Abstract
Bacteria, yeast and human cancer cells possess mechanisms of mutagenesis upregulated by stress responses. Stress-inducible mutagenesis potentially accelerates adaptation, and may provide important models for mutagenesis that drives cancers, host pathogen interactions, antibiotic resistance and possibly much of evolution generally. In Escherichia coli repair of double-strand breaks (DSBs) becomes mutagenic, using low-fidelity DNA polymerases under the control of the SOS DNA-damage response and RpoS general stress response, which upregulate and allow the action of error-prone DNA polymerases IV (DinB), II and V to make mutations during repair. Pol IV is implied to compete with and replace high-fidelity DNA polymerases at the DSB-repair replisome, causing mutagenesis. We report that up-regulated Pol IV is not sufficient for mutagenic break repair (MBR); damaged bases in the DNA are also required, and that in starvation-stressed cells, these are caused by reactive-oxygen species (ROS). First, MBR is reduced by either ROS-scavenging agents or constitutive activation of oxidative-damage responses, both of which reduce cellular ROS levels. The ROS promote MBR other than by causing DSBs, saturating mismatch repair, oxidizing proteins, or inducing the SOS response or the general stress response. We find that ROS drive MBR through oxidized guanines (8-oxo-dG) in DNA, in that overproduction of a glycosylase that removes 8-oxo-dG from DNA prevents MBR. Further, other damaged DNA bases can substitute for 8-oxo-dG because ROS-scavenged cells resume MBR if either DNA pyrimidine dimers or alkylated bases are induced. We hypothesize that damaged bases in DNA pause the replisome and allow the critical switch from high fidelity to error-prone DNA polymerases in the DSB-repair replisome, thus allowing MBR. The data imply that in addition to the indirect stress-response controlled switch to MBR, a direct cis-acting switch to MBR occurs independently of DNA breakage, caused by ROS oxidation of DNA potentially regulated by ROS regulators.
Author summary
Mutagenesis mechanisms upregulated by stress responses promote de novo antibiotic resistance and cross resistance in bacteria, anti-fungal-drug resistance in yeasts, and genome instability in cancer cells under hypoxic stress. Stress-induced mutagenesis is implicated as the main source of spontaneous mutagenesis that drives bacterial evolution, and may drive much of evolution generally. A widely useful model mechanism is mutagenic DNA break repair in Escherichia coli, in which activation of two stress responses allows error-prone DNA polymerase in the break-repair replisome and introduce misincorporations, later fixed as mutations. Both stress responses upregulate the error-prone mutagenic DNA polymerase Pol IV (DinB), suggesting that the regulation of mutagenesis to times of stress is accomplished by indirect gene upregulation, followed by DNA polymerase competition. This paper describes the discovery that the stress responses are not sufficient to allow mutagenesis caused by error-prone DNA polymerases in the break-repair replisome—damaged DNA bases must also be present—and that these are caused by reactive oxygen species in starving E. coli. We hypothesize that damaged bases may inhibit the progress of the highly processive and high fidelity replicative DNA polymerase, thus allowing the switch to error-prone DNA polymerases and mutagenesis. These findings suggest the possibility that the spontaneous mutation rate is regulated by the generation and removal of reactive oxygen, a very common byproduct of metabolism.
Introduction
Spontaneous mutations drive development of most cancers and their resistance to therapy, aging, pathogen escape from the immune response and antibiotics, and evolution generally. Although central in all of biology and many aspects of human health, the causes of spontaneous mutagenesis have long been elusive, and generally difficult to assign with confidence. Whereas many ways to increase or induce mutagenesis are known, the origins of spontaneous mutations in most organisms remain speculative [1, 2].
Spontaneous mutations could potentially form by many different mechanisms [1, 2]. An early proposal [3] was that most spontaneous mutations result from spontaneous DNA damage—that repair of damaged DNA is more mutagenic than standard DNA synthesis. The mutagenicity of DNA repair has been borne out, for example, by demonstrations that repair of DNA double-strand breaks (DSBs) is mutagenic in bacteria [4–8], yeast [9], and human cancer [10–12] (reviewed [13–16]). That DNA damage underlies much of spontaneous mutagenesis was supported by discoveries that yeast antimutator mutants, i.e., mutants with lower-than-normal spontaneous mutation rate, carry mutations in DNA-damage-survival genes [17]. These genes encode alternative error-prone DNA polymerases or proteins that assist them, allowing survival of DNA damage by replication over or extension from otherwise-replication-inhibiting damaged DNA bases [18], implying that most spontaneous mutagenesis in yeast results from use of error-prone DNA polymerases during DNA-damage survival. To our knowledge, the only mechanistic detail available on spontaneous mutagenesis mechanisms is that about half of all spontaneous base-substitutions and small insertion/deletions (indels) in starving Escherichia coli form dependently on the proteins used in stress-inducible mutagenic DNA break repair (MBR) [7].
In MBR, alternative error-prone DNA polymerases appear to be switched into the DSB-repair replisome under the control of stress responses [6–8] (reviewed [13, 15, 16]). The central features of this mechanism have been recapitulated biochemically with purified proteins [19]. Repair of DSBs by homologous recombination requires high-fidelity replicative DNA polymerase III in unstressed cells [20]. In MBR, DSB repair uses alternative error-prone DNA polymerases, principally Pol IV (DinB) [21], but also Pols II [22] and V [7, 23], under the control of the SOS DNA-damage response and the general/starvation stress response controlled by the RpoS transcriptional activator. The sequence signature of RpoS-dependent MBR is evident across bacterial genomes [24], implicating this mechanism or similar mechanisms in most bacterial evolution. Moreover many stress-induced mutation mechanisms from bacteria to human cancer cells show similarities to E. coli MBR [13]. From these previous studies, it seemed probable that the critical step leading to MBR, and perhaps spontaneous mutagenesis generally, could be the switch from high-fidelity replicative DNA polymerases to error-prone DNA polymerases, resulting from DNA-polymerase competition promoted by upregulation of those DNA polymerases, in E. coli, by the SOS and general stress responses [13, 15, 16]. Here, we show that, for MBR, it is not sufficient to upregulate mutagenic DNA polymerases and repair DSBs; damaged DNA bases are also required.
Reactive oxygen species (ROS) are among the most common DNA-damaging agents, and are known to promote mutagenesis when in excess [25–27]. ROS attack essentially all macromolecules in cells, including DNA, RNA, proteins, and lipids [28–30]. ROS include superoxide () and its radical derivatives, which are detoxified by superoxide dismutases upregulated in E. coli by the SoxRS oxidative stress response [31]. ROS also include hydrogen peroxide (H2O2) and its radical derivatives including hydroxyl radicals (OH•), which are detoxified by catalases and alkylhydrogenperoxidases upregulated by the E. coli OxyRS oxidative stress response [30–32]. Oxidative damage to proteins is characterized by carbonylation (reviewed by [33]), whereas ROS damage to DNA includes mainly base modifications 7,8-dihydro-8-oxo-deoxyguanosine (8-oxo-dG) and thymine glycol, in addition to other modified bases and nicks [28, 29].
The presence of 8-oxo-dG in DNA results both from incorporation of oxidized dGTP from the deoxynucleotide triphosphate (dNTP) pool and from in situ oxidation of guanine (G) in DNA [34]. 8-oxo-dG is often incorporated opposite to, and templates incorporation of, an adenine (A) leading to A to C and G to T transversion mutations (e.g., [35, 36]). If 8-oxo-dG is incorporated into DNA, it can persist or be excised by base excision repair (BER). Although replication bypass of template 8-oxo-dG has been shown to occur with high efficiency [35, 37], it is found that the eukaryotic replicative polymerase pol δ is transiently inhibited at 8-oxo-G [38]. Pol δ is able to extend from the A:8-oxo-dG base pair, but not from C:8-oxo-dG. A switch to an alternative polymerase, often pol λ, allows extension from C:8-oxo-dG with no substitution mutation [38]. In E. coli, of the alternative DNA polymerases, Pol IV is responsible for most MBR mutations [21], and makes base substitutions and indel mutations, mostly 1 basepair deletions in mononucleotide repeats [39].
Pol IV is reported to make mutations in the absence of induced DNA damage when overproduced [39, 40], and SOS-upregulated levels of Pol IV are required for MBR [21, 41]. Here, we report that SOS- and general-stress-response induced overproduction of Pol IV is not sufficient for Pol IV-dependent MBR. Damaged bases in the DNA must also be present. We hypothesize that damaged bases allow the switch to use of Pol IV during MBR by pausing the replicative polymerase to allow polymerase exchange in the replisome. The findings suggest that spontaneous mutation by MBR is regulated by regulation of the intracellular level of ROS.
Results
MBR assay in E. coli
The E. coli Lac assay [42] for MBR quantifies both indels and gross chromosomal rearrangements (GCRs) as reversions of a lacI-Z+1bp frameshift allele in an F’ conjugative plasmid. Revertants are scored as Lac+ colonies formed over days of starvation on solid lactose minimal medium (e.g., Fig 1A, WT). The leaky lac allele reverts either by compensatory frameshift mutations (indels) or by amplification (GCRs) [43]. When placed under general/starvation-stress-response inducing conditions, or if RpoS is upregulated artificially [6,7], cells switch from faithful repair of DSBs by homologous recombination (HR) to an error-prone HR mechanism that requires the translesion DNA Pol IV, encoded by dinB [6, 7, 21, 44], leading to indel Lac+ revertants. The prevailing hypothesis for the GCR mechanism is that initial duplications of the lacI-Z+1bp allele are formed by a micro-homologous DSB-repair-instigated recombination event, followed by unequal crossing-over to give tandem arrays of 20 or more copies of the leaky lac allele [45, 46]. Both formation of GCRs and single-nucleotide alterations (SNAs, including base substitutions and indels) via MBR require DSBs [4, 6, 7, 47], HR DSB-repair proteins RecA, RuvABC [48, 49], and RecBC [4], activation of the general stress response [50, 51] and, at some genomic loci including lac, the RpoE (σE) unfolded membrane protein response [52]. Formation of SNAs also requires the SOS response, which promotes SNA MBR by its 10-fold transcriptional upregulation of the DinB error-prone translesion polymerase [41]. SOS and DinB play no role in GCR formation [21], which instead requires DNA polymerase I [45, 53].
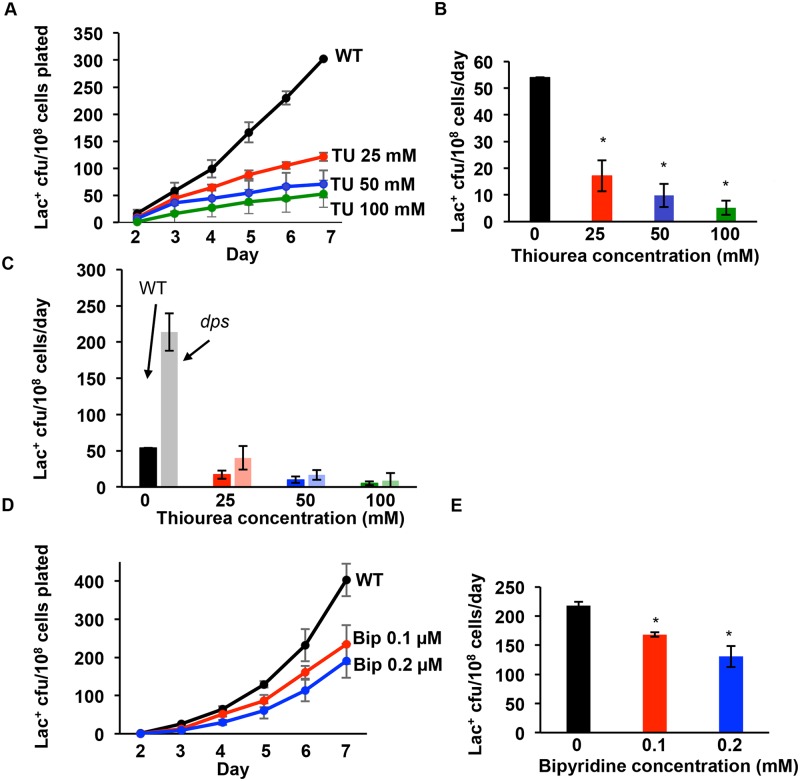
Fig 1. Exogenous ROS reducing agents reduce spontaneous mutagenic break repair.
