Abstract
Mice lacking suppressor of cytokine signaling 3 (SOCS3) exhibited embryonic lethality with death occurring between days 11 and 13 of gestation. At this stage, SOCS3−/− embryos were slightly smaller than wild type but appeared otherwise normal, and histological analysis failed to detect any anatomical abnormalities responsible for the lethal phenotype. Rather, in all SOCS3−/− embryos examined, defects were evident in placental development that would account for their developmental arrest and death. The placental spongiotrophoblast layer was significantly reduced and accompanied by increased numbers of giant trophoblast cells. Delayed branching of the chorioallantois was evident, and, although embryonic blood vessels were present in the labyrinthine layer of SOCS3−/− placentas, the network of embryonic vessels and maternal sinuses was poorly developed. Yolk sac erythropoiesis was normal, and, although the SOCS3−/− fetal liver was small at day 12.5 of gestation (E12.5), normal frequencies of erythroblasts and hematopoietic progenitor cells, including blast forming unit-erythroid (BFU-E) and, colony forming unit-erythroid (CFU-E) were present at both E11.5 and E12.5. Colony formation for both BFU-E and CFU-E from SOCS3−/− mice displayed wild-type quantitative responsiveness to erythropoietin (EPO), in the presence or absence of IL-3 or stem cell factor (SCF). These data suggest that SOCS3 is required for placental development but dispensable for normal hematopoiesis in the mouse embryo.
The eight members of the suppressor of cytokine signaling family of proteins (SOCS1 to -7 and CIS) are characterized by the presence of a centrally located Src homology 2 (SH2) domain, an N-terminal domain of variable length and divergent sequence, and a conserved C-terminal SOCS box domain (1). SOCS1 to -3 and CIS have been shown to participate in a negative feedback loop to regulate cytokine signaling, particularly from the hematopoietin class of cytokine receptors (2). Signaling is initiated on cytokine-dependent receptor aggregation, which activates Janus kinases (JAKs), resulting in tyrosine phosphorylation of the receptor as well as other signaling proteins. Additional signaling molecules, such as the signal transducers and activators of transcription (STATs) are subsequently activated via recruitment to specific receptor phosphotyrosines (3).
The SOCS proteins modulate signal transduction by inhibiting components of the JAK/STAT pathway. Whereas SOCS1 is thought to directly bind the JAK kinases and inhibit their catalytic activity, CIS appears to act by binding to the receptor, preventing recruitment and activation of STATs (reviewed in ref. 2). Recent evidence suggests that SOCS3 action shares aspects of both these mechanisms. Studies of signaling from the GH receptor (GHR; ref. 4), IL-2 receptor beta chain (IL2Rβ; ref. 5), erythropoietin receptor (EPOR; ref. 6), and gp130 (7, 8) suggest that, whereas SOCS3 expression leads to inhibition of JAK kinase activity, SOCS3 does not bind to JAKs with high affinity and depends on the presence of cytokine receptor for this action. The emerging model suggests that SOCS3 relies on interaction with the activated receptor for recruitment to the signaling complex, providing access to the JAK kinases, the activity of which it ultimately inhibits. SOCS proteins may also target associated signaling molecules for degradation. The SOCS box domain has been shown to interact with elongins B and C, proteins involved in the cellular ubiquitination machinery that targets proteins for destruction via the proteasome (9, 10). Thus, SOCS proteins may regulate cytokine responses through control of the turnover of signaling proteins as well as directly inhibiting their catalytic activity or recruitment to the signaling complex.
The physiological significance of SOCS-mediated control of cytokine signaling has emerged from studies in which these regulators have been functionally deleted in mice. SOCS1 is a key modulator of IFN-γ signaling. Mice lacking SOCS1 display deregulated responses to IFN-γ that result in a lethal combination of fatty degeneration and necrosis of the liver, excessive T cell activation, lymphopenia, and hematopoietic infiltration of multiple organs (11–14). In contrast, mice lacking SOCS2 are healthy and fertile, but display excessive growth consistent with a negative regulatory role for SOCS2 in GH and/or insulin-like growth factor (IGF)-I signaling (15). SOCS3 has been implicated in control of cytokine signaling in several biological systems. For example, in addition to the potential roles for SOCS3 implied by its capacity to interact with and inhibit signals from the GHR, IL-2Rβ, EPOR, and gp130 receptors (see above), SOCS3 has also been implicated in processes as disparate as leptin control of energy homeostasis (16) and modulation of intestinal inflammation (17). One report on mice lacking SOCS3 described embryonic lethality with marked erythrocytosis (18). The authors concluded that SOCS3 is an essential physiological regulator of EPO signaling, a model supported by the occurrence of lethal anemia in transgenic mice constitutively expressing SOCS3 (18) and by data showing that SOCS3 binds to and inhibits signaling from the EPO receptor (6).
