Abstract
Duvelisib, an oral dual inhibitor of PI3K-δ and PI3K-γ, is in phase III trials for the treatment of chronic lymphocytic leukemia (CLL) and indolent non-Hodgkin’s lymphoma (iNHL). In CLL, duvelisib monotherapy is associated with high iwCLL and nodal response rates, but complete remissions are rare. To characterize the molecular effect of duvelisib, we obtained samples from CLL patients on the duvelisib phase I trial. Gene-expression studies (RNA seq, Nanostring, Affymetrix array, and real time RT-PCR) demonstrated increased expression of BCL2 along with several BH3-only pro-apoptotic genes. In concert with induction of transcript levels, reverse phase protein arrays and immunoblots confirmed increase at the protein level. The BCL2 inhibitor venetoclax induced greater apoptosis in ex-vivo cultured CLL cells obtained from patients on duvelisib compared to pre-treatment CLL cells from the same patients. In vitro combination of duvelisib and venetoclax resulted in enhanced apoptosis even in CLL cells cultured under conditions that simulate the tumor microenvironment. These data provide a mechanistic rationale for testing the combination of duvelisib and venetoclax in the clinic. Such combination regimen (NCT02640833) is being evaluated for patients with B-cell malignancies including CLL.
Keywords: IPI-145, ABT-199, CLL, combination, BCL2 protein
Introduction
Chronic lymphocytic leukemia (CLL) is an indolent B-cell neoplasm that primarily affects older adults. In 2015, there were an estimated 14,620 new CLL cases and 4,650 deaths in the United States (http://seer.cancer.gov/). CLL is characterized by clonal expansion of CD5+ B-cells in the blood and lymphoid tissues.1, 2 Malignant lymphocytes accumulate partly due to activation of B-cell receptor (BCR) signaling leading to increased proliferation and inhibition of apoptosis.3 In addition to BCR signaling, CLL cells are supported also by the tumor microenvironment, including extensive cytokine and chemokine signaling with T-cells, myeloid cells, and stromal cells.4
BCR pathway signaling seems to be antigen-dependent and antigen binding causes receptor aggregation and phosphorylation of ITAM’s in CD79A and CD79B, which then activates downstream kinases such as SYK, Bruton’s tyrosine kinase (BTK) and PI3K.5 The two PI3K isoforms PI3K-δ and PI3K-γ are preferentially expressed in normal and malignant cells of hematopoietic origin and play an important role in the pathogenesis of CLL, both in malignant cells and in the tumor microenvironment.6 Duvelisib is an oral, small molecule dual inhibitor of PI3K-δ and PI3K-γ.7 Our prior pre-clinical investigations in primary CLL lymphocytes demonstrated that duvelisib inhibited BCR signaling, diminished chemotaxis, reduced pseudoemperipolesis, inhibited cytokine-induced CLL cell proliferation through inhibiting the PI3K-AKT signaling pathway with minimal apoptosis.8 Furthermore, in vitro studies have demonstrated that pharmacological inhibition of PI3K-γ reduces the tumor promoting capabilities of T-cells and myeloid cells in the CLL microenvironment.9 In a phase 1 trial in advanced hematologic malignancies (IPI-145-02; ClinicalTrials.gov: NCT01476657), duvelisib exhibited clinical activity in several indications including CLL10 which was similar to other BCR axis inhibitors such as ibrutinib11, acalabrutinib12, and idelalisib13, 14 indicating importance of this pathway.
A key feature of BCR pathway inhibition in CLL patients is an early and dramatic reduction in lymph node size with a parallel increase of CLL cells in circulation.10–13 BCR inhibitors may also inhibit signalling directly downstream of integrins/chemokine receptors, and this could contribute to lymphocytosis, independent of any effects on the BCR. BCR pathway inhibition does not induce cell death directly but impairs BCR-associated integrin-mediated adhesion and migration leading to CLL cell efflux from lymphoid tissues to peripheral blood8. Nevertheless, the majority of patients treated with BCR pathway inhibitors as monotherapy exhibit a relatively low frequency of complete responses.11, 13, 15–17 Furthermore, prolonged treatment may allow for selection of resistant clones. For example, a proportion of patients on ibrutinib eventually developed resistance due to mutations at the ibrutinib-binding site on BTK (C481) or downstream pathway mutations such as in PLCγ2.18 Thus, opportunities may exist to improve outcomes by deepening response and targeting potential resistance pathways via combination therapies.
The high percentage of malignant cells in the peripheral blood of CLL patients and the treatment-induced lymphocytosis provide the opportunity to easily access tumor cells and explore molecular changes that occur in CLL cells due to duvelisib therapy. The mechanism of BCR inhibitor action appears to be primarily through inhibition of CLL cell proliferation and alteration of the tumor microenvironment. However, cell survival is also a central aspect of CLL pathogenesis. The anti-apoptotic BCL2 protein is highly expressed in CLL and inhibits the activity of pro-apoptotic BH3-only family members.19, 20 In vitro studies have demonstrated that inhibition of PI3K-δ with idelalisib results in apoptotic priming of CLL cells.21 We hypothesized that despite minimal overt CLL cell apoptosis in patients treated with duvelisib, there may nevertheless be effects on apoptotic priming.
To test this hypothesis, we examined the expression of BCL2-family apoptotic regulators in CLL cells of patients enrolled in a phase I trial of duvelisib using various gene expression (RNAseq, Nanostring, Microfluidic chip qRT-PCR, and real-time RT-PCR) and protein expression (reverse phase protein array (RPPA), immunoblots) techniques. We discovered that the anti-apoptotic protein BCL2 and several pro-apoptotic BH3-only family members were upregulated on duvelisib therapy, suggesting a model in which upregulation of pro-apoptotic BH3-only genes might be offset by induction of the anti-apoptotic gene BCL2. We then demonstrated that previous duvelisib treatment enhanced the ability of the BCL2 inhibitor venetoclax (ABT-199) to induce apoptosis in CLL cells. These data provide a mechanistic rationale for investigating the combination of duvelisib and venetoclax in clinical trials.
Materials and Methods
Samples from CLL patients used in this study
Samples during duvelisib therapy
Peripheral blood samples were obtained from patients with CLL enrolled on the phase 1 clinical trial of duvelisib receiving dose ranging from 8–75 mg BID (study IPI-145-02; ClinicalTrials.gov: NCT01476657). The study enrollment completed in January 2014 and included 55 patients with relapsed/refractory (R/R) CLL and 18 patients with treatment-naïve disease. From these patients, 42 paired (baseline and after 7 days of therapy C1D8) were used for RNA seq and nanostring assays (Supplemental Table 1). For RT-PCR, microfluidic chip, RPPA and immunoblot assays, baseline and C1D28 samples were obtained from 16 patients that were treated at MDACC (Supplemental Table 2). These samples were also used for ex vivo incubations with ibrutinib, idelalisib, duvelisib, and venetoclax. Generally, for patients treated with duvelisib therapy, lymphocytosis was observed by week 1 and was maintained almost in all patients until week 4. We selected these time points to determine early and late changes in mRNA and protein levels.
Other CLL samples
For in-vitro study, freshly isolated peripheral blood samples were obtained from CLL patients who were not on duvelisib clinical trial (n=5).
All patients signed written informed consent forms in accordance with the Declaration of Helsinki, and the protocols were approved by the institutional review boards of all clinical sites, including MDACC.
Isolation of CLL cells
Lymphocytes were isolated from PBMCs and cultured in RPMI-1640 medium (HyClone Laboratories, UT) as described before8 but with 10% human serum (Sigma-Aldrich, MO). CLL cells had a purity above 90% and were seeded at 1 × 106 cells per well in 24-well plates. All experiments were performed with fresh samples, and a time-matched control incubated with vehicle (DMSO) was used for each sample.
