Abstract
Endoplasmic reticulum (ER)-plasma membrane (PM) junctions are membrane microdomains important for communication between the ER and the PM. ER-PM junctions were first reported in muscle cells in 1957, but mostly ignored in non-excitable cells due to their scarcity and lack of functional significance. In 2005, the discovery of stromal interaction molecule 1 (STIM1) mediating a universal Ca2+ feedback mechanism at ER-PM junctions in mammalian cells led to a resurgence of research interests toward ER-PM junctions. In the past decade, several major advancements have been made in this emerging topic in cell biology, including the generation of tools for labeling ER-PM junctions and the unraveling of mechanisms underlying regulation and functions of ER-PM junctions. This review summarizes early studies, recently developed tools, and current advances in the characterization and understanding of ER-PM junctions. This article is part of a Special Issue entitled: Membrane contact sites edited by Benoit Kornmann and Christian Ungermann.
1. Introduction
The endoplasmic reticulum (ER) is the largest membrane system in animal cells and constitutes a continuous network extending from the nuclear envelop (NE) to the cell periphery [1]. The ER network undergoes constant remodeling, and can be divided into morphologically distinct subdomains including the NE, sheet-like cisternae, tubules, and dense tubular matrices [2, 3]. The ER harbors biosynthetic activities for membrane and secretory proteins as well as for most lipid species in the cell [1]. In addition, the ER is the main intracellular Ca2+ store in animal cells [4]. To transport lipids and Ca2+ for cell homeostasis and signaling, the ER forms minute membrane junctions with other organelles, where the ER membrane closely apposes other membrane compartments within a 30 nm gap distance [5, 6]. Evidence of ER-organelle junctions was first demonstrated in classical electron microscopy (EM) studies sixty years ago [7, 8]. Recently, these ER-organelle junctions have been shown to support inter-organelle signaling between the ER and the trans-Golgi apparatus, mitochondria, endosomes, and the plasma membrane (PM) in yeast and in mammalian cells [9–24].
In this review, we will focus on ER-PM junctions, subcellular loci where the ER is tethered to the PM and functioning as active interfaces between the intracellular and extracellular environments. ER-PM junctions not only provide a platform for inter-organelle communication, but also serve as direct conduits for inside-out and outside-in signal transduction. Since their initial discovery in 1957, a variety of names have been used to describe ER-PM junctions in different cell types or organisms. The nomenclature includes dyad and triad junctions (or dyads and triads), excitation-contraction (E-C) units and peripheral couplings in muscle cells [25]; subsurface cisterns (SSC’s) in neurons [26]; PM-associated ER and cortical ER in yeast [2, 27, 28]; subrhabdomeric cisternae (SRC) in Drosophila photoreceptor cells [29]; and ER-PM contacts/interactions [16]. Here, we use ER-PM junctions to emphasize that the ER and the PM are joined in both physical proximity and are functionally coupled at these sites.
Based on EM studies, ER-PM junctions are characterized as ER regions that form close appositions with the PM. In general, ER-PM junctions across different cell types share several common features. First, the gap distance between the ER and the PM at ER-PM junctions is within 10 to 30 nm allowing for direct protein-protein/lipid interaction in trans. This gap distance is too narrow to accommodate the presence of ribosomes. However, electron-dense cytosol is often observed in the gap of ER-PM junctions suggesting a local concentration of proteins, lipids or ions. Second, the ER membrane and the PM are aligned in parallel often with a length of approximately 100–400 nm in many cells types. Importantly, no membrane fusion between these two compartments has been reported. Third, most of the EM observations were derived from resting cells, suggesting the presence of pre-existing ER-PM junctions.
We will first discuss early studies demonstrating the important roles of ER-PM junctions in Ca2+ signaling. Next, we will discuss the methods and tools developed for investigating ER-PM junctions. Finally, we will discuss recent advances in understanding the regulation and functions of ER-PM junctions.
2. Important roles of ER-PM junctions in Ca2+ signaling
Ca2+ is a universal second messenger that governs many important cellular functions. Elevation of cytosolic Ca2+ is the key to regulating diverse processes ranging from relatively rapid events, such as muscle contraction and neurotransmitter release, to slower responses such as cell migration, differentiation, and apoptosis [4, 30, 31]. Therefore, cytosolic Ca2+ levels must be dynamically and tightly regulated for cells to precisely execute such diverse functions. During the resting state, cytosolic Ca2+ is maintained at a low level of approximately 50 to 100 nM, which is about 10,000 times lower than the concentration of Ca2+ in the extracellular space (> 1 mM), and that in the ER lumen (~ 400 to 600 µM). This low cytosolic Ca2+ level is maintained by the activities of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and plasma membrane Ca2+-ATPase (PMCA) at the ER and the PM, respectively. With the consumption of ATP, SERCA and PMCA are able to constitutively pump Ca2+ from the cytosol against the steep chemical gradient to the ER and the extracellular space [5, 32]. During Ca2+ signaling, opening of Ca2+ channels at the ER and/or the PM leads to elevation of cytosolic Ca2+ and activation of Ca2+ effectors. Intimate communications between the ER and the PM are required for dynamic regulation of Ca2+ signaling machinery.
2.1. The spatial platform for excitation-contraction (E-C) coupling in muscle cells
ER-PM junctions were studied extensively in muscle cells using EM, and were described as triad junctions in skeletal muscles and dyad junctions in cardiac muscles [33]. These junctions are formed by the sarcoplasmic reticulum (SR, the ER that adopts distinct geometry for contractile functions in muscle cells) making contacts with the tubular invaginations of sarcolemma (the PM in muscle cells) called transvers tubules (T tubules). ER-PM junctions formed with sarcolemma outside of T tubules are called peripheral couplings. The gap distance between the two membranes was measured to be 9 to 12 nm, and electron-dense materials were observed in the gap implicating the enrichment of proteins [34–36]. Interestingly, the gap distance often correlates with different contractile functions. For example, triad junctions with smaller gap distance were observed in fast contraction skeletal muscle fibers, while relaxed junctions were present in slow twitch or cardiac fibers [37]. In addition, the periodic distribution of triad and dyad junctions, along with T tubules, ensures the even proximity of junctions to contractile fibers in muscle cells to maximize the contraction strength [5, 34].
ER-PM junctions are required for contractile functions in muscle cells by serving as a platform for excitation-contraction (E-C) coupling. E-C coupling is mediated by the communication between dihydropyridine receptor (DHPR), a PM-localized voltage-gated Ca2+ channel, and ryanodine receptor (RyR), an SR-localized Ca2+channel. DHPR and RyR are closely apposed to each other in triad and dyad junctions [38–42]. During muscle contraction, DHPR is first activated by membrane depolarization to mediate a Ca2+ influx. The increase in local Ca2+ levels at ER-PM junctions subsequently opens RyR, leading to SR Ca2+ release and a further increase in cytosolic Ca2+ levels, thus triggering muscle contraction. This process that amplifies the increase in cytosolic Ca2+ levels is called Ca2+-induced Ca2+ release [43]. Despite their presence at ER-PM junctions, knockout of DHPRs or RyRs had no effect on the formation of triad junctions in muscle cells, suggesting that they are not required for the formation of these junctions [44–46]. Moreover, co-expression of DHPR and RyR in Chinese hamster ovarian (CHO) cells failed to generate close membrane appositions resembling to triad/dyad junctions in muscle cells [47]. Together, these studies indicated that other structural components are required for formation of ER-PM junctions in muscle cells.
2.2. ER-PM tethers in muscle cells
In 2000, Takeshima and colleagues identified a family of proteins which they named junctophilins (JPH) for their functional roles in ER-PM tethering in muscle cells [48]. JPHs were identified by screening monoclonal antibodies generated from mice immunized with membrane vesicles of rabbit skeletal muscles. Four JPHs have been reported and they are selectively expressed in excitable tissues: JPH-1 and JPH-2 are expressed in both heart and skeletal muscle, and JPH-3 and JPH-4 are highly expressed in brain and neuronal tissues [48, 49]. JPHs are ER transmembrane proteins with eight N-terminal membrane occupation and recognition nexus (MORN) motifs that mediate PM targeting, likely by binding to phospholipids [50, 51]. The ER transmembrane domain and MORN motifs constitute a dual-targeting mechanism for JPHs to bridge the ER and the PM, and form junctions.
Exogenous expression of full-length JPH-1 in Cynops pyrrhogaster (amphibian) embryos was sufficient to generate extensive ER-PM junctions with structural similarities to those in muscle cells [48]. Conversely, cardiac myocytes isolated from JPH-2 null embryos exhibited a 90% reduction of functional dyad junctions. The average length of the remaining junctions in JPH-2 null myocytes was also significantly reduced to 170 ± 60 nm, while in wild-type cells the length was 370 ± 160 nm. These findings demonstrate that JPHs are required for the formation of ER-PM junctions in muscle cells. Notably, ER-PM junctions with an average gap distance of 7.6 ± 0.6 nm were observed in amphibian embryos ectopically expressing JPH1. This gap distance is similar to that of triad junctions in muscle cells lacking RyR, but not to those (~12 nm) found in wild-type muscle cells [46, 48]. These results raised the question of whether JHP-1 is flexible enough to extend up to 12 nm to interact with the PM in the presence of RyRs, or additional components are required to bridge the membranes at triad or dyad junctions. In addition, more relaxed dyad junctions with a gap distance of 30 nm have been reported in embryonic cardiac myocytes [48, 52]. JPH-2-deficient myotubes with a significant reduction in 12-nm dyad junctions appeared to have the 30-nm dyad junctions unaffected. These results indicated that unidentified tethers, in addition to JPH-2, must exist to form the 30-nm junctions during heart development.
Defective ER-PM junctions in muscle cells led to impaired Ca2+ transients in cardiac myocytes, likely the underlying cause of weak heartbeats, cardiac arrest, and eventual embryonic lethality in mice lacking JPH-2 [48]. In addition, myotubes lacking JPH-1 also displayed a severe reduction in store-operated Ca2+ entry (SOCE, another Ca2+ signaling pathway occurring at ER-PM junctions; see Section 2.3), low basal cytosolic Ca2+ levels, and low SR Ca2+ store [53, 54]. These genetic studies further demonstrated that ER-PM junctions are important for Ca2+ signaling in muscle cells.
In early EM studies, ER-PM junctions were also observed in non-muscle cells, including neurons [26, 35], mouse fibroblasts [55], rat Sertoli cells [56], and Xenopus oocytes [57]. ER-PM junctions in these cells displayed structural similarities to triad and dyad junctions including a gap distance of ~20 nm and the presence of electron-dense material in the gap. Unlike muscle cells that adopt extensive triad and dyad junctions to perform contractile functions, non-excitable cells have ER-PM junctions of low abundance, making them difficult to detect. In Xenopus eggs, the abundance of ER-PM junctions appeared to correlate with Ca2+ signaling events during oocyte maturation [57]. Nevertheless, it was difficult to link a particular inter-organelle signaling pathway to ER-PM junctions in non-muscle cells. Therefore, their importance was overlooked for decades. The discovery of stromal interaction molecule 1 (STIM1) activating SOCE at ER-PM junctions revealed the functional significance of ER-PM junctions in non-muscle cells and prompted the re-evaluation of these minute subcellular structures.
2.3. Activation of store-operated Ca2+ entry (SOCE) at ER-PM junctions by STIM1
Stimulation of many cell surface receptors, including G protein-couple receptors and tyrosine kinase-linked receptors, triggers the activation of phospholipase C (PLC). Activated PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) at the PM to generate 1,4,5-triphosphate (IP3), which binds to IP3 receptor (IP3R) in the ER leading to release of ER Ca2+ and an increase in cytosolic Ca2+ level [4]. Because the ER contains a limited Ca2+ store, animal cells have developed an elaborate Ca2+ influx mechanism called SOCE to refill the ER store and to sustain the elevated cytosolic Ca2+ levels.