In this study, we have independently generated mice lacking a functional SOCS3 gene. We show that the death of SOCS3−/− mice at mid-gestation is not linked to defects in the embryo but is associated with abnormalities in specific regions of the placenta. The nature of these defects and our demonstration that SOCS3 is expressed at these placental sites suggests that lethality in SOCS3−/− mice results from placental insufficiency. In contrast to the previous study, we found no evidence of defective erythropoiesis in SOCS3−/− mice.
Materials and Methods
Generation of Targeted Embryonic Stem Cells and Mutant Mice.
A 3-kb PCR product extending 5′ from the SOCS3 ATG initiation codon and a 4.3-kb XbaI-XhoI 3′ SOCS3 fragment (Fig. 1) were cloned into the BamHI and XhoI sites of pβgalpAloxneo (12). This targeting vector was electroporated into C57BL/6 embryonic stem cells (19). Transfected cells were selected in G418, and resistant clones were picked and expanded. Hybridization of HindIII-digested genomic DNA with a 1.5-kb EcoRV-HindIII fragment situated 6-kb 3′ of the SOCS3 gene (Fig. 1) was used to differentiate between the endogenous (20-kb) and targeted (9-kb) SOCS3 alleles. Two independent targeted embryonic stem cell clones were injected into BALB/c blastocysts to generate chimeric mice. Male chimeras were mated with C57BL/6 females to yield heterozygotes for the targeted SOCS3 allele, which were interbred to produce wild-type (SOCS3+/+), heterozygous (SOCS3+/−), and mutant (SOCS3−/−) mice on a C57BL/6 background. The deletion of SOCS3 coding sequence and subsequent inability to produce SOCS3 mRNA in cells from mutant mice was confirmed in nucleic acid blots, which were performed as described (20). Northern blots were probed with a full-length SOCS3 coding region probe and then with a 1.2-kb PstI chicken glyceraldehyde-3-phosphate dehydrogenase (GAPDH) fragment.
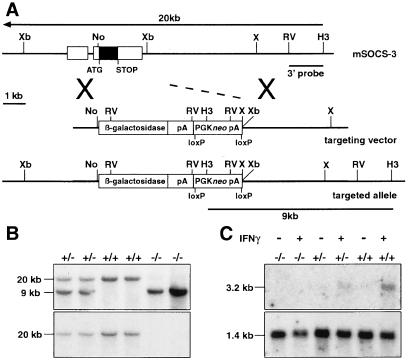
Figure 1.
Disruption of the SOCS3 locus by homologous recombination. (A) The structure of the murine SOCS3 locus is illustrated with exons as raised boxes and the coding region shaded (Xb, XbaI; No, NotI; X, XhoI; RV, EcoRV; H3, HindIII). In the targeted allele, the coding region was deleted and a cassette fusing the lacZ gene to the SOCS3 ATG and including the selectable PGKneo gene was included. (B) Southern blot of genomic DNA from E11.5 embryos collected from a cross between SOCS3+/− mice, including wild-type (+/+), SOCS3+/−, and SOCS3−/− samples. The DNA was digested with HindIII and hybridized with the 3′ probe, which distinguishes between the targeted (9-kb) and endogenous (20-kb) SOCS3 loci. (C) Northern blot of RNA extracted from wild-type (+/+), SOCS3+/−, and SOCS3−/− primary embryo fibroblasts after stimulation with IFN-γ (+) or saline (−). The blot was probed with a SOCS3 coding region probe followed by a GAPDH probe to confirm RNA integrity.
Yolk Sac and Fetal Liver Cultures.
We suspended 5 × 103 or 104 yolk sac or fetal liver cells in 1.5% methylcellulose (Fluka) in Iscove's modified Dulbecco's medium (IMDM) supplemented with 20% FCS and EPO (2 units/ml at maximal concentration) with or without IL-3 (10 ng/ml) and stem cell factor (SCF; 100 ng/ml). After incubation at 37°C for 7 days [blast forming unit-erythroid (BFU-E) and mixed or myeloid colonies] or 2–3 days [colony forming unit-erythroid (CFU-E)] in a humidified atmosphere of 5% CO2 in air, colonies were scored at 35-fold magnification.
Histological Analysis and lacZ Staining.
