Abstract
Objective:
It remains unclear whether drug-resistant temporal lobe epilepsy (TLE) is associated with cumulative brain damage, with no expert consensus and no quantitative syntheses of the available evidence.
Methods:
We conducted a systematic review and meta-analysis of MRI studies on progressive atrophy, searching PubMed and Ovid MEDLINE databases for cross-sectional and longitudinal quantitative MRI studies on drug-resistant TLE.
Results:
We screened 2,976 records and assessed eligibility of 248 full-text articles. Forty-two articles met the inclusion criteria for quantitative evaluation. We observed a predominance of cross-sectional studies, use of different clinical indices of progression, and high heterogeneity in age-control procedures. Meta-analysis of 18/1 cross-sectional/longitudinal studies on hippocampal atrophy (n = 979 patients) yielded a pooled effect size of r = −0.42 for ipsilateral atrophy related to epilepsy duration (95% confidence interval [CI] −0.51 to −0.32; p < 0.0001; I2 = 65.22%) and r = −0.35 related to seizure frequency (95% CI −0.47 to −0.22; p < 0.0001; I2 = 61.97%). Sensitivity analyses did not change the results. Narrative synthesis of 25/3 cross-sectional/longitudinal studies on whole brain atrophy (n = 1,504 patients) indicated that >80% of articles reported duration-related progression in extratemporal cortical and subcortical regions. Detailed analysis of study design features yielded low to moderate levels of evidence for progressive atrophy across studies, mainly due to dominance of cross-sectional over longitudinal investigations, use of diverse measures of seizure estimates, and absence of consistent age control procedures.
Conclusions:
While the neuroimaging literature is overall suggestive of progressive atrophy in drug-resistant TLE, published studies have employed rather weak designs to directly demonstrate it. Longitudinal multicohort studies are needed to unequivocally differentiate aging from disease progression.
Temporal lobe epilepsy (TLE) is the most common drug-resistant epilepsy in adults. Despite randomized controlled trials showing that surgery is the most effective treatment,1,2 an average delay of 20 years passes between initial diagnosis and surgical intervention.3 Epilepsy surgery remains indeed underutilized, with only a fraction of drug-resistant patients being evaluated in tertiary centers.4 This decade-long delay period is associated with increased risk of injury and mortality, affective disturbances, cognitive decline, and marked socioeconomic consequences.5
Evidence for disease progression would provide a strong incentive for targeted screening and accelerated referrals. However, despite the century-old hypothesis that seizures beget seizures6 and studies suggesting that patients with longer epilepsy duration may show a worsening of seizure control, possibly more widespread epileptogenic networks, and increased challenges to maintain adequate quality of life,5 controversy remains as to whether the disease follows a progressive course.
Quantitative MRI lends imaging markers with biological validity and high test-retest reliability, representing an objective framework to test for disease progression. While some studies suggested atrophy related to high seizure frequency and longer epilepsy duration, others did not. To quantitatively synthesize the currently attained level of evidence for progressive atrophy in TLE, we undertook a systematic review and meta-analysis. In addition to integrating findings from hippocampal and whole brain analyses, we provide a systematic overview of methodologic heterogeneity and potential shortcomings of previous work, and outline the need for prospective multicohort study designs to achieve higher levels of evidence for disease progression.
METHODS
Search strategy and selection criteria.
We conducted a systematic review and meta-analysis in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.7 We searched PubMed and Ovid MEDLINE databases on English literature, with no date limits set. We queried the following terms: “temporal lobe epilepsy” or “TLE” and (“MRI” or “magnetic resonance” or “volumetry” or “voxel-based” or “cortical thickness”). The searches were last updated on October 6, 2015. We also carried out manual searches on reference lists of all the included studies and of selected review articles, and a gray literature search through manual consultation of the proceedings of recent annual meetings (2014–2015) of the American Epilepsy Society. Initial screening was performed by one rater (L.C.) for the entire list. Assessment of full-text articles and final study selection was conducted independently by 2 reviewers (L.C. and B.C.B.); disagreement was settled by consensus. Based on 1,000 records screened independently by both L.C. and B.C.B., near-perfect interrater agreement (κ = 0.99) was obtained.
To be selected for quantitative analysis, reports had to meet the following criteria: (1) observational study in human cohorts with drug-resistant TLE, (2) participants evaluated using quantitative MRI methods (i.e., volumetry, voxel-based morphometry, cortical thickness analysis), (3) analyses specifically addressing disease progression (i.e., correlation with epilepsy duration, correlation with seizure counts, longitudinal design) at a hippocampal or whole brain level. Articles were excluded if they involved animals or if they were case series with fewer than 5 individuals.
Data extraction and quality assessment.
To quantitatively synthesize methodologic variability, study designs were categorized as cross-sectional or longitudinal. Within the cross-sectional category, we stratified studies that addressed associations between structural MRI measures and (1) epilepsy duration, (2) frequency/counts of complex partial seizures, (3) frequency/counts of secondarily generalized seizures.
For the included studies, we extracted patient sample characteristics (number of participants, age range, epilepsy duration range), MRI field strength, quantitative MRI methodology, and method for age control (when performed). Each report was assigned to 2 non–mutually exclusive sections: (1) evaluation of cumulative hippocampal atrophy, for which meta-analytical statistical methods were implemented; and (2) assessment of whole brain cumulative atrophy, pertaining to all brain structures other than the hippocampus, for which we summarized evidence using narrative synthesis.