Gene expression analysis
For RNAseq experiments, samples were available from 42 CLL patients on the IPI-145-02 trial at baseline (pre-treatment) and C1D8. RNA was extracted using the Direct-zol Miniprep kit (Zymo Research, #R2050). The samples were shipped to Expression Analysis Inc. (Morrisville, NC) for further processing and sequencing. Based on data quality filters, 31-paired samples were suitable for further analyses. Detailed procedure, data alignment, processing, and analysis are described in the supplemental methods. For further validation, the CLL whole blood RNA sample pairs (baseline and C1D8) were also sent to Labcorp Inc. (Seattle. WA) for gene expression analysis of a custom-made gene panel using NanoString’s nCounter GEx technology.22
To determine mRNA expression of the BCL2 family at baseline and C2D1 in the MDACC subset of CLL patients, a TaqMan Human Apoptosis Array (microfluidic card; Applied Biosystems) was used.23 To further verify, Real-time RT-PCR assay was done in the same 5 samples.
Protein analysis
To determine the protein expression of the BCL2 family, reverse phase protein arrays (RPPA) were performed (n=16) at MDACC Core facility. The first RPPA batch (n=7 samples) tested 141 proteins including the BCL2 family members - BAD.pS112, BAX, BCL-XL, BCL2, and BIM. The second batch (n=9 samples) tested 300 proteins including the additional BCL2 family members BAD.pS155, BAK, BFL1, BID, MCL1, and PUMA. For further validation, immunoblots were performed as described,24 (Supplemental Table 3). Protein band quantification was done using a LI-COR Odyssey CLx Infrared Imaging System.
Ex-vivo CLL cell culture
CLL cells isolated from blood of patients during duvelisib therapy were treated with various pharmacological agents for 24 hours. Sources of these agents are listed in Supplemental Table 3. Cell viability was measured through Annexin V/PI assay24 followed by flow cytometry.
In vitro combination experiments
For in-vitro study, freshly isolated peripheral blood samples were obtained from CLL patients who were not on duvelisib clinical trial (n=5). CLL cells were seeded at 1 × 106 cells/well and treated with either DMSO, 10 μg/mL IgM or a cytokine cocktail containing 1μg/mL sCD40L, 10 ng/mL IL-10, and IL-2 for 4 days8. Our previous study in CLL cells has demonstrated activation of BCR pathway through these stimulation and inhibition of this activation by duvelisib.8 Cells were either untreated or treated with duvelisib for 4 days. Each culture was treated with or without 0.5–10 nM ABT-199 for 12–16 hours and apoptosis was measured. Cell viability was measured through Annexin V/PI assay.24
Statistical analysis
Correlations were derived using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). Statistical tests are indicated in the figure legends. P values were determined using an unpaired or paired student’s t-test. Statistical analyses are based on linear values and for which log 2 data was converted to fold change except for RNAseq and nanostring data where log2 values were compared for statistical analyses. Since same assays were used for statistical comparison, the variance is expected to be similar between the groups that are being statistically compared.
Results
Duvelisib therapy resulted in peripheral blood lymphocytosis
Similar to ibrutinib11 and idelalisib 13, duvelisib also induced lymphocytosis in CLL patients10. In a subset of patients treated at MDACC both absolute lymphocyte count (Figure 1A; n=17) and white blood cell count (Figure 1B; n=17) increased in 14 of 17 individuals after one cycle (28 days) of duvelisib therapy. In three patient samples (#733, #317, #228 Figure 1), one cycle of duvelisib therapy resulted in a decrease in peripheral blood count and total CLL cells. However, for #733 and 317, there was an increase in the lymphocyte count on week 1, followed by a decline. In contrast, for #228, there was a continuous decrease (week 1 onward) (Supplemental Figure 1). The pattern of lymphocytosis was not related to any prognostic or cytogenetic features (Supplemental Table 2).
Figure 1.
Effect of oral duvelisib therapy on peripheral blood absolute lymphocyte count (ALC) and white blood cell (WBC count). Patients with CLL received oral duvelisib therapy. Peripheral blood samples were obtained prior to therapy (baseline) and 28 days after start of therapy. Absolute lymphocyte count and WBC were measured. (A) Changes in ALC and (B) WBC counts from 17 CLL patients on oral duvelisib therapy were plotted prior to treatment (C1D1; cycle 1 day 1) and on day 28 (C2D1; cycle 2 day 1).
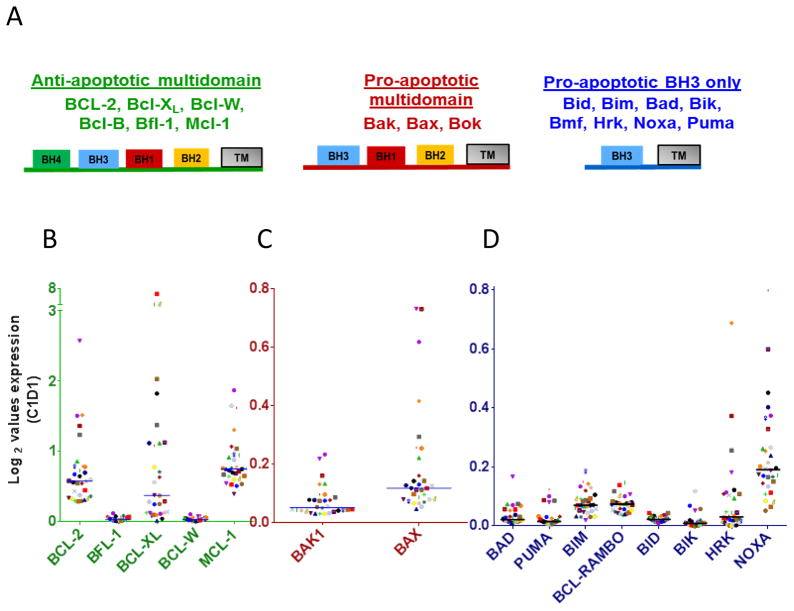
CLL lymphocytes express differential levels of anti- and pro-apoptotic transcripts of BCL2 family members
The BCL2 family plays a major role in CLL pathogenesis and prolonged survival of CLL cells 25,26. This family is divided into anti-apoptotic, pro-apoptotic multi-domain, and pro-apoptotic BH3-only domain subfamilies (Figure 2A). To determine and compare mRNA levels of each Bcl-2 family member, we examined the relative expression levels at baseline by RNA seq analyses. Among the BCL2 family anti-apoptotic members, BCL2, BCL-XL, and MCL1 had higher expression values compared to other anti-apoptotic members (Figure 2B). These three survival proteins mRNA expression level ranged between 0.3 – 2.5 (BCL-2), 0.02 – 6.6 (BCL-XL) and 0.4 – 1.9 (MCL-1). In contrast, multidomain pro-apoptotic family members BAK1 and BAX were always less than 0.8 and between these two BAX was slightly higher (Figure 2C). Among BH3-only proapoptotic family members, NOXA had the highest baseline expression (median 0.19; range 0.05 to 0.80) followed by BIM (median 0.07; range 0.02 to 0.19) and BCL-RAMBO (median 0.07; range 0.04 to 0.15) (Figure 2D). However, all of the BH3 only members were expressed overall at low levels (always less than 0.8) suggesting dominance of BCL-2 family anti-apoptotic transcripts in the CLL cellular milieu prior to duvelisib treatment.
Figure 2.
