With the awareness of an immunological graft-versus-leukemia (GvL) effect being able to eliminate residual leukemic cells, and, therefore, allowing the use of less intensive conditioning regimens, allogeneic hematopoietic stem cell transplantation (HSCT) is now widely used as a curative therapy for many patients with acute myeloid leukemia (AML).1,2 In fact, AML is the most common disorder for which allogeneic HSCT is being used, while matched sibling donor (MSD) or matched unrelated adult donors (MUD) (i.e. match of at least eight out of eight alleles, HLA-A, -B, -C, and -DRB1) are preferentially used to perform allogeneic HSCT.3 It is extremely important to understand the dynamics of the biological age of the hematopoietic system following HSCT because both the age of the transplant donor and of the recipient are related to transplantation success.4 It has been difficult to study biological aging due to a lack of biomarkers of aging. Several previous studies had looked at telomere dynamics in the context of HSCT, but telomere length provides only an incomplete assessment of biological age.5–11 Here we use a DNA methylation (DNAm)-based biomarker of aging, which is referred to as the “epigenetic clock”.12 The recently developed “epigenetic clock” method, which is based on 353 cytosine-guanine sites (CpGs), applies to almost every human tissue and cell type that contains DNA.12 The weighted average of these 353 epigenetic markers gives rise to an estimate of age (in units of years), which is referred to as “DNA methylation age” or as “epigenetic age”. The “epigenetic clock” is thought to capture aspects of biological age, supported by data demonstrating that older epigenetic age of blood is predictive of all-cause mortality.13,14 A detailed review of the literature describing the utility of the “epigenetic clock” is listed in the Online Supplementary Appendix. It is not yet known whether the “epigenetic clock” method is useful in the context of allogeneic HSCT. In this study, we use the “epigenetic clock” method to determine how epigenetically-defined aging dynamics are affected in donor cells post transplant in a large and homogeneous cohort of patients with long-term follow up, and whether these findings can be correlated with the occurrence of graft-versus-host disease (GvHD). A concise description of inclusion criteria for the patients’ samples analyzed, CpG-methylation analysis, and a description of statistical analyses can be found in the Online Supplementary Appendix.
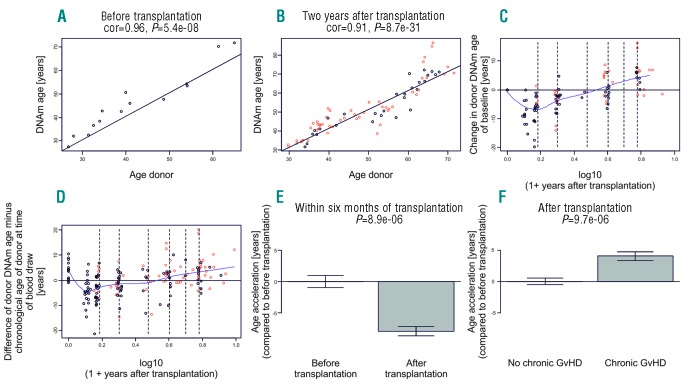
In addition to a DNA sample prior to allogeneic HSCT from each donor, we analyzed donor-derived DNA from 187 peripheral blood mononuclear cell samples obtained from 23 recipients from a mean of seven defined time points (range 86 days to 8.8 years) after transplantation covering a total time span of 16 years. As expected, we observed that DNAm age is highly correlated with chronological donor age before transplantation (Figure 1A). The high correlation between DNAm age and donor age was also observed in DNA samples two years post transplantation (Figure 1B). However, we observed a strong decrease in DNAm age (termed “rejuvenation”) within the first six months of transplantation (Figure 1C, D and E). We measured a decrease in mean DNAm age to a minimum until day +178 (range 86–692 days) after transplantation. Afterwards, significant accelerated epigenetic aging was observed. It has previously been reported that: a) granulocyte colony-stimulating factor (G-CSF) induces rejuvenation of hematopoietic stem cells; and that b) accelerated aging in transplanted patients occurs after a latency period using different methodologies.5,15 Therefore, we calculated aging in all patients after the nadir of rejuvenation had occurred and observed a higher acceleration of DNAm age with 2.4 years per chronological year (P<0.001). Neither donor type (MSD vs. MUD) nor donor age, recipient age, type of GvHD prophylaxis, or occurrence of acute GvHD influenced the aging kinetics observed in the patient cohort. Only occurrence of chronic GvHD was significantly associated with accelerated DNAm age (Figure 1F) (P<0.001). In order to elucidate whether this accelerated epigenetic aging process existed prior to the occurrence of chronic GvHD, we applied a linear mixed model using an interaction term for chronic GvHD. This demonstrated DNAm aging of 5.5 years per chronological year before the onset of chronic GvHD, and DNAm aging of 2.2 years per chronological year after the onset of chronic GvHD (P≤0.001) (Online Supplementary Figure S1). Therefore, we applied a model to predict chronic GvHD by analyzing DNAm aging which indicated the feasibility of using DNAm aging profiles in order to predict the occurrence of chronic GvHD with an area under the curve of 0.97 (Online Supplementary Figure S2), demonstrating that accelerated DNAm age could predict chronic GvHD.
