Abstract
Introduction
Kawasaki disease is an acute febrile systemic vasculitis that predominantly occurs in children below five years of age. Its etiopathogenesis is still not clear, but it is thought to be a complex interplay of genetic factors, infections and immunity.
Areas covered
This review article discusses in detail Kawasaki disease, with particular emphasis on the recent updates on its pathogenesis and upcoming alternate treatment options. Though self-limiting in many cases, it can lead to severe complications like coronary artery aneurysms and thrombo-embolic occlusions, and hence requires early diagnosis and urgent attention to avoid them. Intravenous immunoglobulin (IVIG) with or without aspirin has remained the sole treatment option for these cases, but 10-15% cases develop resistance to this treatment.
Expert Commentary
There is a need to develop additional treatment strategies for children with Kawasaki disease. Targeting different steps of pathogenesis could provide us with alternate therapeutic options.
Keywords: Coronary artery aneurysm, Intravenous immunoglobulin, Kawasaki Disease, Refractory Kawasaki disease, Thrombo-embolic occlusion
1 Introduction
Kawasaki disease (KD), also called mucocutaneous lymph node syndrome, was first described by a Japanese pediatrician, Dr. Tomisaku Kawasaki in 1967 [1]. It is a febrile vasculitis disorder which is usually diagnosed by a cluster of signs and symptoms along with supporting laboratory findings. It is a multisystem disorder, with predilection for small and medium sized arteries, especially coronary arteries [2]. If left untreated, it can lead to various complications like coronary artery aneurysm, thrombosis, stenosis and even sudden death [3]. In fact, KD is considered as the leading cause of acquired heart diseases in developed countries [4]. KD has, therefore, remained an interesting subject of investigation to determine its exact etiology and pathogenesis, and warrants the development of other treatment options apart from intravenous immunoglobulin (IVIG) therapy. There has been considerable research done in this regard and in this article, we have critically reviewed the recent findings on the etiopathogenesis of KD and therapeutic alternatives.
2 Epidemiology
Kawasaki disease predominantly affects young children, mostly below 5 years of age, with 1.5-times higher risk in boys than girls [5]. The epidemiological patterns are quite distinct in different geographical locations, with variations in incidence based on ethnicities and season. Worldwide, the highest incidence has been found in Japan (239/100,000 below 5 years of age), followed by Korea (113.1/100,000) and Taiwan (69/100,000) [6]. In these countries robust nationwide epidemiological surveys for KD are conducted every 2-3 years. Three epidemics of KD have occurred in Japan each in the year 1979, 1982, and 1986 [4]. Epidemiological surveys in Japan have also reported higher incidence in children with parental or sibling with history of KD compared to the general population [7].
In the United States of America (USA), the incidence of KD hospitalizations has been reported to be 19/100,000 in children below 5 years of age [4]. However, clear variation has been noted in different ethnicities. Incidence is 2.5-times more in Asians and Pacific Islanders and 1.5-times more in blacks compared to Caucasians [4]. This is also supported by the fact that Hawaii, which has the highest Asian population in USA, has the highest incidence of KD in the USA with an average of 50.4/100,000 cases over a period of 1996-2006 in children below 5 years of age [8]. In Europe, United Kingdom reported an annual incidence of 8.4/100,000 in children below 5 years of age, while in Denmark and Netherlands it was found to be around 4-5/100,000 in children below 5 years [8].
Interestingly, seasonal variation has also been noted in the trends of KD incidence. While in Japan incidence is higher in January (winters) and July (summer), as also seen in Korea, USA has higher incidence during winter and spring season, with no variation noted in areas like Hawaii possibly due to the tropical climate throughout the year [9-11]. In Europe, incidence is highest in the winters. One hypothesis for this variation was suggested by Singh et al. [5]. These researchers hypothesized that KD might be triggered by an airborne agent in Central Asia which is then blown to different geographical locations to cause KD by gaining entry in the body through the respiratory tract. Therefore, the wind patterns might determine the incidence of KD in different parts of the world. But, this association has to be confirmed by conducting more epidemiological studies across the globe.
3 Diagnosis
Diagnosis of KD is a clinical challenge, given the wide variety of clinical presentations and the similarity to many viral and bacterial illnesses. The clinical sequel occurs in three phases: acute, subacute and convalescent phase [12]. Diagnosis of typical KD is made on the basis of the evidence of fever along with 4 out of the 5 other symptoms (Table 1). All the clinical features might not appear simultaneously, hence it is important to enquire about all the possible symptoms while taking history [13]. Kuo et al [14] have established a mnemonic to aid in memorizing these clinical symptoms. It is described as 1-2-3-4-5, with 1 for one ‘mouth’ (strawberry tongue and fissured lips); 2 for two ‘eyes’ (bilateral conjunctivitis); 3 for three fingers palpation of neck lymph nodes (cervical lymphadenopathy); 4 for limb changes (swelling of hands and feet) and 5 for multiple skin rash throughout the body. This is a simple yet effective way of remembering the variety of symptoms to look in suspicious Kawasaki cases.
Table 1.
Clinical features of classical Kawasaki disease patient and the supporting laboratory findings.
| Classical clinical case definition | Supportive laboratory findings |
|---|---|
Persistent fever for 5 days with additional at least 4 of the 5 following symptoms:
|
|
Acute phase usually lasts for up to 14 days and is characterized by high grade fever (> 38.5 °C), with no response to antipyretics or antibiotics. Conjunctival injection is another common finding, which is usually bilateral, painless, non-exudative with limited involvement of limbic area [13]. Apart from conjunctivitis, patients may also present with other eye symptoms like subconjuctival hemorrhages, uveitis and papilledema. Oropharyngeal mucosal changes include dry and cracked lips, strawberry tongue and non-exudative inflammation of the tongue [15]. Some children can present with cervical adenopathy, which is usually unilateral, mostly involving anterior cervical chain [12]. Rash of KD is non-pruritic, with macular lesions on trunk and extremities. Perineal desquamation is common in the acute phase while periungual desquamation is seen in the subacute phase [16]. Diffuse erythema of the palms and soles with clear distinction from the uninvolved skin and painful swelling of hands and feet is also noticed in the acute phase [13]. Other uncommon symptoms found to be associated with KD in few cases include diarrhea, vomiting, sterile pyuria, dysuria, arthritis, aseptic meningitis [17]. However, these symptoms are not a part of the diagnostic criteria for KD. In patients who have received Bacillus Calmette-Guerin vaccine, erythema, crusting and induration of the skin at the site of vaccination are considered strong specific indicators of KD [13]. Tseng et al [18] recently attempted to correlate the grade of BCG reaction to the severity of systemic involvement in Kawasaki disease. They reported that severe induration in the form of target lesions was associated with highest elevation of liver enzymes, and the risk of coronary artery dilatations and milder induration in the form of a faint rash or a homogenous white area were associated with lesser degree of systemic inflammation in KD. These investigators also indicated that the target lesions could, therefore, even serve as biomarkers of clinical severity of KD [18].
KD has a predilection for cardiovascular complications. During acute phase, valvulitis, myocarditis, pericarditis and KD shock syndrome are commonly seen [12]. Coronary artery aneurysms (CAAs) and dilatation are most often in the subacute to convalescent phase. Almost 20% of the untreated children develop aneurysms [12]. Risk factors for developing aneurysms include: male sex, extremes of age, prolonged fever, delay in diagnosis and treatment [16]. Though involvement of coronary arteries is most common in KD, other arteries that might be affected include axillary, renal and iliac arteries [16].
According to the American Heart Association (AHA) guidelines outlined in 2004, Incomplete KD is the term used for patients with less than 4 positive symptoms along with fever and abnormal lab values, while atypical KD refers to patients with KD who present with rare symptoms like renal impairment [19]. These variations are usually common in younger infants, less than 6 months of age and are at higher risk of CAAs and other complications [13]. Accordingly, AHA recommends that infants less than 6 months of age with fever lasting for more than 7 days, at least 2 classical symptoms of KD and lab values showing systemic inflammation with no apparent alternate explanation should be evaluated by an echocardiograph for incomplete KD [19].
No lab studies are specific for KD, but they can help to rule out KD and predict the outcomes. In majority of the cases, signs of systemic inflammation like high erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are noticed in the acute phase [16]. Other findings include neutrophilic leukocytosis, normocytic normochromic anemia and thrombocytosis [15]. Echocardiography is useful to study in detail the coronary abnormalities. Hyponatremia is reported to predict adverse coronary outcomes [15]. Neutrophils are considered a marker of ongoing inflammation, whereas lymphocytes are markers of immune response. Hence, high neutrophil-to-lymphocyte ratio (NLR) could mean an imbalance between inflammatory and immune response. Ha et al. [20] studied the usefulness of neutrophil to lymphocyte ratio in predicting KD outcomes in 587 patients with KD. They reported that NLR after 2 days of IVIG (Intravenous immunoglobulin) treatment could be helpful in predicting the occurrence of CAAs (p=0.03) and resistance to IVIG (p<0.001). They concluded that NLR above 1 after 2 days of IVIG treatment indicated higher risk of CAAs and IVIG resistance. But this relationship still needs to be evaluated in larger prospective studies.
