Abstract
Mice prefer to mate with individuals expressing different MHC genes from their own. Volatile components presenting MHC-dependent odor types are present in urine and can be detected by mice, as shown by extensive behavioral studies. Similar odor types are suspected to influence human behavior as well. Although a recent report indicates that MHC expression influences the ratio of volatile compounds such as phenylacetic acid, so far no other means than studying the behavior of mice or rats has been available to assess odor types. Here, we report the ability of a gas sensor array (referred to as “electronic nose”) to detect MHC-dependent odor types. The electronic nose consists of an array of chemophysical detectors, in our case quartz crystal microbalances and semiconducting metal-oxide sensors that change frequency or conductivity upon binding of very small numbers of individual molecules present in the gas phase of odorous fluids. The pattern of changes is characteristic for a particular smell. Our electronic nose distinguishes the urine odor types of MHC congenic mouse strains, MHC class I mutant mice, and HLA-A2 transgenic mice. In addition, MHC-dependent odor types can be detected in serum. The device also clearly differentiates between individual odor types of human sera from HLA homozygous individuals; however, HLA expression seems to have only a secondary influence. Thus, odor-type research can now be carried out with an objective and fast through-put system independent of behavioral studies.
The principal function of MHC molecules is to present peptides to T cells (1). MHC class I molecules are expressed on the cell membrane of almost all somatic cells. Typically, MHC I molecules present virus-derived peptides of 8–11 aa to virus-specific cytotoxic T cells. MHC class II molecules are expressed on a subset of cells only, most notably on B cells, dendritic cells, and macrophages. Typically, T-helper cells recognize peptides of 12–25 aa derived from antigen presented on B cell MHC II molecules (2). This induces the T cells to produce signals that activate the B cell to produce antibody. MHC I and II molecules have well-defined peptide receptor specificities that enable binding of peptides with certain sequence patterns. MHC genes are extremely polymorphic; there are, for example, more than 180 alleles at the HLA-B locus (3). This polymorphism is reflected in different peptide receptor specificities for each of the allelic products (4). In consequence, T cells from different individuals recognize a different selection of antigen peptides from the very same pathogen. Thus, pathogens can mutate their MHC-presented peptide sequences to escape T cell recognition in an individual; the same mutations, however, are useless for the pathogen in other individuals with different MHC expression. The extreme polymorphism is, therefore, assumed to have evolved to avoid pathogen escape from immune recognition on the species level (5).
A driving force for establishment and maintenance of MHC polymorphism is probably the “survival of the fittest;” in addition, however, MHC polymorphism, at least in mice, is presumed to be maintained by the mating preference toward MHC-different individuals. This preference is mediated by volatile substances present in the urine, as detected by behavioral studies done with mice and also with rats that were trained to detect odors (6–8). In humans, however, the influence of odor types on mate selection is controversial, at least as based on scientific investigation (9–12). Further investigation of this problem has been hindered by the lack of objectively measurable biochemical parameters reflecting the odor types. Here, we show that a recently developed chemical sensor device, the so-called electronic nose (e-nose), is able to detect individual odor types in mice and human individuals. The influence of MHC genes on the odor type as presented by volatile substances in urine or serum is the subject of our studies described below.
Materials and Methods
Mice.
BALB/c, BALB.B, B10.A, B10.BR, B10.D2, C3H, and C57BL/6 mice were purchased from Charles River Breeding Laboratories or from Harlan Winkelmann (Borchen, Germany); B6.C-H2bm1 (bm1) mice were obtained from The Jackson Laboratory. A2-Kb-transgenic mice were a gift from S. Pascolo (Institute for Cell Biology, Tübingen, Germany). All were maintained or bred in the animal facility at the Institute for Cell Biology and fed with standard diet (SSNIFF, Soest, Germany) and water ad libitum.
Sample Collection.
