Abstract
Importance
Dietary supplements are commonly used by US adults; yet, little is known about recent trends in supplement use.
Objective
To report trends in dietary supplement use among US adults.
Design, Setting, and Participants
Temporal trends in supplement use were evaluated using nationally representative data from the National Health and Nutrition Examination Survey (NHANES) collected between 1999 and 2012. Participants include non-institutionalized adults residing in the US, surveyed over 7 continuous 2-year cycles (sample size per cycle ranged from 4,863 to 6,213).
Exposures
Calendar time, as represented by NHANES cycle.
Main Outcomes/Measures
In an in-home interview, participants were queried on use of supplements in the 30 days prior. This information was used to estimate the prevalence of use within each NHANES cycle, and trends were evaluated across cycles. Results are presented for use of any supplements, use of multivitamins/multiminerals (MVMM), as defined by a product containing ≥10 vitamins and/or minerals, as well as use of individual vitamins, minerals, and non-vitamin, non-mineral supplements. Results are presented overall, and by population subgroup, including age, sex, race/ethnicity, and educational status. All analyses are weighted to be nationally representative.
Results
A total of 37,958 adults were included in the study [weighted mean age, 46.4 years, 52.0% women], with an overall response rate of 74%. Overall, use of supplements remained stable between 1999 and 2012, with 52% of US adults reporting use of any supplements in 2011–2012 (p-trend:0.19). This trend varied by population subgroup. Use of MVMM decreased, with 37% reporting use of MVMM in 1999–2000 and 31% reporting use in 2011–2012 (difference: −5.7%; 95% CI: −8.6%, −2.7%; p-trend<0.001). Vitamin D supplementation from sources other than MVMM increased from 5.1% to 19% (difference: 14%; 95% CI: 12%, 17%; p-trend<0.001) and use of fish oil supplements increased from 1.3% to 12% (difference: 11%; 95% CI: 9.1%, 12%; p-trend<0.001) over the study period, while use of a number of supplements decreased.
Conclusions and Relevance
Among adults in the United States, overall use of dietary supplements remained stable from 1999–2012, use of MVMM decreased, and trends in use of individual supplements varied and were heterogeneous by population subgroups.
Keywords: Adults, Dietary supplements, National Health and Nutrition Examination Survey, NHANES, Prevalence, Trends, United States
INTRODUCTION
Dietary supplement products are commonly used by adults in the United States, with prior research indicating an increase in use between the 1980s and mid-2000s.1,2 Although often taken to improve or maintain health, less than a quarter of all supplement products used are taken at the recommendation of a healthcare provider.3 Instead, their use may be motivated, in part, by evidence suggesting that increased intake of some dietary constituents may be associated with reduced risk of outcomes, including cancer and cardiovascular disease (CVD).4,5 Even so, results from observational studies have yielded mixed results regarding the health benefits of individual supplements or multivitamins/multiminerals (MVMM), and randomized controlled trials (RCTs) have often not supported benefits of these supplements, although the duration of many RCTs may have been insufficient to observe beneficial effects.6–10 Furthermore, some research has indicated that use of selected supplements at high doses may have adverse effects,11 generating some skepticism regarding their use.12
Despite extensive research conducted on the role of dietary supplements in health, little is known about recent trends in supplement use. Most studies describing patterns of use are restricted to older adults, focus on select supplements, are unable to evaluate temporal trends, or do not capture use in the last decade.1,2,13,14 Using data from the National Health and Nutrition Examination Survey (NHANES), this paper therefore seeks to quantify trends in supplement use among US adults from 1999 to 2012, with a focus on use of any supplement products and MVMM, as defined by use of any products containing ≥10 vitamins and/or minerals, as well as use of individual vitamins, minerals, and non-vitamin, non-mineral (NVNM) supplements.
METHODS
Study Population
This study was conducted using data from NHANES, a nationally representative, cross-sectional survey of civilian, non-institutionalized persons living in the United States.15 This analysis includes 7 two-year cycles (1999–2000 to 2011–2012), with 1999–2000 representing the first cycle for which continuous data are available and 2011–2012 representing the most recent cycle for which data are available. Each cycle is an independent sample. Participants provided written informed consent and study procedures were approved by the National Center for Health Statistic’s Research Ethics Review Board. The Memorial Sloan Kettering Cancer Center Institutional Review Board determined that this analysis does not constitute human subjects research, and does not need human subjects approval.
Supplement Use
NHANES participants were first asked if they used any prescription or non-prescription supplements in the prior 30 days. Persons indicating use were asked to show the interviewer the bottles of each supplement product taken (77.4% of supplement products were seen): a supplement product could be a capsule, tablet, pill, softgel, chew/gummy or other product containing one or more supplements. When containers were not seen, participants were asked to recall each product taken. By linking to databases of supplement products and supplement product blends, use of MVMM, vitamins, minerals, and specific supplements was ascertained. More detail has been provided in NHANES documentation,16 and detailed information on how this information was used in analysis is provided in the Supplementary Methods.
From this information, variables were created for use of any supplement product in the prior 30 days, use of ≥4 supplement products, as well as use of any vitamin, any mineral, and use of MVMM (defined as a product containing ≥10 vitamins and/or minerals).17 This definition was created to capture products labeled as multivitamins in the market place (i.e., supplements used by consumers as a non-specific dietary supplement, commonly containing the recommended daily values of multiple vitamins, and typically including minerals, as well). However, this definition did not require any minerals to be in the formulation because several of the most popular multivitamin brands during the period of the surveys did not have minerals. The cutoff of 10 vitamins and/or minerals was used to separate common MVMMs from more specialized supplements such as B-complex supplements and supplements developed for eye and bone health. However, to allow for comparison to prior NHANES analyses, a secondary MVMM variable, defined as products containing ≥3 vitamins and ≥1 mineral, is also presented.3
Variables were also created corresponding to individual vitamins, minerals, and NVNM supplements. To distinguish how supplements are consumed, results are presented both overall (i.e., any use) and exclusive of MVMM supplements (e.g., vitamin D not from MVMM). This is important because supplements consumed as part of MVMM are often consumed at lower doses than they are in individual or focused combination supplements, and because understanding how supplements are consumed can help inform our understanding of trends. Primary analyses focus on any use during the prior 30 days.
Statistical Analyses
The prevalence of supplement use, as determined by use in the prior 30 days, was assessed within each NHANES cycle. Survey-weighted logistic regression was used to calculate a P-trend across cycles; the statistical significance of trends was evaluated using a two-sided test at the α=0.05 level. Ratios for the prevalence of use in 2011–2012 vs. 1999–2000 are presented; in the presentation of results, a ‘decrease’ corresponds to a ratio<1 and p-trend<0.05, an ‘increase’ represents a ratio>1 and p-trend<0.05, and ‘stable’ represents a p-trend≥0.05. Differences, representing the absolute difference in the prevalence of use in 2011–2012 vs. 1999–2000, are also presented.
Because most supplemental vitamins/minerals are consumed in the form of MVMM and total use was of most interest, trends in main tables are shown for total supplement use. However, results are also presented exclusive of MVMM if the trends with and without MVMM markedly varied. Detailed trends, both overall and exclusive of MVMM, are provided in supplementary tables. Throughout the text, the discussion of trends refers to overall trends in any use of a given dietary supplement (regardless of whether consumed as part of a MVMM), unless otherwise noted.
