Key Points
Question
What is the effectiveness of radiofrequency denervation added to a standardized exercise program for patients with chronic low back pain?
Findings
In 3 randomized clinical trials including 681 participants with chronic low back pain originating from the facet joints, sacroiliac joints, or a combination of these or the intervertebral disks, radiofrequency denervation combined with exercise compared with exercise alone resulted in either no significant difference in pain intensity, or a difference smaller than the prespecified minimal clinically important difference after 3 months.
Meaning
The study findings do not support the use of radiofrequency denervation for chronic low back pain originating from these sources.
Abstract
Importance
Radiofrequency denervation is a commonly used treatment for chronic low back pain, but high-quality evidence for its effectiveness is lacking.
Objective
To evaluate the effectiveness of radiofrequency denervation added to a standardized exercise program for patients with chronic low back pain.
Design, Setting, and Participants
Three pragmatic multicenter, nonblinded randomized clinical trials on the effectiveness of minimal interventional treatments for participants with chronic low back pain (Mint study) were conducted in 16 multidisciplinary pain clinics in the Netherlands. Eligible participants were included between January 1, 2013, and October 24, 2014, and had chronic low back pain, a positive diagnostic block at the facet joints (facet joint trial, 251 participants), sacroiliac joints (sacroiliac joint trial, 228 participants), or a combination of facet joints, sacroiliac joints, or intervertebral disks (combination trial, 202 participants) and were unresponsive to conservative care.
Interventions
All participants received a 3-month standardized exercise program and psychological support if needed. Participants in the intervention group received radiofrequency denervation as well. This is usually a 1-time procedure, but the maximum number of treatments in the trial was 3.
Main Outcomes and Measures
The primary outcome was pain intensity (numeric rating scale, 0-10; whereby 0 indicated no pain and 10 indicated worst pain imaginable) measured 3 months after the intervention. The prespecified minimal clinically important difference was defined as 2 points or more. Final follow-up was at 12 months, ending October 2015.
Results
Among 681 participants who were randomized (mean age, 52.2 years; 421 women [61.8%], mean baseline pain intensity, 7.1), 599 (88%) completed the 3-month follow-up, and 521 (77%) completed the 12-month follow-up. The mean difference in pain intensity between the radiofrequency denervation and control groups at 3 months was −0.18 (95% CI, −0.76 to 0.40) in the facet joint trial; −0.71 (95% CI, −1.35 to −0.06) in the sacroiliac joint trial; and −0.99 (95% CI, −1.73 to −0.25) in the combination trial.
Conclusions and Relevance
In 3 randomized clinical trials of participants with chronic low back pain originating in the facet joints, sacroiliac joints, or a combination of facet joints, sacroiliac joints, or intervertebral disks, radiofrequency denervation combined with a standardized exercise program resulted in either no improvement or no clinically important improvement in chronic low back pain compared with a standardized exercise program alone. The findings do not support the use of radiofrequency denervation to treat chronic low back pain from these sources.
Trial Registration
trialregister.nl Identifier: NTR3531
This report describes findings from 3 randomized clinical trials designed to compare the effects of a standardized exercise program with vs without radiofrequency denervation for treatment of chronic low back pain.
Introduction
Low back pain causes more disability than any other condition and has major social and economic consequences.1,2,3 In the Netherlands (16.5 million residents) the cost of low back pain was estimated at €3.5 billion (US $3.9 billion) in 2007, and the majority of the costs were attributable to patients with chronic low back pain. In the United States (326 million residents), the costs of low back pain have not been recently estimated; however, a study by Dieleman et al4 evaluated health care spending from 1996 to 2013 in the United States and estimated the health care spending on low back and neck pain at $87.6 billion.
Potential sources of low back pain of the spinal column include the facet joints, sacroiliac joints, and intervertebral disks. These sources of pain were classified as mechanical low back pain.5,6 Radiofrequency denervation is a commonly used treatment in pain clinics for chronic low back pain. In the United States, facet joint or sacroiliac joint interventions in Medicare recipients increased from approximately 425 000 interventions in 2000 to 2.2 million interventions in 2013.7 Radiofrequency denervation aims to prevent the conduction of nociceptive impulses through the use of an electric current that damages the pain-conducting nerve. The effectiveness of radiofrequency denervation has not been consistently demonstrated. However, there is consensus among anesthesiologists that minimal interventional procedures such as radiofrequency denervation are effective for patients with mechanical low back pain.5 Systematic reviews and multidisciplinary clinical guidelines concluded that there is evidence of very low to moderate quality supporting the effectiveness of radiofrequency denervation in clinical practice for patients with chronic low back pain.5,8,9,10
The aim of this study was to evaluate whether radiofrequency denervation in addition to a standardized exercise program is more effective than the standardized exercise program alone for patients with chronic mechanical low back pain.
Methods
Study Design and Participants
The Cost-Effectiveness of Minimal Interventional Procedures for Patients with Chronic Low Back Pain (Mint) study11 was an initiative to evaluate minimally invasive treatments for patients with spinal column–related chronic low back pain, consisting of 4 trials and an observational study (participants who did not want to be randomized or who did not meet the inclusion criteria for the trials were asked to participate in the observational study, where they received usual care). The full protocol is available in Supplement 1. One trial was designed to evaluate radiofrequency denervation for pain from the intervertebral disks. This trial was prematurely terminated because of a lack of eligible participants. The other 3 trials are presented in this article: (1) the facet joint trial, (2) the sacroiliac joint trial, and (3) the combination trial (facet joint, sacroiliac joint, or the intervertebral disk). The Medical Ethics Committee of the Erasmus University Medical Centre in Rotterdam granted ethical approval. Local research governance was obtained from all participating pain clinics. All participants gave written informed consent.
In 16 multidisciplinary pain clinics in the Netherlands, pain specialists consecutively screened participants with chronic low back pain. Inclusion criteria were pain considered to be related to the facet joint, sacroiliac joint, or a combination of the facet joint, sacroiliac joint, or intervertebral disk; aged 18 to 70 years; and no improvement in symptoms after conservative treatment. Medical history and clinical examination followed a standard format and were performed by experienced clinicians to determine the likely source of the pain. To be considered for a diagnostic sacroiliac joint block, at least 3 of 6 provocation tests (compression test; distraction test; Flexion, Abduction, and External Rotation [FABER] test; Gaenslen test; thigh thrust test; Gillett test) had to have positive results.12,13 Participants with suspected isolated facet joint pain or isolated sacroiliac joint pain received a diagnostic anesthetic block prior to randomization and were only randomized if the diagnostic block was positive. Participants with a suspected combination of sources of pain were randomized based on participant history and physical examination prior to receiving the diagnostic blocks. This choice was made for ethical reasons. It would be unethical to give participants in the study multiple diagnostic blocks (ie, a facet joint diagnostic block, a sacroiliac joint diagnostic block, and a provocative discography) before treatment. Furthermore, it is common practice in Dutch pain clinics for participants with chronic low back pain due to facet joints, sacroiliac joints, or intervertebral disks (based on history taking and physical examination) to start with 1 diagnostic block. If the diagnostic block was positive, the intervention was provided. If the diagnostic block was negative, then another block was provided. If the second diagnostic block was positive, the intervention was provided. If the second diagnostic block was negative, the clinician provided a third block. All participants were considered candidates for intervention based on history taking and physical examination. For this reason, participants were randomized and included in the combination trial after history taking and physical examination, if the pain physician suspected that the pain originated from more than 1 source.
Exclusion criteria for all trials were pregnancy, severe psychological problems (determined with psychological questionnaires), involvement in work-related conflicts or claims; body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) higher than 35; or anticoagulant drug therapy or coagulopathy.
