Key Points
Question
Do individuals with anxiety disorders exhibit dysregulated psychophysiological and neuroimaging profiles during fear conditioning and extinction recall?
Findings
In this cross-sectional, case-control study, 61 individuals with anxiety disorders activated the ventromedial prefrontal cortex less during fear conditioning and extinction recall compared with 21 healthy controls. This hypoactivation was more pronounced in those diagnosed as having multiple anxiety disorders than in those having only one anxiety disorder.
Meaning
Gaining a better understanding of the structures of the fear circuitry that are dysregulated in anxious individuals might help in guiding better treatments targeting the neurobiological features of the disorder.
Abstract
Importance
The fear conditioning and extinction neurocircuitry has been extensively studied in healthy and clinical populations, with a particular focus on posttraumatic stress disorder. Despite significant overlap of symptoms between posttraumatic stress disorder and anxiety disorders, the latter has received less attention. Given that dysregulated fear levels characterize anxiety disorders, examining the neural correlates of fear and extinction learning may shed light on the pathogenesis of underlying anxiety disorders.
Objectives
To investigate the psychophysiological and neural correlates of fear conditioning and extinction recall in anxiety disorders and to document how these features differ as a function of multiple diagnoses or anxiety severity.
Design, Setting, and Participants
This investigation was a cross-sectional, case-control, functional magnetic resonance imaging study at an academic medical center. Participants were healthy controls and individuals with at least 1 of the following anxiety disorders: generalized anxiety disorder, social anxiety disorder, specific phobia, and panic disorder. The study dates were between March 2013 and May 2015.
Exposures
Two-day fear conditioning and extinction paradigm.
Main Outcomes and Measures
Skin conductance responses, blood oxygenation level–dependent responses, trait anxiety scores from the State Trait Anxiety Inventory–Trait Form, and functional connectivity.
Results
This study included 21 healthy controls (10 women) and 61 individuals with anxiety disorders (36 women). P values reported for the neuroimaging results are all familywise error corrected. Skin conductance responses during extinction recall did not differ between individuals with anxiety disorders and healthy controls (ηp2 = 0.001, P = .79), where ηp2 is partial eta squared. The anxiety group had lower activation of the ventromedial prefrontal cortex (vmPFC) during extinction recall (ηp2 = 0.178, P = .02). A similar hypoactive pattern was found during early conditioning (ηp2 = 0.106, P = .009). The vmPFC hypoactivation was associated with anxiety symptom severity (r = −0.420, P = .01 for conditioning and r = −0.464, P = .004 for extinction recall) and the number of co-occuring anxiety disorders diagnosed (ηp2 = 0.137, P = .009 for conditioning and ηp2 = 0.227, P = .004 for extinction recall). Psychophysiological interaction analyses revealed that the fear network connectivity differed between healthy controls and the anxiety group during fear learning (ηp2 range between 0.088 and 0.176 and P range between 0.02 and 0.003) and extinction recall (ηp2 range between 0.111 and 0.235 and P range between 0.02 and 0.002).
Conclusions and Relevance
Despite no skin conductance response group differences during extinction recall, brain activation patterns between anxious and healthy individuals differed. These findings encourage future studies to examine the conditions longitudinally and in the context of treatment trials to improve and guide therapeutics via advanced neurobiological understanding of each disorder.
This cross-sectional, case-control functional magnetic resonance imaging study investigates the psychophysiological and neural correlates of fear conditioning and extinction recall in anxiety disorders and documents how these features differ as a function of multiple diagnoses or anxiety severity.
Introduction
Fear conditioning and extinction paradigms are relevant for studying anxiety disorders. It has been proposed that pathological anxiety could emerge from dysregulated patterns of fear learning and that maintenance of anxiety-related symptoms could be explained by extinction deficits. Until now, this paradigm has been mostly tested in populations with posttraumatic stress disorder (PTSD). At the psychophysiological level, they exhibit generally normal conditioning and extinction learning but impaired extinction recall. During extinction recall, individuals suffering from PTSD exhibit lower activations in brain regions promoting safety signal processing, such as the ventromedial prefrontal cortex (vmPFC) and hippocampus, and they exhibit greater activations in regions promoting fear signal detection, such as the amygdala and dorsal anterior cingulate cortex (dACC).
Until the DSM-5 release, PTSD was considered an anxiety disorder; it is now classified as a trauma and stress–related disorder. However, it remains unclear whether the physiological deficiencies in PTSD are also observed in conditions currently categorized as anxiety disorders. Although PTSD and anxiety disorders present overlapping features—notably, dysregulated fear levels—DSM-5 anxiety disorders have been less studied in the context of conditioning and extinction paradigms. Results of a meta-analysis suggested that anxious individuals exhibit higher fear in response to safety cues during conditioning and higher fear in response to danger cues during extinction. The meta-analysis included study samples with PTSD and dealt with complex clinical portraits (ie, comorbidity from the same or different diagnostic categories). Finally, it remains to be studied whether the magnitude of the pathophysiological deficit differs based on the number of anxiety disorders, without the confounds of other comorbidities.