Pearson correlation coefficient r was extracted as unit of analysis for the subset of studies addressing hippocampal atrophy and its association with epilepsy duration and seizures. When the latter metric was not directly provided, conversions were conducted according to established methods.8 Where both bivariate and partial correlations were reported within the same study, we used the latter. When separate or multiple metrics were reported (e.g., for distinct patient subgroups), weighted averages were generated to obtain a single study-specific measure. Due to considerable between-study variability in the reporting of results, correlations between MRI markers and partial or secondary generalized seizures were pooled and averaged into one single category of seizure estimates.
For data included in the narrative synthesis on whole brain progression, Pearson r, Spearman ρ, or the relevant values of t/z statistics were all equally accepted and extracted as units of analysis.
Quality of evidence was assessed according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework,9 implementing the relevant adjustments suggested for prognostic research studies.10
Data synthesis and analysis.
The meta-analysis for progressive hippocampal atrophy was conducted using the Metafor toolbox11 implemented in R (version 3.2.2; R-project.org), after unbiased estimates of correlation coefficients were obtained to account for its moderate negative bias. We opted for random effects models to account for potential between-study heterogeneity, and fitted meta-regression models to assess (1) effects of epilepsy duration on the hippocampus ipsilateral to the seizure focus, (2) effects of epilepsy duration on the contralateral hippocampus, (3) effects of seizure estimates on the ipsilateral hippocampus, and (4) effects of seizure estimates on the contralateral hippocampus. We also assessed equivalent mixed-effect models with volumetric technique (automated vs manual) as a categorical moderator.
Inspection of funnel plots and Galbraith plots, together with regression tests relating effect size to sample size, addressed small sample and publication bias. The I2 metric was implemented to quantify residual heterogeneity.
We excluded duplicate publications and performed 3 sensitivity analyses, which included only one type of study at a time from a given research group, i.e., the first study, the last study, or the one with the largest sample size.
For the narrative synthesis on whole brain progression, we summarized evidence from previous studies employing voxel-based morphometry, volumetry, cortical thickness mapping, and surface shape analyses. We counted reports of progressive structural changes in patients within the following regional categories, similar to a previous review on whole brain voxel-based morphometric studies12: (1) mesiotemporal, (2) extramesiotemporal, (3) extratemporal, (4) subcortical. Progression was considered as detected in the presence of (1) significant correlations between MRI markers and seizure estimates or epilepsy duration and (2) pertaining to either ipsilateral or contralateral regions of interest. For volumetric studies already included in the meta-analysis on hippocampal findings, the focus was limited to structures other than the hippocampus.
Standard protocol approvals, registrations, and patient consents.
Individual studies were approved by local ethics committees. No additional ethical approval was required for this systematic review and meta-analysis.
RESULTS
Study selection.
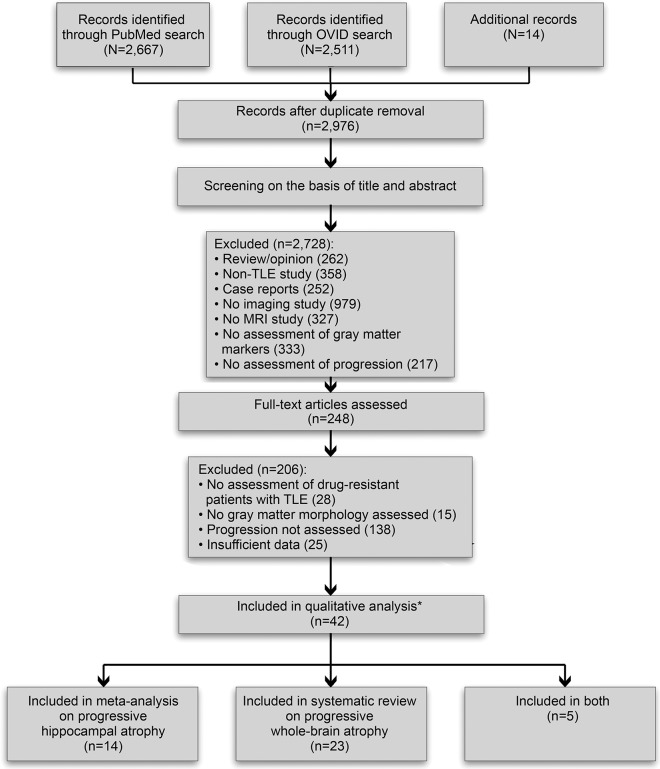
For a flow diagram detailing PRISMA-guided study selection, see figure 1.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for the study screening and inclusion procedures.
*References 16 and 25 refer to the same cohort, for which results are considered once only. Reference 41 contains separate cross-sectional and longitudinal analysis components, and is considered twice. TLE = temporal lobe epilepsy.
Screening.
We identified 2,667 citations through PubMed, 295 additional records through Ovid, and 14 further records by searching reference lists. The title and abstract of the resulting 2,976 citations were screened for eligibility; of these, 2,728 records were excluded based on the following criteria: (1) manuscript type (reviews/opinion papers, non-TLE studies, case reports; n = 872); (2) TLE studies not employing imaging (n = 979); (3) TLE imaging studies not employing MRI (n = 327); (4) MRI studies on TLE not assessing gray matter (n = 333); (5) MRI studies on TLE not assessing progression (n = 217).
Eligibility.
Full-text review of the remaining 248 reports resulted in exclusion of 206 articles based on the following criteria: (1) no assessment of progression (n = 138); (2) sample characteristics (no drug-resistant TLE, pediatric cohort, small sample size; n = 28); (3) no assessment of gray matter morphometry (n = 15); (4) insufficient data (n = 25). The remaining 42 articles were included in the systematic review.
