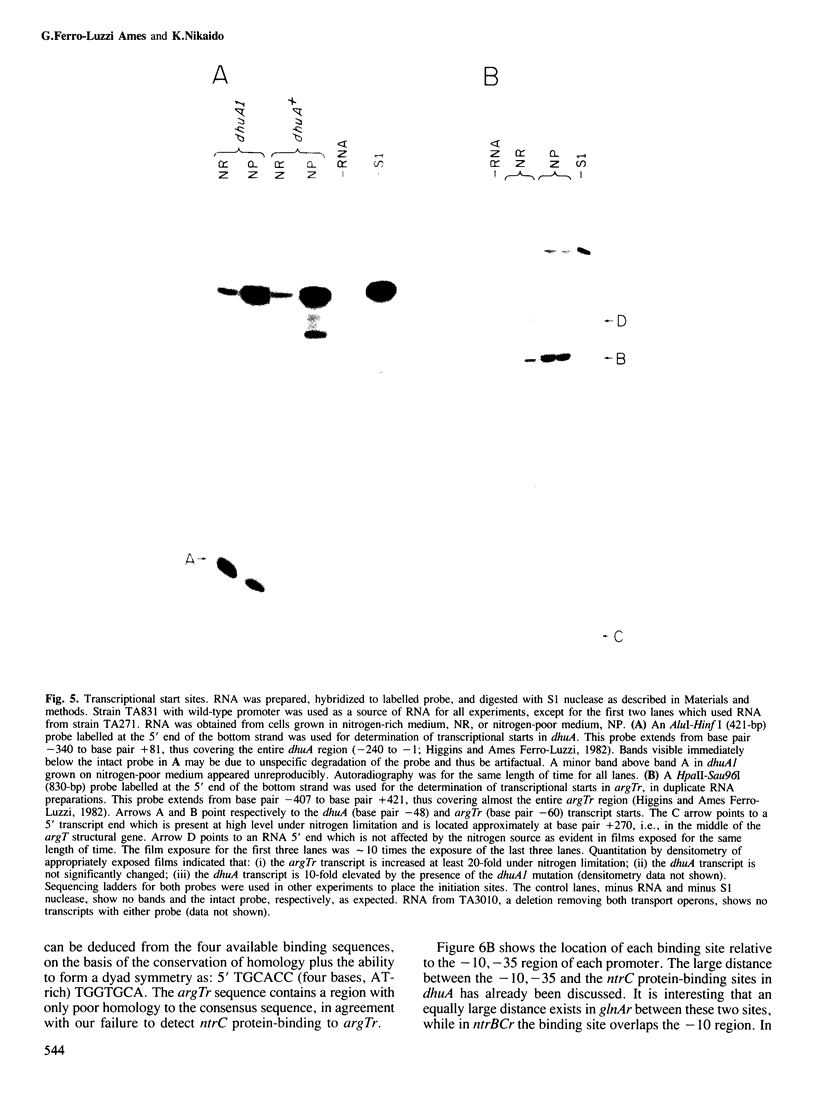
Abstract
We have investigated the DNA-binding ability of a nitrogen regulatory protein, the product of the ntrC gene, to several nitrogen-regulated promoters in Salmonella typhimurium. The ntrC protein is able to bind to the regulatory region (dhuA) of an operon coding for genes involved in the active transport of histidine, but not to another transport-related regulatory region, argTr. It bound to two different sites within the regulatory region of glnA (glutamine synthetase) and to one site in the regulatory region for the ntrBC operon. A consensus sequence has been derived from these four binding sites. The binding sequence displays dyad symmetry, as expected for the dimeric ntrC protein. The relationship of the binding sites to regulation of transcription initiation and termination, and to published homologies within the sequences of regulatory sites for nif genes is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Morales A., Dixon R., Merrick M. Positive and negative control of the glnA ntrBC regulon in Klebsiella pneumoniae. EMBO J. 1984 Mar;3(3):501–507. doi: 10.1002/j.1460-2075.1984.tb01837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon J., Cannon M., Buchanan-Wollaston V., Cannon F. The nif promoters of Klebsiella pneumoniae have a characteristic primary structure. Cell. 1983 Sep;34(2):665–671. doi: 10.1016/0092-8674(83)90399-9. [DOI] [PubMed] [Google Scholar]
- Brown S. E., Ausubel F. M. Mutations affecting regulation of the Klebsiella pneumoniae nifH (nitrogenase reductase) promotor. J Bacteriol. 1984 Jan;157(1):143–147. doi: 10.1128/jb.157.1.143-147.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias A. A., Bastarrachea F. Nucleotide sequence of the glnA control region of Escherichia coli. Mol Gen Genet. 1983;190(1):171–175. doi: 10.1007/BF00330342. [DOI] [PubMed] [Google Scholar]
- Gaillardin C. M., Magasanik B. Involvement of the product of the glnF gene in the autogenous regulation of glutamine synthetase formation in Klebsiella aerogenes. J Bacteriol. 1978 Mar;133(3):1329–1338. doi: 10.1128/jb.133.3.1329-1338.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanau R., Koduri R. K., Ho N., Brenchley J. E. Nucleotide sequence of the control regions for the glnA and glnL genes of Salmonella typhimurium. J Bacteriol. 1983 Jul;155(1):82–89. doi: 10.1128/jb.155.1.82-89.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F. Regulatory regions of two transport operons under nitrogen control: nucleotide sequences. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1083–1087. doi: 10.1073/pnas.79.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Ames G. F. Two periplasmic transport proteins which interact with a common membrane receptor show extensive homology: complete nucleotide sequences. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6038–6042. doi: 10.1073/pnas.78.10.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Haag P. D., Nikaido K., Ardeshir F., Garcia G., Ames G. F. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature. 1982 Aug 19;298(5876):723–727. doi: 10.1038/298723a0. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D. L. A protein that preferentially binds Drosophila satellite DNA. Proc Natl Acad Sci U S A. 1979 Feb;76(2):726–730. doi: 10.1073/pnas.76.