This study aimed to elucidate some of these issues in individuals having anxiety disorders without other comorbidities. Using psychophysiological and neuroimaging tools, this study investigated how anxiety disorders influence the circuitry of fear conditioning and extinction recall. We then examined whether the presence of multiple anxiety disorders influences the circuitry relative to a single disorder. We hypothesized that, relative to healthy controls, individuals with anxiety disorders (1) would have lower differential fear conditioning and deficient extinction recall in terms of skin conductance response (SCR) and (2) would exhibit dysregulated activation patterns in the fear circuitry nodes during fear learning and extinction recall. We also hypothesized that there would be more pronounced dysregulations in those with multiple anxiety disorders. We conclude with a mechanistic focus investigating how activations during fear memory encoding relate to activations during recall.
Methods
Participants
We recruited 61 individuals meeting criteria for at least 1 of the following anxiety disorders: generalized anxiety disorder, social anxiety disorder, specific phobia, and panic disorder (45 had 1 disorder and 16 had ≥2 disorders), with no other current comorbidities. We included previous data from 21 healthy controls who underwent identical experimental procedures with use of the same scanner. For exclusion criteria and a description of the study sample, see the eAppendix in the Supplement.
Procedure
Participants provided written informed consent in accord with the requirements of the Partners Healthcare Institutional Review Board, who approved the study. Participants underwent a Structured Clinical Interview for DSM-IV with one of us (N.B.L.) to determine the diagnoses and eligibility. They filled out the State Trait Anxiety Inventory–Trait Form (STAI-T), examining self-reported anxiety levels. Participants underwent a 2-day fear conditioning and extinction paradigm in a functional magnetic resonance imaging scanner that included conditioned stimuli (CS) (eAppendix in the Supplement). On day 1, fear conditioning occurred, during which 2 cues (CS+) were reinforced and 1 cue (CS−) was not. This conditioning was followed by extinction learning, where 1 CS+ and the CS− were presented. The next day, extinction recall was tested, where the extinguished CS+ (CS + E), the nonextinguished CS+ (CS + NE), and the CS− were presented (details are provided in the eAppendix in the Supplement).
Data Processing
Skin conductance response and imaging data were computed using the previously used methods. Details are provided in the eAppendix in the Supplement.
Analytic Approach
For conditioning, equal numbers of trials are used for CS+ and CS−. However, 2 CS+s and only one CS− are used, suggesting that there might be more habituation to CS− (16 trials of 1 cue) relative to CS+ (16 trials based on 2 cues). We performed analyses to assess habituation effects between groups (eAppendix in the Supplement).
For conditioning, a stimulus (CS+ vs CS−) × time (early vs late) × group (healthy vs anxiety) analysis of covariance (ANCOVA) was performed on SCR. For the imaging analysis, between-group differences were investigated for early, late, and all conditioning. Additional analyses examined whether both groups had similar fear extinction levels (eAppendix in the Supplement). For extinction recall, a stimulus (CS + E vs CS + NE) × group (healthy vs anxiety) ANCOVA was performed on SCR. Similar analyses were performed for the imaging data.
To investigate differences between single vs multiple anxiety disorders, analyses were repeated for the anxiety cohort alone. For these analyses, group (single vs multiple) was the between-group factor.
To assess associations between anxiety severity and the fear network within the anxiety group, a voxelwise analysis was performed with STAI-T scores as a regressor for early conditioning (CS+ vs CS−), late conditioning (CS+ vs CS−), and extinction recall (CS + E vs CS + NE). Beta weights were extracted from the peak voxel to generate a correlation coefficient.
Psychophysiological interaction (PPI) analyses were performed during early conditioning and extinction recall. For these analyses, vmPFC was used as the seed.
Imaging analyses were performed with an initial threshold of P < .005 and 10 contiguous voxels. Activations detected with that threshold within the fear circuitry (amygdala, hippocampus, insular cortex, ACC, and vmPFC) were then tested for small-volume correction.
Results
Demographics
This study included 21 healthy controls, with a mean (SD) age of 25.8 (4.8) years, 47.6% (10 of 21) of whom were female, and 61 individuals with anxiety disorders, with a mean (SD) age of 30.4 (11.5) years, 59.0% (36 of 61) of whom were female. Healthy controls were younger (t77 = 2.559, P = .01) and more educated (t80 = 1.922, P = .06) than the anxiety group. Both groups had similar shock levels (t73 = 0.346, P = .18) and sex distributions (χ1 = 0.824, P = .36). Analyses comparing these groups were run with (main text) and without (eAppendix in the Supplement) age and educational level as covariates. Analyses comparing the single disorder group with the multiple disorders group did not include covariates because the groups did not statistically differ on any demographics.
