Abstract
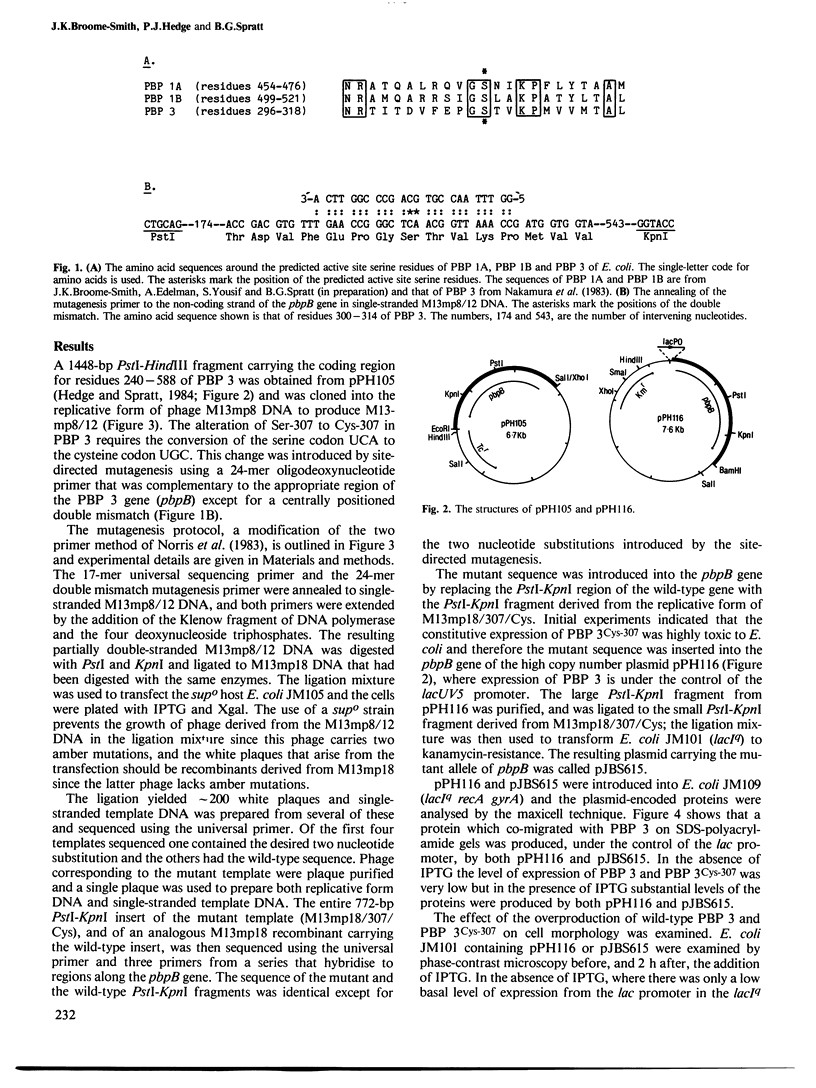
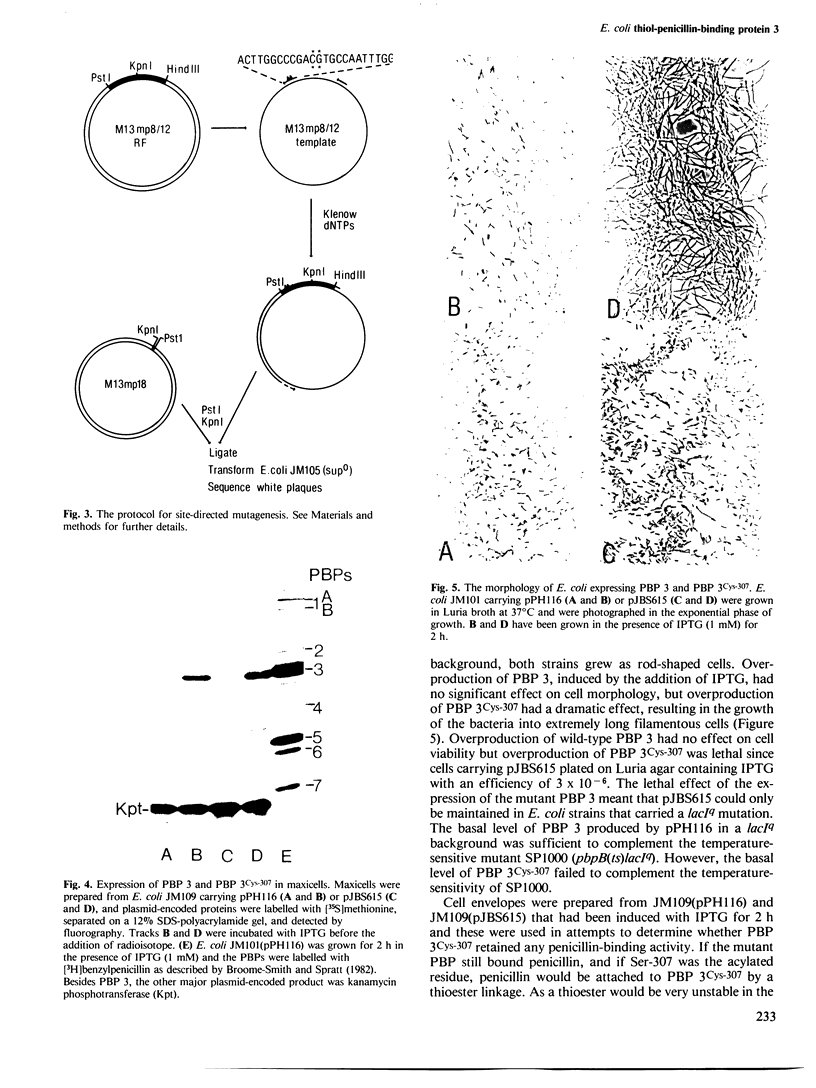
The active site serine residue of penicillin-binding protein 3 of Escherichia coli that is acylated by penicillin (Ser-307) has been converted to a cysteine residue using a simple and efficient two primer method of site-directed mutagenesis. The resulting thiol-penicillin-binding protein 3 was expressed under the control of the lacUV5 promoter in a high copy number plasmid. Constitutive expression of the thiol-enzyme (but not of the wild-type enzyme) was lethal, and the plasmid could only be maintained in E. coli strains that carried the lacIq mutation. Induction of the expression of the thiol-enzyme resulted in inhibition of cell division and the growth of the bacteria into very long filamentous cells. The inhibition of septation was probably due to interference of the function of the wild-type penicillin-binding protein 3 in cell division by the enzymatically inactive thiol-enzyme, and this implies that penicillin-binding protein 3 acts as part of a complex in vivo. We were unable to detect any acylation of the thiol-enzyme by penicillin, but it is not yet clear if this was because the thioester was not formed at an appreciable rate, or if it was formed but was too unstable to be detected by a modified penicillin-binding protein assay.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome-Smith J. K., Spratt B. G. Deletion of the penicillin-binding protein 6 gene of Escherichia coli. J Bacteriol. 1982 Nov;152(2):904–906. doi: 10.1128/jb.152.2.904-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Duez C., Joris B., Frère J. M., Ghuysen J. M., Van Beeumen J. The penicillin-binding site in the exocellular DD-carboxypeptidase-transpeptidase of Actinomadura R39. Biochem J. 1981 Jan 1;193(1):83–86. doi: 10.1042/bj1930083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Duez C., Ghuysen J. M., Vandekerkhove J. Occurrence of a serine residue in the penicillin-binding site of the exocellular DD-carboxy-peptidase-transpeptidase from Streptomyces R61. FEBS Lett. 1976 Nov;70(1):257–260. doi: 10.1016/0014-5793(76)80770-3. [DOI] [PubMed] [Google Scholar]
- Hedge P. J., Spratt B. G. A gene fusion that localises the penicillin-binding domain of penicillin-binding protein 3 of Escherichia coli. FEBS Lett. 1984 Oct 15;176(1):179–184. doi: 10.1016/0014-5793(84)80936-9. [DOI] [PubMed] [Google Scholar]
