Abstract
Sensorimotor integration is the process through which the nervous system creates a link between motor commands and associated sensory feedback. This process allows for the acquisition and refinement of many behaviors, including learned communication behaviors such as speech and birdsong. Consequently, it is important to understand fundamental mechanisms of sensorimotor integration, and comparative analyses of this process can provide vital insight. Songbirds offer a powerful comparative model system to study how the nervous system links motor and sensory information for learning and control. This is because the acquisition, maintenance, and control of birdsong critically depend on sensory feedback. Furthermore, there is an incredible diversity of song organizations across songbird species, ranging from songs with simple, stereotyped sequences to songs with complex sequencing of vocal gestures, as well as a wide diversity of song repertoire sizes. Despite this diversity, the neural circuitry for song learning, control, and maintenance remains highly similar across species. Here, we highlight the utility of songbirds for the analysis of sensorimotor integration and the insights about mechanisms of sensorimotor integration gained by comparing different songbird species. Key conclusions from this comparative analysis are that variation in song sequence complexity seems to covary with the strength of feedback signals in sensorimotor circuits and that sensorimotor circuits contain distinct representations of elements in the vocal repertoire, possibly enabling evolutionary variation in repertoire sizes. We conclude our review by highlighting important areas of research that could benefit from increased comparative focus, with particular emphasis on the integration of new technologies.
Keywords: communication, corollary discharge, learning, sensorimotor, songbird
sensorimotor integration is the process through which the nervous system forges a functional and anatomical link between motor commands and the associated sensory feedback. The comparison of motor commands with sensory feedback serves a variety of functions, including aiding in sensory processing and enabling the learning, refinement, and maintenance of behavior. Because this process of comparing motor commands with sensory reports of the associated outcome is broadly relevant to perception and behavioral learning, the cellular properties and circuits through which the nervous system accomplishes this integration have long been a central focus of neuroscience research (Crapse and Sommer 2008; Diamond et al. 2008; Flanders 2011; Schall 2004; Sommer and Wurtz 2004).
To discern fundamental mechanisms and central themes of how the nervous system compares motor commands and the associated sensory feedback in service of perception and behavior, it is essential to investigate sensorimotor processes across a wide range of species. Indeed, research to date has discovered that a number of mechanisms of sensorimotor integration are shared across a broad diversity of species spanning vertebrate and invertebrate systems (Ahissar and Kleinfeld 2003; Bell 1989; Crapse and Sommer 2008; Poulet and Hedwig 2002; Schall 2004). Our review adopts a comparative approach to reveal general principles underlying sensorimotor integration in songbirds. Songbirds are a powerful model system to uncover sensorimotor processes that underlie behavioral learning and control, and we can apply these principles to understand mechanisms of sensorimotor learning and behavioral control across a wide range of other species. Additionally, insights into the mechanisms of sensorimotor integration can also lead to new translational applications across many diverse fields such as robotics, the development of prosthetic limbs, and the treatment of other sensorimotor pathologies such as stuttering and schizophrenia (Ackerley and Kavounoudias 2015; Feinberg 1978; Feinberg and Guazzelli 1999; Tumanova et al. 2015).
Utility of Sensorimotor Integration
The degree to which sensorimotor integration is used to influence specific functions is a major focus of ongoing research, and our knowledge to date indicates that it plays two important roles in behavior. First, sensorimotor integration is thought to be a means of preserving sensory acuity in the face of sensory feedback that emerges from the animal's own actions. In that paradigm, sensorimotor integration is thought to allow the differentiation of self-generated actions from those actions that result from the presence or actions of others (Bell 1989; Poulet and Hedwig 2002). For example, electric fish are often found in crowded rivers, and they emit electrical signals to locate nearby objects in the water (reviewed by Krahe and Maler 2014). Through a set of specialized receptors that are sensitive to electric signals, the fish's nervous system receives afferent input resulting from self-generated discharges as well as the discharges generated by other nearby fish. In many species, individual fish produce many discharges per second, so the environment is a constant barrage of afferent input from self and others. A primary challenge is to preserve sensory acuity in the midst of that barrage. Comparison of self-generated action vs. the sensory input generated by self and others is thought to play a central role in preserving sensory perception even during self-initiated actions. In particular, by subtracting out the expected sensory feedback from the actual sensory feedback occurring around the time of the action, the fish is able to detect signals in the environment even during the performance of various behaviors.
In addition, the contribution of sensorimotor integration to perception is also highlighted by seminal studies of the visual saccade system in nonhuman primates and the somatosensory cortex of rodents. Motor commands that direct the rapid eye movements called saccades can be integrated with activity in visual cortical areas, and this integration serves to prevent the organism from perceiving the visual world as moving each time that it redirects its gaze (Gandhi and Katnani 2011; Schall 2004; Schiller et al. 1987). Studies in the mouse whisking system demonstrate that motor commands associated with whisker movement are integrated with sensory signals in the somatosensory cortex, and the resulting information is an essential feature of how the mouse uses its whiskers to explore the location, size, and shape of objects (Ahissar and Kleinfeld 2003; Diamond et al. 2008; Kleinfeld et al. 1999).
The second important function of sensorimotor integration is for sensorimotor plasticity and learning. This process entails the comparison of actual sensory feedback with internal representations of the sensory consequences of motor commands. Importantly, there are two types of internal representations of sensory consequences that are important for sensorimotor learning. The first is the expected sensory consequence resulting directly from the motor behavior. It has been proposed that an efference copy of the motor command is sent to an internal model that makes predictions about the sensory consequences associated with muscle states. The comparison of this expected sensory consequence with the actual feedback allows the nervous system to generate a “prediction error” that can be used to update the internal model. For example, acute changes to peripheral muscles could cause a particular motor command to generate a different sensory consequence than expected (based on previous history of behavior). If prediction errors are consistent across initiations of the same action, then those error signals represent a mismatch between the motor command and the associated sensory consequence rather than environmental noise associated with any single initiation. In response to those prediction errors, the nervous system updates the predicted sensory consequence of a particular motor command.
The second type of internal representations of sensory consequences is internal representation of “target” sensory consequences that are important for sensorimotor learning. The comparison of actual feedback with sensory targets allows for the generation of “performance errors,” and information about performance error can then be used to adaptively modify the motor program to improve motor performance. Indeed, sensorimotor learning (e.g., learning how to produce speech, walk, or play an instrument) is characterized by a reduction in the magnitude of discrepancies between the expected and the actual performance based on trial-and-error and reinforcement learning (Broussard and Kassardjian 2004; Schmidt and Lee 2011; Sutton and Barto 1998). Even after mastering a behavior, the nervous system monitors the degree of performance errors and makes fine adjustments to the motor program to maintain a high level of performance. As such, performance error is important information used by the nervous system for motor learning and maintenance. While sensorimotor integration can be strictly defined as the ability to predict the sensory consequences of one's own actions (i.e., the generation of prediction error), we adopt a broader scope and also discuss the comparison of actual sensory feedback to desired sensory consequences (i.e., the generation of performance errors) in our review of sensorimotor integration for birdsong. This is because the use of sensory information during song production to generate adaptive adjustments to the motor commands for birdsong is central to the learning, maintenance, and control of learned behaviors such as birdsong and speech (see below).
A Neural Basis of Sensorimotor Integration
For many years, researchers have investigated how the nervous system compares sensory and motor signals to enable perception and learning. The functions in which sensorimotor integration is thought to play a central role share a common feature in that they each involve comparison of the expected results of a motor action vs. the actual results that are encoded in the sensory feedback. This raises the question of how the nervous system performs that comparison. At its most basic level, each neural circuit that underlies sensorimotor integration accomplishes this task using three fundamental components (Crapse and Sommer 2008). First, there is an efferent component: a neural record of the motor-related command signal that was responsible for the execution of a specific motor action. Second, there is an afferent component: neural activity that encodes specific features of the sensory input that resulted from that action and sensory input that emerged from influences in the surrounding environment. Finally, there must be one or more places where efferent and afferent components are integrated and compared to guide subsequent behavior.
An important question that arises from this framework is, how does the nervous system preserve a record of the motor command so that it can be compared against the ensuing feedback? The answer lies in a mechanism called corollary discharge (Crapse and Sommer 2008). Through collateral projections from an efferent motor pathway onto another pathway that is not itself motor related (i.e., recurrent onto structures other than motor efferents), the nervous system can generate a corollary discharge associated with the motor action that serves to reflect the expected results of the motor action. That record of the motor command can be compared against the resulting sensory feedback to enable sensorimotor comparisons.
Far beyond simple speculation, corollary discharge has been documented in both vertebrate and invertebrate nervous systems (Crapse and Sommer 2008; Poulet and Hedwig 2007). For example, in the electric organ discharge that is used by weakly electric fish to detect properties of their environment, the motor command to initiate an electric organ discharge is generated by a motor command nucleus in the brain. A corollary discharge associated with that command is passed through collateral projections to a collection of cells that will also eventually receive the associated sensory feedback. Specialized receptors in the skin detect the electric status of the environment (both self-generated and external signals) and relay that afferent information to the site of sensorimotor convergence, where it is compared against the corollary discharge generated from the original motor command signal (Bell 1989; Krahe and Maler 2014). This system enables the fish to discern the properties of its environment apart from the effects of its own actions. Such a mechanism is also present in the nervous system of crickets. When crickets rub their legs together to generate sound, a corollary discharge associated with that action is sent to the site where incoming auditory activity arrives. This allows the cricket to remain sensitive to the external auditory environment during self-generated sound production (Poulet and Hedwig 2002, 2006). Each of these examples reveals that sensorimotor comparison through corollary discharge provides organisms with a mechanism to compare the properties of self-generated actions against the properties of the ensuing sensory feedback.
Inhibitory, excitatory, and modulatory connections have been found to mediate the integration of sensory and motor information. For example, integration through inhibitory connections was first shown in the mormyrid weakly electric fish. These studies revealed that motor activity, in the form of a corollary discharge, makes inhibitory connections onto incoming sensory information (Bell 1989; Sommer and Wurtz 2008). Since that discovery, the existence of inhibitory connections from motor areas onto sensory areas has been documented in a range of species. For example, during song production in crickets, the neurons that generate the motor commands for sound production activate a corollary discharge inhibitory neuron, which then causes postsynaptic inhibition of ascending auditory neurons (Poulet and Hedwig 2007). Both excitatory and inhibitory connections have also been shown to contribute to sensorimotor integration in the mouse whisking system through projections from primary motor pathways onto sensory neurons (Kinnischtzke et al. 2014), and modulatory connections in sensorimotor integration have also been shown in other sensorimotor areas in the mouse cortex (Lee et al. 2009; Schubert et al. 2015).
In addition to understanding the anatomical connections and synaptic mechanisms underlying sensorimotor integration, it also essential to understand how neural activity associated with the execution of a motor action is preserved for long enough to be compared against the associated sensory feedback. Motor commands are encoded by action potentials, each of which is ~1 ms in duration. Motor commands course through efferent projections to initiate the movement, resulting in activation of afferents that encode the associated sensory feedback. This afferent activity is also encoded by action potentials that are ~1 ms in duration, but it arrives back at the site of sensorimotor convergence tens of milliseconds after the occurrence of the motor command. Therefore the corollary discharge must be preserved for long enough to serve as a temporal bridge to link the execution of the motor command and the arrival of the associated feedback. This can be accomplished by utilizing polysynaptic pathways or neurons with long axons and slow conduction velocities. Indeed, evidence for such mechanisms has been found. For example, the relic of the motor command is thought to be preserved through a polysynaptic pathway in the visual saccade system of nonhuman primates (Sommer and Wurtz 2002), and in the songbird brain, corollary discharge is persevered for the necessary duration through projections with sufficiently long axons and slow conduction velocities (reviewed by Mooney 2009a; Prather 2013). In addition to understanding these basic principles of sensorimotor integration, it is also critical to reveal the contribution of feedback processing and sensorimotor integration for motor learning.
Sensorimotor Integration in Learned Vocal Communication
One of the most familiar and well-documented forms of feedback-dependent learning is the process through which humans learn the sounds used in vocal communication. Language is the primary means through which humans communicate. Language is predominantly transmitted vocally through speech, which consists of the precise coordination of respiratory, lingual, and other motor behaviors. With proper coordination of speech-related behaviors, a speaker can generate the complex sounds and sequences that compose phonemes, words, and sentences (Kuhl 2010). Speech is a learned behavior that is acquired through imitation of the communicative sounds performed by others. Infants rely on sensory input to form models of the sounds that are eventually produced in speech (Kuhl 2010; Nelken and Bar-Yosef 2008). During early development, infants begin performing their own attempts to match those models (“babbling”). Initially, their imitation of those models is typically quite poor, but through many bouts of feedback-dependent trial-and-error learning, infants gradually become more proficient and eventually learn to imitate those models with exquisite precision (Oller and Eilers 1988; Stoel-Gammon and Otomo 1986). Comparison of motor action and the resulting sensory feedback is essential in the process through which humans acquire and master speech sounds. Indeed, humans that become deaf at a very young age (i.e., when they have been exposed to speech sounds but have had very little time to rehearse their own performance of those sounds) are not capable of mastering the intricate details of clear speech (Ertmer and Goffman 2011; Fitzpatrick et al. 2011; Moeller et al. 2007; Nott et al. 2009; Schauwers et al. 2008; Schick and Moeller 1992; Singleton et al. 1998). Together, these results suggest that the coupling of auditory and motor behaviors is essential early in life for normal speech development (Westermann and Miranda 2004).