Systematic review.
The systematic review included 42 studies13–54 (table e-1 at Neurology.org) conducted between 1997 and 2015. Articles were categorized into those assessing (1) progressive hippocampal atrophy based on quantitative MRI volumetry (n = 19) and (2) whole brain progression (n = 28, 5 also included in no. 1). Two studies16,25 evaluated the same cohort, for which results are reported once only. One study41 reported separate cross-sectional and longitudinal analyses, and each report is counted individually.
Sample and study characteristics.
The 42 analyses included a total of 2,188 patients with TLE (961 male, age 9–74 years, epilepsy duration 0–58 years). Thirty-two studies were based on 1.5T MRI, 2 on 2T,32,43 6 on 3T,49–54 and 1 on 4T37; 1 did not provide field strength information.33
Study design variability.
A cross-sectional design was used in 38/42 (90.5%) studies (hippocampus: 18/19; whole brain: 25/28), while 4 (9.5%) carried out longitudinal analyses (hippocampus: 1/19; whole brain: 3/28).
Among the cross-sectional studies, 35 (92.1%) correlated MRI markers with epilepsy duration, 24 (63.2%) with seizure estimates (counts in 8, frequency in 15, both in 1). Notably, the specific seizure marker differed considerably among articles. Eleven studies performed morphometric correlations with overall seizure estimates13,16,35,37–39,41,48,50,52,54 irrespective of seizure type, whereas 1 considered only complex partial18 and 5 only secondarily generalized seizures.31,33,34,36,40 Separate statistics for complex partial seizures and secondary generalized seizures were reported by 7,14,17,20–22,46,47 2 of which14,21 computed correlations with overall seizure load.
An inherent limitation of all analyses addressing cumulative atrophy is the confounding effect of age (i.e., chronological age), which is highly correlated with disease duration.41 This caveat appears particularly relevant for cross-sectional studies. In 18 of the latter (47.4%),14,16,20–22,24,30,33–38,40,42,50–52 no statistical approaches were implemented to correct for the effects of age. Although the remaining studies addressed aging, no consistent method was chosen: 3 reported no significant effects of age on morphometric measures in controls,15,32,48 3 found no effect of age or no effect of age at epilepsy onset in patients,19,29,53 2 corrected for age at onset,18,28 5 corrected for age in patients,17,26,47,49,54 4 utilized MRI measures adjusted for age13,31,39,46 (based on regression models derived from controls), 1 calculated epilepsy duration/age ratios,23 2 statistically compared chronological age effects between patients and controls.41,45
Among the longitudinal studies, 3 were single-cohort studies of patients without controls (interscan interval ranging from 2.3 to 3.4 years27,41,44). One study followed a multicohort design with a median interscan interval of 3.3 years, but without statistically comparing patients to controls.43 Three analyses reported parametric modulations of change trajectories by measures of seizure frequency.27,43,44
Systematic review and meta-analysis on progressive hippocampal atrophy.
Progression of hippocampal atrophy was assessed in 19 studies involving 979 patients (study sample size mean ± SD 51.5 ± 32.7, range 12–153) (figure 2). Of these, 1 was longitudinal27 and 18 were cross-sectional; of the latter, 8 assessed correlation with epilepsy duration, 3 with seizure estimates, and 7 with both. Hippocampal measures were frequently based on manual volumetry (n = 15, with 5 explicitly mentioning blinded assessment14,16,17,24,50). Four more recent analyses31,34,40,46 used automated approaches.
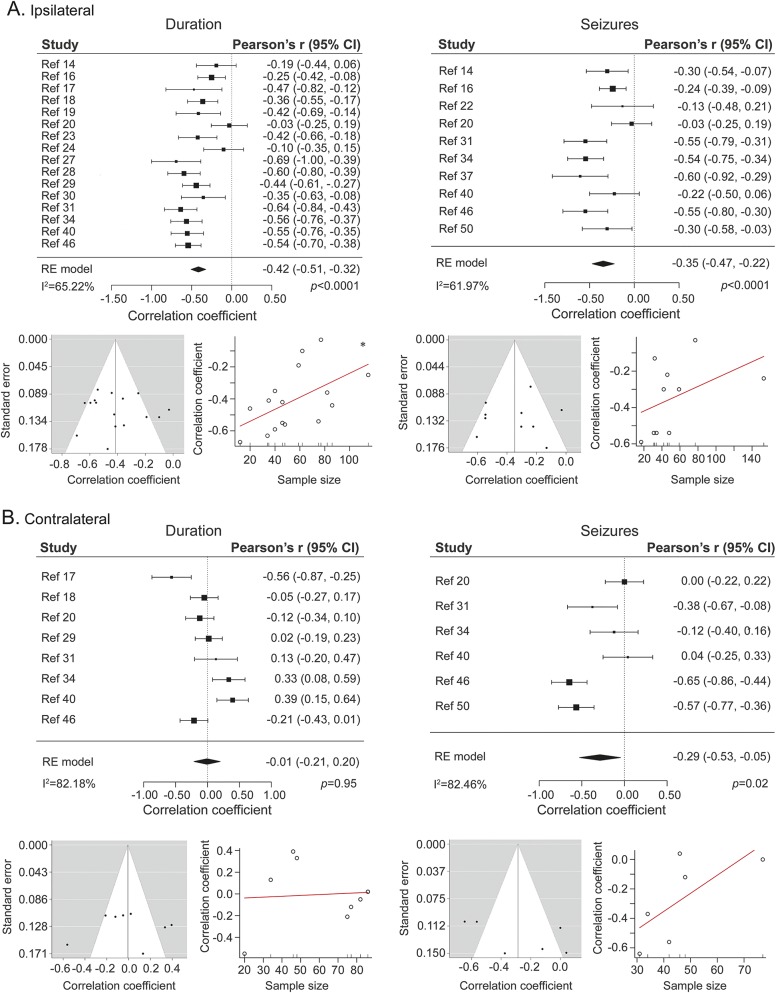
Figure 2. Meta-analysis on progressive hippocampal atrophy.
