Physiol 143: 499–512, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Wu ZY, Chen K, Haendler B, McDonald TV, Bian JS. Stimulation of N-terminal truncated isoform of androgen receptor stabilizes human ether-a-go-go-related gene-encoded potassium channel protein via activation of extracellular signal regulated kinase 1/2. Endocrinology 149: 5061–5069, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Xu H, Guo W, Nerbonne JM. Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J Gen Physiol 113: 661–678, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Xu X, Jiang M, Hsu KL, Zhang M, Tseng GN. KCNQ1 and KCNE1 in the IKs channel complex make state-dependent contacts in their extracellular domains. J Gen Physiol 131: 589–603, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Xu X, Kanda VA, Choi E, Panaghie G, Roepke TK, Gaeta SA, Christini DJ, Lerner DJ, Abbott GW. MinK-dependent internalization of the IKs potassium channel. Cardiovasc Res 82: 430–438, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Yang PC, Song Y, Giles WR, Horvath B, Chen-Izu Y, Belardinelli L, Rajamani S, Clancy CE. A computational modelling approach combined with cellular electrophysiology data provides insights into the therapeutic benefit of targeting the late Na+ current. J Physiol 593: 1429–1442, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Yang T, Chun YW, Stroud DM, Mosley JD, Knollmann BC, Hong C, Roden DM. Screening for acute IKr block is insufficient to detect torsades de pointes liability: role of late sodium current. Circulation 130: 224–234, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Yang T, Snyders DJ, Roden DM. Ibutilide, a methanesulfonanilide antiarrhythmic, is a potent blocker of the rapidly activating delayed rectifier K+ current (IKr) in AT-1 cells. Concentration-, time-, voltage-, and use-dependent effects. Circulation 91: 1799–1806, 1995. [DOI] [PubMed] [Google Scholar]
- 484.Yang Y, Yang Y, Liang B, Liu J, Li J, Grunnet M, Olesen SP, Rasmussen HB, Ellinor PT, Gao L, Lin X, Li L, Wang L, Xiao J, Liu Y, Liu Y, Zhang S, Liang D, Peng L, Jespersen T, Chen YH. Identification of a Kir3.4 mutation in congenital long QT syndrome. Am J Hum Genet 86: 872–880, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Yang ZK, Boyett MR, Janvier NC, McMorn SO, Shui Z, Karim F. Regional differences in the negative inotropic effect of acetylcholine within the canine ventricle. J Physiol 492: 789–806, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Yarbrough TL, Lu T, Lee HC, Shibata EF. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ Res 90: 443–449, 2002. [DOI] [PubMed] [Google Scholar]
- 487.Yarotskyy V, Gao G, Du L, Ganapathi SB, Peterson BZ, Elmslie KS. Roscovitine binds to novel L-channel (CaV1.2) sites that separately affect activation and inactivation. J Biol Chem 285: 43–53, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Yarotskyy V, Gao G, Peterson BZ, Elmslie KS. The Timothy syndrome mutation of cardiac CaV1.2 (L-type) channels: multiple altered gating mechanisms and pharmacological restoration of inactivation. J Physiol 587: 551–565, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, Dolmetsch RE. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471: 230–234, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci 23: 7577–7585, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Yu H, Lin Z, Mattmann ME, Zou B, Terrenoire C, Zhang H, Wu M, McManus OB, Kass RS, Lindsley CW, Hopkins CR, Li M. Dynamic subunit stoichiometry confers a progressive continuum of pharmacological sensitivity by KCNQ potassium channels. Proc Natl Acad Sci USA 110: 8732–8737, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Yu H, Lin Z, Xu K, Huang X, Long S, Wu M, McManus OB, Le Engers J, Mattmann ME, Engers DW, Le UM, Lindsley CW, Hopkins CR, Li M. Identification of a novel, small molecule activator of KCNQ1 channels. In: Probe Reports from the NIH Molecular Libraries Program. Bethesda, MD: NIH, 2010. [PubMed] [Google Scholar]
- 493.Yue L, Feng J, Li GR, Nattel S. Transient outward and delayed rectifier currents in canine atrium: properties and role of isolation methods. Am J Physiol Heart Circ Physiol 270: H2157–H2168, 1996. [DOI] [PubMed] [Google Scholar]
- 494.Yue Y, Castrichini M, Srivastava U, Fabris F, Shah K, Li Z, Qu Y, El-Sherif N, Zhou Z, January C, Hussain MM, Jiang XC, Sobie EA, Wahren-Herlenius M, Chahine M, Capecchi PL, Laghi-Pasini F, Lazzerini PE, Boutjdir M. Pathogenesis of the Novel Autoimmune-Associated Long-QT Syndrome. Circulation 132: 230–240, 2015. [DOI] [PubMed] [Google Scholar]
- 495.Zareba W, Moss AJ, Locati EH, Lehmann MH, Peterson DR, Hall WJ, Schwartz PJ, Vincent GM, Priori SG, Benhorin J, Towbin JA, Robinson JL, Andrews ML, Napolitano C, Timothy K, Zhang L, Medina A. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol 42: 103–109, 2003. [DOI] [PubMed] [Google Scholar]