Diagnostic Blocks
For the facet joints,14 a 22-gauge needle was inserted to the posterior primary root of the spinal nerve (medial branch) under C-arm fluoroscopy. L3-4, L4-5, and L5-6 were selected for diagnostic blocks. The lateral image was checked to confirm the correct position of the needle, after which 0.5 mL of 2% lidocaine was injected.
For the sacroiliac joints,14 a 25-gauge needle was inserted 3 mm to 10 mm laterally of the sacral foramina S1-3 under fluoroscopy. The correct depth of the needle was confirmed laterally, after which 0.5 mL of 2% lidocaine was injected. The dorsal ramus of L5 was also blocked as described in the Spinal Intervention Society guidelines using 0.5 mL of 2% lidocaine.
The blocks were considered positive if the participant reported 50% or more pain reduction within 30 to 90 minutes after the block.
The current standard for diagnosing discogenic pain is pressure-controlled provocative discography using strict criteria and at least 1 negative control level.15
Randomization and Masking
Participants were randomized using a computerized random number generator (Alea II, Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital), accessed through a password-protected website and maintained independently. Randomization was performed at the individual level by means of block randomization (block size = 4), prestratified for pain clinic. Participants were allocated (1:1) to receive either radiofrequency denervation with a standardized exercise program (intervention group) or a standardized exercise program alone (control group).
Participants and caregivers were not blinded. The Dutch Ministry of Health, Welfare, and Sport requested a pragmatic trial in which existing, commonly applied treatment options would be compared. Data handling, analysis, and interpretation of results were conducted blind to treatment allocation. All participants were sequentially assigned unique numbers. Participants’ expectations and satisfaction16,17 were measured to evaluate a possible risk of bias due to a nonblinded study design.
Interventions
Standardized Exercise Program
All participants received a program based on the Dutch physical therapy guidelines18 in 1 of 102 participating physical therapy practices. The 8- to 12-hour programs focused on quality of movement and behavior, and took place during a 3-month intervention period. More details are available in the study protocol, which is available in Supplement 1. If necessary, participants were referred to psychological care.
Radiofrequency Denervation
Within 1 week after the first exercise session the intervention group received radiofrequency denervation. The technical details of the radiofrequency denervation procedures are included in the eAppendix in Supplement 2.19,20,21,22
Co-Interventions
In both treatment groups, participants were asked to refrain from co-interventions during the intervention period of 3 months (duration of the standardized exercise program). Co-interventions that were not allowed included (but were not limited to) surgery; manual therapy; chiropractic therapy; a change in current, back pain–related medication; or newly prescribed medication. Analgesics were not prescribed, but over-the-counter medication was allowed. Co-interventions or recurrence of the radiofrequency denervation was allowed after the intervention period of 3 months. These interventions were recorded. Psychological care was not considered a co-intervention and was provided when needed to participants in either treatment group.
Outcomes
The primary outcome was pain intensity, measured on an 11-point numerical rating scale (NRS; a score of 0 indicates no pain; 10 indicates worst pain imaginable) 3 months after the intervention.23
Secondary outcomes were global perceived recovery,16 participant satisfaction17 (both measured by the 7-point, categorical Global Perceived Effect scale; a score of 1 indicates fully recovered; 4 indicates no change; 7 indicates worse than ever), functional status (measured by Oswestry Disability Index [ODI]; a score of 0 indicates no restrictions in daily activities; 100 indicates most restrictions in daily activities),24 health-related quality of life (measured by the 3-level EuroQol 5D Health Questionnaire [EQ-5D-3L]; a score of 0 indicates worst imaginable health state; 1 indicates best imaginable health state),25 general health (measured by RAND 36-Item Health Survey [Rand-36], a score of 0 indicates lowest general health score; 100 indicates highest general health score),26 and chronic pain experiences (measured by the West Haven-Yale Multidimensional Pain Inventory; a score of 0 indicates lowest score; 6 indicates highest score).27
The minimal clinically important difference in pain for participants with chronic low back pain was estimated at 2 points or more of the 10-point NRS, a difference of 20 points on the 100-point ODI, and between 0.09 and 0.28 points on the EQ-5D-3L utility score between 0 and 1.28,29 No minimal clinically important differences are known for the other secondary outcomes.
All outcome measures were registered using web-based questionnaires, which were sent at baseline and 3-, 6-, 9-, and 12-month follow-up. Pain intensity, global perceived recovery, and health-related quality of life were also assessed at 3-week follow-up and 6-week follow-up.
Sample Size Calculation
A clinically relevant mean difference of 2 points or more on the NRS28 for pain intensity (SD, 4) was used for the sample size calculation. With a power of 0.9, a 2-sided α of .05, and a correlation of 0.5 for repeated measurements, 85 participants per group were needed. Anticipating potential study withdrawal (20%), a minimum of 204 participants per trial was needed.
Statistical Analyses
Effects were estimated using a maximum likelihood estimation for longitudinal mixed-effects model, under “missing at random” assumptions, including a term for pain clinic, if necessary, based on the likelihood ratio test.30 We used a generalized linear mixed model (logit link) for the post hoc analysis of treatment response for dichotomized outcomes. The same multilevel structure was used for both models. All analyses were conducted in accordance with the intention-to-treat principle.
Regression coefficients or odds ratios (ORs) with 95% CIs were calculated; ORs were converted to relative risks (RRs) using the method of Zhang et al31: RR = OR/[(1 − prevalence in control group) + (prevalence in control group × OR)]. We adjusted for the outcome parameter at baseline, and age, sex (self-reported), BMI, education, smoking, marital status, back pain complaint history, and participant expectations. The effect of interest was the time × treatment interaction. Regression coefficients can be interpreted as mean differences between interventions compared with baseline. Additionally, we calculated the number needed to treat and the unadjusted risk differences as absolute differences between groups. Data were compared between complete and incomplete cases to identify possible selective dropout.
Treatment success for the global perceived recovery was defined as “much recovery” or “complete recovery.” In post hoc analyses, treatment success in pain reduction was defined as either more than 30% or 2 points reduction or more on the NRS pain scale.
No adjustments for multiple comparisons were made. Findings for the secondary outcomes should be interpreted as exploratory.
In 2 sensitivity analyses, participants marked as participants who had protocol violations, and participants who received radiofrequency denervation during follow-up were excluded from the analyses. Additionally, data were compared between complete and incomplete cases. We used MLwiN software (University of Bristol), version 2.22, for the effects models (2-sided significance P < .05).
Results
In total, 251 patients were included in the facet joint trial, 228 patients in the sacroiliac joint trial, and 202 in the combination trial (Figure 1, Figure 2, and Figure 3). The 681 randomized participants had a mean age of 52.2 years, 421 participants were women (61.8%), and the mean baseline pain intensity was 7.1 on the NRS scale. Another 5168 patients were included in the observational part of Mint study.
Figure 1. Flow of Patients Through Enrollment in the 3 Randomized Clinical Trials.
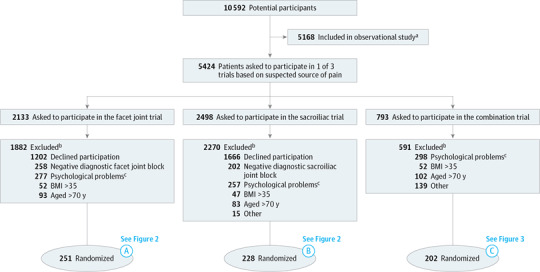
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared).
aObservational study was performed alongside randomized clinical trials; results from the observational study are not reported in this article.
bParticipants not eligible for participation due to 1 positive exclusion criterion or more could be included in the observational study.
cParticipants were excluded based on psychological problems, assessed by validated questionnaires.
Figure 2. Flow of Patients Through the Facet Joint and Sacroiliac Joint Trials.