Embryos and placentas from timed matings of SOCS3+/− mice (day of plug = day 0) were fixed in a solution of 0.2% glutaraldehyde and 1% formaldehyde, and lacZ activity was detected as described (21). Paraffin-embedded lacZ-stained embryos and placentas were serially sectioned, and alternate sections were stained with nuclear fast red or hematoxylin and eosin. Embryos were genotyped by extraction of yolk sac DNA (22) for PCR with an oligonucleotide set that allowed distinction between wild-type and targeted SOCS3 alleles: 5′-CTT CAT GCC ATG ACG TCT GTG ATG C-3′, 5′-GCC TTC GAG GCT GTC TGA AGA TGC-3′, and 5′-GAA GCT GAC TCT AGA CGT TG-3′. PCR was performed for 30 cycles of 96°C for 30 s, 62°C for 30 s, and 72°C for 1 min before visualization of the amplification products on 2% agarose gels with ethidium bromide staining.
Derivation of Primary Embryonic Fibroblasts.
Embryos from timed matings of SOCS3+/− mice were collected at day 11.5 of gestation (E11.5) and dissected, and the trunks were individually treated with trypsin before disaggregation by pipetting. The cells were plated in DMEM containing 10% FCS and passaged as necessary when confluent. Yolk sacs were collected in parallel for genotyping. Near confluent cultures of primary embryonic fibroblasts from each of SOCS3−/−, SOCS3+/−, and wild-type embryos were stimulated for 1 h with 100 ng/ml IFN-γ. Cells were harvested for extraction of total cellular RNA and assessment of SOCS3 expression by Northern blotting as described (20) using a full-length SOCS3 coding region fragment as hybridization probe.
Results
Embryonic Lethality in Mice Lacking SOCS3.
A targeting vector for the deletion of the SOCS3 coding sequence was constructed (Fig. 1) and transfected into embryonic stem cells. The vector was designed so that homologous recombination with an endogenous SOCS3 locus would delete the entire SOCS3 coding sequence and place the lacZ gene under the transcriptional control of the SOCS3 locus. When offspring of mice heterozygous for the disrupted SOCS3 allele were genotyped at weaning, no SOCS3−/− mice were present consistent with embryonic or perinatal lethality. Over 1,000 weanlings from these crosses have now been genotyped, all of which were SOCS3+/− or wild type. Examination of SOCS3+/− mice revealed no apparent abnormalities. When embryos from SOCS3+/− crosses were genotyped at E10.5, viable mice of each genotype were present in numbers consistent with normal Mendelian inheritance of the disrupted SOCS3 allele (Fig. 1 and Table 1). Similar analysis at E11.5 to E15.5 revealed selective death of SOCS3−/− embryos between E11 and E13 (Table 1). To confirm that gene targeting had generated a null allele, primary embryonic fibroblasts were derived from SOCS3−/− and control embryos and then stimulated with IFN-γ. Although SOCS3 RNA was induced in wild-type embryos, consistent with previous data (23), no SOCS3 RNA was present in IFN-γ-stimulated SOCS3−/− fibroblasts, and an intermediate level was induced in SOCS3+/− cells (Fig. 1). Reverse transcription–PCR analysis of RNA purified from E11.5 embryos confirmed the absence of SOCS3 expression in SOCS3−/− mice (data not shown).
Table 1.
Embryonic lethality in SOCS3−/− mice
| Age of embryos | No. of embryos*SOCS3+/+:SOCS3+/−:SOCS3−/− | Proportion of SOCS3−/− embryos, % | Viability of SOCS3−/− embryos, % |
|---|---|---|---|
| E9.5 | 20:38:19 | 25 | 100 |
| E10.5 | 5:10:8 | 35 | 100 |
| E11.5 | 52:69:26 | 18 | 33 |
| E12.5 | 41:72:25 | 18 | 50 |
| E13.5 | 12:19:2 | 6 | 0 |
| E14.5 and 15.5 | 6:10:0 | 0 | 0 |
Number of embryos of each genotype from matings of SOCS3+/− parents are shown.
Placental Abnormalities in SOCS3−/− Embryos.
Initial microscopic inspection and histological analysis of E9.5–E13.5 SOCS3−/− embryos indicated that, from E10.5, many SOCS3−/− embryos were slightly smaller than their wild-type or SOCS3+/− littermates (Fig. 2). However, no gross defects were seen, and extensive histological analysis failed to reveal any anatomical abnormalities that might have been responsible for the embryonic lethality. This result prompted us to examine placentas from SOCS3−/− embryos and their littermates. Embryos and placentas from timed matings were stained for lacZ activity as a surrogate marker of SOCS3 transcription and then serially sectioned for morphological analysis. Only placentas obtained from viable embryos, as judged by the presence of a beating heart and absence of signs of necrosis, were studied.