The SOCE mechanism was first proposed based on the observation that the level of ER Ca2+ negatively operated a Ca2+ influx from the extracellular space [58]. Subsequent studies using thapsigargin (TG), a SERCA inhibitor that results in ER Ca2+ store depletion without activation of IP3R [59], demonstrated that ER Ca2+ depletion is sufficient to activate SOCE [60, 61]. Together, these observations led to the hypothesis that a Ca2+ sensor detects reduction in ER Ca2+ levels and then initiates a signaling cascade to open a Ca2+ channel at the PM leading to Ca2+ influx. The Ca2+ current elicited by SOCE, namely Ca2+ release-activated Ca2+ (CRAC) current or ICRAC, with characteristics of high Ca2+ selectivity over monovalent cations and extreme low conductance, was first reported in mast cells and T cells [62–64]. Although the characteristics of ICRAC explicitly defined the criteria for identifying the activators of SOCE, the mechanisms that link ER Ca2+ store depletion to SOCE across the PM remained unclear for decades. In 2005, the discovery of STIM1 as the ER Ca2+ sensor, and a year later, the identification of Orai1 as the PM SOC channel, provided the first two clues to understand the mechanisms of SOCE [65–69].
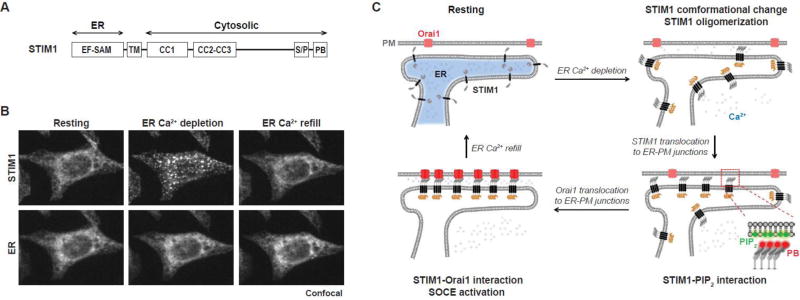
STIM1 was identified by RNA interference screens for SOCE regulators from two independent groups [65, 66]. STIM1 is an ER transmembrane protein containing an N-terminal Ca2+-sensing EF hand-SAM (EF-SAM) domain in the ER lumen, cytosolic coiled-coil domains, a serine and proline rich region, and a polybasic motif at the very C-terminus (Fig. 1A). In the resting state, STIM1 binds to Ca2+ via its luminal EF hand and localizes diffusely throughout the ER (left panels, Fig. 1B). Ca2+-bound STIM1 is inactive given the evidence that disruption of STIM1-Ca2+ binding by mutation of a key residue in the EF hand resulted in constitutive SOCE [65, 70]. ER Ca2+ depletion induces STIM1 translocation to ER-PM junctions, a process also called STIM1 puncta formation based on the initial finding that cells overexpressing fluorescent protein-tagged STIM1 showed bright fluorescence signals in puncta near the cell periphery and adhesion surface [65] (middle panels, Fig. 1B). A later EM study in Jurkat T cells confirmed that STIM1 redistributed from the global ER network to ER regions closely apposed to the PM corresponding to ER-PM junctions following ER Ca2+ store depletion [71]. ER Ca2+ refill enables STIM1 to re-associate with Ca2+, return to the inactive state, and vacate ER-PM junctions (right panel, Fig. 1B).
Fig. 1. Activation of SOCE at ER-PM junctions by STIM1.
(A) A diagram of the domain structure of human STIM1. EF-SAM, EF hand and sterile alpha motif; TM, transmembrane; CC, coiled-coil domain; S/P, serine and proline rich region; PB, polybasic motif.
(B) Bottom-section confocal images of a HeLa cell co-expressing YFP-STIM1 and a CFP-tagged ER luminal marker. The cell was imaged at the resting state (left), after 5 µM 2',5'-di (tert-butyl)-1,4-benzohydroquinone (BHQ) treatment to deplete ER Ca2+ store (middle), and 3 min after BHQ washout to allow ER Ca2+ store refill (right).
(C) Schematic representation of SOCE activation. In the resting state, STIM1 binds to Ca2+ and localizes diffusely throughout the ER. Following ER Ca2+ depletion, dissociation of Ca2+ from STIM1 triggers STIM1 conformational change and oligomerization. STIM1 oligomers translocate to ER-PM junctions by binding to PM PIP2 via its PB domain. STIM1 at ER-PM junctions subsequently recruits Orai1 and activates SOCE to sustain elevated cytosolic Ca2+ levels and refill ER Ca2+ store.
Further investigations revealed that when ER Ca2+ is depleted, STIM1 rapidly undergoes conformational changes and forms oligomers, likely resulting from the partial unfolding and aggregation of the Ca2+-free EF-SAM domain [72–74]. Oligomerization of STIM1 is a crucial step in SOCE activation. This idea is supported by the observation that rapamycin analogs-induced oligomerization of STIM1 fusion proteins, containing an FK506 binding protein (FKBP) motif or a FKBP-rapamycin binding (FRB) motif in place of the EF-SAM domain, was sufficient to trigger SOCE without ER Ca2+ depletion [75, 76]. STIM1 oligomers translocate to ER-PM junctions by binding to PIP2 at the PM via the C-terminal polybasic motif [72, 77–79]. Deletion of this polybasic motif resulted in defective STIM1 translocation [72]. The absence of STIM1 at ER-PM junctions in resting cells suggests that either the positive-charged residues in the STIM1 polybasic tail are structurally hindered, or their binding affinity to the PM is too weak to retain STIM1 at ER-PM junctions. Thus, oligomerization likely mediates the exposure of the positive-charged residues and/or creates a stronger PM binding motif that enables STIM1 oligomers to localize to ER-PM junctions.
The close apposition between the ER membrane and the PM at ER-PM junctions allows STIM1 in the ER to directly interact with the PM-localized SOC channel Orai1. The minimal domain of STIM1 that binds and activates Orai1 has been identified by several groups. This domain corresponding to the CC2-CC3 region of STIM1 is called CAD (CRAC activation domain, amino acid 342 to 448) [80], SOAR (STIM1-Orai activating region, amino acid 344 to 442) [81], or Ccb9 (amino acid 339 to 444) [82]. Expression of the CAD/SOAR/Ccb9 domain of STIM1 led to constitutive activation of ICRAC in the absence of ER Ca2+ depletion, indicating that STIM1 is a direct activator of Orai1.
The crystal structure of the Drosophila Orai protein revealed a hexameric assembly of the SOC channel [83]. Consistently, atomic force microscopy revealed that human Orai1 assembles as a hexamer and STIM1 binds to Orai1 hexamer with six-fold symmetry [84]. A recent study using Orai1 concatemers further supported a hexameric stoichiometry for the SOC channel [85]. It has been postulated that Orai1 diffuses passively into ER-PM junctions, and is then trapped and activated by prior translocated STIM1 to elicit SOCE. This hypothesis is supported by the facts that Orai1 is diffusely distributed in the PM and the ICRAC activation is delayed following STIM1 translocation to ER-PM junctions [80, 86, 87]. The molecular mechanism by which STIM1 activates Orai1 at ER-PM junctions during SOCE is summarized in Fig. 1C, and in several recent review articles [88–91].
2.4. Physiological importance of SOCE
The importance of SOCE has been demonstrated in mouse genetic studies as well as in human patients lacking functional STIM1 or Orai1. Mice lacking STIM1 showed perinatal lethality [92–95]. STIM1-deficient mice that managed to survive also exhibited severe growth retardation and muscle fatigue [93, 94]. Loss of Orai1 in mice displayed phenotypes similar to those in STIM1-deficient mice [96]. The functions of immune cells were mostly abolished in these animals because their activation is highly dependent on SOCE. For example, mast cells lacking STIM1 or Orai1 showed impaired activation of nuclear factor of activated T-cells (NFAT), reduced degranulation, and defective cytokine production [92, 97]. Similarly, T cells from STIM1- and Orai1-defecient mice displayed a reduction in SOCE and cytokine production [95, 98]. T cell-specific ablation of STIM1 also resulted in a lymphoproliferative phenotype and a selective decrease in regulatory T cells [95]. The phenotypes in immune cells acquired from mouse genetic studies were consistent with the severe combined immunodeficiency (SCID) symptoms in human patients lacking normal STIM1 and Orai1 functions [96]. Of notice, STIM1-deficient mice displayed skeletal myopathy, and STIM1 haploinsufficient mice also exhibited increased susceptibility to fatigue [93], reminiscent of muscle defects in human patients lacking functional STIM1 or Orai1 [96]. In addition, STIM1 and Orai1 are highly expressed in human and mouse skeletal muscle [93, 97–99]. These observations indicated that SOCE is required for muscle cell functions. Interestingly, SOCE in muscle cells occurs remarkably faster than in non-excitable cells [100]. STIM1-L, a muscle-specific variant of STIM1, is pre-localized at ER-PM junctions and thus may provide an explanation for faster SOCE activation in muscle cells [101]. In summary, these genetic studies and clinical observations indicate the importance of SOCE in development, immune cell activation, as well as maintaining muscle cell functions.
3. Visualization and characterization of ER-PM junctions
The discovery of STIM1 as an ER Ca2+ sensor and an activator for SOCE resulted in a series of studies aimed at deciphering the molecular mechanisms underlying this pivotal yet not-fully-understood cellular process. Concurrently, the finding that STIM1 interaction with Orai1 occurs at ER-PM junctions generated new research interests in inter-organelle signaling at these subcellular loci that had long been overlooked. New EM studies and the development of novel imaging tools have been applied to gain insights into the structure, formation, dynamics, and functions of ER-PM junctions in mammalian cells as we summarized in this chapter.
3.1. EM characterization of ER-PM junctions after the discovery of STIM1
After the discovery of STIM1, ER-PM junctions were re-examined using EM by several groups. In 2006, Wu and colleagues characterized ER-PM junctions in detail in Jurkat T cells in the resting state and following ER Ca2+ store depletion [71]. Due to the lack of a specific marker for ER-PM junctions, a horseradish peroxidase (HRP)-tagged ER luminal marker was applied to label the ER. ER tubules in close apposition to the PM within 50 nm were defined as ER-PM junctions. They showed the presence of ER-PM junctions in resting cells. The average length of a single ER-PM junction was approximately 150 nm, and the gap distance between the ER and the PM at ER-PM junctions was in the range of 10 to 25 nm. By measuring the total length of ER-PM junctions and the circumference of the entire cell, they further demonstrated that only 4% of the PM was covered by ER-PM junctions in resting Jurkat T cells. Following ER Ca2+ depletion by TG, ER-PM junctions-covered PM was up-regulated to 6% due to an increase in number but not in size of ER-PM junctions. Overexpression of HRP-tagged STIM1 did not change the number of ER-PM junctions. However, TG-induced STIM1 translocation to ER-PM junctions resulted in a significant increase in the length of individual junctions to almost 300 nm. Together, these results indicated that ER-PM junctions exist prior to the activation of SOCE, and are dynamically regulated during ER Ca2+ depletion and STIM1 translocation.
In 2009, Orci and colleagues examined ER-PM junctions in HeLa cells by applying conventional post-staining EM to label membrane compartments [102]. Similar to Jurkat T cells, TG treatment resulted in an increase of PM coverage of ER-PM junctions from 0.23% to 1.24% in HeLa cells. Overexpression of STIM1 led to a marked induction of PM coverage to 1.40% and to 5.44% in unstimulated and in TG-treated HeLa cells, respectively. In STIM1-overexpressing cells, the average length of single ER-PM junctions was 200 to 400 nm, and the average gap distance was 8.3 ± 0.3 nm with large variation ranging from 1.3 to 14.7 nm. A similar gap distance of 11.3 ± 2.9 nm was observed in cryosections. Interestingly, ER-PM junctions were more often distributed to the basal-lateral membranes of the cell, suggesting that specific PM features are involved in determining the formation of ER-PM junctions even in poorly polarized HeLa cells.