Given the high rate of cardiac complications in KD, usefulness of cardiac biomarkers in KD is also being evaluated. One such biomarker that appears to be highly promising is N-terminal pro-B-type natriuretic peptide (NT- proBNP) [21]. This biomarker is synthesized by ventricular cardiomyocytes and is an indicator of cardiomyocyte stress [22]. Elevated levels of NT-proBNP are found to be associated with diastolic dysfunction. A recent meta-analysis to determine the usefulness of proBNP in the diagnosis of KD by Lin et al [22] concluded that it is a specific (pooled specificity 0.72) and moderately sensitive (pooled sensitivity 0.89) diagnostic tool for KD, which could be helpful for recognizing KD in patients with undifferentiated febrile illness. Another study by Ye et al [23] found it to be highly useful for establishing response to IVIG treatment with higher levels noticed in patients who were unresponsive to IVIG treatment (area under the curve 0.73).
For patients with coronary artery lesions, regular monitoring is performed by conventional techniques like myocardial perfusion imaging and CT angiography [24]. Cardiac MRI is a newer technique that has been studied in recent for following up the patients and has the advantage of being radiation-free and non-invasive imaging technique [25]. It can provide information about both structure and function of cardiac tissue [23]. Tacke et al [24] performed a comprehensive MRI in sixty-three patients with KD and compared the findings to echocardiography results in these patients and reported favorable performance of cardiac MRI. Mavrogeni et al [26] similarly reported that cardiac MRI could be useful in acute phase of KD for identifying myocardial inflammation, left ventricular function, lesions of coronary artery lumen and vessel wall, while in chronic phase it might be helpful in risk stratification and treatment guidance.
4 Etiopathogenesis
The etiopathogenesis of KD has been studied extensively but is still not fully understood. It appears to be an interplay of genetic susceptibility and infectious trigger followed by an abnormal immune response.
4.1 Genetic susceptibility
Genetic susceptibility is one of the areas of ongoing investigation in KD given the specific patterns of occurrence of this condition in different ethnic groups and races. Epidemiological studies have shown highest incidence in Japan, Korea and Taiwan [8] and Asian races in the USA [4]. In Japan, it has been reported that there is ten-times more risk of KD in siblings of patients with KD than general population and two-times higher risk in children with parental history of KD [7]. These differences in the family and races suggest that genetic factors could play a strong role in the pathogenesis of KD.
Initial studies on the genetic basis of KD were focused on Human Leukocyte Antigen (HLA) and found HLA-DRB1, HLA B5, Bw51 and Bw44 to be associated with KD susceptibility [27]. There are many ongoing investigations to detect candidate genes for KD. These studies are being conducted not only to identify the genetic relationship of KD but also to provide insight to its etiopathogenesis [28]. With the beginning of Genome Wide Association studies, considerable progress has been achieved to identify potential loci of susceptibility. Some of the most widely studied genes to be found consistently associated with KD include inositol 1,4,5-triphosphate 3-kinase (ITPKC), caspase-3 (CASP3), B lymphocyte kinase (BLK), CD40, and HLA.
The ITPKC gene codes for one of the three isoenzymes of inositol 1,4,5-triphosphate 3-kinase that is involved in the Ca2+/nuclear factors of activated T- cells (NFAT) signaling pathway in T cells [29]. It functions as a negative regulator of T cell activation [29]. Onouchi et al in 2008 reported that single nucleotide polymorphisms (SNPs) in ITPKC on chromosome 19q13.2 were associated with susceptibility to KD and risk of coronary artery abnormalities in Japanese and American children (rs28493229) [30]. Other variants found to be associated with KD were SNP rs2720378 and rs2290692 [31]. Polymorphism of ITPKC might result in increased activation of T cells and consequently increased release of interleukin-2 (IL-2). This could lead to prolonged expression of T cells during acute phase of KD and cause vascular endothelial cell injury and subsequently, increase the severity of KD as well risk of coronary artery lesions [21, 23].
Caspase3 (CASP3) is a part of Activation Induced Cell Death (AICD) pathway of apoptosis. It carries out apoptosis of immature cells [31]. It cleaves Inositol 1,4,5 triphosphate receptor, type 1 in apoptotic T cells and thereby serves as the positive regulator of Ca2+/NFAT pathway [28]. Onouchi et al carried out a potential candidate gene study and reported a SNP within CASP3 gene (rs113420705) located on 4q34-35 to be associated with KD [32]. This finding was again consistent in both Japanese and American population. It has been suggested that the G to A substitution within exon1 of CASP3 reduces the binding of NFAT to DNA around the SNP, decreasing the transcription of CASP3 mRNA. This inhibits T cell apoptosis and causes prolonged activation of immune cells which in turn, might increase the susceptibility to KD. But, this CASP3 polymorphism was not found to be associated with the risk of CAAs or response to IVIG treatment [29, 31].
Another gene found to be strongly associated with KD is the Fc fragment of IgG, low affinity IIa, receptor (FCGR2A) gene [29]. It is expressed on the surface of immune cells like, dendritic cells, macrophages, monocytes and neutrophils and transduces activation signals into cells on binding with immune complexes [27]. The A to G substitution of the SNP in FGR2A (rs1801274) alters the translation of the 131st amino acid from histidine (H) to arginine (R) [33]. This polymorphic form now has lower binding affinity to IgG2, that could be related to more immune activation during the acute phase of KD [33].
B-lymphocyte kinase (BLK) SNPs have also been implicated in KD based on studies in Japanese, Taiwanese, Korean and Asian population [29]. BLK is a tyrosine kinase expressed mainly on the B cells where it is involved in signal transduction [27]. Two GWAS studies carried out independently in Japan and Taiwan reported considerable association of KD with FAM167A-BLK region on chromosome 8p23-p22. This suggests that the involvement of B cell might be in the pathogenesis of KD. Also, in animal studies it was found that BLK is required for maturation of IL 17 producing γδT cells in mice [27]. In acute phase of KD, high levels of IL-17 are reportedly which might be related to BLK polymorphism [27]. However, function of FAM167A still remains unclear [33].
The involvement of the Transforming growth factor-β (TGF-β) signaling pathway in KD susceptibility, disease outcome and response to treatment was shown by Shizimu et al [34]. TGF-β is involved in maintaining a balance of pro- and anti-inflammatory T cells and therefore, genetic variation in TGF-β pathway could lead to an imbalance between inflammatory and regulatory T cells by influencing Foxp3 expression mediated by SMAD3 and NFAT [6, 7, 21]. KD susceptibility was associated with one SNP each in TGF-β2 (rs 2796817) and TGFβR2 (rs 11466480) and 5 in SMAD3 [29].
Other genes found to be associated with KD susceptibility include CD40L, HLA DQB2, HLA DOB, KCNN2, DEL1, COB2 [27, 29].
4.2 Infections
Though the exact etiology of KD is unknown, it is considered to have an infectious trigger followed by the activation of immune system in a genetically susceptible individual [35]. This theory is also supported by results from epidemiological studies. Firstly, similar to viral infections, incidence of KD is higher in winter and spring seasons [17]. Like infections, there is occurrence of epidemics and clusters of cases [36]. KD is also self-resolving in 1-3 weeks and the most common age of acquiring KD is 6 months to 5 years when the susceptibility of the child to infections is also the highest [36]. Cases are rarely seen below 6 months of age which could be attributed to the passive transmission of maternal immunoglobulins providing protection to the child at an early age, which gradually decreases over the time [37]. Recently, Nagao et al also reported that KD has a similar mode of transmission to infections upon close contact with infected persons [38].
Recent studies have shown that bacterial superantigens might be related to the causation of KD. This is based on the similarity of clinical presentations in the two groups like strawberry tongue and desquamation of hands and feet [36]. It has been proposed that Staphylococcal Toxic Shock Syndrome toxin (TSST-1) and Streptococcal pyogenic toxins might act as superantigens that initiate an immune response which could lead to the occurrence of KD [2]. Another study also reported increased IgM antibodies to these superantigens in KD that further supports the hypothesis [39]. It has been suggested that superantigens bind to Vβ region of T cell receptor, and induce the release of immunological mediators like IL-6, TNF-α, TGF-β that are found to be elevated in KD [40]. But, it is still not clear whether these bacterial antigens activate immune system and cause KD by T cells directly or indirectly by T cell-mediated B cell differentiation [2].
Viral etiology has also been considered for KD pathogenesis. It has been found that in acute phase, there is infiltration of CD8+ T lymphocytes, IgA plasma cells and macrophages in the coronary arteries, which is similar to the observations in any acute viral infection [2]. However, in peripheral blood there is predominance of CD4+ T lymphocytes which could be possibly explained by the transmission of CD8+ T lymphocytes to the infected tissues [2]. Apart from this, in autopsy specimens of KD cases, electron microscopy studies have shown cytoplasmic inclusion bodies in ciliated bronchial epithelium with aggregates of RNA and viral protein [2]. This suggests that KD could be initiated by an acute viral infection of the respiratory system which later on leads to dysregulated immune response resulting in KD [2,27].
Till now, no specific infectious agent has been found to be consistently associated with KD. However, many case reports have linked KD with many viral agents like Mycoplasma pneumoniae [41], Cytomegalovirus [42], adenovirus, rhinovirus, enterovirus [43], bocavirus [44].