(A) Thiourea (TU) reduces Lac reversion dose-dependently. Representative experiment showing the effects of varying doses of TU on the yield of Lac+ mutant cfu of SMR4562. (B) Quantification of mutation rates from 3 experiments, days 3 to 7. Adding 25, 50, and 100 mM TU to plates reduced mutagenesis 62% ± 5.6%, 82% ± 5.6% and 90% ± 4.3% respectively (p = 8.3x10-5, 1.7x10-5, and 5.2x10-5, compared with 0mM TU, Student’s 2-tailed t-test). (C) TU eliminated the hypermutation seen in Δdps strain cells dose-dependently. (D) 2'2-bipyridine (bip) reduces MBR dose-dependently. Representative experiment. (E) Either 0.1 or 0.2 μM bip reduced mutagenesis by 23% ± 4.0% and by 40% ±7.8%, respectively (p = 0.007 and 0.02, Student’s 2-tailed t-test). * indicates significantly different from the samples with zero dose of TU or bip. Strains used: wild type, SMR4562; Δdps, PJH2608.
Reduction of ROS through exogenous agents reduces MBR
We reduced ROS levels in cells undergoing MBR using 2,2’-bipyridine (bipyridine) and thiourea (TU), chemical agents commonly used to reduce ROS levels in living cells. Both agents enter cells and prevent ROS formation (bipyridine) or quench ROS (TU) [54]. Bipyridine chelates ferrous iron and prevents it from catalyzing ROS-forming Fenton reactions [55]. TU quenches hydroxyl radicals after formation, and prevents them from damaging macromolecules [56]. We added varying amounts of bipyridine (0.1 uM and 0.2 μM) or TU (25, 50 and 100 mM) to solid minimal lactose medium on which MBR occurs, and found that Lac+ mutation rates were reduced dose-dependently by either ROS reducer, with a 90% ± 4.3% reduction at 100 mM TU (Fig 1A and 1B) (p = 5.2x10-5, Student’s 2-tailed t-test). TU also prevented the previously reported increase in MBR in cells lacking Dps [57], a stationary-phase nucleoid-compaction protein that protects DNA against ROS, again in a dose-dependent manner (Fig 1C). There is a 40% ± 7.8% reduction caused by 0.2 μM bipyridine (Fig 1D and 1E) (p = 0.02, Student’s 2-tailed t-test). Neither bipyridine nor TU affected viability of Lac- mutation-reporter cells over the duration of incubation, nor did they affect the time required for formation of Lac+ colonies under precise reconstructions of experimental conditions (S1 Fig), indicating that mutation rate, not ability of treated cells to form colonies, was reduced by lowering ROS levels. All reductions in mutagenesis affected SNAs and amplification proportionately (an example is shown in Fig 2A). These data imply that ROS are required for MBR and suggest that Dps might inhibit mutagenesis by preventing ROS damage to DNA [57].
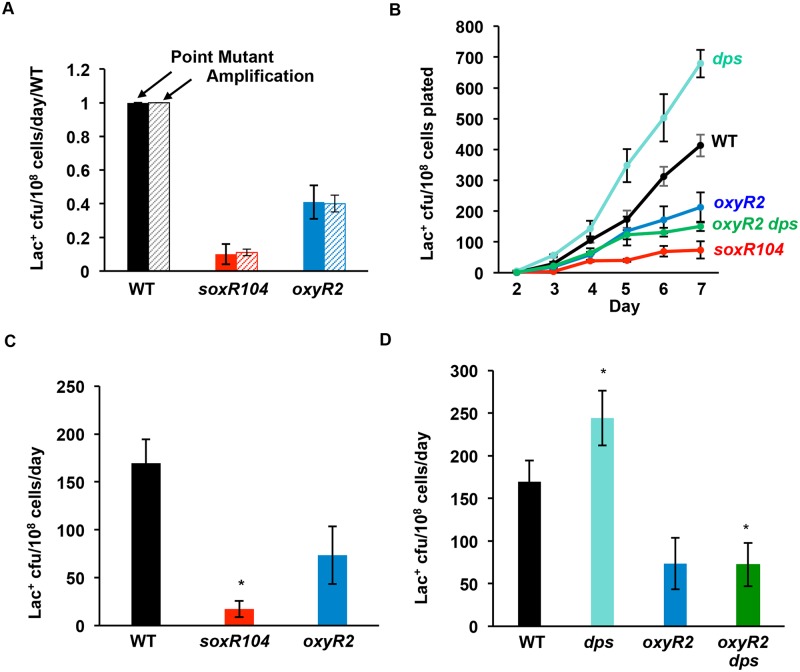
Fig 2. Constitutively active ROS detoxifying responses reduce MBR.
(A) Indels (point mutations) (SNAs) and amplification (GCR), depicted here normalized to the wild-type, are reduced proportionately by soxR104 and oxyR2 alleles, which constitutively activate the SoxR and OxyR responses respectively. Indels (SNAs) and GCR were distinguished (Methods) in all experiments, and no disproportionate effects were detected. (B) Representative experiment. (C) Quantification of MBR rates from three experiments: soxR104 reduced MBR by 90% ± 5.5% (p = 0.004, Student’s 2-tailed t-test). oxyR2 reduced MBR but not quite significantly at the 5% level when tested in the wild-type (59 ± 11%), p = 0.07, Student’s 2-tailed t-test). (D) oxyR2 reverses the hypermutation phenotype of Δdps cells. * indicates significantly different from wild-type (p ≤ 0.05). Wild-type, SMR4562, black; soxR104, PJH2947, red; oxyR2, PJH3278, blue; Δdps, PJH2608, pale green; oxyR2 Δdps, PJH3295, dark green. The data indicate that ROS are required for mutation formation, and that the hypermutation seen in Δdps cells depends on decreased protection from ROS.
Production of ROS-detoxifying enzymes reduces MBR
We reduced ROS levels in cells undergoing MBR by constitutive overexpression of either of two E. coli oxidative stress responses: the SoxRS response and the OxyRS response [31, 32]. Whereas each regulon responds to the presence of ROS in the cell, they employ different enzymes that detoxify different radical species. The SoxR response upregulates superoxide dismutases that inactivate superoxides and their derivatives [31], and the OxyR response upregulates catalases and alkylhydroperoxidases that inactivate hydrogen peroxide and its radical derivatives including hydroxyl radicals [32]. We used the oxyR2 [58] and soxR104 [59] alleles (separately), which cause constitutive expression of each response at induced levels, in the absence of oxidative inducers, to reduce ROS levels normally present during MBR. soxR104 reduced Lac+ MBR mutation rate, conferring a 90 ± 5.5% reduction (Fig 2B and 2C) (p = 0.004, Student’s 2-tailed t-test). Depression of MBR by oxyR2 (59 ± 11%) was not quite significant at the 5% level (p = 0.07, Student’s 2-tailed t-test) when tested in the wild-type strain. However, oxyR2 completely eliminated the Δdps-mediated increase in Lac+ MBR, showing a 70 ± 7.8% reduction in mutation frequency (Fig 2B and 2D), (p = 0.014, Student’s 2-tailed t-test) to the same level as the strain with oxyR2 alone (p = 0.98, Student’s 2-tailed t-test). The data indicate that ROS are required for MBR generally, and we infer that the mechanism of Dps inhibition of MBR is its protection of DNA against ROS.
ROS are required for stress-induced mutagenesis
We removed ROS from cells by overexpressing sodB, which encodes a superoxide dismutase. We used a plasmid from the mobile plasmid library [60] that carries each gene under the control of an isopropyl β-D-1-thiogalactopyranoside (IPTG)-inducible promoter in cells used for the Tet MBR assay [7]. The Tet MBR assay reports on indel mutations that revert a chromosomal tetA +1bp frameshift allele during starvation in liquid minimal medium. In the Tet assay, DSBs are induced near a chromosomal tet mutation-reporter gene by the I-SceI site-specific double-strand endonuclease expressed in F-plasmid-free cells during starvation. tet reversion in this assay requires the key MBR proteins, as in Lac+ MBR, including RpoS, the SOS response regulators, Pol IV, and DSB-repair proteins [6, 7, 16]. The Tet assay differs from the Lac MBR assay not only in having no F-plasmids present, but also in having no selection for the reverted allele during the starvation stress; Tet-resistant (TetR) revertants are selected as TetR cfu after the cells are rescued from starvation in liquid. The Tet assay does not measure GCR. We found that over-expression of sodB reduced TetR MBR mutant frequencies 76% ± 12%, (Fig 3, 0.003, Student’s 2-tailed t-test). These data show, by enzymatic removal of ROS, that ROS are required for starvation-stress-induced MBR. We conclude that MBR requires ROS for mutagenesis.
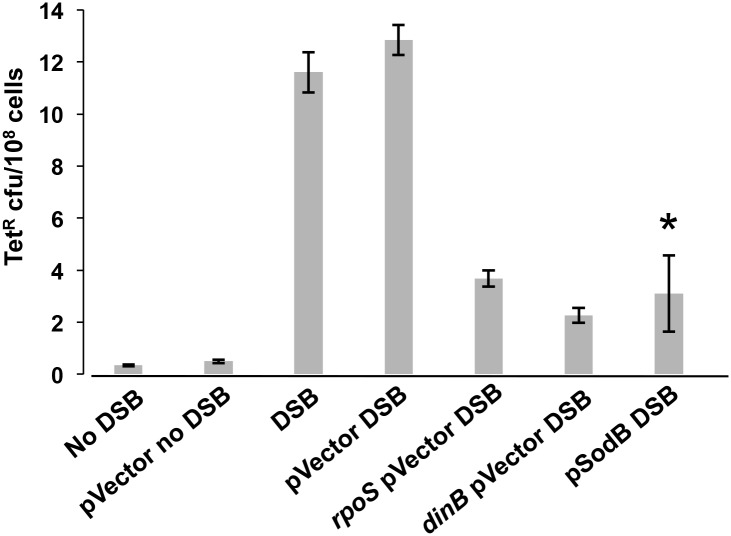
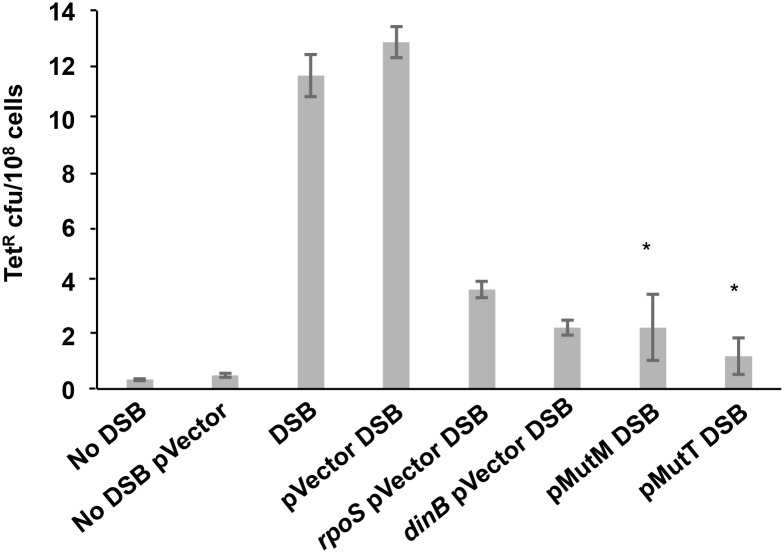
Fig 3. DSBs do not substitute for ROS in MBR.
In the Tet MBR assay, DSBs are induced in starved cells by induction of I-SceI double-strand endonuclease cleavage at an I-SceI cutsite near the chromosomal tet indel mutation-reporter gene [6, 7]. SodB overexpression is induced by IPTG, and reduces TetR mutant frequency even when DSBs are provided by I-SceI. Thus, I-SceI cannot substitute for ROS, and ROS promote MBR other than or in addition to by causing DSBs. * significantly different from the isogenic empty-vector control (pVector) at the 5% level. ΔrpoS and dinB50 positive-control strains with empty vector and DSB induction demonstrate RpoS- and DinB-dependent MBR, here and in Fig 5. Strains used: no DSB, SMR10798; no DSB pVector, PJH3237; DSB, SMR10866; DSB pVector, PJH3232; DSB ΔrpoS pVector, PJH3240; DSB dinB50 pVector, PJH3231; DSB pSodB, PJH3257.
ROS promote MBR other than by formation of DSBs
We eliminated several possible mechanisms for the requirement for ROS in MBR. Repair of oxidized DNA bases might lead to formation of spontaneous DSBs that instigate MBR [4, 6, 7, 47]. Base excision repair proceeds by excision of the damaged base by specific DNA glycosylases forming an abasic (AP) site, followed by nicking at that site by an AP endonuclease. If not repaired, the nick could produce a one-ended DSB when replicated, via replication fork breakage [61]. Using the Tet MBR assay, we find that even in the presence of an I-SceI-endonuclease-generated DSB, over-expression of sodB still reduced TetR MBR as described above (Fig 3). We show that induction of DSBs by I-SceI is effective when SodB is overexpressed (S2 Fig). We conclude that ROS promote MBR other than or in addition to by promoting spontaneous DSBs.
