Abstract
Background
The klotho gene was identified as an “aging-suppressor” gene that accelerates arterial calcification when disrupted. Serum and vascular klotho levels are reduced in patients with chronic kidney disease, and the reduced levels are associated with arterial calcification. Intake of eicosapentaenoic acid (EPA), an n-3 fatty acid, reduces the risk of fatal coronary artery disease. However, the effects of EPA on arterial calcification have not been fully elucidated. The aim of this study was to determine the effect of EPA on arterial calcification in klotho mutant mice.
Methods and results
Four-week-old klotho mutant mice and wild-type (WT) mice were given a diet containing 5% EPA (EPA food, klotho and WT: n = 12, each) or not containing EPA (control food, klotho and WT: n = 12, each) for 4 weeks. Calcium volume scores of thoracic and abdominal aortas assessed by computed tomography were significantly elevated in klotho mice after 4 weeks of control food, but they were not elevated in klotho mice after EPA food or in WT mice. Serum levels of EPA and resolvin E1, an active metabolite of EPA, in EPA food-fed mice were significantly increased compared to those in control food-fed mice. An oxidative stress PCR array followed by quantitative PCR revealed that NADPH oxidase-4 (NOX4), an enzyme that generates superoxide, gene expression was up-regulated in arterial smooth muscle cells (SMCs) of klotho mice. Activity of NOX was also significantly higher in SMCs of klotho mice than in those of WT mice. EPA decreased expression levels of the NOX4 gene and NOX activity. GPR120, a receptor of n-3 fatty acids, gene knockdown by siRNA canceled effects of EPA on NOX4 gene expression and NOX activity in arterial SMCs of klotho mice.
Conclusions
EPA prevents arterial calcification together with reduction of NOX gene expression and activity via GPR120 in klotho mutant mice.
Introduction
Vascular calcification increases with aging and is highly prevalent in patients with atherosclerosis, diabetes mellitus and chronic kidney disease (CKD) [1]. Coronary artery calcium assessed by computed tomography (CT) provides independent incremental information in addition to traditional risk factors for the prediction of coronary heart disease and all-cause mortality [2, 3].
The klotho gene was identified as an “aging-suppressor” gene in mice, and it was shown that disruption of the gene results in acceleration of arterial calcification [4]. We and other investigators have reported that expression levels of serum and local vascular klotho are reduced in patients with CKD and that the decrease in expression level of klotho is associated with arterial calcification and stiffness in patients with CKD [5–7].
Intake of eicosapentaenoic acid (EPA), an n-3 fatty acid, reduces the risk of fatal coronary artery disease [8]. Several studies have revealed that EPA prevents vascular calcification. EPA attenuates arterial medial calcification in warfarin-induced rat models.[9] EPA prevents vascular calcification by inhibiting palmitic acid-induced mineralization of human arterial smooth muscle cells (SMCs) [10]. However, the effects of EPA on arterial calcification such as an association with klotho have not been fully elucidated.
The aim of this study was to determine the effect of EPA on arterial calcification assessed by CT in klotho mutant (kl/kl) mice. Furthermore, since oxidative stress is associated with the development of vascular calcification [11, 12], we assessed the effects of EPA on gene expression related to oxidative stress in SMCs of kl/kl mice.
Materials and methods
Animals
Klotho homozygous mutant (kl/kl) mice were purchased from CLEA Japan. Four-week-old klotho mutant (kl/kl) mice (n = 24, 12 males & 12 females) and wild-type (WT) mice (n = 24, 12 males & 12 females) were given a diet containing 5% EPA (Mochida Pharmaceutical Co. Ltd) (EPA food, klotho and WT: n = 12, each) or not containing EPA (control food, klotho and WT: n = 12, each) for 4 weeks. All animal protocols were approved and conducted according to the recommendations of Okayama University on Animal Care and Use. The animal procedures performed conform to the NIH guidelines (Guide for the Care and Use of Laboratory Animals).
CT Image acquisition and aortic calcification volume quantification
The mice were anesthetized with inhalation of isoflurane. Image acquisitions were performed using multi-detector CT (FX3000 Pre-Clinical Imaging System, TriFoil Imaging Inc.) before and after 4 weeks of feeding. All images were acquired during an inspiratory breath hold, with tube voltage of 120 kV and single-slice thickness of 192 μm.
Calcium volume score of the thoracic and abdominal aorta was calculated by multiplying the number of voxels (Vn) with the voxel volume (Vv)[13] using the volume-rendering method by extracting the area ≥400 Hounsfield units within the entire aorta.
Serum levels of EPA, arachidonic acid, inorganic phosphorus, calcium and resolvin E1
Serum levels of EPA and arachidonic acid (AA) were measured by gas chromatographic assay (SRL Inc. Tokyo). Serum inorganic phosphorus (Pi) levels were measured by method using molybdate (SRL Inc. Tokyo). Serum levels of calcium (Ca) were determined by method using arsenazo III (SRL Inc. Tokyo). Serum mouse resolvin E1 levels were measured using a commercially available enzyme-linked immunosorbent assay kit (MyBioSource Inc., San Diego, USA).
