Abstract
Neutrophils are critical for the rapid eradication of bacterial pathogens, but they also contribute to the development of multiple organ failure in sepsis. We hypothesized that increasing early recruitment of neutrophils to the focus of infection will increase bacterial clearance and improve survival. Sepsis was induced in mice, using cecal ligation and puncture (CLP); blood samples were collected at 6 and 24 h; and survival was followed for 28 d. In separate experiments, peritoneal bacteria and inflammatory cells were measured. Septic mice predicted to die based on IL-6 levels (Die-P) had higher concentrations of CXCL1 and CXCL2 in the peritoneum and plasma compared with those predicted to live (Live-P). At 6 h, Live-P and Die-P had equivalent numbers of peritoneal neutrophils and bacteria. In Die-P mice the number of peritoneal bacteria increased between 6 and 24 h post-CLP, whereas in Live-P it decreased. The i.p. injection of CXCL1 and CXCL2 in naive mice resulted in local neutrophil recruitment. When given immediately after CLP, CXC chemokines increased peritoneal neutrophil recruitment at 6 h after CLP. This early increase in neutrophils induced by exogenous chemokines resulted in significantly fewer peritoneal bacteria by 24 h [CFU (log) = 6.04 versus 4.99 for vehicle versus chemokine treatment; p < 0.05]. Chemokine treatment significantly improved survival at both 5 d (40 versus 72%) and 28 d (27 versus 52%; p < 0.02 vehicle versus chemokines). These data demonstrate that early, local treatment with CXC chemokines enhances neutrophil recruitment and clearance of bacteria as well as improves survival in the CLP model of sepsis.
Neutrophils are a key part of innate immunity, the first line of defense against invading microorganisms (1, 2). Their arsenal of proteases and reactive oxygen species makes them very efficient at killing bacteria, but these same defenses may also harm host tissues (3, 4). During sepsis, neutrophil-induced damage has been associated with the development of multiple organ failure (5). Consequently, attempts to reduce neutrophil number or activity to prevent tissue injury could result in unchecked pathogen proliferation. In animal models of sepsis, reducing the neutrophil recruitment to the focus of infection has improved survival in some studies (6, 7), but in others it was detrimental (8). Studies have also tested the idea of globally increasing the number of neutrophils to augment the innate defenses against bacteria. These investigations used G-CSF to release neutrophils from the bone marrow, but results in both human and animals were contradictory regarding whether this improves survival (9–11). It remains unclear how to manipulate neutrophils in sepsis to enhance survival.
To perform their function, neutrophils must migrate from the circulation to the site of infection in response to chemotactic factors, including complement peptide C5a, leukotriene B4, platelet-activating factor, formyl-methionyl-leucyl-phenylalanine, and CXC chemokines, with CXCL8 (IL-8) being a very powerful chemotactic mediator (12–14). Mice do not have CXCL8, but two functional homologs have been described: CXCL1 (KC) and CXCL2 (macrophage-inflammatory protein-2) (15, 16). In septic patients, neutrophil chemotaxis is impaired as their surface expression of the receptor through which chemokines mediate this effect, CXCR2, is reduced (17, 18). This reduction in chemotaxis has been demonstrated in animal models of sepsis as well (19, 20). At the same time, the capacity for neutrophils to adhere to endothelial cells in sepsis is increased owing to neutrophil overexpression of the β-integrin CD11b (17). Furthermore, neutrophil apoptosis is reduced in septic patients (21, 22). All these data suggest that in sepsis neutrophils are inadequately recruited to the site of infection owing to reduced chemotaxis. However, they do have increased adherence to the endothelium, live longer, and could become activated in the wrong location by circulating pro-inflammatory mediators, which could lead to organ injury.
We hypothesized that increased local recruitment of neutrophils to the site of infection early in the evolution of sepsis would result in increased clearance of bacteria, leading to improved survival. The murine cecal ligation and puncture (CLP) model of peritonitis-induced sepsis was used to establish first whether mice predicted to live or die recruit neutrophils to the peritoneum and control bacteria differently. In the second part of the study, we tested whether increased early recruitment of neutrophils to the site of infection would decrease bacterial load and improve survival. This was accomplished with i.p. injections of CXCL1 and CXCL2 chemokines immediately after CLP.
Materials and Methods
Animals
Female ICR mice (Harlan-Sprague Dawley, Indianapolis, IN) were used. Mice were acclimated to our housing room for at least 24 h before surgeries in a temperature-controlled room with a diurnal cycle of 12 h light and 12 h dark. They were provided food and water ad libitum for the entire duration of the experiment. The experiments were approved by Boston University Animal Care and Use Committee.
Sepsis model
CLP, which results in peritonitis, was performed as first described (23), with minor modifications (24). Following ligation, the cecum was double punctured with a 16-gauge needle to induce ~50% mortality. Some of the mice received either 200 μl saline or a combination of 500 ng CXCL2 and 50 ng CXCL1 resuspended in saline to a total volume of 200 μl by i.p. injection immediately following closure of the peritoneal cavity. Mice received pain medication for the first 2 d post-CLP (buprenorphine 0.05 mg/kg every 12 h) and antibiotic treatment for the first 5 d post-CLP (imipenem 25 mg/kg every 12 h). Survival was followed in some groups of mice for 28 d, whereas other groups were sacrificed at discrete time points. Mice sacrificed at 6 h received the first dose of drugs, and those at 24 h received the first 3. The decision to sacrifice mice at 6 or 24 h was made before the experiment was started.
Sampling
Sampling was performed only once in all the mice, at either 6 or 24 h post-CLP, according to presurgery planning. A 20-μl sample of blood was collected by facial vein puncture, followed by aspiration using a pipette and an EDTA (169 mM tripotassium salt) rinsed tip. The blood was diluted 1/10 in PBS with 1/50 EDTA and centrifuged for 5 min at 1000 × g at 4°C, and the plasma was collected for IL-6 and CXCL1 (KC) ELISA and multiplex measurements. The cells were resuspended in Hemavet diluent and used to obtain an automated complete blood count with differential on a Hemavet instrument (CDC Technologies, Oxford, CT). In the mice that were sacrificed, following anesthesia blood was also collected from the retro-orbital venous plexus, and after sacrifice the peritoneal cavity was opened and lavaged. The first lavage was performed with a 1-ml aliquot of warm HBSS (Mediatech, Herndon, VA), followed by a second lavage with 25 ml -the same solution. Part of the 1-ml lavage (100 μl) was used for bacteria cultures. Then the two lavage fluids were centrifuged separately and the supernatant from the first wash was stored at −20°C for subsequent measurement of cytokine concentration ([C]) by multiplex and ELISA for CXCL1, as well as urea nitrogen concentration ([UN]). The supernatant from the second wash was discarded and the two pellets were resuspended in HBSS and combined. A total cell count was performed with a Beckman-Coulter particle counter model ZF (Coulter Electronics, Hialeah, FL), and a differential count was obtained by counting 300 cells on cytospin slides stained with Diff-Quick (Baxter, Detroit, MI). The total numbers of neutrophils and macrophages in the peritoneal cavity were then calculated.
Peritoneal neutrophil recruitment to exogenous CXC chemokines
Mice were injected i.p. with saline (200 μl), low-dose chemokines (100 ng CXCL2 plus 10 ng CXCL1), or high-dose chemokines (500 ng CXCL2 plus 50 ng CXCL1). After 4 h blood was collected via the facial vein, peritoneal lavage was performed as described earlier, and a total and differential count of the cells recovered was done. The cell pellet was used for myeloperoxidase (MPO) activity determination.
