ABSTRACT
Many fungi can develop on building material in indoor environments if the moisture level is high enough. Among species that are frequently observed, some are known to be potent mycotoxin producers. This presence of toxinogenic fungi in indoor environments raises the question of the possible exposure of occupants to these toxic compounds by inhalation after aerosolization. This study investigated mycotoxin production by Penicillium brevicompactum, Aspergillus versicolor, and Stachybotrys chartarum during their growth on wallpaper and the possible subsequent aerosolization of produced mycotoxins from contaminated substrates. We demonstrated that mycophenolic acid, sterigmatocystin, and macrocyclic trichothecenes (sum of 4 major compounds) could be produced at levels of 1.8, 112.1, and 27.8 mg/m2, respectively, on wallpaper. Moreover, part of the produced toxins could be aerosolized from the substrate. The propensity for aerosolization differed according to the fungal species. Thus, particles were aerosolized from wallpaper contaminated with P. brevicompactum when an air velocity of just 0.3 m/s was applied, whereas S. chartarum required an air velocity of 5.9 m/s. A. versicolor was intermediate, since aerosolization occurred under an air velocity of 2 m/s. Quantification of the toxic content revealed that toxic load was mostly associated with particles of size ≥3 μm, which may correspond to spores. However, some macrocyclic trichothecenes (especially satratoxin H and verrucarin J) can also be found on smaller particles that can deeply penetrate the respiratory tract upon inhalation. These elements are important for risk assessment related to moldy environments.
IMPORTANCE The possible colonization of building material by toxinogenic fungi in cases of moistening raises the question of the subsequent exposure of occupants to aerosolized mycotoxins. In this study, we demonstrated that three different toxinogenic species produce mycotoxins during their development on wallpaper. These toxins can subsequently be aerosolized, at least partly, from moldy material. This transfer to air requires air velocities that can be encountered under real-life conditions in buildings. Most of the aerosolized toxic load is found in particles whose size corresponds to spores or mycelium fragments. However, some toxins were also found on particles smaller than spores that are easily respirable and can deeply penetrate the human respiratory tract. All of these data are important for risk assessment related to fungal contamination of indoor environments.
KEYWORDS: indoor air, mycotoxins, exposure, aerosolization, wallpaper, fungi, indoor air quality, filamentous fungi
INTRODUCTION
In industrialized countries, people spend most of their time inside buildings (1). Many physical, chemical, and microbiological pollutants can have detrimental effects for occupants, such as allergies or infections (2, 3). Among the microorganisms that are present in indoor environments, micromycetes are ubiquitous and capable of growing on most construction and decoration materials under appropriate environmental conditions (4–6). Thus, it is estimated that in northern Europe and North America, 20 to 40% of buildings display macroscopically visible fungal growth (7, 8).
Among the fungal species commonly observed in such habitats, some are known to produce toxic secondary metabolites called mycotoxins (4, 9, 10). For instance, Aspergillus versicolor, a potent producer of sterigmatocystin (STG), is one of the most frequent fungal contaminants of indoor environments that can be found on building materials, in dust, and in air samples (4, 11). On the other hand, Stachybotrys chartarum is often isolated from building materials in homes that have suffered water damage (12–14). This species is known to be able to produce different toxic compounds belonging to the family of macrocyclic trichothecenes (MCT) (satratoxin G [SG] and H [SH], roridin L2 [RL2], and verrucarin J [VerJ]) (15, 16). In the same way, Penicillium brevicompactum, a species able to produce mycophenolic acid (MPA), has also been frequently identified in indoor environments (17).
Such observations raise the question of the possible exposure of occupants to these toxic compounds by contact or inhalation following their aerosolization. Indeed, it has been shown that mycotoxins can be found in fungal spores (9) and could therefore subsequently be inhaled (18, 19).
To evaluate the presence of these contaminants in indoor environments, some studies have measured mycotoxins on contaminated materials (20–23) or settled dust (24, 25). Thus, STG could be found in more than 20% of analyzed samples. Similarly, MCT were also found on material samples taken from water-damaged homes (13).
However, the toxin quantification from material or settled dust does not predict the airborne toxic load or toxin quantities potentially inhaled by the occupants. Indeed, the relationship between contaminated surfaces, mycotoxin production, and transfer to the air of these toxic substances is poorly documented. Most studies have focused primarily on aerosolization of conidia or fungal fragments (26–28) without associating them with mycotoxins. Only one previous work demonstrated the presence of MCT in highly respirable particles (<1 μm) (29). In that study, the authors demonstrated that while passing over cellulose ceiling tiles contaminated with a toxinogenic strain of S. chartarum, house air could be contaminated with MCT, which is related to aerosolization of fungal particles but also due to the presence of toxins on particles smaller than spores.
Within this context, the aim of our study was to quantify mycotoxin production by three fungal species often present in indoor environments, P. brevicompactum, A. versicolor, and S. chartarum, during their growth on wallpaper. We also aimed to evaluate possible aerosolization of produced toxins as a function of both air velocity and size of the released particles. Wallpaper was chosen since it has been shown that this substrate is favorable for mycotoxin production (13, 14, 21). Moreover, this material is often used in indoor furnishing and is therefore in direct contact with indoor air.
We demonstrate here that part of mycotoxins produced on wallpaper during fungal growth can be aerosolized following air velocities that can be encountered in buildings. Toxic load is mostly observed on particles whose size corresponds to spores, but some toxins could also be found in easily respirable particles of less than 2 μm.
RESULTS
Growth and toxinogenesis of fungal strains on wallpaper.
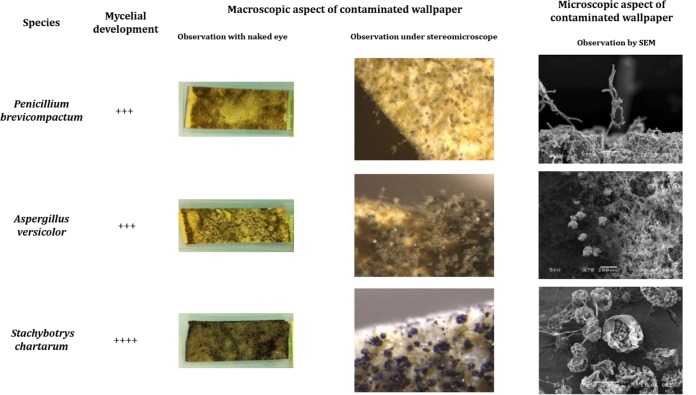
After 10 days of incubation at 25°C, the three tested fungal species grew and sporulated on wallpaper. Nevertheless, some differences could be observed between species (Fig. 1).
FIG 1.