Groups of 3–5 mice were placed in a standard metabolism cage and left overnight with water ad libitum but without food. Discharged urine was separated from feces and collected in a glass beaker. Urine was transferred the next morning into a glass vial and immediately stored at −79°C. Mice were transferred back to standard conditions for a minimum of 3 days before the next urine collection. Several urine collections from individual mouse groups were pooled afterward. Thus, samples consisted of urine from 3–5 mice collected during a period of 6–15 days. Blood samples were obtained by heart puncture after cervical dislocation.
Human serum samples were obtained from HLA-homozygous healthy male donors of the blood bank. Blood samples were stored at −79°C.
Sample Preparation.
To minimize the loss of volatile compounds, samples were processed as rapidly as possible. Five hundred microliters of thawed urine or serum was transferred into 10-ml glass vials. Vials were immediately sealed with a gas-tight silicone/polytetrafluoroethylene septum (Roth, Karlsruhe, Germany). Sample vials for e-nose measurements were placed into the headspace oven. For GC/MS analysis, 400 μl of sample was prepared in the same way.
Chemical Sensor Device (E-Nose).
The e-nose as a gas sensor array is composed of a chemical sensor system (hardware) and pattern recognition software. The set-up comprises the hybrid Modular Sensor System (MOSES) connected to a headspace sampler for sample preparation (13). The sensor system is equipped with two different modules, each of them based on a different transducer principle. The quartz microbalance (QMB) module consists of eight individual sensors with varying polymer coatings. These mass-sensitive quartz crystals change their fundamental frequency according to the mass increase of absorbed molecules. Advantages of the QMB sensors are good reproducibility and long-term stability. The second module contains eight semiconducting metal oxide (MOX)-based gas sensors, which are selected on the basis of different sensitivity and selectivity. The interactions between analyte molecules and the sensors are more complex if compared with QMB sensors. They involve reactions with oxygen on the sensor surface, which lead to a change of the free charge carrier concentrations in the conducting metal oxide. Advantages of these sensors are high sensitivities (14) and long duration (15). The sample's headspace (volume of 3 ml) is carried through the measurement chambers of the modules sequentially. The sensor modules are represented by software objects. A controller is used to control the timing and to collect the data from all modules. It uses a script language to describe its measurement task, which transfers the parameters to the modules, starts the measurement on the sensors, and reports the results to an external computer. The whole measurement time for one sample is 10 min (including the time until the sensor reaches the original baseline). The exposure to the analytes is less than 2 min. The external computer extracts from the incoming data stream the features for the subsequent pattern recognition (more details see below), performs the pattern recognition itself, including statistical evaluation, and displays and stores the results. The software running on the external PC uses an internal representation of the hardware modules for the feature extraction. As each hardware module is represented by a corresponding software object, different feature extraction algorithms can be applied for each individual module. Even within one module, different feature extraction algorithms can be applied to the individual sensory elements. As algorithm for the subsequent pattern recognition, principal component analysis (PCA) is applied and fully integrated into the software.
Headspace Analysis.
Headspace sampling is a means of introducing the volatile components from a liquid sample into a gas chromatograph (GC) for analysis when the original sample cannot be injected into the GC (16). Examples of headspace applications are the analysis of organics in urine, blood, etc. The sample placed in a sealed vial is heated, allowing the volatile components to escape from the sample to form a gaseous headspace above the liquid. After a preprogrammed heating time, the headspace gas is extracted from the vial and injected into a GC for analysis. The whole procedure is fully automated; thus, it ensures the high reproducibility and comparability of the analyses. The GC/MS data evaluation was performed by the Hewlett–Packard MSD Productivity CHEMSTATION software (Rev. B.00.01), which enabled qualitative and quantitative predictions by taking the retention time and the peak areas into account. To identify the peaks, pure compounds were chromatographed, and the National Institute of Standards and Technology (Gaithersburg, MD) mass spectrum library served as a reference data bank.
Pattern Recognition.