The uneven distribution of ER-PM junctions was also demonstrated by Lur and colleagues in pancreatic acinar cells, a cell type specialized in secretion [103]. In these cells, ER-PM junctions were mostly found in the basal-lateral regions of the cells but not in the regions with secretory granules. These observations suggest that the spatial organization of ER-PM junctions and other PM functional domains are cooperatively regulated. In addition, STIM1 overexpression resulted in a 2-fold increase in the density of ER-PM junctions, while the gross appearance of these junctions was not affected. Unexpectedly, TG treatment failed to trigger any change in the density of ER-PM junctions in non-transfected and in STIM1-overexpressing acinar cells. The ER in pancreatic acinar cells is mostly organized into ribosome-bound cisternae to maximize the protein-producing activity and secretory functions. As a result, the ability to generate new ribosome-free ER-PM junctions in these cells may be limited.
A more recent cryo-electron tomography study by Fernandez-Busnadiego and colleagues further revealed the ultra-structural features in the gaps of ER-PM junctions [104]. They observed an intermediate density between the membranes at ER-PM junctions in COS-7 cells overexpressing extended synaptotagmin-like proteins E-Syt1 or E-Syt3 (see Section 4.2). The intermediate density appeared to consist of interconnected globular structures as demonstrated by a higher-resolution tomography dataset. Interestingly, the intermediate density was absent at the ER-PM junctions in non-transfected or STIM1-overexpressing COS-7 cells after ER Ca2+ store depletion. Instead, numerous filamentous structures were found spanning the gap between the ER and the PM. In non-transfected neurons, native ER-PM junctions with intermediate density and with filamentous structures were both observed. These results suggest that there are structurally distinct ER-PM junctions in the same cell.
In the same year, Perni and colleagues used transmission and freeze-fracture EM to examine the patterning of STIM1 and Orai1 at ER-PM junctions during SOCE in HEK293 cells [105]. Their results confirmed STIM1 oligomerization and Orai1 targeting to ER-PM junctions upon ER store depletion. This study also revealed that Orai1 channels are in particles ~7.6 nm in diameter, which is comparable to the size of Drosophila Orai channel measured by structural analysis [83], in both resting and store-depleted cells.
3.2. ER-PM junctions monitored by ER-labeling in live cells
EM studies provide ultra-structural insights of ER-PM junctions. Nevertheless, fixation and extensive sample processing, which are time-consuming and may compromise the integrity of native cellular structures, are required to visualize ER-PM junctions using this approach. Methods enabling detection of ER-PM junctions in live cells will not only facilitate further studies of these subcellular structures, but will also provide new information regarding regulation and function.
The active form of STIM1 can localize to ER-PM junctions. However, expression of this STIM1 mutant triggered constitutive SOCE leading to activation of many Ca2+-sensitive processes [65, 70]. Expression of ER-PM tethering proteins such as E-Syt2 (see Section 4.2) can selectively label ER-PM junctions. Nonetheless, Giordano et al. observed artificial expansion of ER-PM junctions from 2% to over 40% PM occupancy in E-Syt2-overexpressing cells [16]. Therefore, markers that selectively label ER-PM junctions with minimal perturbations to cells are needed to study ER-PM junctions.
Since ER-PM junctions exist in resting cells, it is expected that these structures can be detected by monitoring ER regions very close to the PM in cells transfected with a fluorescent protein-tagged ER marker. Total internal reflection fluorescence (TIRF) microscopy, which generates an evanescent field that excites fluorescent molecules within ~100 nm of the PM [106], is suitable for selective detection of ER signals in close proximity to the PM. When imaging live cells transfected with a fluorescent protein-tagged ER marker using TIRF microscopy, a portion of ER signals appeared to move in and out of the evanescent field, corresponding to cortical ER tubules not forming junctions with the PM. Amongst the background of dynamic ER signals, some bright punctate structures remained stable for more than 10 minutes (indicated by arrows in the top panels of Fig. 2A). These stable ER puncta correspond to ER-PM junctions since STIM1 reversibly translocated into these loci when ER Ca2+ was depleted (Fig. 2A, bottom panels). Notably, the same ER puncta remained stable after ER Ca2+ refill that reverted STIM1 translocation, indicating that these stable ER puncta are not randomly formed and are likely maintained by molecular tethers. The absence of STIM1 at ER-PM junctions before ER Ca2+ depletion and after ER refill suggested that these junctions are maintained by STIM1-independent mechanisms. Consistently, recent studies demonstrated that STIM1 cytosolic regions are likely to adopt a closed conformation with the PM targeting motif hindered [107], suggesting that STIM1 is unable to mediate ER-PM tethering in resting cells. Overall, stable ER-PM junctions in resting cells can be tracked using a fluorescent ER marker combined with live-cell TIRF microscopy. Nonetheless, detection of bona fide ER-PM junctions among dynamic cortical ER tubules requires taking a series of live cell images to identify stable structures using TIRF microscopy, sacrificing temporal resolution in studying dynamic regulation of ER-PM junctions.
Fig. 2. Visualization of ER-PM junctions using ER-labeling or MAPPER.
(A) TIRF images (top) of a HeLa cell co-expressing an YFP-tagged ER luminal marker and mCherry-STIM1. The cell was imaged at time zero (left), 5 min after 5 µM BHQ treatment to induce STIM1 translocation (middle), and 5 min after BHQ washout (right). Arrows indicate stable ER puncta corresponding to ER-PM junctions. Schematic diagrams (bottom) depicting STIM1 translocation to ER-PM junctions. The scale bar represents 2 µm.
(B) Domain structure of MAPPER. GFP, green fluorescence protein; TM, transmembrane; FRB, FKBP12-rapamycin binding; PB, polybasic motif from the small G protein Rit.
(C) Bottom-section confocal images of a HeLa cell co-expressing MAPPER and a mCherry-tagged ER luminal marker. The scale bar represents 2 µm. © Chang et al. and Cell Reports, 2013. Originally published in Cell Reports, 5 (2013) 813–825.
(D) TIRF image of a HeLa cell co-expressing MAPPER and a mCherry-tagged ER luminal marker. The scale bar represents 2 µm. © Chang et al. and Cell Reports, 2013. Originally published in Cell Reports, 5 (2013) 813–825.
(E) STIM1 translocation induced by 1µM thapsigargin (TG) monitored by TIRF microscopy in a HeLa cell co-expressing MAPPER and mCherry-STIM1. The scale bar represents 2 µm. © Chang et al. and Cell Reports, 2013. Originally published in Cell Reports, 5 (2013) 813–825.
(F) Density of ER-PM junctions labeled by ER marker or MAPPER in HeLa cells monitored by TIRF microscopy. Mean ± SD is shown. © Chang et al. and Cell Reports, 2013. Originally published in Cell Reports, 5 (2013) 813–825.
3.3. Chemical-inducible markers for ER-PM junctions
In 2007, Varnai and colleagues established a method to label ER-PM junctions in live cells by applying a chemical-inducible approach [108]. In this method, a FKBP-fused fluorescent protein was targeted to the inner leaflet of the PM, and a FRB-fused fluorescent protein was targeted to the cytosolic surface of the ER. It is reasoned that rapamycin-triggered hetero-dimerization of FKBP and FRB proteins could only occur at regions where the ER and the PM are in close proximity. As expected, both proteins rapidly translocated to the same puncta from their original localizations and colocalized with activated STIM1 following rapamycin treatment. These puncta eventually expanded into stable patches with the size of several micrometers, indicating artificial expansion of ER-PM junctions. By manipulating the length of these two synthetic proteins, they found that Orai1 is likely to interact with a protein complex that bulges into the cytoplasm with a size estimated to be larger than 9 nm but smaller than 14 nm. Interestingly, STIM1 could fit into the 9-nm ER-PM junctions suggesting that its cytosolic region, although long, is likely flexible.
In 2010, Lavieu and colleagues also used a rapamycin-induced dimerization approach to label ER-PM junctions [109]. Instead of bridging the ER to the PM in trans as described above, this study employed rapamycin-induced dimerization of chimeric ER transmembrane proteins. These ER chimeras contain the last transmembrane domain of the yeast protein Ist2 (a multi-transmembrane protein involved in ER-PM tethering in yeast cells, see Section 4.1), an FKBP or an FRB, and the PM-targeting cytosolic tail of Ist2. In resting cells, these chimeras showed diffuse ER localization. Following rapamycin analog treatment, these ER chimeras first concentrated in intracellular puncta, and then accumulated to the cell periphery with possible localization at ER-PM junctions.
These pioneer studies exploiting the idea of visualizing of ER-PM junctions using synthetic tools without activating or perturbing cellular functions led to several interesting observations. Nonetheless, these approaches require transfection of two constructs and treatment of pharmacological reagents, which may restrict the applications in investigating the dynamic properties and functions of ER-PM junctions. It should be noted that rapamycin can activate the mTOR pathway and therefore not suitable for applications longer than several minutes.
3.4. Visualization of ER-PM junctions using MAPPER
In 2013, our group developed a genetically-encoded marker for ER-PM junctions named “MAPPER” for “membrane-attached peripheral ER” [15], based on the subcellular targeting mechanisms of STIM1 (Liou et al., 2007). MAPPER contains the signal peptide (SP) and the transmembrane domain of STIM1 for targeting to the ER membrane (Fig. 2B). At its very C-terminus, MAPPER contains the phosphoinositide-binding polybasic motif of the small G protein Rit for PM binding [110]. An FRB domain for potential recruitment of FKBP-fusion proteins to ER-PM junctions was introduced in the cytosolic region of MAPPER. Moreover, several flexible and helical linkers were incorporated into the cytosolic region of MAPPER to ensure that expression of this marker did not restrict or expand the gap distance of ER-PM junctions in mammalian cells, which ranges from 10 to 25 nm based on EM studies [71].
When MAPPER-transfected HeLa cells were examined using confocal microscopy, many bright puncta were observed near the adhesion surface of each cell (Fig. 2C). The localization of MAPPER and an ER luminal marker appeared dramatically different. Nonetheless, all puncta detected by MAPPER were overlaid with the ER marker indicating that MAPPER puncta localized at the ER. The overlay of MAPPER and the ER marker was better illustrated using TIRF microscopy (Fig. 2D), in which MAPPER signals only colocalized with ER puncta but not the cortical ER tubules in the background. Compared with the ER marker, MAPPER greatly enhanced the identification of ER-PM junctions with minimal background signal from the rest of the ER. The localization of MAPPER at ER-PM junctions was further confirmed by EM [15].
Because MAPPER contains all the necessary components for ER-PM targeting, this method only requires transfection of MAPPER without further manipulation of cells to visualize ER-PM junctions. MAPPER can be easily applied to many imaging techniques including confocal microscopy, TIRF microscopy, EM, and super-resolution imaging. A light-inducible version of MAPPER, named LiMETER, has also been developed as an optogenetic tool to reversibly label ER-PM junctions [111]. Although both MAPPER and STIM1 localize to ER-PM junctions by binding to PM phosphoinosides, expression of MAPPER does not perturb STIM1 translocation (Fig. 2E) and SOCE [15]. These observations indicate that MAPPER selectively labels ER-PM junctions with minimal perturbations to cells, and can therefore be applied to study functions and dynamic regulation of ER-PM junctions.
The density of ER-PM junctions detected by MAPPER was approximately 0.2 junctions per µm2, similar to the density of ER-PM junctions detected by ER-labeling in live cells as described in section 3.2 (Fig. 2F). These results indicate that MAPPER can be used to monitor existing ER-PM junctions without creating new ones. Because the average adherent surface area of HeLa cells is approximate 1,000 µm2, it is estimated that there are ~200 ER-PM junctions at the bottom of a HeLa cell based on the density of 0.2 per µm2. It should be noted that MAPPER may not be suitable for studying the formation of ER-PM junctions in small interfering RNA (siRNA) or pharmacological inhibitor-treated cells since MAPPER may rescue the formation of ER-PM junctions in these cells by providing constitutive tethering, similar to the artificial ER-PM tethers reported in fission yeast [112].