Macroscopic, under stereomicroscope, and SEM observations of mycelial growth on wallpaper contaminated with different species. ++++, colonization of whole sample; +++, development on about 4/5 of the sample.
P. brevicompactum colonized almost the entire surface of wallpaper, with a loosened mycelium. Numerous large and compact penicilli were observed under stereomicroscope and, at microscopic level, long terverticillate conidiophores with adjoined branches sometimes bent away from the axis. Inflated metulae bore divergent phialide clusters and very long, dry, and disordered chains of spores.
As for P. brevicompactum, A. versicolor growth covered almost all the sample but with a heterogeneous density. Stereomicroscope examination revealed a dense field of aerial and closely interwoven hyphae bearing conidiophores. Classical microscopic features were observed: radiate and biseriate conidial heads, closely packed metulae, and phialides bearing short chains of spores.
S. chartarum displayed an intense and regular growth, with abundant hyphae colonizing the whole sample's surface with many sporulated heads. Conidiophores were simple or branched. Phialides, organized in clusters, bore black ellipsoidal conidia agglomerated by a slimy coating.
Mycotoxin measurements revealed that all three species produced mycotoxin(s) during their growth on wallpaper (Table 1). STG was produced in larger quantities, at more than 110 mg/m2. The four analyzed MCT were also found. SH was the most abundant one, followed by SG and RL2. Only small amounts of VerJ were measured after growth of S. chartarum ST82 on wallpaper.
TABLE 1.
Toxin production on wallpaper contaminated by three different toxigenic fungal strainsc
| Species | Toxina | Initial concn (T0) (mg/m2)b | Concn after 10 days (mg/m2) | P value |
|---|---|---|---|---|
| P. brevicompactum | MPA | 0.21 ± 0.09 | 1.8 ± 0.86 | <0.0001 |
| A. versicolor | STG | 0.12 ± 0.004 | 112.1 ± 30.08 | 0.0008 |
| S. chartarum | Total MCT | 1.7 | 27.8 | |
| RL2 | 0.3 ± 0.01 | 5.9 ± 1.04 | <0.0001 | |
| VerJ | 0.08 ± 0.02 | 0.6 ± 0.18 | <0.0001 | |
| SG | ND | 7.1 ± 3.92 | 0.0143 | |
| SH | 1.3 ± 0.33 | 14.2 ± 6.97 | 0.0018 |
MPA, mycophenolic acid; STG, sterigmatocystin; MCT, macrocyclic trichothecenes; RL2, roridin L2; VerJ, verrucarin J; SG, satratoxin G; SH, satratoxin H.
ND, not detected.
Concentration results are expressed as means of 3 determinations ± standard deviations.
Aerosolization of particles from wallpaper.
To define the conditions leading to particle aerosolization from a substrate as a function of the fungal species, contaminated wallpaper samples were submitted to increasing aeraulic stresses. It appeared that for P. brevicompactum, an air velocity of 0.3 m/s was sufficient to aerosolize some particles from the substrate. An increase in air velocity increased the number of aerosolized particles from wallpaper without any modification of the bioaerosol profile (see the supplemental material). For A. versicolor, an air velocity of 2 m/s was required to aerosolize a significant number of particles from substrate. In order to compare aerosolization of these two species, an airflow of 2 m/s was applied on contaminated wallpaper to further characterize bioaerosols and airborne mycotoxins.
In contrast, for S. chartarum, air speed of almost 6 m/s was needed to observe an aerosolization of particles. This air velocity was therefore applied for toxin measurement.
Characterization of bioaerosols.
For P. brevicompactum, the application of an air velocity of 2 m/s led to the release of a total number of particles of 5.6 × 104 from moldy wallpaper. They were distributed mainly in fine aerosols with an optical diameter of about 100 nm (maximal concentration, 103 particles/liter), and particles with optical diameter between 2 and 8 μm (maximal concentration, 2.3 × 103 particles/liter).
For A. versicolor, an air velocity of 2 m/s allowed the aerosolization of a total number of 1.5 × 104 counted particles that were mostly made of fine aerosols with optical diameter of about 100 nm (maximal concentration, 1.2 × 103 particles/liter) and a few particles with optical diameter between 2 and 8 μm (maximal concentration, 700 particles/liter).
For S. chartarum, application of an air speed of almost 6 m/s led to the overall production of 7 × 103 counted particles from substrate, with a polydispersed distribution of particle sizes. The production of submicrometer particles represented 77.5% of the total airborne particles.
The distribution of particle size in bioaerosols obtained from the three species is represented in Fig. 2.
FIG 2.
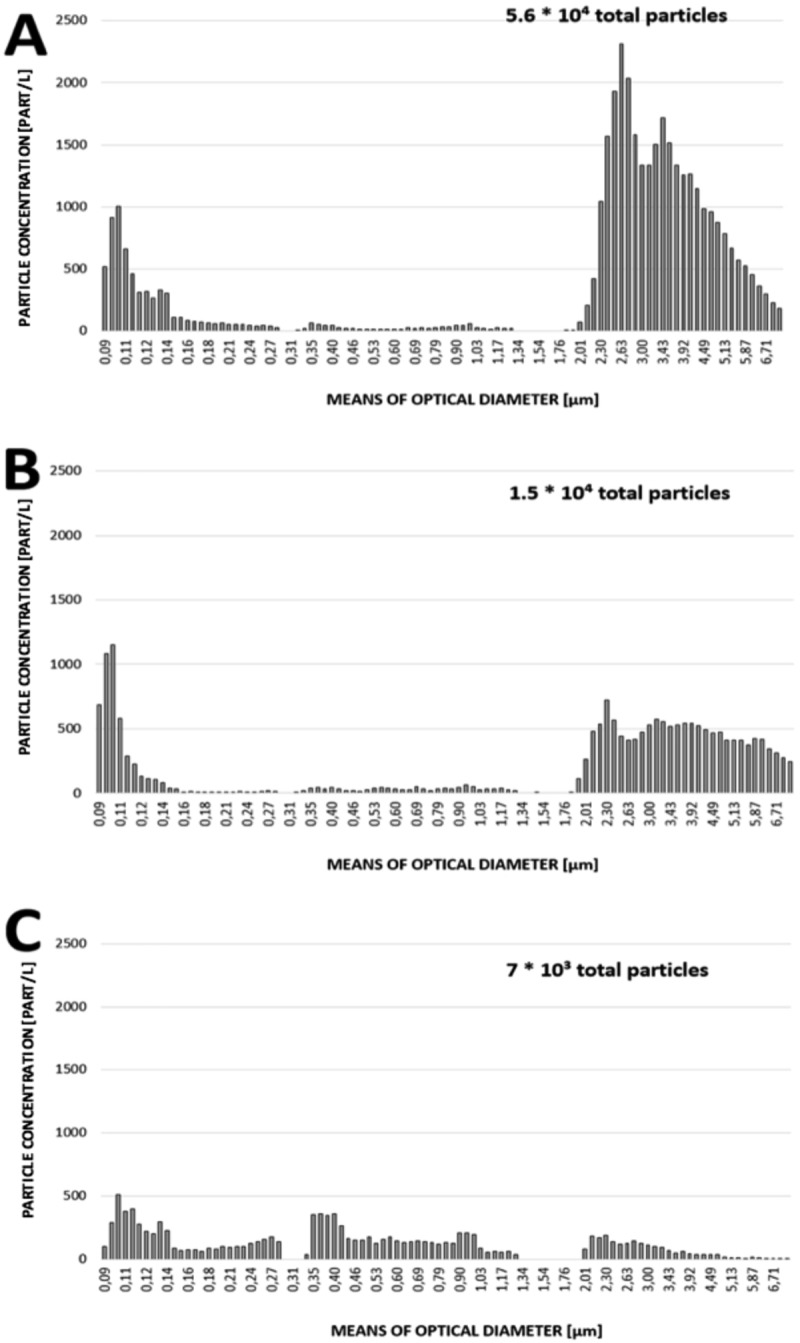
Granulometric profiles of aerosols from P. brevicompactum (A), A. versicolor (B), and S. chartarum (C) following aeraulic solicitations on contaminated wallpaper with airflows of 2, 2, and 6 m/s, respectively.