As data are presented on a large number of supplements, the discussion focuses on supplements fitting the following criteria: i) a high prevalence of use (>10% during any cycle), and ii) a large change in use over the study period (>3-fold change). Given potential heterogeneity by population subgroup, results are presented by age (20–39y, 40–64y, ≥65y), sex, self-reported race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American), and education (highest attained degree: <high school, high school/some college, ≥4-year college)
Sensitivity analyses are presented for regular use (defined as use ≥15 days/month) and age-adjusted trends. To address whether the trends were consistent over the study period, joinpoint regression analyses were conducted and the annual percentage change (APC) before and after potential inflection points were calculated using Joinpoint Regression Program Software (version 4.2.0, National Cancer Institute).18
NHANES is a stratified, complex, multistage probability-based survey that oversamples certain groups, and as such, all participants are assigned analytic weights to account for their unequal sampling probability and non-response. Analyses were conducted using Stata 13.1 (College Station, TX).
RESULTS
Over these 7 NHANES cycles, 38,024 adults aged ≥20y completed an in-home interview, where supplement use was ascertained. Sixty-six persons were excluded due to missing information on supplement use, leaving 37,958 persons (sample size per cycle: 4,863–6,213). The response rate was 73.6%, with the cycle-specific response rate ranging from 67.4%–78.3%.19
In 2011–2012, 52% (95% CI:49%, 55%) of US adults reported using any supplement product in the prior 30 days (Table 1). Supplement use was associated with several socio-demographic variables. For example, supplement use increased with age, with 72% of adults ages 65+ reporting use (95% CI: 69%, 75%), as compared to 40% of adults ages 20–39 (95% CI: 35%, 45%). Women (58%; 95% CI: 55%, 62%) were also more likely to use supplements than men (45%; 95% CI: 42%, 49%). Use was highest among non-Hispanic whites (58%; 95% CI: 55%, 62%), and lowest among Mexican-Americans (29%; 95% CI: 25%, 33%). There was an educational gradient, with use highest among the most highly educated: 65% (95% CI: 61%, 69%) of those with ≥4-year college degree reported use, as compared to 37% (95% CI: 34%, 40%) of those with <a high school degree.
Table 1.
Supplement Use in Prior 30 Days Among US Adults,a by Population Characteristics: 2011–2012
| N | Any Supplement Use
|
||
|---|---|---|---|
| n | % (95% CI)b | ||
| OVERALL | 5,556 | 2,715 | 52 (49, 55) |
|
| |||
| Age group | |||
| 20–39 | 1,957 | 718 | 40 (35, 45) |
| 40–64 | 2,352 | 1,171 | 54 (50, 58) |
| ≥65 | 1,247 | 826 | 72 (69, 75) |
| Sex | |||
| Female | 2,819 | 1,548 | 58 (55, 62) |
| Male | 2,737 | 1,167 | 45 (42, 49) |
| Race/ethnicity | |||
| Non-Hispanic white | 2,038 | 1,169 | 58 (55, 62) |
| Non-Hispanic black | 1,455 | 640 | 41 (37, 45) |
| Mexican-American | 540 | 177 | 29 (25, 33) |
| Other Hispanic | 578 | 243 | 38 (33, 42) |
| Non-Hispanic Asian | 793 | 404 | 52 (45, 58) |
| Other/mixed race | 152 | 82 | 48 (41, 55) |
| Education | |||
| <High school | 1,330 | 510 | 37 (34, 40) |
| High school | 1,168 | 511 | 46 (42, 50) |
| Some college | 1,657 | 826 | 51 (47, 56) |
| ≥4-year college | 1,397 | 866 | 65 (61, 69) |
| Self-reported health status | |||
| Excellent | 470 | 251 | 61 (54, 67) |
| Very good | 1278 | 707 | 58 (53, 62) |
| Good | 1885 | 884 | 47 (43, 50) |
| Fair/Poor | 1069 | 489 | 49 (43, 54) |
Among adults ages ≥ 20 years.
Data are weighted to be nationally representative.
Overall, there was no change in overall supplement use, with 52% reporting use in 1999–2000 and in 2011–2012 (difference:0.0%; 95% CI: −3.6%, 3.6%) (Table 2). There was also no change in use of ≥4 supplement products, with 9.9% reporting use of 4 supplement products in 2011–2012 (difference:1.1%; 95% CI: −0.8%, 3.0%). However, use of MVMM, as defined by a product containing ≥10 vitamins and/or minerals, decreased from 37% to 31% (difference: −5.7%; 95% CI: −8.6%, −2.7%). The trend in MVMM use also significantly decreased when alternatively defined by use of products containing ≥3 vitamins and ≥1 mineral, with use decreasing from 39% to 36% (difference: −3.6%; 95% CI: −6.8%, −0.4%). Use of any vitamin remained stable, with 48% (95% CI: 45%, 50%) reporting use in 2011–2012, although use of any vitamin, excluding MVMM, increased. Use of any mineral/element decreased from 42% to 39% (difference: −3.0%; 95% CI: −6.5%, 0.4%), although use of mineral/elements was stable when excluding MVMM.
Table 2.
Trends in Overall Supplement Use, as well as Use of MVMM, Vitamins and Minerals, and Common MVMM Components: US Adults,a 1999–2000 through 2011–2012b,c
| 30-day prevalence of use % (95% confidence interval [CI])d
|
p-trend | 2011–2012 vs 1999–2000 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1999–2000 (n=4,863) |
2001–2002 (n=5,396) |
2003–2004 (n=5,028) |
2005–2006 (n=4,972) |
2007–2008 (n=5,930) |
2009–2010 (n=6,213) |
2011–2012 (n=5,556) |
Ratio | Difference | ||
| SUMMARY USE | ||||||||||
|
| ||||||||||