ROS do not promote MBR via oxidation of proteins
ROS damage proteins, lipids, RNA and DNA [30]. Oxidation of proteins, which causes carbonyl groups, can inhibit protein function (reviewed by [33]). We used a mutation that reduces cellular levels of carbonylated proteins: rpsL141, which encodes a hyper-accurate ribosomal protein that reduces intracellular carbonylated protein content [62]. We found that Lac assay MBR rates were unaffected by rpsL141 (Fig 4A and 4B), implying that promotion of MBR by ROS is not mediated by oxidation of proteins.
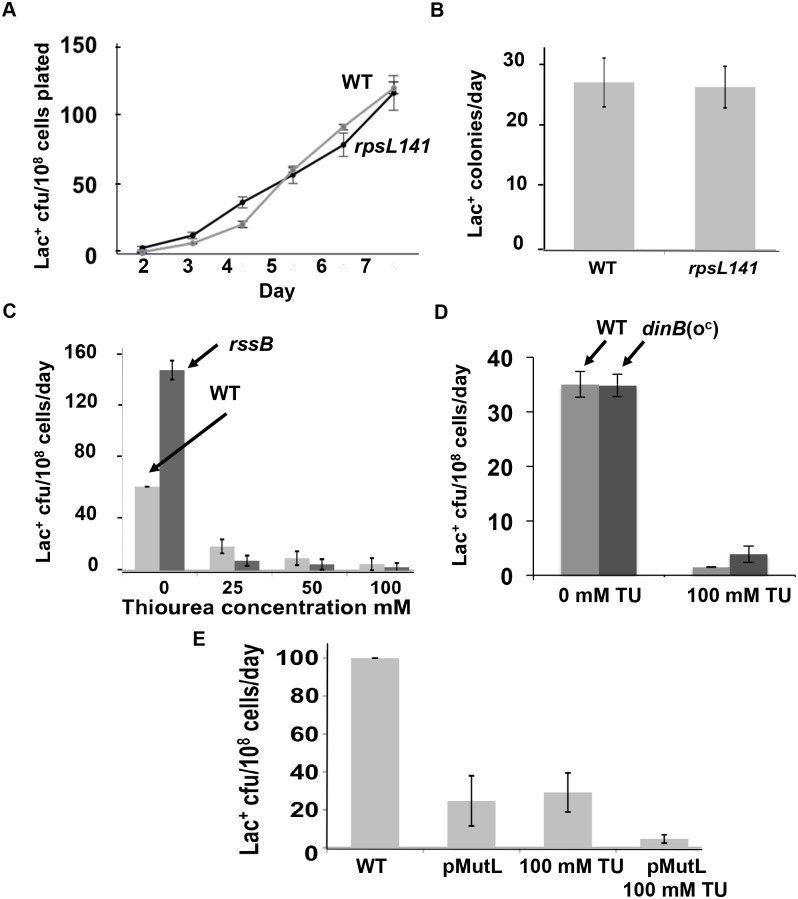
Fig 4. ROS promote MBR other than or in addition to by upregulation of the RpoS or SOS responses, oxidation of proteins, or saturation of mismatch repair.
(A, B) The hyper-accurate ribosomal protein allele rpsL141 [62] does not reduce MBR, implying that ROS promote MBR other than by producing carbonylated proteins, which are reduced by this allele. (A) Representative experiment. (B) Mean ± SEM of three experiments. (C) Artificial upregulation of RpoS levels in ΔrssB strains does not restore MBR in TU-treated cells, implying that ROS promote MBR other than or in addition to by upregulation of RpoS. (D) An operator-constitutive dinB(oc) allele, which substitutes for the SOS response in MBR [41], does not substitute for depletion of ROS by TU. (E) Over-expression of mutL, the limiting mismatch repair (MMR) component in MBR [63], causes additive reduction of MBR with TU treatment, implying that TU and MMR reduce MBR by different pathways, indicating that ROS promote MBR other than by saturation of MMR capacity. Strains used: wild type (WT) SMR4562; rpsL141, PJH3178 ΔrssB, SMR12566; dinB(oc) SMR10308; PBADMutL, SMR15378. PBADMutL was derepressed by absence of glucose in the medium.
ROS promote MBR other than via induction of the general or SOS stress responses
We excluded the possibility that ROS promote MBR by inducing the RpoS response by assaying MBR in an rssB mutant in the presence of TU. RssB targets RpoS, the transcriptional activator of the general stress response, for degradation by the ClpXP protease, so that when rssB is deleted, RpoS levels increase, artificially upregulating the RpoS response [64]. If ROS promoted MBR solely by inducing the general stress response, ΔrssB would be expected to counter the anti-MBR effect of TU treatment. We find that TU treatment reduced MBR dose-dependently in ΔrssB cells as in wild-type cells (Fig 4C), (p = 0.16; 0.92; 0.95, comparing WT with rssB at 25, 50, and 100 mM TU, respectively; Student’s 2-tailed t-test). We conclude that ROS probably promote MBR other than or in addition to by activation of the general stress response.
The SOS DNA-damage response promotes MBR via its 10-fold transcriptional upregulation of Pol IV error-prone DNA polymerase, and is not needed for MBR in cells with an operator-constitutive (constitutively over-expressing) dinB allele [41]. We find that the dinB(oc) allele did not counter TU treatment [Fig 4D, p = 0.001 for wild-type (WT) versus WT +100mM TU; p = 0.0003 comparing dinB(oc) with dinB(oc) +100mM TU, Student’s 2-tailed t-test]. By contrast wild-type and the dinB(oc) strain are not different [p = 0.95; and p = 0.10 for WT with TU versus dinB(oc) with TU, Student’s 2-tailed t-test], implying that ROS promote MBR other than or in addition to by activation of the SOS response.
Oxidative damage promotes MBR other than by titration of mismatch repair
We tested the possibility that excessive base damage from ROS might overwhelm mismatch repair, making it unable to correct base misincorporations by DinB. MutL, a required component of mismatch repair, was shown to become limiting during MBR [63] and for polymerase errors generated by a hyper-mutator Pol III [65]. We combined TU treatments with overproduction of MutL, to test whether oxidized DNA bases saturate mismatch repair capacity during MBR. Previously, increasing mismatch repair capacity by overproducing MutL reduced Lac+ MBR approximately 4-fold [63]. If ROS promoted MBR by saturating mismatch repair capacity, MutL overproduction and TU inhibition of MBR would be expected have an epistatic relationship, implying their action in the same pathway. By contrast, we found that 100mM TU combined with MutL overproduction reduced Lac+ MBR additively (Fig 4E), P = 0.005, 0.002, 0.838, and 0.035 for WT compared with MutL overproduction; WT compared with TU; WT + TU compared with MutL overproduction; and WT + TU compared with MutL overproduction + TU, respectively, Student’s 2-tailed t-test. The data imply that ROS promote MBR other than by causing saturation of mismatch repair.
ROS promote MBR via persistent oxidized guanine in DNA
We found that removing oxidized guanine from DNA reduces MBR. E. coli has three proteins that specifically reduce the mutagenic effects of 8-oxo-dG. MutM is a DNA glycosylase that excises 8-oxo-dG from DNA, MutY is another glycosylase that excises mispaired adenine opposite to G or 8-oxo-dG, and MutT hydrolyses 8-oxo-dGTP in the nucleotide triphosphate pool, reducing incorporation of 8-oxo-dG into DNA. We (separately) overproduced MutM, MutT, and MutY in Tet MBR assay cells using mobile plasmid library plasmids [60], and measured TetR mutant frequencies. We detected no decrease in cell viability during these experiments or in growth of strains containing these plasmids induced with 1mM IPTG (S3 Fig). We found that overproduction of MutM and MutT reduced TetR MBR 82% ± 9.7% and 91% ± 5.3%, respectively, compared with the vector-only control (p = 0.0015 and 0.0002, Student’s 2-tailed t-test) (Fig 5). ΔrpoS and dinB positive-control strains confirm that mutagenesis is via the canonical MBR pathway. In eight repeated experiments we observed a small, but not significant, decrease in Tet MBR with MutY overproduction (32% ± 9.5% decrease, p = 0.15, Student’s 2-tailed t-test), suggesting that mispaired adenines have, at most, a minor effect on promotion of MBR. We conclude that persistent unrepaired 8-oxo-dG in DNA is required for MBR, and that ROS promote MBR via the presence of 8-oxo-dG in DNA. Furthermore, because the increased removal of 8-oxo-dG from DNA eliminates most mutagenesis, we conclude that whereas oxidized proteins or lipids, or oxidative lesions in RNA might play a role in MBR, it is at most a small one. The strong effect of overproduction of MutT, the 8-oxo-dGTP diphosphatase, implies that the MBR-promoting 8-oxo-dG in DNA results mainly from incorporation from the nucleotide pool rather than in situ oxidation of DNA.
Fig 5. Persistent oxidized guanine in DNA is required for MBR.
Overproduction of MutM 8-oxo-dG DNA glycosylase and MutT 8-oxo-GTP diphosphatase nucleotide triphosphate pool sanitizer reduces MBR in the Tet assay. Overproduction of MutM and MutT reduced TetR mutant frequency 82% ± 9.7% and 91% ± 5.3%, respectively compared with the vector control (p = 0.0015 and 0.0002, Student’s 2-tailed t-test). The data indicate, first, that 8-oxo-dG in DNA is required for MBR, second, that the effects of 8-oxo-dG on MBR result mainly from incorporation of 8-oxo-dG from the dNTP pool, and third, that 8-oxo-dG must remain in DNA for MBR to occur, because if 8-oxo-dG is removed by the MutM DNA glycosylase, MBR is reduced. * significantly different from the empty vector control (pVector) at the 5% level. Strains used: No DSB, SMR10798; no DSB pVector, PJH3237; DSB, SMR10866; DSB pVector, PJH3232; DSB rpoS pVector PJH3240; DSB dinB50 pVector PJH3231; DSB pMutM, PJH3233; DSB pMutT, PJH3256.
Other base damage in DNA can substitute for 8-oxo-dG in MBR
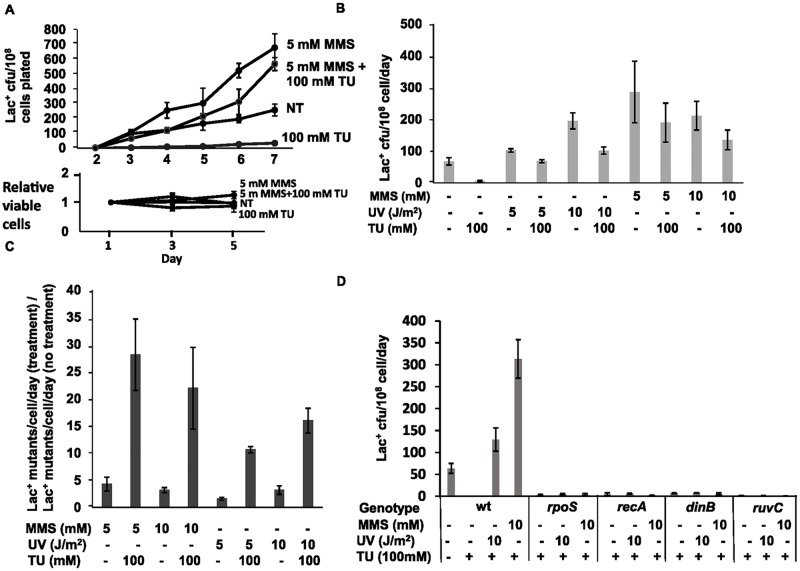
We tested the hypothesis the 8-oxo-dG in DNA might promote MBR by pausing the high-fidelity replicative DNA Pol III, active in DSB repair [20], which might allow Pol IV and other alternative DNA polymerases to switch into the active position in the repair replisome. If this were the case, then 8-oxo-dG would not be required as a specific intermediate in MBR; any fork-stalling DNA-base damage traversed or extended from by translesion DNA polymerases would be expected to substitute for 8-oxo-dG. We tested whether other persistent DNA damage could bypass the requirement for ROS and 8-oxo-dG in MBR by using methyl methanesulfonate (MMS), which methylates DNA, forming mainly N7-methyldeoxyguanosine and N3-methyldeoxyadenosine [66], and ultraviolet-C light (UV-C), which creates pyrimidine dimers and 6–4 photoproducts (reviewed by [67]), all of which stall the replicative DNA Pol III and can be traversed by translesion DNA polymerases [68–70]. We pulse-treated starved cells with nonlethal doses of MMS or UV-C before plating on minimal lactose medium with 100mM TU to scavenge ROS. Whereas both MMS and UV-C increased basal mutation rates slightly (3-fold for each treatment, Fig 6A and 6B), the increase in mutagenesis seen with TU + MMS or UV treatments compared with TU alone was many-fold greater (Fig 6C), 22 ± 7.8-fold for 10mM MMS (p = 0.02, Student’s 2-tailed t-test), and the increase is 16 ± 2.3-fold for 10 J/m2 UV, (p = 0.001, Student’s 2-tailed t-test). The data show a robust return to mutagenesis caused by those base-damaging agents after removal of ROS. The data imply that other base damages can substitute for 8-oxo-dG in mutagenesis. MMS or UV treatment completely countered the effect of 100 mM TU (Fig 6B). Further, we show that the mutagenesis restored by MMS or UV in the absence of ROS is “on-pathway” MBR, in that mutagenesis depends completely on RecA, RpoS, Pol IV, and RuvC (Fig 6D). These data show that MMS and UV did not activate an alternative mutagenesis mechanism(s), but rather restored MBR to ROS-depleted TU-treated cells.