Pooled effect size for the ipsilateral (A) and contralateral (B) hippocampus for studies that assessed the effects of epilepsy duration (left) and of seizure estimates (right) on hippocampal volumes. For each subsection, funnel plots (left side, relating study-wise effect size to standard error) and graphs for regression tests (right side, relating effect size to sample size) are provided. CI = confidence interval.
Fifteen of the cross-sectional studies and the longitudinal study reported significant progressive atrophy ipsilateral to the seizure focus (in relation to epilepsy duration in 12/15 and in relation to seizure estimates in 7/10, for the cross-sectional analyses) with a pooled effect size of r = −0.42 (16 studies, 95% confidence interval [CI] −0.51 to −0.32; p < 0.0001; I2 = 65.22%; figure 2A) for epilepsy duration and of r = −0.35 (10 studies, 95% CI −0.47 to −0.22; p < 0.0001; I2 = 61.97%; figure 2A) for seizure estimates. Nine studies (47.4%) also assessed contralateral effects, with 3 reporting significant progressive atrophy (1/8 with respect to epilepsy duration, 2/6 in relation to seizure estimates). Pooled effect size was r = −0.01 (8 studies, 95% CI −0.21 to 0.20; p = 0.95; I2 = 82.18%; figure 2B) for epilepsy duration and r = −0.29 (6 studies, 95% CI −0.53 to −0.05; p = 0.02; I2 = 82.46%; figure 2B) for seizure estimates.
Mixed-effect models revealed a significant moderator effect of volumetric technique (automated vs manual) for results reported on the ipsilateral hippocampus, both for correlational analyses with epilepsy duration (p = 0.02; amount of variance accounted for: 46.55%, I2 of mixed-effect model: 49.56%) and those with seizure estimates (p = 0.03; variance = 59.16%, I2 = 39.30%). In both cases, studies that employed automated segmentations detected stronger correlations than those based on manual segmentations. While a moderation by volumetric technique was also seen for correlations between epilepsy duration and volume of the contralateral hippocampus, this was in the opposite direction, i.e., studies based on manual volumetry reported larger effects than those based on automated techniques (p = 0.049; variance = 23.77%, I2 = 77.64%). Conversely, volumetric technique did not moderate the relation between seizure estimates and contralateral hippocampal volumes (p = 0.56; I2 = 85.45%).
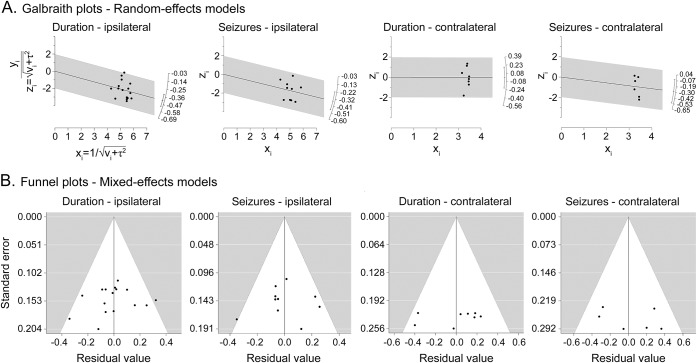
Funnel plots for random and mixed-effects models (figures 2 and 3, respectively) appeared symmetric, Galbraith plots did not indicate the presence of outliers in effect size (figure 3), and there was no evidence of publication bias. Regression tests showed a small yet marginally significant effect of sample size on the effect size of the correlation between epilepsy duration and ipsilateral hippocampal volume (p = 0.03).
Figure 3. Galbraith (radial) plots for random effects models and funnel plots for mixed-effect models.
(A) Galbraith (radial) plots for random effects models and (B) funnel plots for mixed-effect models. For mixed-effects models, study moderator is represented by volumetry technique (automated vs manual).
The 3 sensitivity analyses yielded results that were similar to the overall analyses. In the analysis of the first published study per author group for the ipsilateral hippocampus, the pooled effects sizes for epilepsy duration were r = −0.38 (p < 0.0001, 11 studies; I2 = 67.47%), and r = −0.35 for seizure estimates (p < 0.0001, 7 studies; I2 = 64.05%). Virtually identical effect sizes were seen when assessing the last study per author group (r = −0.38, p < 0.0001, 11 studies, I2 = 65.38% for epilepsy duration; r = −0.29, p = 0.0002, 7 studies, I2 = 60.49% for seizure estimates) and the study with the largest number of participants per author group (r = −0.36, p < 0.0001, 11 studies, I2 = 59.96% for epilepsy duration; r = −0.34, p = 0.0002, 7 studies, I2 = 70.38% for seizure estimates).
Patient inclusion criteria varied considerably among studies (table e-2). To decrease heterogeneity and enhance population representativeness for the pooled patient sample, we conducted an additional analysis focusing only on articles reporting on patients evaluated for epilepsy surgery. This yielded a similar effect size as the analyses above for the effect of epilepsy duration on the ipsilateral hippocampus (r = −0.38, p < 0.0001, I2 = 67.11% based on 9 studies), though evaluating the effect of seizure estimates was impeded by the small number of studies (n = 2). Conversely, it was not possible to separately analyze patients with hippocampal pathology and those with normal-appearing hippocampi, as studies generally included mixed samples comprising both subgroups with varying MRI diagnostic criteria.
