Abstract
Respiratory syncytial virus (RSV) is the most common respiratory pathogen in infants and young children worldwide. Lower respiratory tract infection due to RSV is one of the most common causes of hospitalization for infants, especially those born premature or with chronic lung or heart disease. Furthermore, RSV infection is an important cause of morbidity in adults, particularly in the elderly and immunocompromised individuals. The acute phase of this infection is often followed by episodes of wheezing that recur for months or years and usually lead to a physician diagnosis of asthma. RSV was discovered more than 50 years ago, and despite extensive research to identify pharmacological therapies, the most effective management of this infection remains supportive care. The trial of a formalin-inactivated RSV vaccine in the 1960s resulted in priming the severe illness upon natural infection. Currently, Palivizumab is the only available option for RSV prophylaxis, and because of restricted clinical benefits and high costs, it has been limited to a group of high-risk infants. There are several ongoing trials in preclinical, Phase-I, -II, or -III clinical stages for RSV vaccine development based on various strategies. Here we review the existing available prophylactic options, the current stages of RSV vaccine clinical trials, different strategies, and major hurdles in the development of an effective RSV vaccine.
Introduction
Respiratory syncytial virus (RSV) is one of the leading causes of lower respiratory tract infection (LRTI) worldwide [1]. Historically known as a major cause of bronchiolitis in infants, RSV is now recognized as an important cause of morbidity in adults, particularly in the elderly and immunocompromised individuals [2]. In the United States every year, RSV causes approximately 2.1 million outpatient visits among children younger than 5 years old [3], and 177,000 hospitalizations and 14,000 deaths among adults older than 65 years [2,4]. Epidemiologic data have shown that early life RSV LRTI is a risk factor for persistent wheeze later in life [5].
RSV is a single-stranded RNA virus of the Paramyxoviridae family and contains 10 genes encoding 11 proteins including: small hydrophobic (SH) protein, nucleocapsid associated proteins N, P, L, M2-1, and M2-2, the matrix protein M, nonstructural proteins NS1 and NS2, glycoprotein (G), which mediates viral attachment to the host cell, and fusion (F) protein, which enables fusion of virus with the host cell, thus making it a promising vaccine target. There are two antigenic subtypes of RSV, characterized as A and B, which differ in disease severity mostly based on reactivity to antibodies and amino acid sequence of the RSV G protein [6].
RSV infection is transmitted by direct or indirect contact with nasal or oral secretions. Reinfection is common and prior infection does not result in persistent immunity. The clinical symptoms of RSV usually begin 4–6 days after an incubation period with nasal congestion, rhinorrhea, fever, or cough. In some patients, the disease course may progress to lower airway causing tachypnea, wheeze, increased work of breathing, hypoxemia, or respiratory distress resulting in an emergency room visit or hospital admission [7]. Approximately 20% of infants manifest an apnea episode as the first symptom of infection [8]. While the course of infection and symptoms generally follow a similar pattern in all children, the disease severity is highly variable. Young infants, infants born at a gestational age less than 29 weeks, individuals with chronic lung disease, cyanotic heart disease, complex chronic conditions, hematopoietic cell transplant recipients, or hematologic malignancies are more at risk of severe disease or death [9–11]. Cystic fibrosis has also been recognized as a risk factor for RSV infection [12].
RSV was first isolated in 1955 from nasal secretions of young chimpanzees with rhinorrhea and was named chimpanzee coryza agent (CCA) [13]. A formalin-inactivated RSV (FI-RSV) vaccine was developed and tested in 1966, which unfortunately resulted in a catastrophic vaccine failure, as during that RSV season, 80% of infants given the vaccine developed severe bronchiolitis or pneumonia, compared to only 5% for the placebo group, and two of the vaccinated infants died. The etiology of vaccine-enhanced disease (VED) remains controversial. The autopsies of the two children that died showed increased eosinophil infiltration of the lung parenchyma [14], which indicates a Th2-biased immune response. Further reviewing of autopsies showed enhanced neutrophils and mononuclear cells and high titers of RSV in the lungs, which made the role of eosinophil in VED less critical, but confirmed the role of Th2-associated immune responses [15].
Since then, RSV research has greatly expanded, but the burden of RSV continues to grow worldwide and to date no effective RSV treatment is available aside from supportive care [16]. Different vaccine-development strategies are being explored at different phases of clinical trials. Here we briefly review current and candidate antiviral drugs, prophylactic agents, ongoing vaccine trials and some of the major hurdles that have impeded progress in the development of an effective RSV vaccine.
Antiviral therapy
Ribavirin is a synthetic nucleoside analog with broad in vitro activity against a variety of both RNA and DNA viruses. In 1993, the American Academy of Pediatrics (AAP) Committee on Infectious Diseases supported the use of Ribavirin, which has remained the only antiviral agent licensed for the therapy of severe RSV infections [17]. However, the recommendation changed to “may be considered” in 1996. The use of Ribavirin has been limited to immunocompromised patients with RSV due to the inconvenient route of delivery, which requires prolonged aerosol administration; risks for potential toxicity, such as teratogenic effects during pregnancy; cost of therapy; and need for hospital admission.
Two other RSV inhibitors, GS-5806 and ALS-008176, have both recently completed Phase II clinical trials and appear to be promising antiviral agents. GS-5806, a small oral molecule, blocks viral fusion. GS-5806 treatment resulted in significant reductions of the RSV viral load, the total weight of mucus produced, the total symptom score, and without serious adverse events [18]. ALS-008176, an orally bioavailable prodrug of a cytidine nucleoside analogue, resulted in a reduction of viral load without serious adverse events [19]. However, in this study the baseline of preexisting immune memory of the subjects was not assessed, which could have an effect on the outcome [20].