Fig 6. Base-damage treatments substitute for ROS-induced 8-oxo-dG in MBR.
(A) Methyl methanesulphonate (MMS) or UV-C light restore mutagenesis to ROS-scavenged cells. Representative experiment. ROS are reduced with 100 mM thiourea (TU); NT, no treatment. Below is a representative example of viability data showing that TU treatment and MMS/UV pulse treatment do not affect tester-cell viability during the mutagenesis assay. (B) Quantification from multiple experiments. Non-lethal doses of MMS (5 mM and 10 mM) or ultraviolet C light (UV) (5 and 10 J/m2) restored mutagenesis to cells treated with high levels of TU. For 5 J/m2 UV, there is an 11 ± 0.6-fold increase (p = 0.0004, Student’s 2-tailed t-test), compared with TU treatment with no mutagen. For 10 J/m2 UV, the increase is 16 ± 2.3-fold (p = 0.001, Student’s 2-tailed t-test; for 5mM MMS, 28 ± 6.6-fold (p = 0.049, Student’s 2-tailed t-test) and for 10mM MMS, 22 ± 7.8-fold (p = 0.02, Student’s 2-tailed t-test). (C) MMS and UV treatments have much stronger effects on Lac+ mutant formation when ROS are scavenged by TU than when they are not. Although both treatments increased basal Lac+ mutation rates, 3 ± 0.5-fold with 10 mM MMS and 3.0 ± 0.8-fold with 10 J/m2 UV, the increases caused by MMS or UV were much greater in TU treated than untreated cells, indicating that MMS and UV treatments specifically substitute for the requirement for ROS (p = 0.006 for both, Student’s 2-tailed t-tests, when comparing mutagen +TU/NT+TU with mutagen only/NT with no mutagen). Data are from Fig 6B. (D) The mutagenesis restored to ROS-depleted cells by UV and MMS is on-pathway MBR, requiring RpoS, RecA, DinB and RuvC. Thus, the requirement for damaged bases in DNA for MBR is not specific to 8-oxo-dG, but can be provided by other replication-hindering DNA damage. Strains used: Wild-type (WT), SMR4562; ΔrpoS, PJH1399; recA, SMR4610; dinB50, SMR5889; ruvC, SMR6906.
Both MMS and UV-C might induce oxidative damage in addition to their more common lesions. UV-C can induce 8-oxo-dG formation in HeLa cells and in DNA, although 8-oxo-dG induction was about 2000-fold lower than pyrimidine dimer induction [71]. MMS induces ROS in yeast [72], however, this requires the presence of glucose in the medium [73, 74], and glucose is absent from the medium of starved E. coli cells, making this unlikely. In Fig 1D we used a high dose (100mM) of TU to scavenge ROS and found that the four-fold increase in MBR caused by Δdps is completely blocked by 100mM TU. Given the expected much greater levels of ROS in Δdps than WT cells and the ability of TU to quench the ROS in Δdps cells, these data suggest that there is still substantial ROS quenching capacity when TU is applied to wild-type cells. All of these data imply that MMS and UV-C promoted MBR not by promoting 8-oxo-dG in DNA, but rather by their abundant standard base damages. The slightly lower mutagenesis seen in the presence of TU for all mutagen treatments in Fig 6B (p = 0.01, 0.03, 0.40, and 0.20, Student’s 2 tailed t-test for 5 J/m2 UV, 10 J/m2 UV, 5 mM MMS, and 10 mM MMS, respectively, in the presence versus the absence of TU) might reflect a low level of MBR from oxidative damage when UV and MMS are used. However, most MBR in MMS or UV treated cells arises from non-oxidative base damage, which is not prevented by TU. These data demonstrate that persistent damaged bases in DNA are needed generally for MBR, and imply that the DNA substrate for MBR is more than a simple DSB undergoing HR repair: that, additionally, damaged DNA bases must be present in the DNA for MBR to occur.
Discussion
Discovered in E. coli, MBR is an important model molecular mechanism of mutagenesis later discovered in other bacteria, yeasts, flies, and human cells and cancers (reviewed [13]). Until this report, the E. coli MBR mechanism was defined by its requirements for various trans-acting components, but only a single central cis-acting DNA component: DNA DSBs, which are required for spontaneous MBR [4, 6, 7, 47] and at which mutagenesis is focused [6, 8]. The trans-acting proteins are those of DSB repair, low-fidelity translesion DNA polymerases, transiently insufficient mismatch repair and stress-response regulators (reviewed [13]), including a large gene network, in which most act upstream of the stress-response regulators in stress sensing and signal transduction that activates the RpoS, SOS and membrane stress responses [75]. Supporting this binary view, although transcriptional R-loop RNA/DNA hybrids and the Mfd RNA polymerase translocase are also required for MBR [47], they and the membrane stress response [45] promote MBR by promoting spontaneous DSBs at some loci, and are not needed when endonuclease-generated DSBs are provided [47, 52]. By contrast, the data presented here show that a separate, additional and unrelated DNA substrate and event must occur for MBR: damaged DNA bases. We found that spontaneously, during starvation-induced MBR, ROS-promoted 8-oxo-dG in DNA underlies most MBR (Figs 1–3 and 5), and that 8-oxo-dG does not promote MBR solely by generation of DSBs (Figs 3 and 5), and so is a distinct, new cis-acting DNA component of the MBR molecular mechanism. This mechanism applies both to SNA and GCR MBR mechanisms (Fig 2A). Other roles for ROS in stress response activation, protein oxidation, and mismatch repair saturation were shown not to underlie the ROS role in MBR (Fig 4). Because the persistent 8-oxo-dG in DNA that drives spontaneous MBR during starvation can be substituted by other generic damaged bases caused by UV light or the alkylating agent MMS (Fig 6), the data indicate that damaged bases generally are required for MBR, and in a role separable from specifically oxidized guanine.
Whereas previously we found that the stationary-phase upregulated ROS-detoxifying protein Dps inhibits MBR [57], here we find that the Dps inhibition of MBR is consistent with its effects on ROS reduction (Figs 1C and 2D).
Model: Damaged bases promote DNA polymerase switching to license MBR—SNAs
We suggest a model in which the role of damaged bases in MBR is to allow the switch from high-fidelity DNA Pol III [20] to low-fidelity translesion DNA polymerases during homologous recombinational DSB repair, thus allowing MBR. A requirement for Pol III pausing to allow a switch to Pol IV has been shown previously [76–78]. A simple example of this idea is illustrated in Fig 7 for the formation of SNAs. The general model is that replication stalling at base damages that are not easily replicated or extended from by Pol III allows switching to alternative DNA polymerases, some of which are mutagenic, by pausing the replisome so that the DNA polymerases can switch.
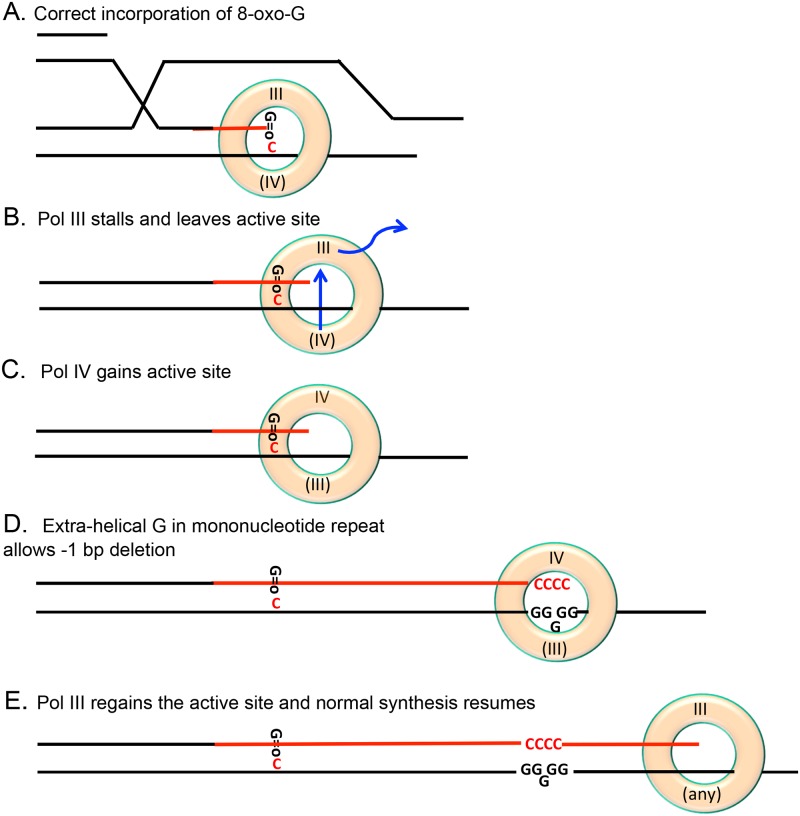
Fig 7. Model: Replisome pausing at damaged bases allows DNA polymerase switching and MBR: Example of oxidized bases allowing Pol IV-dependent indels at non-damaged sites downstream.
This example illustrates a general model in which the switch from the processive, high-fidelity DNA Pol III used in DSB repair [20] to any of the alternative mutagenic DNA polymerases results from Pol III pausing at a damaged base, either in the template or newly added to the 3’-primer end. One way that 8-oxo-dG could allow Pol IV-mediated MBR mutations (at other DNA bases) is shown here. (A) A D-loop made during DSB-repair of a one-ended DSB has invaded a sister DNA molecule (parallel lines, base-paired DNA) and primed new synthesis (red line) using Pol III. Pol III inserts 8-oxo-dG, here opposite a template C [38], or could stop after inserting C at 8-oxo-dG in the template (not illustrated). (B) Pol III pauses because it extends poorly from an 8-oxo-dG primer end paired with template C [38]. We suggest that the pausing allows Pol III to leave the active position on the β-clamp, and an alternative DNA polymerase on the β-clamp (Pol IV/DinB shown here) to move into the β-clamp active position. (C) Pol IV in the active position extends synthesis from the 8-oxo-dG primer opposite template C (or, not illustrated, bypasses an 8-oxo-dG in the template strand, inserting the correct base or an incorrect base), and continues synthesis making indel (and/or base substitution) errors at mononucleotide repeat sequences, at which it is most error-prone. (D) Eventually Pol IV pauses because of its limited processivity [39], allowing Pol III to resume. Black lines represent pre-existing nucleotide chains and red lines represent new synthesis. The β-clamp is represented as an orange doughnut. The β-clamp site with the active DNA polymerase is illustrated at the top of the β-clamp, and the inactive DNA Pol binding site at the bottom in parenthesis. Blue arrows in (B) indicate movements of polymerases on and off the β-clamp. G = o indicates 8-oxo-dG.
Damaged bases can be repaired in double-stranded DNA, but in single-stranded DNA at the replication fork they must be bypassed by translesion synthesis (TLS) [79]. TLS often uses alternative DNA polymerases to synthesize across damaged bases or to extend from the resultant mismatches after incorporation [80]. TLS polymerases include the Y-family polymerases Pol IV/DinB and Pol V in E. coli. DNA polymerases in the replisome and in repair complexes are attached to the β processivity clamp, which is the structural homolog of proliferating cell nuclear antigen (PCNA) in yeast and mammalian cells [81]. In both E. coli and eukaryotes, replicative polymerases might pause when they encounter a damaged base, either before incorporation or before extension, allowing a TLS polymerase that is already loaded on the β-clamp [77] to move onto the DNA primer end and synthesize past the damaged base or to extend beyond it. In the version of this general model shown in Fig 7, we show Pol IV (DinB) replacing Pol III in the active position on the beta clamp (Fig 7B). The replicative Pol III can incorporate 8-oxo-dG opposite A (not illustrated) or C (Fig 7A) [38]. Assuming that findings about eukaryotic polymerases [38] can be applied to E. coli, Pol III would then pause transiently because of poor ability to extend synthesis from the 8-oxo-dG paired with template C [38]. Alternatively Pol III could pause at an 8-oxo-dG in the template strand paired with C on the primer (not illustrated) [38]. In these cases, we suggest that stalling of Pol III allows it to be shifted out of the β processivity clamp site carrying the active DNA polymerase (top of β-clamp doughnut shown Fig 7), and a DNA polymerase in the clamp inactive position (bottom of β-clamp, Fig 7) switched into the active position (Pol IV shown switching places with Pol III, Fig 7B and 7C). With Pol IV in the β-clamp active DNA polymerase position, synthesis can resume from the 8-oxo-dG primer opposite template C (red line, Fig 7C) or from C across from 8-oxo-dG in the template (not illustrated), and base-substitution and/or indel mutations can be made downstream after the switch, for example when Pol IV encounters a run of Gs (Fig 7D and 7E), a slippery template sequence at which Pol IV makes errors with high probability [72]. Pol IV is processive for about 400 nucleotides [39]. Pol IV and its orthologous Y-family DNA polymerases in other species including human can make indel mutations because their active sites can accommodate extrahelical bases in mononucleotide repeats in the template strand, and thus insert fewer nucleotides than are on the template [82, 83]. Their most common errors are base substitutions, however. The data presented here reveal that the availability of alternative DNA polymerases is not sufficient to allow mutagenesis, which requires polymerase switch, supporting models like this one.