Bacterial cultures
For bacterial culturing, 100 μl fluid obtained at the first peritoneal lavage was diluted with HBSS 1:10–1:106, and 33 μl each dilution was cultured on 5% sheep blood agar plates (Fisher Scientific, Pittsburgh, PA). Plates were incubated at 37°C in aerobic or anaerobic conditions for 24 h, and then the number of CFUs was determined.
MPO assay
The MPO assay was performed as previously described, with minor modifications (25). The combined cell pellet was resuspended in hexadecyltrimethylammonium bromide buffer and sonicated 3 times for 10 s each, with placement on ice between pulses. The supernatant was diluted 1:5 in 10 mM citrate buffer, pH 5. On a 96-well plate, two wells for each sample (background) received 75 μl this diluted supernatant and 150 μl stop solution (ice-cold 4N H2SO4). Another two wells received 75 μl the same diluted supernatant and 75 μl substrate solution (0.3 mM 3,3′,5,5′-tetramethylbenzidine, 120 mM resorcinol, and 0.007% H2O2 in double distilled H2O), and the plate was incubated in the dark for 2 min, followed by the addition of 150 μl stop solution. The absorbance was read at 450 nm, and data were expressed as ΔOD (the difference between the average absorbance of the two substrate wells and the two background wells for the same sample). To test whether neutrophils recruited by chemokine injection maintain the granular content necessary to kill bacteria, the MPO activity was also expressed on a per cell basis. The ΔOD for peritoneal samples following chemokine injection was corrected for the cells that were already present in the peritoneum in the absence of treatment by subtracting the average ΔOD for the saline group. This value was divided by the number of neutrophils recruited in the respective mouse minus the average number of neutrophils present in the saline group.
ELISA and microarray immunoassay
An aliquot of the 1/10 diluted plasma collected at 6 and 24 h was used to determine the IL-6 concentration by ELISA, as previously described (26). CXCL1 concentration was also determined by ELISA in plasma as well as the supernatant from the first peritoneal lavage.
Aliquots of 1/10 plasma and supernatant from the first peritoneal lavage were also used to measure several cytokine concentrations (list provided in Table I, plus CXCL2) by microarray immunoassay as previously described (27), with some modifications. The primary (capture) Ab was spotted in quadruplicates for each of the cytokines on the bottom of a 96-well plate. The plate was blocked with Odyssey Blocking Buffer (LI-COR Biotechnology, Lincoln, NE) at room temperature with shaking for 1 h, then washed. Samples and standards were diluted in a 50/50 Odyssey Blocking Buffer/1× PBS solution and loaded on the plate to incubate for 2 h; then the plate was washed. A mixture of detection Abs diluted in the same solution as the samples was then loaded and incubated for 2 h, followed by a plate wash. Streptavidin-IRDye 800CW was diluted in the same solution and incubated with shaking in the dark for 30 min. Plates were washed, dried, and scanned on the Odyssey Li-Cor machine (LI-COR) and analyzed with the SearchLight Array Analyst software (Pierce Biotechnology, Woburn, MA).
Table I.
Cytokine concentrations in the peritoneum at 6 and 24 h post-CLP
| Cytokines | Peritoneal Cavity (ng/ml)
|
|||
|---|---|---|---|---|
| 6 h
|
24 h
|
|||
| Live-P | Die-P | Live-P | Die-P | |
| Proinflammatory | ||||
| IL-1β | 2.0 ± 0.4 | 2.5 ± 0.3b | 1.7 ± 0.2 | 2.2 ± 0.2 |
| IL-2 | 0.25 ± 0.06 | 0.28 ± 0.04 | 0.66 ± 0.1a,c | 0.45 ± 0.09 |
| IL-6 | 98.2 ± 42.3a | 250.9 ± 79.2 | 5.8 ± 0.7a | 47.2 ± 11.6a |
| IL-12 | 0.8 ± 0.1 | 1.1 ± 0.1b | 0.6 ± 0.1 | 0.9 ± 0.1b |
| IL-17 | 0.44 ± 0.08a | 0.72 ± 0.11 | 0.29 ± 0.03a | 0.37 ± 0.03a |
| IFN-γ | 1.4 ± 0.2 | 1.8 ± 0.4 | 0.9 ± 0.1 | 3.4 ± 1.7b |
| CCL3 (MIP-1α) | 11.1 ± 1.9 | 16.2 ± 1.9 | 9.2 ± 1.3 | 43.8 ± 14.4a−c |
| CCL2 (MCP-1) | 60.8 ± 16.4a | 119.8 ± 27.8 | 22.0 ± 2.9a | 17.0 ± 3.8a |
| TNF-α | 0.15 ± 0.03 | 0.26 ± 0.03b,c | 0.11 ± 0.01 | 0.24 ± 0.03b,c |
| ICAM-1 | 6.5 ± 1.2 | 9.1 ± 1.4 | 7.0 ± 0.7 | 8.8 ± 0.9 |
| Anti-inflammatory | ||||
| IL-1ra | 19.0 ± 3.2 | 22.5 ± 3.1 | 8.2 ± 0.8a,c | 13.6 ± 1.6b,c |
| IL-4 | 1.6 ± 0.4 | 2.4 ± 0.4c | 0.6 ± 0.1a,c | 0.8 ± 0.1a,c |
| IL-10 | 7.5 ± 1.2 | 17.7 ± 3.2b,c | 3.1 ± 0.8 | 16.0 ± 2.8b,c |
| TNF-srI | 3.9 ± 0.7 | 6.0 ± 0.8b,c | 3.3 ± 0.3 | 5.6 ± 0.5b,c |
| TNF-srII | 27.0 ± 4.8 | 33.5 ± 4.4 | 16.0 ± 1.6a,c | 20.6 ± 2.0a |
Concentrations were calculated as described in Materials and Methods (at 6 h n = 9 for Live-P and 11 for Die-P; at 24 h n = 24 for Live-P and 13 for Die-P). Results are shown as mean ± SEM.
p < 0.05 when compared with Die-P at 6 h.
p < 0.05 when compared with Live-P at 24 h.
p < 0.05 when compared with Live-P at 6 h.
Urea nitrogen measurement and determination of the peritoneal concentration of cytokines prior to lavage
Measuring cytokine concentrations in the supernatant of the first peritoneal lavage ([C]perit-measured) does not reflect on the concentration that existed in the peritoneal fluid prior to laparotomy ([C]perit-actual) because we do not know the original peritoneal volume (V1). The original volume is probably small, and diluting it with the 1 ml HBSS used for the initial lavage (V2) could substantially reduce the original concentrations. However, [C]perit-actual can still be calculated by relying on the principle used in peritoneal dialysis; i.e., the concentration of a small molecular weight solute such as urea nitrogen equilibrates between the plasma and peritoneal compartments. This principle has been similarly used to calculate the actual concentration of molecules recovered by bronchoalveolar lavage in humans (28, 29). The concentration of urea nitrogen in plasma ([UN]plasma) and in the supernatant of the first peritoneal wash ([UN]perit) was measured using a kit from Pointe Scientific (Canton, MI). Because blood flow is stopped and the time required to perform the lavage is short (seconds), the quantity of urea nitrogen was assumed to be the same in the peritoneum before lavage ([UN]plasma × V1) and after ([UN]perit × (V1 + V2)). This can be expressed as the following equation:
For the same reasons, the quantity of a cytokine in the peritoneum was also assumed to remain the same before ([C]perit-actual × V1) and after lavage ([C]perit-measured × (V1 + V2)), resulting in the following equation:
Using these two equations, we obtained a formula to calculate [C]perit-actual:
[C]perit-measured was determined by multiplex or ELISA in the supernatant of the first lavage, and [C]perit-actual was calculated for all the peritoneal cytokine concentrations that are presented in this study.