Healthy Group vs Anxiety Group
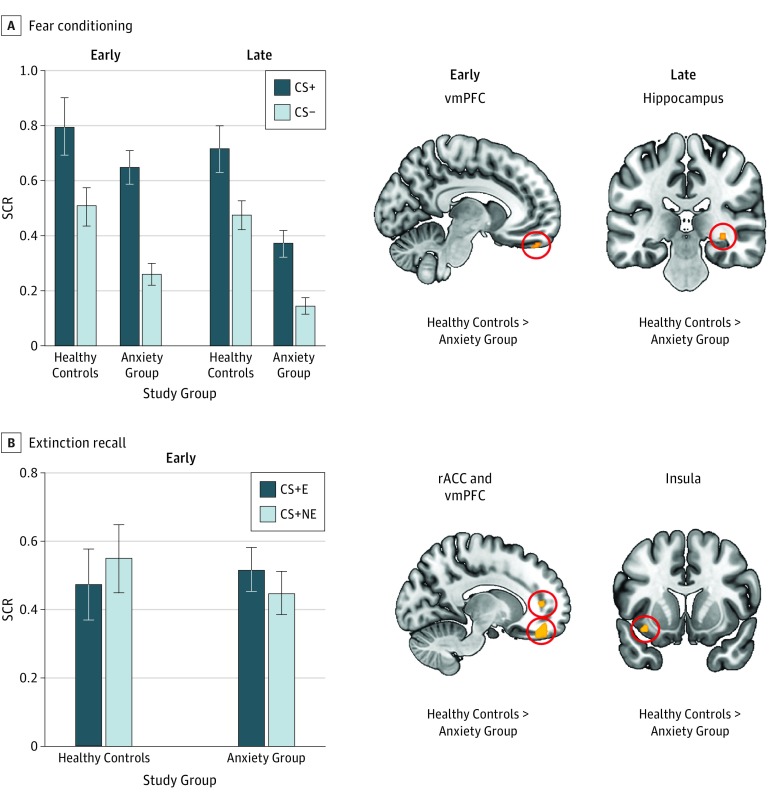
During conditioning (Figure 1A), the SCR ANCOVA yielded a marginal effect of stimulus (F1,77 = 3.009, P = .09, ηp = 0.038), an effect of group (F1,77 = 11.126, P = .001, ηp = 0.126), and a time × group interaction (F1,77 = 5.110, P = .03, ηp = 0.062), where ηp is partial eta squared. Compared with the anxiety group, healthy controls exhibited greater vmPFC activation during early conditioning (Montreal Neurological Institute x, y, z coordinates [hereafter MNI] −8, 50, −28; cluster size of 12; t72 = 2.99; P = .009 familywise error; ηp = 0.106) and greater hippocampal activation during late conditioning (MNI 32, −30, −6; cluster size of 10; t72 = 2.99; P = .007 familywise error; ηp = 0.113). Both groups underwent successful extinction learning (eAppendix in the Supplement).
Figure 1. Comparison of Healthy Controls and Individuals With Anxiety Disorders During Fear Conditioning and Extinction Recall.
Shown are skin conductance response (SCR) and voxelwise analyses contrasting healthy controls with individuals with anxiety disorders. A, On the left, SCR is shown during early and late conditioning as a function of conditioned stimuli (CS) type and group. On the right, significant group differences are shown in terms of brain activation during the early CS+ vs CS− contrast and late CS+ vs CS− contrast from fear conditioning. B, On the left, SCR is shown during early extinction recall as a function of CS type and group. On the right, brain regions are shown for which the 2 groups differed during extinction recall for the CS + E vs CS + NE contrast. Hot colors indicate greater activation in healthy controls relative to the anxiety group. P = .005 is used for all results. Error bars are SEM. CS+ indicates cues that were partially reinforced with a shock; CS−, cue that was never reinforced with a shock; CS + E, extinguished CS+; CS + NE, nonextinguished CS+; rACC, rostral anterior cingulate cortex; and vmPFC, ventromedial prefrontal cortex.
For extinction recall, the SCR ANCOVA did not yield any significant results, (F1,67≤1.109 for all, P ≥ .30 for all, ηp≤0.016 for all). Relative to healthy controls, the anxiety group exhibited less activation in the rostral ACC (rACC) (MNI −12, 44, 8; cluster size of 26; t63 = 3.21; P = .007 familywise error; ηp = 0.117), the vmPFC (MNI −14, 46, −18; cluster size of 169; t63 = 3.41; P = .02 familywise error; ηp = 0.178), and the insular cortex (MNI −36, 10, −12; cluster size of 18; t63 = 3.44; P = .003 familywise error; ηp = 0.136).
Number of Anxiety Disorders
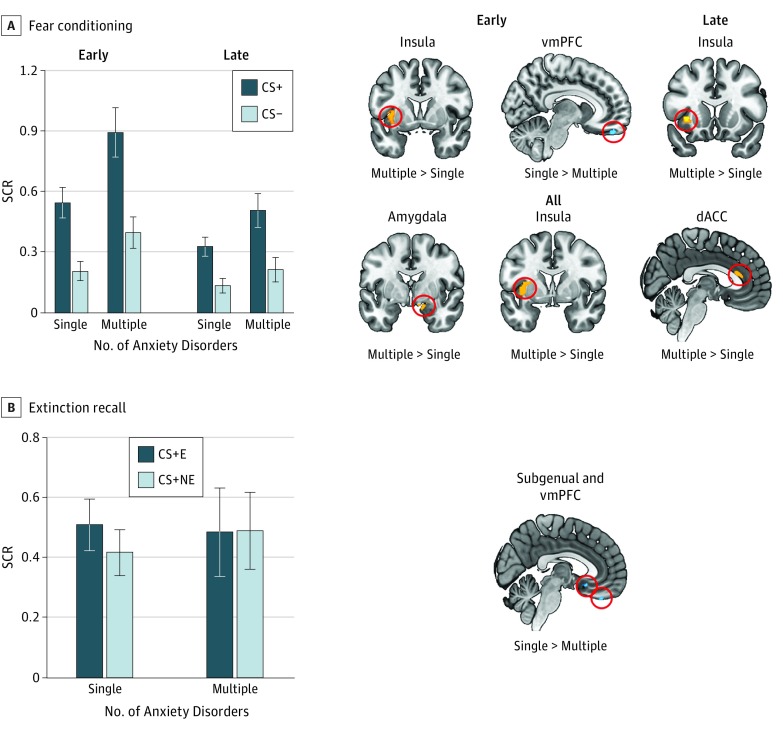
During conditioning (Figure 2A), the SCR ANOVA yielded a main effect of stimulus (F1,58 = 46.005, P < .001, ηp = 0.442), a main effect of time (F1,58 = 36.010, P < .001, ηp = 0.383), a main effect of group (F1,58 = 5.671, P = .02, ηp = 0.089), a marginal time × group interaction (F1,58 = 3.580, P = .06, ηp = 0.204), and a time × stimulus interaction (F1,58 = 14.876, P < .001, ηp = 0.058). During early conditioning, the multiple disorders group had greater insular cortex activation (MNI −36, 2, 4; cluster size of 67; t56 = 4.21; P = .001 familywise error; ηp = 0.179) but less vmPFC activation (MNI 8, 48, −26; cluster size of 33; t56 = 3.19; P = .009 familywise error; ηp = 0.137) relative to the single disorder group. For late conditioning, the multiple disorders group activated the insular cortex (MNI −34, 10, 4; cluster size of 42; t56 = 3.28; P = .008 familywise error; ηp = 0.162) more than the single disorder group. When examining conditioning across all trials, the multiple disorders group had greater activation in the amygdala (MNI 14, −2, −18; cluster size of 86; t56 = 3.99; P = .002 familywise error; ηp = 0.231), the insular cortex (MNI −36, 0, 4; cluster size of 219; t56 = 3.91; P = .006 familywise error; ηp = 0.228), and the dACC (MNI 0, 28,16; cluster size of 59; t56 = 3.39; P = .008 familywise error; ηp = 0.151).