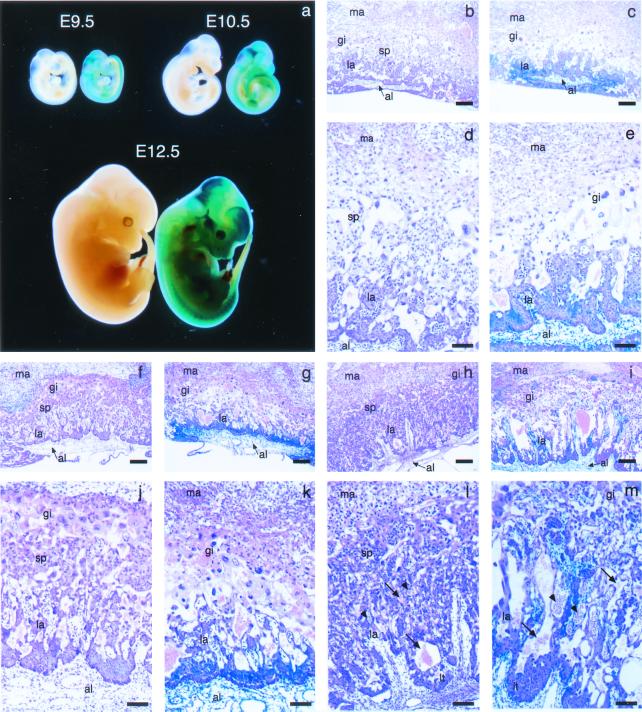
Figure 2.
Placental abnormalities in SOCS3−/− embryos. (a) Viable E9.5, E10.5, and E12.5 SOCS3 null embryos and their wild-type littermates. Embryos were stained for lacZ expression. The SOCS3 null embryos are to the right of their wild-type littermates. (b–m) Histological sections of the placentas from these embryos. Placentas were stained for lacZ and then sectioned and further stained with hematoxylin and eosin (H&E). The lacZ-staining pattern was detectable in the H&E-stained sections and was confirmed by staining near-adjacent sections with nuclear fast red (not shown). Temporal profile of placental development in wild-type (b, d, f, h, j, and l) and SOCS3 null (c, e, g, k, i, and m) placentas at E9.5 (b–e) and E10.5 (f, g, j, and k) and E12.5 (h, i, l, and m) is shown. There is a paucity of spongiotrophoblast in the SOCS3 null placentas. Higher power (d, e, and j–m) reveals lacZ staining of the labyrinthine trophoblast and the allantois in the SOCS3 null placentas. Some lacZ staining is also detected in the walls of embryonic vessels penetrating the labyrinth. LacZ staining is not seen in wild-type embryos. At E12.5, embryonic (arrowheads) and maternal (arrows) vessels within the labyrinth are dilated. Al, allantois; gi, trophoblast giant cells; la, labyrinth; lt, labyrinthine trophoblasts; ma, maternal decidua basalis; sp, spongiotrophoblast. Scale bars for b, c, and f—I = 100 μm; for d, e, and j—m = 10 μm.
At E9.5, there was a marked decrease in the spongiotrophoblast layer in SOCS3−/− placentas and an accompanying increase in the number of giant trophoblast cells. The allantois was attached to the chorion normally, but there was a delay in chorioallantoic branching and a decrease in vascularity of the labyrinth (Fig. 2 b–e). The defects in trophoblast giant cell, spongiotrophoblast and labyrinthine layers persisted in E10.5–12.5 SOCS3−/− placentas (Fig. 2 f–m). Instead of the sparse, discontinuous trophoblast giant cell layer present in control placentas, giant trophoblast cells were increased in number. Spongiotrophoblast cells were markedly reduced. The labyrinthine trophoblast layer was thickened, but the labyrinth was thinner. Embryonic vessels were present in the labyrinthine layer of SOCS3−/− mice. However, unlike placentas from wild-type littermates, in which the embryonic blood vessels were elongated and radiated uniformly from the chorionic plate into the labyrinthine layer, the network of embryonic vessels and maternal sinuses was poorly developed in mutant placentas. By E12.5, there was obvious dilation of maternal and embryonic vessels and pooling of maternal blood in the labyrinthine layer of the mutant placentas (Fig. 2 h, i, k, l, and m). This phenotype was similar on inbred C57BL/6 or hybrid C57BL/6 × 129Sv (B6129) strain backgrounds.
Whole mount lacZ staining of SOCS3−/− placentas indicated SOCS3 transcription in the allantoic mesenchyme and the endothelium of embryonic vessels penetrating the chorionic plate. Expression was also seen in the chorionic plate trophoblast. No lacZ staining was seen in wild-type placental sections (Fig. 2). Extensive staining was also evident in the embryo (Fig. 2a), including the yolk sac and embryonic vasculature, neuroepithelium, nerve ganglia, precartilagenous condensations, and limb buds (L.R., unpublished results). Staining was stronger in SOCS3−/− tissues compared with those in SOCS3+/− mice, although patterns of expression were the same.
Embryonic Hematopoiesis in SOCS3−/− Mice.