Airborne mycotoxins.
The aerosolization of mycotoxins from wallpaper was measured following exposure to air velocities of 2, 2, and 6 m/s for P. brevicompactum, A. versicolor, and S. chartarum, respectively. The global mycotoxin loads of aerosols from the three fungal species are reported in Table 2.
TABLE 2.
Global mycotoxin content of aerosols generated from wallpaper
| Species | Toxina | Air velocity (m/s) | Total quantity of airborne toxin (ng) | % emitted toxin |
|---|---|---|---|---|
| P. brevicompactum | MPA | 2 | 271 | 15 |
| A. versicolor | STG | 2 | 179 | 0.2 |
| S. chartarum | Total MCT | 6 | 1,260 | 4.5 |
| RL2 | 64 | 1.1 | ||
| VerJ | 80 | 13.3 | ||
| SG | 102 | 1.4 | ||
| SH | 1,014 | 7.1 |
MPA, mycophenolic acid; STG, sterigmatocystin; MCT, macrocyclic trichothecenes; RL2, roridin L2; VerJ, verrucarin J; SG, satratoxin G; SH, satratoxin H.
All analyzed toxins were found in the aerosols, but it appeared that the percentage of airborne toxins strongly differed between them. Fifteen percent of the MPA present on wallpaper was transferred to air. It represented a total quantity of 271 ng of MPA. In contrast, the percentage of aerosolized STG was only 0.2%. However, since it was the most produced toxin on wallpaper, the total quantity of airborne STG reached almost 180 ng. The proportion of total aerosolized MCT was 4.5%. It has to be noted that even if S. chartarum required a higher air speed to be aerosolized than the two other species, the total quantity of airborne toxins was greatest for that species. Among the 4 analyzed trichothecenes, VerJ was the most aerosolized, with 13.3% of the initial toxic load, followed by SH, SG, and RL2. However, when considering the quantities that were transferred from substrate to air, SH appeared predominant, representing almost 80% of the overall toxic load.
In order to analyze the distribution of mycotoxins as a function of particle size and the subsequent risk of inhalation, the mycotoxin loads of each domain size of released aerosols were quantified, and the results are presented in Table 3.
TABLE 3.
Quantification of mycotoxins on different stages of the Andersen impactor
| Stage | Size range (μm) | Quantity of emitted toxin (ng)a |
||||||
|---|---|---|---|---|---|---|---|---|
| MPA | STG | MCT (total) | RL2 | VerJ | SG | SH | ||
| 1 | >7 | 20.5 | 74.7 | 380.5 | 15.5 | ND | 55.3 | 309.7 |
| 2 | 4.7–7 | 79.6 | 49.8 | 58.7 | 27.6 | ND | 31.1 | ND |
| 3 | 3.3–4.7 | 138.7 | 45.2 | 522.1 | 8.4 | 10.8 | 15.7 | 487.2 |
| 4 | 2.1–3.3 | 26.5 | 9.2 | 4.4 | 4.4 | ND | ND | ND |
| 5 | 1.1–2.1 | 5.8 | ND | 226.3 | 7.2 | 59.8 | ND | 159.3 |
| 6 | 0.65–1.1 | ND | ND | 68.7 | 1.4 | 9.4 | ND | 57.9 |
MPA, mycophenolic acid; STG, sterigmatocystin; MCT, macrocyclic trichothecenes; RL2, roridin L2; VerJ, verrucarin J; SG, satratoxin G; SH, satratoxin H; ND, not detected. MPA produced by P. brevicompactum, STG produced by A. versicolor, and MCT produced by S. chartarum.
MPA was quantifiable on 5 of the 6 considered granulometric ranges, with the maximum (about 140 ng) being associated with the particles collected on the third stage of the impactor, with a granulometric domain between 3.3 and 4.7 μm. For STG, no toxin was found on stages corresponding to the particles with a size of <2.1 μm, and the total mycotoxin load was associated with bigger particles. Almost 95% of the toxic load was associated with particles bigger than 3.3 μm. Macrocyclic trichothecenes produced by S. chartarum and aerosolized from wallpaper were detected on all stages of an Andersen impactor, even on stages 5 and 6, corresponding to submicrometer particles. Nevertheless, 90% of the total toxic load (1,129 ng) was found on stages 1, 3, and 5.
The four analyzed macrocyclic trichothecenes were differently distributed on Andersen impactor stages. RL2 was found on all stages. SG was exclusively found on stages 1 to 3, and SH was found on stages 1, 3, 5, and 6. VerJ was found on stages 3, 5, and 6, with 86% of the total toxic load being associated with the two later stages, whereas no toxin was measured on stage 1, 2, or 4. Stage 3, which corresponds to particles ranging from 3.3 to 4.7 μm, was the most contaminated, with 41% of the total MCT load. It was also the only one containing all four tested macrocyclic trichothecenes.