Overall, the innovative tools and methods described in this chapter enabled the visualization of the small, few, yet extremely important subcellular domains of ER-PM junctions in fixed and live cells. By tracking changes in structure and composition of ER-PM junctions during physiological processes, it can facilitate our understanding of regulation and functions of these membrane microdomains.
4. Recent advances in understanding the regulation and functions of ER-PM junctions
The interest in studying the regulation and functions of ER-PM junctions in mammalian cells has grown tremendously in the last decade, and expanded beyond the field of Ca2+ signaling. Development of imaging methods and tools for investigating ER-PM junctions accelerated the progress in this area of research. In addition, using the genetic model system of yeast cells, several evolutionary-conserved proteins have been identified to mediate tethering and/or functions at ER-PM junctions. In this chapter, we will first review studies of ER-PM junctions in yeast cells, and then discuss recent discoveries of mammalian proteins involved in tethering and inter-organelle signaling at ER-PM junctions.
4.1 ER-PM junctions in yeast cells
The ER in the budding yeast Saccharomyces cerevisiae has a very different organization than that in mammalian cells. It contains two main ER subdomains, the perinuclear ER and the cortical ER, connected by a few cytoplasmic tubules [113]. The cortical ER in yeast forms extensive contacts with the PM, covering ~40% of the PM. In yeast cells expressing an ER marker, the cortical ER appears as sheet-like structures that run in parallel with the PM under fluorescence microscopy [17, 28]. Examined under 3D EM tomography in detail, the cortical ER in yeast cells appeared to consist of ER tubules and highly fenestrated cisternae [2]. 3D image reconstruction from serial ultrathin sections of a yeast cell revealed that the cortical ER forms ~1,100 junctions with the PM in a yeast cell with a gap distance less than 30 nm [27].
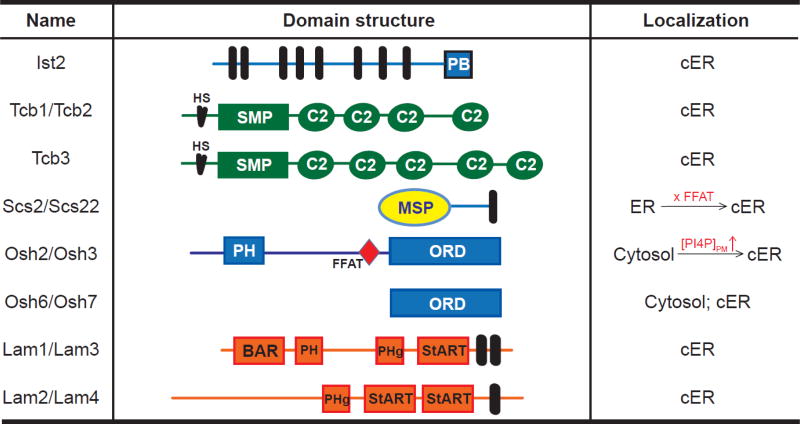
Among the proteins enriched at the cortical ER (Fig. 3), Ist2 is a multi-transmembrane ER protein belonging to the TMEM16/anoctamins protein family [114], and contains a C-terminal polybasic tail that binds to PIP2 at the PM [115]. Ist2 selectively localized at ER-PM junctions in mammalian cells when ectopically-expressed [77]. Tricalbins (Tcbs), including Tcb1, Tcb2, and Tcb3, also selectively localized at the cortical ER in yeast cells [116]. Tcbs contain N-terminal hydrophobic segments for their ER membrane targeting, followed by cytosolic synaptotagmin-like mitochondrial lipid binding protein (SMP) domain and multiple C2 domains. C2 domains comprise the second largest Ca2+ binding module in the proteome after the most common EF hand motif, and many C2 domains are capable of binding to Ca2+ or phospholipids [117]. The SMP domain was identified by protein sequence alignment [118], and was shown to have a role in lipid transfer [119–121]. Studies in yeast showed that all SMP domain-containing proteins localize at ER-organelle contact sites, implicating their common functions at these subcellular structures [116].
Fig. 3. Proteins that localize at yeast cortical ER.
Domain structure of yeast proteins localized at the cortical ER. Black bar, transmembrane; PB, polybasic motif; HS, hydrophobic segments; SMP, synaptotagmin-like mitochondrial lipid binding protein domain; MSP, major sperm protein domain; PH, pleckstrin homology; FFAT, two phenylalanines in an acidic tract motif; ORD, OSBP-related domain; BAR, Bin/Amphiphysin/Rvs domain; PHg, GRAM domains in the Pleckstrin homology domain; StART, Steroidogenic Acute Regulatory Transfer domain; cER, the cortical ER.
Genetic studies have provided insights into the regulation and functions of the cortical ER in yeast cells. Deletion of Scs2/Scs22, the yeast orthologs of mammalian VAMP-associated proteins (VAPs) that localize in the ER providing the ER targeting for other proteins enriched at ER-PM junctions, resulted in a significant decrease in the PM coverage by the cortical ER up to 50% [17, 28]. In addition, the Δtether yeast cells lacking six proteins, including Scs2/Scs22, Ist2, and Tcbs, displayed a dramatic reduction of the cortical ER and an accumulation of cytoplasmic ER structures as revealed by fluorescence microscopy [17]. Further ultrastructural analysis of the Δtether cells using EM showed a nearly 90% decrease from 40 % to 4.8 % in the PM coverage of the cortical ER. Among the three families of proteins, deletion of Tcbs alone had the least effect compared to deletion of Scs2/Scs22 or Ist2. It is noteworthy that constitutive unfolded protein response (UPR) was observed in the Δtether cells. This up-regulated UPR was not due to a defect in protein trafficking from the ER. Instead, it was required for the viability of the Δtether cells.
Therefore, the cortical ER may participate in crucial, yet unidentified, ER functions that suppress UPR in wild-type yeast cells. The best documented function of yeast cortical ER is lipid metabolism. PM-associated ER in yeast cells isolated by biochemical methods showed high capacity of phosphatidylinositol (PI) and phosphatidylserine (PS) synthesis in vitro [27]. A genetics study also demonstrated that yeast cortical ER participates in phosphatidylcholine (PC) synthesis [122]. In addition, yeast cortical ER is important for the metabolism of phosphatidylinositol 4-phohspate (PI4P). An ER-localized phosphatase, Sac1, has been proposed to regulate PM PI4P levels in trans via the cortical ER [18]. Yeast cells lacking Sac1 displayed elevated PI4P levels leading to defects in membrane trafficking, lipid metabolism, and growth [123–125]. The functional significance of Sac1 in higher organisms has been demonstrated by embryonic lethality in mice and in Drosophila lacking Sac1 [126, 127]. Consistent with an important role of the cortical ER in PI4P metabolism regulated by Sac1, a significant elevation of PI4P levels at the PM was observed in the Δtether cells [17, 18].
Several members of the yeast orthologs of oxysterol binding proteins (OSBP)-related proteins (ORPs), including Osh2, Osh3, Osh6, and Osh7, have been shown to be enriched at the cortical ER [128, 129]. Osh proteins contain the lipid-binding ORP domain (ORD) which can simultaneously bind two membranes [129]. The hydrophobic pocket of the ORD domain can bind PI4P and, in many cases, a second lipid in a mutually exclusive manner [130]. In addition to ORD, Osh2 and Osh3 contain a pleckstrin homology (PH) domain and an FFAT (two phenylalanines in an acidic track) motif for dual targeting to ER-PM junctions. The PH domain of Osh3 binds PI4P and mediates the targeting of Osh3 to the PM [18]. The FFAT motif binds Scs2/Scs22 and is important for Osh2 and Osh3 to localize to the cortical ER [128, 131]. It was proposed that high PM PI4P levels recruit and activate Osh3 at the cortical ER, which facilitates Sac1-mediated dephosphorylation of PI4P [18]. Osh6 and Osh7 contribute to ER-to-PM transport of PS [132]. This PS transport is driven by a counterflow transport of PI4P from the PM to the ER, where PI4P is dephosphorylated by ER-localized Sac1 [133].
Furthermore, members of a new family of lipid transfer proteins in the Steroidogenic Acute Regulatory Transfer (StART) superfamily, Lam2 and Lam3, have recently been shown to localize to the cortical ER in yeast cells [134]. These StART-like proteins are ER transmembrane proteins that contain a GRAM domain in the PH superfamily (PHg) domain in addition to the StART domain. StART domains in Lam2 and Lam4 selectively bind sterol. Significant reduction in the transport of exogenous sterols to the ER was observed in yeast cells deficient in Lam1, Lam2, and Lam3, indicating the importance of these proteins in ER-PM sterol transport. It is of interest to investigate whether the mammalian orthologs of these StART-like proteins are involved in sterol transport at ER-PM junctions. Overall, studies in yeast cells have significantly contributed to our understanding of molecular mechanisms underlying regulation and functions of ER-PM junctions.
4.2 Proteins enriched at ER-PM junctions in resting non-excitable mammalian cells
ER-PM junctions exist in non-excitable mammalian cells in the resting state, yet at a much lower abundance compared to those in yeast cells and muscle cells. Proteins enriched at ER-PM junctions in non-excitable mammalian cells are listed in Fig. 4.
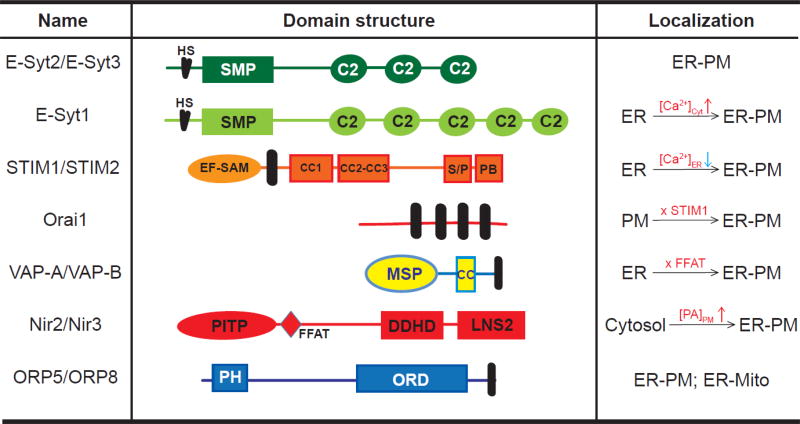
Fig. 4. Proteins that localize at ER-PM junctions in non-excitable mammalian cells.
Domain structure of proteins that localize at ER-PM junctions in mammalian cells. HS, hydrophobic segments; SMP, synaptotagmin-like mitochondrial lipid binding protein domain; EF-SAM, EF hand and sterile alpha motif; Black bar, transmembrane; CC, coiled-coil domain; S/P, serine and proline rich region; PB, polybasic motif; MSP, major sperm protein domain; PITP, PI transfer protein domain; FFAT, two phenylalanines in an acidic tract motif; LNS2, Lipin/Ned1/Smp2 domain. ER-PM, ER-PM junction; ER-Mito, ER-Mitochondria contacts.
The orthologs of yeast Tcbs, E-Syt1, E-Syt2, and E-Syt3, have been identified to function at ER-PM junctions in mammalian cells [16]. E-Syt proteins are ubiquitously expressed, and are named after synaptotagmin because they are membrane-associated proteins with multiple C2 domains [135]. When epitope tagged-E-Syts were overexpressed in cells, E-Syt1 displayed diffuse ER localization, while E-Syt2 and E-Syt3 appeared to localize at the PM with very dim intracellular signals [16, 135]. Further examination of HRP-tagged E-Syt2 and E-Syt3 by EM revealed both proteins are enriched at ER-PM junctions. The PM localization observed by fluorescence microscopy reflected the artificial expansion of ER-PM junctions to ~40% PM occupancy resulting from overexpression of E-Syt2 and E-Syt3 that mediate ER-PM tethering [16]. The very C-terminal C2 domains from both E-Syt2 and E-Syt3 are essential for PM targeting via PIP2 binding [16, 135]. Knockdown of all three E-Syts resulted in ~50% reduction in the number of ER-PM junctions as detected by ER-labeling [16]. Reconstitutions of E-Syt1 and E-Syt2 in triple knockdown cells were sufficient to restore the abundance of ER-PM junctions to the control level, indicting the redundancy of E-Syts in ER -PM tethering. Triple E-Syt knockout animals are viable without obvious defects [136, 137]. These findings indicate that there are tethering mechanisms besides E-Syts maintaining ER-PM junctions.