Immuno-prophylaxis
RSV Immunoglobulin (RSV-IVIG, RespiGam) is a pooled hyperimmune polyclonal immunoglobulin preparation purified from donors with high-RSV-neutralizing antibody titers. When administered, RSV-IVIG significantly reduced hospitalizations in high-risk infants [21]. RSV-IVIG was licensed in 1996, but taken off the market in 2004 because its use was associated with multiple disadvantages, like the need for long intravenous infusion sessions and supervision in a hospital setting, high volume doses resulting in fluid overload in already at-risk infants, and potential risk for blood borne pathogens [22]. Furthermore, immunizations with live-attenuated viruses, such as the measles/mumps/rubella (MMR) vaccine, needed to be postponed until 9 months after RSV-IVIG infusion.
Palivizumab (Synagis), a humanized monoclonal IgG1 antibody produced by recombinant DNA technology that recognizes the F protein of RSV and developed by MedImmune (Gaithersburg, MD, USA) in 1998, has remained the only market-approved protective strategy for RSV in infants at high risk for severe RSV disease [23]. Palivizumab is administered monthly during the RSV season, and has been shown to reduce RSV hospitalizations by 50% compared with placebo. However, its use is currently limited to a subset of high-risk infants due to the high costs. It is currently approved for preterm infants born at <29 weeks of gestation, infants with chronic lung disease (CLD) of prematurity defined as gestational age <32 weeks of gestation and requirement of supplemental oxygen for the first 28 days of life, hemodynamically significant congenital heart disease, and neuromuscular disorders that impair the airway clearance [24].
Motavizumab (MEDI-524, Numax), an affinity-matured derivative of palivizumab, was shown to be more efficient than palivizumab with higher virus neutralizing effects [25]. However, due to cutaneous hypersensitivity reactions in a minority of treated infants, it failed to receive FDA approval [26].
ALX-0171 is an inhaled nanobody, which recently completed Phase I/IIa testing in infants aged 1–24 months hospitalized due to RSV infection and adults (Clinicaltrials.gov identifiers #NCT01483911 and NCT01875926). It reduced viral load and viral replication and had a favorable safety profile. A Phase II clinical trial for children aged 1–24 months started in 2017 (Clinicaltrials.gov identifier #NCT02979431).
REGN2222, a human monoclonal IgG1 antibody against RSV-F, is set to complete Phase III testing (ClinicalTrials.gov identifier #NCT02325791) in preterm infants in April 2017. It was previously shown to be well tolerated in adults.
Summary of current and candidate drugs
Drug candidates for treating RSV infections
Ribavirin: synthetic nucleoside analog antiviral agent.
GS-5806 (Presatovir): inhibitor of the F-protein. Completed a Phase II clinical trial in adults.
ALS-008176: prodrug of a cytidine nucleoside analogue. Completed a Phase IIa clinical trial in adults.
Current treatment or prophylaxis
RSV-IVIG: intravenous immunoglobulin from donors with high titers of RSV-neutralizing antibody, which was not licensed.
Palivizumab (Synagis): monoclonal IgG1 antibody that recognizes the RSV F protein.
Motavizumab (MEDI-524, Numax) affinity-matured derivative of palivizumab, but is not licensed.
ALX-0171: inhaled Nanobody, completed Phase I (NCT01483911 and NCT01875926 in adults), and Phase IIa. A Phase IIb clinical trial for children aged 1–24 months started in 2017.
REGN2222: fully human monoclonal antibody targeting the F protein, evaluated in a Phase III trial in infants < 6 Months (NCT02325791).
RSV vaccines under development
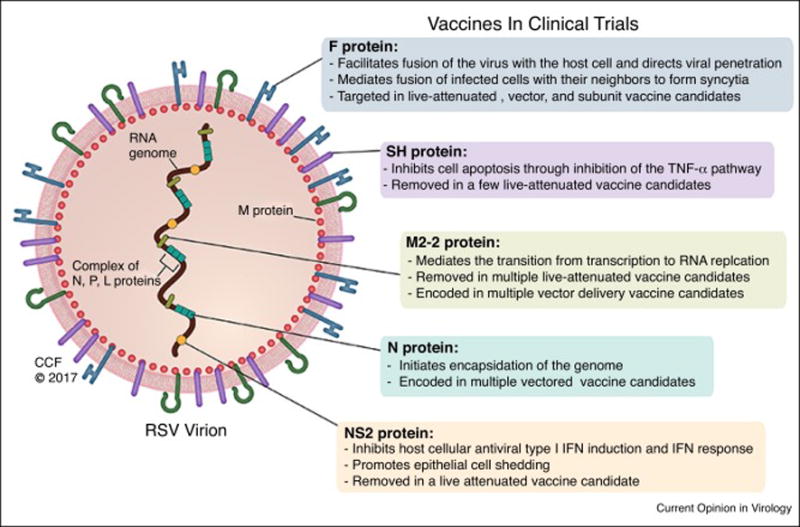
Developing a vaccine against RSV remains a challenge and there is no vaccine for RSV infections on the market as of today. The goal for a vaccine is to induce an immune response to protect against RSV, but at the same time, avoid VED. The particular interest groups for vaccine development are infants and children, elderly, and pregnant women [27]. RSV F comprises a highly conserved amino acid sequence called antigenic site II, between RSV-A and RSV-B antigenic subgroups, and has been considered an important antigen for an RSV vaccine. However, because non-replicating vaccines may elicit enhanced disease in RSV-naïve infants during subsequent infection, replicating or vectored vaccines might be a better choice in this group [28,29]. Additionally, active immunization for infants is challenging due to passive immunity received from the mother. There are a number of different strategies for developing vaccines currently being employed. Some of these strategies have been summarized in Figure 1. Live-attenuated, vector, and protein-based vaccines possess different benefits and drawbacks, and understanding them is essential for vaccine advancement. Because of this, different vaccines may need to be developed for different target populations. The advantages and disadvantages of each vaccine strategy are outlined in Table 1. Table 2 summaries the clinical trials discussed below.