Culture of arterial SMCs
After CT image acquisitions, all animals were anaesthetized and euthanized with an intraperitoneal injection of pentobarbital (50 mg/kg). Then the thoracic and abdominal aortas were removed from kl/kl mice and WT mice. SMCs were isolated from the thoracic and abdominal aortas by the explant culture method as described previously [14–16]. Thoracic and abdominal aortas were disaggregated with collagenase and cut into 2-mm-long sections, and then the adventitia layer was removed. Vessels were plated on a 6-well plate with Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Sigma) and 0.1 mg/mL kanamycin (Sigma) and incubated in a humidified 5% CO2 atmosphere at 37°C. The culture medium was changed every 3 days. After reaching confluence, the cells were subcultured by treatment with trypsin (0.05%)/ethylenediaminetetraacetic acid (EDTA) (0.02%). Cells between passages 3 to 5 were used for all experiments.
Mouse oxidative stress PCR array and quantitative PCR
Oxidative stress-focused gene expression profiling of SMCs of a kl/kl mouse and a WT mouse was performed with the RT2 Profiler PCR Array System using the mouse oxidative stress PCR array (SABiosciences, a QIAGEN company) according to the manufacturer’s instructions. The array measures 84 key genes involved in oxidative stress. Total RNA from arterial SMCs was extracted using RNeasy Mini Kit (QIAGEN). Complementary DNA was synthesized from 1 μg of total RNA using ReverTra Ace (Toyobo Life Science, Tokyo) as prescribed in the manual and subjected to PCR amplification. Expression of mRNA was measured by reverse transcription PCR (RT-PCR) using an ABI PRISM 7300 sequence detector system (Applied Biosystems).
For quantitative PCR, arterial SMCs were reseeded in a 10-cm culture dish at a density of 5 x 104 cells/well. After 24 hours, arterial SMCs were treated with EPA (20 μmol/L) (Sigma) dissolved in DMSO (Sigma) or 0.08% DMSO as a control. After 24 hours of incubation, total RNA was extracted from the SMCs and complementary DNA was synthesized as described above. Quantitative RT-PCR was performed with primers for cytoglobin (Cygb) (PPM28233A, SABiosciences, a QIAGEN company), glutathione peroxidase 3 (GPX3) (PPM06171A, SABiosciences, a QIAGEN company) or GAPDH (PPM02946E, SABiosciences, a QIAGEN company) in combination with RT2 SYBR Green qPCR Master Mix (SABiosciences, a QIAGEN company). Expression of mRNA was measured by RT-PCR using an ABI PRISM 7300 sequence detector system (Applied Biosystems). The quantitative PCR data were processed by a standard curve method. Expression levels were normalized against GAPDH.
RT-PCR and quantitative PCR of NAD(P)H Oxidase 4 (NOX4) gene
Arterial SMCs were reseeded in 10-cm culture dish at a density of 5 x 104 cells/well. After 24 hours, arterial SMCs were treated with EPA (20 μmol/L) (Sigma) dissolved in DMSO (Sigma) or 0.08% DMSO as a control. After 24 hours of incubation, total RNA was isolated from the SMCs using TRIzol (Life Technologies Japan). Reverse transcriptase reactions were performed using a Ready-To-Go T-Primed First-Strand Kit (GE Healthcare Japan, Tokyo, Japan) for first-strand cDNA synthesis. Real-time quantitative PCR was performed using the ABI Prism 7700 sequence detection system (Life Technologies Japan). Data were expressed as copy number relative to that of 18 S rRNA. The primers and probe used for TaqMan analysis of mouse Nox4 were described in our previous report [17]. TaqMan probes consist of the fluorophore 6-carboxyfluorescein (FAM) covalently attached to the 5’ end of the oligonucleotide probe and the quencher tetramethylrhodamine (TAMRA) at the 3’ end. In detail, the primers and probe for mouse NOX4 were as follows: 5’-cctttgcctccattctcaag-3’ (forward primer), 5’-caggtctgcaaaccactcaa-3’ (reverse primer) and 5′-FAM-ctggctgtgcagggacacgc-TAMRA-3’ (TaqMan probe).
Lucigenin chemiluminescence assay of NAD(P)H Oxidase (NOX) activity
Arterial SMCs were prepared in the same manner as that described for quantitative PCR of NOX4. NOX activity of arterial SMCs was measured using lucigenin chemiluminescence (units/min/mg) as described previously [18]. Briefly, proteins from 5 × 104 SMCs were diluted in modified Hepes buffer (140 mmol/L NaCl, 5 mmol/L KCl, 0.8 mmol/L MgCl2, 1.8 mmol/L CaCl2, 1 mmol/L Na2HPO4, 25 mmol/L Hepes, and 1% glucose, pH 7.2) and distributed (100 mg per well) onto a 96-well microplate. NADPH (100 μmol/L) and dark-adapted lucigenin (5 μmol/L; Sigma-Aldrich Japan, Tokyo, Japan) were added just before reading. Lucigenin chemiluminescence was recorded for 5 min and was stopped by the addition of 50 mM Tiron (Sigma-Aldrich Japan, Tokyo, Japan) to observe how the chemiluminescence was detected as superoxide. Lucegenin chemiluminescence was expressed as units per minute per milligram of protein (unit/min/mg). The data are shown as relative chemiluminescence intensity to WT Control. Experiments were performed in triplicate.
