Abstract
Importance
Withdrawal from nicotine is an important contributor to smoking relapse. Understanding how reward-based decision making is affected by abstinence and by pharmacotherapies such as nicotine replacement therapy and varenicline tartrate may aid cessation treatment.
Objective
To independently assess the effects of nicotine dependence and stimulation of the nicotinic acetylcholine receptor on the ability to interpret valence information (reward sensitivity) and subsequently alter behavior as reward contingencies change (cognitive flexibility) in a probabilistic reversal learning task.
Design, Setting, and Participants
Nicotine-dependent smokers and nonsmokers completed a probabilistic reversal learning task during acquisition of functional magnetic resonance imaging (fMRI) in a 2-drug, double-blind placebo-controlled crossover design conducted from January 21, 2009, to September 29, 2011. Smokers were abstinent from cigarette smoking for 12 hours for all sessions. In a fully Latin square fashion, participants in both groups underwent MRI twice while receiving varenicline and twice while receiving a placebo pill, wearing either a nicotine or a placebo patch. Imaging analysis was performed from June 15, 2015, to August 10, 2016.
Main Outcome and Measures
A well-established computational model captured effects of smoking status and administration of nicotine and varenicline on probabilistic reversal learning choice behavior. Neural effects of smoking status, nicotine, and varenicline were tested for on MRI contrasts that captured reward sensitivity and cognitive flexibility.
Results
The study included 24 nicotine-dependent smokers (12 women and 12 men; mean [SD] age, 35.8 [9.9] years) and 20 nonsmokers (10 women and 10 men; mean [SD] age, 30.4 [7.2] years). Computational modeling indicated that abstinent smokers were biased toward response shifting and that their decisions were less sensitive to the available evidence, suggesting increased impulsivity during withdrawal. These behavioral impairments were mitigated with nicotine and varenicline. Similarly, decreased mesocorticolimbic activity associated with cognitive flexibility in abstinent smokers was restored to the level of nonsmokers following stimulation of nicotinic acetylcholine receptors (familywise error–corrected P < .05). Conversely, neural signatures of decreased reward sensitivity in smokers (vs nonsmokers; familywise error–corrected P < .05) in the dorsal striatum and anterior cingulate cortex were not mitigated by nicotine or varenicline.
Conclusions and Relevance
There was a double dissociation between the effects of chronic nicotine dependence on neural representations of reward sensitivity and acute effects of stimulation of nicotinic acetylcholine receptors on behavioral and neural signatures of cognitive flexibility in smokers. These chronic and acute pharmacologic effects were observed in overlapping mesocorticolimbic regions, suggesting that available pharmacotherapies may alleviate deficits in the same circuitry for certain mental computations but not for others.
Trial Registration
clinicaltrials.gov Identifier: NCT00830739
This placebo-controlled crossover study assesses the effects of nicotine dependence and stimulation of the nicotinic acetylcholine receptor on the ability to interpret valence information and subsequently alter behavior as reward contingencies change in a probabilistic reversal learning task.
Key Points
Question
How are reward sensitivity and cognitive flexibility in the mesocorticolimbic system affected by acute abstinence and stimulation of the nicotinic acetylcholine receptors in dependent smokers?
Findings
This placebo-controlled crossover study found a double dissociation between decreased neural signatures of reward sensitivity, which are associated with severity of nicotine dependence but not with the acute effects of nicotine or varenicline tartrate, and behavioral and neural signatures of cognitive flexibility, which were impaired in the abstinent state but restored with stimulation of the nicotinic acetylcholine receptors.
Meaning
Currently available pharmacotherapies appear to alleviate abstinent smokers’ impaired cognitive flexibility but not reward sensitivity.
Introduction
Although 70% of adult smokers want to quit smoking, most attempts to quit fail within the first week. A major cause of relapse is the tobacco abstinence syndrome, characterized by deficits in cognitive, affective, and reward processing. Like other drugs of abuse, nicotine engages the mesocorticolimbic (MCL) system, which consists primarily of striatal and prefrontal brain areas targeted by midbrain dopamine (DA) neurons. Nicotine indirectly stimulates DA neurons through agonist effects on nicotinic acetylcholine receptors (nAChRs). During the development of nicotine dependence, neuroplastic changes occur throughout the MCL circuitry to downregulate levels of DA; drug administration becomes necessary to reach a baseline state, while drug abstinence leads to a hypodopaminergic withdrawal state.
Mesocorticolimbic circuitry subserves reward-based decision making, and its dysregulation in early abstinence likely contributes to relapse. Probabilistic reversal learning (PRL) captures 2 crucial components of reward-based decision making: processing rewarding vs punishing outcomes (reward sensitivity), and deciding when to change one’s behavior in the face of negative outcomes vs when to maintain a previous choice (cognitive flexibility). In a PRL task, participants update reward contingencies based on uncertain information and adjust responses accordingly. This task relies on MCL circuitry, which is modified by acute and chronic nAChR stimulation. However, how reward sensitivity and cognitive flexibility are affected by the deficit state that characterizes early abstinence and how such deficits may be mitigated by available pharmacotherapies remain unknown.
Probabilistic reversal learning is a promising measure of cognitive flexibility and perseveration in addiction, with impaired performance in alcohol, cocaine, and amphetamine dependence. Rodent studies indicate that chronic administration of nicotine impairs cognitive flexibility during reversal learning and that acute delivery of nicotine or varenicline tartrate alleviates withdrawal-induced reversal learning deficits in nicotine-dependent animals. Despite relevance to nicotine withdrawal and subsequent relapse, to our knowledge, the interacting effects of nicotine dependence and acute stimulation of nAChRs on reward sensitivity and cognitive flexibility during PRL have not been characterized in humans.