Base-line expression levels of anti-apoptotic and pro-apoptotic Bcl-2 family transcripts in primary CLL lymphocytes. (A) Schema of BCL2 sub-families reflecting anti-and pro-apoptotic molecules. (B) Anti-apoptotic Bcl-2 family members (C) Pro-apoptotic multi-domain and (D) Pro-apoptotic BH3 only protein transcripts in peripheral blood CLL cells. CLL cells were obtained prior to any therapy and transcript levels were measured in isolated RNA through RNAseq method as described under Methods and Supplementary Material. The transcripts that are not shown were not in the available assay.
Duvelisib therapy is associated with gene expression changes in BCL2 family members
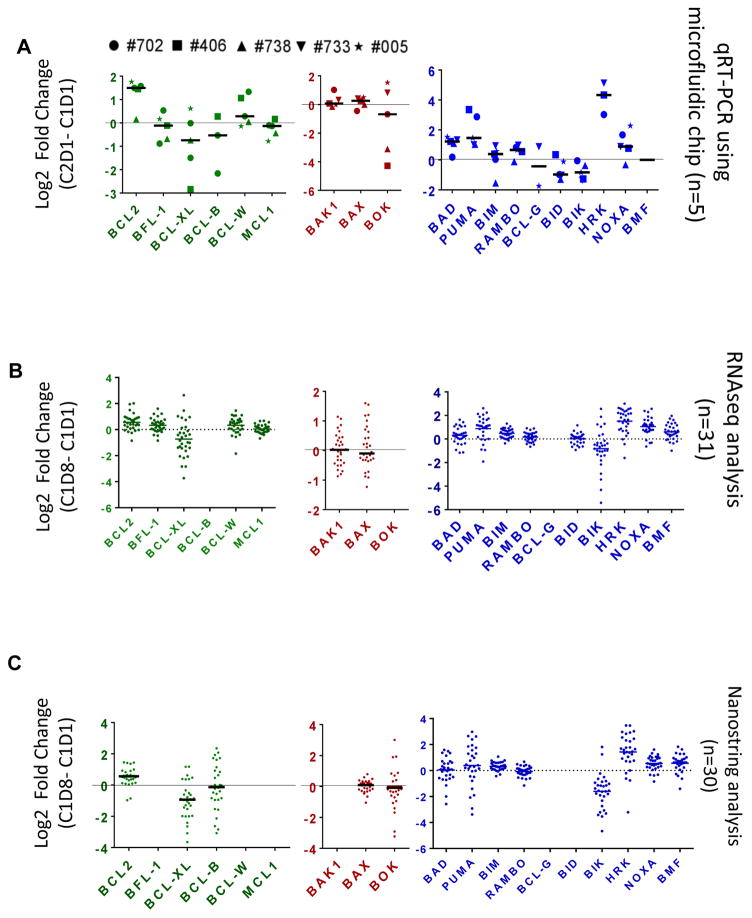
To test whether duvelisib treatment altered expression of BCL2 family members, microfluidic chip qRT-PCR was performed on RNA isolated from peripheral blood cells of 5 R/R CLL patients at baseline and after 28 days of treatment. Six BCL2 family transcripts exhibited a statistically significant change from baseline (Figure 3A, Supplemental Table 4). The anti-apoptotic gene BCL2 exhibited increased expression after duvelisib treatment (mean fold ± SEM: 2.81±0.4; p=0.0020; n=5). In parallel, BAD, BIM, BCL-RAMBO, and HRK displayed an increase in expression on duvelisib treatment while BIK exhibited decreased expression. No statistically significant change was observed in mRNA levels of multidomain pro-apoptotic proteins. The increase in BCL2 transcript level was further confirmed in five patient samples using a real-time RT-PCR assay (Supplemental Figure 2).
Figure 3.
Effect of oral duvelisib therapy on expression of transcripts of BCL2 family in CLL lymphocytes after 7 days (C1D8; cycle 1 day 8) and 28 days (C2D1; cycle 2 day 1) of oral duvelisib intake. (A) Real-Time Quantitative Reverse Transcription PCR (qRT-PCR) using microfluidic chip analysis showing fold change in BCL2 family mRNA expressions before and after 28 days of duvelisib therapy from 5 patient samples. (B) RNAseq analysis and (C) Nanostring quantitation showing log2 fold change in BCL2 family mRNA expressions in CLL B cells isolated from patients (n=31) before and after 7 days of duvelisib therapy. CLL cells were obtained before and after duvelisib therapy and transcript levels were measured in isolated RNA through different methods as described under Methods and Supplementary Material. Detailed information regarding median value and p values is provided in the Supplemental Table 4. The transcripts that are not shown were not available in the assay.
To validate these findings, BCL2 family expression was examined in a larger sample set of 31 CLL patients enrolled on the clinical trial using two assays. First, RNAseq was performed, and normalized FPKM (fragments per kilobase of exon per million fragments) for BCL2 family genes was examined for change from baseline after 8 days of treatment. Similar to the microfluidic qRT-PCR, the 31 patient sample set exhibited increased expression of BCL2, PUMA, BIM, HRK, and NOXA as well as decreased expression of BIK with duvelisib treatment (Figure 3B, Supplemental Table 4). In contrast to the microfluidic chip data, BFL-1, BCL-W, and BMF exhibited increased expression, while BCL-XL decreased.
As an orthogonal approach to confirm the RNAseq findings, the NanoString approach confirmed increased expression of BCL2, BIM, BMF, HRK, and NOXA as well as decreased expression of BIK and BCL-XL (Figure 3C; Supplemental Table 4). In summary, in at least 2 of the 3 independent gene expression methods used, there was an increase of only one anti-apoptotic members (BCL2) and four pro-apoptotic BH3-only members (BIM, NOXA, BMF, and HRK) after both 7 days and 28 days of duvelisib treatment. BIK and BCL-XL were the only BCL2 family members that exhibited a significant decrease in expression.
Expression of BCL2 and several BH3-only proteins increased in CLL cells after duvelisib therapy
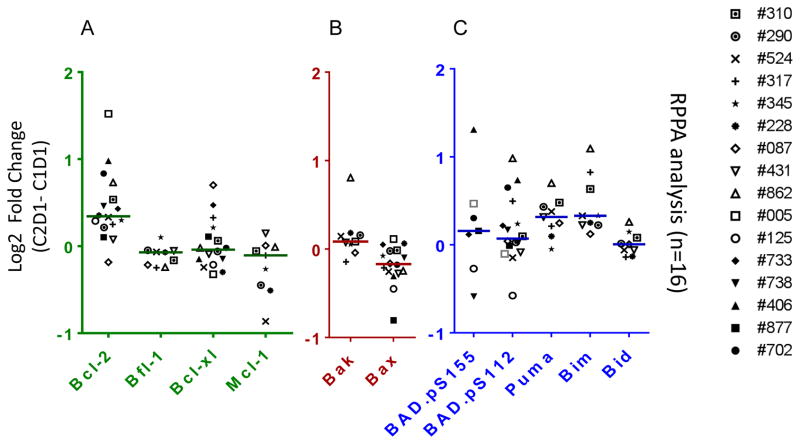
To determine if the mRNA expression changes were recapitulated at the protein level, expression of BCL2 family proteins was measured before treatment and after 28 days of duvelisib treatment using RPPA and immunoblots. Of the four anti-apoptotic proteins analyzed using RPPA, only BCL2 protein was increased significantly (median change = 1.26 fold; n=16; p=0.001; unpaired student t test). To verify quantitation, we compared Bcl-2 fold change in 15 patient samples after normalizing with either vinculin or with actin. Data showed a strong linear relationship with r2 value of 0.939 (p = 0.0001). To demonstrate further reliability of the quantitation, we performed two separate immunoblots from four patient samples and calculated increase in Bcl-2 protein level after duvelisib therapy from each gel. There was concordance in quantitation from 2 gels (r2 = 0.999; p = <0.0001). The other three anti-apoptotic proteins were either not changed (BCL-XL; median= 0.97; p=0.540; n=16) or were slightly decreased (BFL-1; median=0.95; p=0.011; n=9 and MCL-1; median=0.93; p=0.046; n=9) (Figure 4A; Supplemental Table 4). Moreover, among pro-apoptotic multidomain members of the family, transcript levels were generally unchanged, expression of BAX protein was slightly decreased (median=0.89; p=0.002; n=16; Figure 4B) possibly reflecting posttranslational modifications. BH3-only members measured using RPPA (BAD.p155, BAD.p112, BID, BIM, PUMA), both BIM (1.34-fold; p=0.00002, n=9) and PUMA (1.24-fold; p=0.002; n=9) levels were significantly increased (Figure 4C; Supplemental Table 4) and reflected the previous observations at the transcript level.
