Abstract
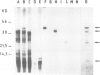
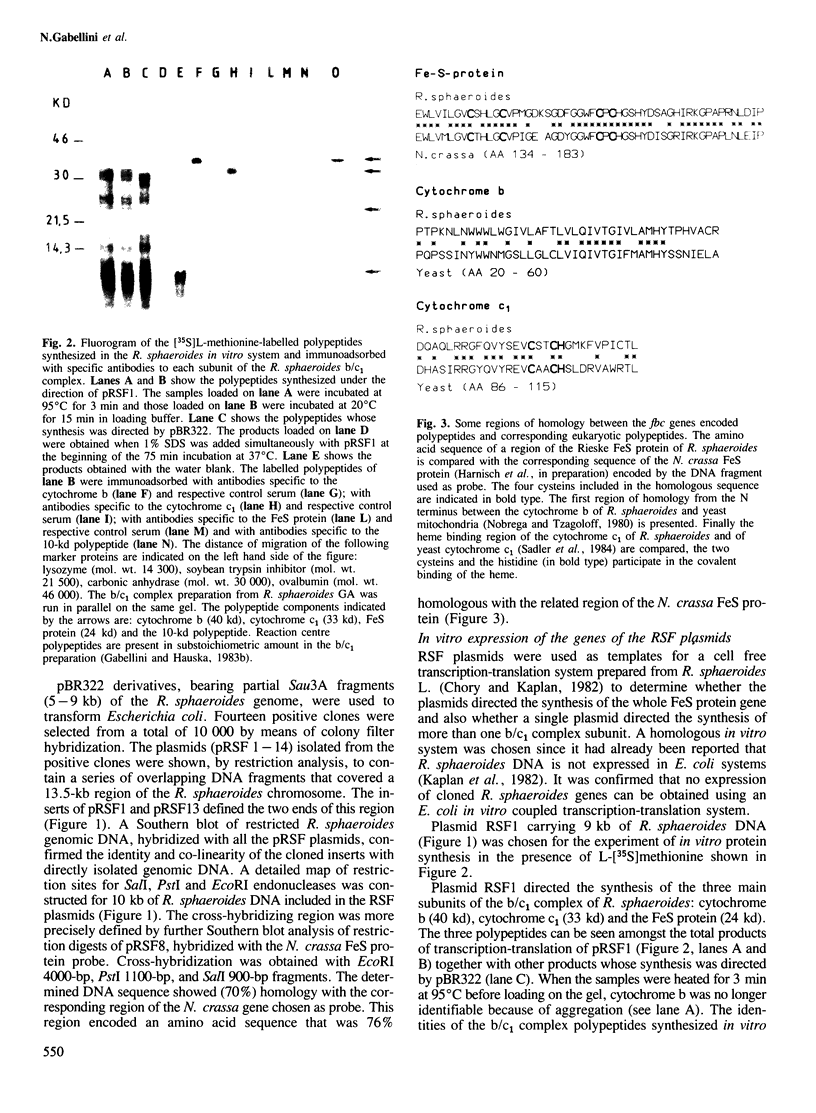
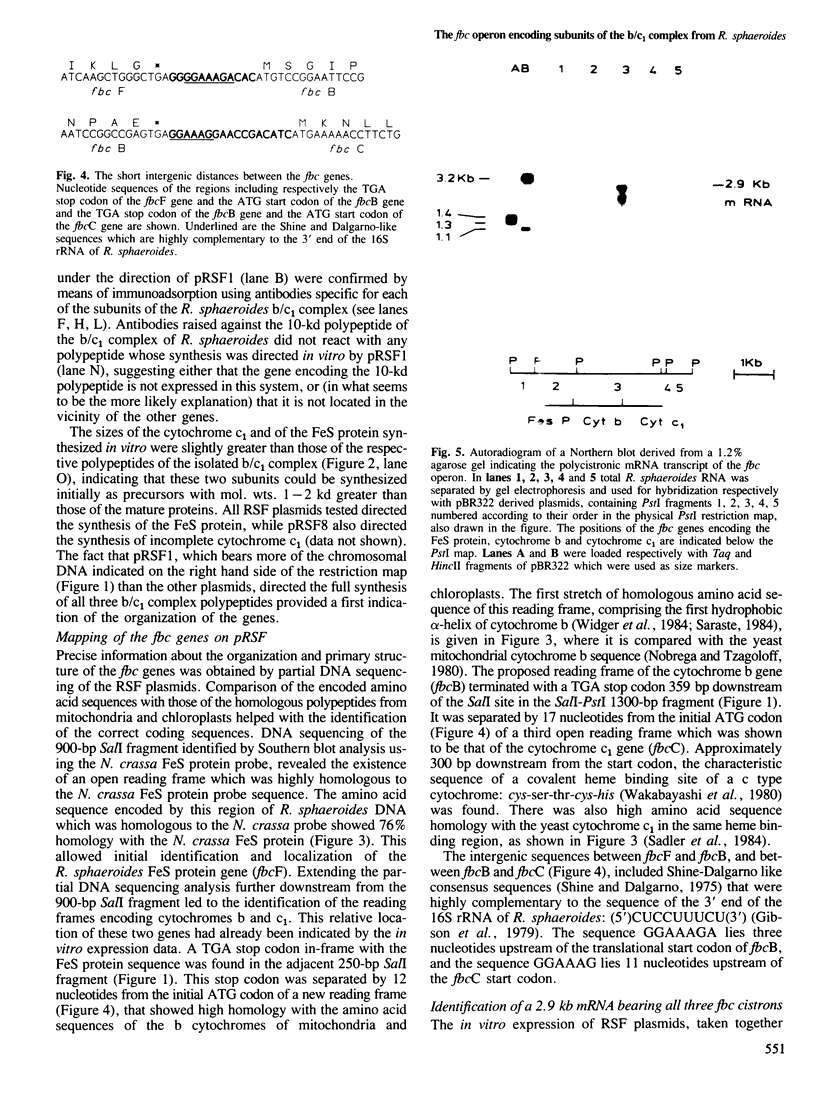
The gene for the FeS protein of the Rhodopseudomonas sphaeroides b/c1 complex was identified by means of cross-hybridization with a segment of the gene encoding the corresponding FeS protein of Neurospora crassa. Plasmids (pRSF1-14) containing the cross-hybridizing region, covering in total 13.5 kb of chromosomal DNA, were expressed in vitro in a homologous system. One RSF plasmid directed the synthesis of all three main polypeptides of the R. sphaeroides b/c1 complex: the FeS protein, cytochrome b and cytochrome c1. The FeS protein and cytochrome c1 were apparently synthesized as precursor forms. None of the pRSF plasmids directed the synthesis of the 10-kd polypeptide found in b/c1 complex preparations. Partial sequencing of the cloned region was performed. Several sites of strong homology between R. sphaeroides and eukaryotic polypeptides of the b/c1 complex were identified. The genes encode the three b/c1 polypeptides in the order: (5') FeS protein, cytochrome b, cytochrome c1. The three genes are transcribed to give a polycistronic mRNA of 2.9 kb. This transcriptional unit has been designated the fbc operon; its coding capacity corresponds to the size of the polycistronic mRNA assuming that only the genes for the FeS protein (fbcF), cytochrome b (fbcB) and cytochrome c1 (fbcC) are present. This could indicate that these three subunits constitute the minimal catalytic unit of the b/c1 complex from photosynthetic membranes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D. G. Cloning and amplified expression of the tyrosyl-tRNA synthetase genes of Bacillus stearothermophilus and Escherichia coli. Eur J Biochem. 1982 Jul;125(2):357–360. doi: 10.1111/j.1432-1033.1982.tb06691.