Abstract
Synthesis of cyclin in serum-stimulated quiescent 3T3 cells increases shortly before DNA synthesis after 10 h of stimulation, reaching a maximum after 16 h. Inhibition of DNA synthesis by hydroxyurea does not affect the increase of cyclin following stimulation, as determined by quantitative two-dimensional gel electrophoresis. The levels of cyclin decrease dramatically at the end of the S-phase. Cells kept in the presence of hydroxyurea (G1/S boundary) do not show this decrease in cyclin, indicating that its amounts are regulated by events occurring during the S-phase. Immunofluorescence studies of serum-stimulated quiescent cells in the presence of hydroxyurea, using proliferating cell nuclear antigen (PCNA) autoantibodies, confirm the results obtained by protein analysis. They also reveal that there are dramatic changes in the nuclear distribution of cyclin and that these depend on DNA synthesis or events occurring during the S-phase. Cyclin (PCNA) is no longer detectable at the end of the S-phase. However, pulse-chase experiments indicate that this protein is very stable, suggesting that it possibly interacts with other macromolecules rendering it inaccessible to the antibody. These results strengthen the notion that cyclin is an important component of the events leading to DNA replication and cell division.
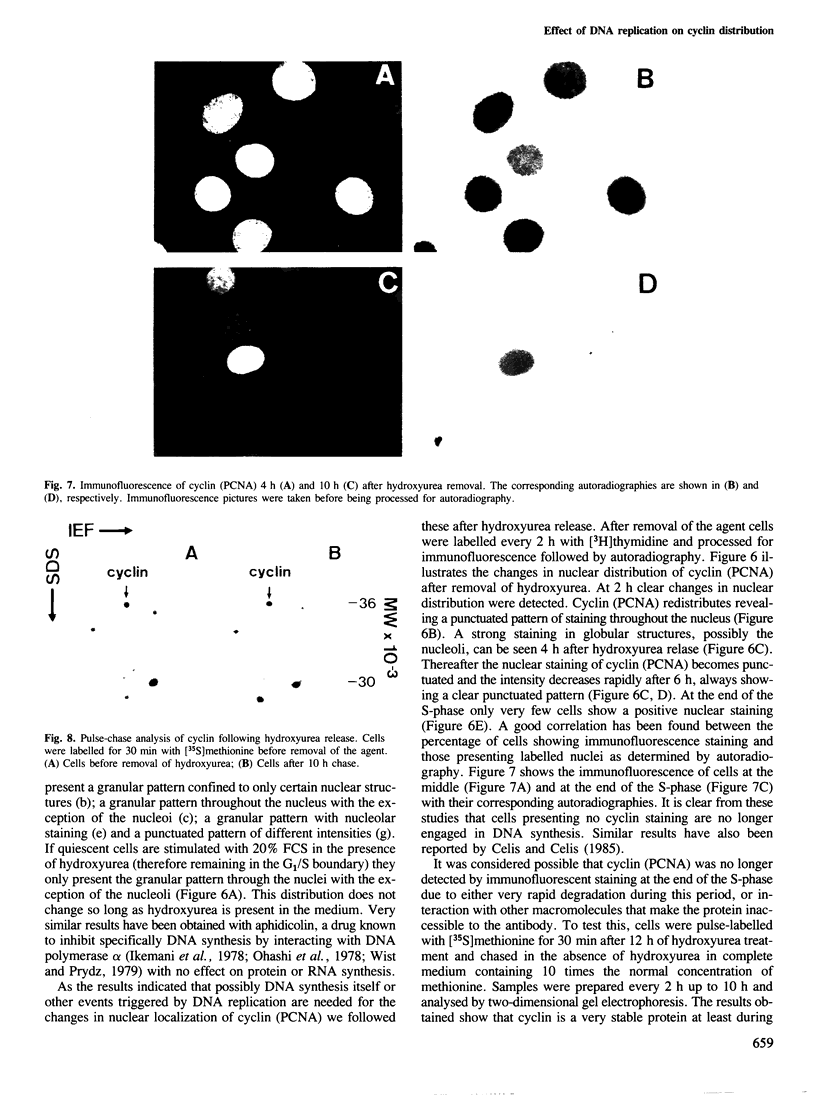
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L., Lindsay J. G. Hydroxyurea reversal of inhibition and use as a cell-synchronizing agent. J Biol Chem. 1967 Mar 25;242(6):1314–1317. [PubMed] [Google Scholar]
- Bravo R., Celis A., Mosses D., Celis J. E. Distribution of HeLa cells polypeptides in cytoplasts and karyoplasts. Cell Biol Int Rep. 1981 May;5(5):479–489. doi: 10.1016/0309-1651(81)90175-2. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Gene expression in normal and virally transformed mouse 3T3b and hamster BHK21 cells. Exp Cell Res. 1980 Jun;127(2):249–260. doi: 10.1016/0014-4827(80)90430-9. [DOI] [PubMed] [Google Scholar]
- Bravo R. Coordinated synthesis of the nuclear protein cyclin and DNA in serum-stimulated quiescent 3T3 cells. FEBS Lett. 1984 Apr 24;169(2):185–188. doi: 10.1016/0014-5793(84)80315-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Bellatin J., Larsen P. M., Arevalo J., Celis J. E. Identification of a nuclear and of a cytoplasmic polypeptide whose relative proportions are sensitive to changes in the rate of cell proliferation. Exp Cell Res. 1981 Dec;136(2):311–319. doi: 10.1016/0014-4827(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Celis J. E. Gene expression in murine hybrids exhibiting different morphologies and tumorigenic properties. Carcinogenesis. 1981;2(8):769–782. doi: 10.1093/carcin/2.8.769. [DOI] [PubMed] [Google Scholar]