Figure 4.
Effect of oral duvelisib therapy on expression of BCL2 family proteins in CLL lymphocytes after 28 days (C2D1; cycle 2 day 1) of oral duvelisib intake. (A–C) Reverse phase protein array (RPPA) analysis showing log2 difference in BCL2 family proteins expressions in CLL B cells isolated from patients before and after 28 days of duvelisib therapy (n=16). CLL cells were obtained pre and post duvelisib therapy and protein was extracted. Protein extracts were analyzed through reverse-phase protein array as described in the Methods section. Proteins that are not included were not available in the assay. Detailed information regarding median value and p values is provided in the Supplemental Table 4.
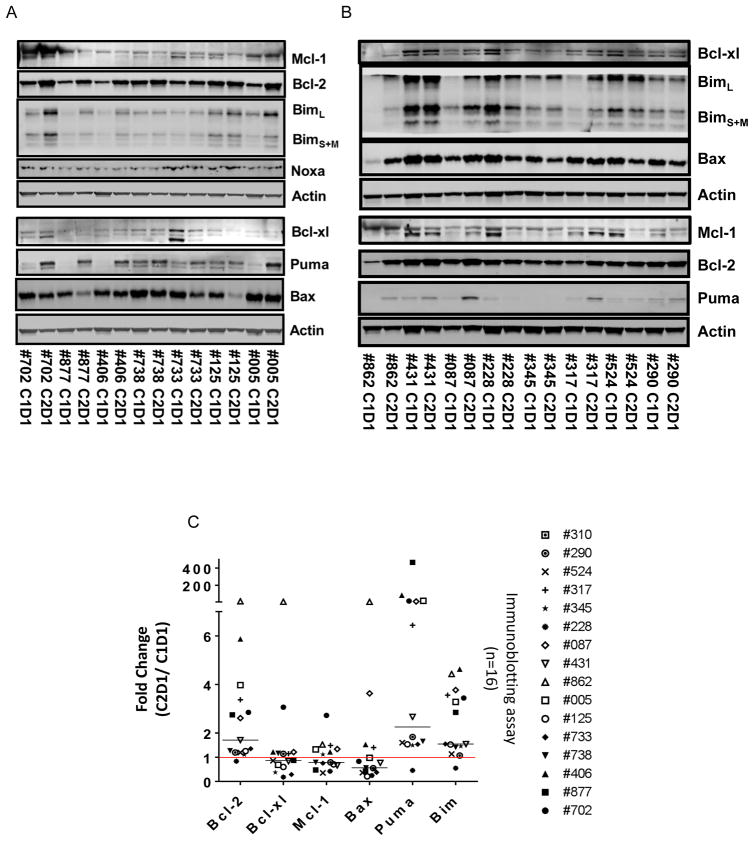
To extend and confirm these results, immunoblot analysis of BCL2, BCL-Xl, MCl-1, BIM, PUMA, and BAX proteins was performed using the same lysates in 16 patients (Figure 5A (n=7) and 5B (n=8); one not shown). The immunoblots suggested that while Bcl-2, PUMA, and Bim protein levels were increased after one cycle of duvelisib therapy, other proteins remained at similar level or decreased. To confirm, immunoblots were quantitated and normalized with the actin loading control protein and graphed (Figure 5C). BCL2 expression was increased significantly (median=1.7 fold change; p=0.0338) reflecting the observations in RPPA (Figure 5C; r=0.57; p=0.029; n=14; correlation graph not shown). Similarly, pro-apoptotic BH3-only members BIM (median=1.54-fold change; p=0.0003), and PUMA (median=2.25 fold increase; p=0.021) were significantly increased. There was not a significant change in BAX (median= 0.56; p=0.47). In summary, levels of anti-apoptotic BCL2 protein as well as pro-apoptotic BH3 members BIM and PUMA were increased by both RPPA and immunoblot after one cycle (28 days) of duvelisib treatment.
Figure 5.
Effect of oral duvelisib therapy on expression of BCL-2 family proteins in CLL lymphocytes before therapy (C1D1) and after 28 days (C2D1) of oral duvelisib intake. (A and B) Immunoblots of expression level of BCL2, Bim, Puma, and Bax proteins in CLL cells from patients before and after 28 days of oral duvelisib intake (n=15). Actin was used as normalization protein. These proteins were selected based on their changes as determined by the RPPA assay, available antibodies, and availability of amount of CLL samples. (C) Quantitation of these blots for all the patient samples analyzed (n=16). Statistical analyses were conducted to obtain p values that are included in the text.
The BCL2 antagonist venetoclax increases apoptosis of CLL cells from duvelisib treated patients
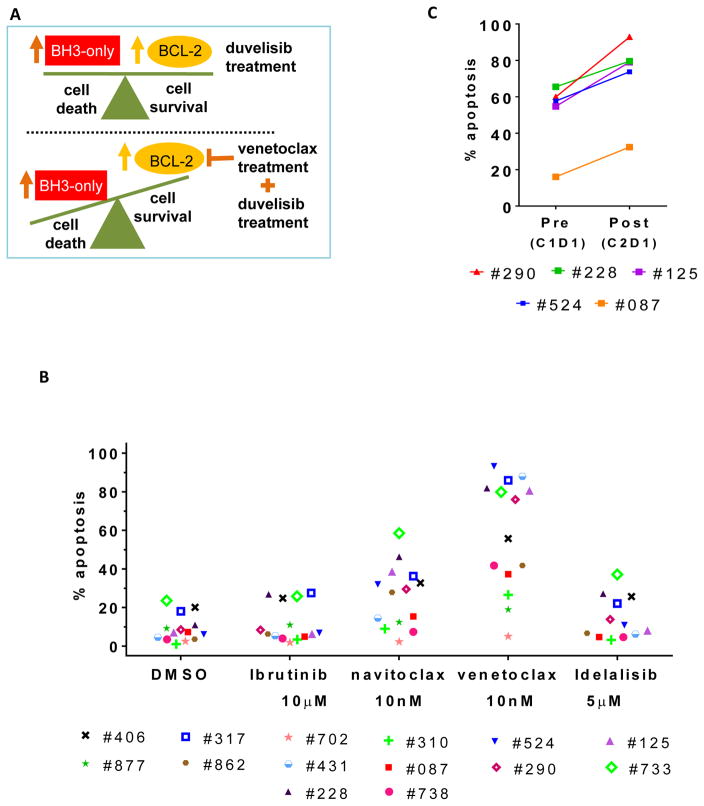
Although duvelisib treatment is associated with increased expression of several pro-apoptotic factors in CLL cells, apoptosis might be inhibited by a concomitant up-regulation of the anti-apoptotic protein, BCL2. In this setting, inhibition of BCL2 by venetoclax may tip the balance toward tumor cell death (Figure 6A). Hence, we evaluated CLL cells from 14 duvelisib treated patients for ex vivo sensitivity to BCL2 inhibition after one cycle (28 days) of duvelisib therapy (Figure 6B). Cells after duvelisib therapy were incubated in vitro with DMSO (control), BCR-pathway inhibitors such as ibrutinib and idelalisib or Bcl-2 antagonists (navitoclax and venetoclax). Samples from duvelisib treated patients exhibited a significant increase in the percent of apoptotic cells upon treatment with the selective BCL2 antagonist venetoclax (median 66%; p=<0.0001) compared to DMSO control. A smaller increase was observed with the BCL2/BCL-XL inhibitor ABT-737 (median 29%; p=0.002; paired student t-test). In contrast, ex-vivo treatment with additional BCR pathway inhibitors did not have a significant effect on apoptosis (Figure 6B).