Auditory feedback and sensorimotor integration play important roles in not only the developmental acquisition of speech but also the preservation of those sounds throughout adulthood. For example, adults who lose their hearing later in life experience gradual deterioration of the clarity of their speech after hearing loss (Cowie and Douglas-Cowie 1992; Waldstein 1990). Feedback-dependent changes in speech are also evident in response to acute manipulations of auditory feedback. Perturbations of auditory feedback, such as changes in the pitch or timing of auditory feedback, can lead to real-time changes to the spectral structure or temporal patterning of speech (Donath et al. 2002; Houde and Jordan 2002; Larson et al. 2000; Purcell and Munhall 2006; Stuart et al. 2002; Tourville et al. 2008; Xu et al. 2004). For example, delayed auditory feedback leads to the slowing down of speech and the repetition or abnormal sequencing of speech syllables (Howell and Archer 1984; Lee 1950; Stuart et al. 2002). Together, these findings reveal that auditory feedback is an essential component of both juvenile development and adult preservation of learned speech patterns.
Across a suite of specialized brain areas, vocal motor commands are compared with the resulting auditory feedback to learn and preserve the performance of speech sounds (reviewed by Hickok and Rogalsky 2011). Injury or other changes to the function of those brain areas can lead to a wide range of speech deficits. For instance, patients with Parkinson's disease are impaired not only in articulation but also in their real-time adaptation to alteration of the pitch of auditory feedback (Mollaei et al. 2013, 2016). This inability to make appropriate vocal changes in response to changes in auditory feedback reveals that the process of sensorimotor integration in speech is altered in the brains of these patients, and it highlights the potential contribution of dopaminergic circuits to feedback processing (e.g., Gadagkar et al. 2016; Hoffmann et al. 2016). Despite studies linking localized brain injury or degeneration to changes in speech control and learning, little is known about the cellular and neurophysiological mechanisms underlying sensorimotor integration for speech learning and control in humans.
Songbirds as a Model of Learned Vocal Communication
For ethical and technical reasons, researchers have turned to an animal model of human speech to gain deeper understanding of the neural mechanisms of sensorimotor learning. Songbirds and other vocal learning birds (i.e., parrots and hummingbirds) have become the dominant animal model system to study sensorimotor contributions to vocal learning, plasticity, and control and to generate and test models of sensorimotor integration for speech. This is because songbirds learn their songs in a manner that is very similar to how humans acquire their speech and use auditory feedback to control their vocalizations (Brainard and Doupe 2000a; 2002; Doupe and Kuhl 1999). Songbirds are one of very few groups of animals that undergo vocal learning, and they are by far the most experimentally tractable (Doupe and Kuhl 1999; Jarvis 2004; Petkov and Jarvis 2012). Songbirds undergo sensory-dependent vocal learning during juvenile development, wherein juvenile birds memorize the sounds in the song(s) of one or more adult conspecifics (Brainard and Doupe 2002; Doupe and Kuhl 1999; Prather 2013). After that period of sensory learning, juveniles begin to produce their own vocalizations, and the rudimentary vocalizations that are produced during those early attempts to sing are highly variable and unstructured. Through vocal practice, juveniles refine their song(s) until their vocalizations closely resemble the memorized song(s) (Brainard and Doupe 2000a, 2002; Mooney et al. 2008; Tchernichovski et al. 2001). Importantly, that pattern of refinement is intimately dependent on auditory feedback. The importance of auditory feedback in song development has been demonstrated by removing auditory feedback during the period of vocal practice. Across the diversity of avian vocal learners in which the contribution of auditory feedback to vocal development has been examined, juveniles that are deprived of auditory feedback during vocal practice fail to converge onto a song that closely resembles that of their tutors (Brainard and Doupe 2002; Dooling et al. 1987; Konishi 1965a; Marler and Waser 1977; Mooney 2009b). This indicates that sensory feedback, especially auditory feedback, is critical for the refinement of vocalizations during song development.
As in humans, songbirds also continue to rely on sensory feedback to maintain the structure of their learned vocalizations throughout adulthood (Brainard and Doupe 2002; Lombardino and Nottebohm 2000; Nordeen and Nordeen 1992; Woolley and Rubel 1997). For example, similar to how deafening leads to the gradual deterioration of speech in humans (Cowie and Douglas-Cowie 1992; Waldstein 1990), deafening adult zebra finches and Bengalese finches also leads to gradual deterioration of song performance (Nordeen and Nordeen 1992; Okanoya and Yamaguchi 1997; Woolley and Rubel 1997). In addition to this effect of prolonged changes in feedback, brief perturbations of auditory feedback acutely affect the sequencing and timing of syllables (Cynx and Von Rad 2001; Osmanski and Dooling 2009; Sakata and Brainard 2006, 2008, 2009), just as brief perturbations of auditory feedback induce real-time changes to speech (Purcell and Munhall 2006; Tourville et al. 2008). In a further parallel with human speech, distorting auditory feedback in a manner that alters the bird's perception of the pitch of individual syllables leads to compensatory changes in the frequency of song components (Sober and Brainard 2009). Together, these findings highlight the similarities in the dependence of birdsong and speech on sensory feedback, and they emphasize the utility of songbirds to investigate the neural basis of vocal learning and communication (reviewed by Mooney et al. 2008).
Finally, songbirds are excellent for revealing fundamental mechanisms of sensorimotor integration because of the enormous diversity in vocal behavior across the thousands of songbird species. For example, the number of songs learned and produced by an individual varies extensively across songbird species, with some species producing only a single song (e.g., zebra finches and Bengalese finches) and other species capable of producing tens to hundreds of different song types (e.g., song sparrows, nightingales, mockingbirds, and brown thrashers; Catchpole and Slater 2008). Furthermore, the sequencing of individual song elements (called “notes” or “syllables”) also ranges from highly stereotyped in species such as the white-crowned sparrow and zebra finch, to moderately complex in the Bengalese finch and sedge warbler, to highly complex in species such as robins (Catchpole and Slater 2008; Dobson and Lemon 1979; Okanoya 2004; Sossinka and Bohner 1980). As such, songbirds provide an excellent opportunity to reveal how sensorimotor mechanisms underlie variation in vocal learning as well as variation in behavioral complexity (Brenowitz and Beecher 2005; Jarvis et al. 2005). Such broad variation in behavior could be associated with the evolution of unique mechanisms of sensorimotor integration. Alternatively, this behavioral diversity could emerge by activating a common set of circuits in distinct ways. To distinguish between these possibilities, we must investigate the mechanisms through which sensorimotor integration contributes to vocal learning across a variety of species.
The Song System: A Neural Basis of Feedback-Dependent Behavior
Just as humans possess neural circuits that are specialized for speech, songbirds possess a collection of interconnected brain sites that are highly specialized for song (Brainard and Doupe 2002; Brenowitz and Beecher 2005; Doupe and Kuhl 1999; Prather 2013). This neural circuitry, collectively called the “song system,” is present across songbird species and highly similar in structure across songbird species with different song organizations and learning strategies (Brenowitz and Beecher 2005; Jarvis 2004). While this broad structural similarity suggests that shared neural mechanisms are activated in different ways to produce different vocal behaviors, fine-scale functional analyses are required to analyze how the nervous system solves the different sensorimotor challenges associated with different song strategies.
The song system consists of two main pathways (Fig. 1). First, neurons in the nucleus interfacialis of the nidopallium (NIf) project to neurons in the nucleus HVC (abbreviation used as a proper name; analogous to the human supplementary motor area or premotor cortex), which in turn projects to neurons in the robust nucleus of the arcopallium (RA; analogous to vocal regions of the primary motor cortex). Thereafter, RA neurons project to brain stem motor neurons that control respiration and muscles of the vocal organ. This pathway, called the song motor pathway (SMP), encodes the motor commands for song and sends commands to the vocal organ and the respiratory muscles that are required to produce songs.
Fig. 1.
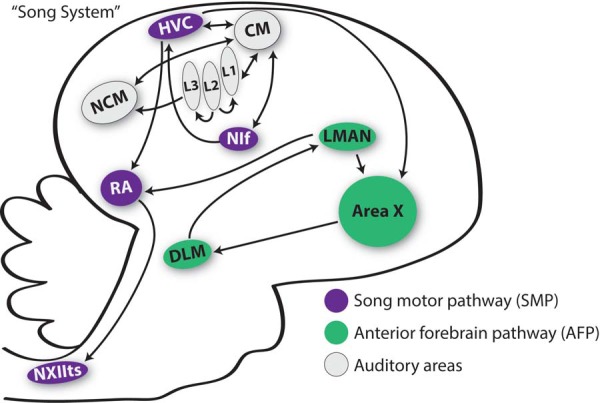
Schematic of the song system and other specialized areas of the songbird brain. HVC (formerly the high vocal center, now represented using simply the abbreviation) is a sensorimotor site that projects into two important pathways. First is the song motor pathway (SMP; purple), which is important in the performance of song behavior. Second is the anterior forebrain pathway (AFP; green), which is important for song learning. Each of those pathways converges onto the vocal motor nucleus RA (the robust nucleus of the arcopallium). Secondary auditory areas caudal mesopallium (CM; white) and caudomedial portion of the nidopallium (NCM; white) are not considered part of the canonical song system, but they also play important roles in song learning and perception. Area X, Area X of the medial striatum; DLM, dorsolateral medial nucleus of the thalamus; LMAN, lateral portion of the magnocellular nucleus of the anterior nidopallium; NIf, nucleus interfacialis; NXIIts, tracheosyringeal portion of the 12th cranial nerve; L1–L3, subregions of Field L, the primary auditory thalamorecipient region of the nidopallium.
Importantly, neural populations in the SMP not only generate the motor commands for song but also receive sensory inputs, in particular inputs from the auditory system. Broadly speaking, auditory information that is transduced in the ear travels to the auditory forebrain via brain stem, midbrain, and thalamic processing areas (Butler et al. 2011; Fortune and Margoliash 1992; Krützfeldt et al. 2010a, 2010b; Wild et al. 2010). Field L is the primary forebrain recipient of ascending thalamic information (Karten 1968; Karten and Hodos 1967; Krützfeldt et al. 2010a, 2010b; Wild et al. 2010) and can be subdivided into three distinct sections: L1, L2, and L3 (Fortune and Margoliash 1992). L2 is the primary recipient of projections from the thalamus, and it sends projections to the neighboring regions L1 and L3 (Butler et al. 2011). Subregions L2 and L3 project to the secondary auditory area, the caudomedial nidopallium (NCM), whereas subregions L1 and L2 project to another secondary auditory area, the caudal mesopallium (CM; Butler et al. 2011). The CM sends auditory information to HVC and NIf as well as to a region in the intermediate arcopallium (AIV; Mandelblat-Cerf et al. 2014). Because HVC and NIf receive auditory information and generate motor commands for song, these nuclei have traditionally been considered as the primary loci for sensorimotor integration for song.
The first key insight into the role of the song system in song performance was the finding that HVC is essential for song performance. If either HVC or its downstream target in the SMP is damaged, then the bird is still able to vocalize, in the form of calls, but the bird can no longer perform the learned vocalizations that characterize song (Nottebohm and Arnold 1976). Since then, various studies have revealed the contribution of neurons in the SMP and AFP neurons to sensorimotor integration for song learning, perception, and control (reviewed by Bolhuis and Eda-Fujiwara 2003; Bolhuis and Moorman 2015; Mooney et al. 2008). In addition, neurophysiological recordings in awake, singing birds revealed that HVC and NIf neurons are active during song production (Lewandowski et al. 2013; McCasland 1987). Additionally, neurophysiological studies of auditory responses revealed that HVC and NIf neurons were selective responsive to auditory stimuli, supporting a contribution of HVC and NIf to sensorimotor integration (e.g., Janata and Margoliash 1999; Katz and Gurney 1981; Lewicki and Konishi 1995). For example, neurons in HVC were active when the bird sang and when that same self-generated song was presented as an auditory stimulus to awake and anesthetized birds (Katz and Gurney 1981; McCasland and Konishi 1981). Intracellular recordings further revealed that auditory activity of individual HVC neurons was sensitive to the sequence in which individual song elements were presented in the auditory stimulus (Lewicki and Konishi 1995). Later experiments also revealed that the auditory selectivity of HVC neurons changes throughout the course of song learning and development, being more selective for tutor song in early development and more selective for self-produced song near the end of juvenile development (Nick and Konishi 2005; Volman 1993). While auditory responses in awake birds are generally less robust than auditory responses in anesthetized birds (Coleman et al. 2007; Schmidt and Konishi 1998), studies in awake birds highlight the potential role of HVC neurons in sensory processing for sensorimotor integration. Thus the auditory and vocal representation of the bird's own vocalizations can provide insights into how sensory and motor stimuli arriving into one and the same nucleus could be integrated and modified to influence downstream targets. Further research into the synaptic mechanism(s) through which vocal and auditory connections influence certain cells or downstream targets can provide evidence of the functional role of sensorimotor integration in learning.