Several mammalian ORPs have been shown to localize at ER-PM junctions in resting cells [138]. Similar to Osh6 in yeast, mammalian ORP5 and ORP8 can mediate PI4P/PS countertransport at ER-PM junctions [139, 140]. ORP5 and ORP8 are ER transmembrane proteins that not only localize at ER-PM junctions but also at ER-mitochondria contacts [141]. Knockdown of ORP5 and ORP8 did not affect ER-PM junctions based on EM analysis. Therefore, ORP5 and ORP8 are unlikely to be dedicated ER-PM tethers like E-Syt2 and E-Syt3.
4.3 Proteins inducibly localized at ER-PM junctions for inter-organelle signaling
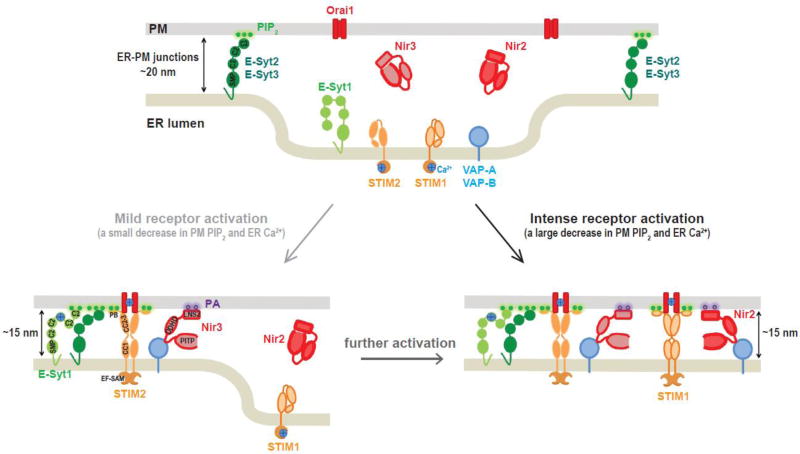
ER-PM junctions are ideal loci for local and efficient communications between the ER and the PM. This is especially important for Ca2+ signaling and for transport of specific lipids via the non-vesicular route [6]. STIM1 and its family member STIM2, are ER membrane proteins that translocate to ER-PM junctions in response to a decrease in ER Ca2+ [65, 142] (See Section 2.3). At ER-PM junctions, STIM1 and STIM2 interact with the PM Ca2+ channel Orai1, resulting Orai1 recruitment to ER-PM junctions and activation of Ca2+ influx [142, 143]. In contrast to STIM1, STIM2 translocated to ER-PM junctions upon smaller decreases in ER Ca2+ [142]. Thus, STIM2 maintains Ca2+ homeostasis under basal and mild receptor activation conditions, whereas both STIM1 and STIM2 contribute to SOCE activation at ER-PM junctions during intense receptor activation (Fig. 5).
Fig. 5. Model depicting dynamic localization of proteins at ER-PM junctions during receptor activation.
E-Syt2 and E-Syt3 readily localize at ER-PM junctions by binding to PM PIP2 in the resting state. During mild receptor activation, STIM2 responds to a small decrease in ER Ca2+ level to activate Orai1 at ERPM junctions for SOCE. E-Syt1 responds to an increase cytosolic Ca2+ level to mediate a decrease in gap distance at ER-PM junctions. Nir3 responds to a small increase in PA at the PM resulting from PIP2 hydrolysis to mediate PI/PA exchange at ER-PM junctions for PM PIP2 homeostasis. Following further or intense receptor activation, STIM1 and Nir2 translocate to ER-PM junctions to support the homeostatic regulation of the PIP2-Ca2+ signaling system with STIM2, Nir3, and E-Syt1.
While STIM1 and STIM2 accumulation at ER-PM junctions depends on a decrease in ER Ca2+ store, E-Syt1 translocates to ER-PM junctions following an increase in cytosolic Ca2+ level. Unlike E-Syt2 and E-Syt3 that constitutively localize to ER-PM junctions providing tethering, E-Syt1 localizes diffusely in the ER in resting cells. Following an increase in cytosolic Ca2+ during receptor activation, the binding of Ca2+ to the cytosolic C2C domain triggers E-Syt1 translocation to ER-PM junctions via its C2E domain binding to PIP2 at the PM [15, 16, 144]. The translocation of E-Syt1 to ER-PM junctions is essential to reduce the distance between the ER and the PM. This idea, namely enhanced ER-to-PM connection, was reported in live HeLa cells by using MAPPER with different length of cytosolic linkers and TIRF imaging [15]. An EM study confirmed that the gap distance of E-Syt1-dependent ER-PM junctions is shortened from ~20 nm to ~15 nm following the elevation of cytosolic Ca2+ using cryo-electron tomography [104]. The decrease in gap distance of ER-PM junctions mediated by E-Syt1 facilitates the accumulation of Nir2, a PI transfer protein (PITP), at ER-PM junctions [15].
Nir2 and Nir3 (also named as PITPNM1 and PITPNM2, respectively) contain an N-terminal PITP domain, an FFAT motif for ER targeting via binding to VAPs, and C-terminal DDHD and LNS2 domains for PM targeting [15, 145–148]. Overexpressed Nir2 and Nir3 localized primarily in the cytosol in resting cells and translocated to ER-PM junctions following receptor-induced PIP2 hydrolysis [15, 146–148]. PA generated following PIP2 hydrolysis triggered Nir2 and Nir3 targeting to ER-PM junctions [146–148]. Translocation of Nir2 to ER-PM junctions resulted in an accumulation of VAP-A and VAP-B at ER-PM junctions to support the localization of Nir2 [15]. The PM targeting of Nir2 seemed to be a more complex process involving the interaction of both diacylglycerol and PA [148]. Recent studies indicated that Nir2 and Nir3 mediate PI/PA countertransport at ER-PM junctions, delivering PI from the ER to the PM in exchange for PA, which is converted to CDP-DAG for PI synthesis in the ER during receptor activation [148–149]. Selective depletion of PI at the ER membrane disrupted Nir2-mediated PM PIP2 replenishment [147], suggesting that Nir2 transports PI from the ER to the PM for PIP2 resynthesis after hydrolysis to support receptor activation and PIP2-dependent cellular processes. Thus, Nir2 and Nir3 are feedback regulators for PM PIP2 homeostasis by detecting metabolic intermediates generated by PIP2 hydrolysis, translocating to ER-PM junctions, and replenishing PM PIP2 [150, 151]. Consistently, knockdown of Nir2 significantly affected PIP2-associated functions, including receptor-induced Ca2+ signaling, epidermal growth factor (EGF) signaling, cell migration, and epithelial-mesenchymal transition (EMT) [146, 147, 152].
Notably, STIM1/STIM2, E-Syt1, and Nir2/Nir3 inducibly localize to ER-PM junctions in response to different cues: ER Ca2+ depletion for STIM1/STIM2, cytosolic Ca2+ increase for E-Syt1, and PM PA increase following PIP2 hydrolysis for Nir2/Nir3, yet all occur during receptor activation. Similar to STIM1 and STIM2 that work in tandem in response to different levels of ER Ca2+ depletion, Nir3 is more sensitive to PA production than Nir2 and maintains PM PIP2 homeostasis during basal and mild receptor activation conditions [147]. Therefore, during intense receptor activation, STIM1/STIM2, E-Syt1, and Nir2/Nir3 can simultaneously localize at ER-PM junctions to contribute to homeostatic regulation of the PIP2-Ca2+ signaling system [150] (Fig. 5).
4.4 Additional proteins implicated in regulation and functions of ER-PM junctions
In addition to those described in previous sections, several proteins have been implicated in regulation and functions of ER-PM junctions. Junctate was identified as an ER membrane Ca2+ binding protein that is expressed in a variety of tissues [153]. Knockdown of Junctate dramatically reduced the average size and number of ER-PM junctions in HEK293 cells, while overexpression of Junctate resulted in the opposite effect [154]. Junctate contains only 23 amino acids in the cytosolic region, which is likely too short for tethering the ER to the PM. Overexpression of Junctate showed diffuse ER localization with no enrichment at ER-PM junctions [155, 156]. Junctate has been reported to interact with IP3 receptor and STIM1-Orai1 complex to modulate Ca2+ signaling [154–156]. Thus, it is likely that Junctate interacts with other components at ER-PM junctions to provide ER- PM tethering. Recently, several proteins including septins, α-SNAP, SARAF, Surf4, POST, and TMEM110 have been shown to regulate STIM1-Orai1 interaction at ER-PM junctions and to modulate SOCE [111, 157–165]. These proteins may also contribute to the tethering of ER-PM junctions.
ER-PM junctions were first observed in muscle cells and a few years later in neurons. In these excitable cell types, ER-PM junctions are likely to be more prevalent since communication between the PM and the ER Ca2+ store is particularly important to mediate their functions. JPHs (see Section 2.2) can mediate ER-PM tethering in both muscle cells and in neurons. In addition, Kv2.1 voltage-gated potassium channels selectively expressed in the PM of mammalian brain have been shown to localize at ER-PM junctions and contribute to the functions and regulation of neuronal ER-PM junctions [166–168]. Moreover, Sec22b, a non-fusogenic ER SNARE protein which can interact with the PM SNARE Syntaxin 1 at the PM, may contribute to ER-PM tethering and PM expansion in neurons [169]. Recently, TMEM24, a SMP domain-containing ER membrane protein highly expressed in brain, neurons, and insulinoma cells, has been shown to localize to ER-PM junctions and contribute to insulin secretion [170].
In yeast lacking tubule-generating proteins including Rtn1/Rtn2 and DP1/Yop1, extensive cisternae-like ER-PM junctions with a narrower thickness were observed, indicating the involvement of these proteins in shaping ER-PM junctions. Interestingly, Rtn4 knockout mouse fibroblasts with loss of ER tubulation also displayed defective SOCE [171]. It is likely that ER-shaping proteins in mammalian cells also participate in regulating ER-PM junctions.
5. Concluding remarks
It is not possible to cover all the work contributing to our understanding of ER-PM junctions done in the last 60 years since the first description of ER-PM junctions in 1957. It is clear that ER-PM junctions are fundamental to PM-to-ER Ca2+ signaling during E-C coupling in muscle contraction. These subcellular loci are also the platforms for ER-to-PM Ca2+ signaling during SOCE important for maintaining Ca2+ homeostasis and activating signaling events following receptor activation. Moreover, it is well recognized that ER-PM junctions are sites for lipid transport mediated by transfer proteins of various lipids including PI, PI4P, PA, PS, PC and sterol.
The progress in the research of ER-PM junctions is linked to the development of methodologies for investigating the dynamic regulation and functions of these miniature membrane structures. In addition to EM, novel imaging methods and markers have been developed to selectively visualize ER-PM junctions during physiological processes. Furthermore, genetic studies using yeast cells and siRNA-mediated gene knockdown in mammalian cells helped dissect the molecular mechanisms of ER-PM junctions. Nevertheless, there are still many open questions in the field. For example, what is the tethering mechanism maintaining ER-PM junctions in cells deficient in all E-Syts? How does E-Syt1 mediate the decrease in gap distance at ER-PM junctions? Is there a mechanism preventing membrane fusion at ER-PM junctions? Is there a mechanism controlling the distribution of ER-PM junctions in a cell? Are there other signaling events occurring at ER-PM junctions besides transport of Ca2+ and lipids?