To determine whether SOCS3 plays an essential role in hematopoiesis, embryos were analyzed at E10.5, E11.5, and E12.5. Blood islands developed normally in the yolk sacs of SOCS3−/− embryos. Yolk sac cellularity and erythroblast morphology were similar in mice of all genotypes, and the hematopoietic progenitor content of yolk sacs at both E10.5 and E11.5 was also normal in SOCS3−/− mice (Table 2).
Table 2.
Yolk sac hematopoiesis in SOCS3−/− mice
| E10.5
|
E11.5
|
|||||
|---|---|---|---|---|---|---|
| SOCS3+/+ | SOCS3+/− | SOCS3−/− | SOCS3+/+ | SOCS3+/− | SOCS3−/− | |
| Number analyzed | 3 | 2 | 2 | 10 | 19 | 3 |
| Cellularity, ×10−6 | 0.24 ± 0.15 | 0.14 ± 0.01 | 0.18 ± 0.15 | 0.35 ± 0.15 | 0.33 ± 0.16 | 0.20 ± 0.22 |
| Myeloid-CFC, per 1 × 104 | 7 ± 3 | 9.5 ± 5 | 10.5 ± 1 | 6 ± 2 | 4 ± 2 | 5 ± 1 |
| BFU-E, per 1 × 104 | 12 ± 5 | 23 ± 24 | 29 ± 5 | 7 ± 3 | 5 ± 3 | 18 ± 15 |
| Mix-CFC, per 1 × 104 | 4 ± 1 | 4 ± 5 | 4 ± 3 | 2 ± 1.5 | 1 ± 1 | 1 ± 1 |
Results represent the mean ± SD of data from multiple embryos. Triplicate cultures were stimulated with SCF, IL-3, and EPO and incubated for 7 days in a humidified atmosphere of 5% CO2/air at 37°C.
The cellularity of fetal livers from viable SOCS3−/− embryos was similar to that of wild-type and SOCS3+/− embryos at E11.5 (Table 3). As judged by cytology, erythroblast content was similar between genotypes at this time point. Semisolid culture assays revealed that the content of hematopoietic progenitors (CFU-E, BFU-E, myeloid-CFC, and mixed lineage-CFC) was also unaltered in SOCS3−/− fetal livers (Table 3). At E12.5, livers in viable SOCS3−/− embryos appeared small, and the total cellularity of the organ was reduced 10-fold (Table 3). However, the frequency of hematopoietic progenitors among fetal liver cells was similar to that observed for wild-type and SOCS3+/− embryos (Table 3). In addition, the size of the colonies formed from individual progenitor cells was indistinguishable between genotypes. To exclude the possibility of a strain effect interfering with the interpretation of these results, embryos on a mixed B6129 genetic background were also examined, and similar results were observed. In particular, the frequency of hematopoietic progenitor cells was similar between genotypes (Table 3).
Table 3.
Fetal liver hematopoiesis in SOCS3−/− mice
| C57BL/6
|
C57BL/6 × 129/Sv
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| E11.5
|
E12.5
|
E11.5
|
|||||||
| SOCS3+/+ | SOCS3+/− | SOCS3−/− | SOCS3+/+ | SOCS3+/− | SOCS3−/− | SOCS3+/+ | SOCS3+/− | SOCS3−/− | |
| Number analyzed | 18 | 33 | 7 | 15 | 23 | 3 | 2 | 4 | 5 |
| Cellularity, ×10−6 | 0.41 ± 0.24 | 0.47 ± 0.23 | 0.32 ± 0.28 | 3.27 ± 1.52 | 2.87 ± 1.79 | 0.27 ± 0.10 | 0.46 ± 0.11 | 0.6 ± 0.19 | 0.40 ± 0.3 |
| CFU-E, per 5 × 103 | 294 ± 143 | 285 ± 172 | 278 ± 4 | 110 ± 44 | 117 ± 38 | 254 (1) | 356 ± 112 | 464 ± 31 | 278 ± 4 |
| Myeloid-CFC, per 1 × 104 | 16 ± 6 | 14 ± 6 | 12 ± 6 | 14 ± 5 | 15 ± 4 | 9 ± 7 | 18 ± 2 | 18 ± 2 | 16 ± 4 |
| BFU-E, per 1 × 104 | 36 ± 14 | 34 ± 16 | 32 ± 12 | 15 ± 8 | 19 ± 8 | 22 ± 26 | 28 ± 4 | 46 ± 20 | 40 ± 4 |
| Mix-CFC, per 1 × 104 | 8 ± 2 | 6 ± 4 | 2 ± 2 | 4 ± 4 | 7 ± 4 | 3 ± 2 | 6 ± 0 | 6 ± 4 | 4 ± 0 |
Results represent the mean ± SD of data from multiple embryos as indicated. Replicate cultures were stimulated with EPO alone, or SCF, IL-3, and EPO, and incubated for 2–3 days for CFU-E and 7 days for CFC in a humidified atmosphere of 5% CO2/air at 37°C.