| Any supplement product | 52 (49, 54) | 51 (50, 53) | 54 (51, 57) | 54 (51, 56) | 49 (45, 53) | 50 (47, 52) | 52 (49, 55) | .19 | 1.0 (0.93, 1.1) | 0.0 (−3.6, 3.6) |
| Excluding MVMM | 34 (32, 37) | 34 (32, 36) | 36 (33, 39) | 36 (33, 38) | 34 (31, 38) | 34 (32, 36) | 39 (36, 42) | .063 | 1.1 (1.0, 1.3) | 5.0 (1.0, 8.9) |
| ≥4 supplement products | 8.7 (7.5, 10) | 9.8 (8.3, 12) | 10 (8.7, 12) | 9.9 (8.5, 11) | 9.0 (7.1, 11) | 7.8 (6.8, 9.0) | 9.9 (8.6, 11) | .60 | 1.1 (0.92, 1.4) | 1.1 (−0.8, 3.0) |
| MVMMe | 37 (35, 39) | 38 (35, 40) | 38 (36, 41) | 40 (38, 42) | 33 (30, 36) | 32 (31, 34) | 31 (29, 33) | <.001 | 0.85 (0.78, 0.92) | −5.7 (−8.6, −2.7) |
| MVMM (alternative definition)f | 39 (37, 42) | 40 (38, 42) | 42 (40, 44) | 43 (41, 45) | 36 (33, 40) | 37 (35, 38) | 36 (34, 38) | <.001 | 0.91 (0.83, 0.99) | −3.6 (−6.8, −0.4) |
| Any vitamin | 47 (44, 50) | 47 (46, 49) | 49 (46, 52) | 49 (47, 51) | 44 (41, 48) | 45 (43, 47) | 48 (45, 50) | .30 | 1.0 (0.94, 1.1) | 0.8 (−3.1, 4.6) |
| Excluding MVMM | 25 (23, 28) | 27 (25, 28) | 28 (25, 31) | 26 (24, 28) | 26 (23, 29) | 26 (24, 28) | 31 (28, 34) | .047 | 1.2 (1.1, 1.4) | 5.7 (1.9, 9.5) |
| Any mineral/element | 42 (39, 45) | 43 (41, 45) | 45 (43, 48) | 46 (43, 49) | 40 (36, 43) | 39 (38, 41) | 39 (37, 41) | <.001 | 0.93 (0.85, 1.0) | −3.0 (−6.5, 0.4) |
| Excluding MVMM | 18 (16, 20) | 20 (18, 22) | 22 (20, 25) | 20 (18, 22) | 19 (16, 22) | 17 (16, 18) | 18 (17, 20) | .054 | 1.0 (0.89, 1.2) | 0.3 (−2.0, 2.6) |
|
| ||||||||||
| INCREASED | ||||||||||
|
| ||||||||||
| Lutein/zeaxanthin | 8.5 (5.8, 12) | 18 (17, 20) | 22 (20, 24) | 21 (20, 23) | 18 (16, 20) | 12 (11, 13) | 10 (9.2, 12) | .019 | 1.2 (0.82, 1.8) | 1.9 (−1.6, 5.3) |
| Lycopene (men only)* | 1.9 (1.2, 2.9) | 2.9 (2.3, 3.8) | 21 (19, 24) | 25 (23, 27) | 22 (20, 26) | 18 (16, 20) | 16 (14, 18) | <.001 | 8.7 (5.5, 14) | 14 (12, 16) |
| Vitamin B12 (Cobalamin), Excluding MVMM* | 5.7 (4.7, 6.8) | 5.6 (4.8, 6.5) | 5.9 (5.0, 7.0) | 6.8 (5.6, 8.2) | 6.8 (5.6, 8.3) | 6.1 (5.5, 6.7) | 8.1 (6.9, 9.5) | .002 | 1.4 (1.1, 1.8) | 2.4 (0.8, 4.1) |
| Vitamin D, Excluding MVMM* | 5.1 (4.3, 6.1) | 8.7 (7.7, 9.9) | 8.5 (7.1, 10) | 8.6 (7.7, 9.6) | 11 (9.4, 12) | 15 (14, 16) | 19 (17, 22) | <.001 | 3.8 (3.0, 4.6) | 14 (12, 17) |
|
| ||||||||||
| DECREASED | ||||||||||
|
| ||||||||||
| Boron | 24 (22, 26) | 25 (23, 27) | 26 (23, 28) | 25 (23, 27) | 19 (17, 22) | 20 (19, 21) | 17 (16, 18) | <.001 | 0.7 (0.63, 0.78) | −7.2 (−9.6, −4.9) |
| Calcium* | 38 (35, 41) | 39 (37, 41) | 42 (39, 45) | 44 (41, 46) | 36 (33, 40) | 37 (35, 38) | 35 (33, 37) | .002 | 0.93 (0.84, 1.0) | −2.8 (−6.2, 0.5) |
| Chromium | 28 (26, 30) | 30 (28, 32) | 30 (28, 33) | 32 (30, 34) | 28 (25, 31) | 28 (26, 30) | 27 (25, 28) | .041 | 0.94 (0.84, 1.0) | −1.8 (−4.6, 1.0) |
| Copper | 31 (28, 33) | 31 (29, 33) | 32 (30, 34) | 34 (32, 36) | 29 (26, 31) | 29 (27, 31) | 27 (25, 28) | <.001 | 0.87 (0.8, 0.96) | −3.8 (−6.5, −1.1) |
| Iodine | 29 (27, 31) | 29 (27, 31) | 28 (26, 31) | 28 (26, 31) | 22 (20, 25) | 22 (21, 24) | 20 (18, 21) | <.001 | 0.68 (0.61, 0.75) | −9.4 (−11.9, −6.8) |
| Iron* | 31 (27, 34) | 29 (27, 30) | 27 (25, 29) | 27 (24, 30) | 21 (19, 23) | 21 (20, 22) | 19 (17, 20) | <.001 | 0.61 (0.53, 0.7) | −12 (−15, −8.3) |
| Magnesium | 33 (30, 35) | 34 (32, 36) | 35 (33, 38) | 37 (35, 39) | 31 (28, 34) | 30 (29, 32) | 28 (27, 30) | <.001 | 0.87 (0.79, 0.96) | −4.2 (−7.2, −1.1) |
| Manganese | 28 (26, 31) | 30 (28, 32) | 31 (28, 33) | 32 (30, 34) | 28 (26, 31) | 28 (26, 30) | 26 (25, 27) | .009 | 0.91 (0.83, 1.0) | −2.5 (−5.1, 0.2) |
| Molybdenum | 25 (23, 28) | 27 (26, 29) | 27 (25, 30) | 26 (25, 28) | 21 (18, 24) | 21 (20, 23) | 18 (17, 19) | <.001 | 0.71 (0.64, 0.79) | −7.4 (−9.8, −5.0) |
| Phosphorus | 25 (23, 27) | 24 (23, 26) | 25 (22, 27) | 23 (22, 25) | 18 (16, 21) | 18 (17, 20) | 15 (14, 17) | <.001 | 0.61 (0.53, 0.7) | −9.6 (−12, −6.9) |
| Potassium | 28 (26, 30) | 28 (26, 30) | 28 (26, 31) | 28 (26, 30) | 23 (20, 25) | 22 (21, 24) | 18 (16, 19) | <.001 | 0.63 (0.55, 0.71) | −10 (−13, −7.8) |
| Selenium* | 28 (26, 31) | 30 (28, 32) | 30 (28, 33) | 32 (30, 34) | 28 (25, 31) | 27 (26, 29) | 26 (24, 27) | .002 | 0.9 (0.82, 0.99) | −2.8 (−5.4, −0.1) |
| Selenium Excluding MVMM* | 2.6 (2.2, 3.2) | 2.6 (2.1, 3.3) | 2.1 (1.4, 3.1) | 1.5 (1.2, 2) | 1.1 (0.7, 1.6) | 0.9 (0.6, 1.4) | 0.7 (0.4, 1.2) | <.001 | 0.28 (0.16, 0.48) | −1.9 (−2.5, −1.3) |
| Vanadium | 22 (20, 25) | 22 (21, 24) | 24 (21, 26) | 23 (21, 25) | 18 (16, 21) | 18 (17, 20) | 14 (13, 16) | <.001 | 0.65 (0.57, 0.74) | −7.9 (−10, −5.3) |
| Vitamin A* | 36 (33, 38) | 37 (35, 40) | 37 (35, 40) | 39 (37, 42) | 33 (30, 36) | 33 (31, 34) | 31 (30, 33) | <.001 | 0.88 (0.81, 0.96) | −4.3 (−7.2, −1.4) |
| Vitamin B1 (Thiamin) | 37 (35, 40) | 38 (36, 41) | 38 (35, 41) | 40 (38, 43) | 33 (30, 36) | 32 (31, 34) | 30 (28, 32) | <.001 | 0.81 (0.74, 0.89) | −6.9 (−10, −3.8) |
| Vitamin B2 (Riboflavin) | 37 (35, 40) | 38 (36, 40) | 38 (35, 41) | 40 (38, 43) | 33 (30, 36) | 32 (31, 34) | 30 (28, 32) | <.001 | 0.82 (0.75, 0.9) | −6.8 (−9.9, −3.6) |
| Vitamin B3 (Niacin) | 37 (35, 40) | 38 (36, 40) | 38 (35, 41) | 40 (38, 43) | 34 (30, 37) | 33 (31, 35) | 31 (29, 33) | <.001 | 0.84 (0.76, 0.92) | −6.1 (−9.2, −2.9) |
| Vitamin B5 (Pantothenic acid) | 34 (32, 37) | 36 (34, 38) | 36 (33, 38) | 38 (35, 40) | 32 (29, 35) | 31 (29, 33) | 30 (28, 31) | <.001 | 0.87 (0.79, 0.96) | −4.5 (−7.5, −1.4) |