Multivariate data—data that depend on more than one variable—generated by an array of N sensors do not necessarily span the complete N dimensional space defined by the sensors (17). If cross-sensitivities exist between sensors, their output values are at least partially intercorrelated. Thus, the existing linear dependency of sensor signals means that the actual dimensionality of the data space defined by the N sensors is typically smaller than the maximum dimensionality N. PCA is an algorithm used to find the optimum representation of a given data set in a space whose dimensionality is less than N. It is attempted to achieve a reduction to two or three dimensions so that the human mind's limited dimensional perception abilities are sufficient for visualizing similarities or dissimilarities in the analyzed data sets. To fulfil this purpose, not the whole measurement curve of each of the individual 16 sensors is taken into account. Fig. 1 displays in an exemplary manner the data of the QMB and the MOX modules. Instead, only a characteristic parameter (called “feature”) describing one of the curve's properties, e.g., signal height, area under the curve, etc., serves as input for the PCA and is selected upon statistical evaluation (Student's t test). PCA is an unsupervised orthogonal projection algorithm, which means that no input from the operator is needed beyond the raw sensor data. It focuses only on classification tasks; quantitative statements cannot be done by this technique. (A more detailed description about PCA can be found in ref. 18 and at http://nose.uia.ac.be.)
Figure 1.
Measurement curves of MOX (a) and QMB (b) sensors. Both indicate the characteristic curve parameter (here, maximum height of the curve), which served as input for the PCA.
GC/MS.
GC/MS (19) was performed on a Hewlett–Packard gas chromatograph (HP 6890 GC) and a Hewlett–Packard mass selective detector (HP 5973 MSD). The capillary column was 0.32 mm i.d. with an intermediate polarity polysiloxane phase (HP-VOC, Hewlett–Packard). The stationary-phase film thickness was 1.8 μm, and the column's length was 60 m. As carrier gas, helium (purity 6.0, Messer-Griesheim, Krefeld, Germany) was used. The GC/MS system was coupled to a Hewlett–Packard headspace autosampler (HP 7694 HSS) applying the static headspace technique. With the autosampler, 44 samples can be analyzed in one measurement run. The fully automated and reproducible sample uptake is maintained by the carrier gas supply through a thermostated injection needle, sample loop (volume 1 ml), and transfer line to the GC inlet. The mass spectrometer was operated in the scan mode; the mass fragments (m/z) were recorded in the range of 33 to 220 atomic mass units. The molecule fragments were formed by electronic ionization; the electron energy was 70 eV.
Results
Experimental Set-Up.
The e-nose is an array of eight QMBs and eight semiconducting MOXs (13). Molecular components in the gas phase are allowed to pass by the sensors and thus change some of their physical parameters (here, frequency in the case of the QMBs or conductivity for the MOXs). These changes are recorded and analyzed by a computer program as a 16-dimensional signal complex. The device is not able to detect the identity of molecules (i.e., exact chemical structure). However, the device is able to tell the difference in the molecular composition of different substances by a different pattern in the 16-dimensional signal complex. The e-nose thus compares patterns derived from different samples. A PCA serves as an appropriate pattern recognition algorithm. The PCA searches for the sensor signals providing the largest difference between two or more samples (referred to as primary component number 1) and does the same for the second largest difference, and so on. The relative magnitude of the signal for the first and the following components is given in percent. Usually, only the first two components are considered. The differences are then compared in arbitrary units and are plotted in two dimensions; the graphic presentation is commonly called scores plot. The selection of the features serving as input for the PCA was based on a statistical evaluation related to the Student's t test with a confidence level of 95%. The results of the Student's t test are used to determine whether differences of samples are random or not. In total, 153 individual samples consisting of pooled urine from 1–6 mice were investigated with the sensor array. In the case of the MOX sensors, the area under the measurement curve was the most reliable parameter expressing the difference between the samples; in 85% of all cases, the 95% confidence limit requirement was fulfilled. Taking the height of the MOX curves into account, the reliability amounts to 78%. Compared with the MOX sensors, the QMBs hardly contribute to the differentiation of the samples.