To further assess whether the absence of SOCS3 had consequences for hematopoietic progenitor cell growth in vitro, cultures of fetal liver cells were maintained for 3 or 4 weeks. Again, no differences were observed between genotypes with respect to either the number of colonies present, or the sizes of individual colonies in the cultures (data not shown).
Normal EPO Responsiveness in SOCS3−/− Erythroid Progenitor Cells.
Because SOCS3 has been implicated as a negative regulator of erythropoietin signaling (6, 18), experiments were performed to determine whether this role for SOCS3 was of physiological relevance in primary cells. The growth of CFU-E from cultures of E11.5 fetal liver cells stimulated by titrated concentrations of erythropoietin was monitored in parallel for each genotype. In two independent experiments, the responsiveness of SOCS3−/− CFU-E to erythropoietin was identical to that observed for wild-type and SOCS3+/− cells (Fig. 3). Similar results were obtained when erythropoietin responsiveness of BFU-E was assayed in the presence of the synergizing growth factors, stem cell factor and IL-3 (Fig. 3).
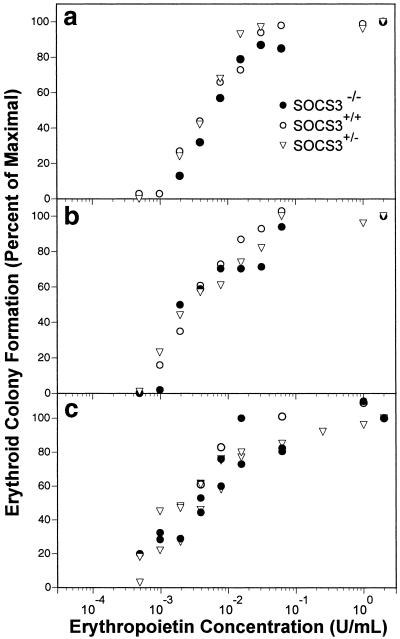
Figure 3.
Erythropoietin responsiveness of SOCS3−/− erythroid progenitor cells. (a and b) Data from two independent experiments are shown. E11.5 fetal liver cells (5 × 103) were cultured for 3 days in various concentrations of erythropoietin. Individual results represent means of duplicate cultures, normalized for CFU-E number in supramaximal concentrations of erythropoietin (2 units/ml). The numbers of CFU-E in cultures containing maximal concentrations of erythropoietin ranged between 264 and 684. (c) E11.5 fetal liver cells (104) were cultured for 7 days in triplicate in stem cell factor, IL-3, and various final concentrations of erythropoietin, and the number of BFU-E (including mix-CFC) enumerated as above. The numbers of BFU-E in cultures containing maximal concentrations of erythropoietin ranged between 26 and 50.
Discussion
We have used gene targeting to generate mice unable to produce SOCS3. Consistent with a previous report (18), we find that SOCS3 is essential for survival during embryogenesis. In the absence of SOCS3, embryos developed apparently normally until E10, but died between E11 and E13 (Table 1). No reproducible embryonic abnormalities were observed in SOCS3−/− mice. Rather, in all SOCS3−/− embryos examined, defects were evident in the developing placenta that would account for their developmental arrest and death.
The chorioallantoic placenta links the embryo with the maternal circulation and is required for embryonic growth and development from E9.5. In mice, there are three distinctive trophoblastic cell structures in the mature placenta that are formed by E10 and persist throughout gestation: an inner labyrinthine layer, an intermediate spongiotrophoblast layer, and an outermost layer of trophoblast giant cells (24). The histology of SOCS3−/− placentas demonstrates that absence of SOCS3 affects both the formation of the spongiotrophoblast and the morphogenesis of the placental labyrinth (Fig. 2). The reduction in spongiotrophoblast suggests an early misallocation or differential proliferation of diploid trophoblast stem cells. In the labyrinthine layer of SOCS3 placentas, vessels enter the labyrinth, but do not sustain further branching. The labyrinth trophoblast remains thickened and poorly differentiated (Fig. 2). Given evidence of SOCS3 expression in labyrinthine trophoblasts and in the allantoic mesenchyme (Fig. 2), defects in either, or both, may affect the normal branching and/or survival of the embryonic labyrinthine vascular system. Abnormalities of the labyrinthine layer can arise as a consequence of abnormalities of the allantoic mesenchyme or of the trophoblast (reviewed in ref. 25). Studies of Mash2 null mutant placentas also showed a reduced spongiotrophoblast layer and increased numbers of giant cells, together with a reduction in the labyrinthine layer (26). Interestingly, chimera studies revealed the absence of spongiotrophoblast to be a primary, intrinsic cellular defect, implying that an intact spongiotrophoblast layer is required for normal development of the labyrinthine trophoblast layer (27). A similar analysis, combining SOCS3−/− with diploid or tetraploid wild-type embryos, should help better define the key roles of SOCS3 in the different placental components affected in SOCS3−/− mice.