Figure 1. Current and future options for RSV treatment or prophylaxis.
Current available RSV therapies include an antiviral agent and immunoprophylaxis with Palivizumab. No RSV vaccine is currently on the market, but diverse vaccine candidates, targeting different proteins within the RSV virion, are undergoing clinical trials.
Table 1.
Advantages and disadvantages of the main strategy categories for RSV vaccine development.
|
Live-attenuated (For young infants and children <24 months of age) |
Advantages | Disadvantages |
|
|
|
|
Vector delivery system (For young infants and children <24 months of age) |
|
|
|
Protein-based (For pregnant women and elderly) |
|
|
Table 2. Recently completed and current RSV vaccine clinical trials.
A wide array of clinical trials – from Phase I to Phase III, with a variety of vaccine strategies - are currently underway or were recently completed.
| Vaccine Type | Mechanism of Action | Clinical Trials |
|---|---|---|
| Live-attenuated | M2-2 gene deletion | LID ΔM2-2 1030s Phase I: NCT02794870, NCT02952339 LID cp ΔM2-2 Phase I: NCT02948127, NCT02890381 |
| SH gene deletion | MEDI–599 Phase I/IIa: NCT00767416 RSV cps2 Phase I: NCT01852266, NCT01968083 |
|
| NS2 gene deletion | RSV ΔNS2 A1313 I1314L Phase I: NCT01893554 |
|
| PIV3 vector* | MEDI–534 Phase I: NCT00345670, NCT00493285 Phase I/IIa: NCT00686075 |
|
| Vector delivery system | Adenovirus vector | GSK3389245A Phase I: NCT02491463 Phase II: NCT02927873 GSK3003891A Phase I: NCT01905215 Phase II: NCT02360475, NCT02956837, NCT02753413, VXA-RSV-f Phase I: NCT02830932 Ad26.RSV.FA2 and Ad35.RSV.FA2 Phase I: NCT02440035, NCT02561871 |
| Modified Vaccinia Ankara vector | PanAd3-RSV and MVA-RSV Phase I: NCT01805921 MVA-BN Phase I: NCT02419391 |
|
| Protein-based | F-protein nanoparticle, with an aluminum hydroxide adjuvant | Nanoparticle vaccine Phase I: NCT02296463, NCT01290419, NCT01709019, Phase II: NCT02247726, NCT01704365, NCT01960686, NCT02593071, NCT02266628, Phase III: NCT02608502, NCT02624947 |
| Post-fusion F glycoprotein, with glucopyranosyl lipid A adjuvant | MEDI-7510 Phase I: NCT02115815 |
MEDI-534 can be categorized as both a live-attenuated and vector vaccine.
Live-attenuated vaccines
The tragic results of the formalin-inactivated pediatric RSV vaccine in the 1960s resulted in studies to develop live-attenuated RSV vaccine candidates. These vaccines are engineered through passaging the live virus at gradually lower temperatures and deleting part of the genome. The goal is that attenuated virus maintains sufficient viral genome RNA replication to illicit enough antibody response in RSV-naïve infants, yet with a low risk of deattenuation and does not lead to the associated harmful effects [30]. Two substantial advantages of a live-attenuated vaccine are that it is, in theory, safe for RSV-naïve infants because it does not exacerbate future exposure to RSV and it can be administered intranasally. Intranasal administration is beneficial because it can mimic the natural infection, but in a milder form, and lead to viral replication in the upper respiratory tract, thereby inducing mucosal immunity and humoral immunogenicity despite the potential presence of maternal antibodies acquired through placental transfer [31].
Several live-attenuated RSV vaccine candidates are currently undergoing or recently completed clinical trials. A group of these vaccine candidates have deletions of a large segment of the M2-2 gene. The M2-2 gene mediates the transition from transcription to RNA replication [32]. In vitro studies have shown that M2-2 gene deletion leads to decreased viral RNA replication, but at the same time increases F and G protein expression through transcription, which means that the virus is adequately attenuated, yet potentially could lead to augmentation of the neutralizing antibody response [32]. A Phase I, placebo-controlled study (ClinicalTrials.gov identifier #NCT02237209) explored the safety of a LID ΔM2-2 vaccine, delivered as nose drops to RSV-seronegative infants ages 6 to 24 months. This vaccine infected the subjects successfully, but the peak vaccine shedding titers were higher than desirable, and therefore the study was stopped prematurely [33]. Multiple modifications to the LID ΔM2-2 have been established since then to combat the high shedding titers and are currently under investigation. For example, LID ΔM2-2 1030s has a mutation conferring temperature sensitivity and was explored in Phase I, placebo-controlled trials in seronegative infants 6 to 24 months of age (ClinicalTrials.gov identifiers #NCT02794870 and #NCT02952339). LID ΔM2-2 1030s was more restricted in replication than LID ΔM2-2 in mice, thereby suggesting that it is more attenuated. LID cp ΔM2-2, also underwent Phase I investigation in the same subject population, and contains 5 amino acid substitutions compared to LID ΔM2-2 (ClinicalTrials.gov identifiers #NCT02948127, and #NCT02890381). The cp mutations were shown to confer a relatively small amount of attenuation in adults and chimpanzees, and are expected to confer a smaller increment of attenuation than 1030s.