Statistical analysis
Statistics were performed using Prism 4 (GraphPad, San Diego, CA). When three or more groups were compared, one-way ANOVA followed by the Newman-Keuls posttest was used if the groups had normal distribution; the Kruskal–Wallis test followed by Dunn’s posttest was used when the distribution was not normal. If two groups were compared, an unpaired t test or a Mann–Whitney U test was used, depending on whether the data were distributed normally. A p value <0.05 was considered statistically significant.
Our laboratory has previously reported on the utility of plasma IL-6 measurement as a predictor of outcome in CLP sepsis (30–32). Using a single cutoff to differentiate the two groups results in a trade-off between the sensitivity and specificity of the test. To reduce the number of false negatives and false positives in the two groups, respectively, we have employed for this study a system with two cutoffs: a low cutoff that gives close to 100% sensitivity and clearly separates the group predicted to live past day 5 post-CLP (Live-P), and a high cutoff that gives close to 100% specificity and clearly separates the group predicted to die by day 5 post-CLP (Die-P). Values in between (indeterminate range) could potentially represent false-negative or false-positive results, and those samples were eliminated from further analysis, a feasible choice for an experimental setup like the one in this study.
To determine these cutoffs, two separate groups of 40 mice each were followed for survival for 28 d after CLP. Blood was collected by facial vein bleeding 6 h post-CLP in one group and 24 h post-CLP in another. The plasma level of IL-6 was measured by ELISA, and the status of the mouse at day 5 post-CLP (dead or alive) was used to generate a receiver operator characteristic analysis. These groups of mice were only used to determine the cutoff values, and none were sacrificed to collect samples. At 6 h, plasma IL-6 levels <12.1 ng/ml gave 95% sensitivity and levels >14.8 ng/ml indicated 90% specificity. With these values, the actual 5-d mortality for the Live-P mice was 5.6% (1 of 18 died), 88.9% for Die-P mice (16 of 18), and 75% for the small, indeterminate group (3 of 4). For the test at 24 h, plasma IL-6 <1.5 ng/ml provided 100% sensitivity for Live-P and levels >12 ng/ml gave 100% specificity. The actual 5-d mortality for Live-P was 0 of 25, 100% for Die-P (9 of 9), and 50% for indeterminate (2 of 4). With the values from these mortality studies, the 20 mice sacrificed at 6 h were separated into 9 Live-P and 11 Die-P (with no animals having IL-6 levels between the 2 cutoffs). The 53 mice sacrificed at 24 h were separated into 29 Live-P and 14 Die-P and 10 mice were eliminated because the IL-6 levels were in the indeterminate range. The IL-6 values used here to predict survival are very similar to those in our previous publication (31) in which an IL-6 cutoff of 13 ng/ml was used to predict, and both studies show an improvement over the prediction capacity originally described by our group (30). The experiments were conducted and samples collected and processed without prior knowledge of whether the mice were in the Live-P, Die-P, or indeterminate group.
For a composite view of the local and systemic pro- and anti-inflammatory environment of the septic mice, average pro- and anti-inflammatory z-scores were calculated. Values recorded for each cytokine were z-score normalized for the group that they belonged to (four groups were obtained for each cytokine by combining the data for Live-P and Die-P mice in the plasma or peritoneal environment, at the 6- or 24-h time point; four other groups were obtained by combining the data for the saline and chemokines mice in a similar manner). The z-score for a cytokine measurement (X) was calculated using the formula z = (X − μ)/σ, where X is the value for that mouse, μ is the mean, and σ is the SD of the group to which X belongs. Each mouse had an average z-score calculated for all the pro- or anti-inflammatory cytokines measured for that mouse. A positive average z-score indicates higher overall levels of pro- or anti-inflammatory mediators when comparing Live-P with Die-P or saline with chemokines.
Results
CXC chemokine levels in the local and systemic environment
Studies from our laboratory and others have shown that peritoneal and systemic levels of CXC chemokines are increased following CLP (7, 33, 34). This study examined whether the chemokine levels or the concentration gradient between the local and systemic environment, the driver of neutrophil recruitment (35), correlates with survival outcome for the septic mice. As peritoneal lavage is a terminal procedure, outcome was based on our biomarker prediction, specifically, the plasma levels of IL-6 (30–32). In separate experiments, mice were sacrificed and samples were collected at either 6 h (the earliest time point when a prediction can be made) or 24 h after being subjected to CLP (a time point that precedes the first death). A consistent pattern emerged, with higher chemokine levels in the mice predicted to die. In the peritoneum and plasma, the levels of CXCL1 (Fig. 1A, 1B) and CXCL2 (Fig 1D, 1E) were higher in the Die-P compared with Live-P mice at 6 h as well as 24 h post-CLP. For Live-P mice, there was a decline of these levels from 6 to 24 h. For the Die-P mice they were higher in plasma at 24 compared with 6 h, whereas there was a slight decreasing trend in the peritoneum.
FIGURE 1.
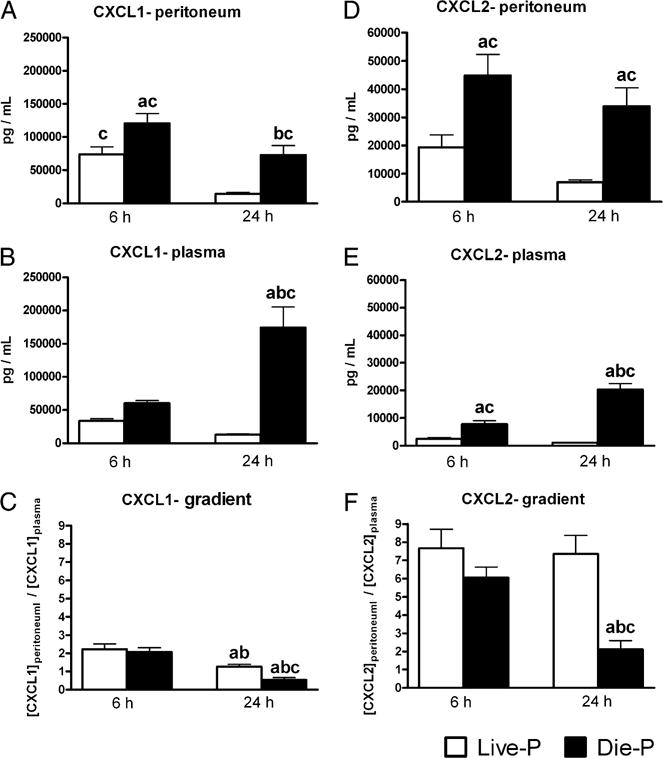
Mice predicted to die in the acute phase of CLP sepsis had higher peritoneal and plasma levels of CXCL1 and CXCL2 than did those predicted to survive. By 24 h post-CLP, mice predicted to die had significantly lower peritoneal to plasma concentration gradients of CXCL1 and CXCL2. CXCL1 was measured by ELISA, and CXCL2 was measured by microarray immunoassay. The peritoneal concentration was calculated as described in the Materials and Methods. The concentration gradient was expressed as the ratio between the concentration of the chemokine in the peritoneum and the concentration in plasma. For A, C, D, and F, at 6 h n = 9 for Live-P and 11 for Die-P, and at 24 h n = 24 for Live-P and 13 for Die-P. For B and E, at 6 h n = 9 for Live-P and 11 for Die-P, and at 24 h n = 29 for Live-P and 14 for Die-P. Results are shown as mean ± SEM. p < 0.05 when compared with Live-P at 6 h (a); Die-P at 6 h (b); Live-P at 24 h (c).