Biochemical analyses and studies of SOCS3 action in overexpression and cell line models provide compelling evidence that SOCS3, like other SOCS family members, can act as a negative feedback regulator of cytokine signaling (2). Knockout studies of SOCS1 and SOCS2 have demonstrated key physiological roles for these family members in modulation of IFN-γ and GH/insulin-like growth factor-I signaling, respectively (14, 15). These data imply that the defects in SOCS3−/− mice may arise from excess signaling from one or more cytokines acting in placental development. Although few studies have examined the effects of cytokine overproduction on the development of the placenta, gene targeting studies have implicated several cytokines in this process. Mice lacking hepatocyte growth factor (HGF) or its receptor c-Met die at E11.5 with defects in the labyrinthine layer, and fibroblast growth factor receptor 2 (FGFR)-deficient mice exhibit lethality at E10.5, with defective chorioallantoic fusion or absence of the labyrinthine layer (reviewed in ref. 25). Leukemia inhibitory factor receptor-null embryos exhibit abnormal, disorganized placentas (28), and mice lacking the epidermal growth factor receptor also display genetic background-dependent defects in the placental labyrinth (29). Future studies to precisely define the physiological role of SOCS3 will require analysis of cytokine signaling in placentas of SOCS3−/− mice.
Previous studies of SOCS3−/− mice reported a marked erythrocytosis associated with the embryonic lethality (18). From our own analyses, we were unable to find evidence for excess erythroid cell production. Microscopic examination revealed no erythrocytosis in SOCS3−/− embryos examined from E9.5 to E13.5. Yolk sac erythropoiesis was normal, and normal numbers of erythroblasts, BFU-E, and CFU-E were evident in the fetal livers of SOCS3−/− mice at E11.5 and E12.5 (Tables 2 and 3). Although the SOCS3−/− fetal liver was abnormally small at E12.5, the frequencies of hematopoietic progenitors were normal, suggesting that the hypocellularity of the liver reflected the developmental arrest of the embryos rather than a specific hematopoietic defect. The absence of an intrinsic defect in SOCS3−/− hematopoietic progenitors is consistent with the report of normal hematopoiesis in irradiated adult recipients of SOCS3−/− fetal liver cell transplants (18). Moreover, we could find no evidence that erythroid progenitor cells have any greater responsiveness to EPO in the absence of SOCS3: both BFU-E and CFU-E from SOCS3−/− mice exhibit identical EPO quantitative responsiveness for colony formation in vitro, in the presence or absence of IL-3 or stem cell factor (Fig. 3). The basis for the discrepancies in erythropoiesis between our mice and those reported previously is unclear. We conclude from our analysis that SOCS3 is essential for placental development but dispensable for normal hematopoiesis in the mouse embryo.
Acknowledgments
We thank Janelle Mighall, Sally Cane, Sandra Mifsud, and Ladina DiRago for technical assistance, Steven Mihajlovic for histology, and Kathy Hanzinikolas for animal husbandry. This work was supported by the National Health and Medical Research Council, Canberra, the Anti-Cancer Council of Victoria, an Australian Government Cooperative Research Centres Program Grant, the National Institutes of Health (Grant CA22556), the J. D. and L. Harris Trust, the Sylvia and Charles Viertal Charitable Foundation, and AMRAD Operations Pty. Ltd., Melbourne. A.R. holds a Neil Hamilton Fairley Fellowship (977211).