Nicotine replacement therapy and varenicline, effective smoking cessation treatments, stimulate α4β2 nAChRs. Nicotine is a full agonist at these nAChRs, and varenicline is a partial agonist at these receptors, partially mimicking the effects of nicotine in its absence while blunting the effects of nicotine in its presence. Although the interacting effects of nicotine and varenicline have been characterized at the receptor level, it is unknown how these drugs act and interact on reward-based decision making and its neural signatures. Here, we used functional magnetic resonance imaging (fMRI) in abstinent smokers and matched nonsmokers during PRL task performance following administration of nicotine, varenicline, neither, or both. We could, therefore, independently assess the effects of acute stimulation of nAChRs between a group of chronically exposed nicotine-dependent participants (with putative neuroplastic circuit alterations) vs a group of nonsmokers. We applied a well-established computational model to participants’ choices to elucidate nicotinic effects on decision making. Given previous research, we hypothesized that behavioral and neural indices of reward sensitivity and cognitive flexibility would be reduced in abstinent smokers. We also expected that acutely abstinent smokers’ deficits would be alleviated by stimulation of nAChRs, consistent with allostatic models of addiction. Lastly, we expected that the effects of nicotine and varenicline on behavior and brain indices would reflect their interacting effects at the nAChR level.
Methods
Participants
Twenty-four cigarette smokers (nicotine-dependent adults smoking ≥10 cigarettes daily for >2 years; 12 women and 12 men) and 20 nonsmokers (adults with no smoking history within the past 2 years and no lifetime daily cigarette use of >1 month; 10 women and 10 men) were recruited at the National Institute on Drug Abuse–Intramural Research Program in Baltimore, Maryland. All 44 participants were right-handed, between 18 and 55 years of age, and healthy, with no reported history of neurologic or psychiatric disorders, contraindications to MRI, or drug dependence (except nicotine in the smokers). Written informed consent was obtained in accordance with the National Institute on Drug Abuse–Intramural Research Program Institutional Review Board.
Experimental Design
The randomized, double-blind, placebo-controlled, crossover design involved 2 drugs: varenicline pills (Chantix; Pfizer) and nicotine patches (NicoDerm CQ; GlaxoSmithKline) (Trial Protocol in Supplement 1). Participants completed 6 fMRI sessions; we report data from the 4 completely counterbalanced sessions conducted from January 1, 2009, to September 29, 2011, crossing factors NICOTINE and VARENICLINE (eAppendix 1 and eFigure 1 in Supplement 2). Participants performed multiple tasks; here, we report PRL data.
PRL Task
Participants completed an event-related PRL task (eAppendix 1 and eFigure 2 in Supplement 2) based on previous studies. Participants aimed to maximize monetary gain by learning which of 2 cues had a high probability (75%) of a $1 reward and a low probability (25%) of a $1 loss, and which cue had the opposite contingencies. After 5 consecutive correct choices or 20 trials, the contingencies were reversed unbeknownst to the participants, who were required to shift responses accordingly. Each scanning session (approximately 35 minutes) consisted of three 120-trial runs. Functional (1104 whole-brain echoplanar imaging scans; repetition time, 2 seconds; echo time, 27 milliseconds) and structural T1-weighted brain images were acquired on a 3-T MRI scanner (3T Siemens Magnetom Allegra) (eAppendix 1 in Supplement 2).
Behavioral Measures
We calculated PRL metrics based on previous work: lose-shift (probability of shifting a response following a loss), win-stay (probability of repeating a response following a win), trials to criterion (mean number of trials after reversal before the participant selected the correct stimulus 5 times consecutively), and number of perseverative errors (selecting the previously rewarded cue at least 3 times following 2 losses after a reversal). We carried out mixed linear models (random intercept) with GROUP as a between-participant factor (smoker vs nonsmoker) and NICOTINE (nicotine vs placebo) and VARENICLINE (varenicline vs placebo) as within-participant factors. We used generalized (binomial dependent variables) and general (continuous dependent variables) mixed models in R using packages afex and phia (https://www.r-project.org/). Interaction effects or trends in the omnibus analysis were followed by within-group analyses.
Computational Modeling
We applied a computational modeling approach previously validated for PRL. Three models were fit: a Rescorla-Wagner model, and 2 Hidden Markov Models, which have been shown to better fit PRL behavior because they capture a crucial task characteristic, namely, that the values of the cues are each other’s inverse (ie, learning that cue A predicts a reward entails learning that cue B predicts a punishment). Effects of GROUP, NICOTINE, and VARENICLINE on free model parameters were analyzed as for the behavioral measures.
Imaging Analysis
Imaging analyses were carried out from June 15, 2015, to August 10, 2016, in Analysis of Functional NeuroImages (AFNI) using standard preprocessing and first-level modeling (eAppendix 1 in Supplement 2). Four event types of interest were modeled (win-stay, lose-stay, and lose-shift), as in previous work. Two contrasts of interest were calculated: reward sensitivity (reward minus punishment) by subtracting lose-stay trials from win-stay and cognitive flexibility (shift minus stay) by subtracting lose-stay from lose-shift trials (eAppendix 1 in Supplement 2).
Mean activity patterns for reward sensitivity and cognitive flexibility were computed with 2-tailed t tests on participants’ beta maps (averaged over sessions). Results were corrected for whole-brain familywise error (α < .05, voxelwise P < .001, cluster size 19 voxels).
Group and drug effects for both contrasts of interest were examined with mixed analyses of variance (between-participant factor GROUP and within-participant factors NICOTINE and VARENICLINE). Significant GROUP interactions were followed by within-group analyses using NICOTINE and VARENICLINE as factors. Given our a priori hypothesis that group and pharmacologic effects would be present in MCL areas, we applied a familywise error correction (α < .05) within a composite mask of interest consisting of the bilateral nucleus accumbens, the caudate, the putamen, the amygdala, the bilateral anterior insula (AI), the anterior cingulate cortex (ACC), and the orbitofrontal cortex (1978 voxels in mask, voxelwise P < .05, cluster size 53 voxels [eFigure 3 in Supplement 2]).