Figure 2. Comparison of One Anxiety Disorder With Multiple Anxiety Disorders During Fear Conditioning and Extinction Recall.
Shown are skin conductance response (SCR) and voxelwise analyses contrasting individuals diagnosed as having one anxiety disorder with those diagnosed as having multiple anxiety disorders. A, On the left, SCR is shown during early and late conditioning as a function of conditioned stimuli (CS) type and group. On the right, significant group differences are shown in terms of brain activation during the early CS+ vs CS− contrast, late CS+ vs CS− contrast, and all CS+ vs CS− contrast from fear conditioning. B, On the left, SCR is shown during early extinction recall as a function of CS type and group. On the right, the brain region is shown for which the 2 groups differed during extinction recall for the CS + E vs CS + NE contrast. Hot colors indicate greater activation in the multiple disorders group relative to the single disorder group. Cold colors indicate greater activation in the single disorder group relative to the multiple disorders group. P = .005 is used for all results. Error bars are SEM. CS+ indicates cues that were partially reinforced with a shock; CS−, cue that was never reinforced with a shock; CS + E, extinguished CS+; CS + NE, nonextinguished CS+; dACC, dorsal anterior cingulate cortex; and vmPFC, ventromedial prefrontal cortex.
During extinction recall, the SCR ANOVA yielded no significant results (F1,48≤0.281 for all, P ≥ .59 for all; ηp≤0.006 for all). The functional magnetic resonance imaging analysis revealed less vmPFC (MNI 0, 38, −30; cluster size of 32; t47 = 3.48; P = .004 familywise error; ηp = 0.227) and less subgenual ACC (sgACC) (MNI 2, 18, −14; cluster size of 23; t47 = 3.11; P = .009 familywise error; ηp = 0.206) activations in the multiple disorders group compared with the single disorder group.
Given the different weights of the specific phobics in the single disorder group (n = 17) compared with the multiple disorders group (only present as comorbidity and not as the main disorder), we conducted analyses to rule out the possibility that the effects obtained were driven only by the specific phobics. Details are provided in the eAppendix in the Supplement.
Correlates of STAI-T Scores During Conditioning and Extinction Recall
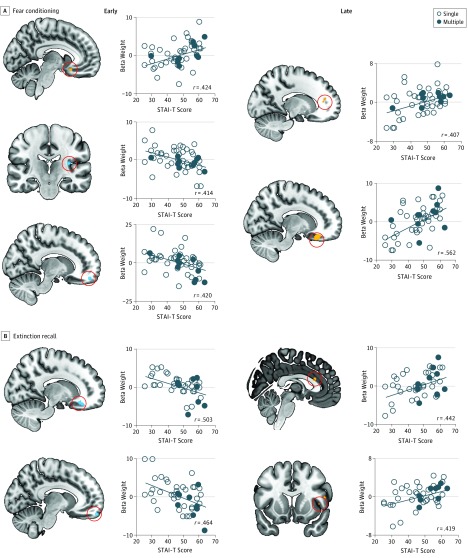
For early conditioning (Figure 3A), STAI-T scores were positively correlated with sgACC activation (MNI −10, 16, −14; cluster size of 25; t47 = 3.21; P = .008 familywise error; r = 0.424) and negatively correlated with insular cortex (MNI −34, −28, 18; cluster size of 31; t47 = 3.16; P = .01 familywise error; r = −0.414) and vmPFC (MNI 14, 54, −18; cluster size of 38; t47 = 3.17; P = .01 familywise error; r = −0.420) activations. During late conditioning, STAI-T scores were positively associated with rACC (MNI −16, 40, 16; cluster size of 18; t47 = 3.05; P = .01 familywise error; r = 0.407) and sgACC (MNI 16, 32, −20; cluster size of 231; t47 = 4.25; P = .001 familywise error; r = 0.562) activations. During extinction recall (Figure 3B), STAI-T scores were negatively associated with sgACC (MNI 16, 34, −18; cluster size of 34; t41 = 3.73; P = .003 familywise error; r = −0.503) and vmPFC (MNI −10, 60, −20; cluster size of 14; t41 = 3.35; P = .004 familywise error; r = −0.464) activations but were positively associated with dACC (MNI 2, 22, 20; cluster size of 23; t41 = 3.15; P = .009 familywise error; r = 0.442) and insular cortex (MNI 44, 12, 4; cluster size of 45; t41 = 2.95; P = .03 familywise error; r = 0.419) activations.