Alternative DNA polymerases in MBR
All three DNA damage-response (SOS)-induced polymerases, Pols II, IV and V, contribute to stress-induced MBR SNA formation [7, 21–23]. Pol IV promotes 85% of indels in Lac MBR, and can increase mutation rates over a thousand-fold in the absence of exogenously induced DNA damage when overproduced in E. coli [40]. The remaining 15% of Lac MBR indels require Pol II [22]. Pol V promotes MBR base substitutions [23] and some indels [7]. GCR, seen as amplifications, does not require any of the SOS-inducible DNA polymerases, but requires DNA Pol I [45]. Thus all stress-induced MBR requires alternative DNA polymerases. Presumably the relative availability of the different DNA polymerases will influence which polymerases are switched into the MBR replisome and replace Pol III at the active position on the beta clamp.
Model: Damaged bases promote DNA polymerase switching to license MBR—GCRs
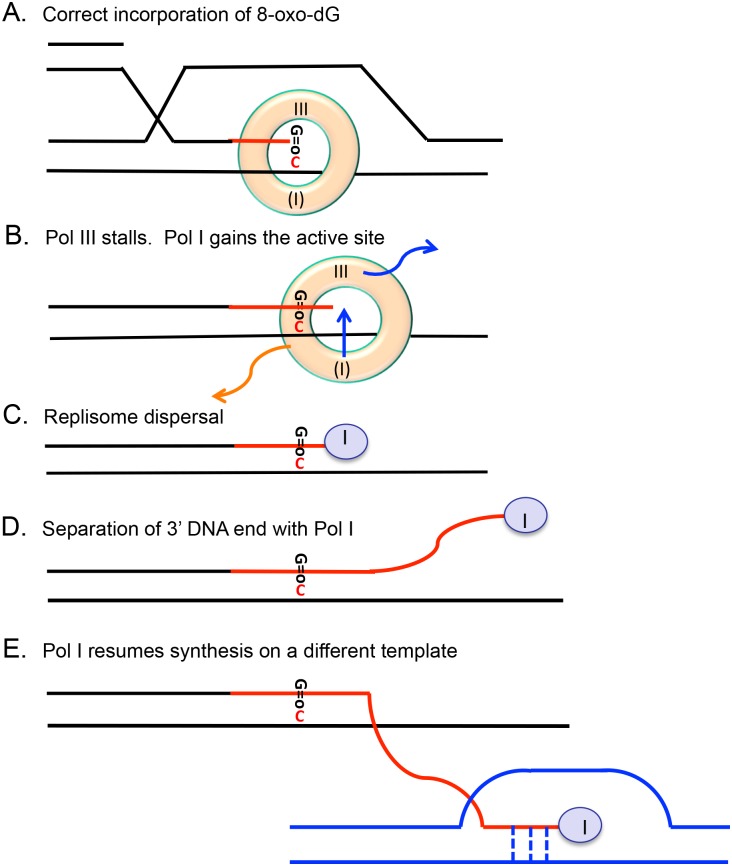
Amplification in the Lac assay is mediated by microhomology [45] and is proposed to arise by polymerase template switching during replication by a microhomology-mediated break-induced replication (MMBIR) mechanism [46, 84]. Because Pol I is required for amplification [53], we suggest that Pol I moves to the beta clamp active position when Pol III is stalled (Fig 8B and 8C), and then mediates template switching creating genome rearrangements. We suggest that when Pol I binds the primer end and the replisome is dispersed (Fig 8B, 8C and 8D), as will happen if the fork is stalled for a time [85], Pol I can mediate template switching using very limited homology (Fig 8D and 8E), as has been observed for the Pol I human ortholog DNA PolQ, another A-family polymerase [86–88]. This mechanism (one specific example of which is illustrated in Fig 8), parallels the model for base-substitution and indel formation (one indel version shown in Fig 7). Dissociation of the primer end with Pol I attached might be achieved if the editing function of Pol I is activated by the mismatch at ROS damaged bases, for example at an 8-oxo-dG:C base pair, causing dissociation of the 3'-end to enable it to attain the nuclease domain [89]. Such dissociation would permit polymerase template switching (Fig 8E), the postulated first step in microhomology-mediated recombination.
Fig 8. A model for template switching leading to chromosomal rearrangement following incorporation opposite 8-oxo-dG.
(A) Pausing of the replication complex after Pol III places 8-oxo-dG opposite C [38] (shown, or after Pol III stops after placing C opposite to 8-oxo-G in the template strand, not illustrated) sometimes leads to (B) exit of Pol III from active position in the β-clamp, and the DNA polymerase from the inactive position (Pol I here) is switched into the active site. (C) Prolonged pausing of the replisome leads to dispersal of the β-clamp. (D) The Pol I-bound primer end can become detached, possibly by activation of the editing function, so that template switching can occur, initiating GCR by MMBIR [45, 46]. (E) In this case the primer binds to a template at a D-loop at a non-homologous position at microhomology (green) or any other single-stranded DNA (not illustrated). Extension by Pol I will stabilize the junction allowing non-homologous recombination, which creates gene duplications, deletions and other GCRs. Conventions are as in Fig 7 except that the orange arrow indicates detachment of the β-clamp, and the vertical dashed bars in part (E) indicate a microhomologous junction.
Other possible ROS-induced mutagenesis mechanisms
Other possible mechanisms that might have explained the role of ROS in MBR have been described, but do not fit the data presented here. First, because oxidation induces nicks and DSBs directly in DNA [90, 91], and both UV and MMS cause DNA breakage [92, 93], together with the established requirement for DSBs for MBR [6], we considered the possibility that ROS were needed for MBR for the provision of DSBs. We answered this by showing that expression of I-SceI double-strand endonuclease did not raise MBR in a strain over-expressing the SodB superoxide dismutase to the level seen in a strain also expressing I-SceI but not over-expressing SodB (Fig 3). This shows that the loss of MBR associated with reduction in endogenous ROS is not suppressed by provision of DSBs, and therefore that ROS has a role in MBR other than or in addition to DSB formation. Similarly, the action of I-SceI did not suppress the reduction in MBR associated with over-expression of MutT (Fig 5), showing that removal of 8-oxo-dGTP from the deoxynucleotide triphosphate pool is not overcome by provision of DSBs. If ROS were required solely for DSB formation we would have seen MBR at wild-type levels in MutT over-expressing strains.
Second, Pol IV overproduction causes cell death by increasing incorporation of 8-oxo-dG into DNA with subsequent increase in DSBs [54]. This is postulated to be because Pol IV is more likely than replicative polymerases to incorporate 8-oxo-dG, which is then removed by base-excision repair in 8-oxo-dG clusters leading to DSBs [54]. This model cannot account for the role of ROS/8-oxo-dG in MBR because the requirement for 8-oxo-dG was other than or in addition to for generation of DSBs (Figs 3 and 5). Also arguing against the Pol IV/8-oxo-dG-DSB model is the observation that amplification also requires ROS (Fig 2A), and neither SOS-induced levels of Pol IV nor Pol IV itself are required for amplification [21]. Thus the model of Foti et al. [54] does not provide an explanation for the requirement for ROS for MBR.
Third, a model for how single-stranded (ss) DNA damage promotes MBR in yeast also does not explain our data in E. coli. In yeast, ssDNA regions experience hypermutability both spontaneously and induced by UV light or MMS treatment [94], with mutagenesis increased by orders of magnitude in ssDNA regions at sites of DSB repair by HR, compared with duplex DNA nearby. The ssDNA at sites of HR DSB repair and at uncapped telomeres involved ssDNA lengths longer than 6kb [95]. A similar mechanism is implicated in human cancer cells [12]. This hypermutability in ssDNA is attributed to the absence of complementary DNA sequence during repair of base lesions formed in the ssDNA, causing their replication by mutagenic translesion DNA polymerases [95]. This model appears not to apply to MBR in E. coli both because the E. coli MBR mutation clusters cover ~100 kb rather than six [8], and because the model offers no explanation for GCR in MBR.
Finally, ROS were reported previously to participate in an RpoS-independent mechanism of starvation-associated mutation in E. coli [96]. In contrast with our findings for RpoS-dependent MBR, in that assay, although mutating mutT, encoding the 8-oxo-G nucleotide pool sanitizer, increased mutagenesis, mutation of mutM, encoding the 8-oxo-dG DNA glycosylase, showed no significant effect, showing no role for 8-oxo-dG in DNA [96, 97], the opposite of our finding (Fig 5). Thus, a different mutagenesis mechanism was at work.
Roles of ROS and damaged bases in spontaneous mutagenesis
Importantly, the mutagenesis-promoting effect of ROS and 8-oxo-dG in DNA reported here is in spontaneous mutation. Although increasing ROS in cells induces mutagenesis (e. g. [25–27]), whether basal levels of spontaneous mutation are also driven by ROS was unknown previously. Previously, the sequence spectrum and frequencies of mutations in anaerobic and aerobic E. coli cultures suggested that spontaneous base substitutions were oxygen-related, via hydroxyl radicals [98]. The data here show directly that spontaneous SNA and GCR MBR in starving cells require ROS and 8-oxo-dG.
A large body of work has established that organisms respond to stress by increasing their mutation rate under the control of stress responses [13] possibly increasing their ability to evolve and adapt [99]. The demonstration that mutation rate in stressed cells additionally requires base damage caused by ROS, together with our previous finding [57] that there is both positive (by H-NS) and negative (by Dps) regulation of ROS, suggest that cells might regulate their spontaneous mutation rates in response to challenging circumstances by regulating/responding to ROS levels. ROS were long regarded as unwanted byproducts of oxidative metabolism, to be avoided because of the damage that they do to macromolecules. They have been held to be a primary cause of aging [100, 101]. However, it is now apparent that ROS are also an integral component of cell physiology [102]. The use of ROS for accelerated evolution in stressed cells described here might be regarded as a parallel with the involvement of ROS in signaling [103] and in the function of the immune system [104]. Conversely, although it might be advantageous for an organism to mutate specifically when under stress [99, 104, 105], it would be to our advantage to stop pathogens from responding to our immune systems and antibiotics with hypermutation, evasion of the immune system and antibiotic resistance. We have suggested that novel “anti-evolvability” drugs might stop stress-induced evolution of pathogens (and cancers) by suppression of the stress-responses that promote mutagenesis [13].
Methods
Construction of Esherichia coli K12 strains
The origin of these strains is listed in Table 1. Strains used for Lac+ MBR assays are isogenic derivatives of SMR4562, an independent construction of FC40 [106]. Genotypes were confirmed either by PCR or by sequencing as necessary. Mobile plasmid library plasmids [60] containing over-expression alleles of mutants under the control of a Ptac promoter were conjugated into SMR10866 and SMR10798, strains expressing the chromosomal I-SceI restriction endonuclease controlled by PBAD, and an I-SceI cutsite, or the enzyme-only control, respectively. Others were constructed by transduction or linear replacement [107] as listed in Table 1. I-SceI cutting was verified by the sensitivity of strains to arabinose that induces I-SceI enzyme.