In addition to the SMP, a separate population of neurons emerges from HVC and projects into a basal ganglia-forebrain circuit called the anterior forebrain pathway (AFP; (Brainard and Doupe 2000b; Kao and Brainard 2006; Scharff and Nottebohm 1991; Woolley and Kao 2015). The AFP is homologous to basal ganglia-cortical-thalamic circuits in mammals and important for song plasticity and control. Importantly, the lateral magnocellular nucleus of the anterior nidopallium (LMAN) sends projections to RA and thus provides another mechanism by which information can influence vocal learning and control. Although it was clear that HVC and associated brain nuclei in the SMP are sensorimotor structures that could serve as sites for comparison of tutor song with the bird's own song and thus influence vocal learning and production, it remained unclear what role the AFP might play in that process. Initial attempts to reveal the role of the AFP in song learning and performance yielded curious results. In contrast to lesions of the SMP, which eliminated adult song performance, lesions to the AFP had little or no effect on adult song (Scharff and Nottebohm 1991; Scharff et al. 2000; Sohrabji et al. 1990). This led to speculation that HVC neurons that project into the AFP (HVCX neurons) may play an inconsequential role. However, when researchers lesioned the AFP in juvenile birds, it became apparent that the AFP plays a central role in song learning (Scharff and Nottebohm 1991; Sohrabji et al. 1990). In particular, lesions of Area X or LMAN (Fig. 1) in juvenile zebra finches prevented juveniles from developing normal species-typical song. Subsequent studies in adult songbirds revealed that the AFP also plays an important role in the plasticity of adult song (reviewed by Brainard and Doupe 2013). Together, these data led to the idea that the pathways that emerge from HVC comprise a neural basis for the integration of sensory and motor activity in service of learned vocal behavior.
More recently, researchers have discovered that structures outside of the canonical song system also play important roles in song learning. For example, neurons in the primary auditory region of songbirds (Field L) could also be important sites of sensorimotor integration and error detection. Individual neurons in Field L are selectively activated when the bird's own song is distorted by simultaneous playback of other sounds (Keller and Hahnloser 2009). Consequently, such neurons were deemed to be error-sensitive neurons that could contribute to song learning. Additionally, neurons in secondary auditory areas such as the CM and the NCM project directly or indirectly to motor areas such as HVC and are necessary for the memorization of tutor song in zebra finches (Bolhuis et al. 2012; London and Clayton 2008) Furthermore, manipulations of activity in the dorsal arcopallium (Ad) and AIV have been found to affect the sensorimotor learning of song and thus could be important sites for sensorimotor integration (Bottjer and Altenau 2010; Mandelblat-Cerf et al. 2014). Thus the songbird forebrain contains multiple sites where sensory and motor-related activity may be compared to affect the acquisition and maintenance of learned behavior.
Benefits of a Comparative Approach to Understanding the Sensorimotor Regulation of Learned Vocal Communication
The vast majority of the insights described in the preceding section emerged from studies of zebra finches. This is largely due to the fact that zebra finches thrive and perform the complete extent of their courtship and reproductive behaviors in the laboratory setting. However, the earliest studies of the role of sensory feedback and the song system in song performance were also conducted in other songbird species such as canaries, chaffinches, and sparrows, and these studies provided an important foundation for subsequent experiments in zebra finches (McCasland and Konishi 1981; Nottebohm and Arnold 1976; Thorpe 1958). We emphasize the importance of this comparative approach because there are limitations associated with relying solely on zebra finches, or any single species, to understand broad themes of sensorimotor learning and control. For example, zebra finch song has a fixed syntax such that song elements are performed in the same sequence during each rendition of adult song (Zann 1996). This stereotypy is advantageous in a number of respects, but it prevents researchers from investigating how the nervous system encodes the variable and complex sequences that are a hallmark of speech in humans and song in other species. In addition, zebra finches have only one song type in their repertoire, preventing researchers from investigating how the nervous system holds a simultaneous representation of an array of learned vocalizations as in human vocabularies or the large repertoires of other songbird species (Brenowitz and Beecher 2005; Catchpole and Slater 2008). These facets of zebra finch song emphasize that even though one species can be especially informative in many regards, it is nonetheless important to adopt a comparative approach to fully understand the role of sensorimotor integration in learned behaviors (Brenowitz and Beecher 2005; Nealen and Schmidt 2002; Sakata and Vehrencamp 2012).
Songbird researchers have repeatedly turned to other species for complementary perspectives on sensory contributions to vocal motor control. For example, the Bengalese finch has been extensively studied by a number of researchers because Bengalese finches produce songs that are more complex in syllable sequencing (Okanoya 2004). Like zebra finches, Bengalese finches produce learned syllables that are sequenced and timed in a precise manner, and the sequencing and timing of Bengalese finch syllables are dependent on auditory feedback (Okanoya and Yamaguchi 1997; Sakata and Brainard 2006; Woolley and Rubel 1997). However, in contrast to zebra finch song, Bengalese finch song contains a complex and variable syntactic structure that more closely resembles the syntactic complexity of human language (Abe and Watanabe 2011; Fujimoto et al. 2011; Okanoya 2004; Sakata and Brainard 2006). In particular, Bengalese finch song contains not only stereotyped sequences but also “branch points,” which are points in the song where syllable sequencing varies from rendition to rendition. As such, the Bengalese finch offers a powerful model system to investigate the contribution of brain mechanisms and sensorimotor integration to a variety of song features, including complex vocal motor sequencing.
The importance of sensorimotor integration in the control of vocal sequencing is exemplified by studies assessing how real-time auditory perturbations of auditory feedback affect vocal control and plasticity (e.g., Sakata and Brainard 2006, 2009; Tumer and Brainard 2007; Warren et al. 2012). Acute perturbations of auditory feedback during ongoing song lead to rapid and localized changes in the sequencing and timing of the individual syllables that compose the song (Sakata and Brainard 2006). In particular, acute perturbations of feedback lead to localized increases in the variability of syllable sequencing and decreases in song tempo. Importantly, the magnitude of these effects of feedback perturbations depends on the complexity (i.e., variability) of the syllable sequences that are targeted for feedback disruption. Feedback perturbations cause greater changes to syllable sequencing and timing at points in the song that contain variable sequences (branch points) than at points that contain stereotyped sequences (Sakata and Brainard 2006). This suggests that sensory feedback has greater real-time contributions to the control of complex vocal sequences than to the control of stereotyped vocal sequences. Additional support for this idea comes from the finding that social context manipulations that increase the stereotypy of syllable sequencing also decrease the effect of feedback perturbations on syllable timing (Sakata and Brainard 2009). The effects of real-time auditory perturbations have not been studied in a wide range of species, and it will be important to test the extent to which auditory feedback contributions to song control vary across species with different levels of sequence complexity (e.g., Cynx and Von Rad 2001; Osmanski and Dooling 2009).
To investigate the influence of auditory feedback on specific aspects of vocal performance and plasticity, researchers have used song-triggered manipulations of sensory feedback to drive changes to song (Ali et al. 2013; Andalman and Fee 2009; Charlesworth et al. 2011, 2012; Tumer and Brainard 2007; Warren et al. 2011, 2012). In such experiments, brief bursts of white noise are played to the bird contingent on what the bird produces, and such contingent reinforcement drives changes to syllable structure, timing, and sequencing. For example, when white noise is played to the bird when it produces a syllable below a specified fundamental frequency, the bird adaptively shifts the pitch of its vocalization up to avoid the white noise playback (Andalman and Fee 2009; Canopoli et al. 2014; Charlesworth et al. 2011, 2012; Warren et al. 2011). Such experiments have also been used to drive changes in the sequencing of syllables in adult Bengalese finch song (Warren et al. 2012). When specific transitions at branch points are targeted for white noise playback, Bengalese finches will shift their transition probabilities away from the targeted syllable transition to adaptively avoid white noise playback. For example, if the bird can transition from syllable A to either syllable B or C and the transition from A → B but not the transition from A → C is targeted for white noise playback, then the bird will gradually change its sequencing at the branch point such that the A → B transition will be produced less frequently and the A → C transition is produced more frequently. Importantly, whereas targeting syllable transitions at branch points leads to adaptive shifts in vocal sequencing, targeting stereotyped transitions does not lead to adaptive changes to sequencing (Warren et al. 2012). These data support the idea that auditory feedback contributes more to the control and plasticity of complex vocal sequences than stereotyped sequences. The degree to which feedback-driven shifts in syllable structure or timing are influenced by sequence complexity remains unknown, but we hypothesize that such shifts will be greater for syllables within complex sequences than for syllables within simpler sequences.
The maintenance of learned song in adulthood relies on auditory feedback, and species differences in the effects of feedback removal seem to covary with species differences in song complexity. Generally speaking, deafening leads to changes to the spectral and temporal components of songs. Adult zebra finch and white-crowned sparrow song, which is syntactically simple, retains much of its species-typical characteristics for months following deafening (Konishi 1965b; Nordeen and Nordeen 1992). However, adult Bengalese finch song, which is more syntactically complex than zebra finch and white-crowned sparrow song, demonstrates a more rapid deterioration of syllable structure and sequencing following deafening (Okanoya and Yamaguchi 1997; Sakata and Brainard 2006; Scott et al. 2000; Woolley and Rubel 1997). Similarly, the temporally complex songs of budgerigars and canaries degrade within days or weeks following deafening (Heaton et al. 1999; Nottebohm and Arnold 1976). These data suggest that species variation in the reliance of adult song maintenance on auditory feedback could be linked to mechanisms underlying sequence complexity (but see Kagawa et al. 2012).
Because the vocal motor control of complex sequences seems to be more reliant on auditory feedback than the control of stereotyped sequences, it is possible that such behavioral differences may be linked to differences in the sensitivity of sensorimotor neurons to auditory feedback. There are numerous studies examining neurophysiological changes in response to feedback perturbations, and those studies suggest possible differences between species. Because HVC activity is tightly associated with both song production and auditory playback of song, this nucleus has been identified as a likely sensorimotor region and has been targeted for many studies across species. For example, auditory feedback signals have been identified in the HVC of adult Bengalese finches (Sakata and Brainard 2008). Specifically, acute perturbations of auditory feedback lead to acute decreases in HVC activity in singing Bengalese finches. In contrast, feedback signals have not been found in HVC or other song control nuclei of zebra finches (Hamaguchi et al. 2014; Kozhevnikov and Fee 2007; Leonardo 2004; Vallentin and Long 2015). Similarly, responses to short-term disruption of auditory feedback were also absent in the HVC of awake, behaving swamp sparrows, a species that, like zebra finches, produces syntactically simple songs (Prather et al. 2008). Together, these data suggest that species differences in the magnitude of auditory feedback signals may be linked to species differences in sequence complexity. This conclusion is based, however, on the comparison of a limited number of species, and we argue that a greater diversity of species needs to be studied. For example, it would be informative to reveal the magnitude of feedback signals in the song system of canaries and budgerigars, whose songs are temporally complex.
Species variation in sensorimotor processes could also be linked to variation in the degree to which sensorimotor neurons encoded auditory information in awake, behaving birds. While off-line mechanisms during sleep contribute to vocal plasticity (reviewed by Margoliash and Schmidt 2010), much of the plasticity stemming from auditory manipulations occurs online (e.g., Andalman and Fee 2009; Sober and Brainard 2009; Tumer and Brainard 2007; Warren et al. 2011, 2012); as such, auditory responsiveness in the awake state may correspond with variation in sensorimotor processes. Neurons in the sensorimotor nucleus HVC robustly and selectively respond to playbacks of the bird's own song (BOS) in awake Bengalese finches (Prather et al. 2008; Sakata and Brainard 2008). Such selective auditory responses in awake, behaving birds have also been observed in zebra finches, canaries, European starlings, song sparrows, and swamp sparrows (Cardin and Schmidt 2003, 2004; George et al. 2005; McCasland and Konishi 1981; Nealen and Schmidt 2002, 2006; Prather 2013; Prather et al. 2008, 2009; Raksin et al. 2012; Rauske et al. 2003; Figs. 2 and 3). However, the magnitude and selectivity of auditory responses of HVC neurons to BOS seem to be attenuated in zebra finches compared with other species such as Bengalese finches and canaries (Prather 2013). Because adult Bengalese finches and canaries are both more sensitive to auditory feedback (i.e., song degrades faster following deafening) than are zebra finches, there appears to be a correspondence between sensorimotor processes and auditory responses in HVC.