Figure 3. Trait Anxiety Prediction of Brain Activation During Fear Conditioning and Extinction Recall in the Anxiety Group.
Shown are voxelwise analyses performed in the anxiety group only with trait anxiety scores as the regressor. A, The regions of the fear network are shown that demonstrated significant association with trait anxiety scores during early and late fear conditioning. B, The regions of the fear network are shown that demonstrated significant associations with trait anxiety scores during early extinction recall. STAI-T indicates State Trait Anxiety Inventory–Trait Form.
PPI Analyses
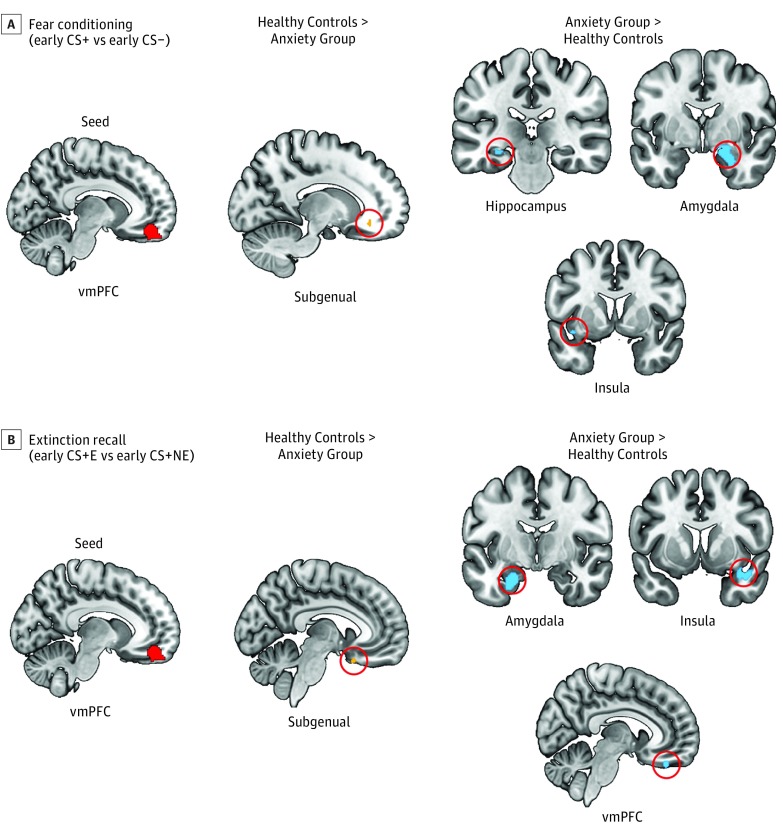
During early conditioning, the vmPFC seed had greater functional connectivity in healthy controls with the sgACC (MNI −14, 34, −12; cluster size of 10; t71 = 3.40; P = .003 familywise error; ηp = 0.095) but greater connectivity in the anxiety group with the hippocampus (MNI −26, −24, −12; cluster size of 17; t71 = 2.81; P = .02 familywise error; ηp = 0.101), the amygdala (MNI 26, −4, −26; cluster size of 384; t71 = 3.71; P = .02 familywise error; ηp = 0.176), and the insular cortex (MNI −38, 6, −12; cluster size of 13; t71 = 2.80; P = .01 familywise error; ηp = 0.088). These results are shown in Figure 4A.
Figure 4. Psychophysiological Interactions With Ventromedial Prefrontal Cortex (vmPFC) as the Seed During Fear Conditioning and Extinction Recall.
Shown are between-group psychophysiological interaction analyses contrasting healthy controls with individuals with anxiety disorders during early fear conditioning (A) and extinction recall (B). Hot colors indicate that the healthy controls exhibited greater connectivity than the anxiety group between the seed and the given region. Cold colors indicate that the anxiety group exhibited greater connectivity than the healthy controls between the seed and the given region. P = .005 is used for all results. CS indicates conditioned stimuli; CS+ indicates cues that were partially reinforced with a shock; CS−, the cue that was never reinforced with a shock; CS + E, extinguished CS+; and CS + NE, nonextinguished CS+.
During extinction recall, the vmPFC seed showed greater functional connectivity in healthy controls with the sgACC (MNI −8, 16, −24; cluster size of 10; t63 = 2.95; P = .007 familywise error; ηp = 0.111) but greater connectivity in the anxiety group with the amygdala (MNI −24, −10, −24; cluster size of 305; t63 = 4.26; P = .002 familywise error; ηp = 0.235), the insular cortex (MNI 40, 10, −22; cluster size of 169; t63 = 3.39; P = .02 familywise error; ηp = 0.158), and the vmPFC (MNI 6, 38, −26; cluster size of 37; t63 = 3.37; P = .006 familywise error; ηp = 0.143). These results are shown in Figure 4B.