Implementation of GRADE recommendations adapted for prognostic research yielded an overall score of 2.5 for evidence pertaining to ipsilateral hippocampal atrophy, and of <1 for contralateral atrophy, reflecting low to moderate and very low confidence in effect estimates, respectively (tables e-3 and e-4).
Narrative synthesis on whole brain progression.
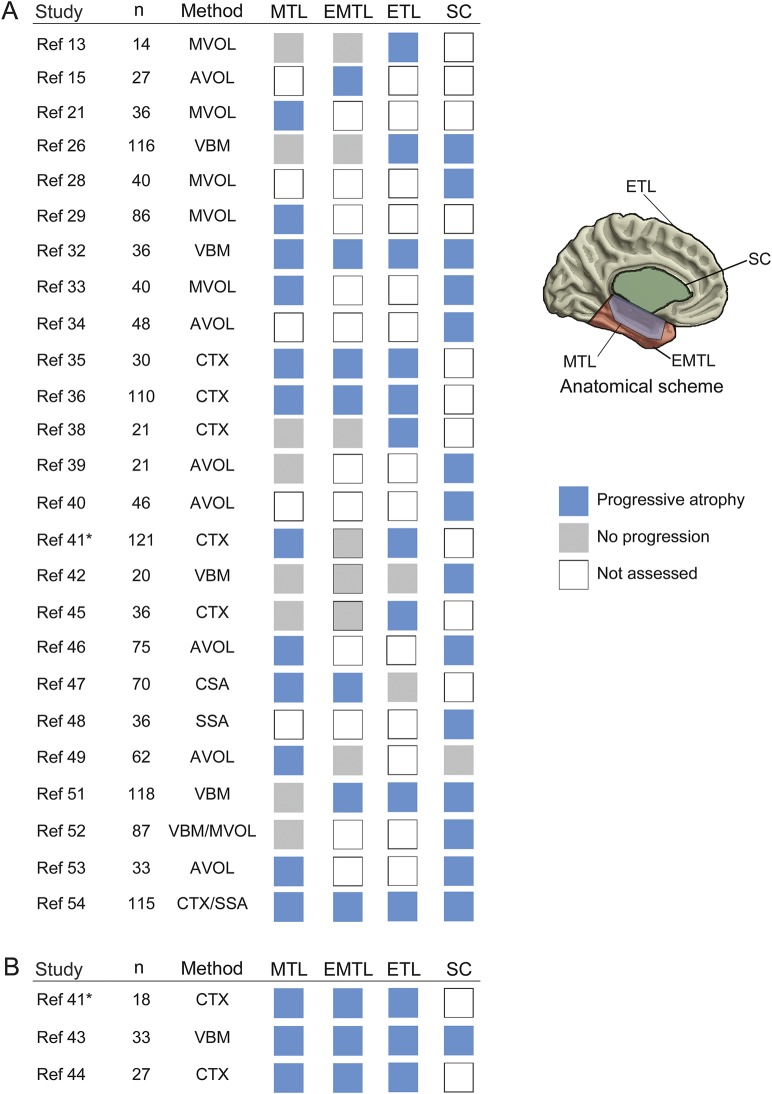
As with the assessment of the hippocampus, most analyses (25/28) followed a cross-sectional design (10 in relation to epilepsy duration and 15 including both seizure counts/frequency and epilepsy duration), while only 3 studies assessed progression longitudinally (2 single-cohort, 1 multicohort study) (figure 4). Patient inclusion criteria showed moderate variability across studies. Evaluation of patients investigated for and/or those who had undergone epilepsy surgery was reported in 19/28 (67.9%) analyses (table e-5). Methods to appraise whole brain changes included multi–region of interest volumetry (n = 13), voxel-based morphometry (n = 6), cortical thickness mapping (n = 8), and surface shape analyses (n = 3) alone or in combination. Progressive atrophy was detected for mesiotemporal regions in 15/23 (65.2%), lateral temporal regions in 10/17 (58.8%), extratemporal cortices in 13/15 (86.7%), and subcortical regions in 15/16 (93.8%), among which thalamus was reported in 12/16 (75%). Having adapted GRADE recommendations for narrative synthesis in prognostic research,10 we obtained an overall score of 1.5, indicative of low overall quality (table e-6).
Figure 4. Systematic review on whole brain studies addressing progressive atrophy.
Studies are divided into (A) cross-sectional and (B) longitudinal analyses. For each study, the sample size and quantitative MRI methods are provided as well as the reported finding (blue: progressive atrophy, gray: no progression, white: progression not assessed) across 4 brain subsystems (mesial temporal lobe [MTL], extramesial or lateral temporal lobe [EMTL], extratemporal cortical areas [ETL], subcortical gray matter [SC]). * Progressive mesio-temporal atrophy further documented at a subregional level in a subsequent analysis,e11 based on a largely overlapping cohort. For further information, see table e-1. AVOL = automated volumetry; CTX = cortical thickness analysis; MVOL = manual volumetry; SSA = surface-based shape analysis; VBM = voxel-based morphometry. McDonald 2008A and 2008B, Alhusaini 2012A and 2012B, and Keller 2015A and B refer to references 38, 39, 46, 47, 52, and 54, respectively.
