Treatment-free remission (TFR) is a new therapy goal for patients with chronic phase chronic myeloid leukemia (CML) receiving tyrosine kinase inhibitors (TKIs), with approximately 40% sustaining deep molecular responses after stopping treatment.1–3 However, it is difficult to predict precisely who will achieve TFR and the subject remains controversial.3 We present data from 64 patients who stopped TKI therapy. Data show a significant association between the type of BCR-ABL1 transcript and age on the probability of TFR.
Subjects were in 1st chronic phase and had a deep molecular response (≥MR4 on the International Scale) for one year or more before stopping TKI therapy, equivalent to the eligibility criteria of the Euro-Ski trial.4 Molecular response level was calculated by standard criteria5 and molecular relapse defined as loss of MR3. Time to molecular relapse was measured from the date of TKI discontinuation to the first of 2 or more consecutive quantitative real-time polymerase chain reaction (qRT-PCR) assessments confirming less than MR3.
Treatment-free remission was defined as the interval between the date of stopping TKI therapy and the date of molecular relapse or, if this did not happen, the date of last contact. Continuous variables were dichotomized to assess prognostic values for TFR using the median value. Sensitivity analysis was performed for these variables excluding outlier values. To explore the impact of age, we interrogated cut-off points at the median age (51 years) and at ages 40 and 60 years. P<0.05 (two-tailed) was considered significant. Potential predictive variables for TFR were analyzed in univariate analysis using the Kaplan-Meier method. Only statistically significant variables were included in multivariate analysis using a Cox proportional hazard regression model.
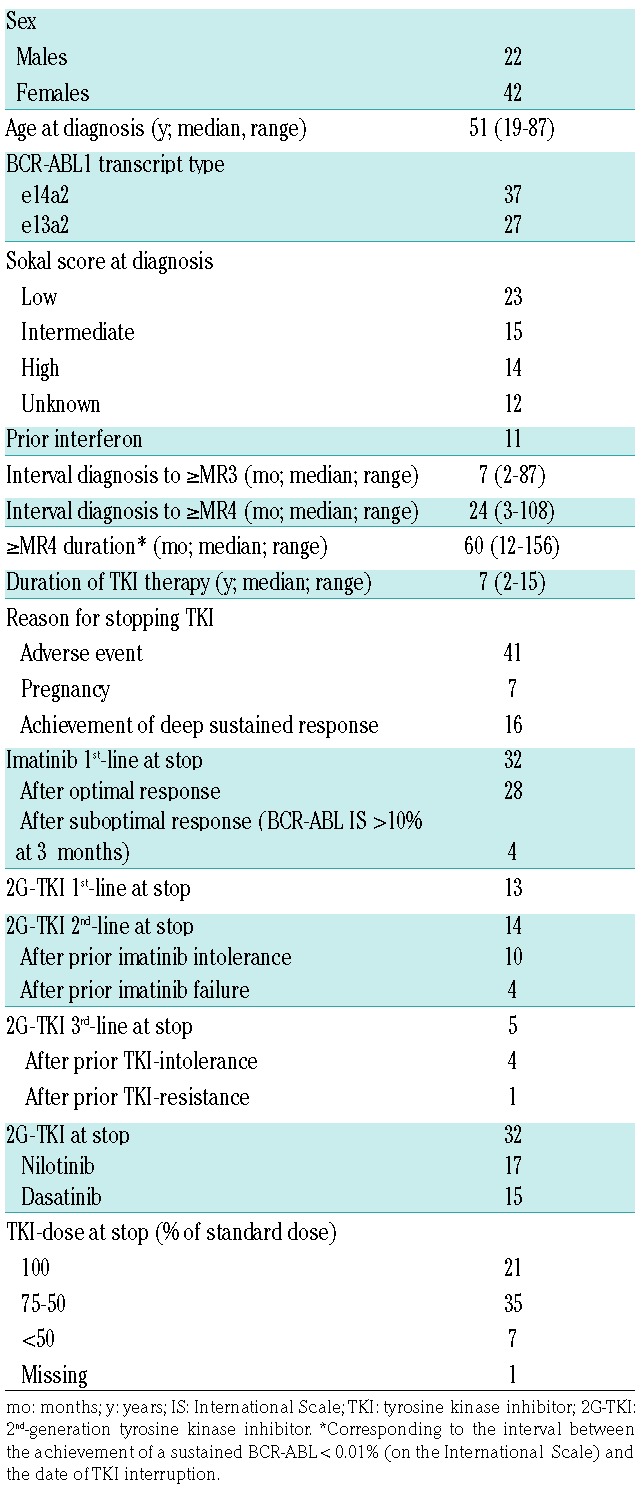
Subject-, disease- and therapy-related variables before stopping TKI therapy are shown in Table 1. Median follow up from stopping TKI therapy was 26 months (range 6–121 months). Forty-one subjects [64%; 95% confidence interval (CI): 53, 75%)] stopped TKI because of intolerance, 7 (11%; 95%CI: 5, 19%) in order to conceive, and 16 (25%; 95%CI: 14, 36%) were elected to stop treatment on achieving a sustained deep response. At the time of discontinuing TKI, 32 subjects (50%; 95%CI: 38, 63%) were receiving imatinib and 32 (50%; 95%CI: 38, 63%), dasatinib or nilotinib. The frequency of patients with e13a2 (42%) or e14a2 transcripts (58%) is similar to that reported within the European LeukemiaNet (ELN) registry, at 45% and 55%, respectively.6
Table 1.