Further development of techniques for studying ER-PM junctions and identification of additional proteins that support and/or mediate communication between the ER and the PM at ER-PM junctions will likely answer some of the above questions and advance our understanding of ER-PM junctions.
Highlights.
ER-PM junctions are essential for Ca2+ signaling and homeostasis.
ER-PM junctions are miniature structures best visualized by electron microscopy.
New methods enabling visualization of ER-PM junctions in live cells are summarized.
Recent studies identifying proteins enriched at ER-PM junctions are discussed.
ER-PM junctions are hubs for non-vesicular transport of phospholipids and sterol.
Acknowledgments
We thank Carlo Quintanilla for carefully reading this manuscript and the members of our lab for valuable discussions. We are grateful to Linda Patterson for administrative assistance. Work in the authors’ lab is supported by NIH grant GM113079 and Welch Foundation Grant I-1789. J.L. is a Sowell Family Scholar in Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baumann O, Walz B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. International review of cytology. 2001;205:149–214. doi: 10.1016/s0074-7696(01)05004-5. [DOI] [PubMed] [Google Scholar]
- 2.West M, Zurek N, Hoenger A, Voeltz GK. A 3D analysis of yeast ER structure reveals how ER domains are organized by membrane curvature. The Journal of cell biology. 2011;193:333–346. doi: 10.1083/jcb.201011039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nixon-Abell J, Obara CJ, Weigel AV, Li D, Legant WR, Xu CS, Pasolli HA, Harvey K, Hess HF, Betzig E, Blackstone C, Lippincott-Schwartz J. Increased spatiotemporal resolution reveals highly dynamic dense tubular matrices in the peripheral ER. Science. 2016;354 doi: 10.1126/science.aaf3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 5.Carrasco S, Meyer T. STIM proteins and the endoplasmic reticulum-plasma membrane junctions. Annu Rev Biochem. 2011;80:973–1000. doi: 10.1146/annurev-biochem-061609-165311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine T. Short-range intracellular trafficking of small molecules across endoplasmic reticulum junctions. Trends Cell Biol. 2004;14:483–490. doi: 10.1016/j.tcb.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Porter KR, Palade GE. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. The Journal of biophysical and biochemical cytology. 1957;3:269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore DH, Ruska H. Electron microscope study of mammalian cardiac muscle cells. The Journal of biophysical and biochemical cytology. 1957;3:261–268. doi: 10.1083/jcb.3.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. The Journal of cell biology. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 11.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. The Journal of cell biology. 2010;190:363–375. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murley A, Lackner LL, Osman C, West M, Voeltz GK, Walter P, Nunnari J. ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. eLife. 2013;2:e00422. doi: 10.7554/eLife.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowland AA, Chitwood PJ, Phillips MJ, Voeltz GK. ER contact sites define the position and timing of endosome fission. Cell. 2014;159:1027–1041. doi: 10.1016/j.cell.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesmin B, Bigay J, Moser von Filseck J, Lacas-Gervais S, Drin G, Antonny B. A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell. 2013;155:830–843. doi: 10.1016/j.cell.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 15.Chang C-L, Hsieh T-S, Yang TT, Rothberg KG, Azizoglu DB, Volk E, Liao J-C, Liou J. Feedback Regulation of Receptor-Induced Ca 2+ Signaling Mediated by E-Syt1 and Nir2 at Endoplasmic Reticulum-Plasma Membrane Junctions. Cell reports. 2013;5:813–825. doi: 10.1016/j.celrep.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Giordano F, Saheki Y, Idevall-Hagren O, Colombo SF, Pirruccello M, Milosevic I, Gracheva EO, Bagriantsev SN, Borgese N, De Camilli P. PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell. 2013;153:1494–1509. doi: 10.1016/j.cell.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manford AG, Stefan CJ, Yuan HL, Macgurn JA, Emr SD. ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev Cell. 2012;23:1129–1140. doi: 10.1016/j.devcel.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144:389–401. doi: 10.1016/j.cell.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Dong R, Saheki Y, Swarup S, Lucast L, Harper JW, De Camilli P. Endosome-ER Contacts Control Actin Nucleation and Retromer Function through VAP-Dependent Regulation of PI4P. Cell. 2016;166:408–423. doi: 10.1016/j.cell.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocha N, Kuijl C, van der Kant R, Janssen L, Houben D, Janssen H, Zwart W, Neefjes J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7-RILP-p150 Glued and late endosome positioning. J Cell Biol. 2009;185:1209–1225. doi: 10.1083/jcb.200811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, Schultz SW, Vietri M, Nisi V, Bucci C, Brech A, Johansen T, Stenmark H. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 2015;520:234–238. doi: 10.1038/nature14359. [DOI] [PubMed] [Google Scholar]
- 22.Alpy F, Rousseau A, Schwab Y, Legueux F, Stoll I, Wendling C, Spiegelhalter C, Kessler P, Mathelin C, Rio MC, Levine TP, Tomasetto C. STARD3 or STARD3NL and VAP form a novel molecular tether between late endosomes and the ER. J Cell Sci. 2013;126:5500–5512. doi: 10.1242/jcs.139295. [DOI] [PubMed] [Google Scholar]
- 23.Eden ER, White IJ, Tsapara A, Futter CE. Membrane contacts between endosomes and ER provide sites for PTP1B-epidermal growth factor receptor interaction. Nat Cell Biol. 2010;12:267–272. doi: 10.1038/ncb2026. [DOI] [PubMed] [Google Scholar]
- 24.Eden ER, Sanchez-Heras E, Tsapara A, Sobota A, Levine TP, Futter CE. Annexin A1 Tethers Membrane Contact Sites that Mediate ER to Endosome Cholesterol Transport. Dev Cell. 2016;37:473–483. doi: 10.1016/j.devcel.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franzini-Armstrong C, Jorgensen AO. Structure and development of E-C coupling units in skeletal muscle. Annual review of physiology. 1994;56:509–534. doi: 10.1146/annurev.ph.56.030194.002453. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbluth J. Subsurface cisterns and their relationship to the neuronal plasma membrane. J Cell Biol. 1962;13:405–421. doi: 10.1083/jcb.13.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pichler H, Gaigg B, Hrastnik C, Achleitner G, Kohlwein SD, Zellnig G, Perktold A, Daum G. A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. European journal of biochemistry/FEBS. 2001;268:2351–2361. doi: 10.1046/j.1432-1327.2001.02116.x. [DOI] [PubMed] [Google Scholar]
- 28.Loewen CJ, Young BP, Tavassoli S, Levine TP. Inheritance of cortical ER in yeast is required for normal septin organization. J Cell Biol. 2007;179:467–483. doi: 10.1083/jcb.200708205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vihtelic TS, Goebl M, Milligan S, O'Tousa JE, Hyde DR. Localization of Drosophila retinal degeneration B, a membrane-associated phosphatidylinositol transfer protein. J Cell Biol. 1993;122:1013–1022. doi: 10.1083/jcb.122.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupont G, Combettes L, Bird GS, Putney JW. Calcium oscillations. Cold Spring Harbor perspectives in biology. 2011;3 doi: 10.1101/cshperspect.a004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophysical journal. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flucher BE. Structural analysis of muscle development: transverse tubules, sarcoplasmic reticulum, and the triad. Developmental biology. 1992;154:245–260. doi: 10.1016/0012-1606(92)90065-o. [DOI] [PubMed] [Google Scholar]
- 35.Henkart M, Landis DM, Reese TS. Similarity of junctions between plasma membranes and endoplasmic reticulum in muscle and neurons. J Cell Biol. 1976;70:338–347. doi: 10.1083/jcb.70.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi AE, Dirksen RT. Sarcoplasmic reticulum: the dynamic calcium governor of muscle. Muscle & nerve. 2006;33:715–731. doi: 10.1002/mus.20512. [DOI] [PubMed] [Google Scholar]
- 37.Franzini-Armstrong C. Studies of the triad. 3. Structure of the junction in fast twitch fibers. Tissue & cell. 1972;4:469–478. doi: 10.1016/s0040-8166(72)80023-5. [DOI] [PubMed] [Google Scholar]
- 38.Loesser KE, Castellani L, Franzini-Armstrong C. Dispositions of junctional feet in muscles of invertebrates. Journal of muscle research and cell motility. 1992;13:161–173. doi: 10.1007/BF01874153. [DOI] [PubMed] [Google Scholar]
- 39.Airey JA, Beck CF, Murakami K, Tanksley SJ, Deerinck TJ, Ellisman MH, Sutko JL. Identification and localization of two triad junctional foot protein isoforms in mature avian fast twitch skeletal muscle. The Journal of biological chemistry. 1990;265:14187–14194. [PubMed] [Google Scholar]
- 40.Fosset M, Jaimovich E, Delpont E, Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. The Journal of biological chemistry. 1983;258:6086–6092. [PubMed] [Google Scholar]
- 41.Yuan SH, Arnold W, Jorgensen AO. Biogenesis of transverse tubules and triads: immunolocalization of the 1,4-dihydropyridine receptor, TS28, and the ryanodine receptor in rabbit skeletal muscle developing in situ. The Journal of cell biology. 1991;112:289–301. doi: 10.1083/jcb.112.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flucher BE, Phillips JL, Powell JA. Dihydropyridine receptor alpha subunits in normal and dysgenic muscle in vitro: expression of alpha 1 is required for proper targeting and distribution of alpha 2. The Journal of cell biology. 1991;115:1345–1356. doi: 10.1083/jcb.115.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endo M. Calcium-induced calcium release in skeletal muscle. Physiological reviews. 2009;89:1153–1176. doi: 10.1152/physrev.00040.2008. [DOI] [PubMed] [Google Scholar]
- 44.Franzini-Armstrong C, Pincon-Raymond M, Rieger F. Muscle fibers from dysgenic mouse in vivo lack a surface component of peripheral couplings. Developmental biology. 1991;146:364–376. doi: 10.1016/0012-1606(91)90238-x. [DOI] [PubMed] [Google Scholar]
- 45.Ikemoto T, Komazaki S, Takeshima H, Nishi M, Noda T, Iino M, Endo M. Functional and morphological features of skeletal muscle from mutant mice lacking both type 1 and type 3 ryanodine receptors. J Physiol. 1997;501(Pt 2):305–312. doi: 10.1111/j.1469-7793.1997.305bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takekura H, Nishi M, Noda T, Takeshima H, Franzini-Armstrong C. Abnormal junctions between surface membrane and sarcoplasmic reticulum in skeletal muscle with a mutation targeted to the ryanodine receptor. Proc Natl Acad Sci U S A. 1995;92:3381–3385. doi: 10.1073/pnas.92.8.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takekura H, Takeshima H, Nishimura S, Takahashi M, Tanabe T, Flockerzi V, Hofmann F, Franzini-Armstrong C. Co-expression in CHO cells of two muscle proteins involved in excitation-contraction coupling. Journal of muscle research and cell motility. 1995;16:465–480. doi: 10.1007/BF00126431. [DOI] [PubMed] [Google Scholar]
- 48.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Molecular cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 49.Nishi M, Sakagami H, Komazaki S, Kondo H, Takeshima H. Coexpression of junctophilin type 3 and type 4 in brain. Brain Res Mol Brain Res. 2003;118:102–110. doi: 10.1016/s0169-328x(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 50.Garbino A, van Oort RJ, Dixit SS, Landstrom AP, Ackerman MJ, Wehrens XH. Molecular evolution of the junctophilin gene family. Physiol Genomics. 2009;37:175–186. doi: 10.1152/physiolgenomics.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma H, Lou Y, Lin WH, Xue HW. MORN motifs in plant PIPKs are involved in the regulation of subcellular localization and phospholipid binding. Cell research. 2006;16:466–478. doi: 10.1038/sj.cr.7310058. [DOI] [PubMed] [Google Scholar]
