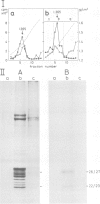
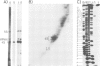
Abstract
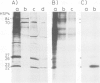
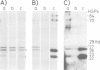
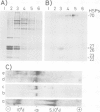
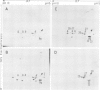
We have identified and characterized a ribonucleoprotein structure from the cytoplasm of Drosophila melanogaster tissue culture cells which is equivalent to the prosome, a recently described ribonucleoprotein particle of duck and mouse cells. During the recovery period following heat shock, the low mol. wt. heat-shock proteins form cytoplasmic ribonucleoprotein particles which co-purify with the Drosophila prosome. Both ribonucleoprotein particles share several structural properties but their protein constituents differ in their metabolism and cellular localization during the heat treatment. We also report the partial nucleotide sequences of several small RNA species associated with the Drosophila prosome. One of them has a strong sequence homology with the U6 mammalian small nuclear RNA.
Full text
PDF
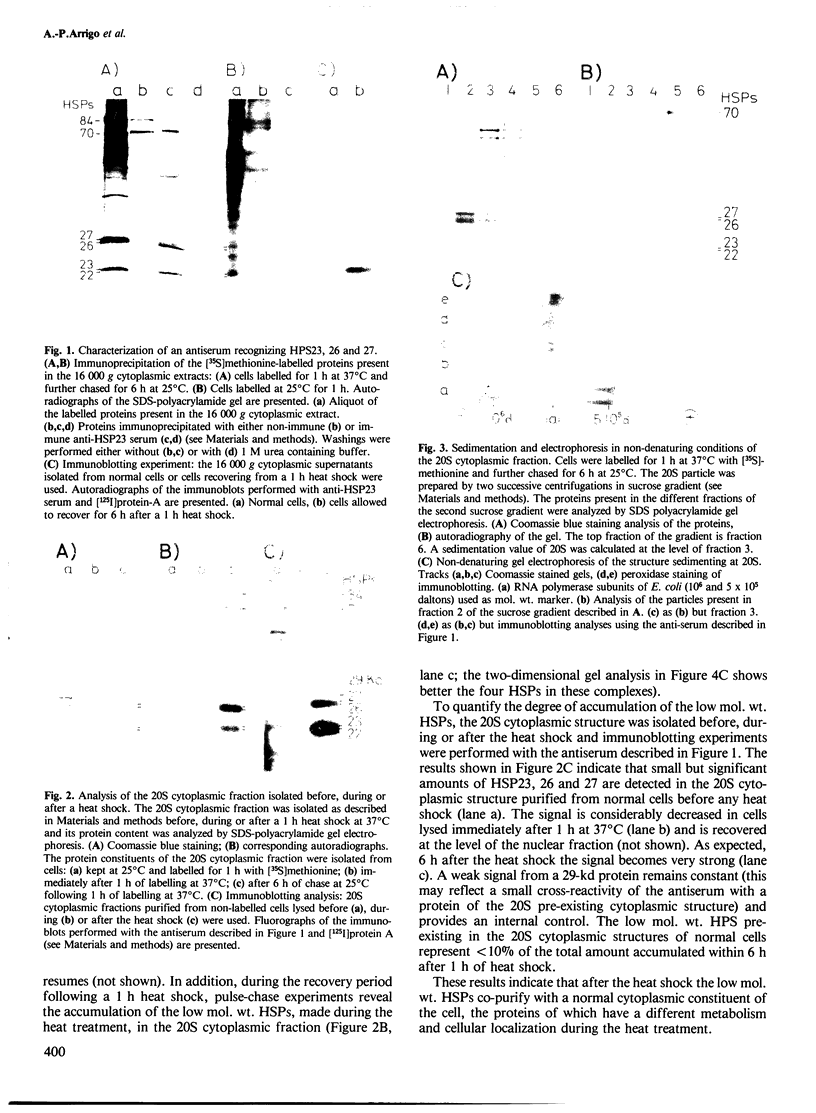
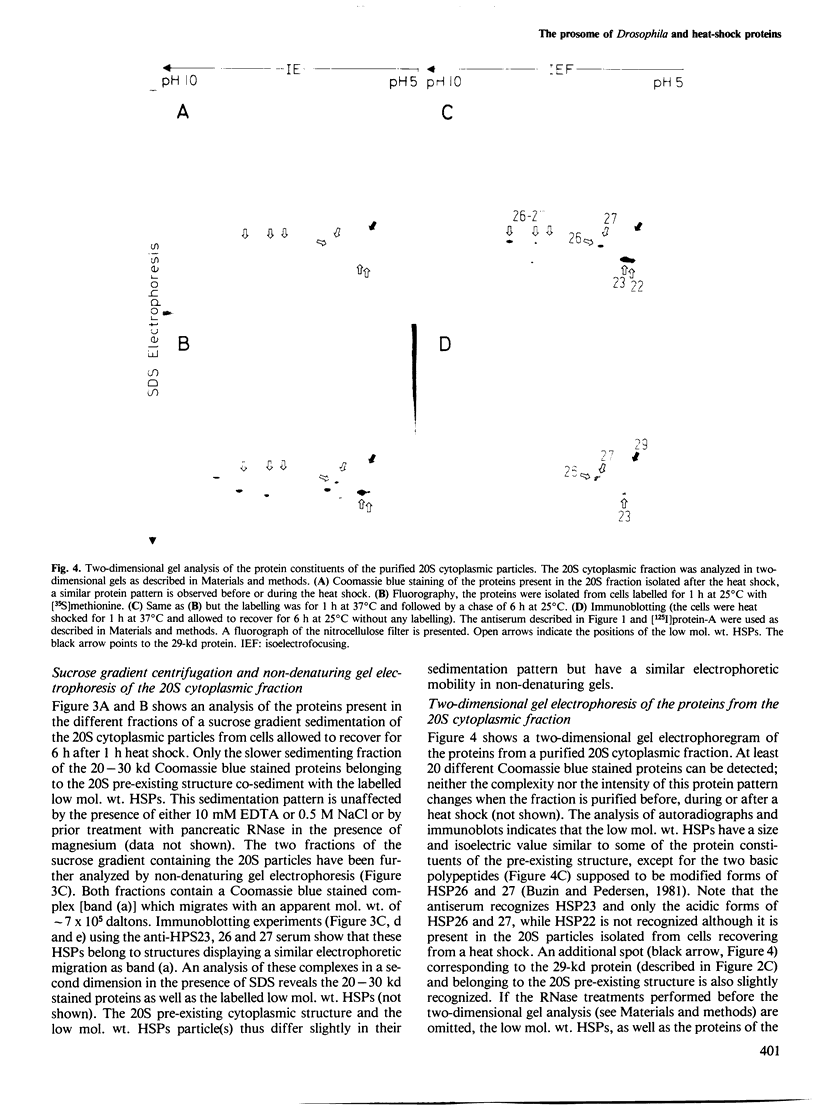



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Ahmad-Zadeh C. Immunofluorescence localization of a small heat shock protein (hsp 23) in salivary gland cells of Drosophila melanogaster. Mol Gen Genet. 1981;184(1):73–79. doi: 10.1007/BF00271198. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Darlix J. L., Spahr P. F. A cellular protein phosphorylated by the avian sarcoma virus transforming gene product is associated with ribonucleoprotein particles. EMBO J. 1983;2(3):309–315. doi: 10.1002/j.1460-2075.1983.tb01424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Fakan S., Tissières A. Localization of the heat shock-induced proteins in Drosophila melanogaster tissue culture cells. Dev Biol. 1980 Jul;78(1):86–103. doi: 10.1016/0012-1606(80)90320-6. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P. Investigation of the function of the heat shock proteins in Drosophila melanogaster tissue culture cells. Mol Gen Genet. 1980;178(3):517–524. doi: 10.1007/BF00337856. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzin C. H., Petersen N. S. A comparison of the multiple Drosophila heat shock proteins in cell lines and larval salivary glands by two-dimensional gel electrophoresis. J Mol Biol. 1982 Jun 25;158(2):181–201. doi: 10.1016/0022-2836(82)90428-4. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Levray M., Bromley P. A., Spahr P. F. Characterization of the genomic RNA from a Rous sarcoma virus mutant temperature sensitive for cell transformation. Nucleic Acids Res. 1979 Feb;6(2):471–485. doi: 10.1093/nar/6.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F. High spontaneous mutation rate of Rous sarcoma virus demonstrated by direct sequencing of the RNA genome. Nucleic Acids Res. 1983 Sep 10;11(17):5953–5967. doi: 10.1093/nar/11.17.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. In vitro culture of Drosophila melanogaster embryonic cells. In Vitro. 1970 Nov-Dec;6(3):162–172. doi: 10.1007/BF02617759. [DOI] [PubMed] [Google Scholar]