Subject-, disease- and therapy-related variables (n=64).
Thirty-seven subjects (58%; 95%CI: 45, 70%) remain in molecular remission at a median of 26 months (range 7–64 months) after stopping TKI therapy. The 3-year actuarial probability of TFR is 53% (95%CI: 38, 66%). Twenty-seven subjects (42%; 95%CI: 30, 55%) had a molecular relapse at a median of four months (range 1–30 months) after stopping TKI therapy.
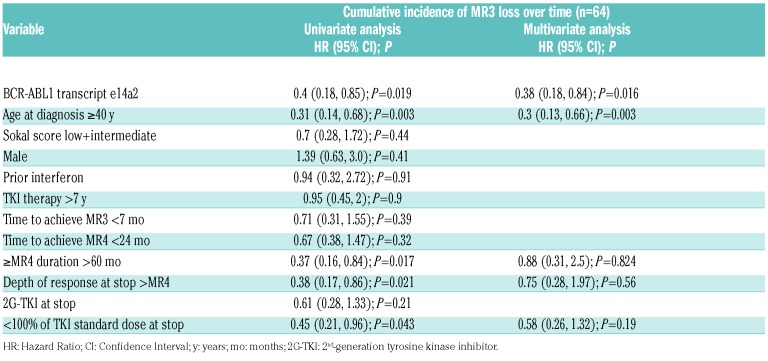
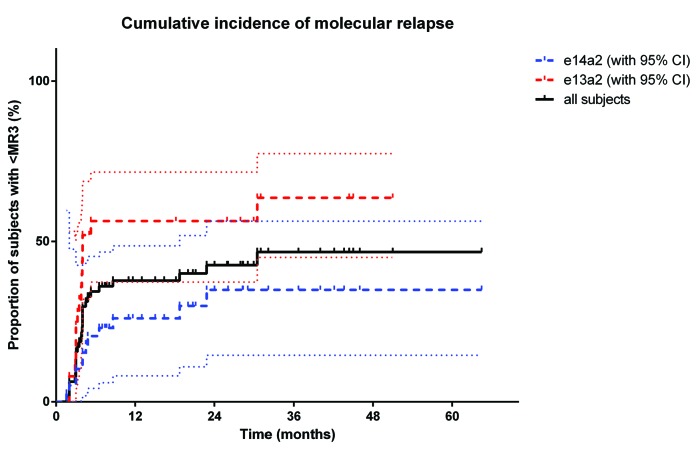
In multivariate analysis of factors found to be predictive of TFR in univariate analysis (i.e. transcript type, age ≥40 years, duration of ≥MR4, depth of response, and percentage of TKI dose at the time of interruption), only e14a2 transcript type [Hazard Ratio (HR) = 0.38 (0.18, 0.84); P=0.016] and age at diagnosis 40 years or over [HR = 0.3 (0.13, 0.66); P=0.003] remained significantly-associated with TFR (Table 2). Figure 1 shows the cumulative incidence of losing MR3 for all subjects and those with e13a2 (64%; 95%CI: 50, 77%) and e14a2 transcripts (35%; 95%CI: 15, 56%).
Table 2.
Univariate and multivariate analysis.
Figure 1.
Cumulative incidence of molecular relapse after tyrosine kinase inhibitor (TKI) interruption. Cumulative incidence of molecular relapse (MR3 loss) after TKI interruption for the entire patient cohort [black line, 46%; 95% Confidence Interval (CI): 31, 60%] and according to transcript type (for e13a2, red dashed line, 64%, 95%CI: 50, 77%; for e14a2, blue dashed line, 35%, 95%CI: 15, 56%). Dotted lines represent confidence intervals.
Twenty-six of 27 subjects with molecular relapse returned to MR3 or over at a median of three months (range 1–9 months) after re-starting TKI therapy. At last follow up, all were alive and in MR3 (5 subjects at a median 6 months of follow up), MR4 (8 subjects at a median 8 months of follow up), or more than MR4 (13 subjects at a median 26 months of follow up after restarting TKI therapy). One patient, who stopped TKI in order to conceive, lost MR3 at 24 weeks of pregnancy. She re-started TKI two months after a normal delivery but has not yet regained MR3 at one month from TKI resumption.