4.3 Immunity
KD is classically believed to occur as a consequence of dysregulated immune system. It has been proposed that KD is similar to autoimmune diseases in pathogenesis [3]. T cells have been primarily implicated in the immunopathogenesis of KD with involvement of inositol 1,4,5-triphosphate 3-kinase (ITPKC) as the main mediator [17]. ITPKC is a negative regulator of T cell activation and loss of its control leads to increased activation of T cells and production of cytokines, which has been shown in KD patients [17]. Onouchi et al [45] identified itpkc_3, a functional SNP in the ITPKC gene on chromosome 19q13.2 to be significantly associated with susceptibility to KD and increased risk of coronary artery lesions. They hypothesized that the C allele might be responsible for the immune hyper reactivity in KD. Wang et al [46] studied the serum samples of KD patients and analyzed the levels of cytokines of Th1 and Th2 cells. They found increased levels of IL-6, IL-20, IFN-γ (interferon γ) and TNF-α (Tumor necrosis factor-α) before IVIG treatment and reported rapid decrease in the serum levels of IL-6, IL-10 and IFN-γ after IVIG treatment [46]. Recently, studies have also shown an imbalance of Th (T helper cells) and Treg cells (regulatory T cells) in acute KD. Th17 cells are pro-inflammatory cells that secrete cytokines like IL-6, TNF-α, and IL-8 after activating neutrophils, monocytes, and fibroblasts [47] while Treg cells are anti-inflammatory that work through secretion of cytokines like IL-10 and TGF-β to control the progression of inflammatory and autoimmune diseases. Jia et al [47] demonstrated that in children with acute KD, levels of Th17 cells and their cytokines (IL-17, IL-6 and IL-23) were significantly upregulated whereas Treg cells and the level of their transcription factor (FoxP3) were significantly downregulated. These investigators then proposed that this imbalance of Th17/ Treg cells might be involved in disturbing the immune homeostasis in KD. This relationship was also shown by Ni et al [48]. Kuo et al [49] performed a meta-analysis of the ITPKC studies carried out in Taiwanese population and reported a significant association between ITPKC allele rs28493229 and KD (Odd's ratio of 1.36 and 95% confidence interval: 1.12-1.66), especially with the risk of aneurysm formation (p=0.001).
NKG2D is expressed by immune cells like NK cells, cytotoxic T cells (CD8+ T cells) and plays a role in immunomodulation by enhancing the activity of these cells [50]. Therefore, its low levels in acute phase of KD might be associated with immune system dysregulation [50]. This association was shown by Ge et al [50]. They analyzed NKG2D expression on CD8+ T cells and CD3-CD56+ NK cells in acute KD cases, and found significantly lower levels than in controls. Additionally, the NKG2D expression was found to be lower in patients with coronary artery lesions compared to those without the coronary artery involvement [50].
Lee et al [51] showed the critical role of IL-1 in the pathogenesis of KD using mice knockout models and antibodies. This then led to clinical trials for the use of IL-1R (Anakinra) in children with KD and is currently in phase IIa of clinical trials [52].
Histological examination of the affected tissue shows both activation and damage of endothelial cells [36]. TNF-α, produced initially by the T lymphocytes and later released by the monocytes/macrophages, is one of the key inflammatory cytokines mediating this process [7]. It facilitates endothelial cell activation via increased expression of adhesion molecules and promotes the release of chemokines required for leukocytes-endothelial cell interaction [7]. TNF-α also stimulates the activity of MMP-9 (matrix metalloproteinase) which in turn leads to breakdown of elastin and aneurysm formation in vessel wall. Therefore, use of TNF-α blockers could be one of the therapeutic strategies in KD patients to prevent aneurysms formation and is undergoing clinical trials [17]. It has also been shown that nitric oxide (NO) production is increased in KD patients and goes down rapidly with IVIG treatment [52]. NO is known to maintain normal vascular tension. High NO concentrations could therefore lead to vessel wall dilatation and lesions [53]. This is mediated by the inducible nitric oxide synthase enzyme (iNOS) present in myocardial cells, vascular smooth muscle cells, leukocytes and inflammatory cells which is upregulated during inflammatory response and in turn, promotes elevation of NO [52,54]. IVIG treatment has been shown to decrease the levels of NO in KD [52,53,55]. The imbalance of various cytokines and other inflammatory markers in KD is summarized in Figure 1.
Figure 1.
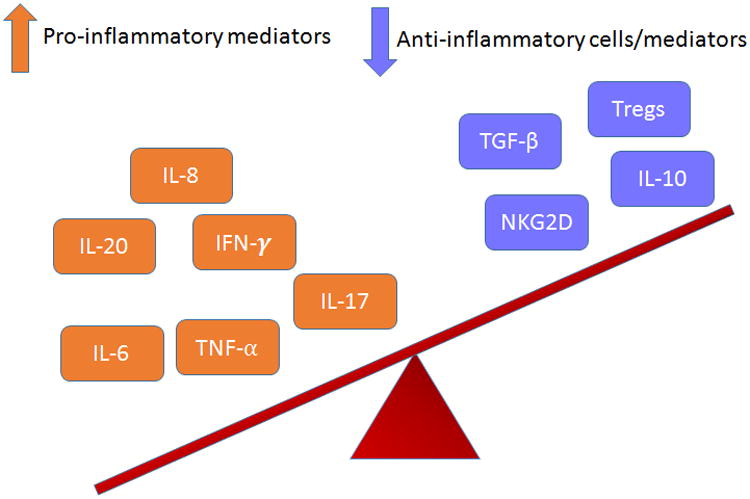
Diagrammatic depiction of the imbalance of the inflammatory markers in Kawasaki Disease. The pro-inflammatory markers are elevated along with downregulation of anti-inflammatory markers, leading to a state of inflammation and damage.
4.4 Perinatal exposure and KD
There has been recent interest to investigate if there are any perinatal factors that might increase the risk of acquiring KD in later life of the child. This hypothesis was recently studied by Hayward et al [56]. They performed a retrospective, case control study and identified 1019 KD patients in the 21-year study period (1987-2007). After adjusting for confounding factors like gender, race and birth year, they found group B streptococcus (GBS) colonization (OR 0.51, 95% Cl: 0.26-0.97), maternal age above 35 years (OR 1.65, 95% CI: 1.20-2.27) and early infant hospitalization (OR 1.42, 95% CI: 1.04-1.93) to be significantly associated with the risk of KD. In particular, early infancy bacterial infections were associated with 2.8-times increased relative risk of KD. One possible mechanism is that the early exposure to infections might weaken the developing immune system in a way that renders it susceptible to KD later in life. Alternatively, these children might be having some immune deficit that could predispose them to both infections in early life and KD at a later age. GBS colonization was associated with a lower risk of KD, which could possibly be explained by the trans-placental transfer of antibodies to the growing fetus that provides protection from KD at later age.
This raises the possibility of KD susceptibility with in utero and early infancy risk factors, but this is only a single study data. More studies should be performed to accurately establish the association between the two.
5 Treatment
5.1 Acute phase treatment
AHA and AAP (American Academy of Pediatrics) recommend a combination of aspirin and IVIG for treating acute KD [57].
5.1.1 IVIG
The first description of the use of IVIG in treating KD dates back to the 1980s by Furusho et al [58]. Since then it has been used successfully in treating majority of the cases of KD and has become the standard of treatment. The best response is seen if IVIG treatment is started within 10 days of the onset of symptoms at a single dose of 2g/kg infusion over 12 hours [59] as per AAP and AHA guidelines.
The mechanism of action of IVIG is still under investigation but the proposed possible mechanisms of action of IVIG include: neutralization of infectious antigens and superantigens, suppression of TNF-α, neutralization of pathogenic autoantibodies, inhibition of inflammatory cytokines release and modulation of B and T cells function [2, 59] (Figure 2).
Figure 2.
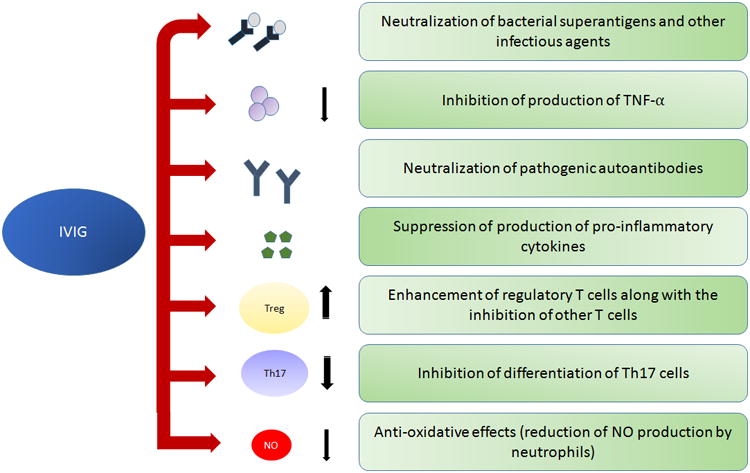
Multiple functions of IVIG in the treatment of Kawasaki disease.
A recent meta-analysis by Chen et al studied the dose-response relationship for IVIG treatment by comparing low dose IVIG (400 mg/kg) given over 4-5 days to a high dose (2g/kg) single infusion [60, 61]. They concluded that single infusion is more efficacious in preventing CAA. Studies have compared the various commercial preparations of IVIG available in the market and reported that using acidic conditions for preserving immunoglobulins has increased risk of CAAs and treatment failure [58]. The efficacy of treating patients with IVIG 10 days after the onset of symptoms is not well established, therefore, it is recommended to start IVIG treatment soon [14]. But, it is still recommended to administer IVIG to patients presenting even later than 10 days if they have ongoing fever or other signs of inflammation, including elevated CRP and ESR [14]. Muta et al reported that IVIG treatment after 10 days is helpful in decreasing the inflammatory process, even though the risk of CAA still remains high [62].