Figure 1.
Epigenetic clock analysis of DNA derived from peripheral blood. (A) DNAm age of the donor before transplantation (y-axis) versus the chronological age of the donor at transplantation (x-axis) derived from 14 donor samples before transplantation. (B) DNAm age two years after transplantation versus the chronological age of the donor. Dots are colored by chronic graft-versus-host disease (GvHD) status (red: chronic GvHD; black: no chronic GvHD). The black line corresponds to y=x. (C and D) The scatter plots demonstrate the effect of the passage of time after transplantation on baseline corrected measures of DNAm age. The x-axis depicts the log (base 10) transformed variable “Years After Transplantation”. To avoid forming the logarithm of zero, one year was added to the variable “Years After Transplantation”. (C) Difference between DNAm age and the DNAm age before transplantation (y-axis). (D) Difference between DNAm age and the chronological age of the donor before transplantation (y-axis). Dots are colored by chronic GvHD status (red: chronic GvHD; black: no chronic GvHD). The dashed vertical lines correspond to half a year, one year, two years, etc. after transplantation. The dip in the blue spline regression line suggests that DNAm age decreases initially. (E) A formal analysis of the rejuvenation effect based only on DNA from peripheral blood samples collected at baseline and within six months post transplantation. (F) Age acceleration effect due to chronic GvHD. The epigenetic measure of age acceleration was defined as residual from a linear model that regressed DNAm age on chronological age in post transplantation samples of subjects without chronic GvHD. The P-values in the bar plot were calculated using a non-parametric group comparison test (Kruskal-Wallis test).
In conclusion, we demonstrate that, after a latency period during which an epigenetic rejuvenation in donor cells occurs, an accelerated epigenetic aging accounting for an incline of 2.4 years per chronological year is initiated. The initially observed rejuvenation may be a compensatory phenomenon related to stressed hematopoiesis early after transplantation and engraftment which has been observed before in models studying telomerase activity and telomere integrity.15 Other analyses were able to demonstrate loss of telomere length after stem cell transplantation but did not demonstrate an equivalent to the rejuvenation seen when using the “epigenetic clock” method used here. Furthermore, they showed a random degree of telomere loss within the first year after transplantation and postulated that this phenomenon might occur only within the first year after transplantation. Thornley et al. even implied that telomere loss after allogeneic transplantation is neither progressive nor sustained.9 However, these studies (in contrast to our work) mostly analyzed pediatric patients receiving bone marrow from related donors and, therefore, without the use of G-CSF mobilized peripheral blood stem cells (PBSCs).6,9–11 Since the nadir of the rejuvenation observed in our analysis occurs after six months, this could also be interpreted as an induction of rejuvenation by the recipient’s bone marrow niche and its stromal cells in combination with the existing initial immunosuppression, especially when considering the high degree of correlation between DNAm age and chronological age of the donor at the time of transplantation. However, induction of rejuvenation by the recipient’s bone marrow could also be a controversial viewpoint considering that the recipients are younger. A recent study postulated that age-associated DNAm is mainly determined by the original donor age and to a lesser extent by the microenvironment of the recipient.7 While Weidner et al. applied a different technique to assess epigenetic age, they corroborate our finding that donor age is the key determinant of DNAm age in hematopoietic cells after allogeneic HSCT.7 In comparison to their analysis, the strength of our study is the homogenous group of patients and donors. Our cohort included only patients with de novo AML (in delineation to therapy-related AML/myeloid neoplasia or secondary AML), only patients transplanted in first complete remission, only with fully matched donors with 10/10 HLA-allele matching, complete donor chimerism after transplantation, no donor-lymphocyte infusion after allogeneic HSCT, no AML relapse, and only patients transplanted with PBSCs. Furthermore, the availability of more time points analyzed after allogeneic HSCT with a mean of seven defined time points up to 8.8 years after allogeneic HSCT in our study, as compared to two time points before and one year after allogeneic HSCT, respectively, means that our study covers