Fig. 2.
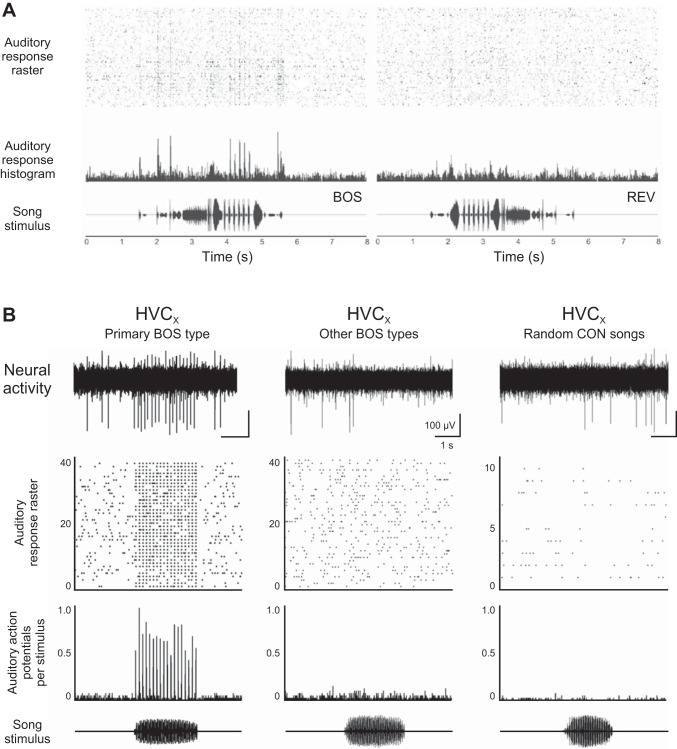
Sensory responses in nucleus HVC are selective at both the population level and the level of individual neurons. A: multiunit recordings from HVC in an awake song sparrow during auditory playback of the bird's own song (BOS) and reversed playback of the BOS (REV). Responses are clearly selective for the BOS, revealing that HVC neurons selectively respond to vocal sequences. Top: raster of auditory responses. Middle: peristimulus time histogram of auditory responses. Bottom: oscillogram of song stimulus. [Adapted from Fig. 4 of Nealen and Schmidt (2006).] B: single-unit activity of an identified HVCX projection neuron is also highly selective in an awake swamp sparrow. This cell reponded strongly to one song in the bird's adult repertoire (primary song type), but there was little or no response to other songs in the bird's adult repertoire (other BOS types) or to randomly selected songs from other conspecifics (random CON song). This cell was responsive to one song type in the bird's adult repertoire. Other HVCX cells in the same bird were selectively responsive to the other elements of the adult repertoire (data not shown). Top: raw trace of a response to a playback; remaining three panels are as in A. [Adapted from Fig. 1 of Prather et al. (2008).]
Fig. 3.
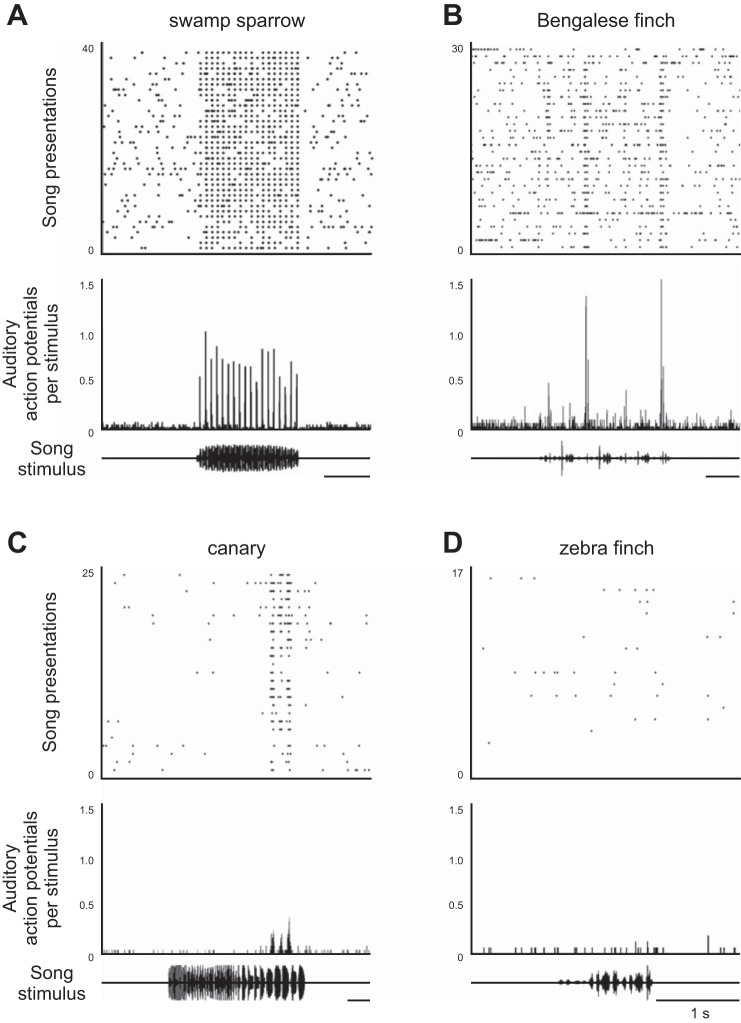
Selective responses of HVCX projection neurons in awake and freely behaving birds are evident across a wide variety of songbird species. A–C: extracellularly recorded activity of HVCX neurons is selective for specific song features of the bird's own song in swamp sparrows (A), Bengalese finches (B), and canaries (C). D: extracellularly recorded HVCX neurons were not strongly responsive to features in the bird's own song in the zebra finch (but see Hamaguchi et al. 2014). Top: raster of auditory responses. Middle: peristimulus time histogram of auditory responses. Bottom: oscillogram of auditory responses. [Adapted from Fig. 3 of Prather (2013).]
In light of possible links between sequence complexity and the dependence on auditory feedback, it is important to reveal the mechanisms underlying the generation and control of complex syllable sequencing (e.g., Bouchard et al. 2015; Fee and Scharff 2010; Jin 2009; Katahira et al. 2013; Warren et al. 2012). Neurophysiological investigations into vocal motor control in the Bengalese finch as well as comparisons between Bengalese and zebra finches have proven important in this regard. Numerous studies have underscored the importance of brain areas in the SMP to syllable sequencing. For example, lesions of NIf lead to significant decreases in the syntactic complexity of adult Bengalese finch song (Hosino and Okanoya 2000). Furthermore, single-unit recordings of HVCX projection neurons in awake Bengalese finches indicate that HVC activity provides information about syllable sequencing (Fujimoto et al. 2011; Prather et al. 2008). Specifically, HVCX activity associated with a focal syllable varies depending on the identity of the syllable preceding and following the focal syllable (Fujimoto et al. 2011; Prather et al. 2008). These data support the ideas that activity in NIf and activity in HVC are both important for the control of syllable sequencing and that species differences in sequence variability could be linked to species differences in the organization and function of nuclei within the SMP.
In addition to neurophysiological recordings, analysis of immediate early gene expression across different social contexts supports the importance of HVC to syllable sequencing. Bengalese finches produce songs with more variable syllable sequencing when singing noncourtship songs in isolation than when producing courtship songs in the presence of females (Hampton et al. 2009; Heinig et al. 2014; James and Sakata 2015; Matheson et al. 2016; Sakata and Brainard 2009; Sakata et al. 2008). Immediate early gene expression in song control nuclei differs when male Bengalese finches produce courtship vs. noncourtship song. In particular, the expression of the immediate early gene EGR-1 in HVC and other portions of the SMP and AFP is higher when Bengalese finches produce noncourtship song than when they produce courtship song (Matheson et al. 2016). EGR-1 expression is also modulated by social context in many of these brain areas in zebra finches (Castelino and Ball 2005; Hara et al. 2007; Jarvis et al. 1998). However, EGR-1 expression in HVC is greater during the performance of noncourtship song than of courtship song in Bengalese finches but not in zebra finches. Similarly, social context affects syllable sequencing in Bengalese finches but not in zebra finches (Fig. 4). These interspecific differences in brain-behavior relationships support the hypothesis that HVC is important for the control of syllable sequencing. Complementary investigations of brain-behavior relationships in other species are necessary to confirm the role of HVC in the generation of sequence complexity and species variation in song organization.
Fig. 4.
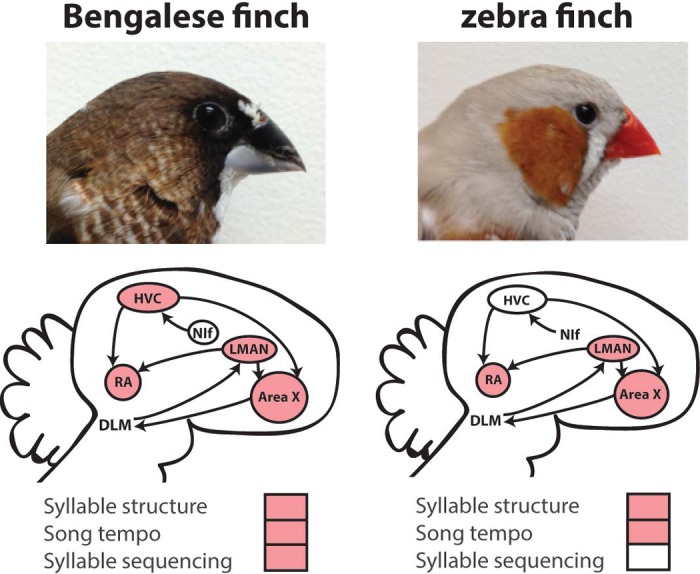
Effects of social context on EGR-1 expression and vocal control in Bengalese and zebra finches. Summarized here are brain areas that show significant differences in EGR-1 expression when birds produce undirected (UD) vs. female-directed (FD) song (light red brain areas). Also depicted are song features that are significantly different when birds produce UD and FD song. In both Bengalese finches (left) and zebra finches (right), EGR-1 expression is greater during UD song than FD song in Area X, LMAN, and RA. However, only in Bengalese finches is EGR-1 expression in HVC greater during UD song. (NIf is not circled in zebra finches because context-dependent changes to EGR-1 expression in NIf have not been analyzed for zebra finches). This species difference in the effects of social context on EGR-1 expression in HVC correlates with species differences in the effects of social context on syllable sequencing, suggesting that evolutionary changes in HVC could mediate species differences in social influences on syllable sequencing.
In addition to allowing for broader inquiries into the neural mechanisms underlying vocal control, one can examine a greater breadth of questions regarding the relationship between the control and plasticity of syllable sequencing, timing, and structure in birds that produce songs with more complex syntactic structure. For example, individual syllables in Bengalese finch song can be embedded within multiple sequences, and the acoustic structure and control of individual syllables vary depending on the sequence in which it is embedded (Hoffmann and Sober 2014; Wohlgemuth et al. 2010). In addition, detailed studies of syllable sequencing and timing in Bengalese finches reveal a significant link between transition probabilities and intersyllable gap durations. Gap durations (i.e., the silent interval between syllables) are generally shorter at stereotyped transitions than at branch point transitions (Matheson and Sakata 2015; Tachibana et al. 2015; Takahasi et al. 2010). Furthermore, as adult Bengalese finches age, syllable sequencing at branch points becomes more stereotyped and gap durations become shorter (James and Sakata 2015; 2014), and the degree to which branch point transitions become stereotyped over time correlates with the degree to which gap durations decrease over time (Matheson and Sakata 2015). The degree to which these relationships hold true in other species remains unclear, but these analyses in Bengalese finches suggest a mechanistic link between the sensorimotor control of syllable sequencing and timing.
Taken together, by adopting a comparative perspective to analyze mechanisms of sensorimotor integration for song plasticity, maintenance, and control, an association between vocal syntactic complexity and sensorimotor processes is revealed. In particular, songbirds that produce songs with more complex syllable sequencing (e.g., Bengalese finches and canaries) seem to be more dependent on auditory feedback than songbirds that produce less complex songs (e.g., zebra finches and white-crowned sparrows). Furthermore, our comparative analysis highlights the potential contribution of sensorimotor processes in HVC to this variation, as HVC neurons appear to be more robustly activated by the sound of the bird's own song and more sensitive to auditory feedback in songbirds with more complex songs.