Neutrophils are recruited to the site of infection in response to concentration gradients of chemokines (35, 36). The peritoneum to plasma gradient of the two chemokines was calculated for the mice in our study to see if there were differences between the Live-P and Die-P groups. A higher gradient would favor recruitment to the peritoneal cavity. For CXCL1 the gradients were found to be similar in Live-P and Die-P at 6 h. At 24 h, the gradients favoring neutrophil recruitment were decreased in both Live-P and Die-P, but significantly more so in Die-P, in which the plasma concentration was actually higher than that in the peritoneum (Fig. 1C). Although the chemotactic gradient for CXCL2 was similar at 6 h for the two groups, by 24 h, the gradient was maintained only in Live-P mice, with Die-P having a 65% reduction (Fig. 1F).
Plasma and peritoneal levels of other cytokines
It was previously reported that post-CLP, mice that die within the first 5 d have higher plasma levels for both pro- and anti-inflammatory cytokines when compared with those that live past that time point (31). In this study, plasma and peritoneal levels of 17 pro- and anti-inflammatory cytokines were measured, and differences between Live-P and Die-P mice were assessed. Data for individual cytokines can be found in Table I (peritoneal concentrations) and Supplemental Table I (plasma concentrations); a global picture of the pro- and anti-inflammatory environment of these mice is presented in Fig. 2, in which a composite score of all the cytokines measured was calculated (see Materials and Methods). This composite score allows an integrated view of the data, rather than focusing on a single mediator. Die-P mice had collectively higher plasma levels for both the pro- and anti-inflammatory cytokines when compared with Live-P at both 6 and 24 h, and the difference between the two was more pronounced at 24 h (Fig. 2A, 2B), confirming previous reports (31). In the peritoneum, Die-P also had higher levels of pro- and anti-inflammatory cytokines when compared with Live-P, and the difference became statistically significant at 24 h (Fig. 2C, 2D). This finding indicates a similarity between the local and systemic environments in septic mice, with higher levels of both pro- and anti-inflammatory mediators in Die-P mice compared with Live-P as early as 6 h post-CLP. The higher levels of CXC chemokines in Die-P mice are a portion of the profile of increased levels of inflammatory mediators that precedes death.
FIGURE 2.
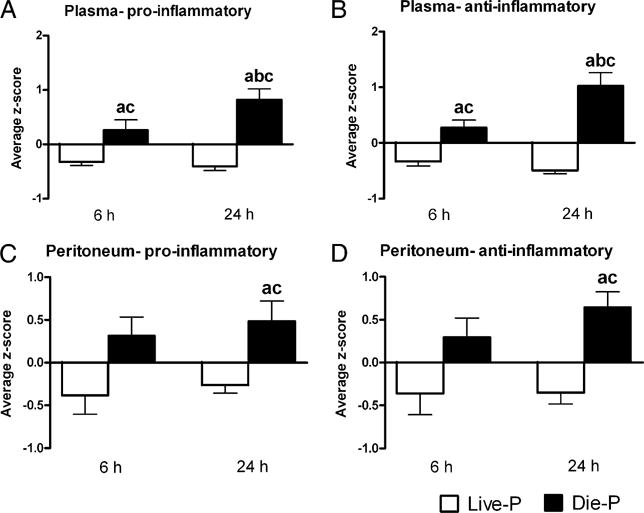
Die-P mice had higher levels of pro- and anti-inflammatory mediators both locally (in the peritoneal cavity) and systemically (in plasma). Average z-scores for all the pro- and anti-inflammatory mediators measured for each mouse were calculated as described in the statistical analysis section of Materials and Methods. For A and B, at 6 h n = 9 for Live-P and 11 for Die-P, and at 24 h n = 29 for Live-P and 14 for Die-P. For C and D, at 6 h n = 9 for Live-P and 11 for Die-P, and at 24 h n = 24 for Live-P and 13 for Die-P. Results are shown as mean ± SEM. p < 0.05 when compared with Live-P at 6 h (a); Die-P at 6 h (b); Live-P at 24 h (c).
Peritoneal neutrophil recruitment and bacterial load
Neutrophils are recruited to a focus of infection to eradicate the pathogen. The relationship between the CXC chemokines, the number of neutrophils recruited to the peritoneum, and the number of peritoneal bacteria was examined. When mice were sacrificed at 6 or 24 h, the peritoneal cavity was lavaged and the numbers of inflammatory cells and bacteria were quantified. The majority of the peritoneal cells were neutrophils (Fig. 3A) or macrophages (Fig. 3C), with the other leukocyte populations accounting for <1% of the cells at these stages in the evolution of sepsis. In naive mice the peritoneal cavity is sterile, with virtually no neutrophils. Within 6 h of CLP, literally millions of neutrophils have been recruited to the site of infection (Fig. 3A). The number of peritoneal neutrophils was similar in Live-P and Die-P at 6 h, but by 24 h it was higher in the Die-P. This higher recruitment in Die-P at 24 h was reflected in the significant drop in circulating numbers of neutrophils compared with all the other groups (Fig. 3B). These data indicate that increased peritoneal neutrophil recruitment was achieved by 24 h in Die-P mice even in the presence of reduced chemotactic gradients by that time. Live-P and Die-P mice had similar numbers of macrophages at 6 h post-CLP (Fig. 3C). By 24 h macrophage numbers were significantly (>3-fold) increased in Die-P compared with Live-P at 24 h and both groups at 6 h.
FIGURE 3.
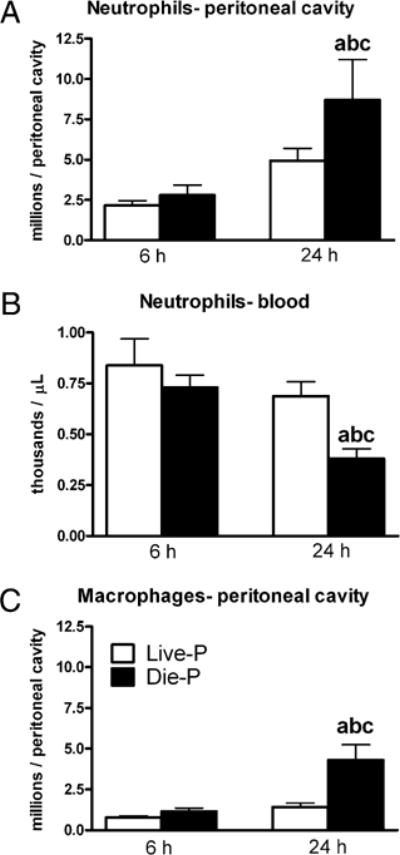
Relation between survival prediction and peritoneal or circulatory numbers of neutrophils. Total numbers of neutrophils (A) and macrophages (C) present in the peritoneum were calculated based on total and differential cell counts. Circulating counts of neutrophils (B) were obtained on a Hemavet instrument. For A, C, at 6 h n = 9 for Live-P and 11 for Die-P, and at 24 h n = 20 for Live-P and 10 for Die-P. For B, at 6 h n = 9 for Live-P and 11 for Die-P, and at 24 h n = 19 for Live-P and 9 for Die-P. Results are shown as mean ± SEM. p < 0.05 when compared with Live-P at 6 h (a); Die-P at 6 h (b); Live-P at 24 h (c).