However, in spite of adequate IVIG treatment, around 5% children develop transient coronary artery dilatation and 1% develop aneurysms [2,36].
5.1.2 Aspirin
Aspirin is used in conjunction with IVIG for its anti-inflammatory effect in the acute phase of KD and for its anti-platelet activity during the sub-acute phase [63][64]. The accepted dosage varies from country to country. While in the USA, it is given at a high dose of 80-100 mg/kg/day as per the AHA guidelines, in Japan it is recommended at a moderate dose of 30-50 mg/kg/day given the risk of hepatic toxicity at higher doses [59, 64]. Studies have reported similar efficacy of the two treatment regimen[65]. According to AHA recommendations, in the acute phase, patient is given 80-100 mg/kg/day in 4 divided doses, followed by 3-5 mg/kg/day during the sub-acute phase of KD for at least 6-8 weeks for its antithrombotic activity [19]. If the patient has coronary artery abnormalities, aspirin is continued at low dose till there is echocardiographic evidence of the resolution of coronary artery lesion, while it is continued for indefinite time if there is evidence of CAA [19]. Patients with giant aneurysms should be started on low molecular weight heparin or warfarin given the risk of thrombotic complications in these cases [59].
Children with KD on treatment with aspirin for prolonged periods have a risk of developing Reye's syndrome if they are also affected by influenza or varicella infections [2]. It is therefore, recommended to use clopidogrel as an alternative to aspirin in such cases [2].
Although it helps to reduce inflammation, aspirin has not been shown to play a role in reducing the frequency of CAA or IVIG resistance in KD [60]. Kuo et al [58] demonstrated that high dose aspirin impairs the decrease in hepcidin level, thereby resulting in lower availability of iron for erythropoiesis and in turn, anemia. They also noted that patients treated with high dose aspirin had persistently elevated CRP levels despite treatment, suggesting no improvement of inflammation with aspirin in KD. Wang et al [55] also reported similar findings. They both therefore, even suggested to completely stop the use of aspirin in the acute phase of KD, but there is a need to carry out more randomized controlled trials in this respect.
5.2 Refractory KD
AHA and AAP define refractory KD as the one where fever persists beyond 36 hours of treatment with IVIG [66]. These patients can then be treated with either a second dose of IVIG or steroids [66]. There are many other upcoming treatments aimed at decreasing inflammation which are discussed in detail below (Table 2).
Table 2. Novel Therapeutics in the treatment of Refractory Kawasaki disease.
| Drug | Dosage | Mechanism of action (MOA) | Potential Side Effects | Evidence | Reference |
|---|---|---|---|---|---|
|
| |||||
| Infliximab | 5mg/kg IV | It binds to and inhibits TNF α, thereby preventing the release of pro- inflammatory cytokines and interleukins. | Infections, hepatomegaly, bradycardia, anemia, neutropenia, leukopenia | 18 of the 20 patients were treated successfully with infliximab, 2 required additional plasma exchange therapy. | [77] |
| In phase III RCT, it did not appear to reduce treatment, although it was helpful in reducing fever duration, few markers of inflammation and left anterior descending coronary artery Z scores [tr6]. | [78] | ||||
|
| |||||
| Etanercept | 0.4-0.8 mg/kg/week for three weeks. | Soluble receptor blockade of TNF α receptors. | Headache, sinus infections, psoriasis, hepatitis B, allergic reactions. | Fifteen patients successfully completed the study. No serious adverse effects were noted. Though etanercept did not significantly improve the treatment outcome, but there was no case of prolonged or recurring fever requiring second dose of IVIG. None of the children developed coronary artery dilation or other abnormalities. | [80] |
|
| |||||
| Steroids Methylprednisolone | Reduces inflammation by preventing the migration of leukocytes and decreasing capillary permeability. | 30 mg/kg IV infused over 2 hrs for 3 days | Sinus bradycardia, hypothermia, hypertension, hypokalemia | Incidence of coronary artery abnormalities was significantly lower in IVIG plus steroids group compared to IVIG and aspirin alone (CI 0.12-0.28, p<0.0001). | [69] |
| Meta-analysis of 9 clinical studies showed that IVMP and steroids combined treatment reduced the risk of coronary artery abnormalities significantly (OR 0.3, 95% CI 0.20-0.46). | [72] | ||||
|
| |||||
| Dexamethasone | 0.3mg/kg/day for 3 days | Patients receiving combined IVIG and dexamethasone treatment had shorter febrile period and hospital stay (p<0.001). Even the risk of coronary artery abnormalities was lower in this group, but it was not statistically significant (p=0.03). | [76] | ||
|
| |||||
| Methotrexate | Inhibits the enzymes dihydrofolate reductase and thymidylate synthase, which play a role in folate synthesis. Might also reduce the release of IL-1 and IL-6 and leukotrienes. | 10 mg/BSA weekly PO | Stomatitis, alopecia, bone marrow suppression, hepatotoxicity | Patients treated with methotrexate following IVIG had lower duration of fever (p=0.023), lower CRP (p<0.001) with no adverse effects. | [93] |
| Case reports showing resolution of symptoms of KD with no adverse effects. | [92,94,95] | ||||
|
| |||||
| Cyclosporine | Inhibits the assembly and release of IL-2, also inhibits the activation of T lymphocytes, thereby suppressing immune activation. | 4 mg/kg/day IV or PO | Hyperkalemia, hypertension, hirsutism, infections, tremors and renal susceptibility | Out of 28 patients of refractory KD, 18 (64.3%) became afebrile within 3 days of cyclosporine treatment, while 6 (21.4%) failed to respond even after 5 days of treatment. | [79] |
|
| |||||
| Cyclophosphamide | Inhibits DNA synthesis and prevents cell division by cross linking DNA strands | 2 mg/kg/day IV | Hemorrhagic cystitis, alopecia, diarrhea, mucositis, bone marrow suppression | 2 patients out of the 5 who developed refractory KD were treated with additional cyclophosphamide in addition to IVIG and reported no progression of coronary aneurysms and no adverse effects. | [82] |
|
| |||||
| Plasma exchange | Centrifugation of blood, followed by discarding of filtered plasma containing the inflammatory cytokines and its replacement with another colloid such as donor plasma or albumin. | - | Transfusion allergic reactions, paresthesia, hypocalcemia, hypotension, bleeding. | Six patients unresponsive to IVIG and infliximab were treated with plasma exchange therapy and they reported complete resolution of symptoms. Even the patients who had coronary artery lesions, later reported suppression or reversal of abnormalities. | [74] |
| 9 children with IVIG resistant KD were treated with plasma exchange and showed good response. Although three patients developed CAA, there was a resolution of these lesions by the end of a year. | [54] | ||||
5.2.1 Corticosteroids
Though steroids are one of the mainstay treatment for various conditions with vasculitis, it was initially avoided for the treatment of KD, based on findings of one report that concluded increased risk of cardiovascular complications with steroids monotherapy [67]. But later studies reported reduced CAA risk on use of steroids along with IVIG and aspirin [68]. One of the largest RCT (Randomized clinical trial) conducted in this regard was in Japan, named RAISE study, randomized controlled trial to Assess Immunoglobulin plus Steroid Efficacy for Kawasaki Disease, which reported improvement in coronary artery outcomes in KD patients on giving prednisolone along with IVIG [69]. Steroids could be helpful in KD by down regulating inflammatory mediators, preventing leukocyte migration and decreasing capillary permeability [70]. Use of intravenous pulsed methylprednisolone (IVMP), 30 mg/kg for 2-3 hours once daily for 2-3 days is the most commonly used steroid regimen [36].
But, controversies still exist regarding the the indications for the use of steroids in KD. Some suggest use of steroids only in cases that fail to respond to >2 infusions of IVIG, while others recommend use of IVIG in cases where the first dose of IVIG itself is unable to bring down inflammation [36]. AHA recommends the use of steroids in patients that fail to respond to 2 or more infusions of IVIG [70]. Ogata et al reported better outcomes in refractory KD patients with combined IVIG and IVMP treatment [71]. Chen et al [72] performed a meta-analysis of clinical trials to compare the efficacy of IVIG plus steroid treatment compared to IVIG alone and concluded that their combined therapy as an initial treatment in KD is more useful in reducing the risk of CAA. IVMP through their anti-inflammatory functions suppress cytokine levels faster than IVIG alone [73]. The usefulness of second dose of IVIG versus steroids in IVIG resistant cases needs to be fully established [14].
The safety of steroids in KD patients was studied by Miura et al [74]. They reported higher incidence of sinus bradycardia and hyperglycemia in patients treated with IVMP compared to those treated with second dose of IVIG. Although these adverse effects were only transient, but indicate the need for monitoring of patients on steroid infusion [75]. Overall IVMP is considered safe as an adjuvant treatment to IVIG alone or along with aspirin [14].
Although with regards to the use of steroids in KD, most of the studies have investigated the use of IVMP, Lim et al [76] studied the use of dexamethasone, a longer acting steroid in KD in a retrospective study. They reported better clinical outcomes on combined use of dexamethasone and high dose IVIG in terms of shorter febrile periods, lesser need for IVIG second dose and shortened hospital stay without worsening of cardiovascular outcomes [76]. Further larger and prospective studies are required to establish the efficacy of steroids as initial or second line treatment and also comparative efficacy of IVMP versus dexamethasone.