more complete aging kinetics after transplantation. This allowed us to identify the described rejuvenation phenomenon occurring early after allogeneic HSCT, and an association of accelerated DNAm and occurrence of chronic GvHD, both findings which are reported for the first time. Other groups demonstrated either pronounced accelerated telomere shortening after related BMT11 or that there is no consistent, but rather a random, pattern of telomere loss within the first year after transplantation, and that its total loss becomes comparable to telomere loss in healthy controls from the second year after transplantation.6,9 Furthermore, they either excluded patients with GvHD in their analyses6 or did not find an association of telomere loss with GvHD.9 Since chronic GvHD is a clinical condition which does not instantly resolve upon treatment, but instead persists and for which symptoms can mostly be only alleviated, it would seem reasonable to speculate that the accelerated epigenetic aging itself triggers onset and development of chronic GvHD. This hypothesis would be further supported by the fact that chronic GvHD occurs when accelerated epigenetic aging is initiated, which is supported by our data. A profound effect of chronological donor age on survival and development of acute GvHD has been published recently.4 However, it has to be emphasized that: a) donor age was not associated with chronic GvHD; and b) age as compared to aging are two distinct biological properties. Overall, our study strongly suggests that epigenetic biomarkers of aging such as the “epigenetic clock” could be useful for predicting adverse outcomes such as chronic GvHD in HSCT patients. However, this needs to be confirmed in prospective studies or in studies comparing different GvHD-preventive allogeneic HSCT regimens.
Supplementary Material
Acknowledgments
We thank Claudia Dill (University Hospital Dresden) and Lorena Vallés Uriarte (University of Kiel) for technical assistance. We thank Catrin Theuser (University Hospital Dresden) for assistance regarding patient data retrieval.
Footnotes
Funding: this work was in part supported by the HLALOSS consortium (funded by the EU via the German Ministry for Education and Research n. 01GU1108A) to MB.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9(10):579–590. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 3.Moore J, Nivison-Smith I, Goh K, et al. Equivalent survival for sibling and unrelated donor allogeneic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2007; 13(5):601–607. [DOI] [PubMed] [Google Scholar]
- 4.Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127(2):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Notaro R, Cimmino A, Tabarini D, Rotoli B, Luzzatto L. In vivo telomere dynamics of human hematopoietic stem cells. Proc Natl Acad Sci USA. 1997;94(25):13782–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rufer N, Brummendorf TH, Chapuis B, Helg C, Lansdorp PM, Roosnek E. Accelerated telomere shortening in hematological lineages is limited to the first year following stem cell transplantation. Blood. 2001;97(2):575–577. [DOI] [PubMed] [Google Scholar]
- 7.Weidner CI, Ziegler P, Hahn M, et al. Epigenetic aging upon allogeneic transplantation: the hematopoietic niche does not affect age-associated DNA methylation. Leukemia. 2015;29(4):985–988. [DOI] [PubMed] [Google Scholar]
- 8.Lowe D, Horvath S, Raj K. Epigenetic clock analyses of cellular senescence and ageing. Oncotarget. 2016;7(8):8524–8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornley I, Sutherland R, Wynn R, et al. Early hematopoietic reconstitution after clinical stem cell transplantation: evidence for stochastic stem cell behavior and limited acceleration in telomere loss. Blood. 2002;99(7):2387–2396. [DOI] [PubMed] [Google Scholar]
- 10.Wynn R, Thornley I, Freedman M, Saunders EF. Telomere shortening in leucocyte subsets of long-term survivors of allogeneic bone marrow transplantation. Br J Haematol. 1999;105(4):997–1001. [DOI] [PubMed] [Google Scholar]
- 11.Wynn RF, Cross MA, Hatton C, et al. Accelerated telomere shortening in young recipients of allogeneic bone-marrow transplants. Lancet. 1998;351(9097):178–181. [DOI] [PubMed] [Google Scholar]
- 12.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marioni RE, Shah S, McRae AF, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin Epigenetics. 2016;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szyper-Kravitz M, Uziel O, Shapiro H, et al. Granulocyte colony-stimulating factor administration upregulates telomerase activity in CD34+ haematopoietic cells and may prevent telomere attrition after chemotherapy. Br J Haematol. 2003;120(2):329–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.