Benefits of a Comparative Approach to Understanding the Sensory Representations of Learned Vocal Communication
Songbird species vary not only in the degree of syntactic complexity of their songs but also in the number of different song types they produce. Songbirds such as the zebra finch and Bengalese finch produce only one song type whereas birds such as lyrebirds and wood thrushes produce hundreds of song types (Brenowitz and Beecher 2005; Catchpole and Slater 2008). One of the fascinating questions that emerges from this comparative perspective of songbirds is how different song types are represented in the brain. Regardless of repertoire sizes, all songbirds depend on sensorimotor processes (e.g., comparisons of produced songs with sensory targets) to produce accurate renditions of their learned songs, and it is important to assess whether species differences in repertoire size are due to the evolution of novel mechanisms or novel functioning of shared mechanisms. Furthermore, understanding the sensory representation of vocal repertoires has implications for understanding the neural basis of vocabularies in language and learning multiple musical instruments.
Swamp sparrows provide an experimentally tractable species for this investigation. Birds in this species typically produce two to five different song types as adults, and the neurophysiological representation of multiple song types has been examined by assessing HVC activity in response to auditory playbacks and during the production of different song types (Mooney et al. 2001; Prather et al. 2008). In an earlier study, Mooney et al. (2001) discovered that individual neurons in the HVC of anesthetized swamp sparrows tend to respond to various song types in the bird's repertoire and that the breadth of stimuli that individual neurons responded to varied across neuron type. Whereas HVC interneurons were generally responsive to all song types produced by the bird, projection neurons (e.g., HVCX neurons) were generally best activated by one song type. How neuronal variation in selectivity is achieved is unknown, but as demonstrated in zebra finches, such selectivity could be sculpted by inhibitory mechanisms (Rosen and Mooney 2003; Vallentin et al. 2016).
Prather et al. (2008) extended the findings of Mooney et al. (2001) by investigating how HVCX neurons in awake swamp sparrows responded to playback of various song types as well as how those same neurons were active during the production of the same song types. Consistent with the previous study, recordings of HVCX neurons in awake, behaving birds revealed that individual HVCX neurons are preferentially activated by the sound of one song type in the bird's adult repertoire. Similarly, individual HVCX neurons were selectively active during the production of one song type. Moreover, there was an invariant association between sensory responses and motor activity: HVCX neurons were preferentially active during the production of the same song type that elicited the most robust auditory responses. Together, these studies in swamp sparrows indicate that HVC neurons encode the song repertoire of individual birds and that this representation is heterogeneously distributed across multiple neuronal populations.
Studies of auditory processing in HVC neurons of other songbird species with repertoires of more than one song type also confirm a distributed representation of song types within HVC. Playbacks of various song types increased the activity of most HVC neurons in awake song sparrows (Nealen and Schmidt 2006). However, as in the swamp sparrow, HVC neurons generally responded most vigorously to one of the song types, and the “preferred” song type varied across sites, suggesting a spatially heterogeneous representation of the bird's repertoire. A study of awake starlings also revealed that most of the HVC neurons that were activated by playbacks of bird's own songs responded to one or a number of song types and that different neurons were preferentially activated by different song types (George et al. 2005). Similar distributed representations of song types were also found in the HVC of canaries, though responses were investigated in anesthetized birds (Lehongre and Del Negro 2011). Neuron type was not identified in these studies, but data from the swamp sparrows suggest that the broadly tuned neurons could be interneurons whereas the cells that respond more selectively to individual song types could represent projection neurons (Mooney et al. 2001; Prather et al. 2008).
Sparrows and other songbird species with multisong repertoires also offer an intriguing opportunity to investigate how “latent” song types are represented in the brain. For example, while adult swamp sparrows, song sparrows, and white-crowned sparrows produce a limited repertoire of song types during the breeding season, juveniles of those species learn additional songs during development and “prune away” many of those songs on the basis of a number of factors, including interactions with conspecifics (Catchpole and Slater 2008; Liu and Nottebohm 2007; Marler 1997). While adult sparrows only produce a subset of the songs that they produced during development, birds do not simply “forget” the songs that they prune away. In strong support for this idea, adult swamp sparrows produce only a small fraction of the songs that they heard or performed during juvenile development, yet HVC neurons in adult swamp sparrows respond to playback of songs that were pruned away or only heard during development (Prather et al. 2010). Furthermore, during the spring period of vocal practice for seasonally reproducing white-crowned sparrows, males reexpress phrases and songs that were dropped in previous years (Hough et al. 2000). These data reveal that males continue to hold a memory of the “dropped” song types and that cells in the SMP contribute to this neural representation and allow for the “reexpression” of dropped song types.
To date, there is only one study that investigated AFP contributions to the representation of multiple song types, including dropped songs (Benton et al. 1998). In this study, adult white-crowned sparrows were held on short days to mimic winter conditions and then transitioned to longer days to mimic the breeding season. Unmanipulated birds demonstrated typical seasonal changes to song and eventually produced the same song they produced in previous years. However, birds in which LMAN was lesioned before the transition to longer days produced songs that more closely resembled songs produced during development. For example, birds with LMAN lesions integrated syllables that were previously only produced during “plastic songs” during development and that were pruned over the course of development. As such, these data suggest that LMAN could be important for suppressing the representation of multiple song types. How LMAN affects the seasonal reexpression of songs remains unknown, but because LMAN activity can indirectly influence HVC activity (Hamaguchi and Mooney 2012; Roberts et al. 2008), it is possible that LMAN lesions may affect song development by modulating HVC circuitry.
Across these songbird species, a picture emerges that the song repertoire of individual birds is heterogeneously represented across populations of HVC neurons. Together with the finding that HVC size and convergence within the SMP are positively related to repertoire size across a wide range of songbird species (Devoogd et al. 1993; Moore et al. 2011), these data highlight the role of HVC in the representation of multiple song types. It is likely that neurons outside the canonical song circuitry also contribute to the representation of song repertoires, but, to date, little is known about how neurons in NCM and CM, for example, represent the bird's own repertoire.
Future Directions to Integrate the Comparative Approach
Studies of songbird neurobiology have always profited from the incorporation of a comparative approach. Since the seminal studies in canaries that helped give rise to the field of songbird neurobiology, studies in finches, sparrows, robins, grosbeaks, starlings, and parrots have been instrumental in revealing the importance of auditory feedback in song learning and maintenance and the neural basis of song learning and performance. While songbird research has become increasingly focused on a single species, the zebra finch, many researchers have returned to the comparative approach and incorporated studies of other species of finches as well as sparrows, starlings, and parrots. Revealing the shared features underlying sensorimotor integration across songbird species is important for revealing mechanisms that could have been selection pressures and for suggesting mechanisms that may play similar roles in other species. Because the underlying components of sensorimotor integration are observed across a wide range of species, mechanisms found to be common across songbirds could also inform understanding of similar mechanisms in other species such as crickets, electric fish, and humans. Furthermore, such mechanistic understanding can also lead to novel translational applications across many diverse fields such as robotics, the development of prosthetic limbs, and the treatment of other sensorimotor pathologies such as stuttering and schizophrenia (Ackerley and Kavounoudias 2015; Feinberg 1978; Feinberg and Guazzelli 1999; Tumanova et al. 2015).
A central conclusion from our comparative analysis of sensorimotor integration is that variation in the dependence of adult song maintenance on auditory feedback is linked to variation in the syntactic complexity of song and/or in auditory responses in HVC. A classic approach to analyze the contribution of auditory feedback to song maintenance is to assess how quickly adult song degrades following deafening. Our comparative analyses suggest that the effect of deafening is more pronounced in songbird species with more complex syllable sequencing and with more robust and selective auditory responses in HVC of awake birds. The effects of adult deafening have been studied in a number of songbird species, and it will be informative to extend this technique to species such as swamp sparrows, song sparrows, and starlings. Swamp sparrows offer a particularly interesting test of the relationships among song complexity, auditory responses in HVC, and the dependence of auditory feedback for adult song maintenance. This is because swamp sparrows produce syntactically simple songs, yet HVC neurons of awake birds demonstrate robust and selective auditory responses to the bird's own song, responses that are more typical of species that produce complex songs (Prather et al. 2008, 2009). On the basis of the relatively simple syntactic structure of swamp sparrow song and the absence of real-time feedback signals in HVC, one might predict that song should remain intact for a relatively long time following deafening. On the other hand, on the basis of the robustness of auditory responses, one would predict that deafening might lead to a relatively rapid degradation of song structure. Consequently, analyzing the rapidity of song degradation following deafening of species such as swamp sparrows would lend insight into whether song complexity or awake auditory responses are more predictive of the dependence on auditory feedback for song maintenance.
On a related note, studying species that produce songs with different degrees of sequence complexity will be important for revealing how sensorimotor processes in the SMP and AFP allow for the evolution and emergence of behavioral complexity (Fig. 1). For example, inactivations of NIf cause decreases in the complexity of syllable sequencing of Bengalese finch song (Hosino and Okanoya 2000), but the contribution of NIf to sequence complexity in other songbirds with variable syntactic structure remains unknown (but see Naie and Hahnloser 2011; Otchy et al. 2015; Piristine et al. 2016). Recently, manipulations of Area X and LMAN have also been found to affect syllable sequencing in zebra finches (see also Hamaguchi and Mooney 2012; Kubikova et al. 2014; Tanaka et al. 2016), and genetic and pharmacological manipulations of Area X and LMAN neurons in other species will be powerful tools for revealing the commonality of AFP contributions to syllable sequencing. Furthermore, dovetailing such experiments of sequence complexity with analyses of feedback processing will also extend our understanding of the relationship between sequence complexity and feedback signals.
The comparative approach can also be used to reveal how the brain encodes the neural representations of song repertoires. For example, recordings from awake juvenile and adult birds could reveal whether the brain uses different strategies to encode a large song repertoire, as in the case of nightingales, vs. a limited repertoire size, such as the song of the zebra finch. Such insights would provide a testable hypothesis about possible ways in which different behaviors and perceptual objects are preserved simultaneously in the human brain. In addition, investigation of the mechanisms underlying species variation in the ability to acquire vocalizations could further enhance our understanding of sensorimotor mechanisms for vocal learning (Beecher and Brenowitz 2005; Catchpole and Slater 2008). Indeed, revealing the genetic and molecular underpinnings of sensitive periods for learning is a central pursuit in neuroscience, and comparative analyses of sensorimotor integration and gene expression between birds that learn and birds that do not learn their songs, or between “close-ended learners” (species that only learn song during a restricted period in development, e.g., zebra and Bengalese finches) and “open-ended learners” (species that continue to learn songs as adults, e.g., canaries, European starlings, and nightingales), can help reveal the genes that could enable or prevent vocal learning (Haesler et al. 2004; Hilliard et al. 2012; Kato and Okanoya 2010; Matsunaga et al. 2008; Pfenning et al. 2014; Thompson et al. 2012). On the basis of findings in finches and sparrows, we hypothesize that HVCX neurons acquire a sensory representation of learned songs, regardless of age, and that this sensory representation is important for generating performance errors for song learning and maintenance for close-ended and open-ended learners.
Fortunately, the development and application of novel and powerful tools make such experiments possible. For example, techniques such as recording intracellularly from individual neurons in singing birds (Hamaguchi et al. 2014; Long et al. 2010), calcium imaging during juvenile song learning (Graber et al. 2013; Lynch et al. 2016; Markowitz et al. 2015; Peh et al. 2015), optogenetics (Deisseroth 2015; Roberts et al. 2012), and gene manipulation (Haesler et al. 2004; Lai et al. 2001; Liu et al. 2015; Murugan et al. 2013; Tanaka et al. 2016) open the door to testing hypotheses of sensorimotor integration that emerge from comparative studies and to assessing mechanisms across a diversity of songbird species. With the transfer of technologies to the songbird community, it will be even more advantageous to continue to expand the comparative approach to reveal fundamental mechanisms of sensorimotor integration for vocal learning and control. Just as new tools make possible new approaches that were previously inaccessible, the diversity of natural behaviors that are evident across songbird species will make possible investigation of topics that remain inaccessible unless we continue to expand our experimental vision to include a wide diversity of species.
GRANTS
This study was supported by National Science Foundation, Biological Sciences, Division of Integrative Organismal Systems Award IOS-1453084 and Center for Studies in Behavioral Neurobiology (Fonds de recherche du Québec – Santé).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M., L.S.J., J.T.S., and J.F.P. prepared figures; K.M., L.S.J., J.T.S., and J.F.P. drafted manuscript; K.M., L.S.J., J.T.S., and J.F.P. edited and revised manuscript; K.M., L.S.J., J.T.S., and J.F.P. approved final version of manuscript.
