Abstract
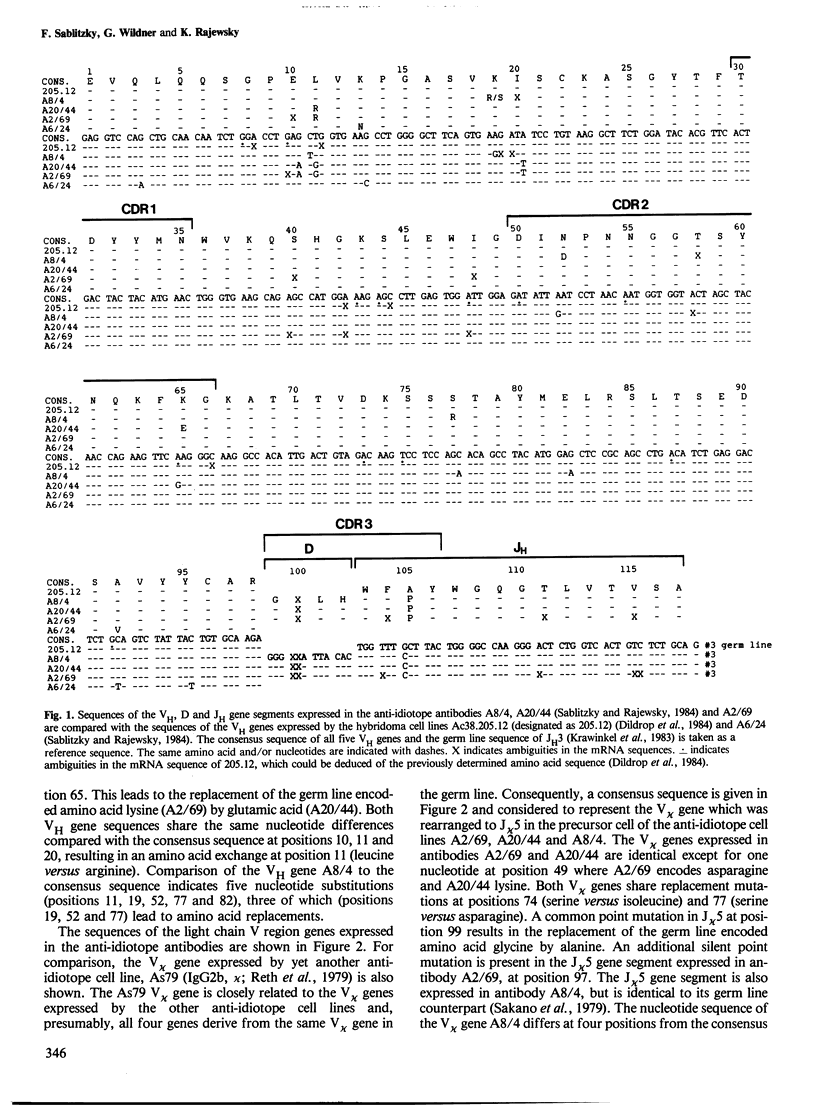
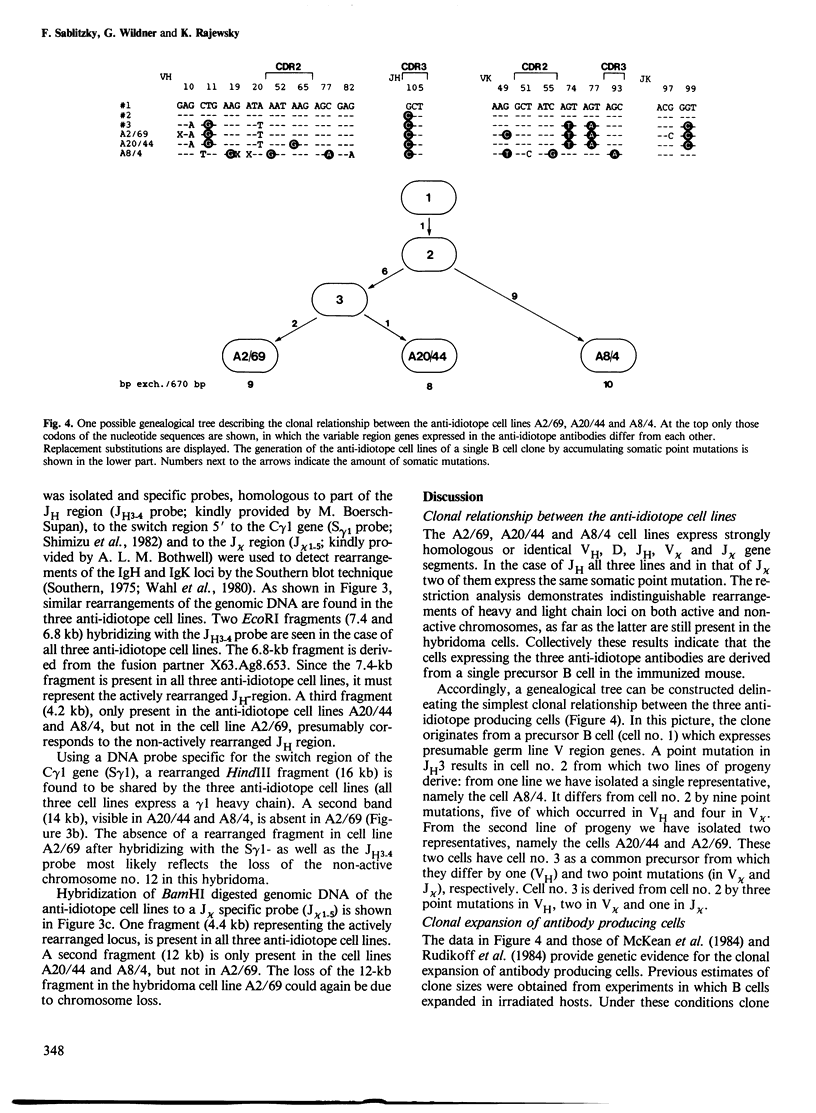
The variable (V) regions of three closely related monoclonal antibodies produced by hybridomas which had been isolated from a single mouse were sequenced at the level of the mRNA. The sequences and the restriction analysis of the immunoglobulin loci carried by the hybridoma cells indicate that the antibodies are derived from cells belonging to a single B cell clone. The sequence data imply a high frequency and stepwise occurrence of somatic point mutations in the expressed V region genes and substantial clonal expansion of B cells in the mouse. The mutations appear to be randomly introduced into heavy and light chain V region genes. Mutations are also seen in the complementarity determining regions which may thus have been involved in the selection of the cells producing the three antibodies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma M., Weiler E. The clonal nature of antibody formation. I. Clones of antibody-forming cells of poly-D-alanine specificity. J Immunol. 1970 Jan;104(1):203–214. [PubMed] [Google Scholar]
- Dildrop R., Bovens J., Siekevitz M., Beyreuther K., Rajewsky K. A V region determinant (idiotope) expressed at high frequency in B lymphocytes is encoded by a large set of antibody structural genes. EMBO J. 1984 Mar;3(3):517–523. doi: 10.1002/j.1460-2075.1984.tb01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildrop R., Brüggemann M., Radbruch A., Rajewsky K., Beyreuther K. Immunoglobulin V region variants in hybridoma cells. II. Recombination between V genes. EMBO J. 1982;1(5):635–640. doi: 10.1002/j.1460-2075.1982.tb01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlyn P. H., Browniee G. G., Cheng C. C., Gait M. J., Milstein C. Complete sequence of constant and 3' noncoding regions of an immunoglobulin mRNA using the dideoxynucleotide method of RNA sequencing. Cell. 1978 Nov;15(3):1067–1075. doi: 10.1016/0092-8674(78)90290-8. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Krawinkel U., Zoebelein G., Brüggemann M., Radbruch A., Rajewsky K. Recombination between antibody heavy chain variable-region genes: evidence for gene conversion. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4997–5001. doi: 10.1073/pnas.80.16.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Hämmerling G. J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978 Jun;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- Reth M., Imanishi-Kari T., Rajewsky K. Analysis of the repertoire of anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in C 57 BL/6 mice by cell fusion. II. Characterization of idiotopes by monoclonal anti-idiotope antibodies. Eur J Immunol. 1979 Dec;9(12):1004–1013. doi: 10.1002/eji.1830091216. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Pawlita M., Pumphrey J., Heller M. Somatic diversification of immunoglobulins. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2162–2166. doi: 10.1073/pnas.81.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablitzky F., Rajewsky K. Molecular basis of an isogeneic anti-idiotypic response. EMBO J. 1984 Dec 1;3(12):3005–3012. doi: 10.1002/j.1460-2075.1984.tb02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Takahashi N., Yaoita Y., Honjo T. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 1982 Mar;28(3):499–506. doi: 10.1016/0092-8674(82)90204-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A. R., Askonas B. A. Senescence of an antibody-forming cell clone. Nature. 1972 Aug 11;238(5363):337–339. doi: 10.1038/238337a0. [DOI] [PubMed] [Google Scholar]