To determine the impact of the recruited phagocytes on the number of peritoneal bacteria, serial dilutions of peritoneal lavage fluid were cultured on blood agar plates under both aerobe and anaerobe conditions (both cultures combined in Fig. 4). At 6 h post-CLP, Live-P and Die-P mice had similar numbers of CFUs in the peritoneum. This finding indicates that the initial infectious inoculum was equivalent in the mice predicted to live and those predicted to die. Despite comparable initial bacterial numbers, by 24 h, the number of peritoneal CFUs decreased in Live-P and increased in Die-P mice. There was a significant, >2 orders of magnitude, difference between the two groups in the number of peritoneal bacteria. Peritoneal bacterial counts were taken for the mice with indeterminate IL-6 levels because the cultures were performed prior to determining the predicted survival status. In confirmation of the intermediate status of this group, the mean CFU counts (5.76) were greater than those in Live-P (4.53) but less than those in Die-P (6.54). These data indicate that in the critical initial response between 6 and 24 h, peritoneal bacterial growth was efficiently controlled by the phagocytes recruited in Live-P mice. Conversely, Die-P mice were unable to stop the growth of bacteria even with increasing number of recruited phagocytes.
FIGURE 4.
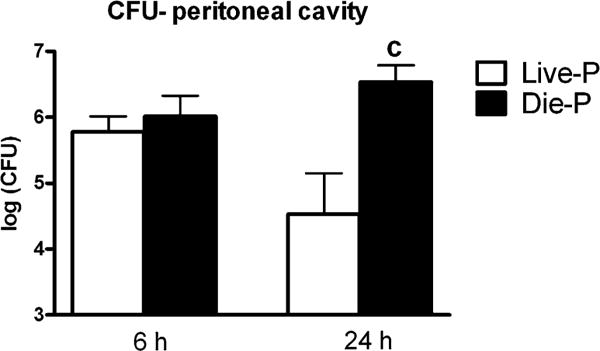
Peritoneal bacterial load in relation to survival prediction. At 6 h post-CLP equivalent numbers of bacteria were present in both groups. The Live-P group controlled the infection and had fewer bacteria at 24 h post-CLP. The counts obtained after incubating in aerobe and anaerobe conditions were added in a single value that was log transformed. At 6 h n = 9 for Live-P and 11 for Die-P, and at 24 h n = 12 for Live-P and 11 for Die-P. Results are shown as mean ± SEM. p < 0.05 when compared with Live-P at 24 h (c).
Enhanced local neutrophil recruitment with exogenous, local chemokines
The results from the first part of the study showed that 6 h post-CLP, regardless of survival outcome, the mice had similar numbers of recruited neutrophils (Fig. 3A) and peritoneal bacteria (Fig. 4). However, the Die-P mice failed to control bacterial growth between 6 and 24 h, even though they actively recruited greater numbers of neutrophils. This raised the possibility that earlier recruitment of neutrophils to the site of an infection could have a potentially beneficial effect. The second part of the study examined whether augmenting early peritoneal neutrophil recruitment would reduce bacterial load and improve survival in the CLP model of sepsis. The i.p. injection of CXCL1 and CXCL2 was performed to examine the feasibility of augmenting local recruitment of neutrophils. The combination of CXCL1 and CXCL2 was used because both were expressed in the peritoneum during CLP-induced sepsis. A small dose-response study was done to determine a concentration that effectively recruits neutrophils without causing degranulation. Normal mice were injected i.p. with either saline, low-dose, or high-dose CXC chemokines and sacrificed 4 h later. The i.p. injection of chemokines resulted in significant recruitment of neutrophils in the peritoneum within 4 h in a dose-dependent manner (Fig. 5A, 5B), with no change in the number of peritoneal macrophages (data not shown). Because the cells had undergone the stress of recruitment, we examined whether they maintained the granular content necessary to kill bacteria, by measuring MPO in the cell pellets recovered from lavage. Fig. 5C shows that chemokine injection increased the total MPO activity of the cells recovered from the peritoneum, with the higher dose resulting in a larger increase. When expressed on a per cell basis, the MPO activity was similar for the two doses (Fig. 5D), indicating that the neutrophils should remain fully equipped to kill bacteria. The number of circulating neutrophils was the same regardless of treatment (data not shown), and the increased peritoneal recruitment in mice receiving chemokines was probably compensated for by release from bone marrow. The higher dose of chemokines was used for future studies because it provides greater recruitment. Results in the literature also indicate a plateau of neutrophil recruitment after a dose of 500 ng CXCL2 (34).
FIGURE 5.
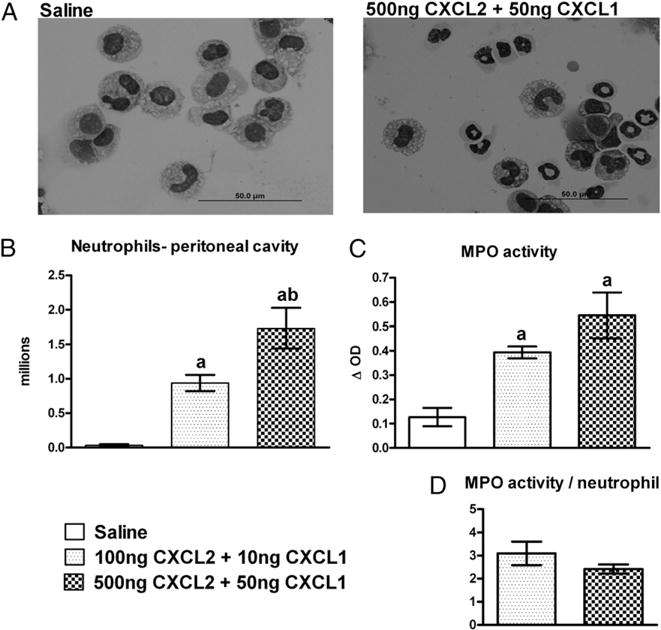
Peritoneal neutrophil recruitment 4 h after local injection of CXCL1 and CXCL2 chemokines. Mice were injected i.p. with saline (200 μl), low-dose chemokines (100 ng CXCL2 + 10 ng CXCL1), or high-dose chemokines (500 ng CXCL2 + 50 ng CXCL1). A shows representative peritoneal lavage cytospin slides from the saline and high-dose chemokine-injected mice (Diff-Quick stain; original magnification ×1000). For B, C, and D, n = 4 for all the groups. Results are shown as mean ± SEM. p < 0.05 when compared with saline (a); 100 ng CXCL2 + 10 ng CXCL1 (b).
CXC chemokine levels after exogenous CXC chemokine administration in CLP mice
CXCL1 and CXCL2 had similar peritoneal and plasma levels 6 h post-CLP in mice receiving chemokines immediately after intervention (chemokines group) when compared with mice that received the saline vehicle (saline group) (Supplemental Fig. 1). By 24 h, there was a slight decrease in the plasma and peritoneal levels of the CXC chemokines for mice receiving the chemokine injection early. Exogenous chemokines did not alter the concentration gradients.
Chemokine treatment influence on the plasma and peritoneal levels of other cytokines
By 24 h, mice that have received chemokines showed lower composite levels of pro- and anti-inflammatory mediators in both plasma and the peritoneal cavity when compared with mice that received saline (Supplemental Fig. 2). However, the early treatment with chemokines did not result in changes in the local or systemic pro- or anti-inflammatory environment that were statistically significant at either 6 or 24 h post-CLP. Levels registered for individual cytokines can be found in Supplemental Tables II and III.