Exploratory Analyses of Associations
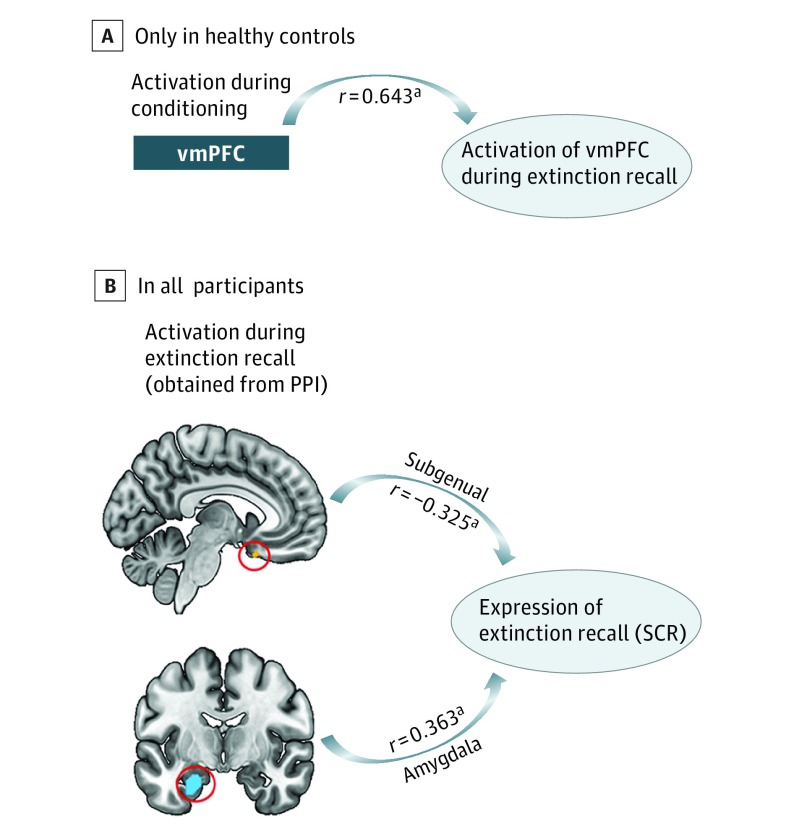
As an exploratory analysis, we examined whether dysregulated activation patterns observed during conditioning could account for or be related to the activation pattern observed in extinction recall. Hippocampus activation during conditioning was not associated with vmPFC activation during recall (r = 0.098, P = .44). There was a suggestion of an association between vmPFC activation during conditioning and vmPFC activation during recall (r = 0.232, P = .06). We examined the pattern within each group and found that it was only present in healthy controls (r = 0.643, P = .004) and not in anxious individuals (r = −0.151, P = .31) (Figure 5A). We tested if similar patterns of associations were present for the SCR data. An exploratory analysis examined correlations between SCR during recall (SCR to the first 4 trials of CS + E minus SCR to the first 4 trials of CS + NE) and the connectivity values between the vmPFC and the following regions: sgACC, amygdala, insular cortex, and vmPFC. The connectivity value between vmPFC and sgACC was negatively associated with SCR (r = −0.281, P = .03), whereas the connectivity value between vmPFC and amygdala was positively associated with SCR (r = 0.271, P = .04) (Figure 5B). The vmPFC-vmPFC (r = 0.101, P = .44) and vmPFC-insula (r = −0.001, P = .99) connectivity values were not associated with SCR.
Figure 5. Exploratory Analyses.
A, Activation in the ventromedial prefrontal cortex (vmPFC) during early fear conditioning is associated with vmPFC activation during extinction recall. This association was only significant in healthy controls and failed to reach significance in the anxiety group. B, Shown are significant associations between brain regions demonstrating significant psychophysiological interaction (PPI) between-group differences during extinction recall and skin conductance response (SCR) during extinction recall. Expression of extinction recall is the computation of SCR to the first 4 trials of extinguished conditioned stimuli minus SCR to the first 4 trials of nonextinguished conditioned stimuli. aP < .05.
Discussion
We recruited individuals diagnosed as having 1 or more anxiety disorders without other comorbid disorders. This sample allowed us to investigate the association between anxiety disorders and fear circuitry across different phases of a conditioning and extinction procedure and to examine whether the number of anxiety disorders differentially influences the circuitry.
During conditioning, SCR was blunted in the anxiety group relative to healthy controls. However, both groups differentiated between the CS+ and the CS−. This blunted pattern seems to be driven by individuals with a single disorder. The literature has suggested larger responses to the CS− in anxious individuals, which could result in lower differential acquisition. Based on our results, the number of diagnoses is an important factor to consider that could be used as an index of clinical severity.
In terms of imaging, individuals with multiple disorders activated more fear encoding and expression regions (amygdala, insular cortex, and dACC) during conditioning. This finding is consistent with studies that have shown hyperactivation of fear-promoting regions during emotional tasks. During early conditioning, the vmPFC was less activated in the anxiety group compared with healthy controls. The number of diagnoses modulated that vmPFC hypoactivation such that individuals having multiple anxiety disorders showed reduced vmPFC activation compared with those having a single disorder. Moreover, vmPFC activation during early conditioning showed a negative correlation with STAI-T scores. These results suggest vmPFC hypoactivation in anxious individuals, an effect that is more pronounced in more severe cases (either greater symptoms or more disorders).