We found that the e14a2 BCR-ABL1 transcript was significantly associated with a higher rate of TFR. Several studies have explored the correlation between BCR-ABL1 transcript type and response to TKI therapy, with the e14a2 transcript reported to predict increased response to imatinib.7 One recent study showed higher rates of MR4.5, better event-free survival and better transformation to blast phase-free survival in subjects with an e14a2 transcript compared with those with e13a2, regardless of initial TKI therapy;8 lower response rates for e13a2 were also found by other authors.6 Another study in subjects receiving first-line imatinib came to the conclusion that different transcript types had no impact on overall survival or CML-related death.9
The association we report of BCR-ABL1 transcript type and TFR, if confirmed, might reflect possible increased tyrosine kinase activity of the e13a2 transcript.7 Alternatively, increased immunogenicity of the e14a2 transcript eliciting a stronger host immune-mediated anti-CML effect is also described, although this seems an unlikely explanation.10–12
Age 40 years or over was also associated with a higher likelihood of TFR after stopping TKI therapy. Recent data suggest that CML patients aged 5–29 years have less frequent cytogenetic and molecular responses to TKI therapy compared with older individuals and an increased risk of transformation to blast phase.13,14 These data are consistent with our findings of an unfavorable impact of age on the probability of TFR. Others report a similar association.15
Our study has important limitations, including its retrospective nature, the small sample size and the fact that a substantial proportion of patients stopped TKI therapy because of intolerance rather than from a planned stopping strategy. Although our conclusions require validation, our data suggest that the presence of e14a2 BCR-ABL1 transcript type and age 40 years or over at diagnosis increase the probability of TFR.
Supplementary Material
Footnotes
Funding: the authors would like to thank the BRC for their support. SC acknowledges Ariodante Mattucci for the informatics support. RG acknowledges support from the National Institute of Health Research (NIHR) Biomedical Research Centre funding scheme.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
Reference
- 1.Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035. [DOI] [PubMed] [Google Scholar]
- 2.Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424–430. [DOI] [PubMed] [Google Scholar]
- 3.Dulucq S, Mahon FX. Deep molecular responses for treatment-free remission in chronic myeloid leukemia. Cancer Med. 2016;5(9):2398–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahon FX, Richter J, Guilhot J, et al. Cessation of Tyrosine Kinase Inhibitors Treatment in Chronic Myeloid Leukemia Patients with Deep Molecular Response: Results of the Euro-Ski Trial. Blood. 2016;128(22):787. [Google Scholar]
- 5.Cross NC, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29(5):999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanfstein B, Lauseker M, Hehlmann R, et al. Distinct characteristics of e13a2 versus e14a2 BCR-ABL1 driven chronic myeloid leukemia under first-line therapy with imatinib. Haematologica. 2014;99(9):1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas CM, Harris RJ, Giannoudis A, et al. Chronic myeloid leukemia patients with the e13a2 BCR-ABL fusion transcript have inferior responses to imatinib compared to patients with the e14a2 transcript. Haematologica. 2009;94(10):1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain P, Kantarjian H, Patel KP, et al. Impact of BCR-ABL transcript type on outcome in patients with chronic-phase CML treated with tyrosine kinase inhibitors. Blood. 2016;127(10):1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfirrmann M, Evtimova D, Saussele S, et al. No influence of BCR-ABL1 transcript types e13a2 and e14a2 on long-term survival: results in 1494 patients with chronic myeloid leukemia treated with imatinib. J Cancer Res Clin Oncol. 2017;143(5):843–850. [DOI] [PubMed] [Google Scholar]
- 10.Clark RE, Dodi IA, Hill SC, et al. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood. 2001;98(10):2887–2893. [DOI] [PubMed] [Google Scholar]
- 11.Rea D, Dulphy N, Henry G, et al. Low natural killer (NK) cell counts and functionality are associated with molecular relapse after imatinib discontinuation in patients (pts) with chronic phase (CP)-chronic myeloid leukemia (CML) with undetectable BCR-ABL transcripts for at least 2 years: preliminary results from immunostim, On Behalf Of STIM Investigators. Blood. 2013;122(21):856.23929834 [Google Scholar]
- 12.Gale RP, Opelz G. Is there immune surveillance against chronic myeloid leukemia? Possibly, but not much. Leuk Res. 2017;59:109–111. [DOI] [PubMed] [Google Scholar]
- 13.Castagnetti F, Gugliotta G, Baccarani M, et al. Differences among young adults, adults and elderly chronic myeloid leukemia patients. Ann Oncol. 2015;26(1):185–192. [DOI] [PubMed] [Google Scholar]
- 14.Pemmaraju N, Kantarjian H, Shan J, et al. Analysis of outcomes in adolescents and young adults with chronic myelogenous leukemia treated with upfront tyrosine kinase inhibitor therapy. Haematologica. 2012;97(7):1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori S, Vagge E, le Coutre P, et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am J Hematol. 2015;90(10):910–914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.