In addition to deleting the M2-2 gene, the SH gene has also been targeted for deletion in live-attenuated vaccine candidates [34]. According to some studies, the SH gene inhibits cell apoptosis through inhibition of the TNF-α pathway [35], while others believe it is involved in viral fusion [36]. The rA2cp248/404/1030ΔSH vaccine, among other mutations, has a complete deletion of the SH gene [29]. However, it was determined that there was restricted antibody response in the subjects, as well as viral phenotypic and genotypic instability primarily due to reversion of the 1030 mutation [29,37]. The MEDI–599 vaccine differs from rA2cp248/404/1030ΔSH by silent nucleotide substitutions throughout the viral genome [29,38]. A Phase I/IIa, placebo-controlled study (ClinicalTrials.gov identifier #NCT00767416), conducted on RSV seronegative infants 5 to <24 months of age and in infants 1 to <3 months of age regardless of baseline serostatus, showed a higher incidence of medically attended LRTI in the experimental group within 28 days after dosing as compared to placebo [38]. The underlining mechanism cannot be explained as the overall number of subjects enrolled in this study was low, and there was no vaccine-associated virus recovered from these subjects. Other ΔSH vaccine candidates include OE4 (RSV-A2-dNS1-dNS2-ΔSH-dGm-Gsnull-line19F) and DB1 (RSV-A2-dNS-ΔSH-BAF), which have both been found to be immunogenic in cotton rats [39,40].
RSV cps2 vaccine shows an increase in stabilization of the 248 mutation and complete stabilization of the 1030 mutation, suggesting there is a smaller likelihood of reverting back to the wild-type genome, as compared to the rA2cp248/404/1030ΔSH virus discussed above. This was attained by replacing amino acids at the 248 and 1030 mutations with leucine and lysine respectively [37,41]. To confirm this, RSV cps2 was shown to be temperature-sensitive and phenotypically and genetically stable in juvenile chimpanzees, as well as attenuated and immunogenic [41]. Two companion Phase I, placebo-controlled studies are currently investigating RSV cps2 vaccines in seronegative infants 6 to 24 months of age (ClinicalTrials.gov identifiers #NCT01852266 and #NCT01968083).
The deletion of the NS2 gene is another potential “knock-out” gene for a live-attenuated vaccine. The RSV NS2 gene is known to promote epithelial cell shedding [42] and Inhibit host cellular antiviral type I IFN induction and IFN response. ΔNS2/Δ1313/1314L, a vaccine candidate with a deleted NS2 gene, is genetically stable and moderately temperature-sensitive [37]. RSV ΔNS2 Δ1313 I1314L vaccine is currently being assessed in a Phase I, placebo-controlled trial in RSV-seropositive children 12 to 59 months of age and RSV-seronegative infants aged 6 to 24 months (ClinicalTrials.gov identifier #NCT01893554). This study is estimated to finish in May 2017.
Using genes from RSV-related viruses, such as parainfluenza, to make a live-attenuated vaccine also appears to be a promising strategy. Three clinical trials– two Phase I, one Phase I/IIa - were recently completed using a MEDI–534 vaccine. MEDI–534 consists of the bovine parainfluenza virus type 3 (PIV3) genome engineered to express RSV F protein [43]. All three were randomized, double-blinded, placebo-controlled, dose-escalation studies. In the first Phase I trial (ClinicalTrials.gov identifier #NCT00345670) healthy RSV and PIV3 seropositive children ages 1–9 were administered MEDI–534. The vaccine had a safety profile similar to placebo, no viral shedding was detected and the vaccine was minimally immunogenic [44], which was a favorable outcome. Ideally, the vaccine dosage should be minimally immunogenic in seropositive children so as to then potentially be satisfactorily attenuated in an RSV-naïve population. To build upon these results, another Phase I trial (ClinicalTrials.gov identifier #NCT00493285) was completed in 2010 with seronegative, healthy children 6 to <24 months of age as subjects. The vaccine’s safety profile was similar to that of placebo, with no vaccine-related adverse effects or illness [44,45]. This suggests that the vaccine is sufficiently attenuated and safe for use in RSV-naïve infants. However, only half of the vaccinated subjects seroresponded to RSV [43], which means that only half of the subjects had RSV-neutralizing antibodies present upon examination. Upon further analysis of virus genome, changes in RSV F gene were observed which could result in reduction in RSV F expression. This raises questions about the genetic stability of the vaccine, also could affect the immunogenicity of the vaccine [43]. A Phase I/IIa trial (ClinicalTrials.gov identifier #NCT00686075) evaluated the safety, tolerability, immunogenicity and viral shedding of MEDI–534 in unscreened infants 2 months of age and seronegative children <24 months of age. A detailed report of the findings has not been published yet.
Vector delivery systems
There are multiple vaccine candidates that take advantage of adenoviruses. These vaccines contain inserted portions of RSV F, N, and M2-1 proteins within an adenovirus or another non-pathogenic virus genome [46] that can act as immune potentiators of delivery systems. The N and M2-1 proteins may improve immune response due to their large amounts of T cell epitopes [47]. Similar to a live virus, vector vaccines increase mucosal IgA and cellular immune responses, yet without the possibility of insufficient attenuation [47]. In addition, adjuvant properties of these vectors could potentially enhance the immune response to the vaccine [48]. GlaxoSmithKline’s ChAd155-RSV (GSK3389245A) and GSK3003891A are RSV vaccine candidates encoded by a chimpanzee-derived adenovector and currently being tested in multiple Phase II trials in subjects aged 18 to 45 years and/or in RSV-seropositive infants aged 12 to 17 months. Another adenoviral-vector based – and oral - RSV vaccine candidate, VXA-RSV-f, expressing the F-protein and a dsRNA adjuvant, is currently being tested in a Phase I, placebo-controlled, dose-ranging study, using subjects aged 18 to 49 years.
Adenoviruses of serotype 35 (Ad35) and serotype 26 (Ad26) are engineered to comprise a nucleotide sequence encoding RSV F protein, which showed efficacy against RSV in mice and cotton rats [49]. Two Phase I, placebo-controlled studies assessed the administration of Ad26.RSV.FA2, given either once or twice, followed by Ad35.RSV.FA2 – or vice versa - to adults aged 18 to 50 years. The first of these studies was completed in June 2016, but no results have been published at this time.