| Vitamin B6 (Pyridoxine) | 38 (36, 41) | 39 (37, 41) | 39 (37, 42) | 41 (39, 44) | 35 (32, 38) | 34 (32, 36) | 32 (30, 35) | <.001 | 0.85 (0.78, 0.93) | −5.6 (−8.8, −2.4) |
| Vitamin B9 (Folic acid) | 38 (35, 40) | 38 (36, 41) | 39 (36, 42) | 41 (38, 44) | 35 (32, 38) | 34 (32, 36) | 33 (30, 35) | <.001 | 0.87 (0.78, 0.95) | −5.1 (−8.5, −1.7) |
| Vitamin B12 (Cobalamin)* | 38 (35, 40) | 39 (37, 41) | 39 (36, 41) | 41 (39, 44) | 36 (32, 39) | 35 (33, 37) | 35 (33, 37) | .001 | 0.92 (0.85, 1.0) | −2.9 (−6.1, 0.2) |
| Vitamin C | 42 (39, 44) | 42 (40, 45) | 43 (40, 46) | 45 (42, 47) | 38 (34, 41) | 37 (35, 38) | 36 (34, 38) | <.001 | 0.86 (0.79, 0.93) | −5.9 (−9.0 −2.8) |
| Vitamin E | 41 (38, 43) | 41 (40, 43) | 42 (39, 45) | 42 (40, 45) | 35 (32, 38) | 34 (32, 36) | 34 (32, 36) | <.001 | 0.83 (0.76, 0.91) | −6.9 (−10, −3.6) |
| Zinc | 35 (32, 38) | 36 (34, 38) | 37 (35, 39) | 39 (37, 42) | 32 (29, 35) | 32 (30, 33) | 30 (28, 32) | <.001 | 0.86 (0.78, 0.94) | −5.0 (−8.1, −1.8) |
|
| ||||||||||
| STABLE | ||||||||||
|
| ||||||||||
| Biotin | 30 (27, 32) | 30 (28, 32) | 31 (28, 34) | 33 (31, 35) | 29 (26, 33) | 30 (28, 32) | 29 (27, 31) | .51 | 0.98 (0.89, 1.1) | −0.5 (−3.5, 2.5) |
| Calcium, Excluding MVMM* | 13 (12, 15) | 15 (14, 17) | 17 (15, 20) | 16 (14, 18) | 15 (12, 17) | 13 (13, 14) | 14 (12, 15) | .15 | 1.0 (0.86, 1.2) | 0.4 (−1.9, 2.8) |
| Choline | 5.1 (4, 6.3) | 5.1 (4.1, 6.3) | 4.5 (3.8, 5.4) | 6.3 (5, 7.8) | 4.6 (3.6, 5.7) | 4.7 (4.1, 5.5) | 6.3 (5.5, 7.3) | .23 | 1.2 (0.95, 1.6) | 1.3 (−0.2, 2.7) |
| Inositol | 4.9 (4, 6) | 5.2 (4.2, 6.4) | 4.7 (3.9, 5.5) | 6.3 (5.1, 7.7) | 5.1 (4, 6.4) | 4.6 (4, 5.2) | 6.5 (5.5, 7.6) | .16 | 1.3 (1.0, 1.7) | 1.5 (0.1, 3.0) |
| Iron, Excluding MVMM* | 2.9 (2.6, 3.2) | 3.1 (2.5, 3.9) | 3.1 (2.5, 3.8) | 3.2 (2.4, 4.3) | 3.8 (3, 4.8) | 2.9 (2.4, 3.5) | 3 (2.5, 3.7) | .65 | 1.1 (0.85, 1.3) | 0.2 (−0.5, 0.8) |
| Lycopene Excluding MVMM (men only)* | - | - | 0.8 (0.4, 1.5) | 0.7 (0.3, 1.3) | 0.6 (0.4, 1.1) | 0.5 (0.2, 0.9) | 0.7 (0.3, 1.4) | .24 | 5.6 (1.3, 24) | 0.5 (0.1, 1.1) |
| Vitamin A, Excluding MVMM* | 3.1 (2.7, 3.5) | 4.1 (3.3, 5.1) | 3.2 (2.6, 3.9) | 3.4 (2.7, 4.2) | 3 (2.4, 3.7) | 2.8 (2.1, 3.7) | 3.5 (2.8, 4.4) | .43 | 1.1 (0.88, 1.5) | 0.4 (−0.4, 1.3) |
| Vitamin D* | 37 (34, 39) | 38 (36, 40) | 39 (36, 42) | 41 (39, 43) | 36 (33, 39) | 39 (37, 41) | 40 (38, 43) | .14 | 1.1 (1.0, 1.2) | 3.9 (0.5, 7.4) |
| Vitamin K | 24 (22, 26) | 24 (23, 26) | 27 (24, 29) | 28 (26, 30) | 25 (22, 27) | 25 (24, 27) | 23 (22, 25) | .53 | 0.97 (0.86, 1.1) | −0.8 (−3.6, 2.0) |
ABBREVIATIONS: MVMM (Multivitamin/multimineral)
Indicates that the trend markedly varied for overall use and use excluding MVMM, and therefore both trends are presented. Detailed information on each trend, both overall and exclusive of MVMM is presented in eTable 2.
Among adults ages ≥ 20 years.
Results are presented for any use of each individual supplement, regardless of whether taken as an individual supplement or as a combination supplement, such as a MVMM. Results are also presented exclusive of MVMM if the two trends markedly varied. In these cases, both results (inclusive and exclusive of MVMM) are marked by a star so as to indicate that results are presented both ways.
A ‘decrease’ corresponds to a ratio<1 and p-trend<0.05, an ‘increase’ represents a ratio>1 and p-trend<0.05, and ‘stable’ represents a p-trend≥0.05.
Data are weighted to be nationally representative. If the relative standard error exceeds 30%, data are suppressed (as indicated by a dash), consistent with NHANES reporting guidelines.20
Defined as supplement products containing ten or more vitamins and/or minerals. This is the primary definition used in this paper, and is the variable used when defining use exclusive of MVMM elsewhere in the paper and tables.
Defined as supplements containing three or more vitamins and 1 or more minerals
There was a decrease in use of several vitamins and minerals overall (Table 2), including vitamin C, vitamin E, and selenium. The decreases in these supplements persisted when excluding MVMM (eTable 1). Although use of most vitamins and minerals decreased, this was not universal. For example, vitamin D, the most commonly used vitamin in 2011–2012, remained stable overall; however, use excluding MVMM increased almost 4-fold (5.1% to 19%; difference:14%; 95% CI:12%, 17%). Use of lycopene-containing supplements (mostly MVMM) increased almost 9-fold (1.9% to 16% among men; difference:14%; 95% CI:12%, 16%); use of lycopene also increased among women, although use was markedly lower than among men.
Use of several NVNM supplements increased (Table 3; eTable 2). Omega-3 fatty acids increased almost 7-fold, with 1.9% of adults reporting use in 1999–2000 and 13% in 2011–2012 (difference:11%; 95% CI:9.4%, 13%). Omega-3 fatty acids were the most commonly used NVNM in 2011–2012. This trend was primarily driven by the 9-fold increase in fish oil (1.3% to 12%; difference:11%; 95% CI:9.1%, 12%), although use of alpha-linolenic acid/flaxseed also increased. Omega-6 and omega-9 fatty acid supplement use increased, as did use of co-enzyme Q10, cranberry, green tea/epigallocatechin gallate (EGCG), methylsulfonylmethane (MSM), and probiotics.
Table 3.