DISCUSSION
The possible implication of mycotoxins in some disorders observed in occupants of moldy homes is a growing public health question worldwide (30–32). Indeed, the risk of exposure to those fungal toxic metabolites by inhalation emerged in the late 1990s, when macrocyclic trichothecenes produced by S. chartarum were implicated in the appearance of pulmonary hemorrhages in infants in the United States (33). More recently, these mycotoxins were also suspected to play a role in sick building syndrome (34, 35). However, data on the direct relationship between mycotoxin production on materials and their transfer to air are missing and therefore do not allow precise risk assessment. That is why the present study aimed to evaluate the ability of mycotoxins produced by A. versicolor, P. brevicompactum, and S. chartarum to be aerosolized from moldy wallpaper.
First, we investigated the ability of these three toxinogenic species to grow and produce toxic compounds on wallpaper. This material, frequently used for indoor decoration, allowed both mycelial growth and sporulation under conditions that can be considered “worst case” but that can be encountered in homes (25°C, humidity, and darkness), especially behind furniture and/or during warm seasons. This is in agreement with surveys reporting frequent contamination of such materials by molds, particularly in cases of water damage (14, 21, 36, 37). Of note, for P. brevicompactum, some morphological features were peculiar when this species was grown on wallpaper compared to agar culture medium. Indeed, a usual feature of a P. brevicompactum colony on agar medium is dominance of a dense felt of large and compact conidiophores and the velutinous characteristic of the thallus, with only few trailings (38). On wallpaper, the colony displayed more abundant aerial mycelia, with conidiophores borne by aerial hyphae. Such a structure may have an important role in facilitating the aerosolization of fungal structures.
Wallpaper also allowed mycotoxin production by the tested species. Concentrations as high as 112 mg/m2, 14 mg/m2, and 7 mg/m2 were found for STG, SH, and SG, respectively. These findings are in agreement with previous studies about the production on wallpaper of SG and SH by Gottschalk et al. (14) and of STG by Polizzi et al. (21).
The investigation of the aerosolization of mycotoxins produced on wallpaper first showed that aerosolization of particles from substrate strongly differed from one species to another, possibly related to mycelium organization and conidial structures. As an illustration, both A. versicolor and P. brevicompactum are fungal species characterized by the presence of small and light spores organized in chains at the extremities of phialides (39). For these two species, an air velocity of 2 m/s, which matches the air speed observed due to mechanical and natural ventilation in tertiary buildings (28), allowed the aerosolization of numerous particles from wallpaper. These particles were distributed in two main categories: one including very small particles (<0.15 μm), and the second including particles ranging from 2 to 6 μm. This second group may correspond to spores, groups of spores, or mycelium debris (38, 39), in agreement with previous data on aerosolization of these fungal species (40–42). One can note that for P. brevicompactum, the total number of particles aerosolized from substrate was higher than for A. versicolor. This is related to the disposition of spores on mycelial structures. In P. brevicompactum, long chains of spores are borne by aerial conidiophores and may easily be aerosolized. For A. versicolor, the spore chains are shorter and located on tight and compact phialides, making them mildly more difficult to aerosolize from material.
For S. chartarum, a higher air velocity was required for aerosolization from substrate. An air velocity of about 6 m/s is more frequently encountered outdoors but could also be encountered in buildings due to mechanical ventilation (28). The use of fans may also generate airflows able to aerosolize S. chartarum. Of note, the total number of airborne particles was lower than that observed for other species, and this may explain why this fungal species is not commonly observed in air samples and is more frequently found by direct examination of building materials (12–14). In cases of sufficient airflow, a polydispersed particle cloud was generated from S. chartarum-contaminated substrate. There was an important cluster made of particles ranging from 0.4 to 1 μm, which are therefore smaller than spores. It might correspond to microfungal particles, debris of wallpaper released from substrate due to the cellulolytic activity of S. chartarum, or exudate droplets from culture (43). Such findings are important, since these small particles could easily deeply penetrate the human respiratory tract in cases of inhalation.
All tested mycotoxins were found in aerosols generated from moldy wallpaper, and the proportion transferred to air varied with fungal species. MPA was the most aerosolized, with 15% of the produced toxin. This is related to the higher facility of P. brevicompactum to be aerosolized from substrate than other species. In contrast, the proportion of airborne STG was low (0.2%). Since numerous particles can be released from substrate contaminated with A. versicolor, this suggests that STG could be located in fungal parts that are strongly adherent to the substrate and probably mainly present/located in mycelia (5, 7). However, considering that STG was the major produced toxin, the quantity of airborne STG was comparable to that of MPA.
For MCT, even if the required air speed for aerosolization was higher, it has to be highlighted that the four analyzed toxins were found in aerosols, and the total aerosolized toxic load was 5 times higher than that of other toxins.
The analysis of the toxin distribution according to the aerosol profile and size of released particles also provided some important information. For MPA and STG, the maximal toxic load was found on particles whose size corresponds to spores, groups of spores, or mycelium debris. However, a low proportion of MPA was also found on particles smaller than spores. It could be related to the excretion of part of the toxin in exudate droplets, as previously demonstrated for other penicillia (44). The excreted toxin could be then adsorbed on small particles of dust.
The distribution of MCT was different. Toxins were found on all stages of the Andersen impactor, even those collecting particles smaller than spores. This result is in agreement with a study by Brasel et al. (29). As for P. brevicompactum, it might be the result of the excretion of MCT by fungus in droplets outside the mycelia (43) and their adsorption on dust particles or wallpaper debris generated by the cellulolytic activity of S. chartarum.
Of note, the analyzed MCT differed regarding their distribution in the various particle sizes. This result suggests that the different MCT analyzed in this study could be differently distributed/excreted within fungal structures. Further studies are required to characterize the distribution of macrocyclic trichothecenes in S. chartarum mycelia. It would help in a better understanding of the biosynthetic pathway and processing of these compounds in fungal cells.
All these results on the aerosolization of mycotoxin according to particle size bring an important insight into risk assessment and possible subsequent toxicity after inhalation. Although no clear dose-effect relationship has been established for these mycotoxins in cases of inhalation, it has been demonstrated that intranasal exposure could be highly toxic. For instance, Carey et al. (45) showed that exposure to 5 μg of SG for 4 days led to widespread apoptosis of olfactory sensory neurons and to epithelial and olfactory nerve atrophy, as well as acute neutrophilic rhinitis in rhesus monkeys.
This study demonstrated that during their growth on wallpaper, P. brevicompactum, A. versicolor, and S. chartarum, which are frequently found indoors, produce mycotoxins. These toxins can subsequently be aerosolized, at least partly, from moldy material. This transfer to air requires air velocities that can be encountered in buildings, since they correspond to the movement of people in a room (0.2 m/s), air speed in ceiling diffusers (2 m/s), slamming doors, air drafts from opening the window, and mechanical ventilation (6 m/s).