This type of e-nose is typically used for the objective detection of odors in foodstuff and other items of forensic importance (20–23). The e-nose, for example, is able to qualitatively distinguish between different kinds of olive oils (24), different brands of coffee (25), and different beers (26), and also can distinguish the smell of car interiors (27). The philosophy behind the e-noses is not to replace well established techniques such as GC/MS. They are not able to describe an odor by certain attributes (e.g., fruity, green, rancid, etc), nor can they state the composition of the evaluated odor. Gas sensor arrays have to be recognized as complementary tools for particular practical applications, which allow a rapid screening and are normally easy to handle. According to the application, the transducers and their sensitive layers can be selected. In all cases, an accurate optimization of the measuring conditions (sample collection, temperature and humidity control, working conditions of the sensors) is acquired. In addition, an appropriate recalibration procedure has to be developed to assure the comparability of temporally different data because sensor long-term drifts or fluctuations are still an unavoidable problem if using e-noses over a longer period.
Odor Differences Between Male and Female Urine.
To obtain first insights into the sensitivity of the e-nose toward different odor components of mouse urine, we analyzed the urine of male and female C57BL/6 mice, which are expected to smell differently. The results clearly indicated that the e-nose is able to detect the difference (data not shown). The odor components showing the most marked difference between the sexes account for 79.1% of the sensor signals and are very similar for the different individual samples tested. To exclude sexual influences on odor types, all of the following experiments were performed with male urine (unless stated otherwise).
Odor of Unrelated H2 Haplotypes.
Urine of mice expressing unrelated H2 haplotypes was tested. The e-nose could clearly differentiate between BALB/c (H2d), BALB.B (H2b), and C3H (H2k) urine (Fig. 2). All of these strains differ in their principal component (86.4%); C3H differs from the two BALB strains also in the second component. Because BALB/c and BALB.B mice differ only in the MHC region, the results indicate already that H2 genes influence odor types profoundly, as detected by the e-nose. To substantiate this notion, a number of H2 congenic strains were analyzed (Fig. 3). B6 (alleles at loci K, A, E, D:bbbb) and B10.A (kkkd) mice clearly differed in the principal component (96.9%, 94.5%, 96.0%, or 90.8%). The same is true for the difference between B6 and B10.D2 (dddd). On the other hand, B10.A, B10.D2, and B10.BR (kkkk) are closer together but are still different from each other (Fig. 3 b–d), although in some experiments this is the case only in the second component. Thus, H2 genes contribute to the principal odor component detectable by the e-nose. Because B10.BR (kkkk) and B10.A (kkkd) express the same H2 class II genes but differ in class I, it can be concluded that class I gene products contribute to the odor type.
Figure 2.
E-nose measurement of urine from H2-different mice. Scores plot of a PCA. The x axis shows principal odor component 1; the y axis shows principal component 2 (arbitrary units). Each dot represents a pool of urine from five [BALB/c (H2d) and BALB.B (H2b)] or six [C3H (H2k)] male mice collected during 3 nights. Two samples from each pool were analyzed; the two dots representing one pool are marked by an ellipse.
Figure 3.
E-nose measurement of urine from H2-congenic mice. (a) Urine pools of five male C57BL/6 and five male B10.A collected during 2 nights; (b–d) three independent measurements of urine from male B10.A (kkkd), B10.D2 (dddd), B10.BR (kkkk), and C57BL/6 (bbbb) mice. (b) Pools of urine of five males from each strain collected during 5 nights; (c) urine of five males from each strain collected during 4 nights; (d) urine of five males from each strain collected during 2 nights.
Odor of F1, Mutant, and Transgenic Mice.
Next, the dominance of H2-dependent odor types was tested. As depicted in Fig. 4 a and b, the urine of (B6 × BALB/c) F1 mice scored between both parental strains in relation to the principal component 1. Thus, odor type expression appears to be codominant. To clearly attribute the odor-type influence to MHC class I genes, the urine of B6.C-H2bm1 (different from B6 only at three amino acid exchanges in the H2-K gene) and transgenic mice expressing α1 and α2 domains of the human HLA-A2 heavy chain was tested. In both cases, the primary component (74.7% and 99.0%, respectively) indicated a strong difference (Fig. 4 c and d).