Trends in Use of Non-vitamin, Non-mineral Specialty Supplements among US Adultsa: 1999–2000 through 2011–2012*,b
| 30-day prevalence of use % (95% confidence interval [CI])c
|
Prevalence in 2011–12 vs. 1999–2000 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1999–2000 (n=4,863) |
2001–2002 (n=5,396) |
2003–2004 (n=5,028) |
2005–2006 (n=4,972) |
2007–2008 (n=5,930) |
2009–2010 (n=6,213) |
2011–2012 (n=5,556) |
p-trend | Ratio | Difference | |
| INCREASED | ||||||||||
|
| ||||||||||
| Co-enzyme Q10 | 1.1 (0.8, 1.7) | 1.5 (1.0, 2.1) | 2.5 (2.0, 3.1) | 3.9 (3.3, 4.6) | 2.8 (2.2, 3.7) | 2.6 (2.1, 3.2) | 2.8 (2.1, 3.7) | <.001 | 2.4 (1.5, 3.9) | 1.6 (0.7, 2.5) |
| Cranberry | - | 1.2 (0.8, 1.9) | 1.3 (1.0, 1.7) | 1.9 (1.4, 2.6) | 1.7 (1.2, 2.3) | 1.5 (1.1, 1.9) | 2.4 (1.8, 3.1) | <.001 | 6.1 (3.0, 12.3) | 2.0 (1.3, 2.7) |
| Green tea/EGCG | 0.8 (0.5, 1.2) | 1.6 (1.2, 2.1) | 2.8 (2.1, 3.7) | 5.5 (4.3, 6.9) | 3.3 (2.7, 4.0) | 3.2 (2.5, 4.1) | 2.9 (2.0, 4.2) | <.001 | 3.7 (2.1, 6.4) | 2.1 (1.0, 3.3) |
| Methylsulfonylmethane (MSM) | 0.5 (0.3, 0.9) | 1.0 (0.8, 1.4) | 1.9 (1.2, 3.0) | 1.9 (1.5, 2.4) | 2.4 (1.8, 3.1) | 1.8 (1.5, 2.3) | 1.9 (1.5, 2.4) | <.001 | 3.6 (2.0, 6.4) | 1.4 (0.8, 1.9) |
| Omega-3 fatty acids of any type | 1.9 (1.4, 2.6) | 2.8 (2.0, 3.8) | 4.1 (3.1, 5.3) | 7.1 (5.6, 9.0) | 11 (8.9, 13) | 11 (10, 13) | 13 (11, 15) | <.001 | 6.8 (4.9, 9.3) | 11 (9.4, 13) |
| Fish oil/EPA/DHA/DPA | 1.3 (0.9, 1.9) | 1.7 (1.1, 2.5) | 3.0 (2.3, 3.9) | 5.5 (4.0, 7.6) | 9.2 (7.5, 11) | 10 (9.1, 12) | 12 (11, 14) | <.001 | 9.1 (6.2, 13.5) | 11 (9.1, 12) |
| Alpha-linolenic acid/flaxseed | - | 1.0 (0.8, 1.4) | 1.4 (0.9, 2.2) | 2.1 (1.6, 2.7) | 2.8 (2.1, 3.7) | 1.8 (1.4, 2.3) | 2.1 (1.5, 2.8) | <.001 | 2.6 (1.3, 5.0) | 1.3 (0.5, 2.0) |
| Omega-6 fatty acids | 0.7 (0.4, 1.2) | 0.9 (0.6, 1.3) | 1.4 (0.8, 2.4) | 2.4 (1.9, 3.1) | 2.4 (1.8, 3.0) | 1.5 (1.1, 2.1) | 2.2 (1.6, 2.9) | <.001 | 3.1 (1.6, 6.0) | 1.5 (0.8, 2.2) |
| Omega-9 fatty acids | 0.6 (0.3, 1.0) | 0.7 (0.4, 1.1) | 1.1 (0.6, 1.9) | 1.7 (1.2, 2.4) | 2.1 (1.5, 2.8) | 1.2 (0.9, 1.6) | 1.8 (1.3, 2.5) | <.001 | 3.2 (1.7, 6.0) | 1.2 (0.5, 1.9) |
| Probiotic | - | 1.0 (0.6, 1.4) | 0.9 (0.6, 1.4) | 1.8 (1.5, 2.2) | 0.8 (0.6, 1.2) | 1.3 (1.1, 1.6) | 2.2 (1.6, 2.9) | .003 | 2.4 (1.2, 4.9) | 1.3 (0.4, 2.1) |
|
| ||||||||||
| DECREASED | ||||||||||
|
| ||||||||||
| Echinacea | 1.9 (1.4, 2.5) | 1.3 (1.0, 1.8) | 1.3 (0.9, 2.0) | 1.5 (1.1, 2.0) | 1 (0.8, 1.3) | 0.6 (0.4, 1.0) | 0.7 (0.5, 1.1) | <.001 | 0.38 (0.22, 0.65) | −1.2 (−1.8, −0.6) |
| Garlic | 4.6 (4.1, 5.3) | 3.6 (2.9, 4.5) | 2.6 (2.0, 3.5) | 2.9 (2.3, 3.7) | 2.1 (1.5, 3.0) | 1.7 (1.4, 2.2) | 1.5 (1.1, 1.9) | <.001 | 0.31 (0.24, 0.41) | −3.2 (−3.9, −2.5) |
| Gingko biloba | 4.6 (3.7, 5.7) | 5.0 (4.0, 6.3) | 2.9 (2.3, 3.7) | 3.7 (3.1, 4.4) | 2.5 (1.8, 3.5) | 2.4 (1.9, 3.0) | 2.0 (1.4, 2.7) | <.001 | 0.43 (0.28, 0.64) | −2.6 (−3.8, −1.5) |
| Ginseng | 6.0 (4.8, 7.4) | 7.0 (5.9, 8.4) | 4.1 (3.4, 5.0) | 4.2 (3.5, 4.9) | 2.4 (1.6, 3.5) | 1.9 (1.4, 2.6) | 1.6 (1.1, 2.2) | <.001 | 0.26 (0.18, 0.39) | −4.4 (−5.8, −3.0) |
| Para-aminobenzoic acid (PABA) | 4.5 (3.5, 5.7) | 4.1 (3.4, 4.9) | 3.1 (2.4, 3.9) | 3.4 (2.5, 4.7) | 1.9 (1.4, 2.7) | 1.3 (1.1, 1.6) | 2.2 (1.6, 3.0) | <.001 | 0.49 (0.33, 0.72) | −2.3 (−3.5, −1.0) |
|
| ||||||||||
| STABLE | ||||||||||
|
| ||||||||||
| Amino acids | 4.5 (3.4, 5.9) | 4.7 (4.1, 5.4) | 4.3 (3.5, 5.2) | 5.0 (4.2, 5.9) | 3.8 (2.9, 4.8) | 3.4 (2.9, 3.9) | 4.3 (3.6, 5.1) | .11 | 0.96 (0.69, 1.3) | −0.2 (−1.6, 1.2) |
| Bilberry | 0.6 (0.4, 1.2) | 1.6 (1.0, 2.5) | 1.6 (1.1, 2.4) | 3.0 (2.5, 3.7) | 1.7 (1.3, 2.2) | 1.1 (0.8, 1.6) | 1.4 (0.9, 2.2) | .47 | 2.1 (1.0, 4.5) | 0.7 (0.0, 1.5) |