Most of the aerosolized toxic load is found in particles whose size corresponds to spores or mycelium fragments. However, for MPA, and mainly MCT, toxins were found also on particles smaller than spores that could be easily inhaled by occupants and deeply penetrate the respiratory tract. It seems important to take these data into consideration for risk assessment related to fungal contamination of the indoor environment and the possible toxicity associated with inhalation of these toxins.
MATERIALS AND METHODS
Mycotoxin standards.
Mycophenolic acid (MPA), sterigmatocystin (STG), verrucarin A (VerA), o-methylsterigmatocystin (o-mSTG), and mycophenolic acid-d3 (MPA-d3) were purchased from Sigma (Saint-Quentin-Fallavier, France). Standards of satratoxin G (SG), satratoxin H (SH), roridin L2 (RL2,) and verrucarin J (VerJ) were a gracious gift from J. J. Pestka (Department of Microbiology and Molecular Genetics, Michigan State University, MI, USA).
All standards were dissolved in methanol (MeOH) to obtain stock solutions that were stored at −20°C, as recommended by the manufacturer.
Solvents and reagents.
All reagents and solvents were purchased from ICS (Lapeyrouse-Fossat, France) and were analytical grade. Acetonitrile (AcN) used for mobile phase was liquid chromatography-mass spectrometry (LC-MS) grade and purchased from Thermo Fischer Scientific (Illkirch, France), and water was obtained from an ultrapure water (18.2 MΩ) system (Elga LabWater, Veolia, Anthony, France).
Wallpaper (WP) (papier peint BLANC BLA 0 INSP; Leroy Merlin) was purchased in a specialized store. The material, visually clean and dry, was cut into 2 by 5-cm pieces and then sterilized by autoclaving at 121°C for 20 min before use (27, 46).
Fungal strains.
P. brevicompactum strain IBT 23078 was a gracious gift from J. B. Nielsen (Technical University of Denmark, Lyngby, Denmark), A. versicolor NCPT 54 was a gift from O. Puel (INRA, Toulouse, France), and the S. chartarum 82 (ST82) strain was previously isolated in our laboratory (47). These three strains were selected for their ability to produce mycophenolic acid, sterigmatocystin, and macrocyclic trichothecenes, respectively. All strains were maintained in the laboratory on malt extract agar (MEA; Biokar, France) at 4°C and were regularly checked for viability by culturing on MEA.
Growth and toxinogenesis of fungi on wallpaper.
The fungal strains were grown on potato dextrose agar (PDA; Biokar, France) for 14 days at 25°C to obtain highly sporulating cultures. Spores were harvested by flooding the plate with 10 ml of Tween 80 (0.05%). Spores were suspended by smoothly scraping the medium with a sterile inoculating loop, and liquid was then collected. Spore concentration was measured by direct counting on a counting cell (Malassez cell; CML, Nemours, France). Spore suspensions were then diluted to reach a concentration of 2 ×106 spores/ml, and contamination was achieved by applying 500 μl of those suspensions dropwise (106 spores/sample) on the decorative side of sterile wallpaper. This contamination level was previously identified as sufficient to observe fungal development within few days (47).
Contaminated wallpaper pieces (2 by 5 cm) were placed in flasks, on a layer of 2 cm of glass beads and 8 ml of sterile water in order to maintain the moisture level at saturation throughout the test, and incubated for 10 days at 25°C in darkness. After incubation, fungal growth was assessed by visual examination of samples (importance of colonized surface). Both hyphal development and density of sporulated conidial heads on the whole sample surface (10 cm2) were observed by examination under stereomicroscope (magnification, ×12 to ×120) (Olympus SZX9) and under scanning electron microscopy (SEM) (Jeol JSM 5600LV) (magnification, ×40 to ×30,000).
Some samples were used for mycotoxin determination, whereas others, incubated under the same conditions, were used for aerosolization, as described below.
The initial mycotoxin baseline due to inoculum deposit on materials (= T0 value) was measured using samples that were frozen immediately after spore deposit without incubation to avoid fungal growth.
All analysis were done in triplicate, and three independent experiments were carried out.
Aerosolization of mycotoxins from wallpaper.
To evaluate the aerosolization of particles and toxins from wallpaper, a specific experimental device capable of producing controlled air velocities over contaminated substrates was developed. The principle of this device is shown in Fig. 3. To ensure the safety of the operator, the entire assembly was placed in a microbiological safety cabinet.
FIG 3.
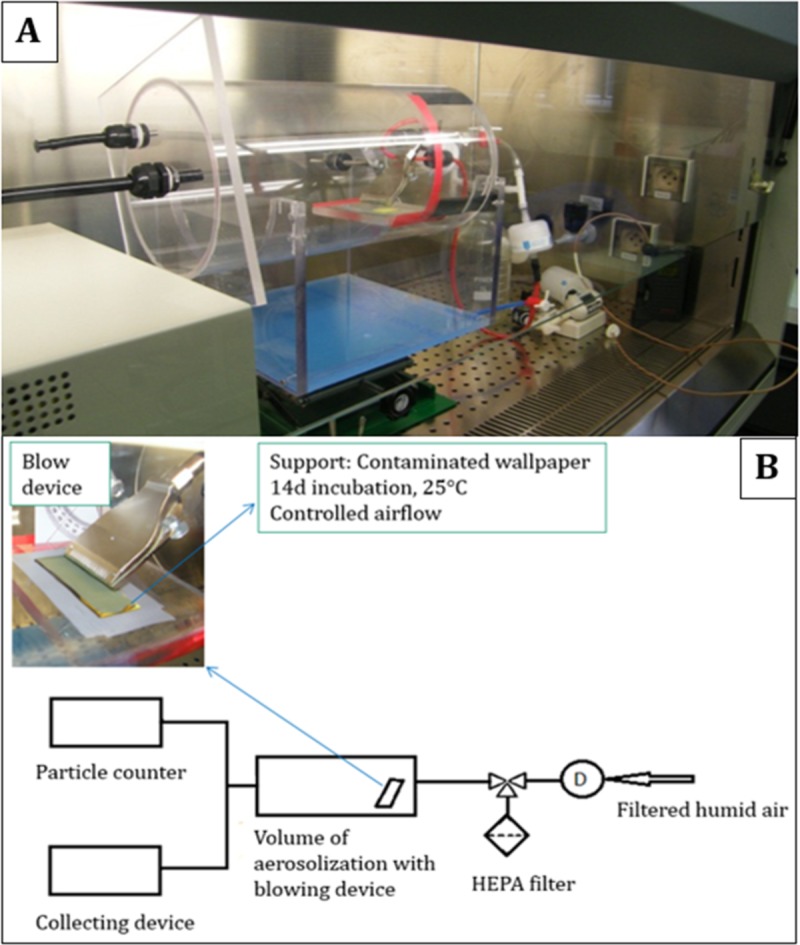
Experimental assembly used for aerosolization of mycotoxins from wallpaper (A) and schema of the experimental assembly (B). HEPA, high-efficiency particulate air.