aParticipants received RF treatment other than their randomized assignment.
bStudy withdrawals were not cumulative.
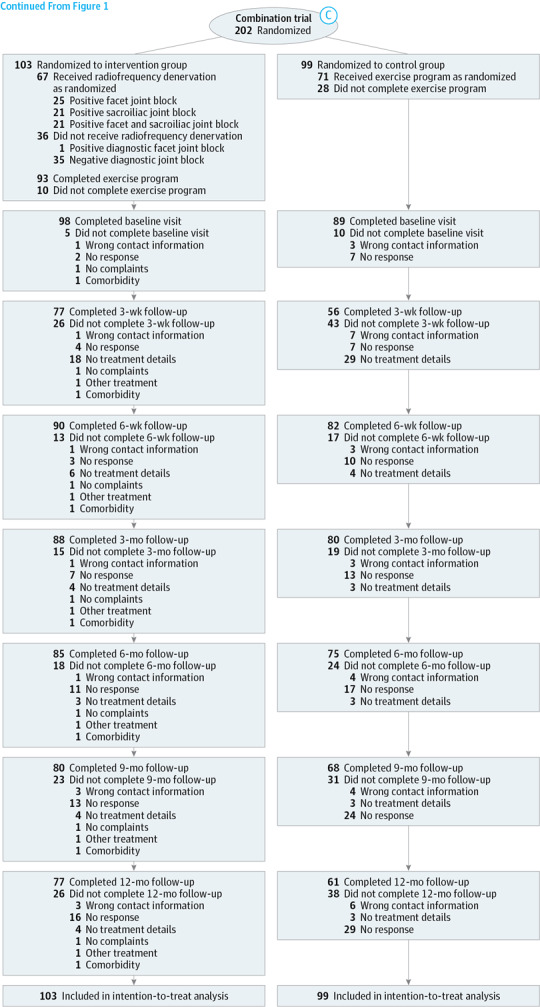
Figure 3. Flow of Patients Through the Combination Trial.
Facet Joint Trial
Study Participants
Between January 1, 2013, and June 3, 2014 (the inclusion period for the facet joint trial), 931 participants received a diagnostic facet joint block. Patients with a negative result for the diagnostic facet joint block (n = 258) were excluded. Patients with psychological problems (n = 277), older than 70 years (n = 93), or with a BMI higher than 35 (n = 52) were followed up in the observational study. The inclusion criteria were met by 251 participants for the facet joint trial and were randomized to the intervention group (n = 125) and control group (n = 126) (Figure 1 and Figure 2).
Baseline characteristics were comparable across groups (Table 1). However, participants in the intervention group had a first low back pain episode 12 years prior compared with 8 years prior in the control group.
Table 1. Baseline Characteristics of Participants With Chronic Low Back Pain.
| Characteristicsa | Facet Joint Trial | Sacroiliac Joint Trial | Combination Trial | |||
|---|---|---|---|---|---|---|
| Intervention (n = 125) |
Control (n = 126) |
Intervention (n = 116) |
Control (n = 112) |
Intervention (n = 103) |
Control (n = 99) |
|
| Age, mean (SD), y | 52.98 (11.48) | 52.60 (10.79) | 51.58 (10.94) | 51.13 (12.22) | 50.80 (11.33) | 53.31 (10.35) |
| Women, No. (%) | 65 (55.56) | 60 (51.72) | 87 (74.35) | 79 (75.96) | 64 (65.31) | 66 (74.15) |
| BMI, mean (SD) | 26.77 (5.17) | 27.62 (4.27) | 26.73 (4.17) | 26.76 (4.53) | 26.84 (3.82) | 26.43 (4.25) |
| Smoker, No. (%) | 34 (29.05) | 34 (29.05) | 29 (26.61) | 31 (29.81) | 23 (23.46) | 26 (29.21) |
| Education level, No. (%)b | ||||||
| Low | 57 (48.72) | 64 (55.17) | 59 (54.13) | 53 (50.96) | 52 (53.06) | 43 (48.31) |
| Moderate | 35 (29.99) | 34 (29.31) | 32 (29.36) | 32 (30.76) | 33 (33.67) | 32 (35.96) |
| High | 21 (17.95) | 16 (13.79) | 18 (16.51) | 18 (17.31) | 12 (12.24) | 14 (15.73) |
| Married or living with a partner, No. (%) | 93 (79.49) | 98 (84.48) | 85 (79.61) | 82 (79.61) | 66 (67.35) | 68 (76.40) |
| Having a paid job, No. (%) | 64 (54.70) | 66 (56.80) | 66 (60.55) | 50 (48.07) | 48 (48.97) | 44 (48.44) |
| History of back pain, median (IQR), mo | ||||||
| Time since first experience with low back pain | 146.00 (49.75-267.67) |
100.33 (36.5-186.30) |
97.33 (37.51-228.12) |
65.08 (27.08-144.21) |
120.58 (37.32-222.04) |
97.33 (32.33-192.58) |
| Time since first current episode with low back pain | 31.33 (12.17-103.42) |
26.73 (10.54-73.00) |
30.33 (12.17-76.03) |
24.33 (12.17-66.58) |
36.50 (12.17-121.67) |
32.33 (8.00-97.19) |
| Origin of back pain, No. | ||||||
| Facet and sacroiliac joint | 69 | 70 | ||||
| Facet and disc | 18 | 18 | ||||
| Sacroiliac joint and disc | 6 | 1 | ||||
| Facet and sacroiliac joint and disc | 3 | 6 | ||||
| Unknown | 7 | 4 | ||||
| CEQ score, mean (SD)c | ||||||
| Credibility | 21.36 (3.92) | 19.47 (5.49) | 21.36 (4.51) | 19.88 (5.31) | 20.10 (4.70) | 17.07 (5.99) |
| Expectancy | 18.97 (4.59) | 17.36 (5.20) | 18.75 (4.99) | 18.23 (5.31) | 16.88 (5.78) | 14.38 (6.24) |
| Pain intensity score in the past week, mean (SD)d | 7.14 (1.38) | 7. 19 (1.29) | 7.17 (1.65) | 7.06 (1.43) | 7.19 (1.43) | 7.43 (1.41) |
| Functioning score, mean (SD)e | 35.07 (14.66) | 34.39 (12.24) | 38.07 (14.07) | 33.70 (14.43) | 39.06 (14.03) | 37.20 (13.74) |
| Quality-of-life score, mean (SD)f | 0.52 (0.26) | 0.54 (0.26) | 0.50 (0.27) | 0.56 (0.27) | 0.49 (0.28) | 0.52 (0.28) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CEQ, credibility expectancy questionnaire.
Results are presented of the 233 participants in the facet joint trial, 207 participants in the sacroiliac joint trial, and 187 participants in the combination trial who had complete baseline data.
Education levels: low indicates preschool, primary school, or lower secondary school; moderate indicates higher secondary school or undergraduate; high indicates tertiary, university, or postgraduate.
A higher score indicates more credibility in the effectiveness of treatment or higher expectations about the treatment (score range, 0-27).
Measured by numeric rating scale (score range, 0-10); a higher score indicates more severe pain intensity.
Measured by Oswestry Disability Index (score range, 0-100); a higher score indicates worse functioning.
Measured by EuroQol-5D (score range, 0-1); a higher score indicates better quality of life.
Complete data on pain intensity, functional status, and global perceived recovery after 3 months was obtained from 233 participants (93%). Complete outcome data on all follow-up points during the year were obtained from 179 participants (71%). Participants with complete data were older, more often nonsmokers, were more likely to have a partner, had a higher BMI, and had low back pain complaints for a longer period (eTable 1 in Supplement 2).
Twelve participants in the control group received radiofrequency denervation within the first 3 months and were marked as participants who had protocol violations. Ten participants (8%) in the intervention group and 11 participants (9%) in the control group received psychological care during the 3-month intervention period.