| Bromelain | 0.8 (0.5, 1.3) | 1.4 (1.0, 2.0) | 0.8 (0.6, 1.3) | 1.6 (1.3, 2.1) | 1.2 (0.8, 1.8) | 1.1 (0.8, 1.5) | 1.0 (0.6, 1.6) | .88 | 1.2 (0.62, 2.3) | 0.2 (−0.4, 0.8) |
| Chondroitin | 1.8 (1.3, 2.6) | 2.4 (2.0, 2.9) | 3.1 (2.0, 4.7) | 3.6 (2.9, 4.6) | 3.3 (2.6, 4.3) | 2.8 (2.4, 3.3) | 2.2 (1.7, 2.9) | .30 | 1.2 (0.77, 1.9) | 0.4 (−0.5, 1.3) |
| Fiber | 2.3 (1.7, 3.0) | 2.5 (2.2, 2.9) | 2.5 (1.9, 3.2) | 3.5 (2.6, 4.7) | 3.3 (2.6, 4.4) | 2.0 (1.6, 2.6) | 2.4 (1.6, 3.5) | .95 | 1.0 (0.63, 1.7) | 0.1 (−1.1, 1.2) |
| Ginger | 1.7 (1.2, 2.4) | 2.0 (1.5, 2.7) | 1.4 (1.0, 1.9) | 2.1 (1.6, 2.9) | 1.5 (1.1, 2.0) | 1.4 (1.0, 2.1) | 1.5 (1.1, 2.0) | .24 | 0.89 (0.55, 1.4) | −0.2 (−0.9, 0.6) |
| Glucosamine | 2.9 (2.3, 3.8) | 3.5 (2.9, 4.2) | 4.9 (3.5, 6.7) | 5.2 (4.2, 6.5) | 4.6 (3.7, 5.8) | 3.9 (3.3, 4.7) | 3.5 (2.9, 4.2) | .37 | 1.2 (0.87, 1.6) | 0.6 (−0.4, 1.5) |
| Grape seed | 1.8 (1.1, 2.9) | 2.7 (1.8, 4.1) | 1.8 (1.4, 2.4) | 2.2 (1.8, 2.7) | 1.3 (1.0, 1.8) | 2.2 (1.7, 2.8) | 1.7 (1.0, 2.8) | .42 | 0.97 (0.47, 2.0) | −0.1 (−1.3, 1.2) |
| Quercetin | 0.8 (0.6, 1.1) | 1.3 (0.9, 1.8) | 1.3 (0.8, 2.0) | 1.5 (1.2, 1.9) | 0.9 (0.7, 1.2) | 1.1 (0.8, 1.4) | 1.0 (0.6, 1.7) | .79 | 1.2 (0.66, 2.3) | 0.2 (−0.4, 0.8) |
| Saw palmetto (men only) | 2.5 (1.8, 3.6) | 3.2 (2.6, 4.1) | 4.2 (2.9, 5.9) | 4.3 (3.4, 5.5) | 2.3 (1.6, 3.2) | 2.2 (1.7, 2.9) | 2.7 (2.2, 3.4) | .075 | 1.1 (0.71, 1.6) | 0.2 (−0.9, 1.2) |
| Soy | - | 1.7 (1.2, 2.4) | 1.9 (1.6, 2.4) | 2.0 (1.3, 3.0) | 1.9 (1.4, 2.7) | 1.4 (1.0, 1.9) | 1.7 (1.3, 2.2) | .84 | 1.3 (0.66, 2.5) | 0.4 (−0.5, 1.3) |
ABBREVIATIONS: DHA (docosahexaenoic acid); DPA (docosapentaenoic acid); EGCG (epigallocatechin gallate); EPA (eicosapentaenoic acid); MVMM (Multivitamin/multimineral)
Detailed information on each trend, both overall and exclusive of MVMM is presented in eTable 3
Among adults ages ≥ 20 years.
A ‘decrease’ corresponds to a ratio<1 and p-trend<0.05, an ‘increase’ represents a ratio>1 and p-trend<0.05, and ‘stable’ represents a p-trend≥0.05.
Data are weighted to be nationally representative.
- Dash represents suppressed data due to unstable data, if the relative standard error exceeds 30%, consistent with NHANES reporting guidelines.40
Use of Echinacea decreased, as did use of garlic, gingko biloba, ginseng, and para-aminobenzoic acid (PABA). Use of glucosamine and chondroitin did not significantly change, nor did use of amino acids or fiber.
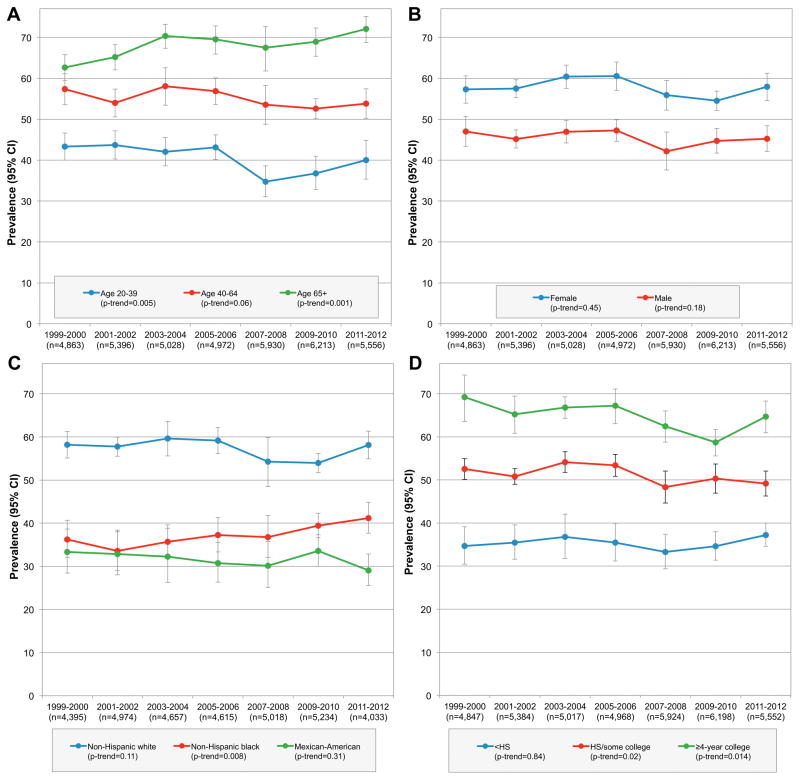
Trends by age, sex, race/ethnicity, and education were also evaluated (eTables 3–6). Any supplement use decreased among adults ages 20–39y, was stable among ages 40–64y, and increased among adults ages 65+y (eTable 3; Figure 1). Although women reported more supplement use than men, trends were stable in both groups groups (eTable 4; Figure 1). Use of any supplements remained stable among non-Hispanic whites and Mexican-Americans, but increased among non-Hispanic blacks (eTable 5; Figure 1). Use of supplements remained stable among those with less than a high school education, but decreased among those in higher education groups (eTable 6; Figure 1).
Figure 1.
Trends in Any Supplement Use, by Age, Sex, Race/Ethnicity, and Education
Abbreviations: HS (high school); MVMM (multivitamin/multimineral); data are weighted to be nationally representative.
The overall decrease in use of MVMM was largely driven by a decrease among adults ages 40–64y, with no change observed among older adults (eTable 3; eFigure 1). Use of MVMM decreased significantly among non-Hispanic whites, but remained stable among non-Hispanic blacks and Mexican-Americans (eTable 5; eFigure 1). Use of MVMM decreased for both men and women (eTable 4; eFigure 1) and for all education groups (eTable 6; eFigure 1).