5.2.2 TNF-α inhibitors
In view of the role of TNF-α in the pathogenesis of KD as well as coronary artery dilatations, it is plausible to use its inhibitors in the primary therapy for KD or in refractory cases. TNF-α antagonists can be subdivided into 2 categories: monoclonal antibodies (infliximab and adalimumab) and soluble receptors (Etanercept) [66].
Infliximab, a chimeric monoclonal antibody has been reported in many studies to be useful for the treatment of refractory KD. Mori et al [77] conducted an open labelled trial for infliximab in patients refractory to IVIG treatment. The 18 of the 20 patients were successfully treated with infliximab without any adverse effects. Tremoulet et al [78] have conducted a large randomized placebo controlled clinical trial for the use of infliximab in addition to the standard treatment in acute KD. They have completed phase III clinical trial and reported that infliximab helped to reduce fever, markers of inflammation, IVIG reaction rates but had no effects on treatment resistance [78]. It was found to be safe and well tolerated [78]. Infliximab is usually administered at the dose of 5 mg/kg and response is seen within 24 hours of the treatment [59].
Abciximab, a monoclonal antibody against GpIIb-IIIa receptors has been used for the treatment of acute and sub-acute cases of KD with coronary artery aneurysms [79]. Williams et al [79] reported that addition of abciximab to standard therapy lead to greater reduction in the aneurysm diameter and hence, better outcomes. They also concluded that abciximab helps to promote vascular remodeling and hence decreases the risk of CAAs [79]. However, larger clinical trials are yet to be undertaken in this regard.
Etanercept, was used as an adjunctive therapy to IVIG for the first time in a clinical trial by Choueiter et al [80] where they reported favorable response in children at the dose of 04-0.8 mg/kg/week for 3 weeks. Based on these findings, a multicenter, double blinded, placebo controlled randomized clinical trial, named the EATAK trial, was initiated in 2011 to assess the efficacy of etanercept as an adjunctive treatment to IVIG in refractory KD [66].
5.2.3 Plasma exchange
In KD, inflammatory mediators are elevated and their elevation is a risk factor for developing CAAs. So, plasma exchange (PE), which removes various inflammatory cytokines could serve as a promising treatment option for preventing CAAs. It has been successfully used in many refractory KD cases. Mori et al [81] reported that early introduction of PE therapy, by 10th day, can help to reduce the risk of CAA in these patients based on their findings in 46 children treated with PE after failure of response to second dose of IVIG.
Fujimaro et al [64] used plasma exchange in 9 IVIG resistant cases, and reported decreased levels of IL-6, IL-17, G-CSF, TNF-α, TNFR1, TNFR2 after 4-5 days of plasma exchange therapy with no adverse effects in any case.
Duration of PE depends on many factors which includes resolution of symptoms like fever and normalization of inflammatory markers [64]. Generally, it is administered for 3-4 days although Fujimaro et al found it to be effective when given for 4-5 days [64].
Matsui et al [82] used a combination of IVMP and PE in a IVIG-resistant case. Two doses of IVIG followed by PE and IVMP from 10th to 14th day of illness resulted in considerable improvement in clinical symptoms as well as improvement of inflammatory markers, IL-6, sTNF αR type 1 and type 2 levels. Sonoda et al [83] used a stepwise treatment of IVIG, infliximab and PE to treat the KD patients. They selected 76 patients unresponsive to 2 doses of IVIG and treated them with infliximab first, followed by PE for those who did not even respond to infliximab (6 out of 76 patients). This resulted in the resolution of inflammation, with disappearance of fever and improvement of laboratory values [83]. Based on these findings, these investigators concluded that this stepwise protocol for refractory KD patients was highly effective. But, further large scale, multicenter, prospective studies are required to establish the success of the combination therapy.
5.2.4 Cyclosporine
Cyclosporine acts as a calcineurin inhibitor [84]. Since recent studies have shown the involvement of IPTKC/calcineurin pathway in the pathogenesis of KD, therefore, blocking of this pathway could be a useful treatment option for refractory KD patients. Cyclosporine would therefore, block the release of IL-2 which is a cytokine signaling molecule [70]. It is also postulated to inhibit T cell differentiation, thereby modulating the cell-mediated immunity and reducing inflammation in KD [70]. There have been conflicting results regarding the efficacy of cyclosporine in KD treatment. Kuijpers et al [85] reported no improvement in clinical outcomes in a 16-month old child with refractory KD. Suzuki et al demonstrated cyclosporine to be a safe and promising treatment option for refractory KD patients [86]. They conducted a prospective cases series in Japan and reported effective treatment of IVIG resistant cases with oral cyclosporine at the dose of 4-8 mg/kg/day in 18 of the 24 patients [86]. A phase III clinical trial of using IVIG and cyclosporine in severe KD (KAICA Trial) is also ongoing in Japan [87]. Patients are randomized to receive either oral cyclosporine (5mg/kg/day for 5 days) and high dose IVIG (2g/kg for 24 hours) plus aspirin 30 mg/kg/day, or high dose IVIG and aspirin at the same dose [87].
But, calcineurin inhibitors can be toxic to the endothelium and therefore should not be routinely administered but could be considered as a potential treatment option in certain cases [61, 88]. Further studies are required to find the optimal dose, safety and efficacy of cyclosporine in KD [14].
5.2.5 Cyclophosphamide
Cyclophosphamide is an immunosuppressive drug that is believed to reduce inflammation in KD. It inhibits DNA synthesis and cross linking of DNA strands, thereby preventing cell division [89]. This leads to cell death and is believed to involve cytokines. Inflammation is reduced when these immune cells are suppressed [70]. There have not been many studies with respect to cyclophosphamide use in KD. Wallace et al [90] performed a retrospective analysis of treatment of children with KD. Out of the 65 children in the study, 5 children had persistent KD even after 2 doses of IVIG. Four of these cases were treated with pulse IVMP (30 mg/kg/day) and 2 with cyclophosphamide (2 mg/kg/day). These patients did not have any progression of coronary artery aneurysms or mortality. No adverse effects of cyclophosphamide were reported. Further studies are required to establish the efficacy of this drug in KD treatment.
5.2.6 Methotrexate
Methotrexate (MTX) is primarily used as an anticancer drug. It is an antimetabolite that inhibits the enzyme Dihydrofolate reductase required for DNA synthesis and replication [91]. In KD, methotrexate (MTX) is believed to lower the C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and block the release of IL-1 and IL-6 [70]. Lee et al [92] first reported the successful use of methotrexate in a 6-year old child with refractory KD. This child did not respond to multiple courses of IVIG and was later started on methotrexate (10 mg/body surface area, weekly once) along with dexamethasone (0.6 mg/kg, daily) on day 38 of illness. This resulted in gradual subsiding of the inflammation. Lee et al [93] later performed a prospective study with 17 patients who were refractory to initial IVIG treatment. These patients were then treated with low dose methotrexate (10 mg/ body surface area, once a week) till the CRP values were normalized. It was found that fever dropped significantly within first week of treatment with MTX and even CRP was significantly lower after 1 week of treatment with MTX (8.9 mg/dl vs 1.2 mg/dl). No adverse effects were noted. Later few case reports have also reported successful resolution of KD with MTX without any adverse effects [94, 95].
6 Expert Commentary
Kawasaki disease has emerged as the major cause of acquired heart diseases in children in industrialized countries. The pathogenesis is still, however, unclear. Recent reports have associated genetic susceptibility to its causation. Many case reports have attributed its occurrence to different infectious agents but none of them have been found to be consistently associated and hence, requires further research. Dysregulation of the immune system is known to occur in Kawasaki disease but the underlying mechanisms warrants further investigation and currently is the subject of research for many investigators. As per treatment, aspirin and IVIG combined treatment has shown positive results in most of the acute cases. But, in refractory cases, IVIG is not of much use. In order to prevent coronary complications, multiple drugs are being examined and have shown positive results, but large scale prospective studies are still required to establish their safety and efficacy in the treatment of refractory KD.
7 Five-year view
With the ongoing research and clinical trials regarding the pathogenesis and effective drugs to be used in KD, in the upcoming years, we are expecting advancement in the field especially in regard to the role of genes, infections and immunity in the causation of KD. With a possibility of various multicenter, larger, prospective studies for the use of alternative drugs to IVIG and aspirin and also in refractory KD, more options for the treatment of KD would hopefully be available to the clinicians. Detailed information on potential adverse effects and safety of all these therapeutic alternatives would facilitate the decision process in the treatment plan depending on individual patient requirements. This will further be able to reduce the incidence of coronary and other complications associated with KD.
Key issues.
Kawasaki disease is the leading cause of acquired heart diseases in children living in industrialized countries.
The pathogenesis is still unclear, though there appears to be an interplay of genetic susceptibility, infections and immune dysregulation.
Genome wide association studies have helped to recognize many genes that may confer susceptibility to Kawasaki disease.
Perinatal factors might also influence the risk of occurrence of Kawasaki disease.
IVIG and aspirin remain the mainstay therapy for acute phase treatment.
There is no drug currently available to decrease the risk of coronary artery abnormalities or reverse the already occurred damage. Obviously, additional studies are urgently warranted in this area.
Acknowledgments
Funding: This work was supported by research grants R01 HL116042, R01 HL112597, and R01HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA.