Results
Behavioral Measures
Complete demographic and behavioral data are given in eTables 1 and 2 in Supplement 2; checks for pharmacologic effectiveness, order effects, and task engagement are in eFigures 4, 5, and 6 in Supplement 2. One participant’s data were removed from all analyses owing to poor data quality. Because nonsmokers were younger (mean [SD] age, 30.4 [7.2] years) than smokers (mean [SD] age, 35.8 [9.9] years; P = .04), age was included as a covariate when comparing groups in all behavioral and imaging analyses.
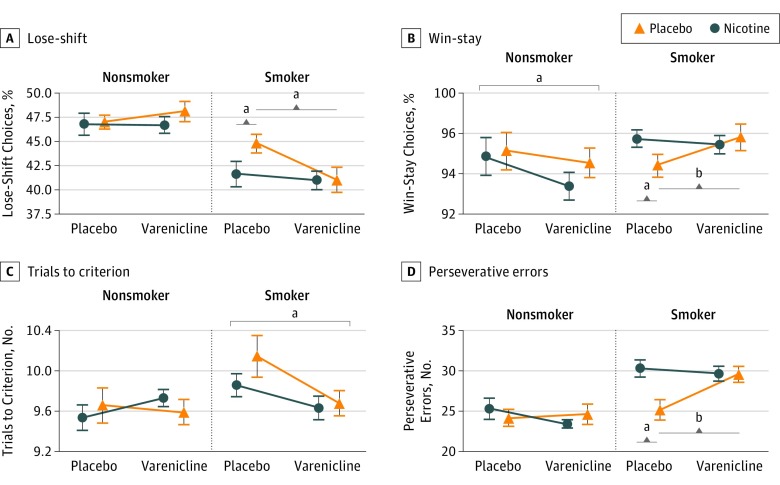
Lose-shift probability (Figure 1A) showed significant NICOTINE (χ21 = 3.79; P = .05) and GROUP × VARENICLINE effects (χ21 = 4.89; P = .022), driven by NICOTINE (χ21 = 3.99; P = .05) and VARENICLINE (χ21 = 7.78; P = .006) effects in smokers and their absence among nonsmokers. Win-stay probability (Figure 1B) showed GROUP × NICOTINE (χ21 = 5.17; P = .04), GROUP × VARENICLINE (χ21 = 9.136; P = .003), and NICOTINE × VARENICLINE (χ21 = 6.80; P = .02) interactions. Abstinent smokers stayed less after a win compared with when they were administered nicotine (χ21 = 7.84; P = .01) or varenicline (χ21 = 8.53; P = .007), while nonsmokers receiving varenicline stayed less after a win (χ21 = 5.29; P = .02). Varenicline effects on trials to criterion (Figure 1C) differed between groups (χ21 = 4.41; P = .04), whereby smokers receiving varenicline required fewer trials (χ21 = 6.08; P = .02); neither drug affected nonsmokers. Finally, there was a NICOTINE × VARENICLINE interaction on the number of perseverative errors (Figure 1D; χ21 = 7.65; P = .01). Counterintuitively, abstinent smokers made more perseverative errors when receiving nicotine (χ21 = 7.65; P = .01) or varenicline (χ21 = 11.01; P = .002) than when they were abstinent. Nonsmokers again showed no behavioral differences following any drug manipulation.
Figure 1. Performance on the Probabilistic Reversal Learning Task as a Function of Smoking Group and Nicotinic Manipulation (Nicotine and Varenicline Tartrate).
A, Acutely abstinent smokers were more likely to shift responses following a loss (lose-shift choice) compared with smokers who receive nicotine or varenicline, while neither drug affected lose-shift behavior in nonsmokers. B, Smokers receiving nicotine or varenicline repeated responses after a win (win-stay choices) more than acutely abstinent smokers. Nonsmokers receiving varenicline showed a decrease in win-stay choices. C, Smokers receiving varenicline required fewer trials to reach criterion than smokers not receiving varenicline. Nicotine did not affect trials to criterion in smokers, and neither drug had an effect in nonsmokers. D, Acutely abstinent smokers made fewer perseverative errors than smokers receiving either nicotine or varenicline. Neither nicotine nor varenicline affected perseverative errors in nonsmokers. Varenicline was given as varenicline tartrate. For an explanation of lose-shift and win-stay, see the Behavioral Measures subsection of the Methods section. Error bars indicate SEM.
aP < .05.
bP < .01.
Computational Modeling
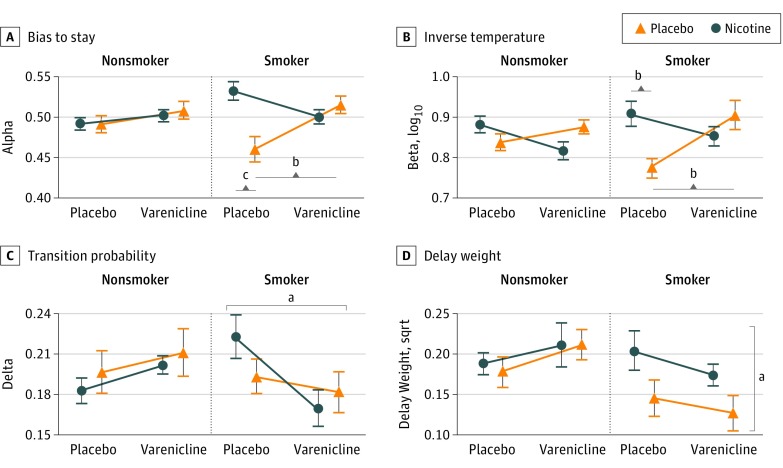
The best-fitting model was a Hidden Markov Model with 4 free parameters (Hidden Markov Model 2; eAppendices 1 and 2, eTables 3-5, eFigures 7 and 8 in Supplement 2): bias toward staying (alpha), inverse temperature (beta), which captures sensitivity to available evidence, perceived probability of a reversal (delta), and perceived increased chance of a reversal as more trials take place since the last reversal (delay-weight; Figure 2).
