Abstract
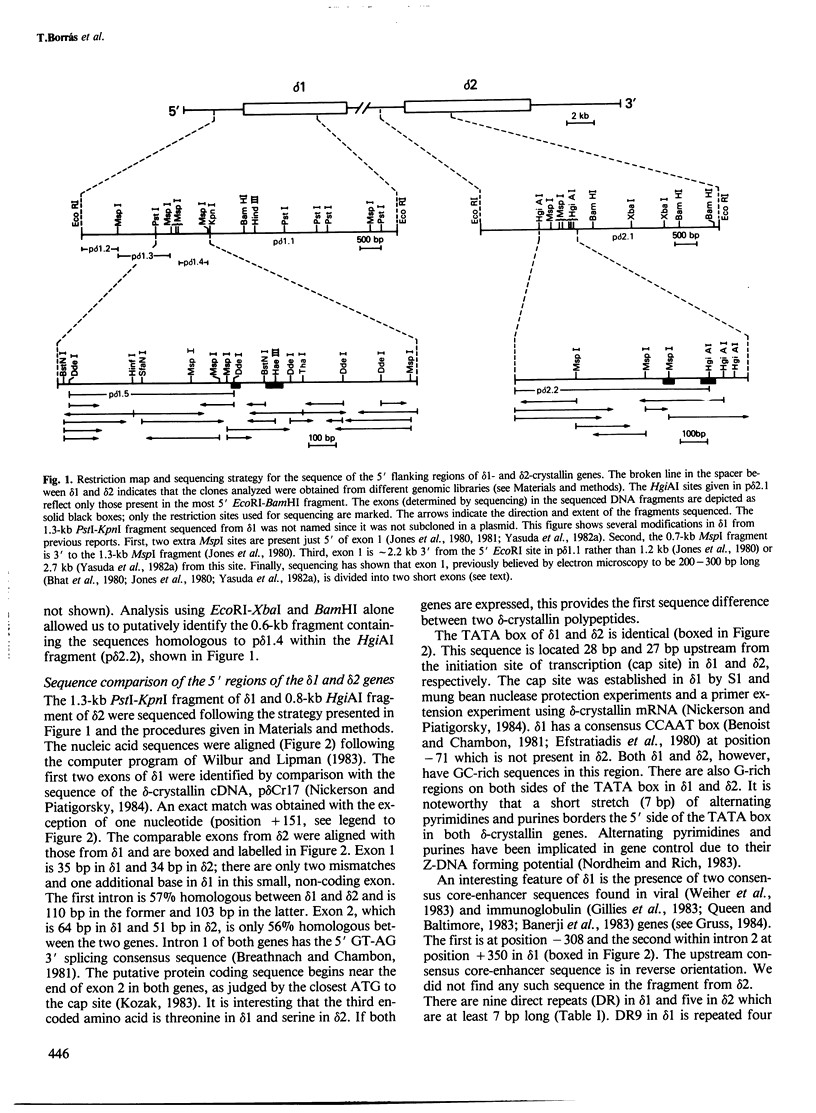
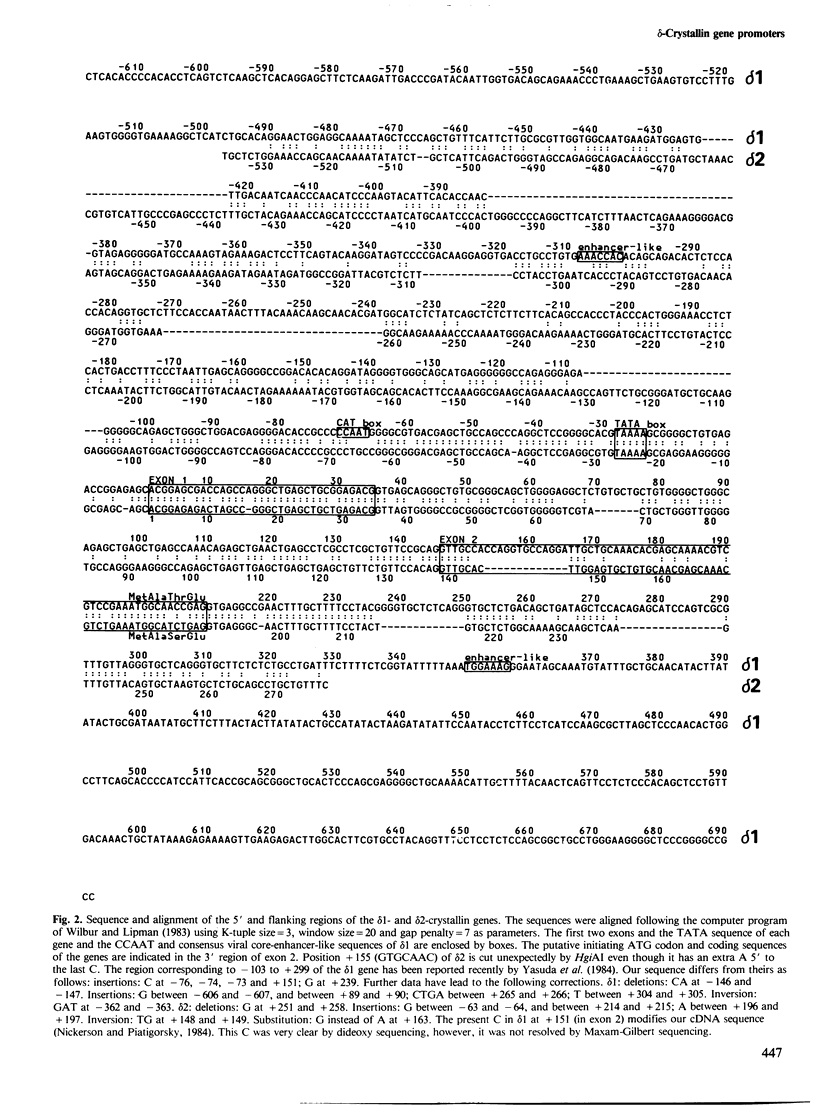
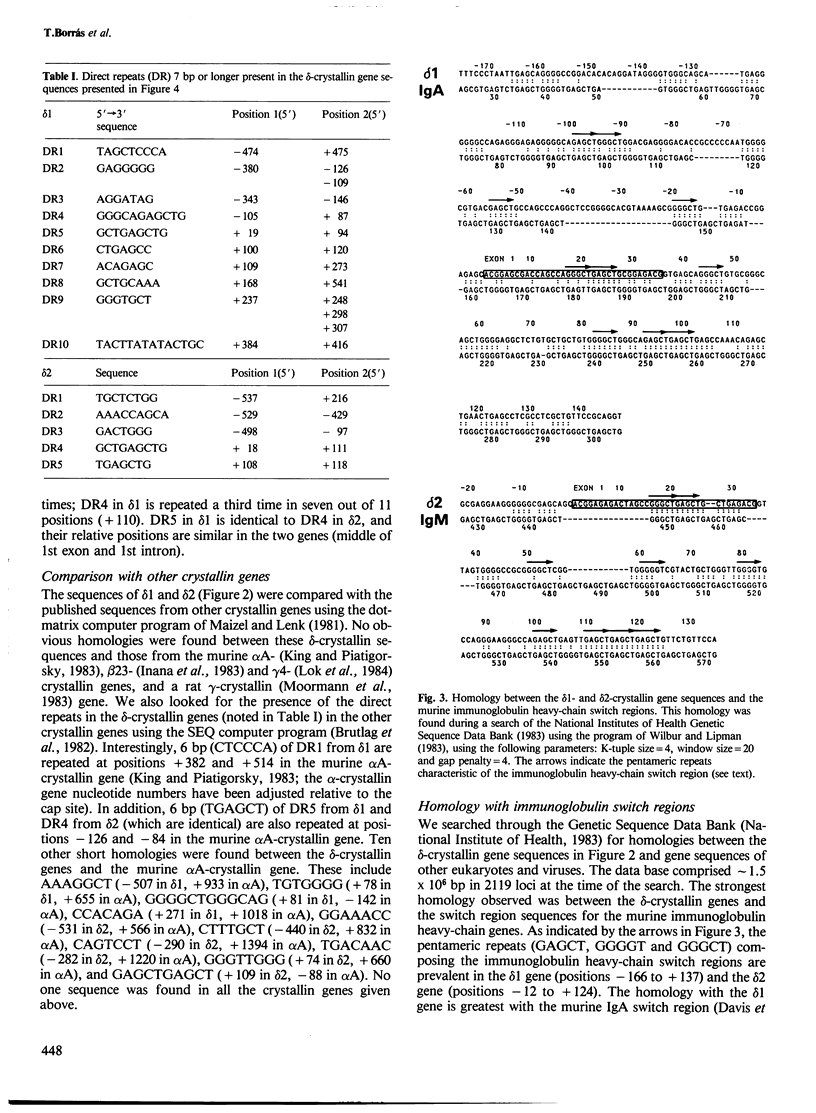
There are two delta-crystallin genes (delta 1 and delta 2) in the chicken which are oriented with the same transcriptional polarity (5' delta 1-delta 2-3') and are separated by approximately 4.2 kb of DNA. Existing evidence indicates that delta 1 is very active in the embryonic lens; in contrast, delta 2 appears very inactive, if expressed at all. We have sequenced the 5' regions of delta 1 and delta 2 and tested their ability to promote chloramphenicol acetyltransferase (CAT) activity using the pSVO-CAT expression vector in transfected embryonic lens epithelia. The sequence data establish that the previously reported delta-crystallin cDNAs were derived from mRNAs encoded in delta 1 and not in delta 2. The transfection experiments indicated that the delta 1 promoter is appreciably stronger than the delta 2 promoter. Interestingly, a consensus CCAAT sequence (position - 71) and two consensus viral core-enhancer-like sequences (positions - 308 and +350) are confined to the more active, delta 1 gene. delta 1 and delta 2 have similar TATA boxes (TAAAA) at positions - 28 and - 27, respectively. Exon 1 in delta 1 (35 bp) and in delta 2 (34 bp) are extremely homologous, containing just two mismatches; exon 2 in delta 1 (64 bp) and in delta 2 (51 bp) are only 56% homologous and contain the putative translation initiating codon and three encoded amino acids. Unexpectedly, intron 1 of both genes has numerous pentameric repeats present in the murine immunoglobulin heavy-chain switch regions (GAGCT, GGGGT and GGGCT). Other direct repeats were found in delta 1 and delta 2, and short homologies were noted between the chicken delta-crystallin and murine alpha A-crystallin genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi M., Alcala J. R., Maisel H. Delta-crystallin synthesis by the adult chicken lens. Exp Eye Res. 1981 Feb;32(2):251–254. doi: 10.1016/0014-4835(81)90013-0. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Jones R. E., Sullivan M. A., Piatigorsky J. Chicken lens crystallin DNA sequences show at least two delta-crystallin genes. Nature. 1980 Mar 20;284(5753):234–238. doi: 10.1038/284234a0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H. The vertebrate eye lens. Science. 1977 Jul 8;197(4299):127–138. doi: 10.1126/science.877544. [DOI] [PubMed] [Google Scholar]