Footnotes
Declaration of Interests: The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Kontopoulou T, Kontopoulous DG, Vaidakis E, Mousoulis GP. Adult Kawasai disease in a European patient: a case report and review of literature. J Med Case Rep. 2015;9:75. doi: 10.1186/s13256-015-0516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2**.Alexoudi I, Kanakis M, Kapsimali V, Vaiopoulos G. Autoimmun Rev. 9. Vol. 10. Elsevier B.V.; 2011. Kawasaki disease: Current aspects on aetiopathogenesis and therapeutic management; pp. 544–7. Internet. Available from: http://dx.doi.org/10.1016/j.autrev.2011.04.005 This article provides detailed information about the factors associated with pathogenesis and treatment of Kawasaki disease, as well as ongoing trials for the newer therapeutic options being explored. [DOI] [PubMed] [Google Scholar]
- 3.Guo MMH, Tseng WN, Ko CH, Pan HM, Hsieh KS, Kuo HC. Th17- and Treg-related cytokine and mRNA expression are associated with acute and resolving Kawasaki disease. Allergy. 2015;70(3):310–8. doi: 10.1111/all.12558. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25585854. [DOI] [PubMed] [Google Scholar]
- 4.Uehara R, Belay ED. Epidemiology of Kawasaki Disease in Asia, Europe, and the United States. J Epidemiol. 2012;22(2):79–85. doi: 10.2188/jea.JE20110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. 2015;100(11):1084–8. doi: 10.1136/archdischild-2014-307536. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26111818 This article has dicussed in detail the epidemiological characteristics of Kawasaki disease in different parts of the world. [DOI] [PubMed] [Google Scholar]
- 6.Greco A, De Virgilio A, Rizzo MI, Tombolini M, Gallo A, Fusconi M, et al. Autoimmun Rev. 8. Vol. 14. Elsevier B.V; 2015. Kawasaki disease: An evolving paradigm; pp. 703–9. Internet. Available from: http://dx.doi.org/10.1016/j.autrev.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Oharaseki T, Yokouchi Y. Update on etio and immunopathogenesis of Kawasaki disease. Curr Opin Rheumatol. 2014;26(1):31–6. doi: 10.1097/BOR.0000000000000010. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24247115. [DOI] [PubMed] [Google Scholar]
- 8.Luca NJ, Yeung RS. Epidemiology and management of Kawasaki disease. Drugs. 2012;72(8):1029–38. doi: 10.2165/11631440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Lin MC, Lai MS, Jan SL, Fu YC. J Chinese Med Assoc. 2. Vol. 78. Elsevier Taiwan LLC and the Chinese Medical Association; 2015. Epidemiologic features of Kawasaki disease in acute stages in Taiwan, 1997-2010: Effect of different case definitions in claims data analysis; pp. 121–6. Internet. Available from: http://dx.doi.org/10.1016/j.jcma.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holman RC, Christensen KY, Belay ED, Steiner CA, Effler PV, Miyamura J, et al. Racial/ethnic differences in the incidence of Kawasaki syndrome among children in Hawaii. Hawaii Med J. 2010;69(8):194–7. Internet. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3118023&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 11.Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, et al. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25(3):239–45. doi: 10.2188/jea.JE20140089. Internet. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4341001&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss PF. Pediatric vasculitis. Pediatr Clin North Am. 2012;59(2):407–23. doi: 10.1016/j.pcl.2012.03.013. Internet. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22560577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayers S, Shulman ST, Paller AS. Kawasaki disease: Part I. Diagnosis, clinical features, and pathogenesis. J Am Acad Dermatol. 2013;69(4):501, e1–501.e11. doi: 10.1016/j.jaad.2013.07.002. Internet. Available from: http://dx.doi.org/10.1016/j.jaad.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Kuo HC, Yang KD, Chang WC, Ger LP, Hsieh KS. Pediatr Neonatol. 1. Vol. 53. Elsevier Taiwan LLC; 2012. Kawasaki disease: an update on diagnosis and treatment; pp. 4–11. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22348488. [DOI] [PubMed] [Google Scholar]
- 15.Patel RM, Shulman ST. Kawasaki disease: a comprehensive review of treatment options. J Clin Pharm Ther. 2015;40(6):620–5. doi: 10.1111/jcpt.12334. Internet. Available from: http://doi.wiley.com/10.1111/jcpt.12334. [DOI] [PubMed] [Google Scholar]
- 16.Scuccimarri R. Pediatr Clin North Am. 2. Vol. 59. Elsevier Inc; 2012. Kawasaki Disease; pp. 425–45. Internet. Available from: http://dx.doi.org/10.1016/j.pcl.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Dimitriades VR, Brown AG, Gedalia A. Kawasaki disease: Pathophysiology, clinical manifestations, and management. Curr Rheumatol Rep. 2014;16(6):423. doi: 10.1007/s11926-014-0423-x. [DOI] [PubMed] [Google Scholar]
- 18.Tseng HC, Ho JC, Guo MMH, Lo MH, Hsieh KS, Tsai WC, et al. Bull's eye dermatoscopy pattern at bacillus Calmette-Guérin inoculation site correlates with systemic involvements in patients with Kawasaki disease. J Dermatol. 2016:1–7. doi: 10.1111/1346-8138.13315. Internet. Available from: http://doi.wiley.com/10.1111/1346-8138.13315. [DOI] [PubMed]
- 19.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Kawasaki Disease A Statement for Health Professionals From the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association Endorsed by the American Academy of Pediatrics. 2004;114(6):1708–33. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 20.Ha KS, Lee J, Jang GY, Lee JH, Lee KC, Son CS, et al. Value of Neutrophil-Lymphocyte Ratio in Predicting Outcomes in Kawasaki Disease. Am J Cardiol. 2015;116(2):301–6. doi: 10.1016/j.amjcard.2015.04.021. Internet. Available from: http://dx.doi.org/10.1016/j.amjcard.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Parthasarathy P, Agarwal A, Chawla K, Tofighi T, Mondal TK. Upcoming biomarkers for the diagnosis of Kawasaki disease: A review. Clin Biochem. 2015;48(16–17):1188–94. doi: 10.1016/j.clinbiochem.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Lin KH, Chang SS, Yu CW, Lin SC, Liu SC, Chao HY, et al. Usefulness of natriuretic peptide for the diagnosis of Kawasaki disease: a systematic review and meta-analysis. BMJ Open. 2015;5(4):e006703. doi: 10.1136/bmjopen-2014-006703. Internet. Available from: http://bmjopen.bmj.com/content/5/4/e006703.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye Q, Shao WX, Shang SQ, Zhang T, Hu J, Zhang CC. A comprehensive assessment of the value of laboratory indices in diagnosing Kawasaki disease. Arthritis Rheumatol. 2015;67(7):1943–50. doi: 10.1002/art.39112. [DOI] [PubMed] [Google Scholar]
- 24.Dietz SM, Tacke CE, Kuipers IM, Wiegman A, de Winter RJ, Burns JC, et al. Cardiovascular imaging in children and adults following Kawasaki disease. Insights Imaging. 2015;6(6):697–705. doi: 10.1007/s13244-015-0422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tacke CE, Kuipers IM, Groenink M, Spijkerboer AM, Kuijpers TW. Cardiac magnetic resonance imaging for noninvasive assessment of cardiovascular disease during the follow-up of patients with kawasaki disease. Circ Cardiovasc Imaging. 2011;4(6):712–20. doi: 10.1161/CIRCIMAGING.111.965996. [DOI] [PubMed] [Google Scholar]
- 26.Mavrogeni S, Papadopoulos G, Hussain T, et al. Int J Cardiovasc Imaging. 2013;29(8):1787–1798. doi: 10.1007/s10554-013-0276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KY, Kim DS. Recent Advances in Kawasaki Disease. Yonsei Med J. 2016;57(1):15–21. doi: 10.3349/ymj.2016.57.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lv YW, Wang J, Sun L, Zhang JM, Cao L, Ding YY, et al. Understanding the pathogenesis of Kawasaki disease by network and pathway analysis. Comput Math Methods Med. 2013;2013:17–9. doi: 10.1155/2013/989307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Yoon KL. Update of genetic susceptibility in patients with kawasaki disease. Korean J Pediatr. 2015;58(3):84–8. doi: 10.3345/kjp.2015.58.3.84. This study presented the results from Genome Wide Association Studies indicating the role of various possible genes in susceptibility to Kawasaki disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onouchi Y, Gunji T, Burns JC, Shimizu C, Jane W, Fukushima Y, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40(1):35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Lou J, Zhong R, Qi Y, Shen N, Lu X, et al. The roles of Ca2+/NFAT signaling genes in Kawasaki disease: single- and multiple-risk genetic variants. Sci Rep. 2014;4:5208. doi: 10.1038/srep05208. Internet. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4047536&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onouchi Y, Ozaki K, Buns JC, Shimizu C, Hamada H, Honda T, et al. Common variants in CASP3 confer susceptibility to Kawasaki disease. Hum Mol Genet. 2010;19(14):2898–906. doi: 10.1093/hmg/ddq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onouchi Y. Genetics of Kawasaki disease: what we know and don't know. Circ J. 2012;76(7):1581–6. doi: 10.1253/circj.cj-12-0568. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22789975. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu C, Jain S, Davila S, Hibberd ML, Lin KO, Molkara D, et al. Transforming Growth Factor-β Signaling Pathway in Patients With Kawasaki Disease. Circ Cardiovasc Genet. 2011;4(1):16–25. doi: 10.1161/CIRCGENETICS.110.940858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yim D, Curtis N, Cheung M, Burgner D. Update on Kawasaki disease: Epidemiology, aetiology and pathogenesis. J Paediatr Child Health. 2013;49(9):704–8. doi: 10.1111/jpc.12172. [DOI] [PubMed] [Google Scholar]