The developed assembly presents a cylindrical volume of 10.5 liter equipped with a blowing device provided with filtered humidified air (50% relative humidity [RH] at 22°C) to ensure the aerodynamic stresses on contaminated material. The blowing device placed in the closed space consists of 16 semicircular holes of 1 mm diameter (PNR Industries, Collègien, France). It was placed so that the air stream forms an angle of 45° with respect to the contaminated material. Moreover, the assembly is leveled so the distance between the bottom of the blowing nozzles and the fungal cultures was 1 cm (Fig. 3). Characterization of the air speed over the substrate as a function of the flow from the blowing device was performed (see the supplemental material).
Different increasing air velocities were first tested to define air speeds allowing significant particle aerosolization from substrates for the three fungal species (see the supplemental material). Once air velocity was defined for each fungal species, the characterization of aerosol was done following air jets of 5 s each that were repeated until the measured concentration of aerosolized particles decreased to 1 particle/liter.
Physical characterization of the produced aerosols was carried out using an optical counter (model 3340; TSI) set at 0.1 liters/min. An Andersen multistage impactor (Tisch Environmental, OH, USA) was used for capturing particles according to 6 ranges of size and aerodynamic characteristics. Each stage of the impactor was equipped with a fiberglass disk to collect particles and allow mycotoxin determination, as described below. Filters were placed on support whose thickness preserved the right distance between the orifice inlet of the impactor and the filter.
Mycotoxin determination.
Four MCT (SG, SH, VerJ, and RL2), MPA, and STG were extracted from samples (wallpaper and fiberglass disks) by gentle mechanical agitation on an agitation table (HS 501 digital reciprocating shaker; IKA, Grosseron, France) in chloroform-methanol (2:1). Mycophenolic acid-d3 and o-methyl sterigmatocystin were added at known concentrations before starting extraction in order to serve as internal standards for MPA and STG, respectively. For MCT, verrucarin A was chosen as an internal standard, as already described (47).
After 4 h, extracts were centrifuged for 5 min at 3,500 rpm and passed through a phase separator (PS) filter (Whatman 1 PS). The filtered extracts were evaporated to dryness and suspended in 1 ml of methanol.
Quantification of mycotoxins was performed using an Acquity ultraperformance liquid chromatography (UPLC) system coupled to a Xevo triple quadrupole mass spectrometer (Waters, Milford, MA, USA). The desolvation temperature and nitrogen flow rate were set at 650°C and 800 liters/h, respectively. Argon was used as the collision gas at a flow rate of 0.12 ml/min.
Mycotoxins (5 μl of samples) were eluted on an Acquity BEH C18 column (2.1 by 100 mm, 1.7 μm; Waters), with an AcN-H2O gradient (t[0 to 0.5 min)], 10% AcN; t[0.5 to 4 min], 90% AcN) at a flow rate of 0.35 ml/min. Quantification was carried out by multiple-reaction monitoring (MRM) mode in positive electrospray ionization (ESI+). The MRM transitions, cone voltage, and collision energies used for the different toxins are listed in Table 4. Chromatographic data were monitored using the MassLynx 4.1 software (Waters, Milford, MA, USA).
TABLE 4.
MRM transitions, cone voltages, and collision energies used for mycotoxin detection
| Toxin | Molecular wt | Parent ion | MRM fragment | Cone voltage (V) | Collision energy (eV) |
|---|---|---|---|---|---|
| MPA | 320 | 321 | 159 | 16 | 36 |
| 321 | 207 | 16 | 22 | ||
| STG | 324 | 325 | 115 | 40 | 64 |
| 325 | 310 | 40 | 24 | ||
| RL2 | 530 | 553 | 249 | 42 | 16 |
| 553 | 305 | 42 | 26 | ||
| SG | 544 | 545 | 81 | 20 | 34 |
| 545 | 231 | 20 | 16 | ||
| SH | 528 | 529 | 249 | 24 | 16 |
| 551 | 303 | 48 | 28 | ||
| VerJ | 484 | 523 | 151 | 46 | 32 |
| 523 | 293 | 46 | 34 |
Limits of detection (LOD) were determined from 3 injections of the mycotoxin standards at the lowest concentration that could be detected with a signal-to-noise ratio of ≥3. They were 1 ng/ml for MPA and STG, 0.2 ng/ml for RL2, 5 ng/ml for VerJ, and 10 ng/ml for SH and SG. The limit of quantification (LOQ) was determined and validated for the lowest concentration of the calibration curve chosen for its relevance to mycotoxin investigation on wallpaper. The LOQs were set at 10 ng/ml for MPA, STG, RL2, and VerJ and 100 ng/ml for SG and SH.
For all analyzed toxins, the percentage of aerosolized toxin from contaminated substrates was calculated as follows: % airborne toxin = [quantity of airborne toxin (ng)/quantity of produced toxin on WP sample (ng)] × 100.
Statistical analysis.
Data were analyzed with GraphPad Prism statistical software version 4.0. Student's t test was used to analyze the differences between the initial concentration of toxins on materials (T0) and toxin concentrations after the incubation period. The differences were considered to be statistically significant when the P value was <0.05.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. J. Pestka (Department of Microbiology and Molecular Genetics, Michigan State University, MI, USA) for toxin standards, J. B. Nielsen (Technical University of Denmark, Lyngby, Denmark) for the P. brevicompactum strain, and O. Puel (INRA, UMR Toxalim) for the A. versicolor strain.
This work was financed by the French Ministry of Ecology, Sustainable Development and Energy (PRIMEQUAL project DSC-BIO/2013-121), the French Environment and Energy Management agency (ADEME), and the Scientific and Technical Centre for Building (CSTB) (Ph.D. grant for B. Aleksic).
Brankica Aleksic performed experiments and participated in manuscript preparation and revision; Marjorie Draghi and Sebastien Ritoux designed the device to study the aerosolization of particles and mycotoxins from moldy wallpaper; Sylviane Bailly did the morphological analysis of wallpaper after fungal development and participated in the development of the analytical method for mycotoxin measurement; Marlène Lacroix did the toxin measurements on both wallpaper and aerosols; Isabelle P. Oswald participated in the overall supervision of the project and redaction of the article; Jean-Denis Bailly and Enric Robine supervised the work and participated in redaction of the article; and Enric Robine took the SEM photos.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01001-17.