REFERENCES
- Abe K, Watanabe D. Songbirds possess the spontaneous ability to discriminate syntactic rules. Nat Neurosci 14: 1067–1074, 2011. doi: 10.1038/nn.2869. [DOI] [PubMed] [Google Scholar]
- Ackerley R, Kavounoudias A. The role of tactile afference in shaping motor behaviour and implications for prosthetic innovation. Neuropsychologia 79, Part B: 192–205, 2015. doi: 10.1016/j.neuropsychologia.2015.06.024. [DOI] [PubMed] [Google Scholar]
- Ahissar E, Kleinfeld D. Closed-loop neuronal computations: focus on vibrissa somatosensation in rat. Cereb Cortex 13: 53–62, 2003. doi: 10.1093/cercor/13.1.53. [DOI] [PubMed] [Google Scholar]
- Ali F, Otchy TM, Pehlevan C, Fantana AL, Burak Y, Ölveczky BP. The basal ganglia is necessary for learning spectral, but not temporal, features of birdsong. Neuron 80: 494–506, 2013. doi: 10.1016/j.neuron.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc Natl Acad Sci USA 106: 12518–12523, 2009. doi: 10.1073/pnas.0903214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher MD, Brenowitz EA. Functional aspects of song learning in songbirds. Trends Ecol Evol 20: 143–149, 2005. doi: 10.1016/j.tree.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Bell CC. Sensory coding and corollary discharge effects in mormyrid electric fish. J Exp Biol 146: 229–253, 1989. [DOI] [PubMed] [Google Scholar]
- Benton S, Nelson DA, Marler P, DeVoogd TJ. Anterior forebrain pathway is needed for stable song expression in adult male white-crowned sparrows (Zonotrichia leucophrys). Behav Brain Res 96: 135–150, 1998. doi: 10.1016/S0166-4328(98)00011-4. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Eda-Fujiwara H. Bird brains and songs: neural mechanisms of birdsong perception and memory. Anim Biol 53: 129–145, 2003. doi: 10.1163/157075603769700331. [DOI] [Google Scholar]
- Bolhuis JJ, Gobes SM, Terpstra NJ, den Boer-Visser AM, Zandbergen MA. Learning-related neuronal activation in the zebra finch song system nucleus HVC in response to the bird's own song. PLoS One 7: e41556, 2012. doi: 10.1371/journal.pone.0041556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Moorman S. Birdsong memory and the brain: in search of the template. Neurosci Biobehav Rev 50: 41–55, 2015. doi: 10.1016/j.neubiorev.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Altenau B. Parallel pathways for vocal learning in basal ganglia of songbirds. Nat Neurosci 13: 153–155, 2010. doi: 10.1038/nn.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard KE, Ganguli S, Brainard MS. Role of the site of synaptic competition and the balance of learning forces for Hebbian encoding of probabilistic Markov sequences. Front Comput Neurosci 9: 92, 2015. doi: 10.3389/fncom.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nat Rev Neurosci 1: 31–40, 2000a. doi: 10.1038/35036205. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature 404: 762–766, 2000b. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature 417: 351–358, 2002. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Translating birdsong: songbirds as a model for basic and applied medical research. Annu Rev Neurosci 36: 489–517, 2013. doi: 10.1146/annurev-neuro-060909-152826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Beecher MD. Song learning in birds: diversity and plasticity, opportunities and challenges. Trends Neurosci 28: 127–132, 2005. doi: 10.1016/j.tins.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Broussard DM, Kassardjian CD. Learning in a simple motor system. Learn Mem 11: 127–136, 2004. doi: 10.1101/lm.65804. [DOI] [PubMed] [Google Scholar]
- Butler AB, Reiner A, Karten HJ. Evolution of the amniote pallium and the origins of mammalian neocortex. Ann N Y Acad Sci 1225: 14–27, 2011. doi: 10.1111/j.1749-6632.2011.06006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canopoli A, Herbst JA, Hahnloser RH. A higher sensory brain region is involved in reversing reinforcement-induced vocal changes in a songbird. J Neurosci 34: 7018–7026, 2014. doi: 10.1523/JNEUROSCI.0266-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness, and suppressed by arousal. J Neurophysiol 90: 2884–2899, 2003. doi: 10.1152/jn.00391.2003. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Auditory responses in multiple sensorimotor song system nuclei are co-modulated by behavioral state. J Neurophysiol 91: 2148–2163, 2004. doi: 10.1152/jn.00918.2003. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Ball GF. A role for norepinephrine in the regulation of context-dependent ZENK expression in male zebra finches (Taeniopygia guttata). Eur J Neurosci 21: 1962–1972, 2005. doi: 10.1111/j.1460-9568.2005.04028.x. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Birdsong: Biological Themes and Variations. Cambridge, UK: Cambridge University Press, 2008. doi: 10.1017/CBO9780511754791. [DOI] [Google Scholar]
- Charlesworth JD, Tumer EC, Warren TL, Brainard MS. Learning the microstructure of successful behavior. Nat Neurosci 14: 373–380, 2011. doi: 10.1038/nn.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth JD, Warren TL, Brainard MS. Covert skill learning in a cortical-basal ganglia circuit. Nature 486: 251–255, 2012. doi: 10.1038/nature11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Roy A, Wild JM, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. J Neurosci 27: 10024–10036, 2007. doi: 10.1523/JNEUROSCI.2215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie R, Douglas-Cowie E. Postlingually Acquired Deafness: Speech Deterioration and the Wider Consequences. New York: Mouton de Gruyter, 1992. doi: 10.1515/9783110869125. [DOI] [Google Scholar]
- Crapse TB, Sommer MA. Corollary discharge circuits in the primate brain. Curr Opin Neurobiol 18: 552–557, 2008. doi: 10.1016/j.conb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynx J, Von Rad U. Immediate and transitory effects of delayed auditory feedback on bird song production. Anim Behav 62: 305–312, 2001. doi: 10.1006/anbe.2001.1744. [DOI] [Google Scholar]
- Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci 18: 1213–1225, 2015. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoogd TJ, Krebs JR, Healy SD, Purvis A. Relations between song repertoire size and the volume of brain nuclei related to song: comparative evolutionary analyses amongst oscine birds. Proc Biol Sci 254: 75–82, 1993. doi: 10.1098/rspb.1993.0129. [DOI] [PubMed] [Google Scholar]
- Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat Rev Neurosci 9: 601–612, 2008. doi: 10.1038/nrn2411. [DOI] [PubMed] [Google Scholar]
- Dobson CW, Lemon RE. Markov sequences in songs of American thrushes. Behaviour 68: 86–105, 1979. doi: 10.1163/156853979X00250. [DOI] [Google Scholar]
- Donath TM, Natke U, Kalveram KT. Effects of frequency-shifted auditory feedback on voice F0 contours in syllables. J Acoust Soc Am 111: 357–366, 2002. doi: 10.1121/1.1424870. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Gephart BF, Price PH, McHale C, Brauth SE. Effects of deafening on the contact call of the budgerigar, Melopsittacus-undulatus. Anim Behav 35: 1264–1266, 1987. doi: 10.1016/S0003-3472(87)80190-2. [DOI] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci 22: 567–631, 1999. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Ertmer DJ, Goffman L. Speech production accuracy and variability in young cochlear implant recipients: comparisons with typically developing age-peers. J Speech Lang Hear Res 54: 177–189, 2011. doi: 10.1044/1092-4388(2010/09-0165). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Scharff C. The songbird as a model for the generation and learning of complex sequential behaviors. ILAR J 51: 362–377, 2010. doi: 10.1093/ilar.51.4.362. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull 4: 636–640, 1978. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Guazzelli M. Schizophrenia: a disorder of the corollary discharge systems that integrate the motor systems of thought with the sensory systems of consciousness. Br J Psychiatry 174: 196–204, 1999. doi: 10.1192/bjp.174.3.196. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick EM, Crawford L, Ni A, Durieux-Smith A. A descriptive analysis of language and speech skills in 4- to 5-yr-old children with hearing loss. Ear Hear 32: 605–616, 2011. doi: 10.1097/AUD.0b013e31821348ae. [DOI] [PubMed] [Google Scholar]
- Flanders M. What is the biological basis of sensorimotor integration? Biol Cybern 104: 1–8, 2011. doi: 10.1007/s00422-011-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Cytoarchitectonic organization and morphology of cells of the field L complex in male zebra finches (Taenopygia guttata). J Comp Neurol 325: 388–404, 1992. doi: 10.1002/cne.903250306. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Hasegawa T, Watanabe D. Neural coding of syntactic structure in learned vocalizations in the songbird. J Neurosci 31: 10023–10033, 2011. doi: 10.1523/JNEUROSCI.1606-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadagkar V, Puzerey PA, Chen R, Baird-Daniel E, Farhang AR, Goldberg JH. Dopamine neurons encode performance error in singing birds. Science 354: 1278–1282, 2016. doi: 10.1126/science.aah6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci 34: 205–231, 2011. doi: 10.1146/annurev-neuro-061010-113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George I, Cousillas H, Richard JP, Hausberger M. State-dependent hemispheric specialization in the songbird brain. J Comp Neurol 488: 48–60, 2005. doi: 10.1002/cne.20584. [DOI] [PubMed] [Google Scholar]
- Graber MH, Helmchen F, Hahnloser RH. Activity in a premotor cortical nucleus of zebra finches is locally organized and exhibits auditory selectivity in neurons but not in glia. PLoS One 8: e81177, 2013. doi: 10.1371/journal.pone.0081177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C. FoxP2 expression in avian vocal learners and non-learners. J Neurosci 24: 3164–3175, 2004. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi K, Mooney R. Recurrent interactions between the input and output of a songbird cortico-basal ganglia pathway are implicated in vocal sequence variability. J Neurosci 32: 11671–11687, 2012. doi: 10.1523/JNEUROSCI.1666-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi K, Tschida KA, Yoon I, Donald BR, Mooney R. Auditory synapses to song premotor neurons are gated off during vocalization in zebra finches. eLife 3: e01833, 2014. doi: 10.7554/eLife.01833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton CM, Sakata JT, Brainard MS. An avian basal ganglia-forebrain circuit contributes differentially to syllable versus sequence variability of adult Bengalese finch song. J Neurophysiol 101: 3235–3245, 2009. doi: 10.1152/jn.91089.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci 25: 3406–3416, 2007. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton JT, Dooling RJ, Farabaugh SM. Effects of deafening on the calls and warble song of adult budgerigars (Melopsittacus undulatus). J Acoust Soc Am 105: 2010–2019, 1999. doi: 10.1121/1.426734. [DOI] [PubMed] [Google Scholar]
- Heinig A, Pant S, Dunning J, Bass A, Coburn Z, Prather JF. Male mate preferences in mutual mate choice: finches modulate their songs across and within male-female interactions. Anim Behav 97: 1–12, 2014. doi: 10.1016/j.anbehav.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Rogalsky C. What does Broca's area activation to sentences reflect? J Cogn Neurosci 23: 2629–2631; discussion 2632–2635, 2011. doi: 10.1162/jocn_a_00044. [DOI] [PubMed] [Google Scholar]
- Hilliard AT, Miller JE, Horvath S, White SA. Distinct neurogenomic states in basal ganglia subregions relate differently to singing behavior in songbirds. PLOS Comput Biol 8: e1002773, 2012. doi: 10.1371/journal.pcbi.1002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann LA, Saravanan V, Wood AN, He L, Sober SJ. Dopaminergic contributions to vocal learning. J Neurosci 36: 2176–2189, 2016. doi: 10.1523/JNEUROSCI.3883-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann LA, Sober SJ. Vocal generalization depends on gesture identity and sequence. J Neurosci 34: 5564–5574, 2014. doi: 10.1523/JNEUROSCI.5169-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosino T, Okanoya K. Lesion of a higher-order song nucleus disrupts phrase level complexity in Bengalese finches. Neuroreport 11: 2091–2095, 2000. doi: 10.1097/00001756-200007140-00007. [DOI] [PubMed] [Google Scholar]
- Houde JF, Jordan MI. Sensorimotor adaptation of speech. I: compensation and adaptation. J Speech Lang Hear Res 45: 295–310, 2002. doi: 10.1044/1092-4388(2002/023). [DOI] [PubMed] [Google Scholar]
- Hough GE II, Nelson DA, Volman SF. Re-expression of songs deleted during vocal development in white-crowned sparrows, Zonotrichia leucophrys. Anim Behav 60: 279–287, 2000. doi: 10.1006/anbe.2000.1498. [DOI] [PubMed] [Google Scholar]
- Howell P, Archer A. Susceptibility to the effects of delayed auditory feedback. Percept Psychophys 36: 296–302, 1984. doi: 10.3758/BF03206371. [DOI] [PubMed] [Google Scholar]