Modified Vaccinia Ankara (MVA), a non-replicating viral vector, is also being evaluated in two different vaccine candidates. PanAd3-RSV and MVA-RSV were both safe and effective in cotton rats, mice, and calves [50] and the vaccine appears to elicit both cellular and humoral responses in a primate model [46]. A vaccine based on the RSV viral proteins F, N and M2-1 encoded by Simian Adenovirus (PanAd3-RSV) and modified Vaccinia Ankara (MVA-RSV) recently completed a Phase I trial in subjects 18 to 75 years of age (ClinicalTrials.gov identifier #NCT01805921). Most adverse effects were mild to moderate, self-limiting at the site of injection and the study concluded that the vaccine was safe and immunogenic [51]. Despite the promising results, no current clinical trial is investigating the vaccine in seronegative infants. MVA-BN (modified Vaccinia Ankara - Bavarian Nordic) is another MVA-based vaccine undergoing investigation shown to be an effective vector by inserting genes driven by the same promoter [52]. It was recently tested in a Phase I, placebo-controlled trial in 18 to 65 year-olds (ClinicalTrials.gov identifiers #NCT02419391). The company announced that vaccinated subjects had high levels of RSV-neutralizing antibodies with no serious adverse events. There is another ongoing Phase II trial with adult subjects aged 55 and older (ClinicalTrials.gov identifier # NCT02873286).
Protein-based vaccines
While live-attenuated or vector vaccines hold the most promise for infants, pregnant women and the elderly are not susceptible to enhanced RSV disease like infants, and therefore RSV protein-based vaccines are likely the most effective candidates. RSV-neutralizing antibody has been shown to be transplacentally transferred from mother to fetus, thereby immunizing the fetus in utero through passive immunity [53]. Consequently, maternal immunization could increase levels of antibody for transplacental transfer [54]. Furthermore, results from experimental studies showed that RSV infection during pregnancy alters the offspring’s’ postnatal immunity, and airway hyperresponsiveness [55]. These findings further elucidate the importance of maternal immunization in preventing RSV infection during infancy and subsequent sequela of infection.
Protein-based vaccine candidates include whole-inactivated viruses, subunit antigens, and particle-based vaccines. There are currently multiple protein-based vaccine candidates undergoing clinical trials. The particle-based vaccines have different sizes and act as adjuvant allowing slow release of the antigen over time [48]. Testing on Novavax’s RSV F-protein nanoparticle vaccine, which consists of nearly the full-length F glycoprotein, was recently completed in different clinical trials and remains ongoing in a couple of other trials. The testing has been conducted both with and without an aluminum hydroxide adjuvant. While most of the trials have had subjects 18 years of age and older, one study (ClinicalTrials.gov identifiers #NCT02296463) had enrolled subjects 24 to <72 months of age. This nanoparticle vaccine prompted transplacental antibody transfer within a guinea pig model, as well as in preliminary studies in humans [56]. Results from the studies showed that, in women 18 to 35 years of age, there were no vaccine-related serious adverse effects and there was a significant increase in RSV/A and RSV/B microneutralization antibodies by day 56 [57]. A Phase III study is evaluating the vaccine in third-trimester pregnant humans aged 18 to 40 years. Another Phase I trial is currently evaluating the safety and immunogenicity of the vaccine in adults ages 60 years and older, who were administered the vaccine – or placebo – in the previous year. Novavax announced in September 2016 that this nanoparticle vaccine failed to be effective in a Phase III trial in subjects 60 years and older. The status of the remaining trials is unknown at this time.
During viral fusion and entry, the F protein transitions from a metastable pre-fusion conformation to a stable post-fusion conformation. Most in vitro RSV-neutralizing antibodies target the prefusion conformation of the F protein. However, the prefusion conformation F protein is likely to prematurely refold into the stable postfusion conformation, both in solution and on the surface of the virions [58]. MEDI-7510 is a subunit RSV vaccine candidate that contains the post-fusion F glycoprotein, with or without a glucopyranosyl lipid A (a synthetic TLR-4 agonist) adjuvant [59]. A Phase I, placebo-controlled study evaluated the safety and immunogenicity of MEDI-7510 in adults 60 years or older (ClinicalTrials.gov identifier #NCT02115815). This study concluded that immune response in subjects who received MEDI-7510 with the adjuvant was significantly higher than for placebo and the safety profile of the vaccine was similar to that of placebo [60]. A Phase IIb trial (ClinicalTrials.gov identifier # NCT02508194) was terminated in November 2016, but a reason for this was not stated.
Conclusions
RSV is one of the most common causes of lower respiratory diseases in infants, young children, and the elderly. However, treatment is limited to supportive care such as supplemental oxygen, mechanical ventilation for severely ill patients, using bronchodilators, corticosteroids, and ribavirin, which are often not effective. Palivizumab has relatively reduced hospitalization in high-risk infants, however its use has been restricted to a subgroup of high-risk infants due to the high cost. A safe and efficacious treatment is not widely available, and currently, there is no licensed vaccine to prevent RSV infection. This lack of vaccine options is partly due to the challenging task of finding the right equilibrium between adequate immunogenicity and attenuation of the vaccine strain in case of live attenuated vaccines. Moreover, non-replicating vaccines can enhance RSV infection in RSV-naïve infants. Therefore, it may be necessary to develop separate vaccines for each at-risk population: neonates and young children, pregnant women, and the elderly. Maternal immunization with a nonreplicating vaccine may provide protection during the first few months of life to decrease the RSV associated morbidity and mortality.