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chory J., Kaplan S. The in vitro transcription-translation of DNA and RNA templates by extracts of Rhodopseudomonas sphaeroides. Optimization and comparison of template specificity with Escherichia coli extracts and in vivo synthesis. J Biol Chem. 1982 Dec 25;257(24):15110–15121. [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabellini N., Bowyer J. R., Hurt E., Melandri B. A., Hauska G. A cytochrome b/c1 complex with ubiquinol--cytochrome c2 oxidoreductase activity from Rhodopseudomonas sphaeroides GA. Eur J Biochem. 1982 Aug;126(1):105–111. doi: 10.1111/j.1432-1033.1982.tb06753.x. [DOI] [PubMed] [Google Scholar]
- Gabellini N., Hauska G. Characterization of cytochrome b in the isolated ubiquinol-cytochrome c2 oxidoreductase from Rhodopseudomonas sphaeroides GA. FEBS Lett. 1983 Mar 7;153(1):146–150. doi: 10.1016/0014-5793(83)80136-7. [DOI] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5066–5070. doi: 10.1073/pnas.75.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R., Stephens P. E. Molecular cloning of the pyruvate dehydrogenase complex genes of Escherichia coli. J Gen Microbiol. 1980 Dec;121(2):277–292. doi: 10.1099/00221287-121-2-277. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hauska G., Hurt E., Gabellini N., Lockau W. Comparative aspects of quinol-cytochrome c/plastocyanin oxidoreductases. Biochim Biophys Acta. 1983 Jul 15;726(2):97–133. doi: 10.1016/0304-4173(83)90002-2. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Gabellini N., Shahak Y., Lockau W., Hauska G. Extra proton translocation and membrane potential generation--universal properties of cytochrome bc1/b6f complexes reconstituted into liposomes. Arch Biochem Biophys. 1983 Sep;225(2):879–885. doi: 10.1016/0003-9861(83)90101-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melandri A. B., Zannoni D. Photosynthetic and respiratory electron flow in the dual functional membrane of facultative photosynthetic bacteria. J Bioenerg Biomembr. 1978 Aug;10(3-4):109–138. doi: 10.1007/BF00743056. [DOI] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Pratt C. Kinetics and regulation of cell-free alkaline phosphatase synthesis. J Bacteriol. 1980 Sep;143(3):1265–1274. doi: 10.1128/jb.143.3.1265-1274.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIESKE J. S., ZAUGG W. S., HANSEN R. E. STUDIES ON THE ELECTRON TRANSFER SYSTEM. LIX. DISTRIBUTION OF IRON AND OF THE COMPONENT GIVING AN ELECTRON PARAMAGNETIC RESONANCE SIGNAL AT G = 1.90 IN SUBFRACTIONS OF COMPLEX 3. J Biol Chem. 1964 Sep;239:3023–3030. [PubMed] [Google Scholar]