Figure 2. Model Parameters for the Hidden Markov Model as a Function of Smoking Group and Nicotinic Receptor Manipulation (Nicotine and Varenicline Tartrate).
A, Acutely abstinent smokers were biased toward shifting responses (alpha < 0.5), and this bias to shift was remedied with nicotine and varenicline. Nonsmokers showed no bias. B, When smokers received nicotine or varenicline, their choice behavior was more sensitive to the available evidence (higher inverse temperature) compared with acute abstinence from nicotine. Neither drug affected inverse temperature in nonsmokers. C, Smokers receiving varenicline perceived the probability of a reversal to be lower than smokers receiving a placebo pill. Neither nicotine nor varenicline affected transition probability in nonsmokers. D, Smokers with a placebo patch took the time since the last reversal into account less than smokers with a nicotine patch, while varenicline did not affect this measure. Nonsmokers’ ability to factor in the time since the last reversal was not affected by either drug. Error bars indicate SEM; sqrt, square root.
aP < .05.
bP < .01.
cP < .001.
Bias to stay (Figure 2A) was significantly affected by GROUP, NICOTINE, and VARENICLINE (3-way interaction: χ21 = 6.68; P = .004), driven by the partial agonist NICOTINE × VARENICLINE interaction in smokers: strong independent effects of nicotine (χ21 = 19.88; P < .001) and varenicline (χ21 = 11.28; P = .002), but no significant effects in combination. That is, abstinent smokers were biased toward shifting (ie, alpha <0.5), but nicotine-sated smokers were biased toward staying (alpha >0.5). Inverse temperature (Figure 2B) showed NICOTINE (χ21 = 10.86; P = .005), VARENICLINE (χ21 = 4.36; P = .03), and NICOTINE × VARENICLINE effects (χ21 = 13.32; P < .001) across groups. Abstinent smokers’ decisions were less sensitive to available evidence, and reliance on evidence increased with nicotine (χ21 = 9.94; P = .003) or varenicline (χ21 = 9.57; P = .004). Transition probability (Figure 2C) showed a GROUP × VARENICLINE effect (χ21 = 6.26; P = .02), driven by a reduced perceived transition probability in smokers receiving varenicline (χ21 = 6.12; P = .01). Finally, delay-weight (Figure 2D), which reflects how much participants consider that contingencies regularly reverse, showed an overall NICOTINE trend (χ21 = 3.32; P = .07), driven by abstinent smokers, who weighted the time since last reversal less (χ21 = 4.48; P = .045).
Imaging Results
Reward Sensitivity (Win-Stay − Lose-Stay)
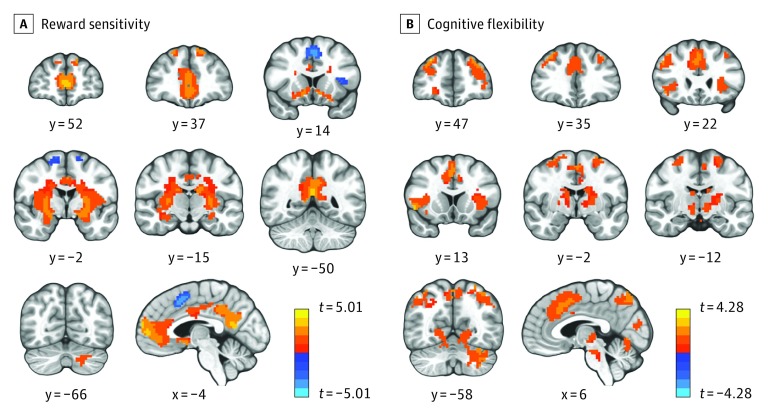
Across sessions, the ventromedial prefrontal cortex, posterior cingulate cortex, bilateral nucleus accumbens, putamen, caudate, left intraparietal sulcus, bilateral secondary visual cortex, and right cerebellum responded more to rewards than punishments (Figure 3A and eTable 6 in Supplement 2). Conversely, salience network areas (dorsal ACC [dACC] and AI) were more active when processing punishments than rewards, consistent with previous work.
Figure 3. Whole-Brain Activation to Reward Sensitivity and Cognitive Flexibility Contrasts Across Groups and Conditions.
A, Increased activation to positive vs negative outcomes in the ventromedial prefrontal cortex, posterior cingulate cortex, and ventral and dorsal striatum, as well as the superior frontal gyrus and left cerebellum. Activity decreases in anterior insula (AI), and the dorsal anterior cingulate cortex (dACC; warm colors [positive t values]: reward > punishment; cool colors [negative t values]: punishment > reward). B, Activation was greater preceding a shift than a stay in the AI, dACC, dorsal striatum, dorsolateral prefrontal cortex, posterior parietal cortex, occipital cortex, and cerebellum. Radiologic convention: left side of the image is the right side of the brain. Threshold levels have been increased to P < .00001 (A) and P < .0001 (B) with a cluster size of 70 voxels to allow for a better visualization of the results (see eFigure 9A and 9B in Supplement 2 for results corrected at familywise error–corrected P < .05). The x and y refer to the location of the slices in the Talairach coordinate system; and the t refers to the t value.
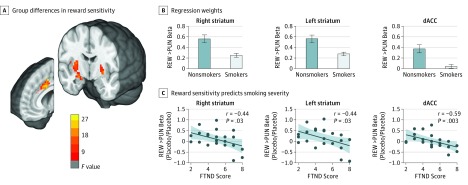
Effects of GROUP, NICOTINE, and VARENICLINE were assessed within the a priori region-of-interest mask (Figure 4A and B, and eTable 6 in Supplement 2). Smokers showed significantly lower reward sensitivity than did nonsmokers in the dACC and dorsal striatum, extending into the right amygdala. Unexpectedly, reward sensitivity was not modulated by nicotine or varenicline. However, consistent with a chronic effect of nicotine dependence, regression weights in abstinence (taking a placebo pill and wearing a placebo patch) correlated negatively with addiction severity in smokers (assessed with the Fägerstrom Test for Nicotine Dependence; Figure 4C).