DISCUSSION
We carried out a systematic review and meta-analysis of MRI morphometry studies addressing cumulative effects of atrophy in drug-resistant TLE. Quantitative synthesis of study design variability revealed that an overwhelming majority of previous work was based on cross-sectional inference, which related MRI markers to estimates of disease duration or seizure frequency. Meta-analytical modeling showed more marked atrophy in the ipsilateral hippocampus, with moderate effect sizes, in patients with longer epilepsy duration and more frequent seizures. Notably, several sensitivity analyses that excluded repeated studies from the same institution indicated virtually identical effects for ipsilateral hippocampal atrophy. While across-technique heterogeneity and the inconsistent reporting styles precluded the application of meta-analytical modeling of progression beyond the hippocampus, a narrative synthesis emphasized the occurrence of changes often extending to extratemporal and subcortical regions. Cumulative thalamic damage represented a consistent finding, in line with evidence indicating a central role of thalamotemporal connections in the pathologic network of TLE.55
The evaluated studies exhibited high heterogeneity with respect to inclusion criteria. First, patients were labelled as drug-resistant, uncontrolled, refractory, medically intractable, or chronic. The definition of drug-resistant epilepsy has posed considerable challenges, resulting in diverse formulations until unifying efforts of an International League Against Epilepsy task force in 2010.56 Most studies (76.2%) included in our systematic assessment were conducted before 2010, and only 2 more recent reports classified patients according to these criteria. Variability in patient eligibility criteria increases heterogeneity. To adjust for this potential limitation, we assessed effects of epilepsy duration on ipsilateral hippocampal volumes in studies that included only patients evaluated for epilepsy surgery. Effects in this more restrictive assessment were virtually identical to those of the overall analysis. Second, studies differed with regards to the range of hippocampal anomalies in patients. Of note, the functional implications of progressive hippocampal damage may be equally important in patients presenting with already marked atrophy as in those with normal volumes. Correlations have in fact been documented between hippocampal volumes and preoperative memory scores,57 and increasing evidence suggests that preoperative volumes may relate to postoperative memory performance.58
It is crucial to emphasize that the predominating cross-sectional design confounds between- and within-subject effects and, thus, does not directly address progression. Moreover, it does not permit a direct control of aging-related effects, as chronological age and epilepsy duration are highly correlated. Cross-sectional investigations are also not adequately tailored to capture the effects of initial precipitating events, including prolonged febrile seizures or traumatic brain injury, on acute gray matter loss, its evolution over time, and their relationship with epilepsy severity. Conversely, longitudinal studies can directly assess within-subject trajectories, and dissociate pathologic change from aging if both patients and controls are included. The few longitudinal studies performed to date were sensitive in detecting cumulative atrophy across all lobes in patients. Moreover, these studies showed additional modulation of progression by seizure estimates in some regions, supporting a more detrimental disease course in patients with frequent seizures.43,44 Notably, no study has dissociated longitudinal effects in patients and controls. One study separately tracked change in both cohorts,43 but did not directly test for progression, i.e., by statistically comparing longitudinal trajectories. A previous report, however, compared aging effects between patients and controls cross-sectionally,41 and observed more marked effects in the former. These findings overall point to more severe age-related thinning in patients, with longitudinal assessments documenting effects of 0.02–0.05 mm/year,41,44 while reported rates of annual age-related change in healthy adults range between 0.001 and 0.008 mm.59,60
Widespread and potentially progressive atrophy may result from a complex combination of the effects of recurrent seizures and neuronal disconnection.41 Moreover, several studies reported temporo-limbic morphometric abnormalities in asymptomatic relatives of patients with TLE,e1–e4 indicating that genetic factors may also contribute to atrophy. Due to our inclusion criteria, we could not compare drug-resistant to well-controlled patients. Previous research has suggested potential effects of medication, including phenytoin and sodium valproate, on gray matter measurements.e5–e6 Drug-related effects might thus be relevant to explain progressive structural damage in well-controlled patients.e7–e8 On the other hand, recent evidence points to an association between longstanding TLE and tau pathology, a common sign of established neurodegenerative diseases, in the ipsilateral temporal lobe.e9 By potentially providing mechanistic insights into the cascade leading from repeated seizures to neuronal loss, the latter finding may offer a framework for conceptualizing progressive atrophy in uncontrolled TLE as a neurodegenerative process.e10
In drug-resistant TLE, evidence exists in favor of the efficacy and superiority of surgical procedures compared to medical treatment.1,2 Advantages of surgery are not limited to seizure control, but also encompass decreased mortality as well as improvements in psychosocial functioning and quality of life. Yet, epilepsy surgery is still underimplemented4 and referrals for evaluation tend to occur several years after medication failure.3 To provide evidence for disease progression beyond effects of aging and to identify underlying factors, longitudinal imaging studies are needed that follow both patient and control cohorts. Quantitative MRI contrasts, such as T1 and T2 relaxometry, may provide additional information on the effects of disease on microstructural integrity of the gray and white matter not captured by conventional weighted images. Arguably, longitudinal studies face logistic challenges, high costs, and risk of attrition. As the necessity for surgical treatment in drug-resistant cohorts may preclude tracking within-patient change over considerable periods of time, structured and accelerated designs to systematically enroll patients at different time points in their disease course would be a feasible strategy. These studies may also help to discriminate patients with a benign disease course from those with aggressive progression, aiding patient stratification and contributing to the identification of disease biomarkers with validity at the individual level.