- Brahma S. K., Starre H. Studies on biosynthesis of soluble lens crystallin antigens in the chick by isoelectric focusing in thin-layer polyacrylamide gel. Exp Cell Res. 1976 Jan;97:175–183. doi: 10.1016/0014-4827(76)90666-2. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brutlag D. L., Clayton J., Friedland P., Kedes L. H. SEQ: a nucleotide sequence analysis and recombination system. Nucleic Acids Res. 1982 Jan 11;10(1):279–294. doi: 10.1093/nar/10.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. E. DNA sequences mediating class switching in alpha-immunoglobulins. Science. 1980 Sep 19;209(4463):1360–1365. doi: 10.1126/science.6774415. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Genis-Galves J. M., Maisel H., Castro J. Changes in chick lens proteins with aging. Exp Eye Res. 1968 Oct;7(4):593–602. doi: 10.1016/s0014-4835(68)80014-4. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Birnstiel M. L. Identification of regulatory sequences in the prelude sequences of an H2A histone gene by the study of specific deletion mutants in vivo. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1432–1436. doi: 10.1073/pnas.77.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P. Magic enhancers? DNA. 1984;3(1):1–5. doi: 10.1089/dna.1.1984.3.1. [DOI] [PubMed] [Google Scholar]
- Hansen J. N. Use of solubilizable acrylamide disulfide gels for isolation of DNA fragments suitable for sequence analysis. Anal Biochem. 1981 Sep 1;116(1):146–151. doi: 10.1016/0003-2697(81)90337-7. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Dilley K. J. Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract. Exp Eye Res. 1976 Jan;22(1):1–73. doi: 10.1016/0014-4835(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Hawkins J. W., Nickerson J. M., Sullivan M. A., Piatigorsky J. The chicken delta-crystallin gene family. Two genes of similar structure in close chromosomal approximation. J Biol Chem. 1984 Aug 10;259(15):9821–9825. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Inana G., Piatigorsky J., Norman B., Slingsby C., Blundell T. Gene and protein structure of a beta-crystallin polypeptide in murine lens: relationship of exons and structural motifs. Nature. 1983 Mar 24;302(5906):310–315. doi: 10.1038/302310a0. [DOI] [PubMed] [Google Scholar]