Figure 4.
E-nose measurement of F1, H2 class I mutant, and HLA-A2 transgenic mice. (a) Pools of urine from two female (C57BL/6 × BALB/c)F1, five female C57BL/6, and five female BALB/c collected during 2 nights; (b) pools of urine from two female (C57BL/6 × BALB/c)F1, five female C57BL/6, five female BALB/c, and five female C3H collected during 4 nights; (c) pools of urine from four male B6.C-H2bm1, four male C57BL/6, and five male B10.D2 collected during 4 nights; (d) pools of urine from eight male HLA-A2A2KbH2−/− mice and eight male C57BL/6 collected during 1 night.
Odor Components in Serum.
Because the odor type is detected in urine, it is likely that the responsible substances are also found in blood. Indeed, odor components from serum of BALB.B, BALB/c, and B6 mice are clearly distinguished by the e-nose (Fig. 5a). All three samples differ at the principal component (94.4%). A strong difference between BALB.B and B6 indicates a profound influence of non-MHC genes in addition to MHC, as also observed with urine samples of BALB.B and B6 (data not shown).
Figure 5.
E-nose measurement of mouse and human serum. (a) Serum of BALB.B, BALB/c, and B6 mice (punction of one mouse per strain); blood was split into two samples of 150 μl each. (b) Serum of eight HLA homozygous male individuals; serum was split into two samples.
HLA-dependent odors are suspected to be contained in human sweat. However, the data leading to this conclusion are not very convincing (9). To test whether the e-nose could help solve this problem, and because the serum of mice does contain MHC-dependent odor components, we analyzed the serum of eight HLA homozygous male individuals (Fig. 5b). Each sample was tested twice to control for reproducibility of the assay. The e-nose indeed could distinguish between the eight individual odor types by primary and secondary components. Three of the individuals were of the HLA-A1, HLA-B8 haplotype. They were similar with regard to the second component but different in the first. Thus, these preliminary data show that individual human odor types can be detected by the e-nose in serum. The odor type appears to be influenced by non-MHC as well as MHC genes. Because, in contrast to laboratory mice, the diet of these individuals was not controlled and therefore probably rather different, food intake is likely to be a third major source of odor component difference.
GC/MS Analysis of Volatile Substances.
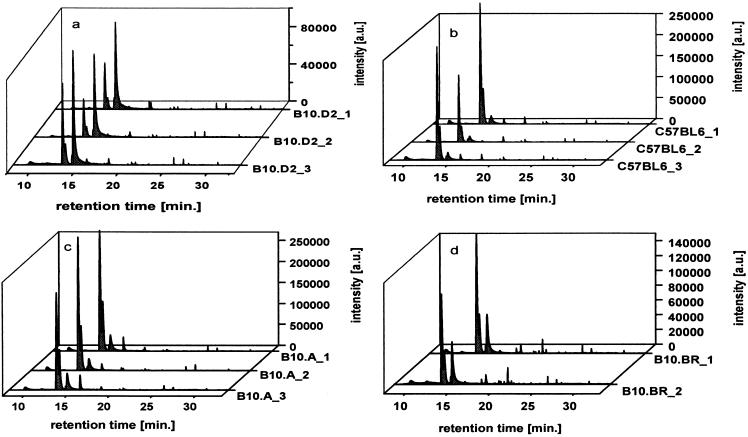
To get an insight into the identity and complexity of the volatile substances measured by the e-nose, the headspaces of urine from four different H2 congenic mouse strains (B10.D2, B6, B10.A, and B10.BR) were subjected to GC, and the separated compounds were detected by MS (Fig. 6). A number of individual components could be detected and partially identified. Similar to previous work (28) that analyzed other mouse strains (B6 and B6-H2k), we did not find obvious signals that are present in one but absent in the other strain. It has to be stressed, however, that the analytical approach used by Singer et al. (28) and the headspace technique presented here differ to a large extent. Especially different are the classes of compounds that can be detected. Headspace analyses naturally focus on volatile components, whereas substances of low volatility such as organic acids are hardly identified. We do confirm, however, that the ratio of volatile substances differs according to H2 expression. For example, the ratio of the peak at 13 min (3-methylbutanal) and the peak at 14 min (2-pentanone) is about 1:1 in B10.D2 (Fig. 6a) but about 20:1 in B6 (Fig. 6b), 10:1 in B10.A (Fig. 6c), and 3:1 in B10.BR (Fig. 6d). As a well established analytical technique, the GC/MS analyses indicate the reproducibility of sample handling. Thus, the results can be correlated with those of the e-nose measurements.