Figure 4. Lower Reward Sensitivity Activity in Smokers Than in Nonsmokers.
A, Group differences (smokers vs nonsmokers) in reward sensitivity in the bilateral dorsal striatum and dorsal anterior cingulate cortex (dACC) within the a priori volume of interest (familywise error–corrected P < .05). Radiologic convention: left side of the image is the right side of the brain. B, Regression weights extracted from the clusters identified in the imaging analysis; error bars indicate the SEM (plotted to aid interpretation only—no statistical inference should be drawn), and the shaded region indicates 95% CI. See eFigure 10 in Supplement 2 for regression weights separating out wins and losses. C, Reward sensitivity contrast weight in the absence of nicotine and varenicline tartrate was associated with severity of nicotine dependence (Fägerstrom Test for Nicotine Dependence [FTND] score). PUN indicates punishment, and REW, reward.
Cognitive Flexibility (Lose-Shift − Lose-Stay)
Across sessions, the dACC, bilateral AI, superior frontal gyrus, superior parietal lobule, caudate, putamen, primary visual cortex, and cerebellum were more active before a shift than before repeating the same response following a negative outcome (Figure 3B and eTable 7 in Supplement 2).
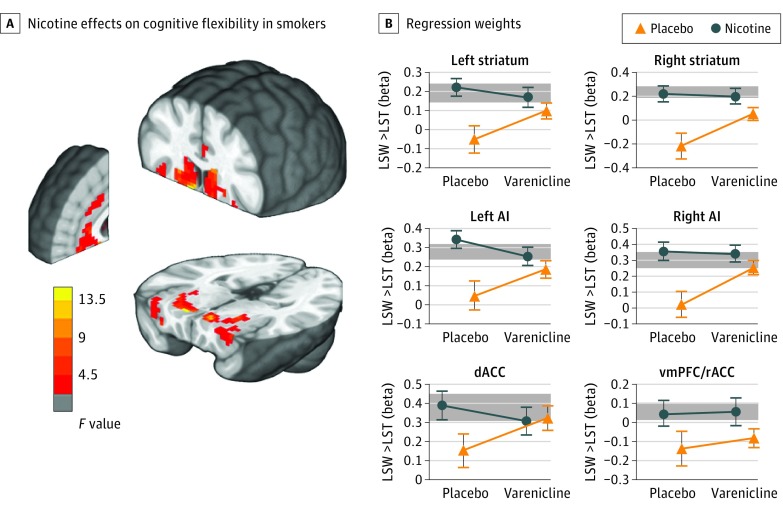
Although the repeated-measures analysis of variance within our region-of-interest volume showed no main GROUP effect, there were significant NICOTINE and GROUP × NICOTINE effects. The follow-up within-group repeated-measures analysis of variance for smokers showed that flexibility-associated activity in the bilateral ventral and dorsal striatum, bilateral AI, ventromedial prefrontal cortex, and dACC was downregulated in abstinence, yet restored with administration of nicotine (Figure 5 and eTable 7 in Supplement 2). In all except the ventromedial prefrontal cortex cluster, extracted regression weights correlated significantly with the bias to shift (α) in our computational model (eFigure 11 in Supplement 2). Although not significant, the pattern of extracted regression weights is consistent with varenicline partial agonist effects (Figure 5B). No effects of either drug were found among nonsmokers.
Figure 5. Nicotine Effects on Cognitive Flexibility Contrast in Smokers.
A, Acute nicotine administration in smokers increases neural signatures of cognitive flexibility in the bilateral striatum, anterior insula (AI), dorsal anterior cingulate cortex (dACC), and ventromedial prefrontal cortex (vmPFC) within a priori masked regions of interest (familywise error–corrected P < .05). Radiologic convention: left side of the image is the right side of the brain. B, Regression weights extracted from the clusters in part A show that activity is reduced in acute abstinence and restored to the level of nonsmokers (shaded band) when nicotine is administered. Although no significant varenicline tartrate main effects or interactions were identified in the imaging contrast, patterns are in line with the interaction of nicotine and varenicline at the receptor level. Error bars indicate SEM (plotted to aid interpretation only—no statistical inference should be drawn). Shaded regions indicate the mean [SEM] of the nonsmokers’ bold responses averaged across conditions. LST indicates lose-stay; LSW, lose-switch; and rACC, rostral anterior cingulate cortex.
Discussion
Chronic exposure to nicotine and subsequent dependence lead to changes in MCL circuitry, which governs reward appraisal and reward-based decision making. Understanding how abstinence and pharmacotherapies such as nicotine replacement therapy and varenicline affect these core MCL functions can inform smoking cessation treatment. Using computational modeling and fMRI in a PRL task, we found that acutely abstinent smokers were excessively flexible (biased to shift their choice) and that neural activity in MCL areas (ACC, bilateral striatum, and AI) was reduced before a behavioral shift. Acute administration of nicotine and varenicline restored these neural and behavioral deficits to levels comparable to those in nonsmokers. In a double-dissociation fashion, smokers’ lower reward sensitivity in the dorsal striatum and dACC was not alleviated following stimulation of the nAChR but was associated with severity of dependence.