Neutrophil recruitment and bacterial growth in mice receiving i.p. chemokines
To ascertain the biological effects of CXC chemokine treatment, the numbers of inflammatory cells and bacteria were measured. Peritoneal neutrophil recruitment was significantly increased in the chemokines compared with saline mice 6 h post-CLP (Fig. 6A). This observation confirms that the treatment results in rapid local recruitment of neutrophils even during sepsis. By 24 h, the number of neutrophils increased significantly in the saline group compared with the same group at 6 h. There was no additional increase at 24 h for the mice in the chemokines group beyond the numbers of neutrophils present by 6 h. The numbers of blood neutrophils were similar for saline and chemokines at 6 h, and both groups had a reduction in circulating neutrophils by 24 h (Fig. 6B). The number of macrophages present in the peritoneum was not affected by the treatment at the 6-h time point, although a slight increase was present at 24 h in the saline group (Fig. 6C).
FIGURE 6.
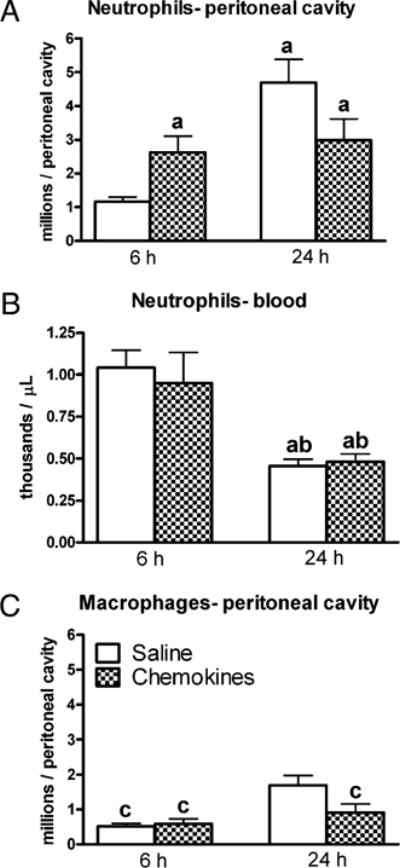
Peritoneal and circulating numbers of neutrophils after injection of chemokines immediately following CLP. Mice were injected i.p. with saline (200 μl) or chemokines (500 ng CXCL2 + 50 ng CXCL1). The i.p. injection of chemokines increased the early, 6 h, recruitment of neutrophils into the peritoneum. For A, C, at 6 h n = 10 for saline and 10 for chemokines, and at 24 h n = 20 for saline and 19 for chemokines. For B, at 6 h n = 9 for saline and 7 for chemokines, and at 24 h n = 27 for saline and 26 for chemokines. Results are shown as mean ± SEM. p < 0.05 when compared with saline at 6 h (a); chemokines at 6 h (b); saline at 24 h (c).
With greater numbers of peritoneal neutrophils following exogenous chemokine treatment, we evaluated the growth of peritoneal bacteria at 6 and 24 h. The number of CFUs at 6 h in the peritoneum of the chemokine-treated mice was similar to that in the saline group (Fig. 7). By 24 h, mice that received exogenous chemokines, and had increased local neutrophil recruitment, showed a clear advantage in the eradication of bacteria. Significantly lower numbers (more than one log difference) are registered for the chemokines group compared with saline at the same time point but also saline at 6 h.
FIGURE 7.
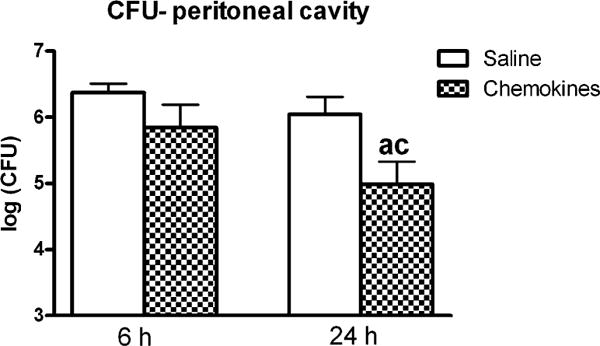
Peritoneal bacterial counts after injection of chemokines immediately following CLP. Mice were injected i.p. with saline (200 μl) or chemokines (500 ng CXCL2 + 50 ng CXCL1). The counts obtained after incubating in aerobe and anaerobe conditions were added in a single value that was log transformed. In mice injected i.p. with exogenous recombinant chemokines, compared with the saline-injected mice, there was a significant reduction in the growth of bacteria. At 6 h n = 10 for saline and 10 for chemokines, and at 24 h n = 20 for saline and 20 for chemokines. Results are shown as mean ± SEM. p < 0.05 when compared with saline at 6 h (a); saline at 24 h (c).
Influence of early neutrophil recruitment boost on survival
Data in Figs. 6 and 7 show that early i.p. injection of chemokines after CLP results in a rapid increase of neutrophil recruitment and subsequent lower bacterial counts in the peritoneal compartment. However, clinical studies have suggested that more neutrophils in the setting of sepsis could have deleterious outcomes (37). Experiments were performed to document if local chemokine treatment translates into a survival benefit. For these studies mice were subjected to CLP, and immediately following surgery they were injected i.p. with either saline or chemokines and then followed for 28-d survival. Chemokine-treated mice had significantly improved survival at 28 d (p = 0.02), with 52% of the mice still alive versus 27% for the saline group (Fig. 8). The largest improvement occurred in the first 5 d of sepsis, with 72% of the chemokines mice alive at day 5 versus 40% for the saline group (p = 0.01). These data demonstrate that increasing the neutrophil recruitment at the locus of infection early in the development of sepsis significantly improves survival.
FIGURE 8.
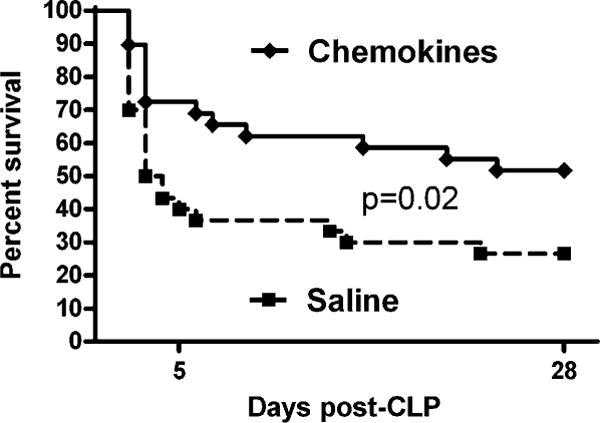
Improved 28-d survival after injection of chemokines immediately following CLP. Mice were injected i.p. with saline (200 μl) or chemokines (500 ng CXCL2 + 50 ng CXCL1). For saline n = 30, and for chemokines n = 29. The log rank test was performed to compare the two survival curves.
Discussion
The most important finding of our study is that by increasing early neutrophil recruitment to the peritoneum, the focus of infection in CLP sepsis, subsequent bacterial growth is better controlled and survival to 28 d is significantly improved. Although intuitive, such an approach is somewhat controversial in sepsis. There is no question that neutrophils represent a powerful defense mechanism against invading microorganisms. Individuals that are neutropenic or those whose neutrophils lack the respiratory burst (e.g., chronic granulomatous disease) or the capacity to kill bacteria because of delayed degranulation (Chédiak-Higashi syndrome) are all prone to bacterial infections (38, 39). However, the capacity to destroy host tissue as well as bacteria has linked neutrophils to the development of multiple organ failure in sepsis and has made them targets for inhibitory treatments.