Expression of G-protein-coupled receptor 120 (GPR120) mRNA
Total RNA was isolated from cultured SMCs using RNeasy Mini Kit (QIAGEN) as previously described [19]. First-strand cDNA was synthesized using ReverTra Ace (Toyobo Life Science, Tokyo). The primers for GPR120 were 5’-ccataaatctagtgctcgct-3’ (forward primer) and 5’-tgcggaagagtcggtagtct-3’ (reverse primer) as previously described [20].
GPR120 knockdown by siRNA
To knock down GPR120, 5 μmol/L small interfering RNA (siRAN, s200889, Ambion) was transfected into mouse vascular smooth muscle cells explanted from the aorta using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s instructions. We confirmed Gpr120 downregulation by the PCR method. Total RNA was extracted using Trizol (Invitrogen) and Purelink RNA Mini Kit (Invitrogen). Complementary DNA was synthesized from 1 μg total RNA using Superscript III with Oligo(dT) primers (Invitrogen) according to the manufacturer’s instructions and subjected to PCR amplification. Taq DNA polymerase (Roche Applied Science) was used for RT-PCR. PCR products were subjected to electrophoresis in 2% agarose gels and stained with ethidium bromide. Primer pairs were as follows: GPR120 forward, tgcccctctgcatcttgttc; GPR120 reverse, cgcgatgctttcgtgatctg; GAPDH forward, catggccttccgtgttccta; and GAPDH reverse, tgcctgcttcaccaccttct. PCR product sizes were 202 bp and 106 bp, respectively, and the annealing temperature was 60°C.
Statistical analysis
Data are expressed as mean ± standard error (SE). Data that were not normally distributed are expressed as median and interquartile range (IQR: 25%-75%). Statistical analysis was performed by Student’s t test or the chi-squared test for paired data or one-way ANOVA with comparison of different groups by Dunnett’s post hoc test. Values of P < 0.05 were considered to be significant.
Results
Changes in calcium volume score
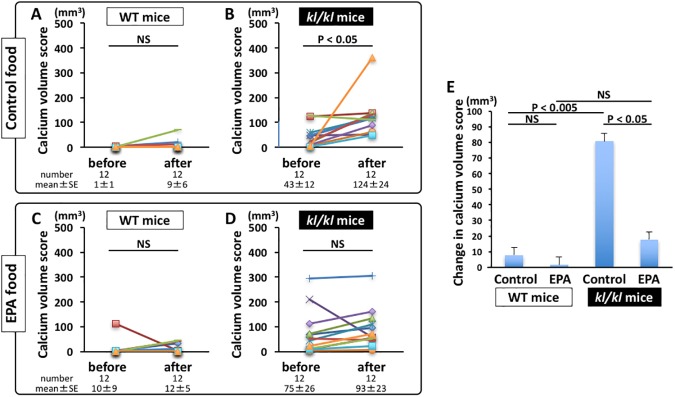
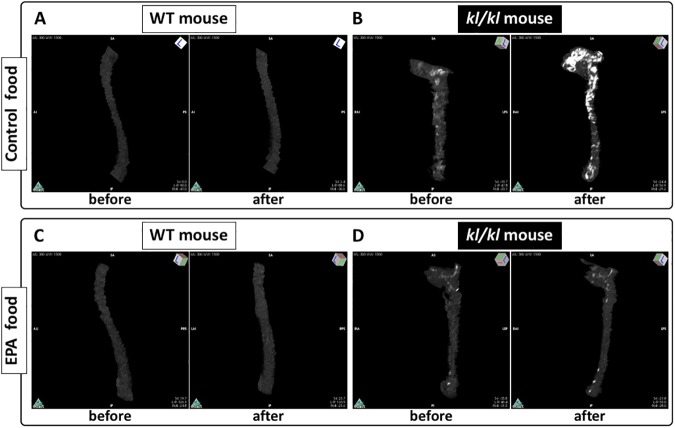
Calcium volume scores were significantly elevated in kl/kl mice after 4 weeks of control food (before vs after feeding, P < 0.05) (Fig 1B), but they were not elevated in kl/kl mice after 4 weeks of EPA food (before vs after feeding, P = NS) (Fig 1D). Calcium volume scores were not changed in WT mice after 4 weeks of control or EPA food (Fig 1A and 1C). The change in calcium volume score in control food-fed kl/kl mice (81±28 mm3) was significantly greater than that in control food-fed WT mice (8±6 mm3, P < 0.005) and that in EPA-fed kl/kl mice (18±17 mm3, P < 0.05) (Fig 1E). Fig 2 and S1 to S4 Movies show representative CT images of the thoracic and abdominal aortas in kl/kl and WT mice. Arterial calcification was elevated in klotho mice after 4 weeks of control food, but EPA prevented arterial calcification in kl/kl mice.
Fig 1. Changes in calcium volume score.