Reversal of Abstinent Smokers’ Increases in Impulsive Choice With nAChR Stimulation
Behavioral results showed that stimulation of the nAChRs affected PRL in smokers but not in nonsmokers. Abstinent smokers committed fewer perseverative errors and were more likely to shift following a loss compared with when they were receiving nicotine or varenicline. Counterintuitively, perseverative errors and lose-shift probabilities among abstinent smokers resembled those among nonsmokers. However, because of the probabilistic task nature, “perseverative” behavior is not intrinsically good or bad: consistently shifting following a loss is suboptimal but counts as a flexible response. In contrast, computational models can capture how the accumulation of evidence informs staying or shifting. Using a previously validated Hidden Markov Model, we found that abstinent smokers were biased toward shifting their responses (alpha) and relied less on the available evidence (beta); that is, abstinent smokers appeared to make more impulsive, rash decisions when facing negative outcomes. This deficit was remedied by stimulation of the nAChR, whereby nicotine and varenicline interacted consistent with their known pharmacologic actions at α4β2 receptors.
Effects of Nicotine Dependence on Reward Sensitivity in the Bilateral Dorsal Striatum and dACC
Dependent smokers had lower neural responses to rewards in the ACC and bilateral dorsal striatum. Although some preclinical evidence shows nicotine-induced reward sensitization, our findings are consistent with a large body of evidence indicating blunted striatal and medial prefrontal responses to monetary and natural rewards in cocaine dependence and nicotine dependence. Strikingly, the observed deficit was not modulated by administration of nicotine or varenicline but was associated with severity of addiction. The absence of acute nicotinic effects is somewhat discrepant with previously reported differences in reward sensitivity between abstinent and sated smoking conditions. However, these studies observed differences in cue reactivity or reward anticipation, while our contrast focused on reward receipt. Rose et al reported lower MCL activity upon reward receipt in smokers vs nonsmokers, which was not remedied by nicotine but was associated with years of smoking. Conversely, in a monetary incentive task, reduced reward anticipation in smokers was mitigated with nicotine. Therefore, there may be a relevant distinction between neural responses to reward-associated cues (anticipation) and neural responses to actual reward receipt, whereby reductions in the former can be remedied by acute stimulation of the nAChRs, while the latter are associated with severity of nicotine dependence. Reduced reward sensitivity may contribute to relapse, especially early in a quit attempt. With prolonged abstinence, the availability of DA at the receptor level normalizes, so future studies should assess whether reward sensitivity is restored over time.
Acute Nicotinic Effects on Neural Correlates of Cognitive Flexibility in Smokers’ MCL System
In stark contrast to reward sensitivity, neural signatures of cognitive flexibility were modulated by acute administration of nicotine (and to some extent varenicline). As hypothesized, neural activity preceding a behavioral change was reduced in abstinent smokers throughout the MCL circuitry: the bilateral ventral and dorsal striatum, the bilateral AI, the ACC, and the ventromedial prefrontal cortex (areas implicated in reinforcement learning and rule switching). When smokers wore a nicotine patch, activity in these areas appeared to normalize, reaching levels comparable to those observed among nonsmokers. Thus, our results support an allostatic account of addiction, whereby chronic exposure to nicotine leads to reduced DA levels in abstinence, which are normalized through stimulation of the nAChRs. Results also dovetail with preclinical studies demonstrating reversal learning deficits accompanied by dorsal striatal brain-derived neurotrophic factor differences in nicotine-dependent rodents, and alleviation of such deficits with nicotine and varenicline. Specifically, the findings of Jackson et al closely track with our results in humans; these authors demonstrated PRL performance decreases in acutely abstinent nicotine-dependent rats, which were alleviated by administration of nicotine and varenicline, while finding no effects of nicotine or varenicline on PRL performance in nicotine-naive rats.
Compulsivity, Excessive Flexibility, and DA Levels
Reversal learning is increasingly used to characterize compulsive disorders, including dependence on alcohol, cocaine, and amphetamines, as well as gambling and binge eating. However, PRL deficits are not always driven by perseveration or inflexibility; in our sample, abstinent smokers shifted responses excessively. Similar excessive flexibility has been documented in binge-eating disorder and cocaine dependence, but cocaine, alcohol, and amphetamine dependence have been linked to decreased flexibility. Very few studies have investigated state effects (abstinence vs satiety) on reversal learning in dependent populations. Probabilistic reversal learning studies in nondependent populations indicate that the effects of DA agonists crucially depend on baseline DA levels, with dopaminergic manipulations improving performance in individuals with low baseline levels of DA but impairing performance in those with high baseline levels of DA. The interacting chronic and acute effects of nicotine exposure found here can be interpreted in light of lowered tonic DA levels in drug dependence and increases in DA following drug administration. Our data are in line with clinical and preclinical work showing abstinence-associated behavioral deficits in reward responsivity in nicotine-dependent individuals and complement results of previous neuroimaging studies showing that nicotine and varenicline can mitigate the effects of abstinence on the limbic system and can influence reward processing.
Limitations
Our study has some limitations. First, we administered only the standard clinical doses of nicotine and varenicline. Although results can be generalized to current clinical applications of nicotine replacement therapy and varenicline, different doses may yield different effects. Future studies might explore dose-response effects on reward-based decision making and its neural correlates. For example, larger nicotine doses in nonsmokers may affect performance, although at the cost of adverse effects. Second, we did not identify significant nicotine-by-varenicline interactions on the cognitive flexibility neuroimaging contrast, but extracted parameter values trended toward the hypothesized nicotine-by-varenicline interaction. This finding may indicate that our analysis was insufficiently powered to detect smaller (partial agonist) varenicline effects, while it did detect larger (full agonist) nicotine effects.
Conclusions
This is the first study, to our knowledge, investigating the effects of chronic nicotine exposure and acute stimulation of the nAChRs on brain and behavioral metrics during reversal learning. We identified behavioral and MCL signatures of dysregulated cognitive flexibility in abstinent smokers, which were restored with nicotine (and to a lesser extent varenicline). Conversely, smokers’ lower neural response to reward was associated with severity of dependence and not remedied with nAChR stimulation. Thus, we found a double dissociation between chronic and acute effects of nicotine on reward sensitivity and cognitive flexibility within overlapping MCL regions. This study highlights the need to dissociate acute drug-associated effects from effects associated with chronic drug dependence, and to consider both mental computations and their anatomical substrate. Finally, the results provide a neural basis for the efficacy of nicotine replacement therapy and varenicline as smoking cessation tools, particularly associated with cognitive flexibility.