REFERENCES
- 1.Brown L. 1983. National radiation survey in the UK: indoor occupancy factors. Radiat Prot Dosimetry 5:203–208. doi: 10.1093/oxfordjournals.rpd.a082694. [DOI] [Google Scholar]
- 2.Le Moullec Y, Squinazi F. 1996. Pollution atmosphérique à l'intérieur des bâtiments: sources expositions et risques sanitaires: analyse bibliographique des études françaises, 1983–1993. Conseil supérieur d'hygiène publique de France, section de l'evaluation des risques de l'environnement sur la santé, Lavoisier-Tec & Doc., Paris, France. [Google Scholar]
- 3.Squinazi F. 2002. La pollution de l'air à l'intérieur des bâtiments (allergènes exclus). Rev Fr Allergol 42:248–255. [Google Scholar]
- 4.Andersen B, Frisvad JC, Sondergaard I, Rasmussen IS, Larsen LS. 2011. Associations between fungal species and water-damaged building materials. Appl Environ Microbiol 77:4180–4188. doi: 10.1128/AEM.02513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen KF, Gravesen S, Nielsen PA, Andersen B, Thrane U, Frisvad JC. 1999. Production of mycotoxins on artificially and naturally infested building materials. Mycopathologia 145:43–56. doi: 10.1023/A:1007038211176. [DOI] [PubMed] [Google Scholar]
- 6.Polizzi V, Adams A, De Saeger S, Van Peteghem C, Moretti A, De Kimpe N. 2012. Influence of various growth parameters on fungal growth and volatile metabolite production by indoor molds. Sci Total Environ 414:277–286. doi: 10.1016/j.scitotenv.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen KF. 2002. Mould growth on building materials. Secondary metabolites, mycotoxins and biomarkers. Ph.D. thesis Technical University of Denmark, Kgs; Lyngby, Denmark. [Google Scholar]
- 8.Moularat S, Robine E, Draghi M, Derbez M, Kirschner S, Ramalho O. 2008. Les moisissures dans les environnements intérieurs et leurs effets sur la santé. Pollut Atmos 50:34–37. [Google Scholar]
- 9.Jarvis BB, Miller JD. 2005. Mycotoxins as harmful indoor air contaminants. Appl Microbiol Biotechnol 66:367–372. doi: 10.1007/s00253-004-1753-9. [DOI] [PubMed] [Google Scholar]
- 10.Verdier T, Coutand M, Bertron A, Roques C. 2014. A review of indoor microbial growth across building materials and sampling and analysis methods. Build Environ 80:136–149. doi: 10.1016/j.buildenv.2014.05.030. [DOI] [Google Scholar]
- 11.Lappalainen S, Kähkönen E, Loikkanen P, Palomäki E, Lindroos O, Reijula K. 2001. Evaluation of priorities for repairing in moisture-damaged school buildings in Finland. Build Environ 36:981–986. doi: 10.1016/S0360-1323(00)00082-2. [DOI] [Google Scholar]
- 12.Bellanger AP, Reboux G, Roussel S, Grenouillet F, Didier-Scherer E, Dalphin JC, Millon L. 2009. Indoor fungal contamination of moisture-damaged and allergic patient housing analysed using real-time PCR. Lett Appl Microbiol 49:260–266. doi: 10.1111/j.1472-765X.2009.02653.x. [DOI] [PubMed] [Google Scholar]
- 13.Bloom E, Nyman E, Must A, Pehrson C, Larsson L. 2009. Molds and mycotoxins in indoor environments: a survey in water-damaged buildings. J Occup Environ Hyg 6:671–678. doi: 10.1080/15459620903252053. [DOI] [PubMed] [Google Scholar]
- 14.Gottschalk C, Bauer J, Meyer K. 2006. Determination of macrocyclic trichothecenes in mouldy indoor materials by LC-MS/MS. Mycotoxin Res 22:189–192. doi: 10.1007/BF02959275. [DOI] [PubMed] [Google Scholar]
- 15.Cabral JPS. 2010. Can we use indoor fungi as bioindicators of indoor air quality? Historical perspectives and open questions. Sci Total Environ 408:4285–4295. [DOI] [PubMed] [Google Scholar]
- 16.Pestka JJ, Yike I, Dearborn DG, Ward MDW, Harkema JR. 2008. Stachybotrys chartarum, trichothecene mycotoxins, and damp building-related illness: new insights into a public health enigma. Toxicol Sci 104:4–26. doi: 10.1093/toxsci/kfm284. [DOI] [PubMed] [Google Scholar]
- 17.Reboux G, Bellanger AP, Roussel S, Grenouillet F, Sornin S, Piarroux R, Dalphin JC, Millon L. 2009. Indoor mold concentration in eastern France. Indoor Air 19:446–453. doi: 10.1111/j.1600-0668.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- 18.Eduard W. 2009. Fungal spores: a critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit Rev Toxicol 39:799–864. doi: 10.3109/10408440903307333. [DOI] [PubMed] [Google Scholar]
- 19.Le Bars J, Le Bars P. 1985. Etude du nuage de spores de Stachybotrys atra contaminant des pailles: risque d'inhalation. Bull Soc Fr Mycol Med 2:321–324. [Google Scholar]
- 20.Charpin-Kadouch C, Maurel G, Felipo R, Queralt J, Ramadour M, Dumon H, Garans M, Botta A, Charpin D. 2006. Mycotoxin identification in moldy dwellings. J Appl Toxicol 26:475–479. doi: 10.1002/jat.1164. [DOI] [PubMed] [Google Scholar]
- 21.Polizzi V, Delmulle B, Adams A, Moretti A, Susca A, Picco AM, Rosseel Y, Kindt R, Van Bocxlaer J, De Kimpe N, Van Peteghem C, De Saeger S. 2009. JEM spotlight: fungi, mycotoxins and microbial volatile organic compounds in mouldy interiors from water-damaged buildings. J Environ Monit 11:1849–1858. doi: 10.1039/b906856b. [DOI] [PubMed] [Google Scholar]
- 22.Täubel M, Sulyok M, Vishwanath V, Bloom E, Turunen M, Järvi K, Kauhanen E, Krska R, Hyvärinen A, Larsson L, Nevalainen A. 2011. Co-occurrence of toxic bacterial and fungal secondary metabolites in moisture-damaged indoor environments. Indoor Air 21:368–375. doi: 10.1111/j.1600-0668.2011.00721.x. [DOI] [PubMed] [Google Scholar]