No treatment-related adverse events were reported during the 1-year follow-up.
Intention-to-Treat Analyses
The mean difference for the primary outcome pain intensity at 3 months was −0.18 (95% CI, −0.76 to 0.40). Results on all other follow-up points are shown in Table 2 and Table 3. The mean difference for functional status at 3 months was −2.45 (95% CI, −5.53 to 1.03); the RR for global perceived recovery at 3 months was 1.35 (95% CI, 0.81 to 2.05). Other follow-up points and secondary outcomes are shown in eTable 2 in Supplement 2.
Table 2. Pain Intensity Score (Primary Outcome)a,b Among Participants With Chronic Low Back Pain.
| Overall Effect | Intervention Group, Mean (95% CI) | Control Group, Mean (95% CI) | Between-Group Difference, Mean (95% CI)c | P Value |
|---|---|---|---|---|
| Facet joint trial, No. of participants | 125 | 126 | ||
| Overall | −0.08 (−0.50 to 0.34) | .71 | ||
| 3 wk | 5.17 (4.73 to 5.61) | 5.92 (5.58 to 6.26) | −0.41 (−1.02 to 0.19) | .18 |
| 6 wk | 5.19 (4.76 to 5.61) | 5.90 (5.53 to 6.26) | −0.38 (−0.96 to 0.20) | .20 |
| 3 mo | 5.01 (4.59 to 5.43) | 5.44 (5.03 to 5.85) | −0.18 (−0.76 to 0.40) | .55 |
| 6 mo | 4.61 (4.18 to 5.04) | 4.84 (4.38 to 5.30) | −0.04 (−0.63 to 0.56) | .91 |
| 9 mo | 4.66 (4.20 to 5.00) | 4.73 (4.24 to 5.22) | 0.19 (−0.41 to 0.80) | .53 |
| 12 mo | 4.49 (4.00 to 4.97) | 4.44 (3.94 to 4.94) | 0.47 (−0.14 to 1.07) | .13 |
| Sacroiliac joint trial, No. of participants | 116 | 112 | ||
| Overall | −0.40 (−0.83 to 0.03) | .07 | ||
| 3 wk | 4.96 (4.51 to 5.40) | 6.00 (5.59 to 6.41) | −0.96 (−1.63 to −0.29) | .005 |
| 6 wk | 5.22 (4.81 to 5.64) | 5.69 (5.31 to 6.08) | −0.53 (−1.17 to 0.10) | .10 |
| 3 mo | 4.77 (4.31 to 5.24) | 5.45 (4.94 to 5.95) | −0.71 (−1.35 to −0.06) | .03 |
| 6 mo | 4.50 (4.01 to 4.98) | 4.78 (4.24 to 5.31) | −0.12 (−0.77 to 0.53) | .73 |
| 9 mo | 5.03 (4.55 to 5.51) | 4.97 (4.39 to 5.56) | 0.16 (−0.51 to 0.83) | .64 |
| 12 mo | 4.65 (4.16 to 5.13) | 4.84 (4.30 to 5.38) | −0.07 (−0.74 to 0.60) | .83 |
| Combination trial, No. of participants | 103 | 99 | ||
| Overall | −0.21 (−0.76 to 0.35) | .47 | ||
| 3 wk | 5.45 (4.95 to 5.95) | 6.40 (5.91 to 6.89) | −0.65 (−1.47 to 0.17) | .12 |
| 6 wk | 5.37 (4.89 to 5.85) | 6.09 (5.65 to 6.52) | −0.40 (−1.14 to 0.34) | .29 |
| 3 mo | 4.77 (4.25 to 5.30) | 5.94 (5.42 to 6.45) | −0.99 (−1.73 to −0.25) | .01 |
| 6 mo | 4.92 (4.39 to 5.44) | 4.95 (4.35 to 5.54) | 0.33 (−0.53 to 1.09) | .39 |
| 9 mo | 5.01 (4.47 to 5.56) | 5.25 (4.65 to 5.86) | −0.05 (−0.82 to 0.73) | .90 |
| 12 mo | 4.85 (4.24 to 5.46) | 4.38 (3.73 to 5.03) | 0.69 (−0.10 to 1.49) | .09 |
Abbreviation: NNT, number needed to treat.
Measured by numeric rating scale (score range, 0-10); a higher score indicates more severe symptoms.
The overall effect measures provide information over the total follow-up time of 12 mo, instead of the time × treatment effects.
Values presented (for mean differences) are model estimates of linear mixed-effects models with a random intercept, and adjusted for outcome at baseline and age, sex, body mass index, education, smoking, marital status, back pain complaint history, and participant expectations. Regression coefficients can be interpreted as mean differences between interventions at a certain follow-up point compared with baseline.
Table 3. Secondary Outcomes Among Participants With Chronic Low Back Paina.
| Overall Effect | Intervention Group, Mean (95% CI) | Control Group, Mean (95% CI) | Between-Group Difference, Mean (95% CI)b | P Value | Risk Difference (95% CI) | NNT |
|---|---|---|---|---|---|---|
| Functioning Score c , d | ||||||
| Facet joint trial, No. of participants | 125 | 126 | ||||
| Overall | 0.04 (−3.02 to 3.10) | .98 | ||||
| 3 mo | 26.03 (23.01 to 29.06) | 28.67 (26.06 to 31.84) | −2.45 (−5.93 to 1.03) | .17 | ||
| 6 mo | 25.38 (22.45 to 28.30) | 27.15 (24.07 to 30.23) | −0.60 (−4.13 to 2.92) | .74 | ||
| 9 mo | 25.74 (22.74 to 28.73) | 24.52 (21.49 to 27.54) | 2.26 (−1.29 to 5.82) | .21 | ||
| 12 mo | 24.59 (21.39 to 27.79) | 25.04 (21.77 to 28.31) | 1.48 (−2.09 to 5.06) | .42 | ||
| Sacroiliac joint trial, No. of participants | 116 | 112 | ||||
| Overall | 0.42 (−2.99 to 3.82) | .81 | ||||
| 3 mo | 27.72 (24.50 to 30.95) | 29.09 (25.47 to 2.71) | −4.20 (−8.39 to −0.00) | .05 | ||
| 6 mo | 25.99 (22.91 to 29.05) | 24.99 (21.45 to 28.52) | 0.07 (−4.16 to 4.30) | .97 | ||
| 9 mo | 28.40 (25.05 to 31.75) | 23.45 (20.00 to 6.91) | 4.45 (0.14 to 8.77) | .04 | ||
| 12 mo | 27.29 (23.89 to 30.69) | 24.49 (20.74 to 28.23) | 2.11 (−2.25 to 6.47) | .34 | ||
| Combination trial, No. of participants | 103 | 99 | ||||
| Overall | 1.90 (−2.96 to 6.76) | .44 | ||||
| 3 mo | 28.00 (24.65 to 31.35) | 33.63 (29.88 to 37.37) | −4.66 (−10.21 to 0.89) | .10 | ||
| 6 mo | 30.24 (26.14 to 34.34) | 28.61 (24.80 to 32.43) | 4.44 (−1.18 to 0.06) | .12 | ||
| 9 mo | 30.73 (26.83 to 34.63) | 28.70 (24.48 to 32.91) | 3.55 (−2.17 to 9.26) | .22 | ||
| 12 mo | 31.20 (27.20 to 35.20) | 24.67 (20.88 to 28.45) | 6.44 (0.61 to 12.26) | .03 | ||
| Global Perceived Recovery e | ||||||
| No. With Treatment Success/Total No. (%) | No. With Treatment Success/Total No. (%) | Relative Risk (95% CI) f | P Value | Risk Difference (95% CI) | NNT | |
| Facet joint trial, No. of participants | 125 | 126 | ||||
| 3 wk | 32/108 (29.63) | 5/101 (4.95) | 5.41 (2.29 to 10.34) | <.001 | 24.68 (15.08 to 34.27) | 4 |
| 6 wk | 35/119 (29.41) | 11/118 (9.32) | 2.71 (1.37 to 4.68) | .005 | 20.09 (10.37 to 29.81) | 5 |
| 3 mo | 43/119 (36.13) | 27/114 (23.68) | 1.35 (0.81 to 2.05) | .24 | 12.45 (0.81 to 24.09) | 8 |
| 6 mo | 46/113 (40.70) | 39/108 (36.11) | 1.04 (0.64 to 1.12) | .85 | 4.59 (−8.21 to 17.41) | NA |
| 9 mo | 41/106 (38.67) | 42/105 (40.00) | 0.81 (0.48 to 0.57) | .35 | −1.33 (−14.50 to 11.86) | NA |