Table 1. Escherichia coli K12 trains used in this study.
| Strain/Plasmid | Genotype | Source/Reference |
|---|---|---|
| CAG12080 | zah281::Tn10 | [108] |
| CH2139 | MG1655 rpsL141 | Christophe Herman |
| FC36 | Δ(lac proB)XIII thi ara RifR | [42] |
| FC40 | FC36 [F'å45 = F' proAB+ lacIq lacI33ΩlacZ] | [42] |
| PJH18 | FC40 [F’ lac-amplified] | [43] |
| PJH33 | FC40 [F’ lac-amplified] | [43] |
| PJH51 | FC40 [F’ lac-amplified] | [43] |
| PJH1390 | SMR4562 ΔrpoS::KanFRT | SMR4562xP1(SMR10336) |
| PJH1399 | SMR4562 ΔrpoS::FRT | PJH1390 x pCP20 |
| PJH1952 | SMR4562 Δdps::KanFRT | SMR4562xP1(JW0797)* |
| PJH2608 | SMR4562 Δdps::FRT | PJH1952 x pCP20 |
| PJH2947 | SMR4562 soxR104 zjc2206::Tn10Kan | SMR4562xP1(JTG1048) [31] |
| PJH2982 | JA200 [pNT3] | [60] |
| PJH3178 | SMR4562 rpsL141 | SMR4562xP1(CH2139) |
| PJH3210 | JA200 [pNT3/sodB] | [60] |
| PJH3211 | JA200[pNT3/mutM] | [60] |
| PJH3231 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRT Δzie3920.5::3ChiKanI-SceIsite Δattλ:: PBADI-SceI dinB50::FRT [pNT3] | SMR10868 conjugated with [pNT3] |
| PJH3232 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRT Δzie3920.5::3ChiKanISceIsite Δattλ:: PBADISceI [pNT3] | SMR10866 conjugated with [pNT3] |
| PJH3233 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRT Δzie3920.5::3ChiKanI-SceIsite Δattλ:: PBADI-SceI [pNT3/mutM] | SMR10866 conjugated with PJH3211 |
| PJH3237 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRTcatFRT Δattλ:: PBADI-SceI [pNT3] | SMR10798 conjugated with [pNT3] |
| PJH3240 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRT Δzie3920.5::3ChiKanI-SceIsite Δattλ:: PBADIsceI rpoS::FRT [pNT3] | SMR10865 conjugated with [pNT3] |
| PJH3255 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRT Δzie3920.5::3ChiKanISceIsite Δattλ:: PBADIsceI [pNT3/mutY] | SMR10866 conjugated with PJH3259 |
| PJH3256 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRT Δzie3920.5::3ChiKanI-SceIsite Δattλ:: PBADI-SceI [pNT3/mutT] | SMR10866 conjugated with PJH3258 |
| PJH3257 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRT Δzie3920.5::3ChiKanISceIsite Δattλ:: PBADI-sceI [pNT3/sodB] | SMR10866 conjugated with [pNT3/sodB] |
| PJH3258 | JA200 [pNT3/mutT] | [60] |
| PJH3259 | JA200 [pNT3/mutY] | [60] |
| PJH3278 | SMR4562 oxyR2 yji-eptC Kan::FRT | SMR4562xP1(SMR20958) |
| PJH3295 | FC40 oxyR2 yji-eptC Kan::FRT Δdps::FRT | PJH2608xP1(SMR20958) |
| SMR601 | ruvC53 eda51::Tn10 | [109] |
| SMR789 | FC40 ruvC53 eda51::Tn10 | FC40 x P1(SMR601) |
| SMR4562 | Independent construction of FC40 | [106] |
| SMR4610 | SMR4562 recA::Tn10dCam | [110] |
| SMR5889 | SMR4562 ΔdinB50::FRT [F' ΔdinB50::FRT] | [41] |
| SMR6906 | SMR4562 ruvC53 eda::Tn10dCam | By linear replacement [107] into SMR789 |
| SMR8847 | SMR4562 [F' zah281::Tn10 Lac+] | SMR4562 x P1(CAG12080) [108] |
| SMR10308 | SMR4562 [F' lafU2::FRTcatFRT dinB21(oc)] | [41] |
| SMR10798 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRTcatFRT Δattλ:: PBADI-sceI | [7] |
| SMR10865 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRT Δzie3920.5::3ChiKanISceIsite Δattλ:: PBADIsceI rpoS::FRT | [7] |
| SMR10866 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRT Δzie3920.5::3ChiKanI-SceIsite Δattλ:: PBADIsceI | [7] |
| SMR10868 | FC36 ΔaraBAD567 Δzie3913.1::tetRtetA+1 FRT Δzie3920.5::3ChiKanI-SceIsite Δattλ:: PBADI-SceI dinB50::FRT | [7] |
| SMR12566 | SMR4562 ΔrssB::tet | [75] |
| SMR15378 | SMR4562 Δattλ:: PBADmutLcat | By replacement of λ prophage [111] in SMR8166 |
| SMR20958 | MG1655 Δattλ::PsulAΩsRBS75mCherry FRT IN-FOG84 | [112] |
*All JW strains are from the Keio Collection, described in [113].
Lac+ MBR assays
Experimental procedures are as described [53]. The strains to be compared are grown at 37° for two days in M9 medium with 0.1% glycerol and thiamine, then mixed with a 25-fold excess of Δlac scavenger cells, and plated in top agar on M9 minimal lactose and thiamine solid medium. Plates are incubated at 37°, and Lac+ revertant colonies counted daily. About half of the colonies appearing on day 2 result from Lac+ mutant cells that arose during growth. Stress-induced mutant colonies appear linearly from day 3 onward. These experiments were continued to day 7 to obtain measurements of lac-amplification, which forms colonies later than SNA mutations [43]. The mutation rate (mutants per cell per day) is taken from the linear part of the curve as the mean and SEM of three or four parallel cultures of each strain. To determine cell viability during the prolonged starvation, the Lac- lawn is sampled at intervals by plating cells on complete medium containing rifampicin, which does not allow growth of the scavenger cells. 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (x-gal) is included in the medium so that only Lac- cells are counted. In all experiments reported, Lac- viable cell counts did not vary significantly over the course of the experiment. Bipyridine and TU were added to the solid lactose minimal medium on which the starved cells were spread. Bipyridine was dissolved in ethanol. TU was dissolved in distilled water. Both solutions were filter-sterilized. Errors on all reported experiments are one standard error of the mean (SEM) of at least three independent experiments of three or four cultures per strain per experiment. To distinguish indel from amplified Lac+ cfu, samples of Lac+ colonies were replated onto rich medium containing x-gal dye on which SNAs produce solid blue colonies and amplified isolates produce sectored blue and white colonies [43]. MutL, expressed from the chromosome regulated by a PBAD promoter, was derepressed by absence of glucose in the medium during growth and on the minimal medium lactose plates.
Chromosomal Tet MBR assays
Experimental procedures are described in [7]. Four cultures of each strain are grown in M9 glycerol liquid medium with 20μg/ml carbenicillin for plasmid maintenance, 50μg/ml proline and with 0.1% glucose to repress PBAD, back-diluted after 12 hours twice and cultured for 84 hours. I-SceI endonuclease is induced by exhaustion of the glucose in the medium. Samples are then plated on complete medium with 0.1% glucose with and without tetracycline to obtain the TetR mutant frequency, and colonies are counted after one day. Activity of I-SceI was confirmed for all cultures during the experiments by their inability to grow with 0.0001% arabinose in M9 glycerol medium with IPTG (S2A Fig). I-SceI cutting was confirmed for each strain by the loss of viability when plated on medium without glucose containing 0.001% arabinose (S2B Fig). Error bars on all reported data are the SEM of at least 3 independent experiments. Genes carried in mobile plasmid library plasmids [60] were introduced to strains containing inducible DSB I-SceI cut-sites and enzyme and induced by adding 1mM IPTG to the liquid cultures during growth. A control strain containing an empty overexpression plasmid vector and an I-SceI cutsite and I-SceI enzyme cassette was also similarly treated with IPTG and plated to control for effects of plasmid expression on DSB induction and growth.
Ultraviolet irradiation and MMS treatment
Immediately prior to plating, aliquots of Lac-assay cells in prolonged stationary phase per the Lac MBR assay, were exposed to either UV-C irradiation or MMS. For MMS treatment, 1 mL of starved cell culture was pulse treated with MMS at 5 or 10mM, freshly diluted in water, for 20 minutes at 37°C. For UV-C irradiation, 1 mL aliquots of each culture were irradiated at either 5 J/m2, or 10 J/m2 using a Stratalinker 2400 UV lamp emitting at 254nm. Viable cell count was determined after MMS and UV treatment to confirm that there was no loss of viability.
Supporting information
Reconstruction experiments, using SMR4562, in which a Lac+ indel revertant and three lac-amplified strains were mixed with Δlac scavenger cells and plated in precise reconstructions of mutant selection conditions show that neither treatment with TU nor 2'2-bipyridine reduces (A) cell viability or (B) the speed of formation of Lac+ revertant colonies under experimental assay conditions. The data indicate that reductions in yields of Lac+ colonies in MBR experiments with TU or bip treatment reflect reduction of mutagenesis, not inability of mutant cells to form colonies in the presence of those ROS-reducing agents. Left panels, bip treatment; right panels, TU treatment.
(DOCX)
(A). Activity of I-SceI was confirmed for all cultures during the experiments by their inability to grow with 0.0001% arabinose in M9 glycerol medium with IPTG (arabinose medium). Only cultures of strains lacking the I-SceI cutsite showed significant growth. (B). I-SceI cutting in the presence of induced mobile plasmids genes was confirmed by their loss of viability at higher arabinose concentrations. Chromosomal PBAD-ISceI cassettes were induced with 0.001% arabinose in the presence and absence of 1 mM IPTG and DSB formation via I-SceI cleavage was measured as the frequency of arabinose-sensitive cfu among total viable cells (assayed on glucose). Student’s t-tests found no significant differences between the frequencies of arabinose-resistance in the presence or absence of IPTG in strains expressing vector only (PJH3232, p = 0.32), pSodB (PJH3257, p = 0.77), pMutT (PJH3256, p = 0.67), and pMutM (PJH3233, p = 0.60) mobile plasmids. Frequencies of arabinose-resistance were taken for three cultures per strain per experiment and frequencies shown are the means of 3 experiments with error bars representing ± SEM. Cells were taken from cultures 52 hours into the Tet assay protocol and plated on M9 minimal medium with 0.1% glucose for viable cell titer, and on M9 minimal medium containing 0.1% glycerol and 0.001% arabinose to induce I-SceI. Other supplements were as described in Methods.