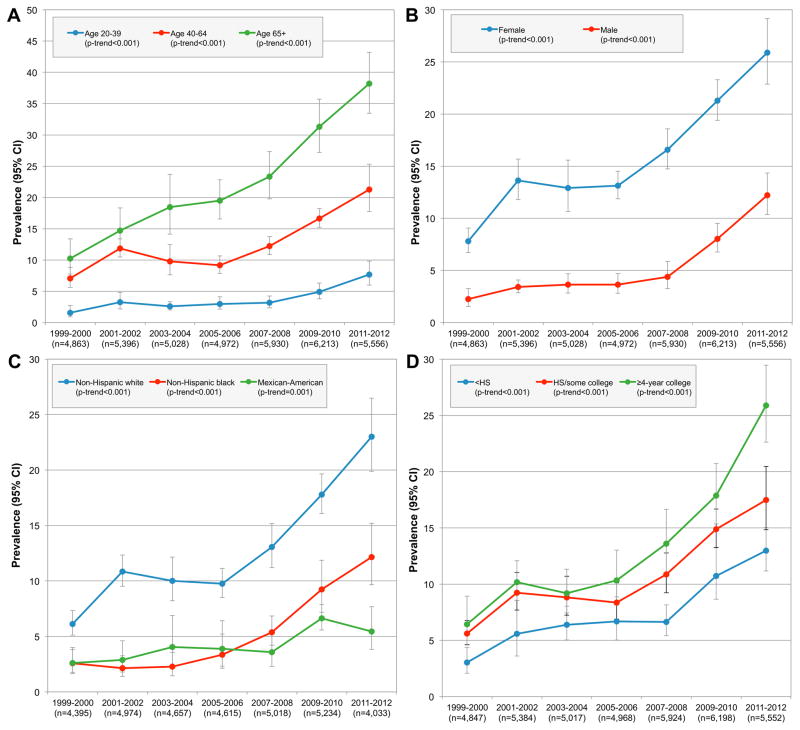
Subgroup-specific estimates are presented in Figure 2 for the most commonly used vitamin/mineral, vitamin D, and in eFigure 2 for the most commonly used NVNM, fish oil. Use of vitamin D (exclusive of MVMM) and fish oil and increased in all population subgroups. However, when including MVMM, increased use of vitamin D was observed only in those aged ≥65y (eTable 3), females (eTable 4), and Non-Hispanic blacks (eTable 5). When exploring use among adult women of childbearing age (20–44y) (eTables 7–8), use of any supplement products and MVMM decreased. Use of folic acid-containing supplements decreased; however, use of folic acid exclusive of MVMM remained stable.
Figure 2.
Trends in Use of Vitamin D (Excluding MVMM), by Age, Sex, Race/Ethnicity, and Education
Abbreviations: HS (high school); MVMM (multivitamin/multimineral); data are weighted to be nationally representative.
Trends were relatively comparable for regular use (eTable 9–10), and when adjusting for age (eTable 11).
No significant joinpoints were observed for overall supplement use or MVMM (eTable 12). However, a significant joinpoint, indicating a change in trend, was observed for a number of supplements. Most notably, lycopene increased dramatically prior to 2003–2004 (APC=130%; 95% CI:62%, 226%), decreasing thereafter (APC=−4.5%; 95% CI: −7.2%, −1.6%).
DISCUSSION
In this large, nationally representative survey of US adults, supplement use remained stable from 1999–2012, with 52% of adults reporting use of supplements in the prior 30 days in 1999–2000 and 2011–2012. Use of MVMM, as defined by a product containing ≥10 vitamins and/or minerals, decreased from 37% to 31%. Trends varied for individual supplements, and across age, sex, race/ethnicity, and education.
The stable trend in overall supplement use stands in contrast to earlier studies reporting their increase between the 1980s and early-2000s.1,2 With the present data, it is clear that the use of supplements among US adults has stabilized. This stabilization appears to be the balance of several opposing trends, with a major contributing downward factor being the decrease in use of MVMM. This trend may reflect increased scrutiny of MVMM,12 following several studies showing no benefit.7,10,20 Furthermore, in the mid-to-late 2000s, several expert bodies released statements or recommendations concluding that there is either insufficient or no evidence to support use of MVMM or supplements to prevent chronic disease.21–23 It is also possible that the economic downturn in the late 2000s may have also impacted trends in use. The Physicians’ Health Study II RCT demonstrated that daily MVMM use modestly reduced total cancer incidence in men;24 however, this study was published in 2012, and would not have influenced our findings.
Beyond the decrease for MVMM, a decrease was also observed in use of most vitamins and minerals, including antioxidants vitamins C, E, and selenium. These trends persisted when limited to non-MVMM supplements, indicating that these downward trends were not solely due to the decrease in MVMM use, but also to the decrease in use of individual or smaller combinations containing these antioxidants. This decrease likely reflects increasing skepticism regarding antioxidant supplements,12 following the publication of studies which demonstrated no or adverse effects on various outcomes.9,11,25–27
Despite the decrease in MVMM and many vitamins/minerals, not all decreased. Although overall vitamin D supplement use remained stable, a marked increase in vitamin D supplement use exclusive of MVMM was observed. This indicates that this increase is not due to increased inclusion of vitamin D in MVMM supplements but rather to an increase in individual vitamin D supplements or more focused combinations. This increase aligns with a growing body of research evaluating the potential beneficial effects of vitamin D on a number of health outcomes, including fractures, cancer, CVD, and multiple sclerosis.28–31 Such research likely increased public awareness about the potential benefits of vitamin D and testing for vitamin D insufficiency,32 contributing to the increase observed here and in a recent study of older adults.14
Use of lycopene-containing supplements also increased. A significant joinpoint for lycopene use was observed in 2005–2006, at which time lycopene was used by 25% of men. This increase in the early 2000s followed well-publicized research suggesting that consumption of lycopene-rich tomato products was associated with reduced risk of prostate cancer.4 This trend was almost entirely driven by MVMM; this increase was due to increased inclusion of lycopene in MVMM formulations, not increased use of individual lycopene supplements.
Use of omega-3 fatty acid-containing supplements also increased; this trend was largely driven by an increase in fish oil supplements, although an increase in alpha-linolenic acid/flaxseed was also observed. This increase, which has been observed previously,13,14 likely reflects increased research and subsequent media attention for the potential benefits of fish/omega-3 fatty acid intake and fish oil supplements on risk of cancer and CVD.5,33,34 The ongoing Vitamin D and Omega-3 Trial (VITAL), when complete, will provide valuable information to guide consumers and health care providers regarding the effects of omega-3 fatty acids and vitamin D.35
Not all NVNM supplements increased; many remained stable and a decrease was observed for several, including those that were amongst the most popular in the early-2000s,36 including ginseng, gingko, and garlic. The decline in gingko may be related to a 2002 RCT observing no effect of ginkgo on memory function, its primary indication.37 Furthermore, while glucosamine and chondroitin remained stable, a significant joinpoint was observed in 2005–2006, after which use declined. This is likely due to the 2006 Glucosamine/Chondroitin Arthritis Intervention Trial, which found no effect of these supplements on joint structure/function.8
The trend in any supplement use varied by age, with use decreasing among young adults, stable among middle-aged adults, and increasing among older adults. Arecent study of adults ages 62–85y, which found that supplement use among older adults rose from 51.8% in 2005–2006 to 63.7% in 2010–2011;14 an increase among older adults was also observed, but this increase does not hold for other age groups. Use of MVMM decreased significantly among non-Hispanic whites and more highly educated groups. The reasons for these patterns are unclear. It is possible that the difference in trends in MVMM use by age group may reflect a cohort effect, but a longer time frame would be needed to address this question. It is also possible that differences in trends by population subgroup reflect that research translates into behavior change more quickly in certain population subgroups,38,39 although differences across subgroups also likely reflect underlying health status and socioeconomic differences.3
This study has several important strengths. This study was conducted using data from a large nationally representative survey. Supplement use was assessed during an in-home interview, in which participants provided detailed information on supplements used over the prior 30 days; supplement bottles were seen for the majority of supplements, likely increasing accuracy of exposure measurement. Furthermore, this data was collected as part of a continuous national survey, allowing for evaluation of trends over a 14-year period. Even so, the present study has important limitations. This is a study of the non-institutionalized, civilian population, and does not capture use among adults in nursing homes or the military. Furthermore, supplements were assessed in the prior 30 days, which may underestimate any use over the year prior for supplements that may vary seasonally, such as those commonly taken for colds; however, this would not influence our ability to detect trends in use over time. This analysis uses the most recently available data (available through 2011–2012), and it is unknown whether or how these findings reflect dietary supplement use at present. Furthermore, this manuscript focuses on any use in the prior 30 days, although sensitivity analyses do include evaluate trends in regular use of supplements. The analyses did not incorporate information on dose, as the intention was to assess prevalence of use in the population, rather than population-level exposure. It also did not incorporate information about adherence. Fifth, the serial cross-sectional study design precluded longitudunal measurement of supplement use within the same individuals.