A and B, Calcium volume scores in wild-type (WT) mice (A) and klotho mutant (kl/kl) mice (B) before and after 4 weeks of control food (n = 12, each). C and D, Calcium volume scores in WT mice (C) and kl/kl mice (D) before and after 4 weeks of EPA food (n = 12, each). E, Changes in calcium volume score in WT mice and kl/kl mice before and after 4 weeks of control and EPA food (n = 12, each).
Fig 2. Representative CT images of thoracic and abdominal aortas in klotho mutant (kl/kl) and wild-type (WT) mice.
A and B, a WT mouse (A) and a kl/kl mouse (B) before and after 4 weeks of control food (S1 and S2 Movies). C and D, a WT mouse (C) and a kl/kl mouse (D) before and after 4 weeks of EPA food (S3 and S4 Movies).
Serum levels of EPA, AA, P, Ca and resolvin E1
Serum levels of EPA, arachidonic acid (AA), inorganic phosphorus (P) and calcium (Ca) (Table 1). Serum levels of EPA in EPA-fed kl/kl and WT mice were significantly increased compared to those in control-fed kl/kl and WT mice, and serum levels of AA in EPA-fed kl/kl and WT mice were significantly decreased compared to those in control-fed kl/kl and WT mice. The ratios of EPA to AA (EPA/AA) in EPA-fed kl/kl and WT mice were significantly larger than those in control-fed kl/kl and WT mice.
Table 1. Serum levels of EPA, AA, P and Ca.
| WT mice | kl/kl mice | |||||
|---|---|---|---|---|---|---|
| Control food | EPA food | P value | Control food | EPA food | P value | |
| EPA (μg/mL) | 7.5 (18.1) | 376.9 (142.0) | < 0.001 | 8.8 (7.9) | 385.8 (169.8) | < 0.001 |
| AA (μg/mL) | 464.3 (224.5) | 32.4 (11.53) | < 0.05 | 387.0 (191.2) | 41.8 (20.0) | < 0.001 |
| EPA/AA | 0.01 (0.05) | 11.13 (4.42) | < 0.01 | 0.02 (0.03) | 8.08 (4.53) | < 0.001 |
| P (mg/dL) | 8.3±0.9 | 7.9±1.4 | NS | 16.4±1.5* | 15.2±1.0# | NS |
| Ca (mg/dL) | 8.9±0.2 | 8.9±0.2 | NS | 9.7±0.3 | 9.3±0.4 | NS |
WT: wild-type, kl/kl: klotho homozygous mutant, EPA: eicosapentaenoic acid, AA: arachidonic acid, P: phosphorus, Ca: calcium, NS: not significant.
*P<0.001: control food-fed WT vs kl/kl mice.
#P<0.001: EPA-fed WT vs kl/kl mice. Data are expressed as median (IQR) or mean ± SE.
Serum levels of P in control-fed klotho mice were increased compared to those in control-fed WT mice. EPA intake did not change the levels in klotho and WT mice. These results indicate that preventative effects of EPA on arterial calcification are not due to effects for negative phosphate balance.
Resolvin E1 is a lipid-derived mediator that is endogenously synthesized from EPA and is generated in response to inflammation and enhances the resolution phase of inflammation [21]. We measured serum levels of resolvin E1 in control food-fed and EPA food-fed WT mice. Serum levels of resolvin E1 in EPA-fed mice (n = 5) were significantly increased compared to those in control-fed mice (n = 6) (control: 1555±95 versus EPA: 1874±56 pg/mL, P = 0.01).
Gene expression and activity of NOX
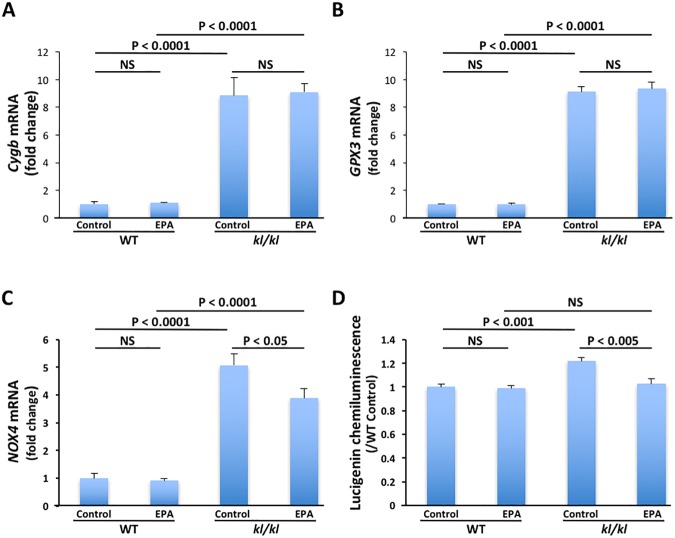
To further assess the mechanism of arterial calcification on kl/kl mice, we investigated the involvement of oxidative stress in arterial SMCs using the mouse oxidative stress and antioxidant defense PCR array of RT2 Profiler PCR Array. Expression levels of apolipoprotein E (Apoe), NOX4, uncoupling protein 2 (Ucp2), heat shock protein 1A (Hspa1a), flavin-containing monooxygenase 2 (Fmo2), cytoglobin (Cygb) and glutathione peroxidase 3 (GPX3) genes in arterial SMCs of a kl/kl mouse were upregulated compared to those in arterial SMCs of a WT mouse (Table 2). To investigate the involvement of the top 2 upregulated genes, we performed quantitative RT-PCR of Cygb, a globin molecule with a protective function during oxidative stress, and GPX3, an enzyme having anti-oxidative activity, in arterial SMCs treated with EPA and not treated with EPA. Expression levels of the Cygb and GPX3 genes were significantly higher in arterial SMCs of kl/kl mice than in those of WT mice (Fig 3A and 3B). However, EPA did not decrease expression levels of the Cygb and GPX3 genes.