- 52.Fawcett DW, McNutt NS. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. The Journal of cell biology. 1969;42:1–45. doi: 10.1083/jcb.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirata Y, Brotto M, Weisleder N, Chu Y, Lin P, Zhao X, Thornton A, Komazaki S, Takeshima H, Ma J, Pan Z. Uncoupling store-operated Ca2+ entry and altered Ca2+ release from sarcoplasmic reticulum through silencing of junctophilin genes. Biophys J. 2006;90:4418–4427. doi: 10.1529/biophysj.105.076570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Ding X, Lopez JR, Takeshima H, Ma J, Allen PD, Eltit JM. Impaired Orai1-mediated resting Ca2+ entry reduces the cytosolic [Ca2+] and sarcoplasmic reticulum Ca2+ loading in quiescent junctophilin 1 knock-out myotubes. The Journal of biological chemistry. 2010;285:39171–39179. doi: 10.1074/jbc.M110.149690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henkart MP, Nelson PG. Evidence for an intracellular calcium store releasable by surface stimuli ifibroblasts (L cells) The Journal of general physiology. 1979;73:655–673. doi: 10.1085/jgp.73.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGinley DM, Posalaky Z, Porvaznik M. Membrane associations between subsurface cisternae of endoplasmic reticulum and the plasma membrane of rat Sertoli cells. Tissue & cell. 1981;13:337–347. doi: 10.1016/0040-8166(81)90009-4. [DOI] [PubMed] [Google Scholar]
- 57.Gardiner DM, Grey RD. Membrane junctions in Xenopus eggs: their distribution suggests a role in calcium regulation. J Cell Biol. 1983;96:1159–1163. doi: 10.1083/jcb.96.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 59.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, Bjerrum PJ, Christensen SB, Hanley MR. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents and actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- 60.Parekh AB, Penner R. Store depletion and calcium influx. Physiological reviews. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- 61.Putney JW, Jr, Bird GS. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocrine reviews. 1993;14:610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- 62.Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell regulation. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Penner R, Matthews G, Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988;334:499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- 64.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 65.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 68.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci U S A. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Y, Srinivasan P, Razavi S, Seymour S, Meraner P, Gudlur A, Stathopulos PB, Ikura M, Rao A, Hogan PG. Initial activation of STIM1, the regulator of store-operated calcium entry. Nature structural & molecular biology. 2013;20:973–981. doi: 10.1038/nsmb.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Putyrski M, Schultz C. Protein translocation as a tool: The current rapamycin story. FEBS Lett. 2012;586:2097–2105. doi: 10.1016/j.febslet.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 77.Ercan E, Momburg F, Engel U, Temmerman K, Nickel W, Seedorf M. A conserved, lipid-mediated sorting mechanism of yeast Ist2 and mammalian STIM proteins to the peripheral ER. Traffic. 2009;10:1802–1818. doi: 10.1111/j.1600-0854.2009.00995.x. [DOI] [PubMed] [Google Scholar]
- 78.Korzeniowski MK, Popovic MA, Szentpetery Z, Varnai P, Stojilkovic SS, Balla T. Dependence of STIM1/Orai1-mediated calcium entry on plasma membrane phosphoinositides. J Biol Chem. 2009;284:21027–21035. doi: 10.1074/jbc.M109.012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walsh CM, Chvanov M, Haynes LP, Petersen OH, Tepikin AV, Burgoyne RD. Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry. Biochem J. 2010;425:159–168. doi: 10.1042/BJ20090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem Biophys Res Commun. 2009;385:49–54. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338:1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Balasuriya D, Srivats S, Murrell-Lagnado RD, Edwardson JM. Atomic force microscopy (AFM) imaging suggests that stromal interaction molecule 1 (STIM1) binds to Orai1 with sixfold symmetry. FEBS Lett. 2014;588:2874–2880. doi: 10.1016/j.febslet.2014.06.054. [DOI] [PubMed] [Google Scholar]
- 85.Yen M, Lokteva LA, Lewis RS. Functional Analysis of Orai1 Concatemers Supports a Hexameric Stoichiometry for the CRAC Channel. Biophysical journal. 2016;111:1897–1907. doi: 10.1016/j.bpj.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madl J, Weghuber J, Fritsch R, Derler I, Fahrner M, Frischauf I, Lackner B, Romanin C, Schutz GJ. Resting state Orai1 diffuses as homotetramer in the plasma membrane of live mammalian cells. The Journal of biological chemistry. 2010;285:41135–41142. doi: 10.1074/jbc.M110.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. The Journal of cell biology. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiological reviews. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frischauf I, Fahrner M, Jardin I, Romanin C. The STIM1: Orai Interaction. Advances in experimental medicine and biology. 2016;898:25–46. doi: 10.1007/978-3-319-26974-0_2. [DOI] [PubMed] [Google Scholar]
- 90.Yeung PS, Yamashita M, Prakriya M. Pore opening mechanism of CRAC channels. Cell Calcium. 2016 doi: 10.1016/j.ceca.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou Y, Cai X, Nwokonko RM, Loktionova NA, Wang Y, Gill DL. The STIM-Orai coupling interface and gating of the Orai1 channel. Cell Calcium. 2017 doi: 10.1016/j.ceca.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 93.Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nature cell biology. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Varga-Szabo D, Braun A, Kleinschnitz C, Bender M, Pleines I, Pham M, Renne T, Stoll G, Nieswandt B. The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J Exp Med. 2008;205:1583–1591. doi: 10.1084/jem.20080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feske S, Picard C, Fischer A. Immunodeficiency due to mutations in ORAI1 and STIM1. Clinical immunology. 2010;135:169–182. doi: 10.1016/j.clim.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gwack Y, Srikanth S, Oh-Hora M, Hogan PG, Lamperti ED, Yamashita M, Gelinas C, Neems DS, Sasaki Y, Feske S, Prakriya M, Rajewsky K, Rao A. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Molecular and cellular biology. 2008;28:5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek MA. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. The Biochemical journal. 2001;357:673–685. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Launikonis BS, Rios E. Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle. J Physiol. 2007;583:81–97. doi: 10.1113/jphysiol.2007.135046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. The Journal of cell biology. 2011;194:335–346. doi: 10.1083/jcb.201012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Orci L, Ravazzola M, Le Coadic M, Shen WW, Demaurex N, Cosson P. From the Cover: STIM1-induced precortical and cortical subdomains of the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2009;106:19358–19362. doi: 10.1073/pnas.0911280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lur G, Haynes LP, Prior IA, Gerasimenko OV, Feske S, Petersen OH, Burgoyne RD, Tepikin AV. Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP(3) receptors. Curr Biol. 2009;19:1648–1653. doi: 10.1016/j.cub.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fernandez-Busnadiego R, Saheki Y, De Camilli P. Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum-plasma membrane contact sites. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E2004–2013. doi: 10.1073/pnas.1503191112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perni S, Dynes JL, Yeromin AV, Cahalan MD, Franzini-Armstrong C. Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry. Proc Natl Acad Sci U S A. 2015;112:E5533–5542. doi: 10.1073/pnas.1515606112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat Rev Mol Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- 107.Maus M, Jairaman A, Stathopulos PB, Muik M, Fahrner M, Weidinger C, Benson M, Fuchs S, Ehl S, Romanin C, Ikura M, Prakriya M, Feske S. Missense mutation in immunodeficient patients shows the multifunctional roles of coiled-coil domain 3 (CC3) in STIM1 activation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:6206–6211. doi: 10.1073/pnas.1418852112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 109.Lavieu G, Orci L, Shi L, Geiling M, Ravazzola M, Wieland F, Cosson P, Rothman JE. Induction of cortical endoplasmic reticulum by dimerization of a coatomer-binding peptide anchored to endoplasmic reticulum membranes. Proc Natl Acad Sci U S A. 2010;107:6876–6881. doi: 10.1073/pnas.1002536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jing J, He L, Sun A, Quintana A, Ding Y, Ma G, Tan P, Liang X, Zheng X, Chen L, Shi X, Zhang SL, Zhong L, Huang Y, Dong MQ, Walker CL, Hogan PG, Wang Y, Zhou Y. Proteomic mapping of ER-PM junctions identifies STIMATE as a regulator of Ca(2)(+) influx. Nat Cell Biol. 2015;17:1339–1347. doi: 10.1038/ncb3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang D, Vjestica A, Oliferenko S. Plasma membrane tethering of the cortical ER necessitates its finely reticulated architecture. Curr Biol. 2012;22:2048–2052. doi: 10.1016/j.cub.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 113.Preuss D, Mulholland J, Kaiser CA, Orlean P, Albright C, Rose MD, Robbins PW, Botstein D. Structure of the yeast endoplasmic reticulum: localization of ER proteins using immunofluorescence and immunoelectron microscopy. Yeast. 1991;7:891–911. doi: 10.1002/yea.320070902. [DOI] [PubMed] [Google Scholar]
- 114.Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins) Physiological reviews. 2014;94:419–459. doi: 10.1152/physrev.00039.2011. [DOI] [PubMed] [Google Scholar]
- 115.Fischer MA, Temmerman K, Ercan E, Nickel W, Seedorf M. Binding of plasma membrane lipids recruits the yeast integral membrane protein Ist2 to the cortical ER. Traffic. 2009;10:1084–1097. doi: 10.1111/j.1600-0854.2009.00926.x. [DOI] [PubMed] [Google Scholar]
- 116.Toulmay A, Prinz WA. A conserved membrane-binding domain targets proteins to organelle contact sites. J Cell Sci. 2012;125:49–58. doi: 10.1242/jcs.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. The Journal of biological chemistry. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 118.Lee I, Hong W. Diverse membrane-associated proteins contain a novel SMP domain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:202–206. doi: 10.1096/fj.05-4581hyp. [DOI] [PubMed] [Google Scholar]