Supplementary Material
GLOSSARY
- CI
confidence interval
- GRADE
Grades of Recommendations, Assessment, Development and Evaluation
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- TLE
temporal lobe epilepsy
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
B.C.B., A.B., N.B., and L.C. formulated the study design. B.C.B. and L.C. performed the literature search, data analysis, and data interpretation, and wrote the paper. N.B. and A.B. provided data interpretation and funding and revised the paper. M.K. provided data interpretation and revised the paper. S.W. consulted on data analysis.
STUDY FUNDING
This research was funded by the Canadian Institutes of Health Research (CIHR MOP-57840 and CIHR MOP-123520). B.C.B. received operating support from CIHR/SickKids. L.C. is funded by a PhD studentship of the Brain Research Trust and acknowledges support from an Ermenegildo Zegna Founder's Scholarship. S.W. received the Hopewell Professorship of clinical neurosciences research.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Wiebe S, Blume WT, Girvin JP, Eliasziw M; Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 2.Engel J Jr, McDermott MP, Wiebe S, et al. ; Early Randomized Surgical Epilepsy Trial (ERSET) Study Group. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 2012;307:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haneef Z, Stern J, Dewar S, Engel J Jr. Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 2010;75:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burneo JG, Shariff SZ, Liu K, Leonard S, Saposnik G, Garg AX. Disparities in surgery among patients with intractable epilepsy in a universal health system. Neurology 2016;86:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiebe S. Burden of intractable epilepsy. Adv Neurol 2006;97:1–4. [PubMed] [Google Scholar]
- 6.Gowers WR. Epilepsy and Other Chronic Convulsive Disorders: Their Causes, Symptoms, and Treatment. London: Churchill; 1881. [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilpin AR. Table for conversion of Kendall tau to Spearman rho within the context of measures of magnitude of effect for metaanalysis. Educ Psychol Meas 1993;53:87–92. [Google Scholar]
- 9.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3: rating the quality of evidence. J Clin Epidemiol 2011;64:401–406. [DOI] [PubMed] [Google Scholar]
- 10.Huguet A, Hayden JA, Stinson J, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst Rev 2013;2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1–48. [Google Scholar]
- 12.Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 2008;49:741–757. [DOI] [PubMed] [Google Scholar]
- 13.Marsh L, Morrel MJ, Shear PK, et al. Cortical and hippocampal volume deficits in temporal lobe epilepsy. Epilepsia 1997;38:576–587. [DOI] [PubMed] [Google Scholar]
- 14.Kalviainen R, Salmenpera T, Partanen K, Vainio P, Riekkinen P, Pitkanen A. Recurrent seizures may cause hippocampal damage in temporal lobe epilepsy. Neurology 1998;50:1377–1382. [DOI] [PubMed] [Google Scholar]
- 15.Lee JW, Andermann F, Dubeau F, et al. Morphometric analysis of the temporal lobe in temporal lobe epilepsy. Epilepsia 1998;39:727–736. [DOI] [PubMed] [Google Scholar]
- 16.Salmenpera T, Kalviainen R, Partanen K, Pitkanen A. Hippocampal damage caused by seizures in temporal lobe epilepsy. Lancet 1998;351:35. [DOI] [PubMed] [Google Scholar]
- 17.Jokeit H, Ebner A, Arnold S, et al. Bilateral reductions of hippocampal volume, glucose metabolism, and Wada hemispheric memory performance are related to the duration of mesial temporal lobe epilepsy. J Neurol 1999;246:926–933. [DOI] [PubMed] [Google Scholar]
- 18.Tasch E, Cendes F, Li LM, Dubeau F, Andermann F, Arnold DL. Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann Neurol 1999;45:568–576. [DOI] [PubMed] [Google Scholar]
- 19.Theodore WH, Bhatia S, Hatta J, et al. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology 1999;52:132–136. [DOI] [PubMed] [Google Scholar]
- 20.Bower SP, Kilpatrick CJ, Vogrin SJ, Morris K, Cook MJ. Degree of hippocampal atrophy is not related to a history of febrile seizures in patients with proved hippocampal sclerosis. J Neurol Neurosurg Psychiatry 2000;69:733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salmenpera T, Kalviainen R, Partanen K, Pitkanen A. Quantitative MRI volumetry of the entorhinal cortex in temporal lobe epilepsy. Seizure 2000;9:208–215. [DOI] [PubMed] [Google Scholar]
- 22.Spanaki MV, Kopylev L, Liow K, et al. Relationship of seizure frequency to hippocampus volume and metabolism in temporal lobe epilepsy. Epilepsia 2000;41:1227–1229. [DOI] [PubMed] [Google Scholar]
- 23.Fuerst D, Shah J, Kupsky WJ, et al. Volumetric MRI, pathological, and neuropsychological progression in hippocampal sclerosis. Neurology 2001;57:184–188. [DOI] [PubMed] [Google Scholar]
- 24.Moran NF, Lemieux L, Kitchen ND, Fish DR, Shorvon SD. Extrahippocampal temporal lobe atrophy in temporal lobe epilepsy and mesial temporal sclerosis. Brain 2001;124:167–175. [DOI] [PubMed] [Google Scholar]
- 25.Salmenpera T, Kalviainen R, Partanen K, Pitkanen A. Hippocampal and amygdaloid damage in partial epilepsy: a cross-sectional MRI study of 241 patients. Epilepsy Res 2001;46:69–82. [DOI] [PubMed] [Google Scholar]
- 26.Keller SS, Wieshmann UC, Mackay CE, Denby CE, Webb J, Roberts N. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry 2002;73:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol 2003;53:413–416. [DOI] [PubMed] [Google Scholar]
- 28.Natsume J, Bernasconi N, Andermann F, Bernasconi A. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology 2003;60:1296–1300. [DOI] [PubMed] [Google Scholar]
- 29.Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology 2005;65:223–228. [DOI] [PubMed] [Google Scholar]