Previous studies performed in social anxiety disorder and generalized anxiety disorder have also reported lower activation in the medial PFC during emotional tasks. Our PPI analyses revealed that this region was more functionally coupled with the sgACC in healthy controls but showed more functional coherence with the hippocampus, amygdala, and insular cortex in the anxiety group. This finding suggests that, for the anxious group, the vmPFC region that showed lower activation during early conditioning was also more coupled with regions known to support fear encoding and processing, which are typically more activated in anxious individuals.
Contrary to our hypothesis, during extinction recall, no SCR deficits were found between the healthy controls and the anxiety group. This result is in contrast with various psychopathological conditions, such as PTSD, obsessive-compulsive disorder, and schizophrenia, in which deficits were noted at the SCR level. This finding is an important psychophysiological distinction between PTSD and the anxiety disorders tested in our study, and it is also contrary to our hypothesis. Despite no group differences for SCR during extinction recall, brain activation patterns differed between the groups. In fact, the healthy controls activated the vmPFC, rACC, and insular cortex more relative to the anxious individuals. This result is consistent with investigations that have shown dysregulated rACC and vmPFC activation patterns in anxious individuals using various emotion regulation tasks. Focusing on the anxiety group, results showed that vmPFC activation was reduced in those with multiple disorders. The regression analyses also revealed a negative correlation between the vmPFC activation and the trait anxiety levels. When looking at PPI analyses with the vmPFC as the seed, we again observed higher functional coherence with the sgACC in healthy controls. On the other hand, the same seed showed more connectivity with the amygdala and insular cortex in the anxiety group, as was the case in the PPI analyses conducted during conditioning, as well as with a vmPFC area. Similar to conditioning, the hypoactive vmPFC region in the anxiety group showed more functional coherence during extinction recall in anxious individuals with fear-promoting regions, which tend to be hyperactive in that same sample.
Similar patterns emerged during early conditioning and extinction recall with regard to vmPFC activation and its modulation by the number of diagnoses and trait anxiety levels, as well as with its functional coherence with the rest of the network. Activation of the vmPFC during early conditioning was positively associated with vmPFC activation during extinction recall but only in healthy controls. These exploratory analyses emphasize the importance of assessing how fear is initially encoded, which seems to influence how the safety memory will be retrieved later. In fact, deficits that have been reported in terms of activation patterns during extinction recall in different disorders might potentially be traced back to dysregulated activation patterns during the initial fear memory formation. In support of this hypothesis, Livneh and Paz showed that the synchronization of amygdala and dACC activity during fear encoding predicts higher resistance to extinction.
As an exploratory analysis, we next examined whether brain activation patterns were associated with fear expression during extinction recall. Although both groups had similar SCR during recall, this measure carries great variability in individuals. The analysis revealed that greater connectivity between the vmPFC and the sgACC, which was more coupled in healthy controls, was associated with better extinction recall. In contrast, the connectivity value between the vmPFC and the amygdala, which was higher in the anxiety group, was associated with worse extinction recall. This finding is in line with animal investigations showing that specific patterns of medial PFC–amygdala correlate with fear expression. These exploratory models highlight the need for studies to further examine such questions with cross-validation techniques in larger sample sizes.
Limitations
Some limitations of this study should be highlighted. First, the anxiety group was older and less educated than the healthy controls. We have covaried for these variables throughout our analyses. We have also rerun all analyses without covariates, and most of our findings remained unchanged. Furthermore, the covariates were not significantly associated with any of our main outcomes (eAppendix in the Supplement). Second, the specific phobics were more represented in the single disorder group, which could suggest that the comparisons made for the number of diagnoses reflect a difference between specific phobia and the other disorders. Our supplemental analyses ruled out this effect by showing that the single disorder group with specific phobia was comparable to the single disorder group without specific phobia and that the differences between the single disorder group and the multiple disorders group remained when excluding individuals with only specific phobia. Third, there are sex differences pertaining to the prevalence of anxiety disorders, and sex hormones modulate extinction learning. We did not assay gonadal hormones, making it impossible to measure their influence. Fourth, we draw some parallels between our findings and those from other clinical study samples, notably PTSD. These inferences are based on patterns emerging from the data, and no direct comparisons were made between these 2 groups statistically.