(DOCX)
Examples from two different experiments. Three 3ml cultures were grown overnight in M9 glucose medium with proline and carbenicillin at 37°, and diluted 100-fold in the same medium with or without IPTG. 200ml of each culture was inoculated in triplicate into each medium, randomized in 96-well plates and change in OD 600 was monitored for 24 h. Most strains show some increase in growth upon induction by IPTG and none shows inhibition. Strains used were those employed in the Tet assay mutation experiments.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Aeronautics and Space Administration through the NASA Astrobiology Institute under Cooperative Agreement No. NNA13AA91A issued through the Science Mission Directorate (PJH), National Institutes of Health Grant R01-GM106373 (PJH), and National Institutes of Health Grant R35-GM122598 (SMR). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Drake JW. General antimutators are improbable. Journal of molecular biology. 1993;229(1):8–13. doi: 10.1006/jmbi.1993.1002 . [DOI] [PubMed] [Google Scholar]
- 2.Smith KC. Spontaneous mutagenesis: experimental, genetic and other factors. Mutation research. 1992;277(2):139–62. . [DOI] [PubMed] [Google Scholar]
- 3.Hastings PJ, Quah SK, von Borstel RC. Spontaneous mutation by mutagenic repair of spontaneous lesions in DNA. Nature. 1976;264(5588):719–22. . [DOI] [PubMed] [Google Scholar]
- 4.Harris RS, Longerich S, Rosenberg SM. Recombination in adaptive mutation. Science. 1994;264:258–60. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SM, Longerich S, Gee P, Harris RS. Adaptive mutation by deletions in small mononucleotide repeats. Science. 1994;265(5170):405–7. . [DOI] [PubMed] [Google Scholar]
- 6.Ponder RG, Fonville NC, Rosenberg SM. A switch from high-fidelity to error-prone DNA double-strand break repair underlies stress-induced mutation. Molecular cell. 2005;19(6):791–804. doi: 10.1016/j.molcel.2005.07.025 . [DOI] [PubMed] [Google Scholar]
- 7.Shee C, Gibson JL, Darrow MC, Gonzales C, Rosenberg SM. Impact of a stress-inducible switch to mutagenic repair of DNAbreaks on mutation in E. coli. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13659–64. doi: 10.1073/pnas.1104681108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shee C, Gibson JL, Rosenberg SM. Two mechanisms produce mutation hotspots at DNA breaks in Escherichia coli. Cell reports. 2012;2(4):714–21. doi: 10.1016/j.celrep.2012.08.033 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strathern JN, Shafer BK, McGill CB. DNA synthesis errors associated with double-strand-break repair. Genetics. 1995;140(3):965–72. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nik-Zainal S, Alexandrov LB, Wedge DC, Van Loo P, Greenman CD, Raine K, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149(5):979–93. doi: 10.1016/j.cell.2012.04.024 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nik-Zainal S, Van Loo P, Wedge DC, Alexandrov LB, Greenman CD, Lau KW, et al. The life history of 21 breast cancers. Cell. 2012;149(5):994–1007. doi: 10.1016/j.cell.2012.04.023 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts SA, Sterling J, Thompson C, Harris S, Mav D, Shah R, et al. Clustered mutations in yeast and in human cancers can arise from damaged long single-strand DNA regions. Molecular cell. 2012;46(4):424–35. doi: 10.1016/j.molcel.2012.03.030 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald D, Hastings PJ, Rosenberg SM. Stress-induced mutagenesis: implications for cancer and drug resistance. Annu Rev Cancer Biol 2017;1:6.1–6.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malkova A, Haber JE. Mutations arising during repair of chromosome breaks. Annual review of genetics. 2012;46:455–73. doi: 10.1146/annurev-genet-110711-155547 . [DOI] [PubMed] [Google Scholar]
- 15.Rogers E, Correa R, Barreto B, Bravo Núñez MA, Minnick PJ, Vera Cruz D, et al. Double-Strand-Break Repair Mutagenesis and Stress In: DeBrujin F, editor. Stress and Environmental Control of Gene Expression in Bacteria. John Wiley and Sons; 2016. doi: 10.1002/97811119004813 [Google Scholar]
- 16.Rosenberg SM, Shee C, Frisch RL, Hastings PJ. Stress-induced mutation via DNA breaks in Escherichia coli: a molecular mechanism with implications for evolution and medicine. Bioessays. 2012;34:885–92. doi: 10.1002/bies.201200050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quah SK, von Borstel RC, Hastings PJ. The origin of spontaneous mutation in Saccharomyces cerevisiae. Genetics. 1980;96(4):819–39. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison A, Christensen RB, Alley J, Beck AK, Bernstine EG, Lemontt JF, et al. REV3, a Saccharomyces cerevisiae gene whose function is required for induced mutagenesis, is predicted to encode a nonessential DNA polymerase. Journal of bacteriology. 1989;171(10):5659–67. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomerantz RT, Kurth I, Goodman MF, O'Donnell ME. Preferential D-loop extension by a translesion DNA polymerase underlies error-prone recombination. Nature structural & molecular biology. 2013;20(6):748–55. doi: 10.1038/nsmb.2573 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motamedi MR, Szigety SK, Rosenberg SM. Double-strand-break repair recombination in Escherichia coli: physical evidence for a DNA replication mechanism in vivo. Genes & development. 1999;13(21):2889–903. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenzie GJ, Lee PL, Lombardo MJ, Hastings PJ, Rosenberg SM. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Molecular cell. 2001;7(3):571–9. . [DOI] [PubMed] [Google Scholar]
- 22.Frisch RL, Su Y, Thornton PC, Gibson JL, Rosenberg SM, Hastings PJ. Separate DNA Pol II- and Pol IV-dependent pathways of stress-induced mutation during double-strand-break repair in Escherichia coli are controlled by RpoS. Journal of bacteriology. 2010;192(18):4694–700. doi: 10.1128/JB.00570-10 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrosino JF, Galhardo RS, Morales LD, Rosenberg SM. Stress-induced beta-lactam antibiotic resistance mutation and sequences of stationary-phase mutations in the Escherichia coli chromosome. Journal of bacteriology. 2009;191(19):5881–9. doi: 10.1128/JB.00732-09 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maharjan R, Ferenci T. Mutational signatures indicative of environmental stress in bacteria. Molecular biology and evolution. 2015;32(2):380–91. doi: 10.1093/molbev/msu306 . [DOI] [PubMed] [Google Scholar]
- 25.Ohno M, Miura T, Furuichi M, Tominaga Y, Tsuchimoto D, Sakumi K, et al. A genome-wide distribution of 8-oxoguanine correlates with the preferred regions for recombination and single nucleotide polymorphism in the human genome. Genome research. 2006;16(5):567–75. doi: 10.1101/gr.4769606 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohno M, Sakumi K, Fukumura R, Furuichi M, Iwasaki Y, Hokama M, et al. 8-oxoguanine causes spontaneous de novo germline mutations in mice. Scientific reports. 2014;4:4689 doi: 10.1038/srep04689 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nature reviews Microbiology. 2010;8(6):423–35. doi: 10.1038/nrmicro2333 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berquist BR, Wilson DM 3rd. Pathways for repairing and tolerating the spectrum of oxidative DNA lesions. Cancer Lett. 2012;327(1–2):61–72. doi: 10.1016/j.canlet.2012.02.001 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demple B, Harrison L. Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem. 1994;63:915–48. doi: 10.1146/annurev.bi.63.070194.004411 . [DOI] [PubMed] [Google Scholar]
- 30.Gutowski M, Kowalczyk S. A study of free radical chemistry: their role and pathophysiological significance. Acta biochimica Polonica. 2013;60(1):1–16. . [PubMed] [Google Scholar]
- 31.Wu J, Weiss B. Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. Journal of bacteriology. 1992;174(12):3915–20. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storz G, Altuvia S. OxyR regulon. Methods in enzymology. 1994;234:217–23. . [DOI] [PubMed] [Google Scholar]
- 33.Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. The EMBO journal. 2005;24(7):1311–7. doi: 10.1038/sj.emboj.7600599 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu AL, Li X, Gu Y, Wright PM, Chang DY. Repair of oxidative DNA damage: mechanisms and functions. Cell biochemistry and biophysics. 2001;35(2):141–70. doi: 10.1385/CBB:35:2:141 . [DOI] [PubMed] [Google Scholar]
- 35.Avkin S, Livneh Z. Efficiency, specificity and DNA polymerase-dependence of translesion replication across the oxidative DNA lesion 8-oxoguanine in human cells. Mutation research. 2002;510(1–2):81–90. . [DOI] [PubMed] [Google Scholar]
- 36.Fowler RG, White SJ, Koyama C, Moore SC, Dunn RL, Schaaper RM. Interactions among the Escherichia coli mutT, mutM, and mutY damage prevention pathways. DNA repair. 2003;2(2):159–73. . [DOI] [PubMed] [Google Scholar]
- 37.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349(6308):431–4. doi: 10.1038/349431a0 . [DOI] [PubMed] [Google Scholar]
- 38.Markkanen E, Castrec B, Villani G, Hubscher U. A switch between DNA polymerases delta and lambda promotes error-free bypass of 8-oxo-G lesions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(50):20401–6. doi: 10.1073/pnas.1211532109 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner J, Fujii S, Gruz P, Nohmi T, Fuchs RP. The beta clamp targets DNA polymerase IV to DNA and strongly increases its processivity. EMBO Rep. 2000;1(6):484–8. Epub 2001/03/27. doi: 10.1093/embo-reports/kvd109 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SR, Maenhaut-Michel G, Yamada M, Yamamoto Y, Matsui K, Sofuni T, et al. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13792–7. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galhardo RS, Do R, Yamada M, Friedberg EC, Hastings PJ, Nohmi T, et al. DinB upregulation is the sole role of the SOS response in stress-induced mutagenesis in Escherichia coli. Genetics. 2009;182(1):55–68. doi: 10.1534/genetics.109.100735 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cairns J, Foster PL. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics. 1991;128(4):695–701. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hastings PJ, Bull HJ, Klump JR, Rosenberg SM. Adaptive amplification: an inducible chromosomal instability mechanism. Cell. 2000;103:723–31. [DOI] [PubMed] [Google Scholar]
- 44.McKenzie GJ, Rosenberg SM. Adaptive mutations, mutator DNA polymerases and genetic change strategies of pathogens. Current opinion in microbiology. 2001;4(5):586–94. . [DOI] [PubMed] [Google Scholar]
- 45.Slack A, Thornton PC, Magner DB, Rosenberg SM, Hastings PJ. On the mechanism of gene amplification induced under stress in Escherichia coli. PLoS genetics. 2006;2(4):e48 doi: 10.1371/journal.pgen.0020048 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hastings PJ, Ira G, Lupski JR. A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS genetics. 2009;5(1):e1000327 Epub 2009/01/31. doi: 10.1371/journal.pgen.1000327 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wimberly H, Shee C, Thornton PC, Sivaramakrishnan P, Rosenberg SM, Hastings PJ. R-loops and nicks initiate DNA breakage and genome instability in non-growing Escherichia coli. Nature communications. 2013;4:2115 doi: 10.1038/ncomms3115 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foster PL, Trimarchi JM, Maurer RA. Two enzymes, both of which process recombination intermediates, have opposite effects on adaptive mutation in Escherichia coli. Genetics. 1996;142(1):25–37. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris RS, Ross KJ, Rosenberg SM. Opposing roles of the holliday junction processing systems of Escherichia coli in recombination-dependent adaptive mutation. Genetics. 1996;142(3):681–91. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Layton JC, Foster PL. Error-prone DNA polymerase IV is controlled by the stress-response sigma factor, RpoS, in Escherichia coli. Molecular microbiology. 2003;50:549–61. doi: 10.1046/j.1365-2958.2003.03704.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lombardo MJ, Aponyi I, Rosenberg SM. General stress response regulator RpoS in adaptive mutation and amplification in Escherichia coli. Genetics. 2004;166(2):669–80. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibson JL, Lombardo MJ, Thornton PC, Hu KH, Galhardo RS, Beadle B, et al. The sigma(E) stress response is required for stress-induced mutation and amplification in Escherichia coli. Molecular microbiology. 2010;77(2):415–30. doi: 10.1111/j.1365-2958.2010.07213.x ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hastings PJ, Slack A, Petrosino JF, Rosenberg SM. Adaptive amplification and point mutation are independent mechanisms: evidence for various stress-inducible mutation mechanisms. PLoS biology. 2004;2(12):e399 doi: 10.1371/journal.pbio.0020399 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–9. doi: 10.1126/science.1219192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mello Filho AC, Hoffmann ME, Meneghini R. Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. The Biochemical journal. 1984;218(1):273–5. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Floyd RA, West MS, Eneff KL, Hogsett WE, Tingey DT. Hydroxyl free radical mediated formation of 8-hydroxyguanine in isolated DNA. Archives of biochemistry and biophysics. 1988;262(1):266–72. . [DOI] [PubMed] [Google Scholar]
- 57.Moore JM, Magnan D, Mojica AK, Bravo Núñez MA, Bates D, Rosenberg SM, et al. Roles of Nucleoid-Associated Proteins in Stress-Induced Mutagenic Break Repair in Starving Escherichia coli. Genetics. 2015;201:1349–62. doi: 10.1534/genetics.115.178970 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christman MF, Storz G, Ames BN. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(10):3484–8. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nunoshiba T, Demple B. A cluster of constitutive mutations affecting the C-terminus of the redox-sensitive SoxR transcriptional activator. Nucleic acids research. 1994;22(15):2958–62. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saka K, Tadenuma M, Nakade S, Tanaka N, Sugawara H, Nishikawa K, et al. A complete set of Escherichia coli open reading frames in mobile plasmids facilitating genetic studies. DNA research: an international journal for rapid publication of reports on genes and genomes. 2005;12(1):63–8. . [DOI] [PubMed] [Google Scholar]