Trial Protocol
eAppendix 1. Methods
eAppendix 2. Results
eFigure 1. Study Design
eFigure 2. Probabilistic Reversal Learning Task and Trial Types
eFigure 3. Composite Mask of the Regions of Interest
eFigure 4. QA: Effectiveness of nicotinic receptor stimulation
eFigure 5. QA: Assessment for task engagement
eFigure 6. QA: Absence of learning effects
eFigure 7. Computational modeling results: Model Comparisons
eFigure 8. Computational modeling results: Example Model Fit
eFigure 9. Imaging results: Task Maps for Reward-Sensitivity and Cognitive Flexibility Contrasts Across Groups and Sessions
eFigure 10. Imaging results: Reward vs Punishment Group Differences
eFigure 11. Imaging results: Cognitive Flexibility is associated with Bias to Shift in Smokers in Areas That Show Decreased Shift-Related activity
eTable 1. Demographics
eTable 2. Behavioral Results
eTable 3. Parameters Estimates and Goodness of Fit Measure for HMM2
eTable 4. Parameters Estimates and Goodness of Fit Measure for HMM1
eTable 5. Parameters Estimates and Goodness of Fit Measure for the Rescorla-Wagner Model
eTable 6. Reward-Sensitivity Contrast: Table of fMRI Results
eTable 7. Cognitive Flexibility Contrast: Table of fMRI Results
eReferences
References
- 1.Center for Disease Control Quitting smoking among adults—United States, 2001–2010. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6044a2.htm. Published November 11, 2011. Accessed November 13, 2016.
- 2.Powell J, Dawkins L, West R, Powell J, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology (Berl). 2010;212(4):537-549. [DOI] [PubMed] [Google Scholar]
- 3.Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2014;76(pt B):581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst M, Matochik JA, Heishman SJ, et al. Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci U S A. 2001;98(8):4728-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedota JR, Sutherland MT, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Reward anticipation is differentially modulated by varenicline and nicotine in smokers. Neuropsychopharmacology. 2015;40(8):2038-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Individual differences in amygdala reactivity following nicotinic receptor stimulation in abstinent smokers. Neuroimage. 2013;66:585-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lesage E, Stein EA. Networks associated with reward In: Pfaff DW, ed. Neuroscience for the 21st Century: From Basic to Clinical. 2nd ed New York, NY: Springer; 2013:1677-1703. [Google Scholar]
- 8.Sutherland MT, Liang X, Yang Y, Stein EA. Beyond functional localization: advancing the understanding of addiction-related processes by examining brain connectivity In: Wilson SJ, ed. The Wiley-Blackwell Handbook on the Neuroscience of Addiction. Hoboken, NJ: Wiley-Blackwell; 2015:472-502. [Google Scholar]
- 9.De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annu Rev Neurosci. 2011;34(1):105-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Exley R, Maubourguet N, David V, et al. Distinct contributions of nicotinic acetylcholine receptor subunit α4 and subunit α6 to the reinforcing effects of nicotine. Proc Natl Acad Sci U S A. 2011;108(18):7577-7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapper AR, McKinney SL, Nashmi R, et al. Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306(5698):1029-1032. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52-58. [DOI] [PubMed] [Google Scholar]
- 14.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweitzer MM, Geier CF, Denlinger R, et al. Blunted striatal response to monetary reward anticipation during smoking abstinence predicts lapse during a contingency-managed quit attempt. Psychopharmacology (Berl). 2016;233(5):751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark L, Robbins T. Decision-making deficits in drug addiction. Trends Cogn Sci. 2002;6(9):361-363. [DOI] [PubMed] [Google Scholar]
- 17.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(11):4563-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):917-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose EJ, Ross TJ, Salmeron BJ, et al. Acute nicotine differentially impacts anticipatory valence- and magnitude-related striatal activity. Biol Psychiatry. 2013;73(3):280-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson SJ, Delgado MR, McKee SA, et al. Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cogn Affect Behav Neurosci. 2014;14(4):1196-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Addicott MA, Baranger DAA, Kozink RV, Smoski MJ, Dichter GS, McClernon FJ. Smoking withdrawal is associated with increases in brain activation during decision making and reward anticipation: a preliminary study. Psychopharmacology (Berl). 2012;219(2):563-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banca P, Harrison NA, Voon V. Compulsivity across the pathological misuse of drug and non-drug rewards. Front Behav Neurosci. 2016;10:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deserno L, Beck A, Huys QJM, et al. Chronic alcohol intake abolishes the relationship between dopamine synthesis capacity and learning signals in the ventral striatum. Eur J Neurosci. 2015;41(4):477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ersche KD, Roiser JP, Abbott S, et al. Response perseveration in stimulant dependence is associated with striatal dysfunction and can be ameliorated by a D2/3 receptor agonist. Biol Psychiatry. 2011;70(8):754-762. [DOI] [PubMed] [Google Scholar]
- 25.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl). 2008;197(3):421-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl). 2012;219(2):607-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patzelt EH, Kurth-Nelson Z, Lim KO, Macdonald AW III. Excessive state switching underlies reversal learning deficits in cocaine users. Drug Alcohol Depend. 2014;134:211-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole RD, Poole RL, Guzman DM, Gould TJ, Parikh V. Contributions of β2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacology (Berl). 2015;232(7):1207-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega LA, Tracy BA, Gould TJ, Parikh V. Effects of chronic low- and high-dose nicotine on cognitive flexibility in C57BL/6J mice. Behav Brain Res. 2013;238:134-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson A, Silk S, Buhidma Y, Shoaib M. Varenicline, the clinically effective smoking cessation agent, restores probabilistic response reversal performance during withdrawal from nicotine. [published online July 20, 2016]. Addict Biol. 2016. doi: 10.1111/adb.12423 [DOI] [PubMed] [Google Scholar]