Conclusions
Our results reveal no SCR deficits for differential acquisition and extinction recall. However, the imaging data suggest that the fear circuitry is dysregulated in individuals with anxiety disorders and that some differences are modulated by the number of disorders or the self-reported anxiety symptoms. The PPI analyses highlighted the importance of investigating the whole fear network and the association between its main nodes because an imbalance in the activation of fear-promoting regions and extinction-promoting regions at different stages throughout the paradigm may synergistically act in conveying a greater vulnerability to anxiety disorders. This study allowed identification of patterns applicable to the DSM-5 category of anxiety disorders that excludes trauma-related and stress-related conditions. Although we used a categorical approach, it would be informative to test similar questions using a dimensional approach. The correlations between the anxiety symptoms and brain activation patterns highlight aspects of the research domain criteria method and the importance of examining more extensively these questions from this approach. From a clinical standpoint, our results provide a rationale for future work in further classifying each anxiety disorder because not all disorders may be equivalent. Understanding the similarities and differences between anxiety disorders may enable neurobiologically driven treatment development and selection tailored to a patient’s diagnosis, comorbidities, and level of anxiety severity.
eAppendix. Supplemental Appendix
References
- 1.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56(1):19-32. [DOI] [PubMed] [Google Scholar]
- 5.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13(11):769-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research: past, present, and future. Biol Psychiatry. 2006;60(4):376-382. [DOI] [PubMed] [Google Scholar]
- 7.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167(6):648-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14(6):417-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42(7):515-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45(9):2019-2033. [DOI] [PubMed] [Google Scholar]
- 12.Garfinkel SN, Abelson JL, King AP, et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34(40):13435-13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin MF, Song H, VanElzakker MB, et al. Association of resting metabolism in the fear neural network with extinction recall activations and clinical measures in trauma-exposed individuals. Am J Psychiatry. 2016;173(9):930-938. [DOI] [PubMed] [Google Scholar]
- 14.Rougemont-Bücking A, Linnman C, Zeffiro TA, et al. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther. 2011;17(4):227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duits P, Cath DC, Lissek S, et al. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress Anxiety. 2015;32(4):239-253. [DOI] [PubMed] [Google Scholar]
- 16.Linnman C, Zeidan MA, Furtak SC, Pitman RK, Quirk GJ, Milad MR. Resting amygdala and medial prefrontal metabolism predicts functional activation of the fear extinction circuit. Am J Psychiatry. 2012;169(4):415-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spielberger CD, Gorsuch RW, Luschene RE. State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 18.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446-454. [DOI] [PubMed] [Google Scholar]
- 19.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62(10):1191-1194. [DOI] [PubMed] [Google Scholar]
- 20.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straube T, Mentzel HJ, Glauer M, Miltner WH. Brain activation to phobia-related words in phobic subjects. Neurosci Lett. 2004;372(3):204-208. [DOI] [PubMed] [Google Scholar]
- 22.Killgore WD, Britton JC, Schwab ZJ, et al. Cortico-limbic responses to masked affective faces across PTSD, panic disorder, and specific phobia. Depress Anxiety. 2014;31(2):150-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klumpp H, Post D, Angstadt M, Fitzgerald DA, Phan KL. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biol Mood Anxiety Disord. 2013;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89(1):273-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34(4):296-302. [PMC free article] [PubMed] [Google Scholar]
- 26.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59(5):424-429. [DOI] [PubMed] [Google Scholar]
- 27.Ball TM, Sullivan S, Flagan T, et al. Selective effects of social anxiety, anxiety sensitivity, and negative affectivity on the neural bases of emotional face processing. Neuroimage. 2012;59(2):1879-1887. [DOI] [PubMed] [Google Scholar]
- 28.Simmons A, Matthews SC, Feinstein JS, Hitchcock C, Paulus MP, Stein MB. Anxiety vulnerability is associated with altered anterior cingulate response to an affective appraisal task. Neuroreport. 2008;19(10):1033-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sripada CS, Angstadt M, Banks S, Nathan PJ, Liberzon I, Phan KL. Functional neuroimaging of mentalizing during the trust game in social anxiety disorder. Neuroreport. 2009;20(11):984-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168(9):968-978. [DOI] [PubMed] [Google Scholar]
- 31.Palm ME, Elliott R, McKie S, Deakin JF, Anderson IM. Attenuated responses to emotional expressions in women with generalized anxiety disorder. Psychol Med. 2011;41(5):1009-1018. [DOI] [PubMed] [Google Scholar]
- 32.Price RB, Eldreth DA, Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Transl Psychiatry. 2011;1:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlund MW, Verduzco G, Cataldo MF, Hoehn-Saric R. Generalized anxiety modulates frontal and limbic activation in major depression. Behav Brain Funct. 2012;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holt DJ, Lebron-Milad K, Milad MR, et al. Extinction memory is impaired in schizophrenia. Biol Psychiatry. 2009;65(6):455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 2012;69(9):893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milad MR, Furtak SC, Greenberg JL, et al. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70(6):608-618. [DOI] [PubMed] [Google Scholar]
- 37.Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Soc Cogn Affect Neurosci. 2009;4(3):257-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livneh U, Paz R. Amygdala-prefrontal synchronization underlies resistance to extinction of aversive memories. Neuron. 2012;75(1):133-142. [DOI] [PubMed] [Google Scholar]
- 39.Karalis N, Dejean C, Chaudun F, et al. 4-Hz oscillations synchronize prefrontal-amygdala circuits during fear behavior. Nat Neurosci. 2016;19(4):605-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8-19. [DOI] [PubMed] [Google Scholar]
- 41.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45(8):1027-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regier DA, Narrow WE, Rae DS. The epidemiology of anxiety disorders: the Epidemiologic Catchment Area (ECA) experience. J Psychiatr Res. 1990;24(suppl 2):3-14. [DOI] [PubMed] [Google Scholar]
- 43.Glover EM, Jovanovic T, Mercer KB, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72(1):19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry. 2013;73(4):371-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang MJ, Zsido RG, Song H, et al. Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry. 2015;15:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milad MR, Zeidan MA, Contero A, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168(3):652-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Appendix