- Knott-Hunziker V., Petursson S., Jayatilake G. S., Waley S. G., Jaurin B., Grundström T. Active sites of beta-lactamases. The chromosomal beta-lactamases of Pseudomonas aeruginosa and Escherichia coli. Biochem J. 1982 Mar 1;201(3):621–627. doi: 10.1042/bj2010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott-Hunziker V., Waley S. G., Orlek B. S., Sammes P. G. Penicillinase active sites: labelling of serine-44 in beta-lactamase I by 6beta-bromopenicillanic acid. FEBS Lett. 1979 Mar 1;99(1):59–61. doi: 10.1016/0014-5793(79)80248-3. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Takagaki Y., Maruyama I. N., Tamaki S., Nishimura Y., Suzuki H., Ogino U., Hirota Y. Mutants of Escherichia coli lacking in highly penicillin-sensitive D-alanine carboxypeptidase activity. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2976–2979. doi: 10.1073/pnas.74.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Maruyama I. N., Soma M., Kato J., Suzuki H., Horota Y. On the process of cellular division in Escherichia coli: nucleotide sequence of the gene for penicillin-binding protein 3. Mol Gen Genet. 1983;191(1):1–9. doi: 10.1007/BF00330881. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Nanci A., Koshland D. E., Jr Properties of thiol-subtilisin. The consequences of converting the active serine residue to cysteine in a serine protease. J Biol Chem. 1968 Dec 25;243(24):6392–6401. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Norris K., Norris F., Christiansen L., Fiil N. Efficient site-directed mutagenesis by simultaneous use of two primers. Nucleic Acids Res. 1983 Aug 11;11(15):5103–5112. doi: 10.1093/nar/11.15.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal I. S., DeGrado W. F., Thomas B. J., Petteway S. R., Jr Purification and properties of thiol beta-lactamase. A mutant of pBR322 beta-lactamase in which the active site serine has been replaced with cysteine. J Biol Chem. 1984 Apr 25;259(8):5327–5332. [PubMed] [Google Scholar]
- Sigal I. S., Harwood B. G., Arentzen R. Thiol-beta-lactamase: replacement of the active-site serine of RTEM beta-lactamase by a cysteine residue. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7157–7160. doi: 10.1073/pnas.79.23.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Deletion of the penicillin-binding protein 5 gene of Escherichia coli. J Bacteriol. 1980 Dec;144(3):1190–1192. doi: 10.1128/jb.144.3.1190-1192.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Yocum R. R., Amanuma H., O'Brien T. A., Waxman D. J., Strominger J. L. Penicillin is an active-site inhibitor for four genera of bacteria. J Bacteriol. 1982 Mar;149(3):1150–1153. doi: 10.1128/jb.149.3.1150-1153.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum R. R., Rasmussen J. R., Strominger J. L. The mechanism of action of penicillin. Penicillin acylates the active site of Bacillus stearothermophilus D-alanine carboxypeptidase. J Biol Chem. 1980 May 10;255(9):3977–3986. [PubMed] [Google Scholar]