- 496.Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K+ current [I(K1)] as revealed by the targeted deletion of the murine Kir2.1 and Kir22 genes. J Physiol 533: 697–710, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Zaydman MA, Cui J. PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front Physiol 5: 195, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Zaydman MA, Silva JR, Delaloye K, Li Y, Liang H, Larsson HP, Shi J, Cui J. Kv7.1 ion channels require a lipid to couple voltage sensing to pore opening. Proc Natl Acad Sci USA 110: 13180–13185, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Zeng H, Lozinskaya IM, Lin Z, Willette RN, Brooks DP, Xu X. Mallotoxin is a novel human ether-a-go-go-related gene (hERG) potassium channel activator. J Pharmacol Exp Ther 319: 957–962, 2006. [DOI] [PubMed] [Google Scholar]
- 500.Zeng J, Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys J 68: 949–964, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol 1: 183–188, 1999. [DOI] [PubMed] [Google Scholar]
- 502.Zhang L, Benson DW, Tristani-Firouzi M, Ptacek LJ, Tawil R, Schwartz PJ, George AL, Horie M, Andelfinger G, Snow GL, Fu YH, Ackerman MJ, Vincent GM. Electrocardiographic features in Andersen-Tawil syndrome patients with KCNJ2 mutations: characteristic T-U-wave patterns predict the KCNJ2 genotype. Circulation 111: 2720–2726, 2005. [DOI] [PubMed] [Google Scholar]
- 503.Zhang L, Timothy KW, Vincent GM, Lehmann MH, Fox J, Giuli LC, Shen J, Splawski I, Priori SG, Compton SJ, Yanowitz F, Benhorin J, Moss AJ, Schwartz PJ, Robinson JL, Wang Q, Zareba W, Keating MT, Towbin JA, Napolitano C, Medina A. Spectrum of ST-T-wave patterns and repolarization parameters in congenital long-QT syndrome: ECG findings identify genotypes. Circulation 102: 2849–2855, 2000. [DOI] [PubMed] [Google Scholar]
- 504.Zhang L, Vincent GM, Baralle M, Baralle FE, Anson BD, Benson DW, Whiting B, Timothy KW, Carlquist J, January CT, Keating MT, Splawski I. An intronic mutation causes long QT syndrome. J Am Coll Cardiol 44: 1283–1291, 2004. [DOI] [PubMed] [Google Scholar]
- 505.Zhang M, Liu J, Tseng GN. Gating charges in the activation and inactivation processes of the HERG channel. J Gen Physiol 124: 703–718, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Zhang X, Szeto C, Gao E, Tang M, Jin J, Fu Q, Makarewich C, Ai X, Li Y, Tang A, Wang J, Gao H, Wang F, Ge XJ, Kunapuli SP, Zhou L, Zeng C, Xiang KY, Chen X. Cardiotoxic and cardioprotective features of chronic beta-adrenergic signaling. Circ Res 112: 498–509, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Zhang Y, Ouyang P, Post WS, Dalal D, Vaidya D, Blasco-Colmenares E, Soliman EZ, Tomaselli GF, Guallar E. Sex-steroid hormones and electrocardiographic QT-interval duration: findings from the third National Health and Nutrition Examination Survey and the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 174: 403–411, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Zhang Z, Zhao Z, Liu Y, Wang W, Wu Y, Ding J. Kinetic model of Nav1.5 channel provides a subtle insight into slow inactivation associated excitability in cardiac cells. PLoS One 8: e64286, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Zhao JT, Hill AP, Varghese A, Cooper AA, Swan H, Laitinen-Forsblom PJ, Rees MI, Skinner JR, Campbell TJ, Vandenberg JI. Not all hERG pore domain mutations have a severe phenotype: G584S has an inactivation gating defect with mild phenotype compared with G572S, which has a dominant negative trafficking defect and a severe phenotype. J Cardiovasc Electrophysiol 20: 923–930, 2009. [DOI] [PubMed] [Google Scholar]
- 510.Zheng R, Thompson K, Obeng-Gyimah E, Alessi D, Chen J, Cheng H, McDonald TV. Analysis of the interactions between the C-terminal cytoplasmic domains of KCNQ1 and KCNE1 channel subunits. Biochem J 428: 75–84, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Zhou J, Augelli-Szafran CE, Bradley JA, Chen X, Koci BJ, Volberg WA, Sun Z, Cordes JS. Novel potent human ether-a-go-go-related gene (hERG) potassium channel enhancers and their in vitro antiarrhythmic activity. Mol Pharmacol 68: 876–884, 2005. [DOI] [PubMed] [Google Scholar]
- 512.Zhou Z, Gong Q, Epstein ML, January CT. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J Biol Chem 273: 21061–21066, 1998. [DOI] [PubMed] [Google Scholar]
- 513.Zou A, Curran ME, Keating MT, Sanguinetti MC. Single HERG delayed rectifier K+ channels expressed in Xenopus oocytes. Am J Physiol Heart Circ Physiol 272: H1309–H1314, 1997. [DOI] [PubMed] [Google Scholar]
- 514.Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 399: 159–162, 1999. [DOI] [PubMed] [Google Scholar]