| 12 mo | 44/103 (42.71) | 40/102 (39.22) | 0.90 (0.55 to 1.33) | .65 | 3.49 (−9.95 to 16.96) | NA |
| Sacroiliac joint trial, No. of participants | 116 | 112 | ||||
| 3 wk | 28/94 (29.78) | 9/88 (10.23) | 2.83 (1.39 to 4.89) | .01 | 19.55 (8.35 to 30.77) | 5 |
| 6 wk | 43/110 (39.09) | 10/95 (10.53) | 3.71 (2.00 to 5.74) | <.001 | 28.56 (17.55 to 39.58) | 4 |
| 3 mo | 43/110 (39.10) | 19/88 (21.59) | 1.87 (1.13 to 2.71) | .02 | 17.51 (4.97 to 30.03) | 6 |
| 6 mo | 46/103 (44.66) | 29/88 (32.95) | 1.26 (0.83 to 1.84) | .21 | 11.71 (−2.03 to 25.44) | NA |
| 9 mo | 36/101 (35.64) | 25/78 (32.05) | 1.13 (0.67 to 1.70) | .62 | 3.59 (−10.35 to 17.54) | NA |
| 12 mo | 49/102 (48.03) | 24/76 (31.78) | 1.46 (0.92 to 2.02) | .10 | 16.25 (2.20 to 30.72) | NA |
| Combination trial, No. of participants | 103 | 99 | ||||
| 3 wk | 17/77 (22.07) | 4/56 (7.14) | 2.23 (0.73 to 5.52) | .15 | 14.93 (3.48 to 25.40) | 6 |
| 6 wk | 25/90 (27.77) | 7/82 (8.54) | 2.41 (0.99 to 4.90) | .05 | 19.23 (8.19 to 30.30) | 5 |
| 3 mo | 30/88 (34.09) | 13/80 (16.25) | 1.99 (0.99 to 3.37) | .06 | 17.84 (5.06 to 30.63) | 5 |
| 6 mo | 30/85 (35.29) | 28/75 (37.33) | 0.76 (0.39 to 1.30) | .36 | −2.04 (−16.97 to 12.90) | NA |
| 9 mo | 29/82 (35.36) | 21/68 (30.88) | 1.11 (0.57 to 1.82) | .73 | 4.48 (−10.61 to 19.57) | NA |
| 12 mo | 26/75 (34.66) | 22/61 (36.06) | 0.91 (0.46 to 1.52) | .76 | −1.40 (−17.65 to 14.76) | NA |
Abbreviations: NA, not applicable; NNT, number needed to treat.
The other secondary outcomes are presented in eTable 2 in Supplement 2.
Values presented (for mean differences) are model estimates of linear mixed-effects models with a random intercept, and adjusted for outcome at baseline and age, sex, body mass index, education, smoking, marital status, back pain complaint history, and participant expectations. Regression coefficients can be interpreted as mean differences between interventions at a certain follow-up point compared with baseline.
Measured by Oswestry Disability Index (score range, 0-100); a higher score indicates worse functioning.
The overall effect measures provide information over the total follow-up time of 12 mo, instead of the time × treatment effects.
Measured by the Global Perceived Effect scale (range, 1-7); a score of 1 to 2 indicates success.
Relative risk was estimated based on the method of Zhang et al.31
Post Hoc Analyses of Treatment Response
No significant differences between the groups were found when success was defined as more than 30% or 2 points reduction or more in pain at 3 months (Table 4).
Table 4. Successful Treatment Effects for Pain Intensity by Study Among Participants With Chronic Low Back Pain.
| Intervention Group, No.With Treatment Success/Total No. (%) |
Control Group, No. With Treatment Success/Total No. (%) |
Relative Risk (95% CI)a | P Value | Risk Difference (95% CI) | NNT | |
|---|---|---|---|---|---|---|
| Facet Joint Trial | ||||||
| Pain intensity reduction >30% | ||||||
| 3 wk | 40/102 (39.22) | 27/100 (27.00) | 1.33 (0.80 to 1.97) | .25 | 12.22 (−0.65 to 25.08) | NA |
| 6 wk | 45/112 (40.17) | 36/114 (31.57) | 1.13 (0.70 to 1.63) | .59 | 8.60 (−3.86 to 21.06) | NA |
| 3 mo | 52/114 (45.61) | 40/111 (36.03) | 1.16 (0.76 to 1.60) | .46 | 9.58 (−3.20 to 22.36) | NA |
| 6 mo | 60/108 (55.56) | 53/105 (50.47) | 1.02 (0.71 to 1.33) | .88 | 5.09 (−8.31 to 18.47) | NA |
| 9 mo | 52/102 (50.98) | 50/102 (49.02) | 1.09 (0.75 to 1.42) | .60 | 1.88 (−11.76 to 15.68) | NA |
| 12 mo | 47/100 (47.00) | 53/99 (53.53) | 0.78 (0.50 to 1.09) | .16 | −6.53 (−20.40 to 7.33) | NA |
| Pain intensity reduction ≥2 points | ||||||
| 3 wk | 56/102 (54.90) | 44/100 (44.00) | 1.17 (0.81 to 1.53) | .36 | 10.90 (−2.81 to 24.61) | NA |
| 6 wk | 57/112 (50.89) | 47/114 (41.23) | 1.09 (0.74 to 1.46) | .65 | 9.66 (−3.27 to 22.60) | NA |
| 3 mo | 64/111 (57.65) | 52/111 (46.85) | 1.07 (0.75 to 1.39) | .68 | 10.80 (−2.25 to 23.87) | NA |
| 6 mo | 68/108 (62.96) | 61/105 (58.09) | 1.00 (0.73 to 1.25) | .98 | 4.84 (−8.25 to 17.98) | NA |
| 9 mo | 56/102 (54.90) | 58/102 (56.86) | 0.90 (0.62 to 1.17) | .47 | −1.96 (−15.59 to 11.66) | NA |
| 12 mo | 55/100 (55.00) | 55/99 (55.56) | 0.76 (0.49 to 1.05) | .11 | −0.56 (−14.37 to 13.26) | NA |
| Sacroiliac Joint Trial | ||||||
| Pain intensity reduction >30% | ||||||
| 3 wk | 41/90 (45.56) | 16/83 (19.27) | 2.35 (1.45 to 3.32) | .001 | 26.29 (12.94 to 39.62) | 4 |
| 6 wk | 43/104 (41.35) | 25/91 (27.47) | 1.49 (0.94 to 2.18) | .08 | 13.88 (0.69 to 27.05) | 7 |
| 3 mo | 48/105 (45.71) | 29/84 (34.52) | 1.33 (0.87 to 1.81) | .16 | 11.19 (−2.74 to 25.13) | NA |
| 6 mo | 50/99 (50.51) | 42/85 (49.41) | 1.01 (0.69 to 1.34) | .94 | 1.10 (−13.40 to 15.58) | NA |
| 9 mo | 39/98 (39.79) | 33/76 (43.42) | 0.88 (0.54 to 1.27) | .53 | −3.63 (−18.39 to 11.14) | NA |
| 12 mo | 48/97 (49.48) | 31/75 (41.33) | 1.15 (0.75 to 1.56) | .48 | 8.15 (−6.79 to 23.09) | NA |
| Pain intensity reduction ≥2 points | ||||||
| 3 wk | 56/90 (62.22) | 30/83 (36.14) | 1.68 (1.25 to 2.05) | .002 | 26.08 (11.68 to 40.47) | 4 |
| 6 wk | 59/104 (56.73) | 40/91 (43.95) | 1.29 (0.97 to 1.59) | .08 | 12.78 (−1.18 to 26.73) | NA |
| 3 mo | 62/105 (59.05) | 40/84 (47.61) | 1.25 (0.94 to 1.52) | .11 | 11.44 (−2.80 to 25.66) | NA |
| 6 mo | 61/99 (61.61) | 47/85 (55.29) | 1.12 (0.85 to 1.35) | .37 | 6.32 (−7.94 to 20.59) | NA |
| 9 mo | 51/98 (52.04) | 41/76 (53.95) | 0.96 (0.68 to 1.22) | .76 | −1.91 (−16.85 to 13.04) | NA |
| 12 mo | 57/97 (58.76) | 41/75 (54.67) | 1.04 (0.76 to 1.30) | .77 | 4.09 (−10.83 to 19.03) | NA |
| Combination Trial | ||||||
| Pain intensity reduction >30% | ||||||
| 3 wk | 23/75 (30.67) | 7/48 (14.58) | 2.39 (1.08 to 4.16) | .03 | 16.09 (1.64 to 30.53) | 6 |