Figure 6.
Ex-vivo pharmacological intervention in duvelisib treated CLL cells with venetoclax (ABT-199). (A) Schematic model representing mechanistic rationale for combining duvelisib and venetoclax. (B) % cell death of lymphocytes obtained from patients (n=14) after 28 days of oral duvelisib intake when incubated with different pharmacological agents (ibrutinib, ABT-737, venetoclax and idelalisib) in RPMI + 10% HS media for 24 hour. (C) % cell death of leukocytes obtained from patients (n=5) before and after 28 days of oral duvelisib intake when incubated with venetoclax (10 nM) in RPMI + 10% HS media for 24 hour. Each pre-treatment and on-treatment sample was normalized to endogenous cell death. Statistical analyses were conducted to obtain p values that are included in the text.
For five patients, viable CLL cells were available both at baseline and after one cycle (28 days) of duvelisib therapy. These patient-matched samples enabled a comparison of the in vitro effect of venetoclax alone (baseline, prior to duvelisib therapy) and combined with duvelisib (on-treatment). Addition of venetoclax induced apoptosis in the baseline samples. Interestingly, in all five patients, the induction of apoptosis was significantly greater in the duvelisib-treatment samples compared with the pre-treatment samples (p=0.004; paired student t test) (Figure 6C). We would like to point out that due to limitation of the available cells, we have data only in 5 patient samples. These limited data, nonetheless, suggest that duvelisib primes the CLL cells for apoptosis-induction with venetoclax.
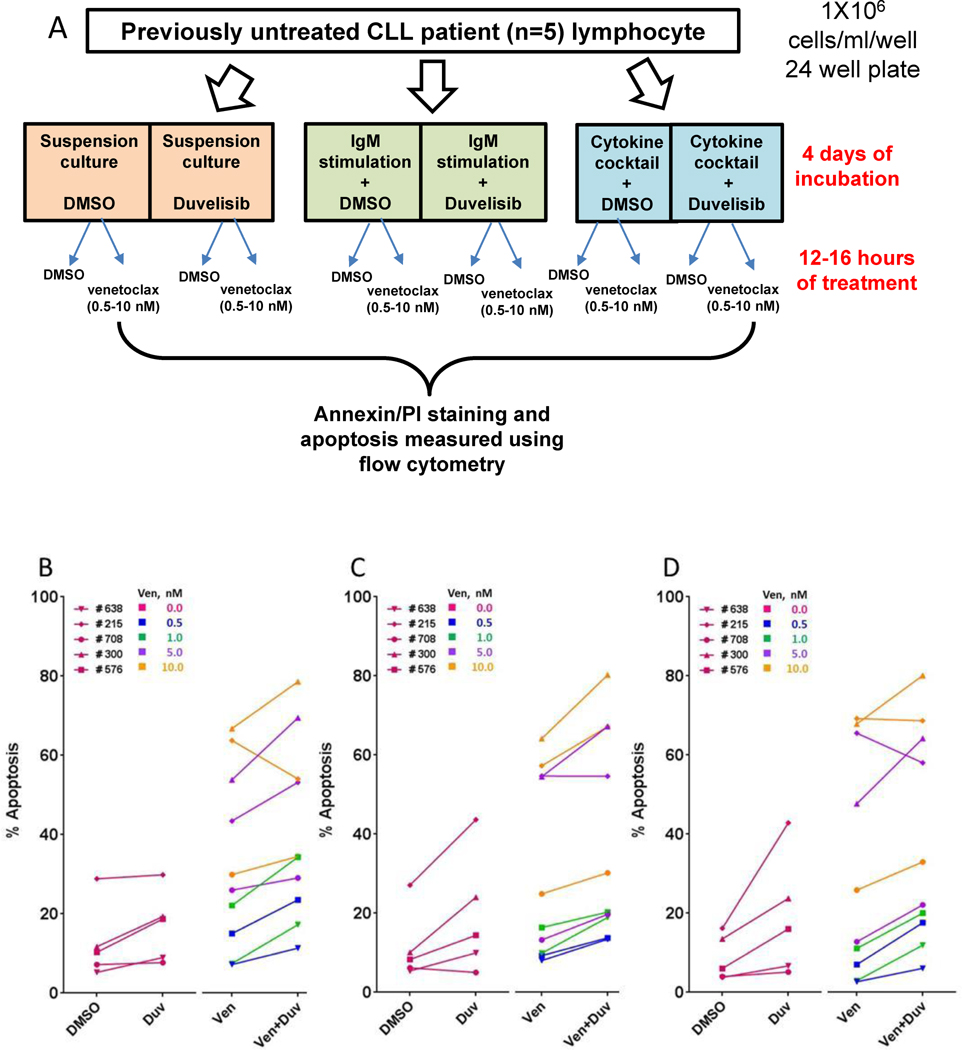
In vitro combination of venetoclax and duvelisib induced more cell death than either drug alone
To examine further the impact of the combination of duvelisib with venetoclax, CLL cells were obtained from patients who were not on any therapy. In these experiments, duvelisib and venetoclax could each be tested during in vitro incubations alone and in combination. We obtained five patients’ lymphocytes and treated with duvelisib in vitro without or with IgM or tumor supportive cytokines as illustrated (Figure 7A). These cells were incubated in vitro with venetoclax and the percentage of apoptotic cells was measured (Figure 7B–D). In vitro treatment of CLL cells with duvelisib followed by venetoclax increased apoptosis compared to venetoclax alone (n= 10 samples; 5 patient done in duplicate; p=0.001; Figure 7B). This observation was not impacted by the culture conditions (+/− BCR pathway activation via IgM, n= 10 samples; p=0.001 Figure 7C, or addition of a proliferation inducing cytokine cocktail- n= 10 samples; p=0.011; Figure 7D). In most samples, the amount of cell death was equal or greater than expected, suggesting the possibility of synergy (Supplemental Table 5).
Figure 7.
Effect of in vitro combination of duvelisib and venetoclax on CLL cell death. In-vitro incubations of CLL cells obtained from previously untreated CLL patients with duvelisib (4 days) and venetoclax (12–16 hours) either alone or in sequential combinations in suspension culture, IgM-stimulated cells, and in media containing cytokine cocktail. (A) Schema to represent processing and culture conditions for in vitro incubation of CLL cells. (B–D) Cell death measurement of CLL cells after incubation with vehicle (DMSO), duvelisib alone, venetoclax alone, and combination of venetoclax and duvelisib in three different culture conditions; (B) suspension culture, (C) soluble IgM-stimulation, (D) cytokine cocktail-stimulation. Statistical analyses were conducted to obtain p values that are included in the text.