Table 2. Genes on RT2 Prodiler PCR-array for which expression is up- or down-regulated in arterial SMCs of kl/kl mice.
| Description | Symbol | Fold Regulation |
|---|---|---|
| Albumin | Alb | -3.398 |
| Amyotrophic lateral sclerosis 2 (juvenile) homolog (human) | Als2 | -1.007 |
| Aldehyde oxidase 1 | Aox1 | -2.090 |
| Adenomatosis polyposis coli | Apc | -1.048 |
| Apolipoprotein E | Apoe | 2.061 |
| Ataxia telangiectasia and rad3 related | Atr | 1.018 |
| Catalase | Cat | -1.376 |
| Chemokine (C-C motif) ligand 5 | Ccl5 | 1.018 |
| Copper chaperone for superoxide dismutase | Ccs | -1.181 |
| Cathepsin B | Ctsb | 1.121 |
| Cytochrome b-245, alpha polypeptide | Cyba | -1.403 |
| Cytoglobin | Cygb | 16.246 |
| Dynamin 2 | Dnm2 | 1.282 |
| Dual oxidase 1 | Duox1 | 1.959 |
| EH-domain containing 2 | Ehd2 | -1.089 |
| Eosinophil peroxidase | Epx | -1.053 |
| Excision repair cross-complementing rodent repair deficiency, complementation 2 | Ercc2 | 1.045 |
| Excision repair cross-complementing rodent repair deficiency, complementation 6 | Ercc6 | -1.154 |
| Fanconi anemia, complementation group C | Fancc | -1.069 |
| Flavin containing monooxygenase 2 | Fmo2 | 3.828 |
| Ferritin heavy chain 1 | Fth1 | 1.034 |
| Glutamate-cysteine ligase, catalytic subunit | Gclc | -2.853 |
| Glutamate-cysteine ligase, modifier subunit | Gclm | 1.125 |
| Glutathione peroxidase 1 | Gpx1 | 1.342 |
| Glutathione peroxidase 2 | Gpx2 | -1.979 |
| Glutathione peroxidase 3 | Gpx3 | 16.279 |
| Glutathione peroxidase 4 | Gpx4 | 1.317 |
| Glutathione peroxidase 5 | Gpx5 | -1.777 |
| Glutathione peroxidase 6 | Gpx6 | -1.204 |
| Glutathione peroxidase 7 | Gpx7 | 1.989 |
| Glutathione reductase | Gsr | 1.039 |
| Glutathione synthetase | Gss | 1.006 |
| Glutathione S-transferase kappa 1 | Gstk1 | -1.072 |
| Glutathione S-transferase, pi 1 | Gstp1 | -1.066 |
| Heme oxygenase (decycling) 1 | Hmox1 | -1.032 |
| Heat shock protein 1A | Hspa1a | 2.692 |
| Isocitrate dehydrogenase 1 (NADP+), soluble | Idh1 | 1.600 |
| Intraflagellar transport 172 homolog (Chlamydomonas) | Ift172 | 1.390 |
| Interleukin 19 | Il19 | -1.147 |
| Interleukin 22 | Il22 | -1.543 |
| Keratin 1 | Krt1 | -1.058 |
| Lactoperoxidase | Lpo | -1.640 |
| Myoglobin | Mb | -3.055 |
| Myeloperoxidase | Mpo | 1.011 |
| Neutrophil cytosolic factor 1 | Ncf1 | -2.478 |
| Neutrophil cytosolic factor 2 | Ncf2 | -1.985 |
| Neuroglobin | Ngb | -1.391 |
| Nitric oxide synthase 2, inducible | Nos2 | 1.016 |
| NADPH oxidase 1 | Nox1 | 1.826 |
| NADPH oxidase 4 | Nox4 | 2.080 |
| NADPH oxidase activator 1 | Noxa1 | 1.127 |
| NADPH oxidase organizer 1 | Noxo1 | -1.116 |
| NAD(P)H dehydrogenase, quinone 1 | Nqo1 | 1.624 |
| Parkinson disease (autosomal recessive, early onset) 7 | Park7 | 1.156 |
| Peroxiredoxin 1 | Prdx1 | -1.205 |
| Peroxiredoxin 2 | Prdx2 | -1.114 |
| Peroxiredoxin 3 | Prdx3 | -1.035 |
| Peroxiredoxin 4 | Prdx4 | 1.067 |
| Peroxiredoxin 5 | Prdx5 | -1.368 |