- 36.Galeotti C, Bayry J, Kone-Paut I, Kaveri SV. Autoimmun Rev. 6. Vol. 9. Elsevier B.V; 2010. Kawasaki disease: Aetiopathogenesis and therapeutic utility of intravenous immunoglobulin; pp. 441–8. Internet. Available from: http://dx.doi.org/10.1016/j.autrev.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KY, Han JW, Lee JS. Kawasaki disease may be a hyperimmune reaction of genetically susceptible children to variants of normal environmental flora. Med Hypotheses. 2007;69(3):642–51. doi: 10.1016/j.mehy.2006.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagao Y, Urabe C, Nakamura H, Hatano N. Predicting the characteristics of the aetiological agent for Kawasaki disease from other paediatric infectious diseases in Japan. Epidemiol Infect. 2016;144(3):478–92. doi: 10.1017/S0950268815001223. Internet. Available from: http://www.journals.cambridge.org/abstract_S0950268815001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsubara K, Fukaya T, Miwa K. Development of serum IgM antibodies against superantigens of Staphylococcus aureus and Streptococcus pyogenes in Kawasaki disease. 2006;143(3):427–34. doi: 10.1111/j.1365-2249.2006.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang CL, Wu YT, Liu CA, Kuo HC, Yang KD. Kawasaki Disease. Pediatr Infect Dis J. 2005;24(11):998–1004. doi: 10.1097/01.inf.0000183786.70519.fa. Internet. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00006454-200511000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Lee MN, Cha JH, Ahn HM, Yoo JH, Kim HS, Sohn S, et al. Mycoplasma pneumoniae infection in patients with Kawasaki disease. Korean J Pediatr. 2011;54(3):123–7. doi: 10.3345/kjp.2011.54.3.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Usta Guc B, Cengiz N, Yildirim SV, Uslu Y. Cytomegalovirus infection in a patient with atypical Kawasaki disease. Rheumatol Int. 2008;28(4):387–9. doi: 10.1007/s00296-007-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang LY, Lu CY, Shao PL, Lee PI, Lin MT, Fan TY, et al. Viral infections associated with Kawasaki disease. J Formos Med Assoc. 2014;113(3):148–54. doi: 10.1016/j.jfma.2013.12.008. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24495555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bajolle F, Meritet JF, Rozenberg F, Chalumeau M, Bonnet D, Gendrel D, et al. Markers of a recent bocavirus infection in children with Kawasaki disease: “A year prospective study”. Pathol Biol. 2014;62(6):365–8. doi: 10.1016/j.patbio.2014.06.002. Internet. Available from: http://dx.doi.org/10.1016/j.patbio.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40(1):35–42. doi: 10.1038/ng.2007.59. Internet. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2876982&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Wang W, Gong F, Fu S, Zhang Q, Hu J. Evaluation of Intravenous Immunoglobulin Resistance and Coronary Artery Lesions In Relation to Th1 / Th2 Cytokine Profiles in Patients With Kawasaki Disease. Arthritis Rhuem. 2013;65(3):805–14. doi: 10.1002/art.37815. [DOI] [PubMed] [Google Scholar]
- 47.Jia S, Li C, Wang G, Yang J, Zu Y. The T helper type 17/regulatory T cell imbalance in patients with acute Kawasaki disease. Clin Exp Immunol. 2010;162(1):131–7. doi: 10.1111/j.1365-2249.2010.04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni CR, Li Q, Xia Y, Wang GB, Yang J, FF L. Regulatory T cell microRNA expression changes in children with acute Kawasaki disease. Clin Exp Immunol. 2014;178:384–93. doi: 10.1111/cei.12418. Internet. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medc&NEWS=N&AN=25039241 http://onlinelibrary.wiley.com/doi/10.1111/cei.12418/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo HC, Yang KD, Juo SHH, Liang CDi, Chen WC, Wang YS, et al. Itpkc single nucleotide polymorphism associated with the kawasaki disease in a taiwanese population. PLoS One. 2011;6(4):2–7. doi: 10.1371/journal.pone.0017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Ge X, Li CR, Yang J, Wang GB. Aberrantly Decreased Levels of NKG2D Expression in Children with Kawasaki Disease. Scand J Immunol. 2013;77(5):389–97. doi: 10.1111/sji.12022. This article provides evidence to the role of NKG2D in causation of Kawasaki disease and the associated coronary artery lesions. [DOI] [PubMed] [Google Scholar]
- 51.Lee Y, Wakita D, Dagvadorj J, Shimada K, Chen S, Huang G, et al. IL-1 Signaling Is Critically Required in Stromal Cells in Kawasaki Disease Vasculitis Mouse Model: Role of Both IL-1α and IL-1β. Arterioscler Thromb Vasc Biol. 2015;35(12):2605–16. doi: 10.1161/ATVBAHA.115.306475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tremoulet AH, Jain S, Kim S, Newburger J, Arditi M, Franco A, Best B, Burns JC. Rationale and study design for a phase I/IIa trial of anakinra in children with Kawasaki disease and early coronary artery abnormalities (the ANAKID trial) Contemp Clin Trials. 2016;48:70–75. doi: 10.1016/j.cct.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song R, Liu G, Li X, Xu W, Liu J, Jin H. Elevated Inducible Nitric Oxide Levels and Decreased Hydrogen Sulfide Levels Can Predict the Risk of Coronary Artery Ectasia in Kawasaki Disease. Pediatr Cardiol. 2015;37(8):322–9. doi: 10.1007/s00246-015-1280-8. Internet. Available from: http://dx.doi.org/10.1007/s00246-015-1280-8. [DOI] [PubMed] [Google Scholar]
- 54.Xiao-hui LI, Chao-ying Z, Jian-xin WU, Ting Z, General M, et al. Committee E. Changes in plasma hydrogen sulfide and nitric oxide levels and. Chinese Med J. 2011;124(21):3445–9. [PubMed] [Google Scholar]
- 55.Wang CL, Wu YT, Lee CJ, Liu HC, Huang LT, Yang KD. Decreased nitric oxide production after intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2002;141(4):560–5. doi: 10.1067/mpd.2002.127505. Internet. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12378198. [DOI] [PubMed] [Google Scholar]
- 56.Hayward K, Wallace Ca, Koepsell T. Perinatal exposures and Kawasaki disease in Washington State: a population-based, case-control study. Pediatr Infect Dis J. 2012;31(10):1027–31. doi: 10.1097/INF.0b013e31825eaed0. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22653485. [DOI] [PubMed] [Google Scholar]
- 57*.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis amd Kawasaki disease, Council on Cardiovascular Disease in the Young,American Heart Association. 2004;110(17):2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. It has provided the nationally accepted guidelines for the diagnosis and management of patients with Kawasaki disease. [DOI] [PubMed] [Google Scholar]
- 58.Kuo HC, Hsu YW, Wu MS, Chien SC, Liu SF, Chang WC. J Microbiol Immunol Infect. 1. Vol. 49. Elsevier Taiwan LLC; 2016. Intravenous immunoglobulin, pharmacogenomics, and Kawasaki disease; pp. 1–7. Internet. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1684118214002278. [DOI] [PubMed] [Google Scholar]
- 59.Bayers S, Shulman ST, Paller AS. J Am Acad Dermatol. 4. Vol. 69. Elsevier Inc; 2013. Kawasaki disease: Part II. Complications and treatment; pp. 513.e1–513.e8. Internet. Available from: http://dx.doi.org/10.1016/j.jaad.2013.06.040. [DOI] [PubMed] [Google Scholar]
- 60.Hsieh K, Weng K, Lin C, Huang T, Lee C, Huang S. Treatment of Acute Kawasaki Disease : Aspirin ' s Role in the Febrile stage revisited. Pediatrics. 2004;114(6):e689–693. doi: 10.1542/peds.2004-1037. [DOI] [PubMed] [Google Scholar]
- 61.Shulman ST. Management of Kawasaki disease. J Pediatr. 1988;113(6):1116–7. doi: 10.1016/s0022-3476(88)80596-1. [DOI] [PubMed] [Google Scholar]
- 62.Muta H, Ishii M, Yashiro M, Uehara R, Nakamura Y. Late Intravenous Immunoglobulin Treatment in Patients With Kawasaki Disease. Pediatrics. 2012;129(2):e291–7. doi: 10.1542/peds.2011-1704. [DOI] [PubMed] [Google Scholar]