| 6 wk | 32/88 (36.36) | 21/72 (29.17) | 1.16 (0.63 to 1.84) | .60 | 7.19 (−7.34 to 21.73) | NA |
| 3 mo | 43/86 (50.00) | 19/72 (26.38) | 1.92 (1.19 to 2.65) | .01 | 23.62 (8.94 to 38.28) | 4 |
| 6 mo | 36/82 (43.90) | 38/68 (55.88) | 0.77 (0.44 to 1.11) | .19 | −11.98 (−27.94 to 3.98) | NA |
| 9 mo | 38/81 (46.91) | 26/61 (42.62) | 1.05 (0.62 to 1.52) | .83 | 4.29 (−12.21 to 20.79) | NA |
| 12 mo | 37/75 (49.33) | 32/56 (57.14) | 0.86 (0.52 to 1.21) | .47 | −7.81 (−25.02 to 9.40) | NA |
| Pain intensity reduction ≥2 points | ||||||
| 3 wk | 32/75 (42.67) | 12/48 (25.00) | 1.67 (0.89 to 2.57) | .10 | 17.67 (1.04 to 34.26) | 5 |
| 6 wk | 44/88 (50.00) | 33/72 (45.83) | 0.96 (0.58 to 1.37) | .83 | 4.17 (−11.38 to 19.71) | NA |
| 3 mo | 48/86 (55.81) | 28/72 (38.88) | 1.32 (0.85 to 1.79) | .20 | 16.93 (1.53 to 32.32) | 5 |
| 6 mo | 49/82 (59.76) | 43/68 (63.23) | 0.91 (0.59 to 1.19) | .54 | −3.47 (−19.10 to 12.14) | NA |
| 9 mo | 48/81 (59.25) | 34/61 (55.73) | 0.98 (0.62 to 1.31) | .91 | 3.52 (−12.91 to 19.95) | NA |
| 12 mo | 41/75 (54.67) | 37/56 (66.07) | 0.80 (0.50 to 1.10) | .21 | −11.40 (−28.16 to 5.35) | NA |
Abbreviations: NA, not applicable; NNT, number needed to treat.
Relative risk was estimated based on the method of Zhang et al.31
Sensitivity Analyses
When participants with protocol violations were excluded from the analysis, the interpretation of the outcomes remained similar (eTable 3 in Supplement 2). After 3 months of follow–up, 31 control group participants received radiofrequency denervation. The analyses were repeated excluding participants receiving the intervention after the 3-month intervention period; this did not alter the results either (eTable 4 in Supplement 2). The complete case analysis showed no significant between-group differences for pain intensity, functional status, and global perceived recovery at 3 months (eTable 5 in Supplement 2).
Sacroiliac Joint Trial
Study Participants
Between January 1, 2013, and July 1, 2014 (the inclusion period for the sacroiliac joint trial), 832 participants received a diagnostic sacroiliac joint block. Patients with a negative result for the diagnostic sacroiliac joint block (n = 202) were excluded. Patients with psychological problems (n = 257), older than 70 years (n = 83), or a BMI higher than 35 (n = 47), or other reasons for not participating in the trial (n = 15) were followed up in the observational study. The inclusion criteria were met by 228 participants for the sacroiliac joint trial and were randomized to the intervention group (n = 116) and the control group (n = 112) (Figure 1 and Figure 2).
Baseline characteristics were comparable across groups (Table 1). However, the first episode of low back pain in the intervention group was 97 months before inclusion compared with 65 months in the control group.
Complete data on pain intensity, functional status, and global perceived recovery after 3 months were obtained from 198 participants (87%). Complete outcome data on all follow-up points during the year were obtained from 134 participants (59%). The participants with complete data were older, more often nonsmokers, were more likely to have a partner, and had low back pain complaints for a longer period (eTable 1 in Supplement 2).
Seven participants in the control group received radiofrequency denervation within the first 3 months and were marked as participants who had protocol violations. Seven participants (6%) in the intervention group and 6 participants (5%) in the control group received psychological care during the 3-month intervention period.
There was 1 registered treatment-related complication (vasovagal reaction to treatment).
Intention-to-Treat Analyses
The mean difference for the primary outcome pain intensity at 3 months was −0.71 (95% CI, −1.35 to −0.06). Results on all other follow-up points are shown in Table 2 and Table 3. The mean difference for functional status at 3 months was −4.20 (95% CI, −8.39 to −0.002); the RR for global perceived recovery at 3 months was 1.87 (95% CI, 1.13 to 2.71). Other follow-up points and secondary outcomes are shown in eTable 2 in Supplement 2.
Post Hoc Analyses of Treatment Response
No significant differences between the groups were found when success was defined as more than 30% or 2 points reduction or more in pain at 3 months (Table 4).
Sensitivity Analyses
When participants who had protocol violations were excluded from the analysis, the interpretation of the outcomes remained similar (eTable 3 in Supplement 2). After 3 months of follow–up, 41 control group participants received radiofrequency denervation. Excluding these from the analysis did not change the long-term results (eTable 4 in Supplement 2). The complete case analysis showed no significant between-group differences for the primary outcomes at 3 months than participants without complete data (eTable 5 in Supplement 2).
Combination Trial
Study Participants
Between January 1, 2013, and October 24, 2014 (the inclusion period for participants in this trial), 793 participants were eligible for this trial. The inclusion criteria were met by 202 participants, and those participants were randomly assigned to the intervention (n = 103) and control group (n = 99). All reasons for exclusions are presented in the flow charts (Figure 1 and Figure 3).
Baseline characteristics were comparable in both groups (Table 1).
Complete data on pain intensity, functional status, and global perceived recovery after 3 months were obtained from 168 participants (83%). Complete data on all follow-up assessments were obtained from 89 participants (44%) on the effect measures. Participants with complete data had low back pain complaints for a longer period, but were similar for all other demographic characteristics (eTable 1 in Supplement 2).