Discussion
Prior studies using real-time RT-PCR or immunoblots have demonstrated that BCL2, BCL-XL, and MCL-1 are expressed in CLL cells19 and play a critical role in tumor cell survival25, 27–30. A multitude of microarray studies demonstrated that the expression of BCL2 is high in CLL compared to other leukemias (Supplemental Figure 3). However, these data do not allow us to compare level of one gene from other due to the methodology that relies on primers and probes which have differential affinity with each gene. In the current study, quantitative comparison by RNAseq demonstrated that at baseline BCL2, BCL-XL, and MCL1 were the most highly expressed anti-apoptotic members (Figure 2). The median values for these three members were 0.58, 0.38, and 0.76, respectively. Importantly, these levels are 10 to 20-fold higher than that of the BH3-only or multi-domain pro-apoptotic members. Sequestration of BH3-only pro-apoptotic proteins by antiapoptotic Bcl-2 family members prevents CLL cell apoptosis31. Hence, the expression of anti-apoptotic BCL2 proteins is likely an important factor in CLL cell survival in the peripheral blood, as stoichiometry of pro and anti-survival proteins and their interactions decide the fate of any cell.29, 32–34 With that respect, immunoprecipitation and immunoblotting of anti- and pro-apoptotic proteins, respectively would be helpful to determine how the stoichiometry is changing in circulating CLL cells after duvelisib therapy.
Interestingly, expression of three of these genes was further elevated with duvelisib treatment (BCL2, NOXA, and BIM). At the protein level BCL2, BIM, and PUMA were significantly elevated with duvelisib treatment. These observations are similar to that of our prior study, in which an increase in BCL2 protein was observed during ibrutinib therapy.35 Combined with the observation that duvelisib does not induce overt apoptosis of CLL cells, we hypothesize that the increase in BCL2 counter-balances the increase in BH3-only family members.
As shown in Figure 1, duvelisib treatment is associated with lymphocytosis. The lymphocytosis is in parallel to a decrease in lymph nodes and concomitant increase in serum cytokines and chemokines.36 These changes are in coincident with the observed increase in BCL2 and BH3-only expression. There are three possible mechanisms for increased expression of BCL2 mRNA and protein levels. First, it has been reported that BCL2 transcript and protein expression is higher in CLL cells residing in lymph node niches compared to the peripheral blood.3, 37 Because duvelisib causes CLL cells to exit the lymph node and enter circulation, it is possible that the change in expression is due to a shift in the CLL cell population. However, augmented expression of BH3-only proteins has not been reported in CLL cells residing in lymph node niches.3 In addition, the duvelisib-induced increase in BCL2 protein expression did not correlate with fold change in absolute lymphocyte count (Pearson r = 0.22; p = 0.44; Supplemental Figure 4). Second, it is possible that CLL cells with lower levels of BCL2 are more vulnerable to duvelisib and were eliminated, leaving a population of cells with higher BCL2 expression. Once again, this postulate does not explain modulation of BH3-only proteins with duvelisib treatment. In addition, the bulk of evidence suggests that BCR pathway inhibitor monotherapy primarily impacts cell proliferation and the tumor microenvironment, but not apoptosis.38, 39 Third, inhibition of PI3K-δ/-γ with duvelisib may modulate molecular signaling leading to induced BCL2 and BH3-only expression in CLL cells. It is known that the PI3K/AKT axis is a major regulator of survival, proliferation, and migration of CLL cells.40 Duvelisib-induced inhibition of phosphorylation of AKT at Ser473 has been shown in CLL cells.8 Furthermore, AKT-induced up-regulation of BCL2 expression through cAMP-response element-binding protein has been reported.41 A possibility also exists that the reason for the increase in pro-apoptotic proteins is simply due to simultaneous increase in the level of anti-apoptotic proteins. This is feasible, however, we see a parallel induction of mRNA also.
Several studies have implicated BIM as being particularly important in the regulation of CLL cell survival.42–44 First, BH3-profiling suggests that the BIM/BCL2 dimer is the target of BCL2 inhibition, with ABT-737 causing displacement of BIM from BCL2 allowing BIM to interact with BAX and promote cell death.20 Second, BIM was phosphorylated in CLL cells treated with IgM or co-incubated with HK cells that simulate the microenvironment.45, 46 Phosphorylation of BIM was associated with a worse prognosis.47, 48 More recently, a mechanistic role of BIM was observed in a study of ibrutinib and venetoclax combinations in CLL and mantle cell lymphoma.49 In the current study, BCL2 and BIM were the only BCL2 family members tested that demonstrated a significant increase in at least two gene expression methods and by both RPPA and immunoblot at the protein level. Furthermore, we observed a strong correlation between the increase in BCL2 and the increase in BIM protein expression with duvelisib treatment (p=0.00045; r = 0.773; n=16; Supplemental Figure 5), which may be consistent with the description of the BCL2/BIM dimer as being of particular importance in regulation of CLL cell survival.
In vitro combination investigations of duvelisib and venetoclax were performed to mimic CLL cells that reside in pseudofollicles which are present in lymph nodes. These cells should have active BCR pathway signature and higher proliferation index. For this purpose, we used two systems to stimulate BCR pathway; first was soluble IgM stimulation. For activation of BCR signaling in CLL cells conventionally several groups3, 50–55 including our group 8, 35, 56 have used soluble anti-IgM. Alternatively, immobilized antiIgM has been used to initiate BCR stimulation in CLL cells31. Second system was IL-2, IL-10 and CD40L cocktail. There are several rationales for this combination; first, CD40L (1 μg/ml) results in activation of BCR pathway.57 Second, combination of IL-2, IL-10, and CD40L is an established in vitro model for CLL cells in pseudofollicles.58 Third, our previous publication demonstrated that this cocktail results in AKT phosphorylation in CLL cells.8 Fourth, this cocktail increases Ki67 indicating activation of proliferation; an activity observed in CLL cells in lymph node or bone marrow niches.8 Finally, duvelisib inhibited this TME-induced malignant B-cell proliferative response.9 Under these BCR-activation and pseudofollicle-mimicking model conditions, the cell death by each drug (duvelisib or venetoclax) and combination resulted in similar response as in the suspension culture suggesting that the combination is able to overcome survival signals.
Using immunoblot assays35 or BH3 profiling technique, the induction of BCL2 protein leading to dependence of CLL cell survival in ibrutinib-treated CLL cells was also recently reported59. Finally, data obtained after idelalisib therapy of CLL patients suggested similar phenomenon (Gandhi, unpublished data). Collectively, these results suggest that CLL cells obtained after lymphocytosis with BCR pathway inhibitors have a molecular signature of increased BCL2.
The upregulation of BCL2 in concert with pro-apoptotic BH3-only proteins raised the possibility that duvelisib treatment might not cause significant apoptosis on its own but might enhance sensitivity to BCL2 inhibition. The BH3 mimetic, navitoclax targets both BCL2 and BCL-XL20, 60 while venetoclax neutralizes only BCL2.61, 62 Venetoclax is a more potent and selective inhibitor,61 showing promising clinical activity for patients with relapsed/refractory CLL.62 Consistent with our hypothesis, cell death induced by venetoclax in ex vivo cultures was found to be greater when CLL cells had been previously exposed to duvelisib. These data are consistent with prior observations that in vitro inhibition of PI3K-δ with idelalisib21, or Bruton’s tyrosine kinase with ibrutinib35 results in apoptotic priming of CLL cells.