Studies have been performed in which the recruitment of neutrophils was inhibited in sepsis models. Neutralizing Abs to CXCL2 (7) or to the chemotactic CXCR2 receptor (6) have improved survival in the CLP model of sepsis. The effect of these treatments was decreased peritoneal neutrophil recruitment in the first study (although the authors did not confirm if the total neutrophil count was decreased or merely the percentage of neutrophils) or just a delay in recruitment for the first 4 h post-CLP in the second study. Peritoneal bacterial growth was not altered in the second study. These studies contrast with our current work, in which augmented neutrophil recruitment improved survival. However, there are important differences in the studies. The administration of the Abs was systemic and the anti-CXCR2 was repeated every 48 h. This may have resulted in prolonged inhibition of neutrophil recruitment to organs away from the focus of infection, preventing organ failure and explaining the survival benefit. A different approach to the same idea of blocking local recruitment of neutrophils to the peritoneum in CLP sepsis has proved detrimental, however (8). The authors have used an Ab against CD18 (a β2 integrin that is important for neutrophil adhesion to the endothelium, a step that precedes trans-migration) to obtain the reduction in recruitment. This treatment resulted in increased numbers of peritoneal bacteria as well as increased neutrophil recruitment and injury in other organs like liver and lungs. These findings are in agreement with our study, because early recruitment of additional neutrophils improved survival.
Using chemokines to increase local neutrophil recruitment could have the potential side effect of increasing circulating numbers of neutrophils and result in their accumulation in other organs, with subsequent damage. Mice transgenic for human IL-8 that have elevated plasma levels of IL-8 do have an elevated neutrophil count (40, 41). However, in our studies with local, peritoneal administration, there were no higher counts of circulating neutrophils in mice receiving chemokines compared with saline for either normal or septic mice receiving the treatment. The improved survival and the paucity of changes in the systemic levels of cytokines following the treatment are also proof that concerns about systemic toxicity did not materialize. Even if the treatment increased the circulating pool of neutrophils, that would not necessarily have translated into organ injury, as proven by trials using G-CSF. Concerns of increased lung injury in nonneutropenic septic patients receiving G-CSF have not been substantiated, but no survival benefit was found either (10, 42). Our study proposes a more targeted approach to neutrophil manipulation in sepsis, with a local increase in recruitment as opposed to global effects given by G-CSF.
In the mice that were predicted to die, there was an increased recruitment of neutrophils in the peritoneum, despite a reduction of the local versus systemic concentration gradient for the CXCL1 and CXCL2 chemokines. Even though reduced, this gradient is still present for CXCL2, and it could be enough to drive the recruitment. Alternatively, a previous study has shown that neutrophil recruitment could happen even in the face of an unfavorable chemotactic gradient (41). The concentrations of CXCL1 and CXCL2 were increased in mice predicted to die in both the peritoneal cavity and the plasma. This finding probably reflects the struggle to recruit more neutrophils from a decreasing circulating pool as the growth of bacteria continues in these animals. The timing of the treatment is probably essential, as it has to be early enough to be able to establish an effective concentration gradient and there must be sufficient numbers of circulating neutrophils to recruit.
In this study the i.p. administration of chemokines was used as a preventive treatment, applied right after the surgery that renders the mice septic. In emergency units, however, it is usually not known how much time has passed since the patient became septic, and it takes additional time to detect the focus of infection. Probing the length of time for the therapeutic window of opportunity for such a treatment will be explored in future studies. It is possible that this interval is short, probably less than a day, as by then neutropenia develops, as well as the reduction in chemotaxis (19, 20), making it difficult to recruit extra neutrophils to the peritoneum from a smaller, more refractory pool of circulating cells. This treatment, though, applied at the right time, could be very important for bacterial control in clinical sepsis, along with the use of antibiotics. We use broad-spectrum antibiotics in our CLP model, and although that prevents bacteremia (43), it does not prevent bacterial growth inside the peritoneum.
An interesting finding from this study was that early (6 h post-CLP) neutrophil recruitment is similar in mice that are going to live and those that are going to die while their peritoneal bacterial load is also equal. A recent review of the CLP model has called for an increased standardization of the murine CLP model (44), and these results confirm that it can be performed such that all mice have a similar initial insult. By 24 h post-CLP, however, mice that are going to die have more neutrophils in the peritoneum compared with those that are going to live, but they also have uncontrolled bacterial growth. This finding indicates that the initial insult is the same, but the trajectory is vastly different. It is possible that the neutrophils of the mice that die are less capable of killing bacteria, either because of reduced phagocytosis, reduced activity of their granular content, or reduced oxidative burst. Qualitative neutrophil diseases are very rare, but it could be possible that a small defect in the host response is exacerbated in sepsis. Alternatively, a reduced capacity of humoral elements to opsonize bacteria or increased bacterial virulence in some mice could also hamper bacterial killing.
Sepsis mortality remains high at around 30% of cases, as very few treatments have been proven to reduce it. The key question is why the mice die during the acute phase of sepsis, and why early recruitment of neutrophils improves survival. The early deaths are probably due to a combination of events, including excessive inflammation and endothelial cell dysfunction, but not excessive cytokine production. Previously, we have shown that nonspecific immunosuppression with glucocorticoids will improve survival in those mice predicted to die, without reducing cytokine levels (32). Maintaining endothelial cell integrity will reduce mortality after CLP-induced sepsis, without decreasing cytokine levels (45). We have also reported that mice dying in the early stages of sepsis gained body weight secondary to edema, which may have been due to disruption of endothelial cell function (30). This early, excessive inflammation is driven by pathogens because antibiotic therapy reduces mortality, again without altering cytokine levels (43). The consistent theme is that improving survival, whether with antibiotics, immunosuppression targeted to those who die, or enhancing endothelial cell function, does not decrease cytokine production. The present study shows that the host’s own weapons, the neutrophils, can be targeted to contain bacteria more efficiently at the focus of infection, also with minimal effect on cytokine levels. Further studies are needed to establish this strategy as a viable treatment choice in human sepsis.
Supplementary Material
Acknowledgments
We thank Dr. Shinichiro Kurosawa for support in designing the method for determination of the peritoneal concentration of cytokines prior to lavage.
This work was supported by National Institutes of Health Grant GM 82962.