Two participants in the control group received radiofrequency denervation, and 2 participants did not receive any treatment. In the intervention group, 11 participants did not receive or it was unknown if they received the standardized exercise program. These 14 participants were considered participants who had protocol violations. Eight participants (8%) in the intervention group and 10 participants (10%) in the control group received psychological care during the 3-month intervention period.
In the intervention group, 35 participants had negative results for diagnostic blocks and did not receive radiofrequency denervation. These participants were still included in the intention-to-treat analyses. The diagnostic block had a positive result for 68 participants, of whom 25 received facet joint radiofrequency denervation, 21 sacroiliac joint radiofrequency denervation, 21 received a combination of radiofrequency denervation treatments (facet and sacroiliac joint radiofrequency denervation), and 1 participant did not receive radiofrequency denervation despite a positive result for the diagnostic block.
One complication was recorded during the 1-year follow-up in the intervention group: a hematoma, causing extra pain. The participant completely recovered.
Intention-to-Treat Analyses
The mean difference for the primary outcome pain intensity at 3 months was −0.99 (95% CI, −1.73 to −0.25). Results on all other follow-up points are shown in Table 2 and Table 3. The mean difference for functional status at 3 months was −4.66 (95% CI, −10.21 to 0.89); the RR for global perceived recovery at 3 months was 1.99 (95% CI, 0.99 to 3.36). Other follow-up points and secondary outcomes are shown in eTable 2 in Supplement 2.
Post Hoc Analyses of Treatment Response
When success was defined as 30% pain reduction (RR, 1.92 [95% CI, 1.19 to 2.65]), there was a statistically significant difference at 3 months favoring the intervention group (Table 4).
Sensitivity Analyses
Excluding participants who had protocol violations from the analysis slightly increased the contrast between the groups, as significantly more people in the intervention group recovered based on global perceived recovery after 3 months (RR, 2.07 [95% CI, 1.02 to 3.43]) (eTable 3 in Supplement 2). After 3 months follow-up, 31 control group participants received radiofrequency denervation. The analyses were repeated without participants receiving the intervention after the 3-month intervention period; this resulted in only minor differences (eTable 4 in Supplement 2). The complete-cases analysis showed no significant between-group differences for the primary outcomes at 3 months (eTable 5 in Supplement 2).
Discussion
In 3 trials, the effects of radiofrequency denervation for participants with chronic low back pain due to facet joints, sacroiliac joints, or a combination of the facet joints, sacroiliac joints, or intervertebral disks in addition to a standardized exercise program were compared with a standardized exercise program alone. The 2 trials assessing radiofrequency denervation for the sacroiliac joints and a combination of the facet joints, sacroiliac joints, or intervertebral disks showed a statistically significant but not clinically important improvement in pain intensity 3 months after the intervention. No clinically important or statistically significant differences between the groups were shown in the trial assessing radiofrequency denervation for facet joint pain. Only small or no effects were found for all secondary outcomes.
Based on this study, radiofrequency denervation is not recommended and should be performed only in a research setting. Patients with chronic low back pain who show no improvement in symptoms after conservative treatment have no clear alternative therapies that have been shown to be effective. Future research regarding the diagnosis and treatment for low back pain in participants with chronic low back pain is necessary and should focus on better participant selection (because there remains a possibility that radiofrequency denervation could be beneficial on a subset of participants) and improvement of the treatment techniques.
Strengths and Limitations
Strengths of these trials are the large sample sizes and stratified randomization that allowed for well-balanced study groups, and the use of outcome measures as recommended by the core outcome set for low back pain research.32 In addition to the primary time point at 3 months, a follow-up of 12 months was included.
This study has several limitations. First, different radiofrequency denervation techniques (cooled radiofrequency denervation, Palisade, and Simplicity III) were used in the sacroiliac joint trial.33,34,35,36 However, the groups were too small for a subgroup analysis. Second, because the aim of the study was to provide evidence of the added value of radiofrequency denervation in a multidisciplinary setting, as done in daily practice, participants and clinicians were not blinded. Evidence suggests that treatment effects for subjective outcomes may be overestimated when outcome assessors (ie, participants, if outcomes are self-reported) are not blinded.37 However, the magnitude of this bias is unknown. The lack of blinding was a significant limitation of the trials, and it is possible that radiofrequency denervation could even be harmful, but the lack of blinding may have made the treatment effect seem null. Also, the short-term differences in global perceived recovery for facet joint and sacroiliac joint radiofrequency denervation in absence of a difference in functional status might be the result of a nonspecific effect due to the nonblinded study design.
Third, a reference standard for diagnosing facet joint or sacroiliac joint pain is not available.14 In this pragmatic study, diagnostic tests that are commonly applied in clinical practice were used. Controversy concerning the ideal threshold value of pain reduction in the diagnostic blocks exists. A 50% cutoff was most frequently used in previous studies38 and in clinical practice. Performing 2 or more independent diagnostic blocks will decrease the false-positive rate, but increase the number of false-negative blocks.38 Furthermore, a clinical trial showed that multiple blocks are not cost-effective.38
Fourth, the generalizability of the results might be reduced by the large number of people excluded for psychological problems. In the Netherlands, participants visiting a pain clinic often have long-lasting persistent low back pain. A large number of these participants have psychological problems. These participants were excluded from this study because in daily practice they are not considered candidates for radiofrequency denervation and will be referred to psychological treatment.
Fifth, in all 3 trials, some control group participants received radiofrequency denervation after the 3-month intervention period (25% in the facet joint trial, 35% in sacroiliac joint trial, and 31% in the combination trial) and some intervention group participants received a second radiofrequency denervation (8% in the facet joint trial, 17% in the sacroiliac joint trial, and 15% in the combination trial). This could have influenced the long-term outcomes. However, sensitivity analyses without these participants showed similar results.
Sixth, in the sacroiliac joint trial, there was a higher dropout in the control group. This could potentially have biased the long-term results.
Seventh, in the combination trial, not all participants in the intervention group received radiofrequency denervation, because they did not respond to the diagnostic block or provocative discography.
Eighth, more missing data were found in the combination trial compared with the other 2 trials. This is a potential limitation, but because of the relatively large number of dropouts at 12 months, the complete case analysis might also be biased. Although we did not define differences between the complete-case analysis and the intention-to-treat analysis using all data, it is possible that completers are different from noncompleters, which could have biased the results of the complete-case analyses.
Ninth, we assessed multiple outcomes and made no adjustment for multiple comparisons, which could have resulted in some statistically significant findings by chance.
Comparison With the Literature
Recent systematic reviews have evaluated the association of radiofrequency denervation with isolated pain sources and showed evidence of low to moderate quality for associations of facet joint radiofrequency with small positive effects on pain and functional status compared with placebo or steroid injections.9,10,11 There is very low to moderate quality evidence and conflicting evidence for sacroiliac joint radiofrequency denervation and radiofrequency denervation in the intervertebral disk.5,9,10,11 In the trials included in these reviews, participants had a baseline pain score of 1 point lower compared with this trial, and the radiofrequency denervation groups decreased more (to 3.3 of 10) than the placebo groups (to 5.0 of 10). In the Mint study, participants in both groups decreased in pain, but both groups continued to have a higher pain level compared with other similar trials.
Conclusions
In 3 randomized clinical trials of participants with chronic low back pain originating in the facet joints, sacroiliac joints, or a combination of facet joints, sacroiliac joints, or intervertebral disks, radiofrequency denervation combined with a standardized exercise program resulted in either no improvement or no clinically important improvement in chronic low back pain compared with a standardized exercise program alone. The findings do not support the use of radiofrequency denervation to treat chronic low back pain from these sources.