Abbreviations
- SOCS3
suppressor of cytokine signaling 3
- BFU-E
blast forming unit-erythroid
- CFU-E
colony forming unit-erythroid
- STAT
signal transducer and activator of transcription
- JAK
Janus kinase
- EPO
erythropoietin
- SCF
stem cell factor
- E
day of gestation
- CFC
colony-forming units
References
- 1.Hilton D J, Richardson R T, Alexander W S, Viney E M, Willson T A, Sprigg N S, Starr R, Nicholson S E, Metcalf D, Nicola N A. Proc Natl Acad Sci USA. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krebs D L, Hilton D J. J Cell Sci. 2000;113:2813–2819. doi: 10.1242/jcs.113.16.2813. [DOI] [PubMed] [Google Scholar]
- 3.Ihle J N. Nature (London) 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 4.Hansen J A, Lindberg K, Hilton D J, Nielsen J H, Billestrup N. Mol Endocrinol. 1999;13:1832–1843. doi: 10.1210/mend.13.11.0368. [DOI] [PubMed] [Google Scholar]
- 5.Cohney S J, Sanden D, Cacalano N A, Yoshimura A, Mui A, Migone T S, Johnston J A. Mol Cell Biol. 1999;19:4980–4988. doi: 10.1128/mcb.19.7.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasaki A, Yasukawa H, Shouda T, Kitamura T, Dikic I, Yoshimura A. J Biol Chem. 2000;275:29338–29347. doi: 10.1074/jbc.M003456200. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz J, Weissenbach M, Haan S, Heinrich P C, Schaper F. J Biol Chem. 2000;275:12848–12856. doi: 10.1074/jbc.275.17.12848. [DOI] [PubMed] [Google Scholar]
- 8.Nicholson S E, De Souza D, Fabri L J, Corbin J, Willson T A, Zhang J G, Silva A, Asimakis M, Farley A, Nash A D, et al. Proc Natl Acad Sci USA. 2000;97:6493–6498. doi: 10.1073/pnas.100135197. . (First Published May 30, 2000; 10.1073/pnas.100135197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamura T, Sato S, Haque D, Liu L, Kaelin W G, Jr, Conaway R C, Conaway J W. Genes Dev. 1998;12:3872–3881. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J G, Farley A, Nicholson S E, Willson T A, Zugaro L M, Simpson R J, Moritz R L, Cary D, Richardson R, Hausmann G, et al. Proc Natl Acad Sci USA. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto T. Proc Natl Acad Sci USA. 1998;95:15577–15582. doi: 10.1073/pnas.95.26.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starr R, Metcalf D, Elefanty A G, Brysha M, Willson T A, Nicola N A, Hilton D J, Alexander W S. Proc Natl Acad Sci USA. 1998;95:14395–14399. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marine J C, Topham D J, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle J N. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 14.Alexander W S, Starr R, Fenner J E, Scott C L, Handman E, Sprigg N S, Corbin J E, Cornish A L, Darwiche R, Owczarek C M, et al. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 15.Metcalf D, Greenhalgh C J, Viney E, Willson T A, Starr R, Nicola N A, Hilton D J, Alexander W S. Nature (London) 2000;405:1069–1073. doi: 10.1038/35016611. [DOI] [PubMed] [Google Scholar]
- 16.Bjorbaek C, Elmquist J K, El-Haschimi K, Kelly J, Ahima R S, Hileman S, Flier J S. Endocrinology. 1999;140:2035–2043. doi: 10.1210/endo.140.5.6736. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, et al. J Exp Med. 2001;193:471–482. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marine J C, McKay C, Wang D, Topham D J, Parganas E, Nakajima H, Pendeville H, Yasukawa H, Sasaki A, Yoshimura A, Ihle J N. Cell. 1999;98:617–627. doi: 10.1016/s0092-8674(00)80049-5. [DOI] [PubMed] [Google Scholar]
- 19.Kontgen F, Suss G, Stewart C, Steinmetz M, Bluethmann H. Int Immunol. 1993;5:957–964. doi: 10.1093/intimm/5.8.957. [DOI] [PubMed] [Google Scholar]
- 20.Alexander W S, Metcalf D, Dunn A R. EMBO J. 1995;14:5569–5578. doi: 10.1002/j.1460-2075.1995.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elefanty A G, Begley C G, Hartley L, Papaevangeliou B, Robb L. Blood. 1999;94:3754–3763. [PubMed] [Google Scholar]
- 22.Robb L, Lyons I, Li R, Hartley L, Kontgen F, Harvey R P, Metcalf D, Begley C G. Proc Natl Acad Sci USA. 1995;92:7075–7079. doi: 10.1073/pnas.92.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song M M, Shuai K. J Biol Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 24.Cross J C, Werb Z, Fisher S J. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 25.Ihle J N. Cell. 2000;102:131–134. doi: 10.1016/s0092-8674(00)00017-9. [DOI] [PubMed] [Google Scholar]
- 26.Guillemot F, Nagy A, Auerbach A, Rossant J, Joyner A L. Nature (London) 1994;371:333–336. doi: 10.1038/371333a0. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Gertsenstein M, Rossant J, Nagy A. Dev Biol. 1997;190:55–65. doi: 10.1006/dbio.1997.8685. [DOI] [PubMed] [Google Scholar]
- 28.Ware C B, Horowitz M C, Renshaw B R, Hunt J S, Liggitt D, Koblar S A, Gliniak B C, McKenna H J, Papayannopoulou T, Thoma B, et al. Development. 1995;121:1283–1299. doi: 10.1242/dev.121.5.1283. [DOI] [PubMed] [Google Scholar]
- 29.Threadgill D W, Dlugosz A A, Hansen L A, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris R C, et al. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]