- 61.Kuzminov A. Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8241–6. Epub 2001/07/19. doi: 10.1073/pnas.131009198 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ballesteros M, Fredriksson A, Henriksson J, Nystrom T. Bacterial senescence: protein oxidation in non-proliferating cells is dictated by the accuracy of the ribosomes. The EMBO journal. 2001;20(18):5280–9. doi: 10.1093/emboj/20.18.5280 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris RS, Feng G, Ross KJ, Sidhu R, Thulin C, Longerich S, et al. Mismatch repair protein MutL becomes limiting during stationary-phase mutation. Genes & Dev. 1997;11:2426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Becker G, Klauck E, Hengge-Aronis R. Regulation of RpoS proteolysis in Escherichia coli: the response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(11):6439–44. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schaaper RM, Radman M. The extreme mutator effect of Escherichia coli mutD5 results from saturation of mismatch repair by excessive DNA replication errors. The EMBO journal. 1989;8(11):3511–6. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beranek DT. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutation research. 1990;231(1):11–30. . [DOI] [PubMed] [Google Scholar]
- 67.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. Washington, D. C.: ASM Press; 2005. [Google Scholar]
- 68.Grzesiuk E, Janion C. The frequency of MMS-induced, umuDC-dependent, mutations declines during starvation in Escherichia coli. Molecular & general genetics: MGG. 1994;245(4):486–92. . [DOI] [PubMed] [Google Scholar]
- 69.Walker GC. SOS-regulated proteins in translesion DNA synthesis and mutagenesis. Trends in biochemical sciences. 1995;20(10):416–20. . [DOI] [PubMed] [Google Scholar]
- 70.Cafarelli TM, Rands TJ, Godoy VG. The DinB*RecA complex of Escherichia coli mediates an efficient and high-fidelity response to ubiquitous alkylation lesions. Environmental and molecular mutagenesis. 2014;55(2):92–102. doi: 10.1002/em.21826 . [DOI] [PubMed] [Google Scholar]
- 71.Zhang X, Rosenstein BS, Wang Y, Lebwohl M, Mitchell DM, Wei H. Induction of 8-oxo-7,8-dihydro-2'-deoxyguanosine by ultraviolet radiation in calf thymus DNA and HeLa cells. Photochemistry and photobiology. 1997;65(1):119–24. . [DOI] [PubMed] [Google Scholar]
- 72.Salmon TB, Evert BA, Song B, Doetsch PW. Biological consequences of oxidative stress-induced DNA damage in Saccharomyces cerevisiae. Nucleic acids research. 2004;32(12):3712–23. doi: 10.1093/nar/gkh696 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitanovic A, Walther T, Loret MO, Holzwarth J, Kitanovic I, Bonowski F, et al. Metabolic response to MMS-mediated DNA damage in Saccharomyces cerevisiae is dependent on the glucose concentration in the medium. FEMS yeast research. 2009;9(4):535–51. doi: 10.1111/j.1567-1364.2009.00505.x . [DOI] [PubMed] [Google Scholar]
- 74.Kitanovic A, Wolfl S. Fructose-1,6-bisphosphatase mediates cellular responses to DNA damage and aging in Saccharomyces cerevisiae. Mutation research. 2006;594(1–2):135–47. doi: 10.1016/j.mrfmmm.2005.08.005 . [DOI] [PubMed] [Google Scholar]
- 75.Al Mamun AA, Lombardo MJ, Shee C, Lisewski AM, Gonzalez C, Lin D, et al. Identity and function of a large gene network underlying mutagenic repair of DNA breaks. Science. 2012;338(6112):1344–8. doi: 10.1126/science.1226683 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heltzel JM, Maul RW, Wolff DW, Sutton MD. Escherichia coli DNA polymerase IV (Pol IV), but not Pol II, dynamically switches with a stalled Pol III* replicase. Journal of bacteriology. 2012;194(14):3589–600. doi: 10.1128/JB.00520-12 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Indiani C, McInerney P, Georgescu R, Goodman MF, O'Donnell M. A sliding-clamp toolbelt binds high- and low-fidelity DNA polymerases simultaneously. Molecular cell. 2005;19(6):805–15. doi: 10.1016/j.molcel.2005.08.011 . [DOI] [PubMed] [Google Scholar]
- 78.Scotland MK, Heltzel JM, Kath JE, Choi JS, Berdis AJ, Loparo JJ, et al. A Genetic Selection for dinB Mutants Reveals an Interaction between DNA Polymerase IV and the Replicative Polymerase That Is Required for Translesion Synthesis. PLoS genetics. 2015;11(9):e1005507 doi: 10.1371/journal.pgen.1005507 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bichara M, Meier M, Wagner J, Cordonnier A, Lambert IB. Postreplication repair mechanisms in the presence of DNA adducts in Escherichia coli. Mutation research. 2011;727(3):104–22. doi: 10.1016/j.mrrev.2011.04.003 . [DOI] [PubMed] [Google Scholar]
- 80.Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, et al. The Y-family of DNA polymerases. Molecular cell. 2001;8(1):7–8. . [DOI] [PubMed] [Google Scholar]
- 81.Fujii S, Fuchs RP. Interplay among replicative and specialized DNA polymerases determines failure or success of translesion synthesis pathways. Journal of molecular biology. 2007;372(4):883–93. doi: 10.1016/j.jmb.2007.07.036 . [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi S, Valentine MR, Pham P, O'Donnell M, Goodman MF. Fidelity of Escherichia coli DNA polymerase IV. Preferential generation of small deletion mutations by dNTP-stabilized misalignment. J Biol Chem. 2002;277(37):34198–207. doi: 10.1074/jbc.M204826200 . [DOI] [PubMed] [Google Scholar]
- 83.Kokoska RJ, Bebenek K, Boudsocq F, Woodgate R, Kunkel TA. Low fidelity DNA synthesis by a Y family DNA polymerase due to misalignment in the active site. J Biol Chem. 2002;277(22):19633–8. doi: 10.1074/jbc.M202021200 . [DOI] [PubMed] [Google Scholar]
- 84.Payen C, Koszul R, Dujon B, Fischer G. Segmental duplications arise from Pol32-dependent repair of broken forks through two alternative replication-based mechanisms. PLoS genetics. 2008;4(9):e1000175 doi: 10.1371/journal.pgen.1000175 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Branzei D, Foiani M. The DNA damage response during DNA replication. Current opinion in cell biology. 2005;17(6):568–75. doi: 10.1016/j.ceb.2005.09.003 . [DOI] [PubMed] [Google Scholar]
- 86.Chan SH, Yu AM, McVey M. Dual roles for DNA polymerase theta in alternative end-joining repair of double-strand breaks in Drosophila. PLoS genetics. 2010;6(7):e1001005 doi: 10.1371/journal.pgen.1001005 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koole W, van Schendel R, Karambelas AE, van Heteren JT, Okihara KL, Tijsterman M. A Polymerase Theta-dependent repair pathway suppresses extensive genomic instability at endogenous G4 DNA sites. Nature communications. 2014;5:3216 doi: 10.1038/ncomms4216 . [DOI] [PubMed] [Google Scholar]
- 88.Wyatt DW, Feng W, Conlin MP, Yousefzadeh MJ, Roberts SA, Mieczkowski P, et al. Essential Roles for Polymerase theta-Mediated End Joining in the Repair of Chromosome Breaks. Molecular cell. 2016;63(4):662–73. doi: 10.1016/j.molcel.2016.06.020 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haber JE. A life investigating pathways that repair broken chromosomes. Annu Rev Genet 2016;50:18.1–.28. doi: 10.1146/annurev-genet-120215-035043 [DOI] [PubMed] [Google Scholar]
- 90.Mahaseth T, Kuzminov A. Cyanide enhances hydrogen peroxide toxicity by recruiting endogenous iron to trigger catastrophic chromosomal fragmentation. Molecular microbiology. 2015;96(2):349–67. doi: 10.1111/mmi.12938 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahaseth T, Kuzminov A. Prompt repair of hydrogen peroxide-induced DNA lesions prevents catastrophic chromosomal fragmentation. DNA repair. 2016;41:42–53. doi: 10.1016/j.dnarep.2016.03.012 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chlebowicz E, Jachymczyk WJ. Repair of MMS-induced DNA double-strand breaks in haploid cells of Saccharomyces cerevisiae, which requires the presence of a duplicate genome. Molecular & general genetics: MGG. 1979;167(3):279–86. . [DOI] [PubMed] [Google Scholar]
- 93.Khan SR, Kuzminov A. Replication forks stalled at ultraviolet lesions are rescued via RecA and RuvABC protein-catalyzed disintegration in Escherichia coli. J Biol Chem. 2012;287(9):6250–65. doi: 10.1074/jbc.M111.322990 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Degtyareva NP, Heyburn L, Sterling J, Resnick MA, Gordenin DA, Doetsch PW. Oxidative stress-induced mutagenesis in single-strand DNA occurs primarily at cytosines and is DNA polymerase zeta-dependent only for adenines and guanines. Nucleic acids research. 2013;41(19):8995–9005. doi: 10.1093/nar/gkt671 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Y, Sterling J, Storici F, Resnick MA, Gordenin DA. Hypermutability of damaged single-strand DNA formed at double-strand breaks and uncapped telomeres in yeast Saccharomyces cerevisiae. PLoS genetics. 2008;4(11):e1000264 doi: 10.1371/journal.pgen.1000264 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bridges BA. The role of DNA damage in stationary phase ("adaptive") mutation. Mutat Res DNA Repair. 1998;408:1–9. [DOI] [PubMed] [Google Scholar]
- 97.Bridges BA. Elevated mutation rate in mutT bacteria during starvation: evidence for DNA turnover? J Bacteriol. 1997;178:2709–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakai A, Nakanishi M, Yoshiyama K, Maki H. Impact of reactive oxygen species on spontaneous mutagenesis in Genes to cells: devoted to molecular & cellular mechanisms. 2006;11(7):767–78. [DOI] [PubMed] [Google Scholar]
- 99.Ram Y, Hadany L. Stress-induced mutagenesis and complex adaptation. Proceedings Biological sciences / The Royal Society. 2014;281(1792). doi: 10.1098/rspb.2014.1025 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. . [DOI] [PubMed] [Google Scholar]
- 101.Zuo L, Zhou T, Pannell BK, Ziegler AC, Best TM. Biological and physiological role of reactive oxygen species—the good, the bad and the ugly. Acta Physiol (Oxf). 2015;214(3):329–48. doi: 10.1111/apha.12515 . [DOI] [PubMed] [Google Scholar]
- 102.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001 . [DOI] [PubMed] [Google Scholar]
- 103.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001 . [DOI] [PubMed] [Google Scholar]
- 104.Bogdan C, Rollinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12(1):64–76. . [DOI] [PubMed] [Google Scholar]
- 105.Ram Y, Hadany L. The evolution of stress-induced hypermutation in asexual populations. Evolution; international journal of organic evolution. 2012;66(7):2315–28. doi: 10.1111/j.1558-5646.2012.01576.x . [DOI] [PubMed] [Google Scholar]
- 106.McKenzie GJ, Harris RS, Lee PL, Rosenberg SM. The SOS response regulates adaptive mutation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(12):6646–51. doi: 10.1073/pnas.120161797 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(12):6640–5. doi: 10.1073/pnas.120163297 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singer M, Baker TA, Schnitzler G, Deischel SM, Goel M, Dove W, et al. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiological reviews. 1989;53(1):1–24. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lopez CR, Yang S, Deibler RW, Ray SA, Pennington JM, Digate RJ, et al. A role for topoisomerase III in a recombination pathway alternative to RuvABC. Molecular microbiology. 2005;58(1):80–101. doi: 10.1111/j.1365-2958.2005.04812.x . [DOI] [PubMed] [Google Scholar]
- 110.Bull HJ, Lombardo MJ, Rosenberg SM. Stationary-phase mutation in the bacterial chromosome: recombination protein and DNA polymerase IV dependence. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8334–41. doi: 10.1073/pnas.151009798 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gumbiner-Russo LM, Lombardo MJ, Ponder RG, Rosenberg SM. The TGV transgenic vectors for single-copy gene expression from the Escherichia coli chromosome. Gene. 2001;273(1):97–104. . [DOI] [PubMed] [Google Scholar]
- 112.Nehring RB, Gu F, Lin HY, Gibson JL, Blythe MJ, Wilson R, et al. An ultra-dense library resource for rapid deconvolution of mutations that cause phenotypes in Escherichia coli. Nucleic acids research. 2015. doi: 10.1093/nar/gkv1131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Molecular systems biology. 2006;2:2006 0008 doi: 10.1038/msb4100050 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reconstruction experiments, using SMR4562, in which a Lac+ indel revertant and three lac-amplified strains were mixed with Δlac scavenger cells and plated in precise reconstructions of mutant selection conditions show that neither treatment with TU nor 2'2-bipyridine reduces (A) cell viability or (B) the speed of formation of Lac+ revertant colonies under experimental assay conditions. The data indicate that reductions in yields of Lac+ colonies in MBR experiments with TU or bip treatment reflect reduction of mutagenesis, not inability of mutant cells to form colonies in the presence of those ROS-reducing agents. Left panels, bip treatment; right panels, TU treatment.
(DOCX)
(A). Activity of I-SceI was confirmed for all cultures during the experiments by their inability to grow with 0.0001% arabinose in M9 glycerol medium with IPTG (arabinose medium). Only cultures of strains lacking the I-SceI cutsite showed significant growth. (B). I-SceI cutting in the presence of induced mobile plasmids genes was confirmed by their loss of viability at higher arabinose concentrations. Chromosomal PBAD-ISceI cassettes were induced with 0.001% arabinose in the presence and absence of 1 mM IPTG and DSB formation via I-SceI cleavage was measured as the frequency of arabinose-sensitive cfu among total viable cells (assayed on glucose). Student’s t-tests found no significant differences between the frequencies of arabinose-resistance in the presence or absence of IPTG in strains expressing vector only (PJH3232, p = 0.32), pSodB (PJH3257, p = 0.77), pMutT (PJH3256, p = 0.67), and pMutM (PJH3233, p = 0.60) mobile plasmids. Frequencies of arabinose-resistance were taken for three cultures per strain per experiment and frequencies shown are the means of 3 experiments with error bars representing ± SEM. Cells were taken from cultures 52 hours into the Tet assay protocol and plated on M9 minimal medium with 0.1% glucose for viable cell titer, and on M9 minimal medium containing 0.1% glycerol and 0.001% arabinose to induce I-SceI. Other supplements were as described in Methods.
(DOCX)
Examples from two different experiments. Three 3ml cultures were grown overnight in M9 glucose medium with proline and carbenicillin at 37°, and diluted 100-fold in the same medium with or without IPTG. 200ml of each culture was inoculated in triplicate into each medium, randomized in 96-well plates and change in OD 600 was monitored for 24 h. Most strains show some increase in growth upon induction by IPTG and none shows inhibition. Strains used were those employed in the Tet assay mutation experiments.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.