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S. G., McFarland N. C., Hui S. P., Esmon B., Ames G. F. Nitrogen control of Salmonella typhimurium: co-regulation of synthesis of glutamine synthetase and amino acid transport systems. J Bacteriol. 1979 Apr;138(1):218–234. doi: 10.1128/jb.138.1.218-234.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S., Burton D., Garcia E., McCarter L., McFarland N. Nitrogen control in Salmonella: regulation by the glnR and glnF gene products. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4576–4580. doi: 10.1073/pnas.76.9.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. S., Ames G. F. Analysis of promoter mutations in the histidine transport operon of Salmonella typhimurium: use of hybrid M13 bacteriophages for cloning, transformation, and sequencing. J Bacteriol. 1984 Sep;159(3):1000–1005. doi: 10.1128/jb.159.3.1000-1005.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- McCarter L., Krajewska-Grynkiewicz K., Trinh D., Wei G., Kustu S. Characterization of mutations that lie in the promoter-regulatory region for glnA, the structural gene encoding glutamine synthetase. Mol Gen Genet. 1984;197(1):150–160. doi: 10.1007/BF00327936. [DOI] [PubMed] [Google Scholar]
- McFarland N., McCarter L., Artz S., Kustu S. Nitrogen regulatory locus "glnR" of enteric bacteria is composed of cistrons ntrB and ntrC: identification of their protein products. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2135–2139. doi: 10.1073/pnas.78.4.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M. J. A new model for nitrogen control. Nature. 1982 Jun 3;297(5865):362–363. doi: 10.1038/297362a0. [DOI] [PubMed] [Google Scholar]
- Ow D. W., Sundaresan V., Rothstein D. M., Brown S. E., Ausubel F. M. Promoters regulated by the glnG (ntrC) and nifA gene products share a heptameric consensus sequence in the -15 region. Proc Natl Acad Sci U S A. 1983 May;80(9):2524–2528. doi: 10.1073/pnas.80.9.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Rothstein D. M., Magasanik B. Complex glnA-glnL-glnG operon of Escherichia coli. J Bacteriol. 1982 Apr;150(1):202–213. doi: 10.1128/jb.150.1.202-213.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Isolation of the nitrogen assimilation regulator NR(I), the product of the glnG gene of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5554–5558. doi: 10.1073/pnas.80.18.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., Brill W. J. Genetics and regulation of nitrogen fixation. Annu Rev Microbiol. 1981;35:207–235. doi: 10.1146/annurev.mi.35.100181.001231. [DOI] [PubMed] [Google Scholar]
- Stern M. J., Higgins C. F., Ames G. F. Isolation and characterization of lac fusions to two nitrogen-regulated promoters. Mol Gen Genet. 1984;195(1-2):219–227. doi: 10.1007/BF00332750. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno-Nishio S., Backman K. C., Magasanik B. Regulation at the glnL-operator-promoter of the complex glnALG operon of Escherichia coli. J Bacteriol. 1983 Mar;153(3):1247–1251. doi: 10.1128/jb.153.3.1247-1251.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno-Nishio S., Mango S., Reitzer L. J., Magasanik B. Identification and regulation of the glnL operator-promoter of the complex glnALG operon of Escherichia coli. J Bacteriol. 1984 Oct;160(1):379–384. doi: 10.1128/jb.160.1.379-384.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]