Figure 6.
GC/MS measurements of urine from H2-congenic strains. Chromatograms of urine pools from four male mice per strain collected during 4 nights. (a) B10.D2, three samples of the pool; (b) C57BL/6, three samples; (c) B10.A, three samples; (d) B10.BR, two samples.
Discussion
Our e-nose distinguishes the odor types of different mouse strains. As expected, the sexes smell differently. If only sex-matched mice are considered, dominant components of the odor-type are MHC-dependent. Another important contribution comes from non-H2 genes. Thus, our approach allows for the objective analysis of odor types independent of behavioral studies with either mice trying to reach a sexual partner or with trained rats that have learned that a certain reaction to a particular odor is awarded by a drink or foodstuff. Our data are in agreement with the behavioral results obtained during the last 20 years, led by Boyse and colleagues (6, 8, 28). Our results demonstrate that H2 congenic and mutant mouse strains do smell differently; for example, B6, B10.A, B10.D2, B10.BR, and B6bm1 all are typed as being different by the e-nose. Thus, H2 class I gene products contribute to odor type (29). This does not indicate, however, that class II has no influence. In addition, non-H2 genes influence the odor type significantly. Most interestingly, the e-nose-detected odor type is present not only in urine but also in serum. For many years, it has been impossible to obtain this information from behavioral studies. Only recently has it been shown that mice are able to distinguish the odor of protease-treated and diluted serum (30). Our e-nose data confirm and extend these results. One caveat here, however, is that we cannot be sure that the MHC-dependent substances recognized by the e-nose are identical to those used by mice to differentiate between the odor types. This problem could be solved once the respective substances are identified (see below).
For humans, HLA-dependent odor is even more difficult to detect. A study suggested that female students prefer the odor of worn T-shirts from HLA different male colleagues (9). Convincing in this study is that women taking the contraceptive pill or during menses do not like the smell of sweaty male T-shirts; influences of HLA on odors were, however, not impressive. Our e-nose is able to tell the difference between individual odor types as present in human serum and thus will allow a larger study to investigate a possible HLA dependency of human odor type.