- Bravo R., Graf T. Synthesis of the nuclear protein cyclin does not correlate directly with transformation in quail embryo fibroblasts. Exp Cell Res. 1985 Feb;156(2):450–454. doi: 10.1016/0014-4827(85)90551-8. [DOI] [PubMed] [Google Scholar]
- Bravo R., Macdonald-Bravo H. Induction of the nuclear protein 'cyclin' in quiescent mouse 3T3 cells stimulated by serum and growth factors. Correlation with DNA synthesis. EMBO J. 1984 Dec 20;3(13):3177–3181. doi: 10.1002/j.1460-2075.1984.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Small J. V., Fey S. J., Larsen P. M., Celis J. E. Architecture and polypeptide composition of HeLa cytoskeletons. Modification of cytoarchitectural polypeptides during mitosis. J Mol Biol. 1982 Jan 5;154(1):121–143. doi: 10.1016/0022-2836(82)90421-1. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Bravo R., Larsen P. M., Fey S. J. Cyclin: a nuclear protein whose level correlates directly with the proliferative state of normal as well as transformed cells. Leuk Res. 1984;8(2):143–157. doi: 10.1016/0145-2126(84)90135-8. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Macdonald-Bravo H., Bravo R. Induction of the nuclear protein cyclin in serum-stimulated quiescent 3T3 cells is independent of DNA synthesis. Exp Cell Res. 1985 Feb;156(2):455–461. doi: 10.1016/0014-4827(85)90552-x. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Miyachi K., Fritzler M. J., Tan E. M. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978 Dec;121(6):2228–2234. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohashi M., Taguchi T., Ikegami S. Aphidicolin: a specific inhibitor of DNA polymerases in the cytosol of rat liver. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1084–1090. doi: 10.1016/0006-291x(78)90298-x. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Takasaki Y., Deng J. S., Tan E. M. A nuclear antigen associated with cell proliferation and blast transformation. J Exp Med. 1981 Dec 1;154(6):1899–1909. doi: 10.1084/jem.154.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki Y., Fishwild D., Tan E. M. Characterization of proliferating cell nuclear antigen recognized by autoantibodies in lupus sera. J Exp Med. 1984 Apr 1;159(4):981–992. doi: 10.1084/jem.159.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Wist E., Prydz H. The effect of aphidicolin on DNA synthesis in isolated HeLa cell nuclei. Nucleic Acids Res. 1979 Apr;6(4):1583–1590. doi: 10.1093/nar/6.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]