Conclusions
Among adults in the United States, overall use of dietary supplements remained stable from 1999–2012, use of MVMM decreased, and trends in use of individual supplements varied and were heterogeneous by population subgroups.
Supplementary Material
KEY POINTS.
Question
As little is known about recent patterns in supplement use, this manuscript describes patterns of dietary supplement use among US adults from 1999–2012.
Findings
In the nationally representative National Health And Nutrition Examination Survey, use of any supplement products remained stable between 1999 and 2012, with 52% of US adults reporting use of any supplements in both 1999–2000 and 2011–2012. Patterns varied by individual supplements, and multivitamin/multimineral use decreased, with 37% reporting use in 1999–2000 and 31% reporting use in 2011–2012.
Meaning
While any use of dietary supplement products remained stable in recent years, patterns varied by individual supplements.
Acknowledgments
This study was supported by the following grants from the National Cancer Institute of the National Institutes of Health: P30CA008748 and K05CA154337. The National Cancer Institute had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Elizabeth D. Kantor (Memorial Sloan Kettering Cancer Center) and Colin D. Rehm (Montefiore Medical Center) contributed equally to the manuscript. Elizabeth D. Kantor and Colin D. Rehm had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. No co-authors have any conflict of interest to declare.
References
- 1.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 2.Kim HJ, Giovannucci E, Rosner B, Willett WC, Cho E. Longitudinal and secular trends in dietary supplement use: Nurses’ Health Study and Health Professionals Follow-Up Study, 1986–2006. J Acad Nutr Diet. 2014;114(3):436–443. doi: 10.1016/j.jand.2013.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173(5):355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94(5):391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 5.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287(14):1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 6.Rautiainen S, Rist PM, Glynn RJ, Buring JE, Gaziano JM, Sesso HD. Multivitamin Use and the Risk of Cardiovascular Disease in Men. J Nutr. 2016 doi: 10.3945/jn.115.227884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuhouser ML, Wassertheil-Smoller S, Thomson C, et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women’s Health Initiative cohorts. Arch Intern Med. 2009;169(3):294–304. doi: 10.1001/archinternmed.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clegg DO, Reda DJ, Harris CL, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808. doi: 10.1056/NEJMoa052771. [DOI] [PubMed] [Google Scholar]
- 9.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sesso HD, Christen WG, Bubes V, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(17):1751–1760. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 12.Guallar E, Stranges S, Mulrow C, Appel LJ, Miller ER., 3rd Enough is enough: Stop wasting money on vitamin and mineral supplements. Ann Intern Med. 2013;159(12):850–851. doi: 10.7326/0003-4819-159-12-201312170-00011. [DOI] [PubMed] [Google Scholar]
- 13.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the Use of Complementary Health Approaches Among Adults: United States, 2002–2012. Natl Health Stat Report. 2015;(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 14.Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in Prescription and Over-the-Counter Medication and Dietary Supplement Use Among Older Adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473–82. doi: 10.1001/jamainternmed.2015.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. NHANES 2003–2004 Public Data General Release File Documentation. Hyattsville, MD: National Center for Health Statistics; 2005. [Accessed May 21, 2015]. Available: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/general_data_release_doc_03-04.pdf. [Google Scholar]
- 16.National Center for Health Statistics. National Health and Nutrition Examination Survey 1999 – 2012 Data Documentation, Codebook, and Frequencies, Dieary Supplement Database-Ingredient Information. Hyattsville, MD: National Center for Health Statistics; 2014. [Accessed August 22, 2016]. Available: http://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/DSII.htm. [Google Scholar]
- 17.Pocobelli G, Peters U, Kristal AR, White E. Use of supplements of multivitamins, vitamin C, and vitamin E in relation to mortality. Am J Epidemiol. 2009;170(4):472–483. doi: 10.1093/aje/kwp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics. NHANES Response Rates and Population Totals. Hyattsville, MD: National Center for Health Statistics; [Accessed May 21 2015]. Available: http://www.cdc.gov/nchs/nhanes/response_rates_cps.htm. [Google Scholar]
- 20.Rautiainen S, Lee IM, Rist PM, et al. Multivitamin use and cardiovascular disease in a prospective study of women. Am J Clin Nutr. 2015;101(1):144–152. doi: 10.3945/ajcn.114.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang HY, Caballero B, Chang S, et al. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: a systematic review for a National Institutes of Health state-of-the-science conference. Ann Intern Med. 2006;145(5):372–385. doi: 10.7326/0003-4819-145-5-200609050-00135. [DOI] [PubMed] [Google Scholar]
- 22.Marra MV, Boyar AP. Position of the American Dietetic Association: nutrient supplementation. J Am Diet Assoc. 2009;109(12):2073–2085. doi: 10.1016/j.jada.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 23.World Cancer Research Fund; American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 2007. [Google Scholar]
- 24.Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308(18):1871–1880. doi: 10.1001/jama.2012.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 27.Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300(18):2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40–49. doi: 10.1056/NEJMoa1109617. [DOI] [PubMed] [Google Scholar]
- 29.Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100(3):746–755. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- 30.Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer. 2014;111(5):976–980. doi: 10.1038/bjc.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 32.Shahangian S, Alspach TD, Astles JR, Yesupriya A, Dettwyler WK. Trends in laboratory test volumes for Medicare Part B reimbursements, 2000–2010. Arch Pathol Lab Med. 2014;138(2):189–203. doi: 10.5858/arpa.2013-0149-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caygill CP, Charlett A, Hill MJ. Fat, fish, fish oil and cancer. Br J Cancer. 1996;74(1):159–164. doi: 10.1038/bjc.1996.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285(3):304–312. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 35.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millen AE, Dodd KW, Subar AF. Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: The 1987, 1992, and 2000 National Health Interview Survey results. J Am Diet Assoc. 2004;104(6):942–950. doi: 10.1016/j.jada.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Solomon PR, Adams F, Silver A, Zimmer J, DeVeaux R. Ginkgo for memory enhancement: a randomized controlled trial. JAMA. 2002;288(7):835–840. doi: 10.1001/jama.288.7.835. [DOI] [PubMed] [Google Scholar]
- 38.McLaren L, McIntyre L, Kirkpatrick S. Rose’s population strategy of prevention need not increase social inequalities in health. Int J Epidemiol. 2010;39(2):372–377. doi: 10.1093/ije/dyp315. [DOI] [PubMed] [Google Scholar]
- 39.Pampel FC, Krueger PM, Denney JT. Socioeconomic Disparities in Health Behaviors. Annu Rev Sociol. 2010;36:349–370. doi: 10.1146/annurev.soc.012809.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2(161):1–24. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.