- Jones R. E., Bhat S. P., Sullivan M. A., Piatigorsky J. Comparison of two delta-crystallin genes in the chicken. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5879–5883. doi: 10.1073/pnas.77.10.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. E., DeFeo D., Piatigorsky J. Transcription and site-specific hypomethylation of the delta-crystallin genes in the embryonic chicken lens. J Biol Chem. 1981 Aug 10;256(15):8172–8176. [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Kondoh H., Yasuda K., Okada T. S. Tissue-specific expression of a cloned chick delta-crystallin gene in mouse cells. Nature. 1983 Feb 3;301(5899):440–442. doi: 10.1038/301440a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok S., Tsui L. C., Shinohara T., Piatigorsky J., Gold R., Breitman M. Analysis of the mouse gamma-crystallin gene family: assignment of multiple cDNAs to discrete genomic sequences and characterization of a representative gene. Nucleic Acids Res. 1984 Jun 11;12(11):4517–4529. doi: 10.1093/nar/12.11.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McAvoy J. W. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J Embryol Exp Morphol. 1978 Jun;45:271–281. [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Moormann R. J., den Dunnen J. T., Mulleners L., Andreoli P., Bloemendal H., Schoenmakers J. G. Strict co-linearity of genetic and protein folding domains in an intragenically duplicated rat lens gamma-crystallin gene. J Mol Biol. 1983 Dec 25;171(4):353–368. doi: 10.1016/0022-2836(83)90034-7. [DOI] [PubMed] [Google Scholar]
- Nickerson J. M., Piatigorsky J. Sequence of a complete chicken delta-crystallin cDNA. Proc Natl Acad Sci U S A. 1984 May;81(9):2611–2615. doi: 10.1073/pnas.81.9.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T., Nakai S., Honjo T. Switch region of immunoglobulin Cmu gene is composed of simple tandem repetitive sequences. Nature. 1981 Aug 27;292(5826):845–848. doi: 10.1038/292845a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Papaconstantinou J. Molecular aspects of lens cell differentiation. Science. 1967 Apr 21;156(3773):338–346. doi: 10.1126/science.156.3773.338. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Delta crystallins and their nucleic acids. Mol Cell Biochem. 1984;59(1-2):33–56. doi: 10.1007/BF00231304. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19(3):134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Webster H. D., Craig S. P. Protein synthesis and ultrastructure during the formation of embryonic chick lens fibers in vivo and in vitro. Dev Biol. 1972 Feb;27(2):176–189. doi: 10.1016/0012-1606(72)90096-6. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- RABAEY M. Electrophoretic and immunoelectrophoretic studies on the soluble proteins in the developing lens of birds. Exp Eye Res. 1962 Jun;1:310–316. doi: 10.1016/s0014-4835(62)80017-7. [DOI] [PubMed] [Google Scholar]