- Sadler I., Suda K., Schatz G., Kaudewitz F., Haid A. Sequencing of the nuclear gene for the yeast cytochrome c1 precursor reveals an unusually complex amino-terminal presequence. EMBO J. 1984 Sep;3(9):2137–2143. doi: 10.1002/j.1460-2075.1984.tb02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M. Location of haem-binding sites in the mitochondrial cytochrome b. FEBS Lett. 1984 Jan 30;166(2):367–372. doi: 10.1016/0014-5793(84)80114-3. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Falk G., Walker J. E. Rhodopseudomonas blastica atp operon. Nucleotide sequence and transcription. J Mol Biol. 1984 Oct 25;179(2):185–214. doi: 10.1016/0022-2836(84)90465-0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S., Matsubara H., Kim C. H., Kawai K., King T. E. The complete amino acid sequence of bovine heart cytochrome C1. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1548–1554. doi: 10.1016/s0006-291x(80)80042-8. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Gay N. J. The unc operon. Nucleotide sequence, regulation and structure of ATP-synthase. Biochim Biophys Acta. 1984 Sep 6;768(2):164–200. doi: 10.1016/0304-4173(84)90003-x. [DOI] [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Herrmann R. G., Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D., Darlison M. G., Wilde R. J., Guest J. R. Nucleotide sequence encoding the flavoprotein and hydrophobic subunits of the succinate dehydrogenase of Escherichia coli. Biochem J. 1984 Sep 1;222(2):519–534. doi: 10.1042/bj2220519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Yu L., Yu C. A. Isolation and properties of the cytochrome B-C1 complex from Rhodopseudomonas sphaeroides. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1285–1292. doi: 10.1016/0006-291x(82)92139-8. [DOI] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]