- James LS, Sakata JT. Vocal motor changes beyond the sensitive period for song plasticity. J Neurophysiol 112: 2040–2052, 2014. doi: 10.1152/jn.00217.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LS, Sakata JT. Predicting plasticity: acute context-dependent changes to vocal performance predict long-term age-dependent changes. J Neurophysiol 114: 2328–2339, 2015. doi: 10.1152/jn.00688.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata P, Margoliash D. Gradual emergence of song selectivity in sensorimotor structures of the male zebra finch song system. J Neurosci 19: 5108–5118, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci 1016: 749–777, 2004. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Güntürkün O, Bruce L, Csillag A, Karten H, Kuenzel W, Medina L, Paxinos G, Perkel DJ, Shimizu T, Striedter G, Wild JM, Ball GF, Dugas-Ford J, Durand SE, Hough GE, Husband S, Kubikova L, Lee DW, Mello CV, Powers A, Siang C, Smulders TV, Wada K, White SA, Yamamoto K, Yu J, Reiner A, Butler AB; Avian Brain Nomenclature Consortium . Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci 6: 151–159, 2005. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron 21: 775–788, 1998. doi: 10.1016/S0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Jin DZ. Generating variable birdsong syllable sequences with branching chain networks in avian premotor nucleus HVC. Phys Rev E Stat Nonlin Soft Matter Phys 80: 051902, 2009. doi: 10.1103/PhysRevE.80.051902. [DOI] [PubMed] [Google Scholar]
- Kagawa H, Takahashi R, Ikebuchi M, Okanoya K. Song complexity and auditory feedback in birds: a comparison between two strains of Bengalese finches with different degrees of song complexity. Zool Sci 29: 645–651, 2012. doi: 10.2108/zsj.29.645. [DOI] [PubMed] [Google Scholar]
- Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol 96: 1441–1455, 2006. doi: 10.1152/jn.01138.2005. [DOI] [PubMed] [Google Scholar]
- Karten HJ. The ascending auditory pathway in the pigeon (Columba livia). II. Telencephalic projections of the nucleus ovoidalis thalami. Brain Res 11: 134–153, 1968. doi: 10.1016/0006-8993(68)90078-4. [DOI] [PubMed] [Google Scholar]
- Karten HJ, Hodos W. A Stereotrucic Atlas of the Brain of the Pigeon. Baltimore, MD: Johns Hopkins, 1967. [Google Scholar]
- Katahira K, Suzuki K, Kagawa H, Okanoya K. A simple explanation for the evolution of complex song syntax in Bengalese finches. Biol Lett 9: 20130842, 2013. doi: 10.1098/rsbl.2013.0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Okanoya K. Molecular characterization of the song control nucleus HVC in Bengalese finch brain. Brain Res 1360: 56–76, 2010. doi: 10.1016/j.brainres.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Katz LC, Gurney ME. Auditory responses in the zebra finch's motor system for song. Brain Res 221: 192–197, 1981. doi: 10.1016/0006-8993(81)91073-8. [DOI] [PubMed] [Google Scholar]
- Keller GB, Hahnloser RH. Neural processing of auditory feedback during vocal practice in a songbird. Nature 457: 187–190, 2009. doi: 10.1038/nature07467. [DOI] [PubMed] [Google Scholar]
- Kinnischtzke AK, Simons DJ, Fanselow EE. Motor cortex broadly engages excitatory and inhibitory neurons in somatosensory barrel cortex. Cereb Cortex 24: 2237–2248, 2014. doi: 10.1093/cercor/bht085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Berg RW, O'Connor SM. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens Mot Res 16: 69–88, 1999. doi: 10.1080/08990229970528. [DOI] [PubMed] [Google Scholar]
- Konishi M. Effects of deafening on song development in American robins and black-headed grosbeaks. Z Tierpsychol 22: 584–599, 1965a. [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol 22: 770–783, 1965b. [PubMed] [Google Scholar]
- Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol 97: 4271–4283, 2007. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- Krahe R, Maler L. Neural maps in the electrosensory system of weakly electric fish. Curr Opin Neurobiol 24: 13–21, 2014. doi: 10.1016/j.conb.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Krützfeldt NO, Logerot P, Kubke MF, Wild JM. Connections of the auditory brainstem in a songbird, Taeniopygia guttata. I. Projections of nucleus angularis and nucleus laminaris to the auditory torus. J Comp Neurol 518: 2109–2134, 2010a. doi: 10.1002/cne.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krützfeldt NO, Logerot P, Kubke MF, Wild JM. Connections of the auditory brainstem in a songbird, Taeniopygia guttata. II. Projections of nucleus angularis and nucleus laminaris to the superior olive and lateral lemniscal nuclei. J Comp Neurol 518: 2135–2148, 2010b. doi: 10.1002/cne.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L, Bosikova E, Cvikova M, Lukacova K, Scharff C, Jarvis ED. Basal ganglia function, stuttering, sequencing, and repair in adult songbirds. Sci Rep 4: 6590, 2014. doi: 10.1038/srep06590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron 67: 713–727, 2010. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413: 519–523, 2001. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Larson CR, Burnett TA, Kiran S, Hain TC. Effects of pitch-shift velocity on voice Fo responses. J Acoust Soc Am 107: 559–564, 2000. doi: 10.1121/1.428323. [DOI] [PubMed] [Google Scholar]
- Lee BS. Effects of delayed speech feedback. J Acoust Soc Am 22: 824–826, 1950. doi: 10.1121/1.1906696. [DOI] [Google Scholar]
- Lee SB, Beak SK, Park SH, Waterhouse BD, Lee HS. Collateral projection from the locus coeruleus to whisker-related sensory and motor brain regions of the rat. J Comp Neurol 514: 387–402, 2009. doi: 10.1002/cne.22012. [DOI] [PubMed] [Google Scholar]
- Lehongre K, Del Negro C. Representation of the bird's own song in the canary HVC: contribution of broadly tuned neurons. Neuroscience 173: 93–109, 2011. doi: 10.1016/j.neuroscience.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Leonardo A. Experimental test of the birdsong error-correction model. Proc Natl Acad Sci USA 101: 16935–16940, 2004. doi: 10.1073/pnas.0407870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski B, Vyssotski A, Hahnloser RH, Schmidt M. At the interface of the auditory and vocal motor systems: NIf and its role in vocal processing, production and learning. J Physiol Paris 107: 178–192, 2013. doi: 10.1016/j.jphysparis.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki MS, Konishi M. Mechanisms underlying the sensitivity of songbird forebrain neurons to temporal order. Proc Natl Acad Sci USA 92: 5582–5586, 1995. doi: 10.1073/pnas.92.12.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WC, Kohn J, Szwed SK, Pariser E, Sepe S, Haripal B, Oshimori N, Marsala M, Miyanohara A, Lee R. Human mutant huntingtin disrupts vocal learning in transgenic songbirds. Nat Neurosci 18: 1617–1622, 2015. doi: 10.1038/nn.4133. [DOI] [PubMed] [Google Scholar]
- Liu WC, Nottebohm F. A learning program that ensures prompt and versatile vocal imitation. Proc Natl Acad Sci USA 104: 20398–20403, 2007. doi: 10.1073/pnas.0710067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardino AJ, Nottebohm F. Age at deafening affects the stability of learned song in adult male zebra finches. J Neurosci 20: 5054–5064, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci 11: 579–586, 2008. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Jin DZ, Fee MS. Support for a synaptic chain model of neuronal sequence generation. Nature 468: 394–399, 2010. doi: 10.1038/nature09514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GF, Okubo TS, Hanuschkin A, Hahnloser RH, Fee MS. Rhythmic continuous-time coding in the songbird analog of vocal motor cortex. Neuron 90: 877–892, 2016. doi: 10.1016/j.neuron.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y, Las L, Denisenko N, Fee MS. A role for descending auditory cortical projections in songbird vocal learning. eLife 3: 02152, 2014. doi: 10.7554/eLife.04371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D, Schmidt MF. Sleep, off-line processing, and vocal learning. Brain Lang 115: 45–58, 2010. doi: 10.1016/j.bandl.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JE, Liberti WA III, Guitchounts G, Velho T, Lois C, Gardner TJ. Mesoscopic patterns of neural activity support songbird cortical sequences. PLoS Biol 13: e1002158, 2015. doi: 10.1371/journal.pbio.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. Three models of song learning: evidence from behavior. J Neurobiol 33: 501–516, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Marler P, Waser MS. Role of auditory feedback in canary song development. J Comp Physiol Psychol 91: 8–16, 1977. doi: 10.1037/h0077303. [DOI] [PubMed] [Google Scholar]
- Matheson AM, Sakata JT. Relationship between the sequencing and timing of vocal motor elements in birdsong. PLoS One 10: e0143203, 2015. doi: 10.1371/journal.pone.0143203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson LE, Sun H, Sakata JT. Forebrain circuits underlying the social modulation of vocal communication signals. Dev Neurobiol 76: 47–63, 2016. doi: 10.1002/dneu.22298. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Kato M, Okanoya K. Comparative analysis of gene expressions among avian brains: a molecular approach to the evolution of vocal learning. Brain Res Bull 75: 474–479, 2008. doi: 10.1016/j.brainresbull.2007.10.045. [DOI] [PubMed] [Google Scholar]
- McCasland JS. Neuronal control of bird song production. J Neurosci 7: 23–39, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland JS, Konishi M. Interaction between auditory and motor activities in an avian song control nucleus. Proc Natl Acad Sci USA 78: 7815–7819, 1981. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller MP, Tomblin JB, Yoshinaga-Itano C, Connor CM, Jerger S. Current state of knowledge: language and literacy of children with hearing impairment. Ear Hear 28: 740–753, 2007. doi: 10.1097/AUD.0b013e318157f07f. [DOI] [PubMed] [Google Scholar]
- Mollaei F, Shiller DM, Baum SR, Gracco VL. Sensorimotor control of vocal pitch and formant frequencies in Parkinson's disease. Brain Res 1646: 269–277, 2016. doi: 10.1016/j.brainres.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollaei F, Shiller DM, Gracco VL. Sensorimotor adaptation of speech in Parkinson's disease. Mov Disord 28: 1668–1674, 2013. doi: 10.1002/mds.25588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learn Mem 16: 655–669, 2009a. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Mooney R. Neurobiology of song learning. Curr Opin Neurobiol 19: 654–660, 2009b. doi: 10.1016/j.conb.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Hoese W, Nowicki S. Auditory representation of the vocal repertoire in a songbird with multiple song types. Proc Natl Acad Sci USA 98: 12778–12783, 2001. doi: 10.1073/pnas.221453298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Prather JF, Roberts T. Neurophysiology of birdsong learning. In: Learning and Memory: A Comprehensive Reference, edited by Eichenbaum H. Oxford, UK: Elsevier, 2008, p. 441–474. doi: 10.1016/B978-012370509-9.00116-9. [DOI] [Google Scholar]
- Moore JM, Székely T, Büki J, Devoogd TJ. Motor pathway convergence predicts syllable repertoire size in oscine birds. Proc Natl Acad Sci USA 108: 16440–16445, 2011. doi: 10.1073/pnas.1102077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan M, Harward S, Scharff C, Mooney R. Diminished FoxP2 levels affect dopaminergic modulation of corticostriatal signaling important to song variability. Neuron 80: 1464–1476, 2013. doi: 10.1016/j.neuron.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naie K, Hahnloser RH. Regulation of learned vocal behavior by an auditory motor cortical nucleus in juvenile zebra finches. J Neurophysiol 106: 291–300, 2011. doi: 10.1152/jn.01035.2010. [DOI] [PubMed] [Google Scholar]
- Nealen PM, Schmidt MF. Comparative approaches to avian song system function: insights into auditory and motor processing. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 188: 929–941, 2002. doi: 10.1007/s00359-002-0357-z. [DOI] [PubMed] [Google Scholar]
- Nealen PM, Schmidt MF. Distributed and selective auditory representation of song repertoires in the avian song system. J Neurophysiol 96: 3433–3447, 2006. doi: 10.1152/jn.01130.2005. [DOI] [PubMed] [Google Scholar]
- Nelken I, Bar-Yosef O. Neurons and objects: the case of auditory cortex. Front Neurosci 2: 107–113, 2008. doi: 10.3389/neuro.01.009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol 62: 231–242, 2005. doi: 10.1002/neu.20087. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol 57: 58–66, 1992. doi: 10.1016/0163-1047(92)90757-U. [DOI] [PubMed] [Google Scholar]
- Nott P, Cowan R, Brown PM, Wigglesworth G. Early language development in children with profound hearing loss fitted with a device at a young age: part I–the time period taken to acquire first words and first word combinations. Ear Hear 30: 526–540, 2009. doi: 10.1097/AUD.0b013e3181a9ea14. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science 194: 211–213, 1976. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Okanoya K. The Bengalese finch: a window on the behavioral neurobiology of birdsong syntax. Ann N Y Acad Sci 1016: 724–735, 2004. doi: 10.1196/annals.1298.026. [DOI] [PubMed] [Google Scholar]
- Okanoya K, Yamaguchi A. Adult Bengalese finches (Lonchura striata var. domestica) require real-time auditory feedback to produce normal song syntax. J Neurobiol 33: 343–356, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Oller DK, Eilers RE. The role of audition in infant babbling. Child Dev 59: 441–449, 1988. doi: 10.2307/1130323. [DOI] [PubMed] [Google Scholar]