Acknowledgments
This work was supported NIH K08 AI112781 (F.R.), and NIH-NHLBI RO1HL061007 (G.P.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geoghegan S, Erviti A, Caballero MT, Vallone F, Zanone SM, Losada JV, Bianchi A, Acosta PL, Talarico LB, Ferretti A, et al. Mortality due to Respiratory Syncytial Virus. Burden and Risk Factors. Am J Respir Crit Care Med. 2017;195:96–103. doi: 10.1164/rccm.201603-0658OC. • Recent paper reporting a prospective, multicenter study on burden and risk factors for mortality due to RSV infection. [DOI] [PubMed] [Google Scholar]
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. •• Highly cited, comprehensive study of the RSV related problems among young children from three geographically diverse populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClure DL, Kieke BA, Sundaram ME, Simpson MD, Meece JK, Sifakis F, Gasser RA, Jr, Belongia EA. Seasonal incidence of medically attended respiratory syncytial virus infection in a community cohort of adults >/=50 years old. PLoS One. 2014;9:e102586. doi: 10.1371/journal.pone.0102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther. 2011;9:731–745. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilca R, De Serres G, Tremblay M, Vachon ML, Leblanc E, Bergeron MG, Dery P, Boivin G. Distribution and clinical impact of human respiratory syncytial virus genotypes in hospitalized children over 2 winter seasons. J Infect Dis. 2006;193:54–58. doi: 10.1086/498526. [DOI] [PubMed] [Google Scholar]
- 7.Piedimonte G. RSV infections: State of the art. Cleve Clin J Med. 2015;82:S13–18. doi: 10.3949/ccjm.82.s1.03. •• Comprehensive overview of RSV structure, virology, clinical manifestation, and its relation to subsequent recurrent wheezing and asthma. [DOI] [PubMed] [Google Scholar]
- 8.Sabogal C, Auais A, Napchan G, Mager E, Zhou BG, Suguihara C, Bancalari E, Piedimonte G. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res. 2005;57:819–825. doi: 10.1203/01.PDR.0000157679.67227.11. [DOI] [PubMed] [Google Scholar]
- 9.Meissner HC. Viral Bronchiolitis in Children. N Engl J Med. 2016;374:62–72. doi: 10.1056/NEJMra1413456. •• A recent paper reviewing pathogenesis of viral bronchiolitis, host and environmental factors. It summarizes recent American Academy of Pediatrics guidelines for diagnosis, management, and prevention of bronchiolitis. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics Committee on Infectious D, American Academy of Pediatrics Bronchiolitis Guidelines C. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:e620–638. doi: 10.1542/peds.2014-1666. [DOI] [PubMed] [Google Scholar]
- 11.Byington CL, Wilkes J, Korgenski K, Sheng X. Respiratory syncytial virus-associated mortality in hospitalized infants and young children. Pediatrics. 2015;135:e24–31. doi: 10.1542/peds.2014-2151. • Using two national data sources, this paper reviews mortality, morbidity and healthcare cost associated with RSV infection in infants and young children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Solis M, Gartner S, Bosch-Gimenez V, Garcia-Marcos L. Is palivizumab effective as a prophylaxis of respiratory syncytial virus infections in cystic fibrosis patients? A meta-analysis Allergol Immunopathol (Madr) 2015;43:298–303. doi: 10.1016/j.aller.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Blount RE, Jr, Morris JA, Savage RE. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med. 1956;92:544–549. doi: 10.3181/00379727-92-22538. [DOI] [PubMed] [Google Scholar]
- 14.Acosta PL, Caballero MT, Polack FP. Brief History and Characterization of Enhanced Respiratory Syncytial Virus Disease. Clin Vaccine Immunol. 2015;23:189–195. doi: 10.1128/CVI.00609-15. •• A review describing the immunological basis and role of T-helper bias on enhanced RSV disease (ERD) resulted from formalin-inactivated RSV vaccine and how it applies to selecting different strategies for current vaccine candidates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudson CJ, Hartwig SM, Meyerholz DK, Varga SM. RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog. 2015;11:e1004757. doi: 10.1371/journal.ppat.1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol. 2011;46:324–347. doi: 10.1002/ppul.21377. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics Committee on Infectious Diseases. Use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics. 1993;92:501–504. [PubMed] [Google Scholar]
- 18.DeVincenzo JP, Whitley RJ, Mackman RL, Scaglioni-Weinlich C, Harrison L, Farrell E, McBride S, Lambkin-Williams R, Jordan R, Xin Y, et al. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 19.DeVincenzo JP, McClure MW, Symons JA, Fathi H, Westland C, Chanda S, Lambkin-Williams R, Smith P, Zhang Q, Beigelman L, et al. Activity of Oral ALS-008176 in a Respiratory Syncytial Virus Challenge Study. N Engl J Med. 2015;373:2048–2058. doi: 10.1056/NEJMoa1413275. [DOI] [PubMed] [Google Scholar]
- 20.DeVincenzo JP, McClure MW, Fry J. ALS-008176 for Respiratory Syncytial Virus Infection. N Engl J Med. 2016;374:1391–1392. doi: 10.1056/NEJMc1516110. [DOI] [PubMed] [Google Scholar]
- 21.Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics. 1997;99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 22.Roymans D, Koul A. Treatment of Respiratory Syncytial Virus Infection: Past, Present and Future. In: Resch B, editor. Human Respiratory Syncytial Virus Infection. InTech; 2011. [Google Scholar]
- 23.American Academy of Pediatrics Committee on Infectious D, American Academy of Pediatrics Bronchiolitis Guidelines C. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420. doi: 10.1542/peds.2014-1665. • The most recent guidelines regarding the use of palivizumab to reduce the risk of RSV infection in high risk infants. Compared to the guidelines issued in 2012, this one further restricts which infants should receive the prophylaxis. Palivizumab was previously recommended to use in infants born at <32 weeks, but is now restricted to infants born at <29 weeks. The use of palivizumab in infants with hemodynamically significant congenital heart disease is recommended for children <12 months old instead of 24 months old. [DOI] [PubMed] [Google Scholar]