- 119.Kopec KO, Alva V, Lupas AN. Homology of SMP domains to the TULIP superfamily of lipid-binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics. 2010;26:1927–1931. doi: 10.1093/bioinformatics/btq326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saheki Y, Bian X, Schauder CM, Sawaki Y, Surma MA, Klose C, Pincet F, Reinisch KM, De Camilli P. Control of plasma membrane lipid homeostasis by the extended synaptotagmins. Nat Cell Biol. 2016;18:504–515. doi: 10.1038/ncb3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schauder CM, Wu X, Saheki Y, Narayanaswamy P, Torta F, Wenk MR, De Camilli P, Reinisch KM. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature. 2014;510:552–555. doi: 10.1038/nature13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tavassoli S, Chao JT, Young BP, Cox RC, Prinz WA, de Kroon AI, Loewen CJ. Plasma membrane--endoplasmic reticulum contact sites regulate phosphatidylcholine synthesis. EMBO reports. 2013;14:434–440. doi: 10.1038/embor.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Foti M, Audhya A, Emr SD. Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Molecular biology of the cell. 2001;12:2396–2411. doi: 10.1091/mbc.12.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. The Journal of biological chemistry. 1999;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- 125.Rivas MP, Kearns BG, Xie Z, Guo S, Sekar MC, Hosaka K, Kagiwada S, York JD, Bankaitis VA. Pleiotropic alterations in lipid metabolism in yeast sac1 mutants: relationship to "bypass Sec14p" and inositol auxotrophy. Molecular biology of the cell. 1999;10:2235–2250. doi: 10.1091/mbc.10.7.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Y, Boukhelifa M, Tribble E, Morin-Kensicki E, Uetrecht A, Bear JE, Bankaitis VA. The Sac1 phosphoinositide phosphatase regulates Golgi membrane morphology and mitotic spindle organization in mammals. Molecular biology of the cell. 2008;19:3080–3096. doi: 10.1091/mbc.E07-12-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wei HC, Sanny J, Shu H, Baillie DL, Brill JA, Price JV, Harden N. The Sac1 lipid phosphatase regulates cell shape change and the JNK cascade during dorsal closure in Drosophila. Curr Biol. 2003;13:1882–1887. doi: 10.1016/j.cub.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 128.Loewen CJ, Roy A, Levine TP. A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. The EMBO journal. 2003;22:2025–2035. doi: 10.1093/emboj/cdg201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schulz TA, Choi MG, Raychaudhuri S, Mears JA, Ghirlando R, Hinshaw JE, Prinz WA. Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J Cell Biol. 2009;187:889–903. doi: 10.1083/jcb.200905007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tong J, Manik MK, Yang H, Im YJ. Structural insights into nonvesicular lipid transport by the oxysterol binding protein homologue family. Biochim Biophys Acta. 2016;1861:928–939. doi: 10.1016/j.bbalip.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 131.Raychaudhuri S, Prinz WA. The diverse functions of oxysterol-binding proteins. Annu Rev Cell Dev Biol. 2010;26:157–177. doi: 10.1146/annurev.cellbio.042308.113334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maeda K, Anand K, Chiapparino A, Kumar A, Poletto M, Kaksonen M, Gavin AC. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature. 2013;501:257–261. doi: 10.1038/nature12430. [DOI] [PubMed] [Google Scholar]
- 133.Moser von Filseck J, Copic A, Delfosse V, Vanni S, Jackson CL, Bourguet W, Drin G INTRACELLULAR TRANSPORT. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science. 2015;349:432–436. doi: 10.1126/science.aab1346. [DOI] [PubMed] [Google Scholar]
- 134.Gatta AT, Wong LH, Sere YY, Calderon-Norena DM, Cockcroft S, Menon AK, Levine TP. A new family of StART domain proteins at membrane contact sites has a role in ER-PM sterol transport. eLife. 2015;4 doi: 10.7554/eLife.07253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Min SW, Chang WP, Sudhof TC. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc Natl Acad Sci U S A. 2007;104:3823–3828. doi: 10.1073/pnas.0611725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sclip A, Bacaj T, Giam LR, Sudhof TC. Extended Synaptotagmin (ESyt) Triple Knock-Out Mice Are Viable and Fertile without Obvious Endoplasmic Reticulum Dysfunction. PloS one. 2016;11:e0158295. doi: 10.1371/journal.pone.0158295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tremblay MG, Moss T. Loss of all 3 Extended Synaptotagmins does not affect normal mouse development, viability or fertility. Cell Cycle. 2016;15:2360–2366. doi: 10.1080/15384101.2016.1203494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Olkkonen VM. OSBP-Related Protein Family in Lipid Transport Over Membrane Contact Sites. Lipid insights. 2015;8:1–9. doi: 10.4137/LPI.S31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349:428–432. doi: 10.1126/science.aab1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sohn M, Ivanova P, Brown HA, Toth DJ, Varnai P, Kim YJ, Balla T. Lenz-Majewski mutations in PTDSS1 affect phosphatidylinositol 4-phosphate metabolism at ER-PM and ER-Golgi junctions. Proc Natl Acad Sci U S A. 2016;113:4314–4319. doi: 10.1073/pnas.1525719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Galmes R, Houcine A, van Vliet AR, Agostinis P, Jackson CL, Giordano F. ORP5/ORP8 localize to endoplasmic reticulum-mitochondria contacts and are involved in mitochondrial function. EMBO reports. 2016;17:800–810. doi: 10.15252/embr.201541108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y, Deng X, Zhou Y, Hendron E, Mancarella S, Ritchie MF, Tang XD, Baba Y, Kurosaki T, Mori Y, Soboloff J, Gill DL. STIM protein coupling in the activation of Orai channels. Proc Natl Acad Sci U S A. 2009;106:7391–7396. doi: 10.1073/pnas.0900293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Idevall-Hagren O, Lu A, Xie B, De Camilli P. Triggered Ca2+ influx is required for extended synaptotagmin 1-induced ER-plasma membrane tethering. The EMBO journal. 2015;34:2291–2305. doi: 10.15252/embj.201591565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amarilio R, Ramachandran S, Sabanay H, Lev S. Differential regulation of endoplasmic reticulum structure through VAP-Nir protein interaction. J Biol Chem. 2005;280:5934–5944. doi: 10.1074/jbc.M409566200. [DOI] [PubMed] [Google Scholar]
- 146.Kim S, Kedan A, Marom M, Gavert N, Keinan O, Selitrennik M, Laufman O, Lev S. The phosphatidylinositol-transfer protein Nir2 binds phosphatidic acid and positively regulates phosphoinositide signalling. EMBO reports. 2013;14:891–899. doi: 10.1038/embor.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chang CL, Liou J. Phosphatidylinositol 4,5-Bisphosphate Homeostasis Regulated by Nir2 and Nir3 Proteins at Endoplasmic Reticulum-Plasma Membrane Junctions. The Journal of biological chemistry. 2015;290:14289–14301. doi: 10.1074/jbc.M114.621375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim YJ, Guzman-Hernandez ML, Wisniewski E, Balla T. Phosphatidylinositol-Phosphatidic Acid Exchange by Nir2 at ER-PM Contact Sites Maintains Phosphoinositide Signaling Competence. Dev Cell. 2015;33:549–561. doi: 10.1016/j.devcel.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yadav S, Garner K, Georgiev P, Li M, Gomez-Espinosa E, Panda A, Mathre S, Okkenhaug H, Cockcroft S, Raghu P. RDGBalpha, a PtdIns-PtdOH transfer protein, regulates G-protein-coupled PtdIns(4,5)P2 signalling during Drosophila phototransduction. J Cell Sci. 2015;128:3330–3344. doi: 10.1242/jcs.173476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chang CL, Liou J. Homeostatic regulation of the PI(4,5)P2-Ca(2+) signaling system at ER-PM junctions. Biochim Biophys Acta. 2016;1861:862–873. doi: 10.1016/j.bbalip.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cockcroft S, Raghu P. Topological organisation of the phosphatidylinositol 4,5-bisphosphate-phospholipase C resynthesis cycle: PITPs bridge the ER-PM gap. Biochem J. 2016;473:4289–4310. doi: 10.1042/BCJ20160514C. [DOI] [PubMed] [Google Scholar]
- 152.Keinan O, Kedan A, Gavert N, Selitrennik M, Kim S, Karn T, Becker S, Lev S. The lipid-transfer protein Nir2 enhances epithelial-mesenchymal transition and facilitates breast cancer metastasis. J Cell Sci. 2014;127:4740–4749. doi: 10.1242/jcs.155721. [DOI] [PubMed] [Google Scholar]
- 153.Treves S, Feriotto G, Moccagatta L, Gambari R, Zorzato F. Molecular cloning, expression, functional characterization, chromosomal localization, and gene structure of junctate, a novel integral calcium binding protein of sarco(endo)plasmic reticulum membrane. The Journal of biological chemistry. 2000;275:39555–39568. doi: 10.1074/jbc.M005473200. [DOI] [PubMed] [Google Scholar]
- 154.Treves S, Franzini-Armstrong C, Moccagatta L, Arnoult C, Grasso C, Schrum A, Ducreux S, Zhu MX, Mikoshiba K, Girard T, Smida-Rezgui S, Ronjat M, Zorzato F. Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. The Journal of cell biology. 2004;166:537–548. doi: 10.1083/jcb.200404079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Srikanth S, Jew M, Kim KD, Yee MK, Abramson J, Gwack Y. Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1) Proc Natl Acad Sci U S A. 2012;109:8682–8687. doi: 10.1073/pnas.1200667109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Treves S, Vukcevic M, Griesser J, Armstrong CF, Zhu MX, Zorzato F. Agonist-activated Ca2+ influx occurs at stable plasma membrane and endoplasmic reticulum junctions. J Cell Sci. 2010;123:4170–4181. doi: 10.1242/jcs.068387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Krapivinsky G, Krapivinsky L, Stotz SC, Manasian Y, Clapham DE. POST, partner of stromal interaction molecule 1 (STIM1), targets STIM1 to multiple transporters. Proc Natl Acad Sci U S A. 2011;108:19234–19239. doi: 10.1073/pnas.1117231108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fujii Y, Shiota M, Ohkawa Y, Baba A, Wanibuchi H, Kinashi T, Kurosaki T, Baba Y. Surf4 modulates STIM1-dependent calcium entry. Biochem Biophys Res Commun. 2012;422:615–620. doi: 10.1016/j.bbrc.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 159.Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E. SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell. 2012;149:425–438. doi: 10.1016/j.cell.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 160.Miao Y, Miner C, Zhang L, Hanson PI, Dani A, Vig M. An essential and NSF independent role for alpha-SNAP in store-operated calcium entry. eLife. 2013;2:e00802. doi: 10.7554/eLife.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maleth J, Choi S, Muallem S, Ahuja M. Translocation between PI(4,5)P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nature communications. 2014;5:5843. doi: 10.1038/ncomms6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Albarran L, Lopez JJ, Amor NB, Martin-Cano FE, Berna-Erro A, Smani T, Salido GM, Rosado JA. Dynamic interaction of SARAF with STIM1 and Orai1 to modulate store-operated calcium entry. Scientific reports. 2016;6:24452. doi: 10.1038/srep24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li P, Miao Y, Dani A, Vig M. alpha-SNAP regulates dynamic, on-site assembly and calcium selectivity of Orai1 channels. Molecular biology of the cell. 2016;27:2542–2553. doi: 10.1091/mbc.E16-03-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Quintana A, Rajanikanth V, Farber-Katz S, Gudlur A, Zhang C, Jing J, Zhou Y, Rao A, Hogan PG. TMEM110 regulates the maintenance and remodeling of mammalian ER-plasma membrane junctions competent for STIM-ORAI signaling. Proc Natl Acad Sci U S A. 2015;112:E7083–7092. doi: 10.1073/pnas.1521924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca entry. Nature. 2013 doi: 10.1038/nature12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fox PD, Haberkorn CJ, Weigel AV, Higgins JL, Akin EJ, Kennedy MJ, Krapf D, Tamkun MM. Plasma membrane domains enriched in cortical endoplasmic reticulum function as membrane protein trafficking hubs. Molecular biology of the cell. 2013;24:2703–2713. doi: 10.1091/mbc.E12-12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cobb MM, Austin DC, Sack JT, Trimmer JS. Cell Cycle-dependent Changes in Localization and Phosphorylation of the Plasma Membrane Kv2.1 K+ Channel Impact Endoplasmic Reticulum Membrane Contact Sites in COS-1 Cells. J Biol Chem. 2015;290:29189–29201. doi: 10.1074/jbc.M115.690198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fox PD, Haberkorn CJ, Akin EJ, Seel PJ, Krapf D, Tamkun MM. Induction of stable ER-plasma-membrane junctions by Kv2.1 potassium channels. J Cell Sci. 2015;128:2096–2105. doi: 10.1242/jcs.166009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Petkovic M, Jemaiel A, Daste F, Specht CG, Izeddin I, Vorkel D, Verbavatz JM, Darzacq X, Triller A, Pfenninger KH, Tareste D, Jackson CL, Galli T. The SNARE Sec22b has a non-fusogenic function in plasma membrane expansion. Nat Cell Biol. 2014;16:434–444. doi: 10.1038/ncb2937. [DOI] [PubMed] [Google Scholar]
- 170.Lees JA, Messa M, Sun EW, Wheeler H, Torta F, Wenk MR, De Camilli P, Reinisch KM. Lipid transport by TMEM24 at ER-plasma membrane contacts regulates pulsatile insulin secretion. Science. 2017;355 doi: 10.1126/science.aah6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jozsef L, Tashiro K, Kuo A, Park EJ, Skoura A, Albinsson S, Rivera-Molina F, Harrison KD, Iwakiri Y, Toomre D, Sessa WC. Reticulon 4 is necessary for endoplasmic reticulum tubulation, STIM1-Orai1 coupling, and store-operated calcium entry. The Journal of biological chemistry. 2014;289:9380–9395. doi: 10.1074/jbc.M114.548602. [DOI] [PMC free article] [PubMed] [Google Scholar]