Our data now allow for a biochemical approach to study the influence of genetically determined odor types on social behavior, including that of humans. Samples determined by the e-nose to differ strongly in odorous substances can now be further analyzed by sensitive molecular identification methods, such as GC followed on-line by MS. Gas sensor arrays can be considered as a kind of screening instrumentation that provides the researcher with rather simple analytical information, e.g., the discrimination of chemically different samples, or the assignment of an unknown sample to a certain class/strain. Because the analyses carried out with e-noses are highly automated (which is also true for the subsequent data evaluation), this technique can be considered as very user-friendly and time-saving. The results of Singer et al. (28) as well as our own results presented here indicate that the abundant volatile substances of urine have the same identity in mice of different MHC haplotypes and show that their relative quantities depend on MHC expression. For example, the peaks for 3-methylbutanal and 2-pentanone occur in a ratio of 1:1 in B10.D2 mice but 20:1 in B6 (Fig. 6 a and b). Thus, the mouse nose and the e-nose might record these ratio differences as a particular odor trait. On the other hand, however, it is possible that the presence of less abundant substances that are more difficult to detect depend on the expression of a particular MHC allele. Indeed, we found evidence for such a substance present in HLA-A2 transgenic mice but absent in their nontransgenic counterparts. The identification of substances that depend on a single gene—in their absolute presence or in their relative quantity—will allow the identification of the relationship between the gene product and the odorous substance. For MHC-dependent odor types, there might be catabolic derivatives from the MHC protein itself, from the peptides bound to it, or from molecules influenced secondarily by MHC expression (30–32). Such molecules could be derived from environmental viruses or bacteria, as MHC expression influences the immune response against these organisms. However, as detected with behavioral studies (8), the odor type of germ-free mice is not different from that of mice living in a normal environment. MHC-influenced molecules other than environmental antigens are T-cell receptors, which are selected on MHC molecules in the thymus, and all of the peptides not binding to the MHC molecules in a cell because these peptides are not protected from rapid degradation.
Thus, our studies might help to identify the odorous substances characterizing individuality. The possibility to detect such substances by our e-nose should help to analyze the influence of genetically different odor types on our societies. It might well be that primary influences on mate choice, for example, cannot be found any more because other sociocultural influences, extended use of perfumed cosmetic products, and inhibitors of sweating overlay the inherent individual odor type. A person's odor still might have secondary influences that are at work in the long run, as precipitated, for example, in divorce rates. Thus, studying the extent of e-nose-detectable odor type differences, or better substances responsible, in couples living in stable relationships vs. divorces might help to solve this issue.
Acknowledgments
We thank G. Nicholson for initial GC experiments, D. Roopenian for mouse urine samples for initial experiments, J. Klein and R. Apfelbach for critically reading the manuscripts and comments, and M. Wandel and L. Yakes for editorial assistance. This work was supported by the Deutsche Forschungsgemeinschaft (Leibniz program to H.-G.R.) and by Lennartz Electronic (Tübingen, Germany) through the donation of a MOSES instrument.
Abbreviations
- PCA
principal component analysis
- MOX
metal oxide
- QMB
quartz microbalance
- e-nose
electronic nose
References
- 1.Rammensee H G, Falk K, Rötzschke O. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- 2.Rammensee H G. Curr Opin Immunol. 1995;7:85–96. doi: 10.1016/0952-7915(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 3.Rammensee H G, Bachmann J, Stevanovic S. MHC Ligands and Peptide Motifs. Heidelberg: Springer; 1997. [Google Scholar]