Abbreviations
- [C]
cytokine concentration
- CLP
cecal ligation and puncture
- Die-P
predicted to die in the first 5 d post-CLP
- Live-P
predicted to survive past day 5 post-CLP
- MPO
myeloperoxidase
- perit
peritoneum
- [UN]
urea nitrogen concentration
- V
volume
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 3.Henson PM, Johnston RB., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J Clin Invest. 1987;79:669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 5.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ness TL, Hogaboam CM, Strieter RM, Kunkel SL. Immunomodulatory role of CXCR2 during experimental septic peritonitis. J Immunol. 2003;171:3775–3784. doi: 10.4049/jimmunol.171.7.3775. [DOI] [PubMed] [Google Scholar]
- 7.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun. 1997;65:3847–3851. doi: 10.1128/iai.65.9.3847-3851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercer-Jones MA, Heinzelmann M, Peyton JC, Wickel D, Cook M, Cheadle WG. Inhibition of neutrophil migration at the site of infection increases remote organ neutrophil sequestration and injury. Shock. 1997;8:193–199. doi: 10.1097/00024382-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa K, Tanaka H, Nakamori Y, Hosotsubo H, Ogura H, Nishino M, Shimazu T, Sugimoto H. Difference in the responses after administration of granulocyte colony-stimulating factor in septic patients with relative neutropenia. J Trauma. 2000;48:814–824. doi: 10.1097/00005373-200005000-00004. discussion 824–815. [DOI] [PubMed] [Google Scholar]
- 10.Root RK, Lodato RF, Patrick W, Cade JF, Fotheringham N, Milwee S, Vincent JL, Torres A, Rello J, Nelson S, Pneumonia Sepsis Study Group Multicenter, double-blind, placebo-controlled study of the use of filgrastim in patients hospitalized with pneumonia and severe sepsis. Crit Care Med. 2003;31:367–373. doi: 10.1097/01.CCM.0000048629.32625.5D. [DOI] [PubMed] [Google Scholar]
- 11.Quezado Z, Parent C, Karzai W, Depietro M, Natanson C, Hammond W, Danner RL, Cui X, Fitz Y, Banks SM, et al. Acute G-CSF therapy is not protective during lethal E. coli sepsis. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1177–R1185. doi: 10.1152/ajpregu.2001.281.4.R1177. [DOI] [PubMed] [Google Scholar]
- 12.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 13.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 14.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 15.Bozic CR, Gerard NP, von Uexkull-Guldenband C, Kolakowski LF, Jr, Conklyn MJ, Breslow R, Showell HJ, Gerard C. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. J Biol Chem. 1994;269:29355–29358. [PubMed] [Google Scholar]
- 16.Modi WS, Yoshimura T. Isolation of novel GRO genes and a phylogenetic analysis of the CXC chemokine subfamily in mammals. Mol Biol Evol. 1999;16:180–193. doi: 10.1093/oxfordjournals.molbev.a026101. [DOI] [PubMed] [Google Scholar]
- 17.Chishti AD, Shenton BK, Kirby JA, Baudouin SV. Neutrophil chemotaxis and receptor expression in clinical septic shock. Intensive Care Med. 2004;30:605–611. doi: 10.1007/s00134-004-2175-y. [DOI] [PubMed] [Google Scholar]
- 18.Adams JM, Hauser CJ, Livingston DH, Lavery RF, Fekete Z, Deitch EA. Early trauma polymorphonuclear neutrophil responses to chemokines are associated with development of sepsis, pneumonia, and organ failure. J Trauma. 2001;51:452–456. doi: 10.1097/00005373-200109000-00005. discussion 456–457. [DOI] [PubMed] [Google Scholar]
- 19.Reddy RC, Narala VR, Keshamouni VG, Milam JE, Newstead MW, Standiford TJ. Sepsis-induced inhibition of neutrophil chemotaxis is mediated by activation of peroxisome proliferator-activated receptor-gamma. Blood. 2008;112:4250–4258. doi: 10.1182/blood-2007-12-128967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rios-Santos F, Alves-Filho JC, Souto FO, Spiller F, Freitas A, Lotufo CM, Soares MB, Dos Santos RR, Teixeira MM, Cunha FQ. Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase-derived nitric oxide. Am J Respir Crit Care Med. 2007;175:490–497. doi: 10.1164/rccm.200601-103OC. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez MF, Watson RW, Parodo J, Evans D, Foster D, Steinberg M, Rotstein OD, Marshall JC. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg. 1997;132:1263–1269. doi: 10.1001/archsurg.1997.01430360009002. discussion 1269–1270. [DOI] [PubMed] [Google Scholar]
- 22.Martins PS, Kallas EG, Neto MC, Dalboni MA, Blecher S, Salomão R. Upregulation of reactive oxygen species generation and phagocytosis, and increased apoptosis in human neutrophils during severe sepsis and septic shock. Shock. 2003;20:208–212. doi: 10.1097/01.shk.0000079425.52617.db. [DOI] [PubMed] [Google Scholar]
- 23.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock —a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 24.Ebong SJ, Call DR, Bolgos G, Newcomb DE, Granger JI, O’Reilly M, Remick DG. Immunopathologic responses to non-lethal sepsis. Shock. 1999;12:118–126. doi: 10.1097/00024382-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Schneider T, Issekutz AC. Quantitation of eosinophil and neutrophil infiltration into rat lung by specific assays for eosinophil peroxidase and myeloperoxidase. Application in a Brown Norway rat model of allergic pulmonary inflammation. J Immunol Methods. 1996;198:1–14. doi: 10.1016/0022-1759(96)00143-3. [DOI] [PubMed] [Google Scholar]
- 26.Nemzek JA, Siddiqui J, Remick DG. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods. 2001;255:149–157. doi: 10.1016/s0022-1759(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 27.Knight PR, Sreekumar A, Siddiqui J, Laxman B, Copeland S, Chinnaiyan A, Remick DG. Development of a sensitive microarray immunoassay and comparison with standard enzyme-linked immunoassay for cytokine analysis. Shock. 2004;21:26–30. doi: 10.1097/01.shk.0000101668.49265.19. [DOI] [PubMed] [Google Scholar]
- 28.Rennard SI, Basset G, Lecossier D, O’Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60:532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- 29.van der Vliet A, O’Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol. 1999;276:L289–L296. doi: 10.1152/ajplung.1999.276.2.L289. [DOI] [PubMed] [Google Scholar]
- 30.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 32.Osuchowski MF, Connett J, Welch K, Granger J, Remick DG. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit Care Med. 2009;37:1567–1573. doi: 10.1097/CCM.0b013e31819df06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebong S, Call D, Nemzek J, Bolgos G, Newcomb D, Remick D. Immunopathologic alterations in murine models of sepsis of increasing severity. Infect Immun. 1999;67:6603–6610. doi: 10.1128/iai.67.12.6603-6610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mercer-Jones MA, Shrotri MS, Heinzelmann M, Peyton JC, Cheadle WG. Regulation of early peritoneal neutrophil migration by macrophage inflammatory protein-2 and mast cells in experimental peritonitis. J Leukoc Biol. 1999;65:249–255. doi: 10.1002/jlb.65.2.249. [DOI] [PubMed] [Google Scholar]
- 35.Call DR, Nemzek JA, Ebong SJ, Bolgos GL, Newcomb DE, Remick DG. Ratio of local to systemic chemokine concentrations regulates neutrophil recruitment. Am J Pathol. 2001;158:715–721. doi: 10.1016/S0002-9440(10)64014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blackwell TS, Lancaster LH, Blackwell TR, Venkatakrishnan A, Christman JW. Chemotactic gradients predict neutrophilic alveolitis in endotoxin-treated rats. Am J Respir Crit Care Med. 1999;159:1644–1652. doi: 10.1164/ajrccm.159.5.9806166. [DOI] [PubMed] [Google Scholar]
- 37.Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–169. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 38.Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab. 1999;68:283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- 39.Assari T. Chronic Granulomatous Disease; fundamental stages in our understanding of CGD. Med Immunol. 2006;5:4. doi: 10.1186/1476-9433-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonet WS, Hughes TM, Nguyen HQ, Trebasky LD, Danilenko DM, Medlock ES. Long-term impaired neutrophil migration in mice overexpressing human interleukin-8. J Clin Invest. 1994;94:1310–1319. doi: 10.1172/JCI117450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remick DG, Green LB, Newcomb DE, Garg SJ, Bolgos GL, Call DR. CXC chemokine redundancy ensures local neutrophil recruitment during acute inflammation. Am J Pathol. 2001;159:1149–1157. doi: 10.1016/S0002-9440(10)61791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson S, Belknap SM, Carlson RW, Dale D, DeBoisblanc B, Farkas S, Fotheringham N, Ho H, Marrie T, Movahhed H, et al. CAP Study Group A randomized controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalized patients with community-acquired pneumonia. J Infect Dis. 1998;178:1075–1080. doi: 10.1086/515694. [DOI] [PubMed] [Google Scholar]
- 43.Newcomb D, Bolgos G, Green L, Remick DG. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock. 1998;10:110–117. doi: 10.1097/00024382-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med. 2010;2:23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