| Peroxiredoxin 6 | Prdx6 | -1.494 |
| Prion protein | Prnp | 1.454 |
| Proteasome (prosome, macropain) subunit, beta type 5 | Psmb5 | -1.160 |
| Prostaglandin-endoperoxide synthase 1 | Ptgs1 | -1.029 |
| Prostaglandin-endoperoxide synthase 2 | Ptgs2 | -2.986 |
| Recombination activating gene 2 | Rag2 | -11.046 |
| RecQ protein-like 4 | Recql4 | 1.091 |
| Stearoyl-Coenzyme A desaturase 1 | Scd1 | 1.491 |
| Serine (or cysteine) peptidase inhibitor, clade B, member 1b | Serpinb1b | 1.938 |
| Solute carrier family 38, member 1 | Slc38a1 | 1.162 |
| Superoxide dismutase 1, soluble | Sod1 | -1.258 |
| Superoxide dismutase 2, mitochondrial | Sod2 | 1.293 |
| Superoxide dismutase 3, extracellular | Sod3 | 1.470 |
| Sequestosome 1 | Sqstm1 | -1.400 |
| Sulfiredoxin 1 homolog (S. cerevisiae) | Srxn1 | -1.423 |
| Thyroid peroxidase | Tpo | -1.223 |
| Thioredoxin 1 | Txn1 | -1.687 |
| Thioredoxin interacting protein | Txnip | -1.203 |
| Thioredoxin reductase 1 | Txnrd1 | -1.399 |
| Thioredoxin reductase 2 | Txnrd2 | -1.518 |
| Thioredoxin reductase 3 | Txnrd3 | 1.316 |
| Uncoupling protein 2 (mitochondrial, proton carrier) | Ucp2 | 2.547 |
| Uncoupling protein 3 (mitochondrial, proton carrier) | Ucp3 | -1.173 |
| Vimentin | Vim | 1.370 |
| Xeroderma pigmentosum, complementation group A | Xpa | 1.372 |
Fig 3. Gene expression of cytoglobin (Cygb), glutathione peroxidase 3 (GPX3) and NADPH oxidase (NOX) and activity of NOX.
A to C, Expression levels of Cygb (A), GPX3 (B) and NOX4 (C) genes in arterial smooth muscle cells (SMCs) of wild-type (WT) and klotho mutant (kl/kl) mice treated with EPA and not treated with EPA (control) (n = 6, each). D, NOX activity in arterial SMCs of WT and kl/kl mice treated with EPA and not treated with EPA (control) (n = 6, each).
NOX is an enzyme that produces superoxide and plays a pivotal role in generation of oxidative stress in vascular SMCs [22]. Other upregulated genes in PCR array are related to proteins that have antioxidant properties. To clarify the involvement of NOX, we performed quantitative RT-PCR of NOX4, a component of NOX, and measured NOX activity in arterial SMCs treated with EPA and not treated with EPA. Expression level of the NOX4 gene and activity of NOX were significantly higher in arterial SMCs of kl/kl mice than in those of WT mice (Fig 3C and 3D). EPA decreased expression levels of the NOX4 gene and NOX activity.
GPR120 and NOX
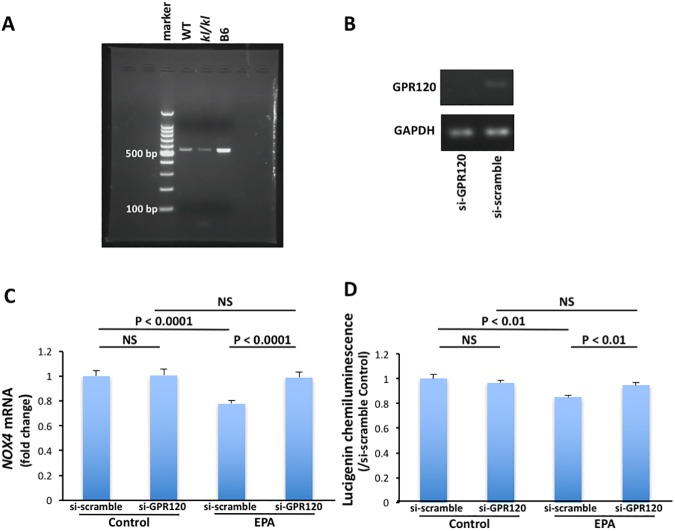
G-protein-coupled Receptor 120 (GPR120) is a receptor for n-3 fatty acids [20]. Activation of GPR120 by n-3 fatty acids inhibits inflammation cascades and preserves insulin sensitivity. We hypothesized that inhibitory effects of EPA on NOX expression and activity might be actions on arterial SMCs via GPR120. mRNA of the gene encoding GPR120 was expressed in arterial SMCs obtained from kl/kl and WT mice (Fig 4A).
Fig 4. GPR120 and NADPH oxidase (NOX) in arterial smooth muscle cells.