- 31.Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of α4β2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28(7):316-325. [DOI] [PubMed] [Google Scholar]
- 32.Hampton AN, Bossaerts P, O’Doherty JP. The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans. J Neurosci. 2006;26(32):8360-8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013;74(7):538-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Stein EA. Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology (Berl). 2013;228(1):143-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroll AJ, Sutherland MT, Salmeron BJ, Ross TJ, Stein EA. Greater externalizing personality traits predict less error-related insula and anterior cingulate cortex activity in acutely abstinent cigarette smokers. Addict Biol. 2015;20(2):377-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen MX, Elger CE, Weber B. Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. Neuroimage. 2008;39(3):1396-1407. [DOI] [PubMed] [Google Scholar]
- 37.O’Doherty JP, Hampton A, Kim H. Model-based fMRI and its application to reward learning and decision making. Ann N Y Acad Sci. 2007;1104(1):35-53. [DOI] [PubMed] [Google Scholar]
- 38.den Ouden HEM, Daw ND, Fernandez G, et al. Dissociable effects of dopamine and serotonin on reversal learning. Neuron. 2013;80(4):1090-1100. [DOI] [PubMed] [Google Scholar]
- 39.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement Rescorla RA, Wagner AR, eds. Classical Conditioning II: Current Research and Theory. New York, NY: Appleton-Century-Crofts; 1972:64-99. [Google Scholar]
- 40.Sohn H, Kim S. Simple reinforcement learning models are not always appropriate. J Neurosci. 2006;26(45):11511-11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162-173. [DOI] [PubMed] [Google Scholar]
- 42.O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(21):7931-7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127. [DOI] [PubMed] [Google Scholar]
- 44.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31(6):1203-1211. [DOI] [PubMed] [Google Scholar]
- 46.Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789-1798. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein RZ, Tomasi D, Rajaram S, et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007;144(4):1153-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin-Soelch C, Leenders KL, Chevalley A-F, et al. Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Res Brain Res Rev. 2001;36(2-3):139-149. [DOI] [PubMed] [Google Scholar]
- 49.Peters J, Bromberg U, Schneider S, et al. ; IMAGEN Consortium . Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry. 2011;168(5):540-549. [DOI] [PubMed] [Google Scholar]
- 50.Sweitzer MM, Geier CF, Joel DL, et al. Dissociated effects of anticipating smoking versus monetary reward in the caudate as a function of smoking abstinence. Biol Psychiatry. 2014;76(9):681-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose EJ, Ross TJ, Salmeron BJ, et al. Chronic exposure to nicotine is associated with reduced reward-related activity in the striatum but not the midbrain. Biol Psychiatry. 2012;71(3):206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rademacher L, Prinz S, Winz O, et al. Effects of smoking cessation on presynaptic dopamine function of addicted male smokers. Biol Psychiatry. 2016;80(3):198-206. [DOI] [PubMed] [Google Scholar]
- 53.Bissonette GB, Roesch MR. Neurophysiology of rule switching in the corticostriatal circuit. Neuroscience. 2017;345:64-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reiter AM, Heinze HJ, Schlagenhauf F, Deserno L. Impaired flexible reward-based decision-making in binge eating disorder: evidence from computational modeling and functional neuroimaging. Neuropsychopharmacology. 2017;42(3):628-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boorman ED, Rushworth MF, Behrens TE. Ventromedial prefrontal and anterior cingulate cortex adopt choice and default reference frames during sequential multi-alternative choice. J Neurosci. 2013;33(6):2242-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camchong J, MacDonald AW III, Nelson B, et al. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry. 2011;69(11):1117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Ruiter MB, Veltman DJ, Goudriaan AE, Oosterlaan J, Sjoerds Z, van den Brink W. Response perseveration and ventral prefrontal sensitivity to reward and punishment in male problem gamblers and smokers. Neuropsychopharmacology. 2009;34(4):1027-1038. [DOI] [PubMed] [Google Scholar]
- 58.Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29(5):1538-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Schaaf ME, van Schouwenburg MR, Geurts DEM, et al. Establishing the dopamine dependency of human striatal signals during reward and punishment reversal learning. Cereb Cortex. 2014;24(3):633-642. [DOI] [PubMed] [Google Scholar]
- 60.Pergadia ML, Der-Avakian A, D’Souza MS, et al. Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA Psychiatry. 2014;71(11):1238-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008;63(11):1061-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Methods
eAppendix 2. Results
eFigure 1. Study Design
eFigure 2. Probabilistic Reversal Learning Task and Trial Types
eFigure 3. Composite Mask of the Regions of Interest
eFigure 4. QA: Effectiveness of nicotinic receptor stimulation
eFigure 5. QA: Assessment for task engagement
eFigure 6. QA: Absence of learning effects
eFigure 7. Computational modeling results: Model Comparisons
eFigure 8. Computational modeling results: Example Model Fit
eFigure 9. Imaging results: Task Maps for Reward-Sensitivity and Cognitive Flexibility Contrasts Across Groups and Sessions
eFigure 10. Imaging results: Reward vs Punishment Group Differences
eFigure 11. Imaging results: Cognitive Flexibility is associated with Bias to Shift in Smokers in Areas That Show Decreased Shift-Related activity
eTable 1. Demographics
eTable 2. Behavioral Results
eTable 3. Parameters Estimates and Goodness of Fit Measure for HMM2
eTable 4. Parameters Estimates and Goodness of Fit Measure for HMM1
eTable 5. Parameters Estimates and Goodness of Fit Measure for the Rescorla-Wagner Model
eTable 6. Reward-Sensitivity Contrast: Table of fMRI Results
eTable 7. Cognitive Flexibility Contrast: Table of fMRI Results
eReferences