The plethora of new, targeted agents for the treatment of CLL opens up many opportunities for improving the depth and durability of response via combination therapy.63 In aggregate, the data presented here are consistent with the model that PI3K-δ/γ inhibition with duvelisib primes CLL cells for apoptosis by upregulating pro-apoptotic BH3-only proteins, but that cell survival is maintained by a concomitant upregulation of BCL2. The addition of venetoclax inhibits BCL2 and tips the balance toward more robust apoptosis. A decrease in MCL1 mRNA and protein after duvelisib therapy, albeit minor, may further sensitize CLL cells to venetoclax as Mcl-1 has been shown to promote maintenance and survival of these malignant lymphocytes.64 This is important because survival signals induced by microenvironment such as soluble IgM8, insoluble IgM31, or CD-40L57 results in an increase in Mcl-1 protein levels in CLL lymphocytes. Venetoclax is effective in relapsed/refractory CLL disease62 including patients with 17p deletion status.65 These data provide a mechanistic rationale for evaluating the combination of duvelisib with venetoclax in the clinic.
Supplementary Material
Acknowledgments
Financial support: This work was supported in part by grant P01CA81534 and cancer center core grant CA16672 from the NCI, DHHS, a sponsored research agreement from Infinity Pharmaceuticals, Inc., and funds from the MD Anderson Moon Shot Program.
We thank Ben Hayes and Mark Nelson to coordinate patient sample distribution and Yuling Chen and Min Fu for patient sample collection.
Footnotes
Conflict-of-interest: KB/VG received sponsored research agreement from Infinity Pharmaceuticals, Inc. MD, TT, EYX, JLK, HMS are employees and shareholders of Infinity Pharmaceuticals, Inc. Other authors declare no competing financial interests.
Author Contributions
VKP., designed and performed most of the experiments presented in this study, analyzed data, and wrote the manuscript. KB. Directed VKP, performed some experiments and helped with writing of the manuscript. MD., TT., EYX., performed RNA Seq and Nanostring experiments. HMS., JK., directed worked done at Infinity and reviewed manuscript., MA., performed all immunoblots, AS. RG., performed some experiments, WGW., SO., NJ., were conducting clinical trials, provided clinical and patient-related information. VG. obtained funding, participated in designing experiments, and reviewed the manuscript.
References
- 1.Montserrat E, Moreno C. Chronic lymphocytic leukaemia: a short overview. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2008 Sep;19(Suppl 7):vii320–325. doi: 10.1093/annonc/mdn460. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. The New England journal of medicine. 2005 Feb 24;352(8):804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 3.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011 Jan 13;117(2):563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Seminars in cancer biology. 2014 Feb;24:71–81. doi: 10.1016/j.semcancer.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson MG, Woods DB, McMahon M, Wahl MI, Witte ON, Kurosaki T, et al. A conditional form of Bruton’s tyrosine kinase is sufficient to activate multiple downstream signaling pathways via PLC Gamma 2 in B cells. BMC immunology. 2001;2:4. doi: 10.1186/1471-2172-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Q, Modi P, Newcomb T, Queva C, Gandhi V. Idelalisib: First-in-Class PI3K Delta Inhibitor for the Treatment of Chronic Lymphocytic Leukemia, Small Lymphocytic Leukemia, and Follicular Lymphoma. Clin Cancer Res. 2015 Apr 1;21(7):1537–1542. doi: 10.1158/1078-0432.CCR-14-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler DG, Faia KL, DiNitto JP, Ali JA, White KF, Brophy EE, et al. PI3K-delta and PI3K-gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem Biol. 2013 Nov 21;20(11):1364–1374. doi: 10.1016/j.chembiol.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Balakrishnan K, Peluso M, Fu M, Rosin NY, Burger JA, Wierda WG, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015 Sep;29(9):1811–1822. doi: 10.1038/leu.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peluso M, Faia K, Winkler D, Patel N, Brophy E, White K, et al. Duvelisib (IPI-145) Inhibits Malignant B-Cell Proliferation and Disrupts Signaling from the Tumor Microenvironment through Mechanisms That Are Dependent on PI3K-δ and PI3K-γ. Blood. 2014;124(21):328–328. 2014-12-06 00:00:00. [Google Scholar]
- 10.O’Brien SPM, Kahl B, Horwitz S, Foss F, Porcu P, et al. Duvelisib (IPI-145), an oral dual inhibitor of PI3K-δ, -γ, is clinically active in patients with relapsed/refractory chronic lymphocytic leukemia. Leukemia & Lymphoma; Abstracts from the XVI International Workshop on Chronic Lymphocytic Leukemia; 2015. [Google Scholar]
- 11.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013 Jul 4;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. The New England journal of medicine. 2016 Jan 28;374(4):323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014 May 29;123(22):3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2014 Mar 13;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. The Lancet Oncology. 2014 Jan;15(1):48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015 Apr 16;125(16):2497–2506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel MR, O’Brien SM, Faia K, White K, Douglas M, Allen K, et al. Early clinical activity and pharmacodynamic effects of duvelisib, a PI3K-{delta},{gamma} inhibitor, in patients with treatment-naive CLL. ASCO Meeting Abstracts 2015. 2015 May 18;33(15_suppl):7074. [Google Scholar]
- 18.Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014 Jun 12;370(24):2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitada S, Andersen J, Akar S, Zapata JM, Takayama S, Krajewski S, et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with In vitro and In vivo chemoresponses. Blood. 1998 May 1;91(9):3379–3389. [PubMed] [Google Scholar]
- 20.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. The Journal of clinical investigation. 2007 Jan;117(1):112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davids MS, Deng J, Wiestner A, Lannutti BJ, Wang L, Wu CJ, et al. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012 Oct 25;120(17):3501–3509. doi: 10.1182/blood-2012-02-414060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008 Mar;26(3):317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 23.Patel V, Chen LS, Wierda WG, Balakrishnan K, Gandhi V. Impact of bone marrow stromal cells on Bcl-2 family members in chronic lymphocytic leukemia. Leukemia & lymphoma. 2014 Apr;55(4):899–910. doi: 10.3109/10428194.2013.819573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel V, Balakrishnan K, Keating MJ, Wierda WG, Gandhi V. Expression of executioner procaspases and their activation by a procaspase-activating compound in chronic lymphocytic leukemia cells. Blood. 2015 Feb 12;125(7):1126–1136. doi: 10.1182/blood-2014-01-546796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Packham G, Stevenson FK. Bodyguards and assassins: Bcl-2 family proteins and apoptosis control in chronic lymphocytic leukaemia. Immunology. 2005 Apr;114(4):441–449. doi: 10.1111/j.1365-2567.2005.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buggins AG, Pepper CJ. The role of Bcl-2 family proteins in chronic lymphocytic leukaemia. Leuk Res. 2010 Jul;34(7):837–842. doi: 10.1016/j.leukres.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Al-Harbi S, Hill BT, Mazumder S, Singh K, Devecchio J, Choudhary G, et al. An antiapoptotic BCL-2 family expression index predicts the response of chronic lymphocytic leukemia to ABT-737. Blood. 2011 Sep 29;118(13):3579–3590. doi: 10.1182/blood-2011-03-340364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepper C, Bentley P, Hoy T. Regulation of clinical chemoresistance by bcl-2 and bax oncoproteins in B-cell chronic lymphocytic leukaemia. British journal of haematology. 1996 Dec;95(3):513–517. doi: 10.1046/j.1365-2141.1996.d01-1927.x. [DOI] [PubMed] [Google Scholar]