A, Expression of the GPR120 gene. Lane 1, maker; lane 2, wild-type (WT) mouse; lane 3, klotho mutant (kl/kl) mouse and lane 4, C57/BL6 mouse (positive control). B, GPR120 downregulation by siRNA. Lane 1, si-GPR120 RNA and lane 2, s-scramble RNA. C, Effects of GPR120 gene knockdown on NOX4 gene expression and NOX activity in arterial SMCs of kl/kl mice (n = 6, each).
Next, we investigated effects of GPR120 gene knockdown on NOX4 gene expression and NOX activity in arterial SMCs of kl/kl mice. GPR120 downregulation by siRNA canceled the effects of EPA on NOX4 gene expression and NOX activity in arterial SMCs of kl/kl mice (Fig 4B, 4C and 4D).
Discussion
Two major findings were obtained in the present study. First, EPA intake prevented arterial calcification in klotho mutant mice. Second, NOX gene expression and activity were elevated in arterial SMCs of klotho mutant mice and EPA reduced them via GPR120.
Elevated oxidative stress is associated with the development of vascular calcification [11, 12]. Oxidative stress induces vascular SMC calcification by upregulation of Runx2, which is mediated by activation of the AKT/FOXO1/3 signaling axis. Although there are several enzymatic sources of reactive oxygen species (ROS) in vascular cells, NOX is thought to be a major enzymatic source of ROS in atherosclerosis [23]. Gao et al reported that klotho deficiency upregulated NADPH oxidase activity and superoxide production in the media of aortas[24]. We also showed that NOX gene expression and activity were elevated in arterial SMCs of klotho mutant mice. Although the precise mechanism underlying the inhibitory effects of EPA on vascular calcification remains unclear, reduction of NOX activity by EPA might play an important role in prevention of vascular calcification in klotho mutant mice.
Changes of NOX4 gene expression and NOX activity were minor in our study using a cell culture system. Another mechanism might also contribute to the prevention of vascular calcification in vivo. Serum levels of resolvin E1, a bioactive metabolite of EPA, were increased in EPA food-fed mice. Resolvin E1 is responsible for facilitating the resolving phase of acute inflammation [25]. Furthermore, resolvin E1 reduces oxidative stress by suppressing NOX activation [26]. These effects might contribute to the prevention of vascular calcification. Further studies are needed to clarify this point.
Coronary artery calcium assessed by multi-detector CT reflects the plaque burden of coronary arteries, and it is an independent predictor of cardiovascular events and is associated with the incidence of congestive heart failure [2, 27]. Furthermore, one of the most appealing features of CT is the potential to detect the progression or regression of coronary atherosclerotic disease noninvasively [28]. CT also enables evaluation of vascular calcification in living mice [29]. We assessed changes in the degree of arterial calcification by multi-detector CT as recent clinical situations in this study. Our study showed that CT is useful for detecting the changes over time and by treatment in coronary calcification of living mice.
In the Japan EPA lipid intervention study (JELIS) in patients with hypercholesterolemia, on-treatment mean plasma EPA concentrations and EPA/AA were 170 μg/mL and 1.21, respectively in patients treated with EPA (1800 mg/day) [30]. Therefore, serum levels of EPA in EPA-fed mice were very high in this study (EPA-fed WT mice, median (IQR): 376.9 (142.0), mean ± SD: 373.4 ± 75.4 μg/mL; EPA-fed kl/kl mice, median (IQR): 385.8 (169.8), mean ± SD: 393.5 ± 150.1 μg/mL), and EPA/AA ratios in EPA-fed mice were also quite high (EPA-fed WT mice, median (IQR): 11.13 (4.42), mean ± SD: 11.57 ± 2.39; EPA-fed kl/kl mice, median (IQR): 8.08 (4.53), mean ± SD: 9.15 ± 3.32). A large amount of EPA might be needed for clinical application of this study. Since it was found in this study that EPA reduced NOX activity via GPR120, novel GPR120 agonists might also be useful for prevention of arterial calcification.
A decrease in the expression level of klotho is associated with arterial calcification and stiffness in patients with CKD [5–7]. Thus, preventative effects of EPA on atrial calcification might be expected in patients with CKD.
In conclusion, EPA intake prevents arterial calcification along with reduction of NOX gene expression and activity via GPR120 in klotho mutant mice.
Supporting information
(MOV)
(MOV)
(MOV)
(MOV)
Acknowledgments
The authors are grateful to Aya Miura, Kaoru Akazawa and Masayo Ohmori for their excellent technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Dr. Nakamura was supported by a grant from Vascular Biology Innovation Conference (4th).