Trial Protocol and Statistical Analysis
eAppendix. Description Radiofrequency Denervation Intervention
eTable 1. Baseline Characteristics of Completers vs Non-Completers
eTable 2. Treatment Effects for Secondary Outcomes Based on Intention-to-Treat Analyses
eTable 3. As-Treated Analysis for Pain Intensity, Functional Status, and Global Perceived Recovery, Without Protocol Violators Based on Intention-to-Treat Analyses
eTable 4. Treatment Effects for Pain Intensity, Functional Status, and Global Perceived Recovery Based on an As-Treated After 3 Months
eTable 5. Treatment Effects for Complete Cases for Pain Intensity, Functional Status, and Global Perceived Recovery Based on Intention-to-Treat Analyses
eReferences.
References
- 1.Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656-664. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010 [correction appears in Lancet. 2013;381(9867):628]. Lancet. 2012;380(9859):2163-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21-24. [DOI] [PubMed] [Google Scholar]
- 4.Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996-2013. JAMA. 2016;316(24):2627-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Zundert J, Hartrick C, Lataster A, Huygen F, Mekhail N, van Kleef MPJ. Evidence-Based Interventional Pain Practice: According to Clinical Diagnoses. Oxford, UK: Wiley-Blackwell; 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bogduk N. The anatomical basis for spinal pain syndromes. J Manipulative Physiol Ther. 1995;18(9):603-605. [PubMed] [Google Scholar]
- 7.Manchikanti L, Hirsch JA, Pampati V, Boswell MV. Utilization of facet joint and sacroiliac joint interventions in Medicare population from 2000 to 2014: explosive growth continues! Curr Pain Headache Rep. 2016;20(10):58. [DOI] [PubMed] [Google Scholar]
- 8.Koes BW, van Tulder M, Lin C-WC, Macedo LG, McAuley J, Maher C. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maas ET, Ostelo RW, Niemisto L, et al. Radiofrequency denervation for chronic low back pain. Cochrane Database Syst Rev. 2015;10(10):CD008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henschke N, Kuijpers T, Rubinstein SM, et al. Injection therapy and denervation procedures for chronic low-back pain: a systematic review. Eur Spine J. 2010;19(9):1425-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maas ET, Juch JN, Groeneweg JG, et al. Cost-effectiveness of minimal interventional procedures for chronic mechanical low back pain: design of four randomised controlled trials with an economic evaluation. BMC Musculoskelet Disord. 2012;13:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RSGM. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368. [DOI] [PubMed] [Google Scholar]
- 13.Hancock MJ, Maher CG, Latimer J, et al. Systematic review of tests to identify the disc, SIJ or facet joint as the source of low back pain. Eur Spine J. 2007;16(10):1539-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogduk N. Practice Guidelines for Spinal Diagnostic and Treatment Procedures. Hinsdale, IL: International Spine Intervention Society; 2004. [Google Scholar]
- 15.Kallewaard JW, Terheggen MA, Groen GJ, et al. 15: Discogenic low back pain. Pain Pract. 2010;10(6):560-579. [DOI] [PubMed] [Google Scholar]
- 16.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73-86. [DOI] [PubMed] [Google Scholar]
- 17.Bombardier C. Outcome assessments in the evaluation of treatment of spinal disorders: summary and general recommendations. Spine (Phila Pa 1976). 2000;25(24):3100-3103. [DOI] [PubMed] [Google Scholar]
- 18.Hoeijenbos M, Bekkering T, Lamers L, Hendriks E, van Tulder M, Koopmanschap M. Cost-effectiveness of an active implementation strategy for the Dutch physiotherapy guideline for low back pain. Health Policy. 2005;75(1):85-98. [DOI] [PubMed] [Google Scholar]
- 19.Cohen SP, Hurley RW, Buckenmaier CC III, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosman ER Jr, Gonzalez CD. Bipolar radiofrequency lesion geometry: implications for palisade treatment of sacroiliac joint pain. Pain Pract. 2011;11(1):3-22. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt PC, Pino CA, Vorenkamp KE. Sacroiliac joint radiofrequency ablation with a multilesion probe: a case series of 60 patients. Anesth Analg. 2014;119(2):460-462. [DOI] [PubMed] [Google Scholar]
- 22.Gauci CA, Jankowiak B. Manual of RF Techniques: A Practical Manual of Radiofrequency Procedures in Chronic Pain Management. Seattle, WA: CoMedical; 2011. [Google Scholar]
- 23.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37(4):378-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25(22):2940-2952. [DOI] [PubMed] [Google Scholar]
- 25.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53-72. [DOI] [PubMed] [Google Scholar]
- 26.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33(5):350-357. [DOI] [PubMed] [Google Scholar]
- 27.Lousberg R, Van Breukelen GJP, Groenman NH, Schmidt AJM, Arntz A, Winter FAM. Psychometric properties of the Multidimensional Pain Inventory, Dutch Language Version (MPI-DLV). Behav Res Ther. 1999;37(2):167-182. [DOI] [PubMed] [Google Scholar]
- 28.Ostelo RWJG, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33(1):90-94. [DOI] [PubMed] [Google Scholar]
- 29.van der Roer N, Ostelo RW, Bekkering GE, van Tulder MW, de Vet HC. Minimal clinically important change for pain intensity, functional status, and general health status in patients with nonspecific low back pain. Spine (Phila Pa 1976). 2006;31(5):578-582. [DOI] [PubMed] [Google Scholar]
- 30.Twisk JWR. Applied Multilevel Analysis: A Practical Guide for medical researchers. Cambridge, UK: Cambridge Univ Press; 2006. [Google Scholar]
- 31.Zhang J, Yu KF. What’s the relative risk? a method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690-1691. [DOI] [PubMed] [Google Scholar]
- 32.Chiarotto A, Deyo RA, Terwee CB, et al. Core outcome domains for clinical trials in non-specific low back pain. Eur Spine J. 2015;24(6):1127-1142. [DOI] [PubMed] [Google Scholar]
- 33.Ferrante FM, King LF, Roche EA, et al. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Reg Anesth Pain Med. 2001;26(2):137-142. [DOI] [PubMed] [Google Scholar]
- 34.Patel N, Gross A, Brown L, Gekht G. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med. 2012;13(3):383-398. [DOI] [PubMed] [Google Scholar]
- 35.Burnham RS, Yasui Y. An alternate method of radiofrequency neurotomy of the sacroiliac joint: a pilot study of the effect on pain, function, and satisfaction. Reg Anesth Pain Med. 2007;32(1):12-19. [DOI] [PubMed] [Google Scholar]
- 36.van Tilburg CW, Schuurmans FA, Stronks DL, Groeneweg JG, Huygen FJ. Randomized sham-controlled double-blind multicenter clinical trial to ascertain the effect of percutaneous radiofrequency treatment for sacroiliac joint pain: three-month results. Clin J Pain. 2016;32(1):921-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahan BC, Cro S, Doré CJ, et al. Reducing bias in open-label trials where blinded outcome assessment is not feasible: strategies from two randomised trials. Trials. 2014;15(1):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen SP, Huang JHY, Brummett C. Facet joint pain—advances in patient selection and treatment. Nat Rev Rheumatol. 2013;9(2):101-116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis
eAppendix. Description Radiofrequency Denervation Intervention
eTable 1. Baseline Characteristics of Completers vs Non-Completers
eTable 2. Treatment Effects for Secondary Outcomes Based on Intention-to-Treat Analyses
eTable 3. As-Treated Analysis for Pain Intensity, Functional Status, and Global Perceived Recovery, Without Protocol Violators Based on Intention-to-Treat Analyses
eTable 4. Treatment Effects for Pain Intensity, Functional Status, and Global Perceived Recovery Based on an As-Treated After 3 Months
eTable 5. Treatment Effects for Complete Cases for Pain Intensity, Functional Status, and Global Perceived Recovery Based on Intention-to-Treat Analyses
eReferences.