- 30.Lin JJ, Salamon N, Dutton RA, et al. Three-dimensional preoperative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology 2005;65:1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seidenberg M, Kelly KG, Parrish J, et al. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia 2005;46:420–430. [DOI] [PubMed] [Google Scholar]
- 32.Bonilha L, Rorden C, Appenzeller S, Coan AC, Cendes F, Li LM. Gray matter atrophy associated with duration of temporal lobe epilepsy. Neuroimage 2006;32:1070–1079. [DOI] [PubMed] [Google Scholar]
- 33.Szabo CA, Lancaster JL, Lee S, et al. MR imaging volumetry of subcortical structures and cerebellar hemispheres in temporal lobe epilepsy. AJNR Am J Neuroradiol 2006;27:2155–2160. [PMC free article] [PubMed] [Google Scholar]
- 34.Pulsipher DT, Seidenberg M, Morton JJ, Geary E, Parrish J, Hermann B. MRI volume loss of subcortical structures in unilateral temporal lobe epilepsy. Epilepsy Behav 2007;11:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JJ, Salamon N, Lee AD, et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex 2007;17:2007–2018. [DOI] [PubMed] [Google Scholar]
- 36.Bernhardt BC, Worsley KJ, Besson P, et al. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. Neuroimage 2008;42:515–524. [DOI] [PubMed] [Google Scholar]
- 37.Cavus I, Pan JW, Hetherington HP, et al. Decreased hippocampal volume on MRI is associated with increased extracellular glutamate in epilepsy patients. Epilepsia 2008;49:1358–1366. [DOI] [PubMed] [Google Scholar]
- 38.McDonald CR, Hagler DJ Jr, Ahmadi ME, et al. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia 2008;49:794–803. [DOI] [PubMed] [Google Scholar]
- 39.McDonald CR, Hagler DJ Jr, Ahmadi ME, et al. Subcortical and cerebellar atrophy in mesial temporal lobe epilepsy revealed by automatic segmentation. Epilepsy Res 2008;79:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seidenberg M, Hermann B, Pulsipher D, et al. Thalamic atrophy and cognition in unilateral temporal lobe epilepsy. J Int Neuropsychol Soc 2008;14:384–393. [DOI] [PubMed] [Google Scholar]
- 41.Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology 2009;72:1747–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brazdil M, Marecek R, Fojtikova D, et al. Correlation study of optimized voxel-based morphometry and (1)H MRS in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Hum Brain Mapp 2009;30:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology 2009;73:834–842. [DOI] [PubMed] [Google Scholar]
- 44.Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology 2010;74:1776–1784. [DOI] [PubMed] [Google Scholar]
- 45.Kemmotsu N, Girard HM, Bernhardt BC, et al. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia 2011;52:2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alhusaini S, Doherty CP, Scanlon C, et al. A cross-sectional MRI study of brain regional atrophy and clinical characteristics of temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res 2012;99:156–166. [DOI] [PubMed] [Google Scholar]
- 47.Alhusaini S, Doherty CP, Palaniyappan L, et al. Asymmetric cortical surface area and morphology changes in mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2012;53:995–1003. [DOI] [PubMed] [Google Scholar]
- 48.Bernhardt BC, Bernasconi N, Kim H, Bernasconi A. Mapping thalamocortical network pathology in temporal lobe epilepsy. Neurology 2012;78:129–136. [DOI] [PubMed] [Google Scholar]
- 49.Keller SS, Schoene-Bake JC, Gerdes JS, Weber B, Deppe M. Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: cross-sectional evidence for progressive neurologic injury. PLoS One 2012;7:e46791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pacagnella D, Lopes TM, Morita ME, et al. Memory impairment is not necessarily related to seizure frequency in mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia 2014;55:1197–1204. [DOI] [PubMed] [Google Scholar]
- 51.Coan AC, Campos BM, Yasuda CL, et al. Frequent seizures are associated with a network of gray matter atrophy in temporal lobe epilepsy with or without hippocampal sclerosis. PLoS One 2014;9:e85843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keller SS, Richardson MP, Schoene-Bake JC, et al. Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Ann Neurol 2015;77:760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan VL, Conrad BN, Abou-Khalil B, Rogers BP, Kang H. Increasing structural atrophy and functional isolation of the temporal lobe with duration of disease in temporal lobe epilepsy. Epilepsy Res 2015;110:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keller SS, Richardson MP, O'Muircheartaigh J, Schoene-Bake JC, Elger C, Weber B. Morphometric MRI alterations and postoperative seizure control in refractory temporal lobe epilepsy. Hum Brain Mapp 2015;36:1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guye M, Regis J, Tamura M, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain 2006;129:1917–1928. [DOI] [PubMed] [Google Scholar]
- 56.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 57.Baxendale SA, Van Paesschen W, Thompson PJ, et al. The relationship between quantitative MRI and neuropsychological functioning in temporal lobe epilepsy. Epilepsia 1998;39:158–166. [DOI] [PubMed] [Google Scholar]
- 58.Trenerry MR, Jack CR Jr, Ivnik RJ, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology 1993;43:1800–1805. [DOI] [PubMed] [Google Scholar]
- 59.Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage 2010;52:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Storsve AB, Fjell AM, Tamnes CK, et al. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neurosci 2014;34:8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.