References
- 1.Virmani R, Joner M, Sakakura K. Recent Highlights of ATVB: Calcification. Arterioscler Thromb Vasc Biol. 2014;34(7):1329–32. doi: 10.1161/ATVBAHA.114.304000 [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49(18):1860–70. doi: 10.1016/j.jacc.2006.10.079 [DOI] [PubMed] [Google Scholar]
- 3.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45. doi: 10.1056/NEJMoa072100 [DOI] [PubMed] [Google Scholar]
- 4.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- 5.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280(4):1015–20. doi: 10.1006/bbrc.2000.4226 [DOI] [PubMed] [Google Scholar]
- 6.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243–55. doi: 10.1161/CIRCULATIONAHA.111.053405 [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, et al. A decreased level of serum soluble klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8(2):e56695 doi: 10.1371/journal.pone.0056695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–8. doi: 10.1016/S0140-6736(07)60527-3 [DOI] [PubMed] [Google Scholar]
- 9.Kanai S, Uto K, Honda K, Hagiwara N, Oda H. Eicosapentaenoic acid reduces warfarin-induced arterial calcification in rats. Atherosclerosis. 2011;215(1):43–51. doi: 10.1016/j.atherosclerosis.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 10.Kageyama A, Matsui H, Ohta M, Sambuichi K, Kawano H, Notsu T, et al. Palmitic acid induces osteoblastic differentiation in vascular smooth muscle cells through ACSL3 and NF-kappaB, novel targets of eicosapentaenoic acid. PLoS One. 2013;8(6):e68197 doi: 10.1371/journal.pone.0068197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283(22):15319–27. doi: 10.1074/jbc.M800021200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng L, Huang L, Sun Y, Heath JM, Wu H, Chen Y. Inhibition of FOXO1/3 promotes vascular calcification. Arterioscler Thromb Vasc Biol. 2015;35(1):175–83. doi: 10.1161/ATVBAHA.114.304786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callister TQ, Cooil B, Raya SP, Lippolis NJ, Russo DJ, Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208(3):807–14. doi: 10.1148/radiology.208.3.9722864 [DOI] [PubMed] [Google Scholar]
- 14.Kouchi H, Nakamura K, Fushimi K, Sakaguchi M, Miyazaki M, Ohe T, et al. Manumycin A, inhibitor of ras farnesyltransferase, inhibits proliferation and migration of rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1999;264(3):915–20. doi: 10.1006/bbrc.1999.1546 [DOI] [PubMed] [Google Scholar]
- 15.Ogawa A, Nakamura K, Matsubara H, Fujio H, Ikeda T, Kobayashi K, et al. Prednisolone inhibits proliferation of cultured pulmonary artery smooth muscle cells of patients with idiopathic pulmonary arterial hypertension. Circulation. 2005;112(12):1806–12. doi: 10.1161/CIRCULATIONAHA.105.536169 [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K, Shimizu J, Kataoka N, Hashimoto K, Ikeda T, Fujio H, et al. Altered nano/micro-order elasticity of pulmonary artery smooth muscle cells of patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140(1):102–7. doi: 10.1016/j.ijcard.2008.11.022 [DOI] [PubMed] [Google Scholar]
- 17.Nagasu H, Satoh M, Kiyokage E, Kidokoro K, Toida K, Channon KM, et al. Activation of endothelial NAD(P)H oxidase accelerates early glomerular injury in diabetic mice. Lab Invest. 2016;96(1):25–36. doi: 10.1038/labinvest.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A. 2006;103(19):7432–7. doi: 10.1073/pnas.0510444103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda T, Nakamura K, Akagi S, Kusano KF, Matsubara H, Fujio H, et al. Inhibitory effects of simvastatin on platelet-derived growth factor signaling in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. J Cardiovasc Pharmacol. 2010;55(1):39–48. doi: 10.1097/FJC.0b013e3181c0419c [DOI] [PubMed] [Google Scholar]
- 20.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–98. doi: 10.1016/j.cell.2010.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyurko R, Van Dyke TE. The role of polyunsaturated omega-3 fatty acid eicosapentaenoic acid-derived resolvin E1 (RvE1) in bone preservation. Crit Rev Immunol. 2014;34(4):347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. [DOI] [PubMed] [Google Scholar]
- 23.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110(10):1364–90. doi: 10.1161/CIRCRESAHA.111.243972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, et al. Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity. Hypertension. 2016;68(5):1191–9. doi: 10.1161/HYPERTENSIONAHA.116.07709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–61. doi: 10.1038/nri2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takamiya R, Fukunaga K, Arita M, Miyata J, Seki H, Minematsu N, et al. Resolvin E1 maintains macrophage function under cigarette smoke-induced oxidative stress. FEBS Open Bio. 2012;2:328–33. doi: 10.1016/j.fob.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osawa K, Miyoshi T, Oe H, Sato S, Nakamura K, Kohno K, et al. Association between coronary artery calcification and left ventricular diastolic dysfunction in elderly people. Heart Vessels. 2016;31(4):499–507. doi: 10.1007/s00380-015-0645-5 [DOI] [PubMed] [Google Scholar]
- 28.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339(27):1972–8. doi: 10.1056/NEJM199812313392703 [DOI] [PubMed] [Google Scholar]
- 29.Wait JM, Tomita H, Burk LM, Lu J, Zhou OZ, Maeda N, et al. Detection of aortic arch calcification in apolipoprotein E-null mice using carbon nanotube-based micro-CT system. J Am Heart Assoc. 2013;2(1):e003358 doi: 10.1161/JAHA.112.003358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itakura H, Yokoyama M, Matsuzaki M, Saito Y, Origasa H, Ishikawa Y, et al. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18(2):99–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(MOV)
(MOV)
(MOV)
(MOV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.