- 63.Rowley AH. The Complexities of the Diagnosis and Management of Kawasaki Disease. Infect Dis Clin North Am. 2015;29(3):525–37. doi: 10.1016/j.idc.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujimaru T, Ito S, Masuda H, Oana S, Kamei K, Ishiguro A, et al. Cytokine. 2. Vol. 70. Elsevier Ltd; 2014. Decreased levels of inflammatory cytokines in immunoglobulin-resistant Kawasaki disease after plasma exchange; pp. 156–60. Internet. Available from: http://dx.doi.org/10.1016/j.cyto.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Rahbarimanesh A, Taghavi-goodarzi M. Comparison of High-Dose versus Low-Dose Aspirin in the Management of Kawasaki Disease. Indian J Pediatr. 2014;81(December):12098. doi: 10.1007/s12098-014-1437-0. [DOI] [PubMed] [Google Scholar]
- 66.Portman MA, Olson A, Soriano B, Dahdah N, Williams R, Kirkpatrick E. Etanercept as adjunctive treatment for acute kawasaki disease: Study design and rationale. Am Heart J. 2011;161(3):494–9. doi: 10.1016/j.ahj.2010.12.003. Internet. Available from: http://dx.doi.org/10.1016/j.ahj.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Kato H, Koike S, Yokoyama T. Kawasaki disease: effect of treatment on coronary artery involvement. Pediatrics. 1979;63(2):175–9. [PubMed] [Google Scholar]
- 68.Adachi S, Sakaguchi H, Kuwahara T, Uchida Y, Fukao T, Kondo N. High regression rate of coronary anuerysms developed in patients with immune globulin resistant Kawasaki disease treated with steroid pulse therapy. Tohoku J Exp Med. 2010;220(4):285–90. doi: 10.1620/tjem.220.285. [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, Kato T, Hara T, Hamaoka K, et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open label, blinded-endpoints trial. Lancet. 2012;379(9826):1613–20. doi: 10.1016/S0140-6736(11)61930-2. [DOI] [PubMed] [Google Scholar]
- 70.Saneeymehri S, Baker K, So TY. Overview of Pharmacological Treatment Options for Pediatric Patients with Refractory Kwasaki Disease. J Pediatr Pharmacol Ther. 2015;20(3):163–77. doi: 10.5863/1551-6776-20.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogata S, Ogihara Y, Honda T, Kon S, Akiyama K, Ishii M. Corticosteroid Pulse Combination Therapy for Refractory Kawasaki Disease: A Randomized Trial. Pediatrics. 2012;129(1):e17–23. doi: 10.1542/peds.2011-0148. [DOI] [PubMed] [Google Scholar]
- 72.Chen S, Dong Y, Yin Y, Krucoff MW. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: a meta-analysis. Heart. 2012:76–82. doi: 10.1136/heartjnl-2012-302126. [DOI] [PubMed] [Google Scholar]
- 73.Weng KP, Ou SF, Lin CC, Hsieh KS. Recent advances in the treatment of Kawasaki disease. J Chin Med Assoc. 2011;74(11):481–4. doi: 10.1016/j.jcma.2011.09.001. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22100015. [DOI] [PubMed] [Google Scholar]
- 74.Miura M, Ohki H, Yoshiba S, Ueda H, Sugaya A, Satoh M, Yamagishi H. Adverse effects of methylprednisolone pulse therapy in refractory Kawasaki disease. Arch Dis Child. 2005;90(10):1096–7. doi: 10.1136/adc.2004.062299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu JJ. Use of corticosteroids during acute phase of Kawasaki disease. World J Clin Pediatr. 2015;4(4):135. doi: 10.5409/wjcp.v4.i4.135. Internet. Available from: http://www.wjgnet.com/2219-2808/full/v4/i4/135.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lim YJ, Jung JW. Clinical Outcomes of Initial Dexamethasone Treatment Combined with a Single High Dose of Intravenous Immunoglobulin for Primary Treatment of Kawasaki Disease. 2014;55(5):1260–6. doi: 10.3349/ymj.2014.55.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mori M, Imagawa T, Hara R, Kikuchi M, Hara T, Nozawa T, et al. Efficacy and limitation of infliximab treatment for children with Kawasaki disease intractable to intravenous immunoglobulin therapy: Report of an open-label case series. J Rheumatol. 2012;39(4):864–7. doi: 10.3899/jrheum.110877. [DOI] [PubMed] [Google Scholar]
- 78.Tremoulet AH, Jain S, Jaggi P, Jimenez-Fernandez S, Pancheri JM, Sun X, et al. Lancet. 9930. Vol. 383. Elsevier Ltd; 2014. Infliximab for intensification of primary therapy for Kawasaki disease: A phase 3 randomised, double-blind, placebo-controlled trial; pp. 1731–8. Internet. Available from: http://dx.doi.org/10.1016/S0140-6736(13)62298-9. [DOI] [PubMed] [Google Scholar]
- 79.Williams RV, Wilke VM, Tani LY, Minich LL. Objective A, Kawasaki A. Does Abciximab Enhance Regression of Coronary Aneurysms Resulting From Kawasaki Disease? 2002;109(1) doi: 10.1542/peds.109.1.e4. [DOI] [PubMed] [Google Scholar]
- 80.Choueiter NF, Olson AK, Shen DD, Portman MA. A prospective open label trial of etanercept as adjunctive Therapy for Kawasaki Disease. J Pediatr. 2010;157(6):960–966. doi: 10.1016/j.jpeds.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mori M, Imagawa T, Katakura S, Miyamae T, Okuyama K, Ito S, Nakamura T, Kimura H, Yokota S. Efficacy of plasma exhange therapy for Kawasaki disease intractable to intravenous gamma-globulin. Mod Rheumatol. 2004;14(1):43–7. doi: 10.1007/s10165-003-0264-3. [DOI] [PubMed] [Google Scholar]
- 82.Matsui M, Okuma Y, Yamanaka J, Uryu H, Sato N, Shichino H, et al. Cytokine. 2. Vol. 74. Elsevier Ltd; 2015. Kawasaki disease refractory to standard treatments that responds to a combination of pulsed methylprednisolone and plasma exchange: Cytokine profiling and literature review; pp. 339–42. Internet. Available from: http://dx.doi.org/10.1016/j.cyto.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 83.Sonoda K, Mori M, Hokosaki T, Yokota S. J Pediatr. 5. Vol. 164. Elsevier Ltd; 2014. Infliximab plus plasma exchange rescue therapy in Kawasaki disease; pp. 1128–1132.e1. Internet. Available from: http://dx.doi.org/10.1016/j.jpeds.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 84.Tremoulet AH, Pancoast P, Franco A, Bujold M, Shimizu C, Onouchi Y, et al. Calcineurin inhibitor treatment of intravenous immunoglubulin resistant Kawasaki disease. J Pediatr. 2012;161(3):506–12. doi: 10.1016/j.jpeds.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuijpers TW, Biezeveld M, Achterhuis A, Kuipers I, Lam J, Hack CE, et al. Longstanding obliterative panarteritis in Kawasaki disease: lack of cyclosporin A effect. Pediatrics. 2003;112(4):986–92. doi: 10.1542/peds.112.4.986. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki h, Terai M, Hamada H, Honda T, Suenaga T, Takeuchi T, Yoshikawa N, Shibuta S, et al. Cyclosporin A treatment for kawasaki disease refractory to initial and additional intravenous immunoglobulin. Pediatr Infect Dis J. 2011;30(10):871–6. doi: 10.1097/INF.0b013e318220c3cf. [DOI] [PubMed] [Google Scholar]
- 87.Aoyagi R, Hamada H, Sato Y, Suzuki H, Onouchi Y, Ebata R, et al. Study protocol for a phase III multicentre, randomised, open-label, blinded-end point trial to evaluate the efficacy and safety of immunoglobulin plus cyclosporin A in patients with severe Kawasaki disease (KAICA Trial) BMJ Open. 2015;5(12):e009562. doi: 10.1136/bmjopen-2015-009562. Internet. Available from: http://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2015-009562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodrigues-Diez R, González-Guerrero C, Ocaña-Salceda C, Rodrigues-Diez RR, Egido J, Ortiz A, et al. Sci Rep. May. Vol. 6. Nature Publishing Group; 2016. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling; p. 27915. Internet. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27295076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al-homsi AS, Roy TS, Cole K, Feng Y, Duffner U. Biol Blood Marrow Transplant. 4. Vol. 21. Elsevier Inc; 2015. Biology of Blood and Marrow Transplantation Post-Transplant High-Dose Cyclophosphamide for the Prevention of Graft-versus-Host Disease; pp. 604–11. Internet. Available from: http://dx.doi.org/10.1016/j.bbmt.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 90.Falcini F. Kawasaki disease. Curr Opin Rheumatol. 2006;18(1):33–8. doi: 10.1097/01.bor.0000197998.50450.f6. [DOI] [PubMed] [Google Scholar]
- 91.Wong PT, Choi SK. Mechanisms and Implications of Dual-Acting Methotrexate in Folate-Targeted Nanotherapeutic Delivery. Int J Mol Sci. 2015;16(1):1772–90. doi: 10.3390/ijms16011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee MS, An SY, Jang GC, Kim DS. A case of intravenous immunoglobulin resistant Kawasaki disease treated with methotrexate. Yonsei Med J. 2002;43(4):527–532. doi: 10.3349/ymj.2002.43.4.527. [DOI] [PubMed] [Google Scholar]
- 93.Lee TJ, Kim KH, Chun JK, Kim DS. Low-dose methotrexate therapy for intravenous immunoglobulin-resistant Kawasaki disease. Yonsei Med J. 2008;49(5):714–8. doi: 10.3349/ymj.2008.49.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim NY, Choi DY, Jung MJ, Jeon IS. A case of refractory Kawasaki disease complicated by peripheral ischemia. Pediatr Cardiol. 2008;29(6):1110–4. doi: 10.1007/s00246-008-9221-4. [DOI] [PubMed] [Google Scholar]
- 95.de Magalhães CMR, Alves NR, de Melo AV, Silveira Junior CA, Nobrega YK, Gandolfi L, et al. Catastrophic Kawasaki disease unresponsive to IVIG in a 3-month-old infant: a diagnostic and therapeutic challenge. Pediatr Rheumatol Online J. 2012;10:28. doi: 10.1186/1546-0096-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]