- 24.Meissner HC. More on Viral Bronchiolitis in Children. N Engl J Med. 2016;375:1200. doi: 10.1056/NEJMc1607283. [DOI] [PubMed] [Google Scholar]
- 25.Weisman LE. Motavizumab, a second-generation humanized mAb for the prevention of respiratory syncytial virus infection in high-risk populations. Curr Opin Mol Ther. 2009;11:208–218. [PubMed] [Google Scholar]
- 26.O’Brien KL, Chandran A, Weatherholtz R, Jafri HS, Griffin MP, Bellamy T, Millar EV, Jensen KM, Harris BS, Reid R, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15:1398–1408. doi: 10.1016/S1473-3099(15)00247-9. [DOI] [PubMed] [Google Scholar]
- 27.Roberts JN, Graham BS, Karron RA, Munoz FM, Falsey AR, Anderson LJ, Marshall V, Kim S, Beeler JA. Challenges and opportunities in RSV vaccine development: Meeting report from FDA/NIH workshop. Vaccine. 2016;34:4843–4849. doi: 10.1016/j.vaccine.2016.07.057. ••• Summary of expert RSV Vaccines Workshop in Bethesda 2016, reviewing challenges in RSV vaccine development, such as clinically indicators of vaccine impact on severity of disease and the risk of enhanced respiratory disease (ERD). [DOI] [PubMed] [Google Scholar]
- 28.Wright PF, Karron RA, Belshe RB, Shi JR, Randolph VB, Collins PL, O’Shea AF, Gruber WC, Murphy BR. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25:7372–7378. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, Polack FP, Randolph VB, Deatly A, Hackell J, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–1104. doi: 10.1086/427813. ••• Comprehensive overview of the advantages and disadvantages, as well as the current clinical trials, for live-attenuated RSV vaccines. [DOI] [PubMed] [Google Scholar]
- 30.Le Nouen C, McCarty T, Brown M, Smith ML, Lleras R, Dolan MA, Mehedi M, Yang L, Luongo C, Liang B, et al. Genetic stability of genome-scale deoptimized RNA virus vaccine candidates under selective pressure. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1619242114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:259–284. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bermingham A, Collins PL. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci U S A. 1999;96:11259–11264. doi: 10.1073/pnas.96.20.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McFarland EKR. In: Phase I Placebo-Controlled Study of the Infectivity, Safety and Immunogenicity of a Single Dose of a Recombinant Live-Attenuated Respiratory Syncytial Virus Vaccine, LID ΔM2-2 1030s, Lot RSV#010A, Delivered as Nose Drops to RSV-Seronegative Infants 6 to 24 Months of Age. Office of Clinical Research Policy and Regulatory Operations (OCRPRO), editor. Division of Clinical Research (DCR), National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH); 2016. pp. 16–20. [Google Scholar]
- 34.Bukreyev A, Whitehead SS, Murphy BR, Collins PL. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1997;71:8973–8982. doi: 10.1128/jvi.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuentes S, Tran KC, Luthra P, Teng MN, He B. Function of the respiratory syncytial virus small hydrophobic protein. J Virol. 2007;81:8361–8366. doi: 10.1128/JVI.02717-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Techaarpornkul S, Barretto N, Peeples ME. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J Virol. 2001;75:6825–6834. doi: 10.1128/JVI.75.15.6825-6834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luongo C, Winter CC, Collins PL, Buchholz UJ. Respiratory syncytial virus modified by deletions of the NS2 gene and amino acid S1313 of the L polymerase protein is a temperature-sensitive, live-attenuated vaccine candidate that is phenotypically stable at physiological temperature. J Virol. 2013;87:1985–1996. doi: 10.1128/JVI.02769-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malkin E, Yogev R, Abughali N, Sliman J, Wang CK, Zuo F, Yang CF, Eickhoff M, Esser MT, Tang RS, et al. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLoS One. 2013;8:e77104. doi: 10.1371/journal.pone.0077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rostad CA, Stobart CC, Gilbert BE, Pickles RJ, Hotard AL, Meng J, Blanco JC, Moin SM, Graham BS, Piedra PA, et al. A Recombinant Respiratory Syncytial Virus Vaccine Candidate Attenuated by a Low-Fusion F Protein Is Immunogenic and Protective against Challenge in Cotton Rats. J Virol. 2016;90:7508–7518. doi: 10.1128/JVI.00012-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stobart CC, Rostad CA, Ke Z, Dillard RS, Hampton CM, Strauss JD, Yi H, Hotard AL, Meng J, Pickles RJ, et al. A live RSV vaccine with engineered thermostability is immunogenic in cotton rats despite high attenuation. Nat Commun. 2016;7:13916. doi: 10.1038/ncomms13916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham CKR. In: A Phase I Study of the Safety and Immunogenicity of a Single Dose of the Recombinant Live-Attenuated Respiratory Syncytial Virus Vaccine RSV cps2, Lot RSV#005A, Delivered as Nose Drops to RSV-Seronegative Infants and Children 6 to 24 Months of Age. Regulatory Compliance and Human Subjects Protection Branch (RCHSPB) Division of Clinical Research (DCR)/Office of the Director (OD) The National Institute of Allergy and Infectious Diseases (NIAID), The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), editor. 2013. pp. 10–20. [Google Scholar]