- Reszelbach R., Shinohara T., Piatigorsky J. Resolution of two distinct embryonic chick delta-crystallin bands by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate and urea. Exp Eye Res. 1977 Dec;25(6):583–593. doi: 10.1016/0014-4835(77)90137-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T., Piatigorsky J. Quantitation of delta-crystallin messenger RNA during lens induction in chick embryos. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2808–2812. doi: 10.1073/pnas.73.8.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. An improved technique for obtaining enhanced infectivity with herpes simplex virus type 1 DNA. J Gen Virol. 1976 Dec;33(3):447–458. doi: 10.1099/0022-1317-33-3-447. [DOI] [PubMed] [Google Scholar]
- Tréton J. A., Shinohara T., Piatigorsky J. Degradation of delta-crystallin mRNA in the lens fiber cells of the chicken. Dev Biol. 1982 Jul;92(1):60–65. doi: 10.1016/0012-1606(82)90150-6. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. A., Piatigorsky J. Comparative and evolutionary aspects of delta-crystallin in the vertebrate lens. Eur J Biochem. 1979 Oct 15;100(2):349–357. doi: 10.1111/j.1432-1033.1979.tb04177.x. [DOI] [PubMed] [Google Scholar]
- Yasuda K., Kondoh H., Okada T. S., Nakajima N., Shimura Y. Organization of delta-crystallin genes in the chicken. Nucleic Acids Res. 1982 May 11;10(9):2879–2891. doi: 10.1093/nar/10.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K., Nakajima N., Isobe T., Okada T. S., Shimura Y. The nucleotide sequence of a complete chicken delta-crystallin cDNA. EMBO J. 1984 Jun;3(6):1397–1402. doi: 10.1002/j.1460-2075.1984.tb01983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Katoh A. Crystallin synthesis by chick lens. II. Changes in synthetic activities of epithelial and fiber cells during embryonic development. Exp Eye Res. 1971 Mar;11(2):184–194. doi: 10.1016/s0014-4835(71)80022-2. [DOI] [PubMed] [Google Scholar]
- Zwaan J., Ikeda A. Macromolecular events during differentiation of the chicken lens. Exp Eye Res. 1968 Apr;7(2):301–311. doi: 10.1016/s0014-4835(68)80081-8. [DOI] [PubMed] [Google Scholar]