- 23.Tuomi T, Reijula K, Johnsson T, Hemminki K, Hintikka EL, Lindroos O, Kalso S, Koukila-Kähkölä P, Mussalo-Rauhamaa H, Haahtela T. 2000. Mycotoxins in crude building materials from water-damaged buildings. Appl Environ Microbiol 66:1899–1904. doi: 10.1128/AEM.66.5.1899-1904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bloom E, Bal K, Nyman E, Must A, Larsson L. 2007. Mass spectrometry-based strategy for direct detection and quantification of some mycotoxins produced by Stachybotrys and Aspergillus spp. in indoor environments. Appl Environ Microbiol 73:4211–4217. doi: 10.1128/AEM.00343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelhart S, Loock A, Skutlarek D, Sagunski H, Lommel A, Farber H, Exner M. 2002. Occurrence of toxigenic Aspergillus versicolor isolates and sterigmatocystin in carpet dust from damp indoor environments. Appl Environ Microbiol 68:3886–3890. doi: 10.1128/AEM.68.8.3886-3890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho SH, Seo SC, Schmechel D, Grinshpun SA, Reponen T. 2005. Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos Environ 39:5454–5465. doi: 10.1016/j.atmosenv.2005.05.042. [DOI] [Google Scholar]
- 27.Górny RL, Reponen T, Grinshpun S, Willeke K. 2001. Source strength of fungal spore aerosolization from moldy building material. Atmos Environ 35:4853–4862. doi: 10.1016/S1352-2310(01)00261-8. [DOI] [Google Scholar]
- 28.Kanaani H, Hargreaves M, Ristovski Z, Morawska L. 2009. Fungal spore fragmentation as a function of airflow rates and fungal generation methods. Atmos Environ 43:3725–3735. doi: 10.1016/j.atmosenv.2009.04.043. [DOI] [Google Scholar]
- 29.Brasel TL, Douglas DR, Wilson SC, Straus DC. 2005. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins on particulates smaller than conidia. Appl Environ Microbiol 71:114–122. doi: 10.1128/AEM.71.1.114-122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. 2009. WHO guidelines for indoor air quality: dampness and mold. World Health Organization, Geneva, Switzerland: http://www.euro.who.int/__data/assets/pdf_file/0017/43325/E92645.pdf?ua=1. [PubMed] [Google Scholar]
- 31.Hope J. 2013. A review of the mechanism of injury and treatment approaches for illness resulting from exposure to water-damaged buildings, mold, and mycotoxins. Sci World J 2013:767482. doi: 10.1155/2013/767482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutarowska B, Piotrowska M. 2007. Methods of mycological analysis in buildings. Build Environ 42:1843–1850. doi: 10.1016/j.buildenv.2006.02.015. [DOI] [Google Scholar]
- 33.Dearborn DG, Yike I, Sorenson WG, Miller MJ, Etzel RA. 1999. Overview of investigations into pulmonary hemorrhage among infants in Cleveland, Ohio. Environ Health Perspect 107:495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hossain MA, Ahmed MS, Ghannoum MA. 2004. Attributes of Stachybotrys chartarum and its association with human disease. J Allergy Clin Immunol 113:200–209. doi: 10.1016/j.jaci.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Kuhn DM, Ghannoum MA. 2003. Indoor mold, toxigenic fungi, and Stachybotrys chartarum: infectious disease perspective fungal organisms in damp buildings. Clin Microbiol Rev 16:144–172. doi: 10.1128/CMR.16.1.144-172.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloom E, Grimsley LF, Pehrson C, Lewis J, Larsson L. 2009. Molds and mycotoxins in dust from water-damaged homes in New Orleans after hurricane Katrina. Indoor Air 19:153–158. doi: 10.1111/j.1600-0668.2008.00574.x. [DOI] [PubMed] [Google Scholar]
- 37.Nunez M, Sivertsen MS, Mattsson J. 2012. Growth preferences on substrate, construction, and room location for indoor moulds and Actinomycetes, session 5H P11. Healthy Buildings, Oslo, Norway. [Google Scholar]
- 38.Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B. 2010. Food and indoor fungi. CBS Laboratory Manual Series, CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- 39.Pitt JI, Hocking AD. 2009. Fungi and food spoilage. Springer US, Boston, MA. [Google Scholar]
- 40.Afanou KA, Straumfors A, Skogstad A, Nilsen T, Synnes O, Skaar I, Hjeljord L, Tronsmo A, Green BJ, Eduard W. 2014. Submicronic fungal bioaerosols: high-resolution microscopic characterization and quantification. Appl Environ Microbiol 80:7122–7130. doi: 10.1128/AEM.01740-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Górny RL, Ławniczek-Wałczyk A. 2012. Effect of two aerosolization methods on the release of fungal propagules from a contaminated agar surface. Ann Agric Environ Med 19:279–284. [PubMed] [Google Scholar]
- 42.Madsen AM, Larsen ST, Koponen IK, Kling KI, Barooni A, Karottki DG, Tendal K, Wolkoff P. 2016. Generation and characterization of indoor fungal aerosols for inhalation studies. Appl Environ Microbiol 82:2479–2493. doi: 10.1128/AEM.04063-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gareis M, Gottschalk C. 2014. Stachybotrys spp. and the guttation phenomenon. Mycotoxin Res 30:151–159. doi: 10.1007/s12550-014-0193-3. [DOI] [PubMed] [Google Scholar]
- 44.Gareis M, Gareis E-M. 2007. Guttation droplets of Penicillium nordicum and Penicillium verrucosum contain high concentrations of the mycotoxins ochratoxin A and B. Mycopathologia 163:207–214. doi: 10.1007/s11046-007-9003-1. [DOI] [PubMed] [Google Scholar]
- 45.Carey SA, Plopper CG, Hyde DM, Islam Z, Pestka JJ, Harkema JR. 2012. Satratoxin-G from the black mold Stachybotrys chartarum induces rhinitis and apoptosis of olfactory sensory neurons in the nasal airways of rhesus monkeys. Toxicol Pathol 40:887–898. doi: 10.1177/0192623312444028. [DOI] [PubMed] [Google Scholar]
- 46.Peitzsch M, Bloom E, Haase R, Must A, Larsson L. 2012. Remediation of mould damaged building materials–efficiency of a broad spectrum of treatments. J Environ Monit 14:908–915. doi: 10.1039/c2em10806b. [DOI] [PubMed] [Google Scholar]
- 47.Aleksic B, Bailly S, Draghi M, Pestka JJ, Oswald IP, Robine E, Bailly JD, Lacroix MZ. 2016. Production of four macrocyclic trichothecenes by Stachybotrys chartarum during its development on different building materials as measured by UPLC-MS/MS. Build Environ 106:265–273. doi: 10.1016/j.buildenv.2016.07.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.