- Osmanski MS, Dooling RJ. The effect of altered auditory feedback on control of vocal production in budgerigars (Melopsittacus undulatus). J Acoust Soc Am 126: 911–919, 2009. doi: 10.1121/1.3158928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otchy TM, Wolff SB, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SM, Ölveczky BP. Acute off-target effects of neural circuit manipulations. Nature 528: 358–363, 2015. doi: 10.1038/nature16442. [DOI] [PubMed] [Google Scholar]
- Peh WY, Roberts TF, Mooney R. Imaging auditory representations of song and syllables in populations of sensorimotor neurons essential to vocal communication. J Neurosci 35: 5589–5605, 2015. doi: 10.1523/JNEUROSCI.2308-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov CI, Jarvis ED. Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front Evol Neurosci 4: 12, 2012. doi: 10.3389/fnevo.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenning AR, Hara E, Whitney O, Rivas MV, Wang R, Roulhac PL, Howard JT, Wirthlin M, Lovell PV, Ganapathy G, Mouncastle J, Moseley MA, Thompson JW, Soderblom EJ, Iriki A, Kato M, Gilbert MTP, Zhang G, Bakken T, Bongaarts A, Bernard A, Lein E, Mello CV, Hartemink AJ, Jarvis ED. Convergent transcriptional specializations in the brains of humans and song-learning birds. Science 346: 1256846, 2014. doi: 10.1126/science.1256846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piristine HC, Choetso T, Gobes SM. A sensorimotor area in the songbird brain is required for production of vocalizations in the song learning period of development. Dev Neurobiol 76: 1213–1225, 2016. doi: 10.1002/dneu.22384. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Hedwig B. A corollary discharge maintains auditory sensitivity during sound production. Nature 418: 872–876, 2002. doi: 10.1038/nature00919. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Hedwig B. The cellular basis of a corollary discharge. Science 311: 518–522, 2006. doi: 10.1126/science.1120847. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Hedwig B. New insights into corollary discharges mediated by identified neural pathways. Trends Neurosci 30: 14–21, 2007. doi: 10.1016/j.tins.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Prather JF. Auditory signal processing in communication: perception and performance of vocal sounds. Hear Res 305: 144–155, 2013. doi: 10.1016/j.heares.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Nowicki S, Anderson RC, Peters S, Mooney R. Neural correlates of categorical perception in learned vocal communication. Nat Neurosci 12: 221–228, 2009. doi: 10.1038/nn.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature 451: 305–310, 2008. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Persistent representation of juvenile experience in the adult songbird brain. J Neurosci 30: 10586–10598, 2010. doi: 10.1523/JNEUROSCI.6042-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell DW, Munhall KG. Compensation following real-time manipulation of formants in isolated vowels. J Acoust Soc Am 119: 2288–2297, 2006. doi: 10.1121/1.2173514. [DOI] [PubMed] [Google Scholar]
- Raksin JN, Glaze CM, Smith S, Schmidt MF. Linear and nonlinear auditory response properties of interneurons in a high-order avian vocal motor nucleus during wakefulness. J Neurophysiol 107: 2185–2201, 2012. doi: 10.1152/jn.01003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauske PL, Shea SD, Margoliash D. State and neuronal class-dependent reconfiguration in the avian song system. J Neurophysiol 89: 1688–1701, 2003. doi: 10.1152/jn.00655.2002. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Gobes SM, Murugan M, Ölveczky BP, Mooney R. Motor circuits are required to encode a sensory model for imitative learning. Nat Neurosci 15: 1454–1459, 2012. doi: 10.1038/nn.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Klein ME, Kubke MF, Wild JM, Mooney R. Telencephalic neurons monosynaptically link brainstem and forebrain premotor networks necessary for song. J Neurosci 28: 3479–3489, 2008. doi: 10.1523/JNEUROSCI.0177-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MJ, Mooney R. Inhibitory and excitatory mechanisms underlying auditory responses to learned vocalizations in the songbird nucleus HVC. Neuron 39: 177–194, 2003. doi: 10.1016/S0896-6273(03)00357-X. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Real-time contributions of auditory feedback to avian vocal motor control. J Neurosci 26: 9619–9628, 2006. doi: 10.1523/JNEUROSCI.2027-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Online contributions of auditory feedback to neural activity in avian song control circuitry. J Neurosci 28: 11378–11390, 2008. doi: 10.1523/JNEUROSCI.3254-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Social context rapidly modulates the influence of auditory feedback on avian vocal motor control. J Neurophysiol 102: 2485–2497, 2009. doi: 10.1152/jn.00340.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Hampton CM, Brainard MS. Social modulation of sequence and syllable variability in adult birdsong. J Neurophysiol 99: 1700–1711, 2008. doi: 10.1152/jn.01296.2007. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Vehrencamp SL. Integrating perspectives on vocal performance and consistency. J Exp Biol 215: 201–209, 2012. doi: 10.1242/jeb.056911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res 44: 1453–1467, 2004. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron 25: 481–492, 2000. doi: 10.1016/S0896-6273(00)80910-1. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci 11: 2896–2913, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwers K, Gillis S, Govaerts PJ. The characteristics of prelexical babbling after cochlear implantation between 5 and 20 months of age. Ear Hear 29: 627–637, 2008. doi: 10.1097/AUD.0b013e318174f03c. [DOI] [PubMed] [Google Scholar]
- Schick B, Moeller MP. What is learnable in manually coded English sign systems? Appl Psycholinguist 13: 313–340, 1992. doi: 10.1017/S014271640000566X. [DOI] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol 57: 1033–1049, 1987. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, Konishi M. Gating of auditory responses in the vocal control system of awake songbirds. Nat Neurosci 1: 513–518, 1998. doi: 10.1038/2232. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Lee T. Motor Control and Learning: A Behavioral Emphasis. Champaign, IL: Sheridan, 2011. [Google Scholar]
- Schubert D, Nadif Kasri N, Celikel T, Homberg J. Impact of monoaminergic neuromodulators on the development of sensorimotor circuits. In: Sensorimotor Integration in the Whisker System, edited by Krieger P and Groh A. New York: Springer, 2015, p. 243–273. doi: 10.1007/978-1-4939-2975-7_11. [DOI] [Google Scholar]
- Scott LL, Nordeen EJ, Nordeen KW. The relationship between rates of HVc neuron addition and vocal plasticity in adult songbirds. J Neurobiol 43: 79–88, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Singleton JL, Supalla S, Litchfield S, Schley S. From sign to word: considering modality constraints in ASL/English bilingual education. Top Lang Disord 18: 16–29, 1998. doi: 10.1097/00011363-199808000-00004. [DOI] [Google Scholar]
- Sober SJ, Brainard MS. Adult birdsong is actively maintained by error correction. Nat Neurosci 12: 927–931, 2009. doi: 10.1038/nn.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol 53: 51–63, 1990. doi: 10.1016/0163-1047(90)90797-A. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science 296: 1480–1482, 2002. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. II. Role of the SC-MD-FEF pathway in corollary discharge. J Neurophysiol 91: 1403–1423, 2004. doi: 10.1152/jn.00740.2003. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Visual perception and corollary discharge. Perception 37: 408–418, 2008. doi: 10.1068/p5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossinka R, Bohner J. Song types in the zebra finch Poephila guttata castanotis. Z Tierpsychol 53: 123–132, 1980. doi: 10.1111/j.1439-0310.1980.tb01044.x. [DOI] [Google Scholar]
- Stoel-Gammon C, Otomo K. Babbling development of hearing-impaired and normally hearing subjects. J Speech Hear Disord 51: 33–41, 1986. doi: 10.1044/jshd.5101.33. [DOI] [PubMed] [Google Scholar]
- Stuart A, Kalinowski J, Rastatter MP, Lynch K. Effect of delayed auditory feedback on normal speakers at two speech rates. J Acoust Soc Am 111: 2237–2241, 2002. doi: 10.1121/1.1466868. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge, MA: MIT Press, 1998. [Google Scholar]
- Tachibana RO, Koumura T, Okanoya K. Variability in the temporal parameters in the song of the Bengalese finch (Lonchura striata var. domestica). J Comp Physiol A Neuroethol Sens Neural Behav Physiol 201: 1157–1168, 2015. doi: 10.1007/s00359-015-1046-z. [DOI] [PubMed] [Google Scholar]
- Takahasi M, Yamada H, Okanoya K. Statistical and prosodic cues for song segmentation learning by Bengalese finches (Lonchura striata var. domestica). Ethology 116: 481–489, 2010. doi: 10.1111/j.1439-0310.2010.01772.x. [DOI] [Google Scholar]
- Tanaka M, Singh Alvarado J, Murugan M, Mooney R. Focal expression of mutant huntingtin in the songbird basal ganglia disrupts cortico-basal ganglia networks and vocal sequences. Proc Natl Acad Sci USA 113: E1720–E1727, 2016. doi: 10.1073/pnas.1523754113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science 291: 2564–2569, 2001. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Meitzen J, Replogle K, Drnevich J, Lent KL, Wissman AM, Farin FM, Bammler TK, Beyer RP, Clayton DF, Perkel DJ, Brenowitz EA. Seasonal changes in patterns of gene expression in avian song control brain regions. PLoS One 7: e35119, 2012. doi: 10.1371/journal.pone.0035119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe WH. The learning of song patterns by birds, with special reference to the song of the chaffinch, Fringilla coelebs. Ibis 100: 535–570, 1958. doi: 10.1111/j.1474-919X.1958.tb07960.x. [DOI] [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage 39: 1429–1443, 2008. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumanova V, Zebrowski PM, Goodman SS, Arenas RM. Motor practice effects and sensorimotor integration in adults who stutter: evidence from visuomotor tracking performance. J Fluency Disord 45: 52–72, 2015. doi: 10.1016/j.jfludis.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature 450: 1240–1244, 2007. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- Vallentin D, Kosche G, Lipkind D, Long MA. Inhibition protects acquired song segments during vocal learning in zebra finches. Science 351: 267–271, 2016. doi: 10.1126/science.aad3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallentin D, Long MA. Motor origin of precise synaptic inputs onto forebrain neurons driving a skilled behavior. J Neurosci 35: 299–307, 2015. doi: 10.1523/JNEUROSCI.3698-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman SF. Development of neural selectivity for birdsong during vocal learning. J Neurosci 13: 4737–4747, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein RS. Effects of postlingual deafness on speech production: implications for the role of auditory feedback. J Acoust Soc Am 88: 2099–2114, 1990. doi: 10.1121/1.400107. [DOI] [PubMed] [Google Scholar]
- Warren TL, Charlesworth JD, Tumer EC, Brainard MS. Variable sequencing is actively maintained in a well learned motor skill. J Neurosci 32: 15414–15425, 2012. doi: 10.1523/JNEUROSCI.1254-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren TL, Tumer EC, Charlesworth JD, Brainard MS. Mechanisms and time course of vocal learning and consolidation in the adult songbird. J Neurophysiol 106: 1806–1821, 2011. doi: 10.1152/jn.00311.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann G, Miranda ER. A new model of sensorimotor coupling in the development of speech. Brain Lang 89: 393–400, 2004. doi: 10.1016/S0093-934X(03)00345-6. [DOI] [PubMed] [Google Scholar]
- Wild JM, Krützfeldt NO, Kubke MF. Connections of the auditory brainstem in a songbird, Taeniopygia guttata. III. Projections of the superior olive and lateral lemniscal nuclei. J Comp Neurol 518: 2149–2167, 2010. doi: 10.1002/cne.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth MJ, Sober SJ, Brainard MS. Linked control of syllable sequence and phonology in birdsong. J Neurosci 30: 12936–12949, 2010. doi: 10.1523/JNEUROSCI.2690-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SC, Kao MH. Variability in action: Contributions of a songbird cortical-basal ganglia circuit to vocal motor learning and control. Neuroscience 296: 39–47, 2015. doi: 10.1016/j.neuroscience.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Rubel EW. Bengalese finches Lonchura striata domestica depend upon auditory feedback for the maintenance of adult song. J Neurosci 17: 6380–6390, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Larson CR, Bauer JJ, Hain TC. Compensation for pitch-shifted auditory feedback during the production of Mandarin tone sequences. J Acoust Soc Am 116: 1168–1178, 2004. doi: 10.1121/1.1763952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann R. The Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford, UK: Oxford University Press, 1996. [Google Scholar]