- Epstein P., Reddy R., Henning D., Busch H. The nucleotide sequence of nuclear U6 (4.7 S) RNA. J Biol Chem. 1980 Sep 25;255(18):8901–8906. [PubMed] [Google Scholar]
- Harada F., Kato N., Nishimura S. The nucleotide sequence of nuclear 4.8S RNA of mouse cells. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1332–1340. doi: 10.1016/0006-291x(80)91620-4. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W., Loche M., Darlix J. L., Cramer R., Türler H., Weil R. Simian virus 40 large tumor antigen: a "RNA binding protein"? Proc Natl Acad Sci U S A. 1982 Feb;79(4):1139–1143. doi: 10.1073/pnas.79.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W., Türler H. Simian virus 40 and polyoma virus induce synthesis of heat shock proteins in permissive cells. Mol Cell Biol. 1983 Jan;3(1):1–8. doi: 10.1128/mcb.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel P. M., Bautz E. K. Heat-shock proteins are associated with hnRNA in Drosophila melanogaster tissue culture cells. EMBO J. 1983;2(5):705–710. doi: 10.1002/j.1460-2075.1983.tb01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levinger L., Varshavsky A. Heat-shock proteins of Drosophila are associated with nuclease-resistant, high-salt-resistant nuclear structures. J Cell Biol. 1981 Sep;90(3):793–796. doi: 10.1083/jcb.90.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirault M. E., Goldschmidt-Clermont M., Moran L., Arrigo A. P., Tissières A. The effect of heat shock on gene expression in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):819–827. doi: 10.1101/sqb.1978.042.01.082. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Steitz J. A. Sequence of U1 RNA from Drosophila melanogaster: implications for U1 secondary structure and possible involvement in splicing. Nucleic Acids Res. 1981 Dec 11;9(23):6351–6368. doi: 10.1093/nar/9.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Okada N., Tani T., Itoh Y., Itoh M. Nucleotide sequences of mouse genomic loci including a gene or pseudogene for U6 (4.8S) nuclear RNA. Nucleic Acids Res. 1981 Oct 10;9(19):5145–5158. doi: 10.1093/nar/9.19.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
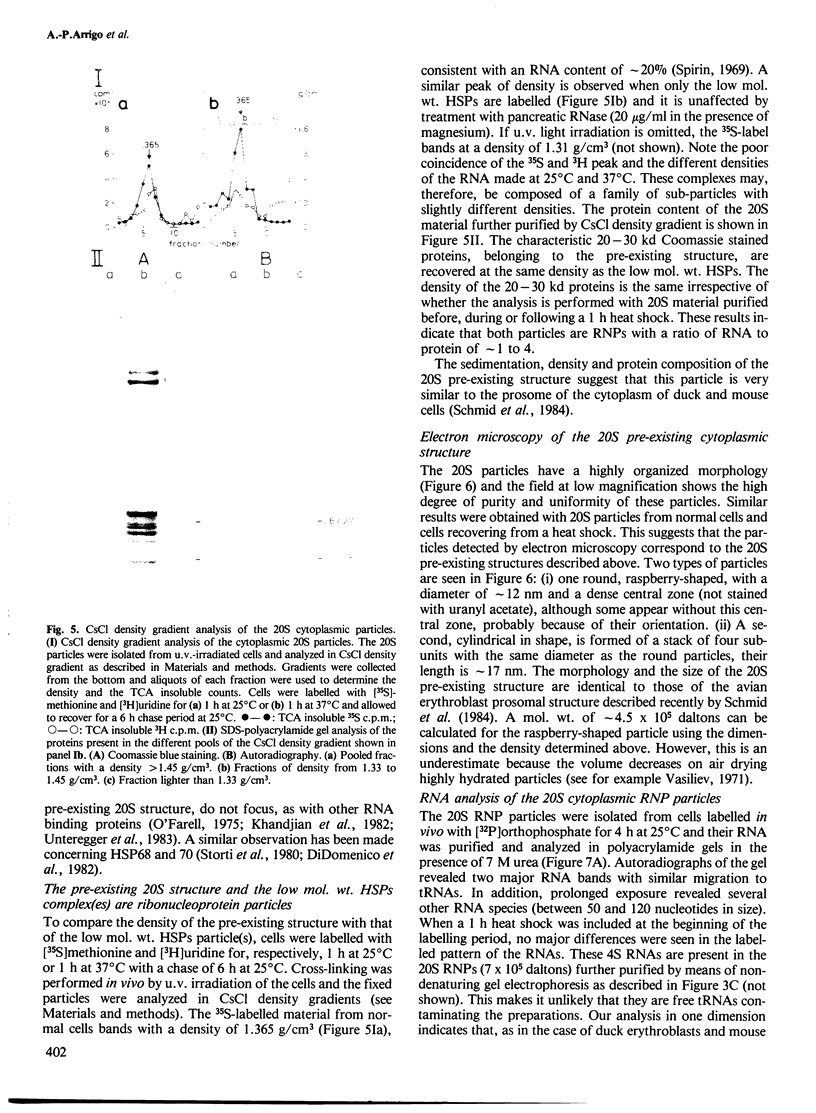
- Schmid H. P., Akhayat O., Martins De Sa C., Puvion F., Koehler K., Scherrer K. The prosome: an ubiquitous morphologically distinct RNP particle associated with repressed mRNPs and containing specific ScRNA and a characteristic set of proteins. EMBO J. 1984 Jan;3(1):29–34. doi: 10.1002/j.1460-2075.1984.tb01757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Sinibaldi R. M., Morris P. W. Putative function of Drosophila melanogaster heat shock proteins in the nucleoskeleton. J Biol Chem. 1981 Nov 10;256(21):10735–10738. [PubMed] [Google Scholar]
- Southgate R., Ayme A., Voellmy R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983 Mar 25;165(1):35–57. doi: 10.1016/s0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Spirin A. S. The second Sir Hans Krebs Lecture. Informosomes. Eur J Biochem. 1969 Aug;10(1):20–35. doi: 10.1111/j.1432-1033.1969.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