- 29.Saxena A, Viswanathan S, Moshynska O, Tandon P, Sankaran K, Sheridan DP. Mcl-1 and Bcl-2/Bax ratio are associated with treatment response but not with Rai stage in B-cell chronic lymphocytic leukemia. American journal of hematology. 2004 Jan;75(1):22–33. doi: 10.1002/ajh.10453. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997 Feb 21;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 31.Bojarczuk K, Sasi BK, Gobessi S, Innocenti I, Pozzato G, Laurenti L, et al. BCR signaling inhibitors differ in their ability to overcome Mcl-1-mediated resistance of CLL B cells to ABT-199. Blood. 2016 Jun 23;127(25):3192–3201. doi: 10.1182/blood-2015-10-675009. [DOI] [PubMed] [Google Scholar]
- 32.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 33.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 34.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, et al. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. The EMBO journal. 2007 Jun 20;26(12):2856–2867. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cervantes-Gomez F, Lamothe B, Woyach JA, Wierda WG, Keating MJ, Balakrishnan K, et al. Pharmacological and Protein Profiling Suggests Venetoclax (ABT-199) as Optimal Partner with Ibrutinib in Chronic Lymphocytic Leukemia. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015 Aug 15;21(16):3705–3715. doi: 10.1158/1078-0432.CCR-14-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douglas M, Allen K, Sweeney J, O’Brien SM, Flinn I, Horwitz SM, et al. Serum chemokines and cytokines in CLL patients treated with duvelisib, a PI3K-{delta},{gamma} inhibitor. ASCO Meeting Abstracts 2015. 2015 May 18;33(15_suppl):7072. [Google Scholar]
- 37.Gilling CE, Mittal AK, Chaturvedi NK, Iqbal J, Aoun P, Bierman PJ, et al. Lymph node-induced immune tolerance in chronic lymphocytic leukaemia: a role for caveolin-1. Br J Haematol. 2012 Jul;158(2):216–231. doi: 10.1111/j.1365-2141.2012.09148.x. [DOI] [PubMed] [Google Scholar]
- 38.Herman SE, Niemann CU, Farooqui M, Jones J, Mustafa RZ, Lipsky A, et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia. 2014 Nov;28(11):2188–2196. doi: 10.1038/leu.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wodarz D, Garg N, Komarova NL, Benjamini O, Keating MJ, Wierda WG, et al. Kinetics of CLL cells in tissues and blood during therapy with the BTK inhibitor ibrutinib. Blood. 2014 Jun 26;123(26):4132–4135. doi: 10.1182/blood-2014-02-554220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nature reviews Drug discovery. 2014 Feb;13(2):140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, et al. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. The Journal of biological chemistry. 2000 Apr 14;275(15):10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 42.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999 Nov 26;286(5445):1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 43.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2004 Apr 20;101(16):6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. The Journal of experimental medicine. 2003 Oct 6;198(7):1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paterson A, Mockridge CI, Adams JE, Krysov S, Potter KN, Duncombe AS, et al. Mechanisms and clinical significance of BIM phosphorylation in chronic lymphocytic leukemia. Blood. 2012 Feb 16;119(7):1726–1736. doi: 10.1182/blood-2011-07-367417. [DOI] [PubMed] [Google Scholar]
- 46.O’Reilly LA, Kruse EA, Puthalakath H, Kelly PN, Kaufmann T, Huang DC, et al. MEK/ERK-mediated phosphorylation of Bim is required to ensure survival of T and B lymphocytes during mitogenic stimulation. Journal of immunology. 2009 Jul 1;183(1):261–269. doi: 10.4049/jimmunol.0803853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003 Oct 2;22(43):6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 48.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochimica et biophysica acta. 2007 Aug;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Portell CA, Axelrod M, Brett LK, Gordon VL, Capaldo B, Xing JC, et al. Synergistic Cytotoxicity of Ibrutinib and the BCL2 Antagonist, ABT-199(GDC-0199) in Mantle Cell Lymphoma (MCL) and Chronic Lymphocytic Leukemia (CLL): Molecular Analysis Reveals Mechanisms of Target Interactions. Blood. 2014;124(21):509. [Google Scholar]
- 50.Guarini A, Chiaretti S, Tavolaro S, Maggio R, Peragine N, Citarella F, et al. BCR ligation induced by IgM stimulation results in gene expression and functional changes only in IgV H unmutated chronic lymphocytic leukemia (CLL) cells. Blood. 2008 Aug 1;112(3):782–792. doi: 10.1182/blood-2007-12-127688. [DOI] [PubMed] [Google Scholar]
- 51.Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009 Mar 26;113(13):3050–3058. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quiroga MP, Balakrishnan K, Kurtova AV, Sivina M, Keating MJ, Wierda WG, et al. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009 Jul 30;114(5):1029–1037. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krysov S, Steele AJ, Coelho V, Linley A, Sanchez Hidalgo M, Carter M, et al. Stimulation of surface IgM of chronic lymphocytic leukemia cells induces an unfolded protein response dependent on BTK and SYK. Blood. 2014 Nov 13;124(20):3101–3109. doi: 10.1182/blood-2014-04-567198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rozovski U, Wu JY, Harris DM, Liu Z, Li P, Hazan-Halevy I, et al. Stimulation of the B-cell receptor activates the JAK2/STAT3 signaling pathway in chronic lymphocytic leukemia cells. Blood. 2014 Jun 12;123(24):3797–3802. doi: 10.1182/blood-2013-10-534073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tavolaro S, Colombo T, Chiaretti S, Peragine N, Fulci V, Ricciardi MR, et al. Increased chronic lymphocytic leukemia proliferation upon IgM stimulation is sustained by the upregulation of miR-132 and miR-212. Genes, chromosomes & cancer. 2015 Apr;54(4):222–234. doi: 10.1002/gcc.22236. [DOI] [PubMed] [Google Scholar]
- 56.Yang Q, Chen LS, Ha MJ, Do KA, Neelapu SS, Gandhi V. Idelalisib impacts cell growth through inhibiting translation regulatory mechanisms in mantle cell lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016 Jun 24; doi: 10.1158/1078-0432.CCR-15-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010 Sep 23;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plander M, Seegers S, Ugocsai P, Diermeier-Daucher S, Ivanyi J, Schmitz G, et al. Different proliferative and survival capacity of CLL-cells in a newly established in vitro model for pseudofollicles. Leukemia. 2009 Nov;23(11):2118–2128. doi: 10.1038/leu.2009.145. [DOI] [PubMed] [Google Scholar]
- 59.Deng J, Isik E, Fernandes SM, Brown JR, Letai A, Davids MS. Ibrutinib Therapy Increases BCL-2 Dependence and Enhances Sensitivity to Venetoclax in CLL. Blood. 2015;126(23):490–490. doi: 10.1038/leu.2017.32. 2015-12-03 00:00:00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial Susceptibility of Chronic Lymphocytic Leukemia to BCL2 Inhibition: Results of a Phase I Study of Navitoclax in Patients With Relapsed or Refractory Disease. Journal of Clinical Oncology. 2012 Feb 10;30(5):488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature medicine. 2013 Feb;19(2):202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 62.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. The New England journal of medicine. 2016 Jan 28;374(4):311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Faia K, White K, Proctor J, Andrade P, Pink M, Rickles R, et al. High throughput in vitro combination sensitivity screen in hematologic malignancies with the phosphoinositide-3 kinase (PI3K)-{delta},{gamma} inhibitor, duvelisib. ASCO Meeting Abstracts 2015. 2015 May 18;33(15_suppl):8559. [Google Scholar]
- 64.Pepper C, Lin TT, Pratt G, Hewamana S, Brennan P, Hiller L, et al. Mcl-1 expression has in vitro and in vivo significance in chronic lymphocytic leukemia and is associated with other poor prognostic markers. Blood. 2008 Nov 1;112(9):3807–3817. doi: 10.1182/blood-2008-05-157131. [DOI] [PubMed] [Google Scholar]
- 65.Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. The Lancet Oncology. 2016 May 10; doi: 10.1016/S1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.