- 4.Falk K, Rötzschke O, Stevanovic S, Jung G, Rammensee H G. Nature (London) 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 5.Klein J, Satta Y, O'hUigin C, Takahata N. Annu Rev Immunol. 1993;11:269–295. doi: 10.1146/annurev.iy.11.040193.001413. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki K, Beauchamp G K, Shen F-W, Bard J, Boyse E A. Proc Natl Acad Sci USA. 1994;91:3735–3738. doi: 10.1073/pnas.91.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penn D, Potts W. Adv Immunol. 1998;69:411–436. doi: 10.1016/s0065-2776(08)60612-4. [DOI] [PubMed] [Google Scholar]
- 8.Yamazaki K, Beauchamp G K, Imai Y, Bard J, Phelan S P, Thomas L, Boyse E A. Proc Natl Acad Sci USA. 1990;87:8413–8416. doi: 10.1073/pnas.87.21.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wedekind C, Seebeck T, Bettens F, Paepke A J. Proc R Soc London Ser B. 1995;260:245–249. doi: 10.1098/rspb.1995.0087. [DOI] [PubMed] [Google Scholar]
- 10.Hedrick P W, Black F L. Am J Hum Genet. 1997;61:505–511. doi: 10.1086/515519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ober C, Weitkamp L R, Cox N, Dytch H, Kostyu D, Elias S. Am J Hum Genet. 1997;61:497–504. doi: 10.1086/515511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wobst B, Zavazava N, Luszyk D, Lange K, Ussat S, Eggert F, Ferstl R, Muller-Ruchholtz W. Genetica. 1998–1999;104:275–283. doi: 10.1023/a:1026487421626. [DOI] [PubMed] [Google Scholar]
- 13.Mitrovics J, Ulmer H, Weimar U, Göpel W. Acc Chem Res. 1998;31:307–315. [Google Scholar]
- 14.Bârsan N, Stetter J R, Findlay J, Göpel W. Anal Chem. 1999;71:2512–2517. doi: 10.1021/ac981246d. [DOI] [PubMed] [Google Scholar]
- 15.Hierlemann A, Schweizer-Berberich M, Weimar U, Kraus G, Pfau A, Göpel W. In: Sensors Update Sensor Technology: Applications–Markets. Baltes H, Göpel W, Hesse J, editors. Weinheim: VCH; 1996. pp. 119–180. [Google Scholar]
- 16.Kolb B, Ettre L S. Static Headspace-Gas Chromatography: Theory and Practice. New York: Wiley; 1997. [Google Scholar]
- 17.Manly B F J. Multivariate Statistical Analysis. London: Chapman & Hall; 1986. [Google Scholar]
- 18.Jurs P C, Bakken A, McClelland H E. Chem Rev. 2000;100:2649–2678. doi: 10.1021/cr9800964. [DOI] [PubMed] [Google Scholar]
- 19.Hübschmann H J. Handbuch der GC/MS –Grundlagen und Anwendung. Weinheim: VCH; 1996. [Google Scholar]
- 20.Schweizer-Berberich M, Vaihinger S, Göpel W. Sens Actuators B. 1994;18–19:282–290. [Google Scholar]
- 21.Stetter J R, Findlay M W, Schroeder K M, Yue C, Penrose W R. Anal Chim Acta. 1993;284:1–11. [Google Scholar]
- 22.Persaud K C, Travers P J. In: Handbook of Biosensors and Electronic Noses. Kress-Royers E, editor. Boca Raton, FL: CRC; 1996. pp. 563–592. [Google Scholar]
- 23.Di Natale C, Macagnano A, Paolesse R, Mantini A, Tarizzo E, D'Amico A, Sinesio F, Bucarelli F M, Moneta E, Quaglia G B. Sens Actuators. 1998;50:246–252. [Google Scholar]
- 24.Gonzalez-Martin Y, Perez-Pavon J L, Moreno B. Anal Chim Acta. 1999;384:83–94. [Google Scholar]
- 25.Gardner J W, Sturmer A V, Tan T T. Sens Actuators B. 1992;6:71–75. [Google Scholar]
- 26.Gardner J W, Bartlett P N. In: Olfaction and Taste XI. Kurihara K, Suzuki N, Ogawa A, editors. Tokyo: Springer; 1994. pp. 690–693. [Google Scholar]
- 27.Kalman E L, Löfvendahl A, Winquist F, Lundström I. Anal Chim Acta. 2000;403:31–38. [Google Scholar]
- 28.Singer A G, Beauchamp G K, Yamazaki K. Proc Natl Acad Sci USA. 1997;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bard J, Yamazaki K, Curran M, Boyse E A, Beauchamp G K. Immunogenetics. 2000;51:514–518. doi: 10.1007/s002510000165. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi M, Yamazaki K, Beauchamp G K, Bard J, Thomas L, Boyse E A. Proc Natl Acad Sci USA. 1981;78:5817–5820. doi: 10.1073/pnas.78.9.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamazaki K, Beauchamp G K, Singer A, Bard J, Boyse E A. Proc Natl Acad Sci USA. 1999;96:1522–1525. doi: 10.1073/pnas.96.4.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown R E, Roser B, Singh P B. Behav Genet. 1989;19:659–674. doi: 10.1007/BF01066029. [DOI] [PubMed] [Google Scholar]