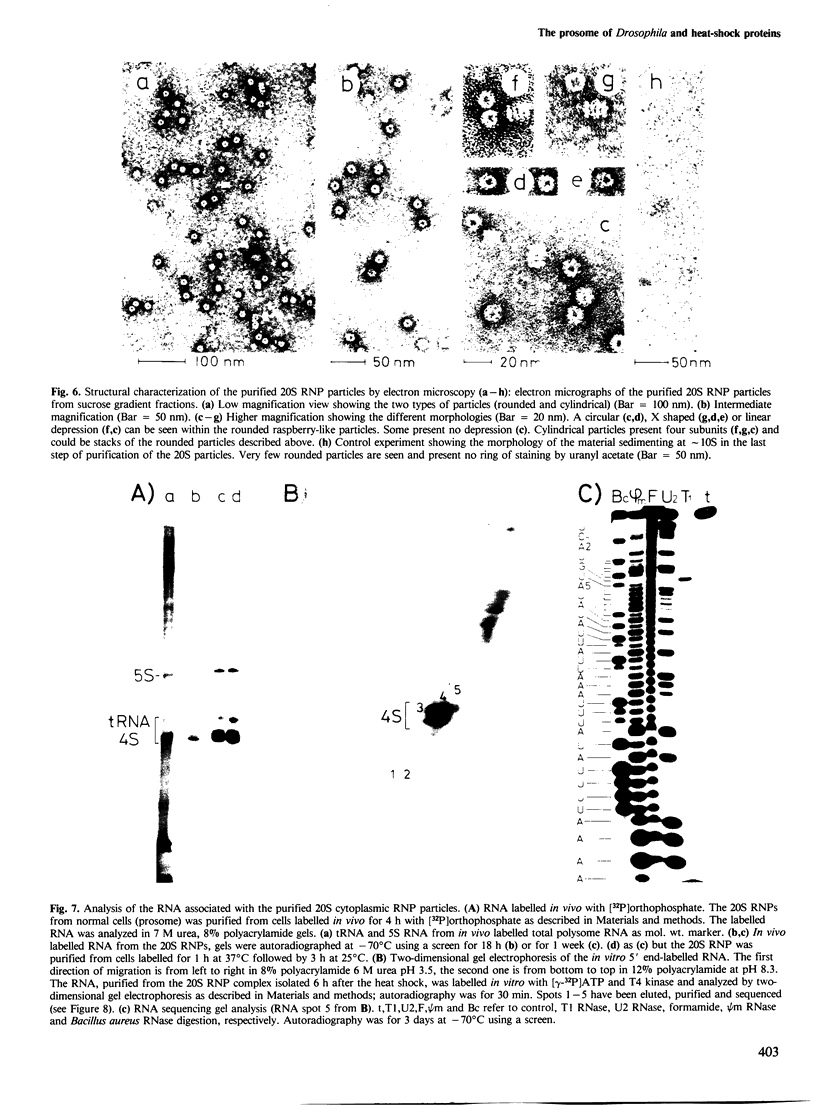
- Vasiliev V. D. Electron microscopy study of 70 S ribosomes of Escherichia coli. FEBS Lett. 1971 May 10;14(4):203–205. doi: 10.1016/0014-5793(71)80617-8. [DOI] [PubMed] [Google Scholar]
- Velazquez J. M., DiDomenico B. J., Lindquist S. Intracellular localization of heat shock proteins in Drosophila. Cell. 1980 Jul;20(3):679–689. doi: 10.1016/0092-8674(80)90314-1. [DOI] [PubMed] [Google Scholar]
- Vincent A., Akhayat O., Goldenberg S., Scherrer K. Differential repression of specific mRNA in erythroblast cytoplasm: a possible role for free mRNP proteins. EMBO J. 1983;2(11):1869–1876. doi: 10.1002/j.1460-2075.1983.tb01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M., Tanguay R. M. Different intracellular distributions of heat-shock and arsenite-induced proteins in Drosophila Kc cells. Possible relation with the phosphorylation and translocation of a major cytoskeletal protein. J Mol Biol. 1982 Dec 5;162(2):365–378. doi: 10.1016/0022-2836(82)90532-0. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Reinders R. J., van Venrooij W. J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur J Biochem. 1980 Nov;112(2):323–330. doi: 10.1111/j.1432-1033.1980.tb07207.x. [DOI] [PubMed] [Google Scholar]
- Zeller R., Nyffenegger T., De Robertis E. M. Nucleocytoplasmic distribution of snRNPs and stockpiled snRNA-binding proteins during oogenesis and early development in Xenopus laevis. Cell. 1983 Feb;32(2):425–434. doi: 10.1016/0092-8674(83)90462-2. [DOI] [PubMed] [Google Scholar]