- 42.Liesman RM, Buchholz UJ, Luongo CL, Yang L, Proia AD, DeVincenzo JP, Collins PL, Pickles RJ. RSV-encoded NS2 promotes epithelial cell shedding and distal airway obstruction. J Clin Invest. 2014;124:2219–2233. doi: 10.1172/JCI72948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang CF, Wang CK, Malkin E, Schickli JH, Shambaugh C, Zuo F, Galinski MS, Dubovsky F, Study G. Tang RS. Implication of respiratory syncytial virus (RSV) F transgene sequence heterogeneity observed in Phase 1 evaluation of MEDI-534, a live attenuated parainfluenza type 3 vectored RSV vaccine. Vaccine. 2013;31:2822–2827. doi: 10.1016/j.vaccine.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Gomez M, Mufson MA, Dubovsky F, Knightly C, Zeng W, Losonsky G. Phase-I study MEDI-534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatr Infect Dis J. 2009;28:655–658. doi: 10.1097/INF.0b013e318199c3b1. [DOI] [PubMed] [Google Scholar]
- 45.Bernstein DI, Malkin E, Abughali N, Falloon J, Yi T, Dubovsky F, Investigators M-C Phase 1 study of the safety and immunogenicity of a live, attenuated respiratory syncytial virus and parainfluenza virus type 3 vaccine in seronegative children. Pediatr Infect Dis J. 2012;31:109–114. doi: 10.1097/INF.0b013e31823386f1. [DOI] [PubMed] [Google Scholar]
- 46.Pierantoni A, Esposito ML, Ammendola V, Napolitano F, Grazioli F, Abbate A, Del Sorbo M, Siani L, D’Alise AM, Taglioni A, et al. Mucosal delivery of a vectored RSV vaccine is safe and elicits protective immunity in rodents and nonhuman primates. Mol Ther Methods Clin Dev. 2015;2:15018. doi: 10.1038/mtm.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31(Suppl 2):B209–215. doi: 10.1016/j.vaccine.2012.11.106. •• Discusses the goals, and safety and efficacy concerns for each target population and the considerations for different vaccination strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorquera PA, Tripp RA. Synthetic Biodegradable Microparticle and Nanoparticle Vaccines against the Respiratory Syncytial Virus. Vaccines (Basel) 2016;4 doi: 10.3390/vaccines4040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Widjojoatmodjo MN, Bogaert L, Meek B, Zahn R, Vellinga J, Custers J, Serroyen J, Radosevic K, Schuitemaker H. Recombinant low-seroprevalent adenoviral vectors Ad26 and Ad35 expressing the respiratory syncytial virus (RSV) fusion protein induce protective immunity against RSV infection in cotton rats. Vaccine. 2015;33:5406–5414. doi: 10.1016/j.vaccine.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 50.Green CA, Scarselli E, Voysey M, Capone S, Vitelli A, Nicosia A, Cortese R, Thompson AJ, Sande CS, de Lara C, et al. Safety and immunogenicity of novel respiratory syncytial virus (RSV) vaccines based on the RSV viral proteins F, N and M2-1 encoded by simian adenovirus (PanAd3-RSV) and MVA (MVA-RSV); protocol for an open-label, dose-escalation, single-centre, phase 1 clinical trial in healthy adults. BMJ Open. 2015;5:e008748. doi: 10.1136/bmjopen-2015-008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green CA, Scarselli E, Sande CJ, Thompson AJ, de Lara CM, Taylor KS, Haworth K, Del Sorbo M, Angus B, Siani L, et al. Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci Transl Med. 2015;7:300ra126. doi: 10.1126/scitranslmed.aac5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timm A, Enzinger C, Felder E, Chaplin P. Genetic stability of recombinant MVA-BN. Vaccine. 2006;24:4618–4621. doi: 10.1016/j.vaccine.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 53.Chu HY, Steinhoff MC, Magaret A, Zaman K, Roy E, Langdon G, Formica MA, Walsh EE, Englund JA. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. J Infect Dis. 2014;210:1582–1589. doi: 10.1093/infdis/jiu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Munoz FM. Respiratory syncytial virus in infants: is maternal vaccination a realistic strategy? Curr Opin Infect Dis. 2015;28:221–224. doi: 10.1097/QCO.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 55.Brown PM, Harford TJ, Agrawal V, Yen-Lieberman B, Rezaee F, Piedimonte G. Prenatal Exposure to Respiratory Syncytial Virus Alters Postnatal Immunity and Airway Smooth Muscle Contractility during Early-Life Reinfections. PLoS One. 2017;12:e0168786. doi: 10.1371/journal.pone.0168786. •• Highlights the effect of RSV infection during pregnancy on offspring. By using a rodent model of RSV infection, this paper investigates the mechanisms by which maternal RSV infection alters postnatal immunity, such as changing lower airway immune cell patterns and increasing contractility of airway smooth muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glenn GM, Fries LF, Smith G, Kpamegan E, Lu H, Guebre-Xabier M, Hickman SP, Flyer D. Modeling maternal fetal RSV F vaccine induced antibody transfer in guinea pigs. Vaccine. 2015;33:6488–6492. doi: 10.1016/j.vaccine.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 57.Glenn GM, Fries LF, Thomas DN, Smith G, Kpamegan E, Lu H, Flyer D, Jani D, Hickman SP, Piedra PA. A Randomized, Blinded, Controlled, Dose-Ranging Study of a Respiratory Syncytial Virus Recombinant Fusion (F) Nanoparticle Vaccine in Healthy Women of Childbearing Age. J Infect Dis. 2016;213:411–422. doi: 10.1093/infdis/jiv406. [DOI] [PubMed] [Google Scholar]
- 58.Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, Bisschop IJ, Widjojoatmodjo MN, Zahn R, Schuitemaker H, McLellan JS, et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun. 2015;6:8143. doi: 10.1038/ncomms9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broadbent L, Groves H, Shields MD, Power UF. Respiratory syncytial virus, an ongoing medical dilemma: an expert commentary on respiratory syncytial virus prophylactic and therapeutic pharmaceuticals currently in clinical trials. Influenza Other Respir Viruses. 2015;9:169–178. doi: 10.1111/irv.12313. • Reviews RSV-specific prophylactic, therapeutic candidates, as well as RSV vaccines that entered clinical trials between 2008 and 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Falloon J, Ji F, Curtis C, Bart S, Sheldon E, Krieger D, Dubovsky F, Lambert S, Takas T, Villafana T, et al. A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine. 2016;34:2847–2854. doi: 10.1016/j.vaccine.2016.04.002. [DOI] [PubMed] [Google Scholar]


